Introduction
Polycythemia vera (PV) is a relatively indolent
myeloid-proliferative neoplasm (MPN) that is characterized by the
clonal proliferation of red blood cells, bone marrow and
megakaryocytes (1,2). In addition, PV has several unique
molecular characteristics, such as mutations in the JAK2V617F gene
or exon 12 (1,2). However, although patients with PV can
have prolonged survival with general treatment (e.g., bleeding
therapy) and medication (e.g., hydroxyurea), PV is generally
incurable (1,2). The clinical manifestations of PV also
lack specificity, typically presenting with visible skin redness,
particularly on the face, neck and extremities (3). Neurological symptoms can include
headache, dizziness and tinnitus (4). In addition, peptic ulcers (5) and water pruritus are common in some
patients because of increased basophil and histamine release
(6). Common complications of PV
can include recurrent ischemic cerebrovascular lesions (7), aquagenic pruritus (6), hypertension (8), pulmonary hypertension (9) and coronary heart disease (8,10).
Since a universally-recognized set of unique clinical
manifestations from PV are not available, missed diagnosis and
misdiagnosis often occur. For example, a previous case report
mentioned that the hypochondromic mass was misdiagnosed as
hepatosplenomegaly caused by PV because the patient had concomitant
polycythemia vera (11). That may
delay the best opportunity and time for treatment.
Patients with PV have an increased risk of venous
and arterial thrombotic events, with an incidence of 0.4-2.8 cases
per 100,000 individuals per year and with a lifetime prevalence of
20-30% in an international study (12,13).
Portal vein thrombosis (PVT) in patients with PV is a common risk
factor for non-cirrhotic portal vein thrombosis (14). PV with PVT may result in cavernous
transformation of the liver, which may lead to collateral
circulation formation, ascites, portal hypertension, biliary
disease, esophagogastric varices and gastrointestinal bleeding
(15-17).
Portal hypertension is an abnormal hemodynamic
syndrome that causes ascites, variceal hemorrhage, hepatic
encephalopathy and other serious complications, the most common of
which is cirrhosis (18). Portal
hypertension can be classified as cirrhotic or non-cirrhotic, where
the latter, non-cirrhotic portal hypertension (NCPH), is the second
leading cause of portal hypertension (19) and can be caused by PVT (20,21).
PVT is a hepatic vascular disease that refers to thrombosis of the
portal vein trunk and/or left and right branches of the portal vein
of whichever cause, with or without thrombosis of the superior
mesenteric vein, inferior mesenteric vein and splenic vein
(12). When PVT occurs, the liver
compensates by increasing hepatic artery blood flow to maintain
normal liver function (22). NCPH
is clinically characterized by features of portal hypertension,
moderate to massive splenomegaly, with or without hypersplenism,
but preserved liver functions (15). Liver pathology of NCPH is
characterized by phlebosclerosis, periportal and perisinusoidal
fibrosis, fibroelastosis, hepatic lobules that are structurally
intact and differential atrophy (15). According to the location of blood
flow resistance, portal hypertension can be classified into
prehepatic, intrahepatic and posthepatic (20,21).
The common pathogenesis of NCPH includes PV (23). PV combined with portal hypertension
is classified as prehepatic NCPH (21). At present, to the best of our
knowledge, there are few studies on patients with PV or NCPH.
Therefore, present study aimed to analyze the clinical data of
patients with PV and NCPH, to enhance the awareness of this
disease.
Patients and methods
Patients
The study was approved by Ethics Committee of
Beijing You'an Hospital Affiliated to Capital Medical University
(Beijing, China) and written informed consent was obtained from all
patients. The age and sex distribution can be seen in Table I. Information of patients with PV
and NCPH was obtained from the electronic medical record system.
Patients with seropositive hepatitis B and C viruses were excluded,
as were patients with a history of alcohol or drug-induced liver
injury, patients who were positive for autoimmune liver
disease-related indicators, and patients with inherited metabolic
liver disease. Specific inclusion and exclusion criteria are
further described subsequently. This was a retrospective study of
cases. The diagnosis of polycythemia vera was a clear diagnosis of
the patient in other hospitals (Beijing Friendship Hospital
Affiliated to Capital Medical University, Beijing, China; Peking
University People's Hospital, Beijing, China). Their diagnoses were
recorded in the electronic medical records system but it was not
clear whether pathological tests were carried out. Patients who
were diagnosed with PV and NCPH and treated in Beijing You'an
Hospital, Capital Medical University (Beijing, China) from January
2010 to March 2022 were included into the present study. The
patient's hospitalization information was collected from the
hospital's medical record database, and the patient's examination
data, including laboratory blood test and imaging data
(hematological examination, the nurse extracted the fasting venous
blood of the patient in the morning on the day after admission and
sent it to the biochemical laboratory; imaging examination, during
the hospitalization of the patient, the imaging physician used a
Siemens AG 64 CT scanner to conduct CT scanning of the abdomen;
electronic gastroscopy, an experienced gastroenterologist completed
the electronic gastroscopy for the patient.), were collected from
the electronic medical record system of Beijing You'an Hospital,
Capital Medical University. All samples were processed at the
Clinical Laboratory Center of Beijing You'an Hospital, Capital
Medical University (Beijing, China). All patients included had
complete biochemical, routine blood and coagulation results. Upper
limb venous blood samples were tested using an automatic
biochemical analyzer (AU400; Olympus Corporation), automatic blood
cell analyzer (BC-5390CRP; Shenzhen Mindray Biomedical Electronics
Co., Ltd.) and an automatic coagulation analyzer (Precil C3510;
Shenzhen Mindray Biomedical Electronics Co., Ltd.). Enhanced
abdominal CT examination was performed, and CT images and reports
were collected from the Imaging Center of Beijing You'an Hospital.
