Introduction
Ischemic heart disease (IHD) is a common
cardiovascular disease. In 2016, the World Health Organization
estimated that there are currently ~126.5 million cases of IHD,
with >9 million deaths of patients with IHD per year worldwide,
making IHD a leading cause of morbidity and mortality worldwide
(1), and therefore it is a great
burden on society and the economy, and has become a major global
public health problem (2,3). For patients with acute myocardial
infarction, immediate and successful myocardial reperfusion is the
most effective strategy to reduce the size of myocardial infarction
and improve clinical efficacy (4).
Surgical treatment, such as coronary artery bypass grafting and
percutaneous coronary intervention, are performed immediately to
restore blood supply in ischemic cardiomyocytes (5,6).
However, reperfusion itself can lead to further cardiomyocyte death
and systolic dysfunction, which is known as ischemia/reperfusion
(I/R) injury, thereby eliminating the benefits of reperfusion
therapy in patients with IHD and leading to secondary myocardial
injury (7,8). Apoptosis is considered to be the main
form of cardiomyocyte death, and cardiomyocyte apoptosis is usually
observed in myocardial I/R injury (9). Therefore, inhibiting I/R
injury-induced apoptosis of cardiomyocytes may be an effective
approach in the treatment of IHD.
Myocardial I/R injury is involved in the activation
of the inflammatory cascade, which serves a role in the acute
expansion of injury and myocardial repair (10-12).
This inflammatory response is associated with the massive
production of a series of mediators that determine the outcome of
reperfusion injury, and these mediators, such as proteases,
chemokines and interleukins, may contribute to cardiomyocyte
apoptosis after I/R injury (13).
Certain inflammatory molecules or pathways may be involved in
mediating apoptosis. Cyclic GMP-AMP synthase (cGAS) is a nuclease
with the function of recognizing cytoplasmic DNA and stimulating
the transcription of the stimulator of interferon genes (STING), as
well as regulating the secretion of type I IFN and other cytokines,
which contribute to the activation of immune responses (14). In innate immune cells, the presence
of cytosolic DNA is sensed by the cGAS-STING signaling pathway,
which initiates cytokine production and apoptosis induction
(15). It has been reported that
cGAS can mediate the activation of Caspase-3-dependent apoptosis by
decreasing Bcl-2 expression and increasing Bax expression (16). It has also been demonstrated that
the cGAS-STING signaling pathway is involved in the development of
I/R-induced neuroinflammation (17). However, to the best of our
knowledge, the role of the cGAS-STING signaling pathway in I/R
injury-induced cardiomyocyte apoptosis is unclear. Therefore, it
was hypothesized that cGAS-STING signaling may be an important
target for preventing myocardial I/R injury.
Scutellarin (SCU), a natural bioactive flavonoid, is
extracted from Erigeron breviscapus (18). It has been demonstrated to have
anti-inflammatory and anti-apoptotic effects (19,20).
It has been reported that SCU can alleviate hypoxia-induced
cerebral injury (21,22). SCU has been demonstrated to protect
rat cortical neurons from oxygen-glucose deprivation-induced
apoptosis (21). SCU treatment has
also been demonstrated to suppress apoptosis after brain I/R
injury, as evidenced by reduced DNA fragmentation, NAD depletion
and mitochondrial dysfunction (22). Wang et al (23) reported that SCU exerted
anti-apoptotic and anti-oxidative stress activity to protect
cardiomyocytes from I/R injury. Xu et al (24) reported that SCU inhibited
activation of the NLR family pyrin domain containing 3 (NLRP3)
inflammasome to attenuate myocardial I/R injury. However, the
mechanisms underlying the protective effect of SCU are not well
understood.
In the present study, C57BL/6 mice were used to
establish an I/R-induced heart injury model and H9c2 cells were
used to construct a hypoxia/reoxygenation (H/R)-induced
cardiomyocyte injury model to explore the effects of SCU on I/R or
H/R injury-induced cardiomyocyte apoptosis and cardiac dysfunction,
and its potential mechanisms.
Materials and methods
Animals and treatment
The animal experiment was approved by the Animal
Ethics Committee of The People's Hospital of Yue Chi County
(Guang'an, China). A total of 20 male C57BL/6 mice (age, 6-8 weeks;
weight, 18-25 g) purchased from Western Biotechnology, Inc. were
randomly divided into four groups: Sham, I/R, SCU and I/R + SCU
group (n=5/group). The mice were treated as follows: In the Sham
group, PBS was injected intraperitoneally 1 h before sham surgery;
in the SCU group, SCU (20 mg/kg) was injected intraperitoneally
each day for a total of 7 days to achieve a stable blood
concentration of the drug (25)
before sham surgery; in the I/R group, PBS was injected
intraperitoneally 1 h before ligation of the left anterior
descending coronary artery; and in the I/R + SCU group, SCU (20
mg/kg) was injected intraperitoneally for 7 days before ligation of
the left anterior descending coronary artery. The mice had free
access to food and water and were housed in an environment at
22±2˚C with a humidity between 40 and 60% and a light-dark cycle of
12 h.
Ischemia/reperfusion (I/R) model
The mice were anesthetized with isoflurane gas (dose
for both induction and maintenance, 2%) and the thorax was opened.
Subsequently, the heart was compressed and quickly positioned in
the third and fourth intercostal spaces. In the sham and SCU
groups, only the chest was opened without proceeding with ligation,
while mice in the I/R and I/R + SCU groups underwent ligation of
the left anterior descending coronary artery for 30 min to mimic
the ischemia phase, and then the ligation line of the left anterior
descending coronary artery was removed for 24 h to mimic the
reperfusion phase, to establish the I/R model.
Echocardiography
Left ventricular function in mice was assessed using
echocardiography (Philips TIS 0.8; Koninklijke Philips N.V.) and an
RMV 707B transducer (frequency, 30 MHz; Siemens AG). The mice were
anesthetized with isoflurane (dose for both induction and
maintenance, 2%) before echocardiography. Images were obtained by
identifying the interventricular septum and the left ventricular
posterior wall. The left ventricular fractional shortening (LVFS;
%) and left ventricular ejection fraction (LVEF; %) were
automatically calculated by echocardiography. Each parameter was
evaluated by calculating the average of four cardiac cycles. All
mice in the present study were sacrificed by cervical dislocation
at the end of this experiment.