Gastroscopy images were collected from the Endoscopy Center of
Beijing You'an Hospital, but no endoscopic histological samples
were collected. All aforementioned examinations and examination
information were recorded in the medical record system, and the
results were collected from this and sorted in the present study. A
total of 5 patients with PV and portal hypertension were included,
of whom 3 were male and 2 were females. The mean age was
63.60±18.33 years. Table I
summarizes the general characteristics of the 5 patients.
 | Table IGeneral data of patients. |
Table I
General data of patients.
| Case no. | Age/Sex | Chief complaint | Medical history | Diagnosis time of
PV | Treatment of PV | Diagnosis time of
portal hypertension |
|---|
| 1 | 83/M | Abdominal
discomfort | Coronary heart
disease, atrial fibrillation, chronic bronchitis. Deny family
history of hypertension, diabetes and liver disease | 1 week | Hydroxyurea | 1 day |
| 2 | 49/F | Abdominal pain | Deny history of
chronic liver disease. No history of long-term medication. No
history of long- term alcohol consumption | During this hospital
stay | Hydroxyurea | During this hospital
stay |
| 3 | 79/M | Swelling of the legs
and feet | Deny history of
hypertension, diabetes and coronary heart disease | 10 years | Bloodletting,
hydroxyurea, Peg-Interferon | 2 years |
| 4 | 66/M | Fatigue and yellowing
of skin and eyes | Cerebral infarction,
hypertension, coronary heart disease, myocardial infarction,
hyperlipidemia, skin ulceration of bilateral lateral malleolus | 19 years | Hydroxyurea,
Peg-Interferon | 4 days |
| 5 | 41/F | Vomiting blood and
black stool | Deny history of
hypertension, diabetes and coronary heart disease. Deny family
history of liver disease | 3 years | Peg-Interferon | 2 months |
Inclusion criteria
Inclusion criteria were set in line with the
diagnostic criteria of PV (2):
Major criteria: i) Hemoglobin >16.5 g/dl in men and hemoglobin
>16.0 g/dl in women; ii) bone marrow biopsy showing
hypercellularity for age with trilineage growth (panmyelosis),
including prominent erythroid, granulocytic and megakaryocytic
proliferation with pleomorphic, mature megakaryocytes (differences
in size); and iii) presence of JAK2V617F or JAK2 exon 12 mutation.
Minor criterion: Subnormal serum erythropoietin level. (Diagnosis
of PV requires meeting either all three major criteria, or the
first two major criteria and the minor criterion). They were also
in line with the diagnostic criteria of NCPH (21), as follows: The presence of
esophageal and gastric varices or imaging manifestations (CT) of
portal hypertension, such as splenomegaly, portal vein widening
and/or collateral circulation.
Exclusion criteria
Serologically positive for hepatitis virus B or C;
with history of alcoholic or drug-induced liver injury; indices
associated with autoimmune liver disease were positive (such as
anti-nuclear antibody, anti-mitochondrial antibody,
immunoglobulin-G4, anti-Ro-52 antibody, scleroderma 70 antigen,
double-stranded-DNA, anti-Jo-1 antibody and anti-nucleosome
antibody); and with genetic metabolic liver disease (if the
patient's medical record information in the medical record system
indicated that genetic testing (ATPase copper transporting β gene
mutation/homeostatic iron regulator gene mutation) had revealed
hereditary liver disease that could lead to portal hypertension,
such as hepatolenticular degeneration, hemochromatosis, etc., the
patient was excluded).
Data analysis
This was a retrospective study. Patient clinical
data, such as sex, age, major complaints, medical history,
laboratory examination, and imaging findings of the abdomen and
gastroscopy, were extracted from the hospital case system. Data
were processed using the SPSS 26.0 software (IBM Corp.). The mean
values of data conforming to a normal distribution are expressed as
mean ± standard deviation. The mean values of data that did not
conform to normal distribution were expressed as median (quartile
1, quartile 3).
Results
General data of patients
NCPH is relatively rare in the clinic (15), the patients included in the present
study were serologically hepatitis virus B- and C-negative and had
no history of alcoholic or drug-induced liver injury. In addition,
their indices associated with autoimmune liver disease were
negative and they had no genetic metabolic liver disease. The
course since definite diagnosis of PV was longer than that of NCPH
in cases 1, 3, 4 and 5; the duration of PV was 1 week, 10 years, 19
years and 3 years, respectively, while that of NCPH was 1 day, 2
years, 4 years and 2 months. The diagnosis of PV and NCPH of case 2
was simultaneous. PV treatment mainly entails the use of
hydroxyurea and interferons (1).
Overall, case nos. 1, 2, 3 and 4 received symptomatic treatment
with hydroxyurea, whilst cases nos. 3 and 4 were treated with
Peg-interferon in the later stages of the disease. By contrast,
case no. 5 was treated with interferon. In the present study, case
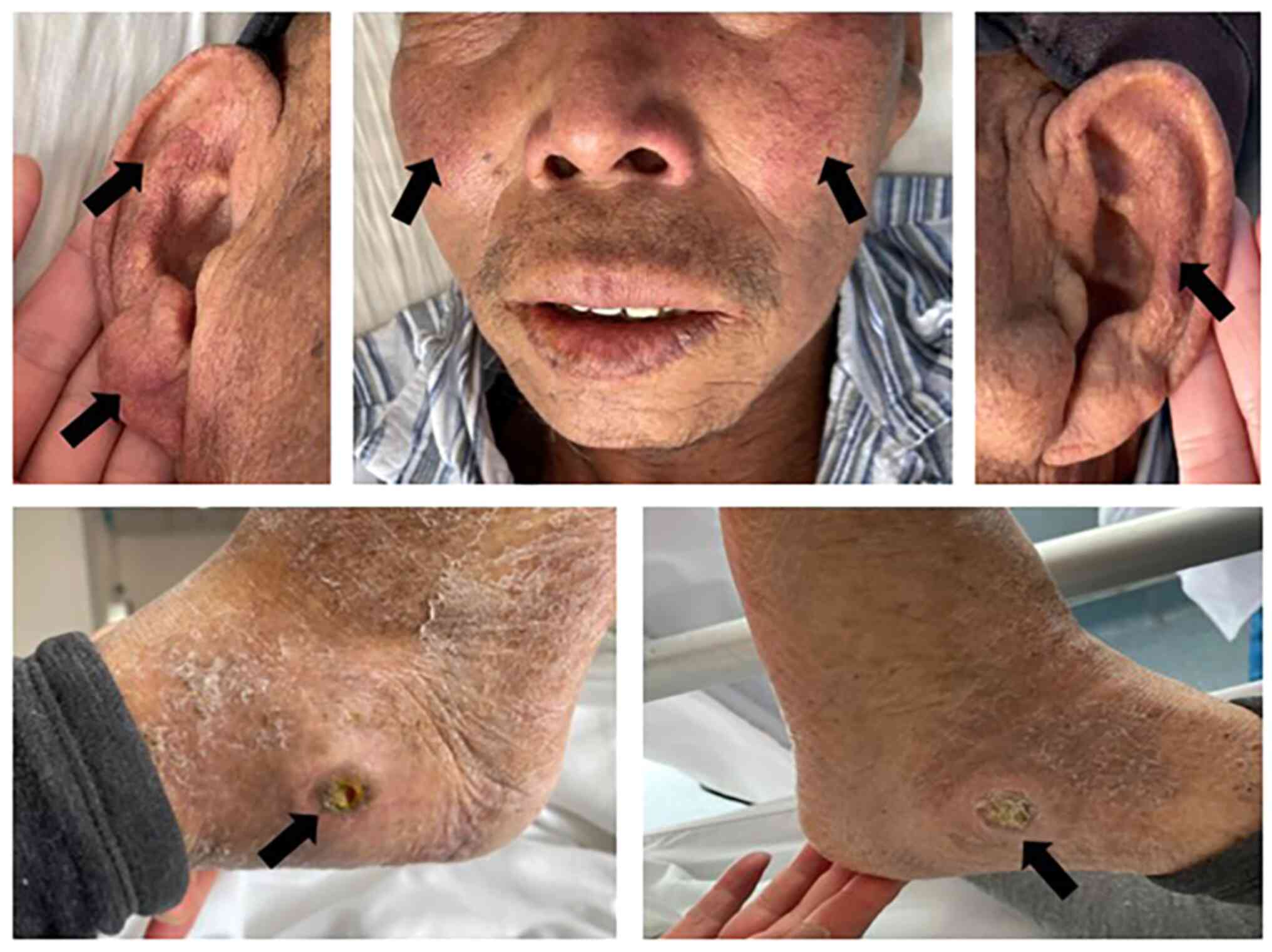
no. 4 showed obvious erythema on the cheek and auricle, where a
history of bilateral lateral ankle joint ulcers was considered to
be associated with the long-term use of hydroxyurea (Fig. 1).