TUNEL staining
Mouse heart tissues were fixed with 4%
paraformaldehyde at room temperature for 24 h, embedded in paraffin
and cut into 5-µm-thick paraffin-embedded sections. The In
Situ Cell Death Fluorescein Kit (Roche Diagnostics) was used to
detect the free 3'-OH chain breaks induced by DNA degradation. The
detailed steps were as follows: The paraffin sections were soaked
in xylene twice (5 min each) and immersed in gradient ethanol (100,
95, 90, 80 and 70%) for 3 min each. Subsequently, the sections were
soaked in steaming water once for 3 min, 3%
H2O2 once for 10 min and rinsed with PBS
twice (5 min each). The sections were placed in a wet box, treated
with 100 µl proteinase K working solution (20 µg/ml) at 37˚C for 15
min and rinsed with PBS twice (5 min each). Subsequently, 50 µl
TUNEL reaction solution (10% TdT + 90% fluorescein labeled dUTP)
was added to the tissue sections on the glass slides, and the glass
slides were covered and incubated at 37˚C for 1.5 h, and
subsequently rinsed with PBS three times (5 min each). DAPI
staining solution (5 µg/ml) was added and incubated at room
temperature for 5 min, and subsequently rinsed with PBS three times
(5 min each). Anti-Fade Mounting Medium (Sangon Biotech Co., Ltd.)
was added. The slides were observed under a fluorescence microscope
with an excitation wavelength of 450-500 nm and a detection
wavelength of 515-565 nm. Five random visual fields were selected
from each slide under the microscope. The TUNEL-positive-stained
cells were counted using ImageJ software (version 1.46; National
Institutes of Health).
Staining with
2,3,5-triphenyltetrazolium chloride (TTC)
The myocardial infarct area was assessed by TTC
staining. After 24 h of reperfusion, the hearts were excised, and
the 2-mm-thick tissue slices were incubated for 20 min in 1% TTC
(MilliporeSigma) at 37˚C, followed by fixation in 4%
paraformaldehyde at room temperature overnight. The images were
captured using a digital camera and the infarct area appeared
white. The ratio of the infarct size (white area) was calculated
using ImageJ software (version 1.46; National Institutes of
Health).
Cell culture and processing
The H9c2 cell line was purchased from American Type
Culture Collection. The cells were cultured in DMEM (low glucose, 5
Mm; Thermo Fisher Scientific, Inc.) with 10% FBS (Thermo Fisher
Scientific, Inc.) at 37˚C, 5% CO2 and saturated
humidity. H9c2 cells were passaged at a ratio of 1:4 in culture
plates. Plates with H9c2 (4x105 cells per well)
cardiomyocytes were then placed in a sealed chamber (Modular
Incubator Chamber MIC1; Billups-Rothenberg, Inc.) filled with 95%
N2 and 5% CO2 to achieve an oxygen-deficient
environment. Ventilation at 5 l/min for 15 min was used to achieve
a 1% oxygen concentration in the chamber at 37˚C. Cells were
incubated with PBS at 37˚C for 3 h, then PBS was removed and
replaced with fresh medium containing 10% FBS. Further incubation
for 3 h in 95% air and 5% CO2 at 37˚C was performed for
reoxygenation. The cells in the control and DMSO groups were kept
in fresh medium containing 10% FBS in 95% air and 5% CO2
at 37˚C. The cells in the RU.521 (10 mM in 1 ml DMSO;
MedChemExpress) group were treated with 1 mmol/l RU.521 for 48 h at
37˚C. The cells in the H-151 (10 mM in 1 ml DMSO; MedChemExpress)
group were treated with 2 µmol/l H-151 for 48 h at 37˚C. The cells
in the SCU (10 mM in 1 ml DMSO; MedChemExpress) group were treated
with 100 µmol/l SCU for 48 h at 37˚C. For the H/R + RU.521 group,
the H/R-injured cells were treated with 1 mmol/l RU.521 for 48 h at
37˚C. For the H/R + H-151 group, the H/R-injured cells were treated
with 2 µmol/l H-151 for 48 h at 37˚C. For the H/R + SCU group, the
H/R-injured cells were treated with 100 µmol/l SCU for 48 h at
37˚C.
Cell viability detection using the
CCK-8 assay
In brief, H9c2 cells (5x103 per well)
were seeded into 96-well microplates, then placed in a sealed
chamber filled with 95% N2 and 5% CO2 at 37˚C
and treated with 0, 25, 50 and 100 µmol/l SCU (10 mM in 1 ml DMSO;
MedChemExpress) for 48 h or treated with 100 µmol/l SCU for 0, 12,
24 and 48 h. A total of 10 µl CCK-8 (Beyotime Institute of
Biotechnology) was added, followed by incubation at 37˚C for 1 h.
The absorbance value of each well was detected at 490 nm using a
microplate reader (Agilent Technologies, Inc.).
Annexin V/PI staining
Flow cytometry was used to detect the apoptosis rate
of H9c2 cells. In brief, the H/R-injured cells were treated with 0,
25, 50 and 100 µmol/l SCU for 24 h. Subsequently, ≥1x105
cells were resuspended in 100 µl binding buffer containing Annexin
V-FITC and PI (Beyotime Institute of Biotechnology) and incubated
at room temperature for 15 min. The BD FACScan™ system
(BD Biosciences) was used to quantify Annexin V-FITC and PI binding
using the channels FL-1 (Annexin V-FITC) and FL-3 (PI), and
analysis was performed using BD CellQuest Pro™ software
(version 5.1; BD Biosciences). Apoptosis was calculated as the sum
of early and late apoptosis.
Western blot analysis
Pre-cooled H9c2 cells at 4˚C and left ventricular
tissue from mice were lysed on ice for 30 min using RIPA Lysis
Buffer (MedChemExpress), which contained 20 mmol/l Tris (pH 7.5),
150 mmol/l NaCl, 1% Triton X-100 and 1% Phosphatase Inhibitor
Cocktail I and III (MedChemExpress). Next, the cell lysis products
were centrifuged for 10 min at 12,700 x g in a 4˚C refrigerated
centrifuge and the supernatants were collected. The protein
concentration was measured using a BCA protein kit (Thermo Fisher
Scientific, Inc.) and the samples were boiled at 100˚C for 5 min. A
total of 30 µg cellular protein/lane were electrophoresed using 10%
SDS-PAGE, and transferred onto a PVDF membrane. The membrane was
blocked for 2 h at room temperature with 5% BSA (Beyotime Institute
of Biotechnology), incubated overnight at 4˚C with primary
antibodies of GAPDH (dilution, 1:10,000; ab181602; Abcam), cGAS
(dilution, 1:1,000; ab252416; Abcam), STING (dilution, 1:1,000;
ab288157; Abcam), Bcl-2 (dilution, 1:1,000; ab196495; Abcam), Bax
(dilution, 1:1,000; ab32503; Abcam) and cleaved Caspase-3
(dilution, 1:1,000; ab184787; Abcam), washed with TBS containing
0.1% Tween 20, and incubated for 2 h with HRP Anti-Rabbit IgG
antibody (dilution, 1:10,000; ab184787; Abcam) at room temperature.