Laboratory tests data of patients
Routine blood examination of four patients (case
nos. 1, 2, 3 and 4) showed an elevation of 2-3 parameters (red
blood cells, white blood cells or platelets). Alanine
aminotransferase (ALT) and aspartate aminotransferase (AST) levels
were normal in all patients except for case 4, whose ALT and AST
levels were elevated. Total bilirubin (TBIL) was elevated in four
patients (case nos. 1, 2, 3 and 4). Albumin (ALB) protein levels
were decreased in four patients (case nos. 1, 3, 4 and 5). Cases 3
and 4 had elevated glutamyl transpeptidase (GGT) and alkaline
phosphatase (ALP) levels and cholestasis. Coagulation function
showed 4 patients (case nos. 1, 2, 3 and 5) had decreased
prothrombin activity (PTA), whilst 3 patients (case nos. 1, 2 and
3) had an elevated international normalized ratio. The Child-Pugh
grade of liver function (24) was
found to be A or B in all five patients (Table II). Case 1 died 9 days after
admission. Cases 2, 3, 4 and 5 were followed up by telephone in
December 2022. Cases 2, 3 and 5 survived, and case 4 died in June
2022.
 | Table IILaboratory test data of each
patient. |
Table II
Laboratory test data of each
patient.
| | Case no. |
|---|
| Laboratory
indices | Reference
range | 1 | 2 | 3 | 4 | 5 |
|---|
| Blood routine
examination | | | | | | |
|
Red blood
cell (x1012/l) | 4.3-5.8 | 7.76 | 6.58 | 4.8 | 5.36 | 3.27 |
|
White blood
cell (x109/l) | 3.5-9.5 | 11.49 | 8.88 | 43.03 | 28.96 | 2.35 |
|
Neutrophils
(%) | 40-75 | 74.8 | 76.2 | 93.2 | 91.4 | 55 |
|
Hemoglobin
(g/l) | 130-175 | 174 | 198 | 127 | 139 | 73 |
|
Platelet
(x109/l) | 100-300 | 455 | 699 | 480 | 782 | 91 |
| Liver function | | | | | | |
|
Alanine
aminotransferase (U/l) | 9-50 | 19 | 9 | 15 | 56 | 10 |
|
Aspartate
aminotransferase (U/l) | 15-40 | 32 | 25 | 23 | 47 | 12 |
|
Total
bilirubin (µmol/l) | 5-21 | 46.9 | 30.8 | 21.2 | 90.5 | 20 |
|
Direct
bilirubin (µmol/l) | <7 | 12 | 7.9 | 9.8 | 65.3 | 4.3 |
|
Albumin
(g/l) | 40-55 | 31.5 | 41.4 | 33.1 | 35.7 | 36 |
|
Glutamyl
transpeptidase (U/l) | 10-60 | 32 | 44 | 75 | 584 | 15 |
|
Alkaline
phosphatase (U/l) | 45-125 | 103 | 52 | 191 | 718 | 45 |
| Coagulation
function | | | | | | |
|
Prothrombin
time (sec) | 9.9-12.8 | 15 | 15 | 16 | 10.7 | 13.9 |
|
Prothrombin
activity (%) | 80-120 | 72.1 | 63 | 60 | 107 | 78.8 |
|
International
normalized ratio | 0.8-1.2 | 1.24 | 1.33 | 1.43 | 0.96 | 1.15 |
|
Activated
partial thromboplastin time (sec) | 25-36.5 | 46.7 | 57.1 | 49.2 | 33.4 | 51.2 |
|
Fibrinogen
(g/l) | 2-4 | 1.47 | 2.69 | 2 | 3.85 | 1.41 |
|
D-Dimer
(µg/l) | <230 | 189 | 162.2 | 557 | 472 | / |
|
Child
grade | | B | A | B | B | A |
Imaging and gastroscopy data of
patients
Gastroscopy revealed moderate and severe esophageal
varices in case nos. 1, 3, 4 and 5. Imaging examination (CT)
revealed all patients had portal hypertension, of which case nos.
2, 4 and 5 had at least a venous emboli formation, whereas case
nos. 2, 3 and 4 had hepatic perfusion abnormalities. All five
patients had splenomegaly, where case nos. 2 and 3 found with
associated spleen infarction. In addition, case nos. 1, 2, 4 and 5
had associated collateral circulation formation. Case nos. 1, 3, 4
and 5 had varying degrees of ascites (Table III).
 | Table IIIImaging and gastroscopy. |
Table III
Imaging and gastroscopy.
| Case no. | Venous embolus | Abnormality of
hepatic perfusion | Splenomegaly | Infarction of
spleen | Ascites | Collateral
circulation | Varicose veins | Other gastroscopic
findings |
|---|
| 1 | Portal and splenic
veins widening | No | Yes | No | Yes | Yes | Esophageal varices
(G3, severe, red-color sign positive) | Portal hypertensive
gastropathy |
| 2 | Emboli of portal
and superior mesenteric vein were extensively formed. Portal
cavernous transformation | Yes | Yes | Yes | No | Yes | Chronic superficial
gastritis | - |
| 3 | Portal vein
widening | Yes | Yes | Yes | Yes | No | Esophageal varices
(G3, severe, red-color sign positive) | Portal hypertensive
gastropathy. Gastric polyp |
| 4 | Emboli of portal
vein, superior mesenteric vein, splenic vein. Portal cavernous
transformation | Yes | Yes | No | Yes | Yes | Esophageal varices
(G2, moderate). Gastric varices | Reflux esophagitis.