The immunoreactivity of the proteins was visualized by
chemiluminescence with immobilon western chemilum HRP substrate
(MilliporeSigma). Signals were detected and analyzed with
ChemiDoc™XRS + with Image Lab™ Software Gel
Imaging System (version 2.0; Bio-Rad Laboratories, Inc.).
Statistical analysis
All experiments were performed in triplicate and the
data are presented as the mean ± SD. Statistical significance was
analyzed using one-way ANOVA followed by Dunnett's test for
comparisons vs. a single control or Tukey's test for comparisons
among multiple groups. GraphPad Prism (version 5.01; GraphPad
Software; Dotmatics) was used to analyze the data. P<0.05 was
considered to indicate a statistically significant difference.
Results
SCU ameliorates I/R injury-induced
cardiac dysfunction and inhibits cardiomyocyte apoptosis
Cardiac function (indicated by LVEF and LVFS) was
measured by echocardiography to determine the effect of SCU
treatment on I/R-injured mice. The results demonstrated that I/R
injury induced a decrease in LVEF and LVFS in mice, which was
ameliorated by SCU treatment (Fig.
1A and B). The effect of SCU
treatment on I/R-induced cardiomyocyte apoptosis was detected using
the TUNEL technique. The results revealed that I/R injury induced
an increase in cardiomyocyte apoptosis in cardiac tissues, which
was ameliorated by SCU treatment (Fig.
1C and D). The effect of SCU
treatment on I/R-induced myocardial infarction (MI) was detected by
TTC staining. The results demonstrated that the I/R-induced
myocardial infarct area was decreased by SCU treatment compared
with the I/R group (Fig. 1E and
F).
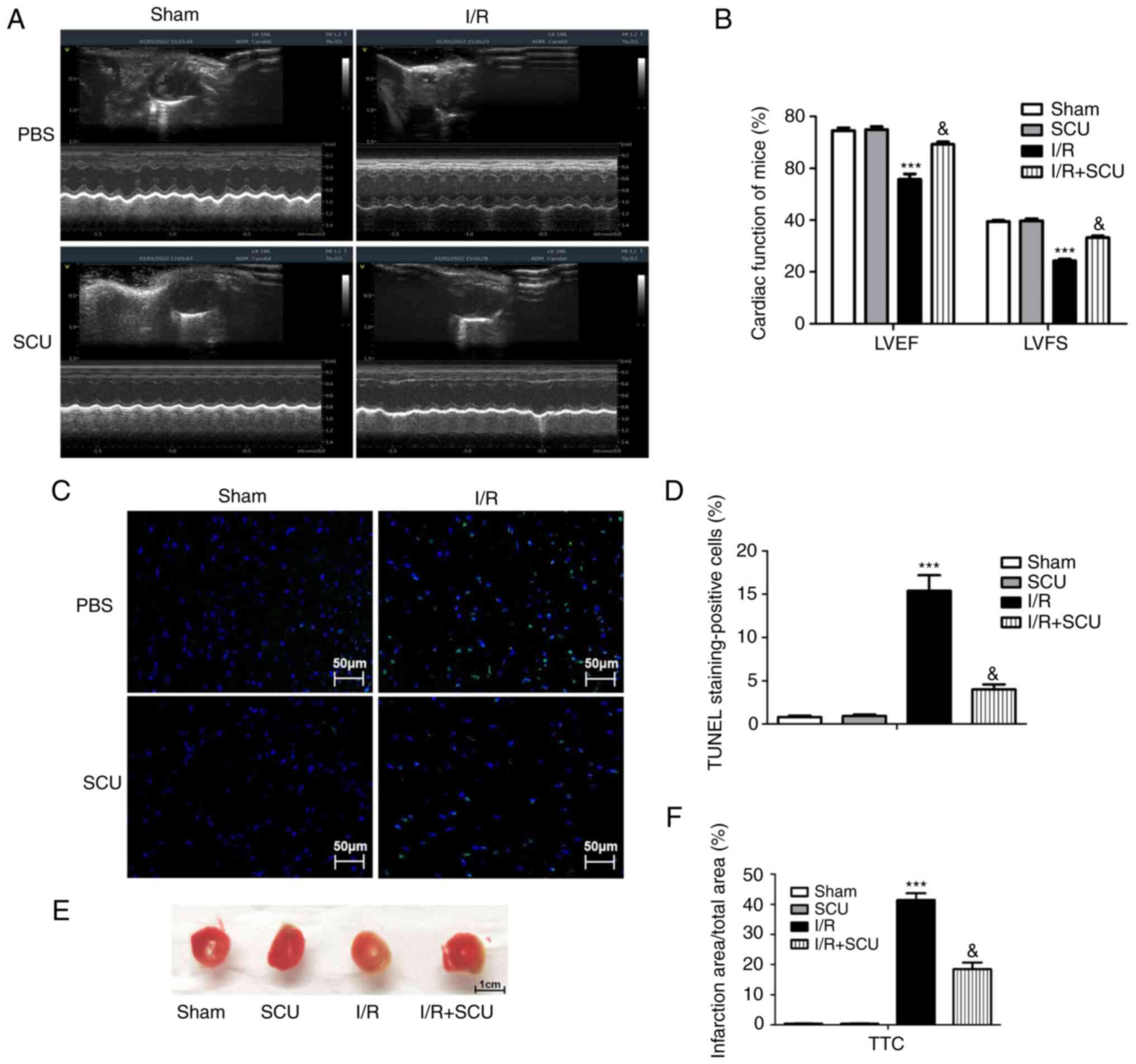 | Figure 1Effect of SCU on cardiac dysfunction
and cardiomyocyte apoptosis in cardiac tissues of I/R-injured mice.