Portal hypertensive gastropathy. Duodenal ulcer (Stage A1) |
| 5 | Emboli of portal
vein. Portal cavernous transformation | No | Yes | No | Yes | Yes | Esophageal varices
(G3, severe, red-color sign positive) | - |
Discussion
PV is an MPN, with a high risk of arteriovenous
thrombosis (2,12,13).
PVT is a common cause of NCPH, which can lead to the clinical
manifestations of portal hypertension, such as collateral
circulation, ascites, esophageal and gastric varices and
gastrointestinal bleeding (15,23).
PVT and a high coagulation state are common in patients with PV
(12). Therefore, the present
study hypothesized that PV can lead to NCPH.
In the present study, cases no. 3 and 4 exhibited
increased D-Dimer levels, whereas three cases (nos. 2, 4 and 5)
showed PVT accompanied by portal vein sponge appearance change
(Table III). Table III specifically records the
imaging findings of each patient. The venous embolus column shows
the diagnosis of portal cavernous transformation. Cases no. 2 and 4
had mesenteric and splenic vein thrombosis, which are risk factors
for portal hypertension. All five cases had splenomegaly, two of
whom (case nos. 2 and 3) had splenic infarction. Chronic
compression of bile ducts by collateral circulation (or ischemia
caused by venule thrombosis) may lead to portal hypertension
cholelithiasis (25,26).
Imaging reports of case no. 4 showed portal
hypertension cholelithiasis with obvious elevations in ALP and GGT
levels. Although the clinical symptoms were not obvious, abnormal
liver function may have been caused by bile duct blockage in case
no. 4. In the present study, the hematological manifestations of
PV-induced NCPH included increased erythrocytes, white blood cells,
hemoglobin and platelets in some of the patients. However,
different patients behaved differently so these were not observed
in all cases. These observations are different from the decreases
in white blood cells and platelets due to hypersplenism in portal
hypertension caused by cirrhosis (27). Among the patients included in the
present study, only case no. 4 showed an increase in transaminases
(ALT, AST, GGT and ALP). In addition, 4 patients (nos. 1, 3, 4 and
5) had slightly reduced ALB levels and 4 patients (nos. 1, 2, 3 and
4) had elevated bilirubin levels. The liver function index Child
grade was A or B, indicating that the liver function damage in
patients with portal hypertension caused by PV was relatively
mild.
The gastroscopy and imaging examinations in the
present study revealed manifestations of esophageal and gastric
varices or portal hypertension, such as splenomegaly, portal vein
widening and/or collateral circulation. The imaging and gastroscopy
examinations provided a reliable basis for the initial diagnosis of
portal hypertension in the patients, especially when the laboratory
examination results were not consistent with clinical experience.
As aforementioned, hyperplenism causes platelets and white blood
cells (27). However, in the
present study, the hematological manifestations of PV-induced NCPH
included increased erythrocytes, white blood cells, hemoglobin and
platelets.
Overall, cases nos. 1, 3, 4 and 5 developed ascites,
whereas cases nos. 1, 2, 4 and 5 showed collateral circulation, all
of which were considered to be associated with PV thrombosis and
disease progression. A previous study has reported that even
without varicose veins at baseline, the probability of developing
varicose veins in patients with non-cirrhotic and non-neoplastic
portal thrombosis is 2% after 1 year and 22% after 5 years
(28). Once portal hypertension
occurs, blood flows to the hepatic sinuses through the lateral
branches of the portal vein or out of the liver through the lateral
branches of the portal vein system, resulting in bleeding from the
gastroesophageal, ectopic or rectal varices (29). Patients with moderate or severe
esophageal varices that are red color sign-positive (RC-positive)
are at higher risk of bleeding (30). In the present study, the esophageal
veins of cases 1, 3 and 5 were severely varicose and RC-positive.
It is recommended that after PV diagnosis,
esophagogastroduodenoscopy (EGD) should be performed relatively
early and regularly. Patients at risk of gastrointestinal bleeding
should be treated prophylactically. Early prevention of primary
esophageal variceal bleeding is recommended (30). However, there is no consensus on
whether active antithrombotic therapy in patients with PV can delay
or prevent venous thrombosis and various downstream complications,
such as visceral infarction, ascites and variceal hemorrhage.