(A) Cardiac function (LVEF and LVFS) of mice in the sham, SCU, I/R
and I/R + SCU groups was detected by echocardiography. (B) LVEF and
LVFS data were analyzed using GraphPad Prism. Data are presented as
the mean ± SD. ***P<0.001 compared with the sham
group; &P<0.001 compared with the I/R group. (C)
Apoptotic cells were detected using a TUNEL assay, and (D) the
TUNEL-positive cell percentage was determined using ImageJ and
analyzed using GraphPad Prism. Scale bar, 50 µm. Data are presented
as the mean ± SD. ***P<0.001 compared with the sham
group; &P<0.001 compared with the I/R group. (E)
Myocardial infarction was detected by TTC staining. (F) Infarct
size (white area) was calculated using ImageJ software and analyzed
using GraphPad Prism. Scale bar, 1 cm. Data are presented as the
mean ± SD. ***P<0.001 compared with the sham group;
&P<0.001 compared with the I/R group. SCU,
scutellarin; LVEF, left ventricular ejection fraction; LVFS, left
ventricular fractional shortening; I/R, ischemia/reperfusion; TTC,
2,3,5-triphenyltetrazolium chloride; ADM, add/drop multiplexer. |
SCU inhibits activation of the
cGAS-STING and Bcl-2/Bax/Caspase-3 signaling pathways in cardiac
tissues of I/R-injured mice
The effect of SCU on the cGAS-STING and
Bcl-2/Bax/Caspase-3 signaling pathways in I/R-injured mice was
assessed. The results revealed that SCU treatment reversed the I/R
injury-induced increase in cGAS, STING and cleaved Caspase-3
expression, as well as the decrease in the Bcl-2/Bax ratio, in
mouse cardiac tissues (Fig.
2).
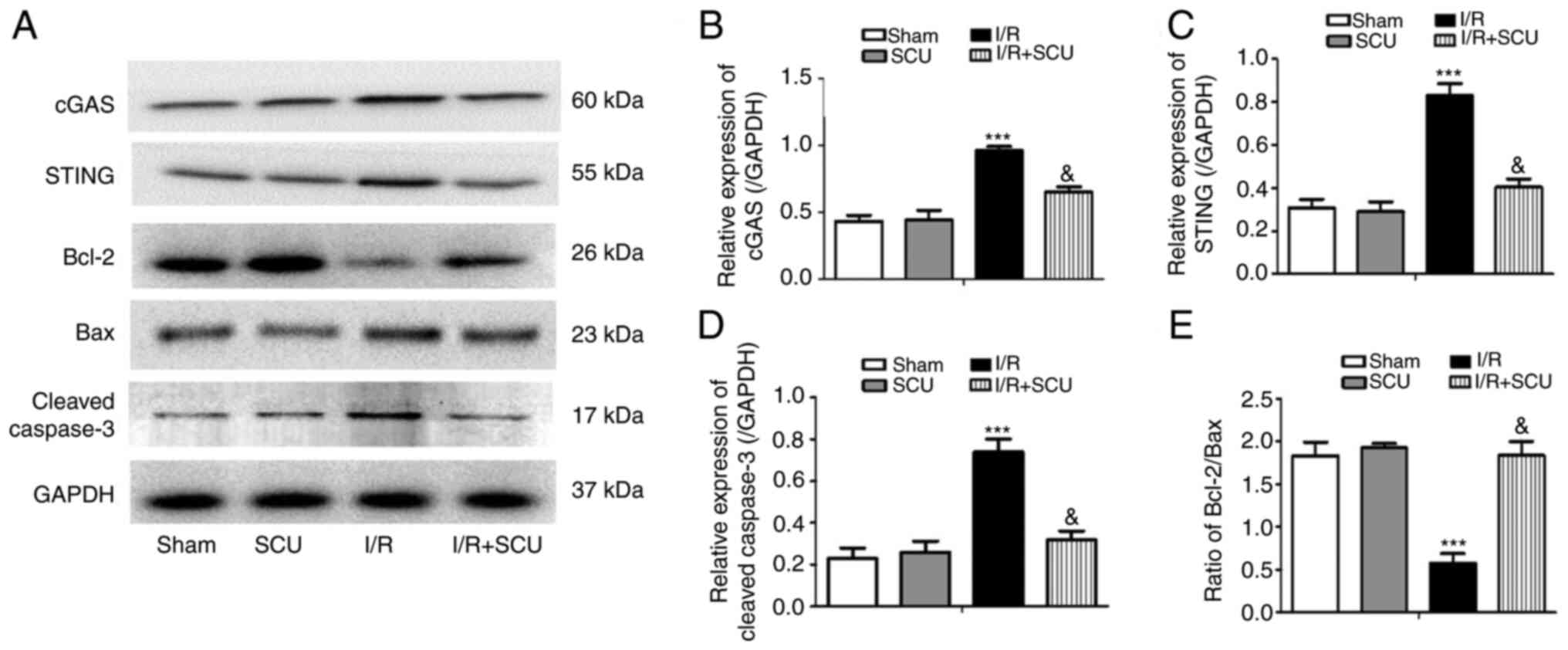 | Figure 2SCU inhibits the activation of the
cGAS-STING and Bcl-2/Bax/Caspase-3 pathways in cardiac tissues of
I/R-injured mice. (A) Western blotting was performed to detect the
expression levels of cGAS, STING, Bcl-2, Bax, cleaved Caspase-3 and
GAPDH in mouse cardiac tissues. Relative expression levels of (B)
cGAS, (C) STING and (D) cleaved Caspase-3, and (E) the Bcl-2/Bax
ratio were calculated from the gray-scan value and analyzed using
GraphPad Prism. Data are presented as the mean ± SD.
***P<0.001 compared with the sham group;
&P<0.001 compared with the I/R group. SCU,
scutellarin; cGAS, cyclic GMP-AMP synthase; STING, stimulator of
interferon genes; I/R, ischemia/reperfusion. |
SCU inhibits cell apoptosis and
activation of the cGAS-STING and Bcl-2/Bax/Caspase-3 signaling
pathways in H9c2 cells after H/R injury
H/R-injured H9c2 cells were treated with SCU (0, 25,
50 and 100 µmol/l) for 48 h and cell viability was detected using a
CCK-8 assay. The cell viability was significantly increased by 50
and 100 µmol/l SCU treatment compared with the H/R group (Fig. 3A). H/R-injured H9c2 cells were
treated with SCU (100 µmol/l) and a CCK-8 assay was used to detect
cell viability at 0, 12, 24 and 48 h. A significant increase in
cell viability from 0 to 48 h was observed in H/R-injured cells
treated with 100 µmol/l SCU compared with that at 0 h (Fig. 3B). The rate of apoptosis in
H/R-injured H9c2 cells was detected by flow cytometry. SCU
treatment significantly decreased the high apoptotic rate of
HR-injured H9c2 cells (Fig. 3C and
D). Since 100 µmol/l SCU had the
highest anti-apoptotic effect in H/R-injured cells, 100 µmol/l SCU
was selected for subsequent experiments.