Inpatients diagnosed with PV in the case system of
Beijing You'an Hospital from January 2010 to March 2022 were
included in the present study. The clinical data for observation
were the data of the patients at their first admission. In cases
nos. 1, 3, 4 and 5 of the present study, the diagnosis of PV was
earlier compared with that of NCPH, whereas in case no. 2 PV and
NCPH were found simultaneously. Although the time and course of
diagnosis of PV were different for the five patients, imaging
examinations all patients indicated portal hypertension. Inpatient
medical records showed that the symptoms of case nos. 2, 3, 4 and 5
were improved and discharged after acid suppression, gastric mucosa
protection, upper gastrointestinal bleeding prevention and liver
lowering enzyme treatment.
Due to their old age, case 1 was admitted to the
hospital with abdominal and chest infections with numerous
underlying diseases (coronary heart disease, atrial fibrillation
and chronic bronchitis). After anti-infection and symptomatic
treatment failed, the patient died clinically on the 9th day after
admission. Case 4 was discharged from hospital after symptom
improvement; however, the family confirmed that the patient had
died during telephone follow-up 6 months later. In the present
study, there was no difference between the treatment of patients
with PV complicated with portal hypertension and that of hepatic
portal hypertension. The present study suggested that clinicians
should further consider whether patients with PV are complicated
with portal hypertension and improve the examination for
corresponding treatment. PV treatment mainly uses hydroxyurea and
interferons (31). Among the five
patients included in the present study, four patients (case nos. 1,
2, 3 and 4) received symptomatic treatment with hydroxyurea, with
case nos. 3 and 4 also treated with interferon at the latter stages
of the disease. However, case no. 5 was treated with interferon
only.
Hydroxyurea is the first-line drug of choice,
whereas Peg-interferon is typically administered to young women of
childbearing age and to patients who show intolerance or resistance
to hydroxyurea (31). Hydroxyurea
is commonly used for treating various blood diseases, such as
sickle cell anemia, thalassemia and chronic myelogenous leukemia.
Long-term use of hydroxyurea can cause skin side effects, such as
diffuse hyperpigmentation, heterochromia dermatitis and skin
atrophy (32). Multiple ulcers
occur in 64% patients treated with hydroxyurea long-term, where the
most common ulcer site is the ankle (32). In a previous study, 30 of 993
patients treated with hydroxyurea for MPN developed painful
ulcerative skin conditions, mainly in the ankle area. A total of 11
patients developed oral ulcers. In addition, 10 patients presented
with non-ulcerative skin conditions, complicated by erythema and
skin infiltration (by inflammatory exudate) (32). Furthermore, long-term sequelae of
drug-induced liver injury can also be observed in portal
hypertension (33). In a previous
report, when thioguanine is used to treat acute and chronic myeloid
leukemia (34) or when the
adjuvant chemotherapy drug oxaliplatin was used, NCPH was found
after several years of administration (35). Since liver injury caused by
hydroxyurea has not been previously reported, portal hypertension
in the patients of the present study was not considered for
drug-induced liver injury.
In conclusion, the patients included in the present
study were serologically hepatitis virus B and C negative, had no
history of alcoholic or drug-induced liver injury, their indices
associated with autoimmune liver disease were negative and had no
genetic metabolic liver disease. The history is only a series of
reports of NCPH caused by PV. However, due to the small sample size
included in the present study, the mechanism of non-sclerosing
portal hypertension caused by PV needs to be further explored.
Analysis of patients' characteristics showed that decompensated
manifestations of portal hypertension caused by PV, such as
splenomegaly, cholecystitis and esophageal varicose veins, were
more common. However, impairments in liver function was mild. After
diagnosing PV, EGD should be performed as early as possible and
regularly, where the primary prevention of esophageal variceal
hemorrhage is particularly important. However, there is no
consensus on whether active antithrombotic therapy in patients with
PV can delay or prevent venous thrombosis and downstream
complications, such as visceral infarction, ascites and variceal
hemorrhage. If portal hypertension and related complications are
consciously prevented in advance during PV, then the adverse
outcomes of the disease may be improved.
Acknowledgements
Not applicable.
Funding
Funding: Funding was provided by Key Medical Major of Beijing
Sailing Plan: Severe Liver Disease with Integrated Traditional
Chinese and Western Medicine (reference no. ZYLX201819) and the
Clinical Efficacy Evaluation of Traditional Chinese Medicine in
reducing the mortality of acute-or-chronic liver failure (reference
no. 2018ZX10725506-002).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LZ, WL and JH were responsible for data acquisition.
LZ, YW and CG were responsible for data interpretation and confirm
the authenticity of all the raw data. CG, WL and JH critically
revised this paper. WL and CG provided theoretical guidance for the
single patient treatment protocol. CG and JH gave final approval of
the version to be published. All authors agreed to be accountable
for all aspects of the work in ensuring that questions related to
the accuracy or integrity of any part of the work are appropriately
investigated and resolved. All authors have read and approved the
final manuscript.
Ethics approval and consent to
participate
The present retrospective study was approved by an
institutional review board of Beijing Youan Hospital, Capital
Medical University (Beijing, China; approval no.