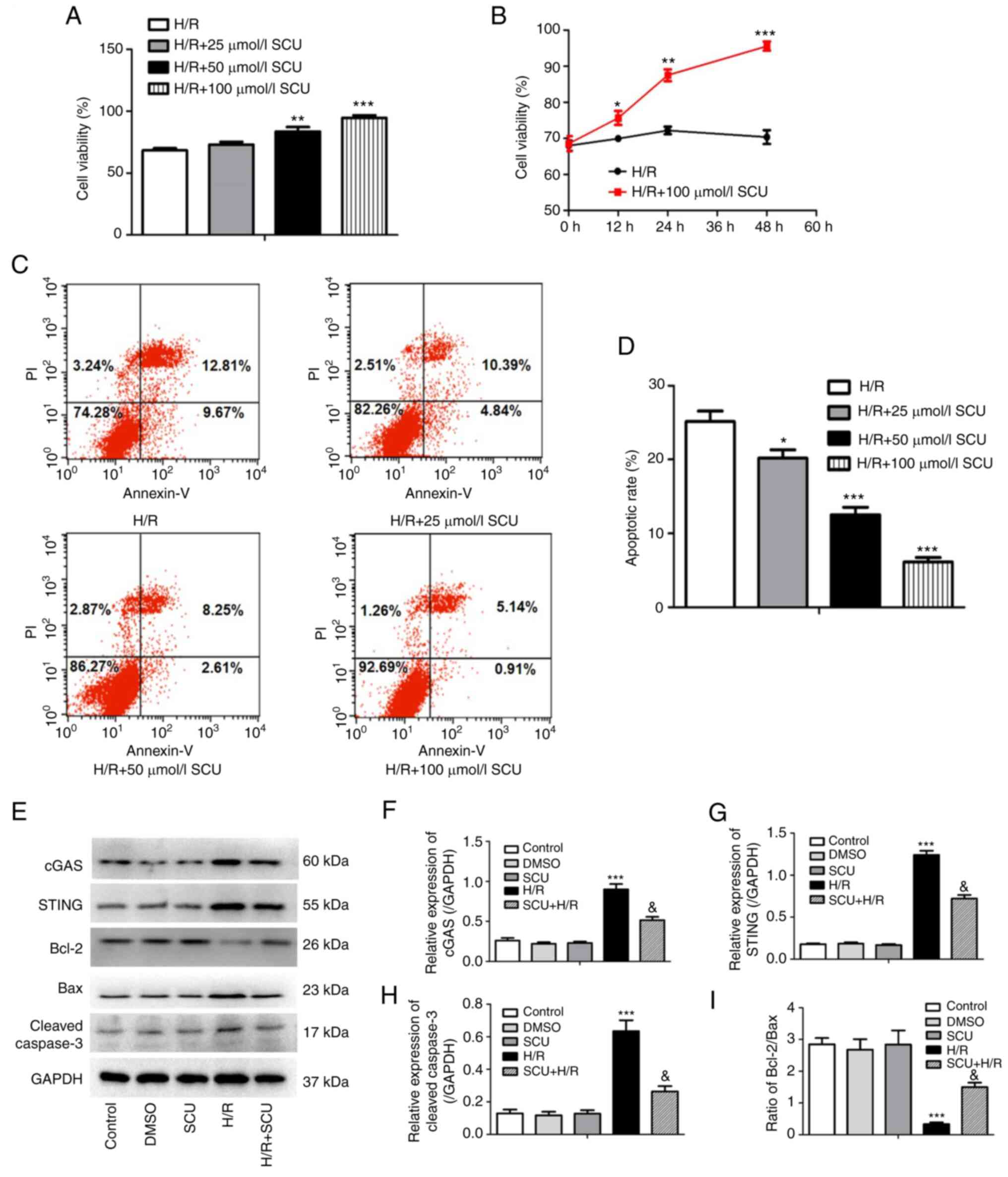 | Figure 3SCU inhibits cell apoptosis and
activation of the cGAS-STING and Bcl-2/Bax/Caspase-3 signaling
pathways in H9c2 cells after H/R injury. (A) After H/R injury, H9c2
cells were exposed to SCU at concentrations of 0, 25, 50 and 100
µmol/l for 48 h, and then a CCK-8 assay was performed to detect
cell viability. **P<0.01 and ***P<0.001
compared with the H/R group. (B) After H/R injury, H9c2 cells were
either exposed or not exposed to SCU (100 µmol/l), and then a CCK-8
assay was performed to detect viability at 0, 12, 24 and 48 h.
*P<0.05, **P<0.01 and
***P<0.001 compared with the 0 h group. (C) After H/R
injury, H9c2 cells were treated with 0, 25, 50 and 100 µmol/l SCU
for 24 h, and then the apoptotic rate of H9c2 cells was determined
by flow cytometry. (D) Apoptosis rate was calculated as the sum of
early and late apoptosis and analyzed using GraphPad Prism. Each
graph represents the results of three independent experiments (data
are presented as the mean ± SD). *P<0.05 and
***P<0.001 compared with the H/R only group. (E)
After H/R injury, H9c2 cells were exposed to 100 µmol/l SCU. The
expression levels of cGAS, STING, Bcl-2, Bax and cleaved Caspase-3
were determined by western blotting, and the relative expression
levels of (F) cGAS, (G) STING and (H) cleaved Caspase-3, and (I)
the Bcl-2/Bax ratio were calculated from the gray-scan value and
analyzed using GraphPad Prism. Each graph represents the
densitometry results of three independent experiments (data are
presented as the mean ± SD). ***P<0.001 compared with
the control group; &P<0.001 compared with the H/R
group. SCU, scutellarin; cGAS, cyclic GMP-AMP synthase; STING,
stimulator of interferon genes; H/R, hypoxia/reoxygenation; CCK-8,
Cell Counting Kit-8. |
Apoptosis and activation of the cGAS-STING signaling
pathway were observed after I/R and/or H/R injury; therefore, the
protein levels of cGAS, STING, Bcl-2, Bax and cleaved Caspase-3 in
H/R-injured H9c2 cells were detected. It was found that 100 µmol/l
SCU significantly decreased the expression levels of cGAS, STING
and cleaved Caspase-3, and increased the ratio of Bcl-2/Bax in H9c2
cells after H/R injury (Fig.