LL-2022-089-K).
Patient consent for publication
Written informed consent was obtained from the
relatives of patient 4. The Ethics Committee of Beijing You'an
Hospital Affiliated to Capital Medical University (Beijing, China)
waived the requirement of informed consent for the other 4 patients
(cases 1, 2, 3 and 5). The study was also approved by the Ethics
committee of Beijing You'an Hospital Affiliated to Capital Medical
University (Beijing, China). The present study was a retrospective
study. Clinical data were collected from medical records of
eligible patients who had been hospitalized in our hospital.
Patients who were diagnosed with PV and NCPH and treated in Beijing
You'an Hospital, Capital Medical University (Beijing, China)
between January 2010 and March 2022 were included into the present
study. The patient's hospitalization information was collected from
the hospital's medical record database, and the patient's
examination data, including laboratory blood test and imaging data,
were collected from the electronic medical record system of Beijing
You'an Hospital Affiliated to Capital Medical University (Beijing,
China). The specific data were collected from the medical record
management center of our hospital. The collected data do not
involve patients' names and other privacy information.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Barbui T, Tefferi A, Vannucchi AM,
Passamonti F, Silver RT, Hoffman R, Verstovsek S, Mesa R, Kiladjian
JJ, Hehlmann R, et al: Philadelphia chromosome-negative classical
myeloproliferative neoplasms: Revised management recommendations
from European LeukemiaNet. Leukemia. 32:1057–1069. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Arber DA, Orazi A, Hasserjian R, Thiele J,
Borowitz MJ, Le Beau MM, Bloomfield CD, Cazzola M and Vardiman JW:
The 2016 revision to the World Health Organization classification
of myeloid neoplasms and acute leukemia. Blood. 127:2391–2405.
2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ongenae K, Janssens A, Noens L, Wieme N,
Geerts ML, Beele H and Naeyaert JM: Erythromelalgia: A clue to the
diagnosis of polycythemia vera. Dermatology. 192:408–410.
1996.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Corredoira Sánchez JC, González López M,
Cortés Laiño JA, Gomara García S, Pérez Alvarez R, Casariego Vales
B, López Alvarez MJ and García Rodríguez JF: Neurologic
manifestations of polycythemia vera. Analysis of 24 cases and
review of the literature. An Med Interna. 7:67–70. 1990.PubMed/NCBI(In Spanish).
|
|
5
|
Torgano G, Mandelli C, Massaro P, Abbiati
C, Ponzetto A, Bertinieri G, Bogetto SF, Terruzzi E and de Franchis
R: Gastroduodenal lesions in polycythaemia vera: Frequency and role
of Helicobacter pylori. Br J Haematol. 117:198–202. 2002.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lelonek E, Matusiak L, Wrobel T and
Szepietowski JC: Aquagenic pruritus in polycythemia vera: Clinical
characteristics. Acta Derm Venereol. 98:496–500. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ong E, Barraco F, Nighoghossian N, Praire
A, Desestret V, Derex L, Vighetto A and Biotti D: Cerebrovascular
events as presenting manifestations of Myeloproliferative Neoplasm.
Rev Neurol (Paris). 172:703–708. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Akdi A, Ozeke O, Karanfil M, Ertem AG,
Yayla C, Demirtas K, Guney T, Unal S and Selcuk MT: Diurnal rhythm
of blood pressure in patients with polycythemia vera. Blood Press
Monit. 25:69–74. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Venton G, Turcanu M, Colle J, Thuny F,
Chebrek S, Farnault L, Mercier C, Ivanov V, Fanciullino R, Suchon
P, et al: Pulmonary hypertension in patients with
myeloproliferative neoplasms: A large cohort of 183 patients. Eur J
Intern Med. 68:71–75. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Rossi C, Randi ML, Zerbinati P, Rinaldi V
and Girolami A: Acute coronary disease in essential thrombocythemia
and polycythemia vera. J Intern Med. 244:49–53. 1998.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Monge M, Vaida I, Modeliar SS, Solanilla
A, Airapetian N, Presne C, Makdassi R, Fournier A and Choukroun G:
Retroperitoneal hematoma compressing a single functioning kidney:
An unusual Cause of obstructive renal failure. Clin Nephrol.
67:318–320. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Cerquozzi S, Barraco D, Lasho T, Finke C,
Hanson CA, Ketterling RP, Pardanani A, Gangat N and Tefferi A: Risk
factors for arterial versus venous thrombosis in polycythemia vera:
A single center experience in 587 patients. Blood Cancer J.
7(662)2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Barbui T, Carobbio A, Rumi E, Finazzi G,
Gisslinger H, Rodeghiero F, Randi ML, Rambaldi A, Gisslinger B,
Pieri L, et al: In contemporary patients with polycythemia vera,
rates of thrombosis and risk factors delineate a new clinical
epidemiology. Blood. 124:3021–3023. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Plessier A, Darwish-Murad S,
Hernandez-Guerra M, Consigny Y, Fabris F, Trebicka J, Heller J,
Morard I, Lasser L, Langlet P, et al: Acute portal vein thrombosis
unrelated to cirrhosis: A prospective multicenter follow-up study.