3E-I).
Inhibition of cGAS inhibits activation
of the cGAS-STING and Bcl-2/Bax/Caspase-3 signaling pathways
induced by H/R injury, similarly to SCU treatment
To explore whether cGAS serves a role in the
protective effective of SCU in cardiomyocytes from H/R
injury-induced apoptosis, the activity of cGAS was inhibited using
RU.521 (a cGAS inhibitor) and the effect was compared with that of
SCU in H9c2 cells after H/R injury. The expression levels of cGAS,
STING, Bcl-2, Bax and cleaved Caspase-3 were detected. H/R injury
increased the expression levels of cGAS, STING and cleaved
Caspase-3 and decreased the ratio of Bcl-2/Bax, and these effects
were significantly reversed by RU.521 treatment, which was similar
to the effect of SCU treatment (Fig.
4).
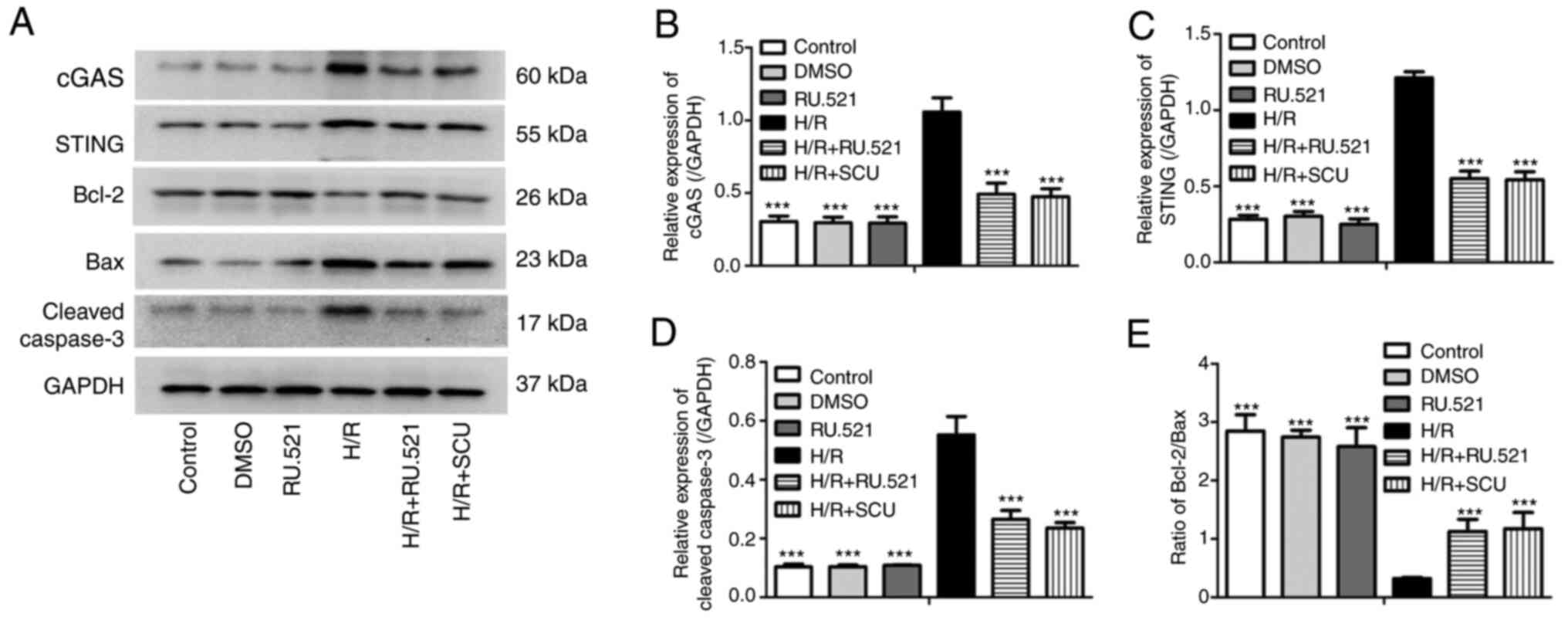 | Figure 4Inhibition of cGAS inhibits
activation of the cGAS-STING and Bcl-2/Bax/Caspase-3 signaling
pathways induced by H/R injury, similarly to SCU treatment. After
H/R injury, H9c2 cells were treated with 100 µmol/l SCU or 1 mmol/l
RU.521 for 48 h. (A) Expression levels of cGAS, STING, Bcl-2, Bax
and cleaved Caspase-3 were examined by western blotting. and the
relative expression levels of (B) cGAS, (C) STING and (D) cleaved
Caspase-3, and (E) the Bcl-2/Bax ratio were calculated from the
gray-scan value and analyzed using GraphPad Prism. Each graph
represents the densitometry results of three independent
experiments (data are presented as the mean ± SD).
***P<0.001 compared with the H/R group. cGAS, cyclic
GMP-AMP synthase; STING, stimulator of interferon genes; H/R,
hypoxia/reoxygenation; SCU, scutellarin. |
Inhibition of STING inhibits
activation of the cGAS-STING and Bcl-2/Bax/Caspase-3 signaling
pathways induced by H/R injury, similarly to SCU treatment
STING is the downstream target of and can be
activated by cGAS (14). However,
it is unclear whether the cGAS-mediated apoptosis of H9c2 cells
after H/R injury is dependent or independent of STING. Therefore,
the STING inhibitor H-151 was used to inhibit STING, and the effect
on H9c2 cells after H/R injury was compared with the effect of SCU.
H/R injury induced upregulation of the expression levels of STING
and cleaved Caspase-3, and reduced the ratio of Bcl-2/Bax, and
these effects were significantly reversed by H-151 treatment, which
was similar to the effect of SCU treatment (Fig. 5). However, H-151 treatment
exhibited no effect on cGAS expression in response to H/R
injury.
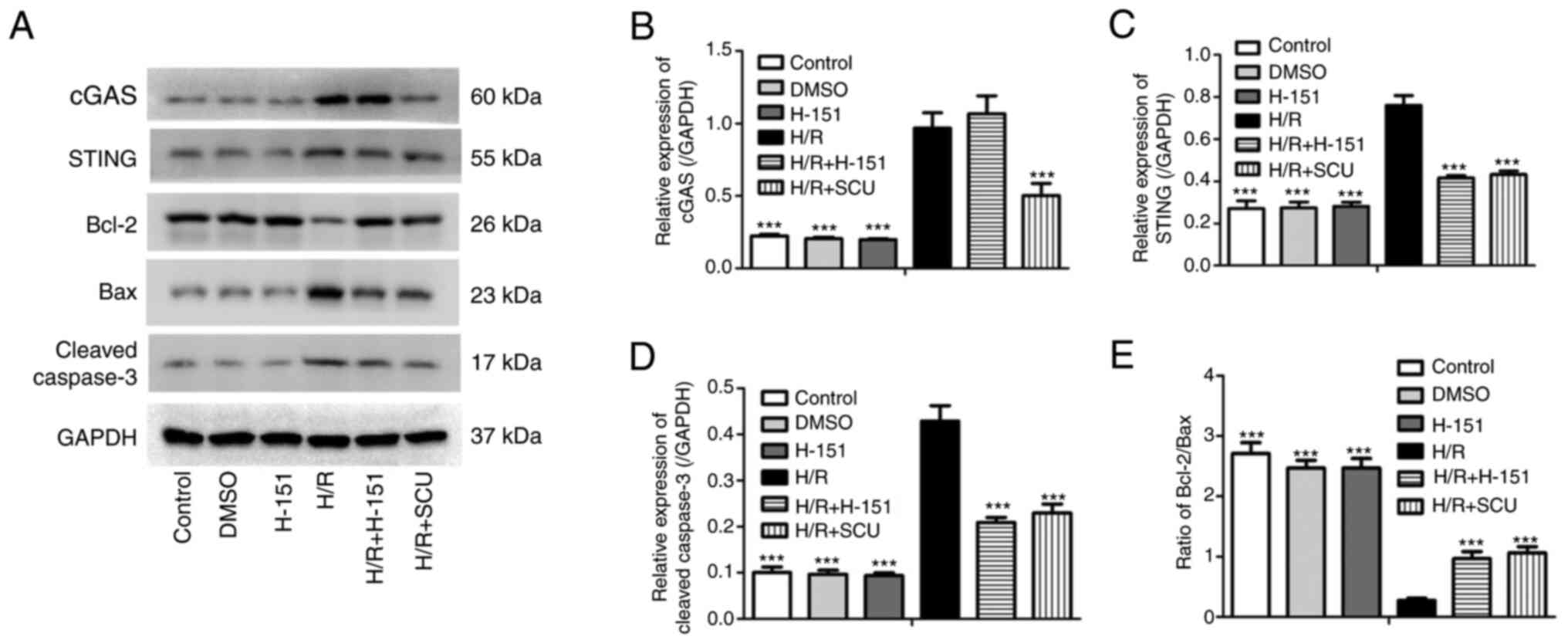 | Figure 5Inhibition of STING inhibits the
activation of the cGAS-STING and Bcl-2/Bax/Caspase-3 signaling
pathways induced by H/R injury, similarly to SCU treatment. After
H/R injury, H9c2 cells were treated with 100 µmol/l SCU or 2 µmol/l
H-151 for 48 h. (A) Expression levels of cGAS, STING, Bcl-2, Bax
and cleaved Caspase-3 were examined by western blotting, and the
relative expression levels of (B) cGAS, (C) STING and (D) cleaved
Caspase-3, and (E) the Bcl-2/Bax ratio were calculated from the
gray-scan value and analyzed using GraphPad Prism. Each graph
represents the densitometry results of three independent
experiments (data are presented as the mean ± SD).
***P<0.001 compared with the H/R group. STING,
stimulator of interferon genes; cGAS, cyclic GMP-AMP synthase; H/R,
hypoxia/reoxygenation; SCU, scutellarin. |
Discussion
The present study revealed that SCU ameliorated I/R
injury-induced cardiac dysfunction, and inhibited cardiomyocyte
apoptosis and activation of the cGAS-STING and Bcl-2/Bax/Caspase-3
signaling pathways in I/R-injured mice. In vitro experiments
demonstrated that SCU significantly increased cell viability and
decreased the apoptosis of H/R-induced H9c2 cells. Furthermore, it
was observed that H/R injury increased the expression levels of
cGAS, STING and cleaved Caspase-3, and decreased the ratio of
Bcl-2/Bax, which was reversed by treatment with SCU and both cGAS
and STING inhibitors.
SCU is a flavone isolated from Stipa barbata
and Erigeron breviscapus, which has been reported to exert a
broad range of cardiovascular pharmacological effects, including
vasodilative, anti-inflammatory, anticoagulative and
antithrombotic, myocardial protection and protection against I/R
injury effects (26). It has been
reported that SCU has an anti-apoptotic effect in cerebral I/R
injury (21,22,25,27).
SCU also inhibits apoptosis and oxidative stress in hepatocytes
after H/R injury (28).
Furthermore, SCU has protective effects in cardiovascular ischemia
in rats (29). Xu et al
(24) reported that SCU suppressed
activation of the NLRP3 inflammasome to protect against myocardial
I/R injury. Wang et al (23) found that SCU treatment protected
cardiomyocytes from I/R injury-induced oxidative stress and
apoptosis. In the present study, it was observed that SCU protected
against cardiomyocyte I/R injury by increasing cell viability and
decreasing apoptosis, and also improved the cardiac function of
I/R-injured mice.
Different cell death forms, such as autophagy,
ferroptosis and apoptosis, contribute to I/R injury. Deng et
al (30) provided evidence
that ferroptosis aggravated intestinal I/R injury. Huang et
al (31) reported that
inhibition of autophagy suppressed I/R injury-induced inflammation
and apoptosis. Apoptosis is a gene-regulated programmed cell death
process, and abnormal regulation of this process has been
associated with a variety of human diseases, including immune and
developmental disorders, neurodegeneration and cancer (32). Members of the Bcl-2 protein family
mainly regulate apoptosis via the mitochondrial pathway (33). The family contains pro-apoptotic
and anti-apoptotic proteins that share four conserved Bcl-2
homology (BH) domains and form complexes by binding to the common
BH3 domain (34). Bcl-2 itself is
a key regulator that inhibits cell death by reducing cell
permeability, cytochrome c release and calcium flow across
endoplasmic reticulum membranes (35). The pro-apoptotic protein Bax of the
Bcl-2 family is also an important regulator of apoptosis, mainly
promoting apoptosis by inducing the release of Caspase-3(36). Therefore, the Bcl-2/Bax ratio
determines the sensitivity of cells to apoptotic stimuli (37). When the Bcl-2/Bax ratio is
increased, apoptosis is inhibited, and when the Bcl-2/Bax ratio is
decreased, apoptosis is promoted (38). The present study demonstrated that
SCU reversed the I/R- or H/R-induced decrease in the Bcl-2/Bax
ratio, which indicated that SCU had a protective role in
apoptosis.