Hepatology. 51:210–218. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Khanna R and Sarin SK: Non-cirrhotic
portal hypertension-diagnosis and management. J Hepatol.
60:421–441. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
European Association for the Study of the
Liver. Electronic address: simpleeasloffice@easloffice.eu.
EASL clinical practice guidelines: Vascular diseases of the liver.
J Hepatol. 64:179–202. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
de Franchis R: Baveno VI Faculty.
Expanding consensus in portal hypertension: Report of the Baveno VI
Consensus Workshop: Stratifying risk and individualizing care for
portal hypertension. J Hepatol. 63:743–752. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sarin SK, Sethi KK and Nanda R:
Measurement and correlation of wedged hepatic, intrahepatic,
intrasplenic and intravariceal pressures in patients with cirrhosis
of liver and non-cirrhotic portal fibrosis. Gut. 28:260–266.
1987.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Garcia-Pagan JC, Hernandez-Guerra M and
Bosch J: Extrahepatic portal vein thrombosis. Semin Liver Dis.
28:282–292. 2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sarin SK and Kumar A: Noncirrhotic portal
hypertension. Clin Liver Dis. 10:627–651, x. 2006.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Schouten JN, Garcia-Pagan JC, Valla DC and
Janssen HL: Idiopathic noncirrhotic portal hypertension.
Hepatology. 54:1071–1081. 2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Vollmar B and Menger MD: The hepatic
microcirculation: Mechanistic contributions and therapeutic targets
in liver injury and repair. Physiol Rev. 89:1269–1339.
2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Intagliata NM, Caldwell SH and Tripodi A:
Diagnosis, development, and treatment of portal vein thrombosis in
patients with and without cirrhosis. Gastroenterology.
156:1582–1599.e1. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Pugh RN, Murray-Lyon IM, Dawson JL,
Pietroni MC and Williams R: Transection of the oesophagus for
bleeding oesophageal varices. Br J Surg. 60:646–649.
1973.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Llop E, de Juan C, Seijo S, Garcia-Criado
A, Abraldes JG, Bosch J and Garcia-Pagan JC: Portal cholangiopathy:
Radiological classification and natural history. Gut. 60:853–860.
2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Dhiman RK, Behera A, Chawla YK, Dilawari
JB and Suri S: Portal hypertensive biliopathy. Gut. 56:1001–1008.
2007.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Spencer RP and Pearson HA: The spleen as a
hematological organ. Semin Nucl Med. 5:95–102. 1975.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Noronha FC, Seijo S, Plessier A,
Silva-Junior G, Turon F, Rautou PE, Baiges A, Bureau C, Bosch J,
Hernandez-Gea V, et al: Natural history and management of
esophagogastric varices in chronic noncirrhotic, nontumoral portal
vein thrombosis. Hepatology. 63:1640–1650. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kovacs TOG and Jensen DM: Varices:
Esophageal, gastric, and rectal. Clin Liver Dis. 23:625–642.
2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Nagashima K, Irisawa A, Kashima K, Sakuma
F, Minaguchi T, Yamamiya A, Yamabe A, Hoshi K, Tominaga K, Iijima M
and Goda K: The risk of bleeding in small/straight esophageal
varices with red color sign on endoscopy: A retrospective analysis
from the natural course. Healthcare (Basel).
10(1193)2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Tefferi A, Vannucchi AM and Barbui T:
Polycythemia vera: Historical oversights, diagnostic details, and
therapeutic views. Leukemia. 35:3339–3351. 2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Latagliata R, Spadea A, Cedrone M, Di
Giandomenico J, De Muro M, Villiva N, Breccia M, Anaclerico B,
Porrini R, Spirito F, et al: Symptomatic mucocutaneous toxicity of
hydroxyurea in Philadelphia chromosome-negative myeloproliferative
neoplasms: The Mister Hyde face of a safe drug. Cancer.
118:404–409. 2012.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Bjornsson ES and Andrade RJ: Long-term
sequelae of drug-induced liver injury. J Hepatol. 76:435–445.
2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Shepherd PC, Fooks J, Gray R and Allan NC:
Thioguanine used in maintenance therapy of chronic myeloid
leukaemia causes non-cirrhotic portal hypertension. Results from
MRC CML. II. Trial comparing busulphan with busulphan and
thioguanine. Br J Haematol. 79:185–192. 1991.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kim HP, Navarro V, Zacks S, Odin J,
Kleiner DE and Hayashi PH: Drug-Induced Liver Injury Network
Investigators. The clinical spectrum and diagnosis of oxaliplatin
liver injury in the era of nonalcoholic fatty liver disease. Clin
Gastroenterol Hepatol. 19:2199–2201. 2021.PubMed/NCBI View Article : Google Scholar
|