The inflammatory reaction is one of the most
important elements in myocardial I/R injury (39). cGAS is a critical cytosolic DNA
sensor with the function of generating cyclic GMP-AMP (40), which further binds and activates
STING (41), inducing a strong
innate immune response. Self-DNA leaked from the nucleus or
mitochondria can also serve as a cGAS ligand to activate this
pathway and trigger extensive inflammatory responses (42). The cGAS-STING signaling pathway is
recognized as a main mediator bridging innate and adaptive immunity
(43,44), which is involved in local tissue
inflammation (45). The cGAS
response to cardiac ischemia is similar to that of a pattern
recognition receptor in the sterile immune response (46). With regard to ischemic MI, cGAS can
sense cytoplasmic DNA released from dying ruptured cells and can
lead to fatal post-MI cardiac inflammation, which can be reversed
by inhibition of the cGAS-STING signaling pathway (47). In the present study, it was
revealed that H/R injury significantly increased the expression
levels of cGAS, STING and cleaved Caspase-3, and decreased the
Bcl-2/Bax ratio, which could be reversed by blocking the cGAS-STING
signaling pathway using RU.521 and H-151. These results indicated
that the cGAS-STING signaling pathway is involved in H/R
injury-induced apoptosis of H9c2 cells.
SCU has been indicated to exhibit an
anti-inflammatory effect in experimentally induced cerebral
ischemia (48). Yuan et al
(49) reported that SCU
effectively suppressed the inflammatory responses in activated
microglia, which were induced by cerebral ischemia, by decreasing
TNF-α expression. It has also been reported that SCU exerts an
anti-inflammatory effect to protect against myocardial I/R injury
by suppressing NLRP3 inflammasome activation (24). Furthermore, it has been
demonstrated that SCU exhibits strong anti-oxidative activity
against ischemic injury (50).
Zhang et al (51) reported
that SCU alleviated cerebral I/R by suppressing oxidative stress
and inflammatory responses via the MAPK/NF-κB signaling pathways in
rats. Wu and Jia (28) reported
that SCU attenuated H/R injury in hepatocytes by inhibiting
apoptosis and oxidative stress. It has also been demonstrated that
cellular oxidative stress activated the cGAS/STING/IFN-I signaling
pathway via FOXO3-regulated lamin post-translational modification
(52). However, to the best of our
knowledge, the effect of SCU on the cGAS-STING signaling pathway
has not been elucidated. The present study revealed that SCU
treatment reversed the I/R injury-induced upregulation of cGAS and
STING, which indicated that SCU can inhibit the cGAS-STING
signaling pathway. Considering the anti-oxidative stress role of
SCU (50) and the activation of
cGAS-STING by oxidative stress (52), inhibition of the cGAS-STING pathway
by SCU may be achieved by inhibiting oxidative stress, which should
be confirmed in future studies.
In summary, to the best of our knowledge, the
present study was the first to demonstrate that SCU ameliorated
cardiac dysfunction and protected cardiomyocytes from I/R
injury-induced apoptosis via deactivation of the cGAS-STING
signaling pathway. Considering the protective effect of SCU on
cardiomyocyte H/R injury, SCU may be a promising agent for the
treatment of IHD in the future.
Acknowledgements
The authors would like to thank the Academy of
Biological Sciences of Chongqing Medical University (Chongqing,
China) for providing experimental sites and equipment.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JKL contributed to performing the experiments and
data analysis. ZPS contributed to performing the experiments. XZH
contributed to data analysis and study design. JKL and XZH confirm
the authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The animal experiment was approved by the Animal
Ethics Committee of The People's Hospital of Yue Chi County
(Guang'an, China; approval no. 21000104).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wei D, Tang L, Su L, Zeng S, Telushi A,
Lang X, Zhang Y, Qin M, Qiu L, Zhong C and Yu J: Edgeworthia
gardneri (Wall.) Meisn. extract protects against myocardial
infarction by inhibiting NF-κB-and MAPK-mediated endothelial
inflammation. Front Cardiovasc Med. 9(1013013)2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Writing Committee for the VISION Study
Investigators. Devereaux PJ, Biccard BM, Sigamani A, Xavier D, Chan
MTV, Srinathan SK, Walsh M, Abraham V, Pearse R, et al: Association
of postoperative high-sensitivity troponin levels with myocardial
injury and 30-day mortality among patients undergoing noncardiac
surgery. JAMA. 317:1642–1651. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Li F, Li J, Li S, Guo S and Li P:
Modulatory effects of Chinese herbal medicines on energy metabolism
in ischemic heart diseases. Front Pharmacol. 11(995)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Keller K, Sagoschen I, Schmitt VH, Münzel
T, Gori T and Hobohm L: Hypothermia and its role in patients with
ST-segment-elevation myocardial infarction and cardiac arrest.
Front Cardiovasc Med. 9(1051978)2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Eltzschig HK and Eckle T: Ischemia and
reperfusion-from mechanism to translation. Nat Med. 17:1391–1401.
2011.PubMed/NCBI View
Article : Google Scholar
|
|
6
|
Khan SU, Rahman H, Arshad A, Khan MU,
Lekkala M, Yang TJ, Mishra A and Kaluski E: Percutaneous coronary
intervention versus surgery in left main stenosis-a meta-analysis
and systematic review of randomised controlled trials. Heart Lung
Circ. 27:138–146. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Heusch G: Cardioprotection: Chances and
challenges of its translation to the clinic. Lancet. 381:166–175.
2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lv B, Zhou J, He S, Zheng Y, Yang W, Liu
S, Liu C, Wang B, Li D and Lin J: Induction of myocardial
infarction and myocardial ischemia-reperfusion injury in mice. J
Vis Exp, 2022.
|
|
9
|
Scarabelli T, Stephanou A, Rayment N,
Pasini E, Comini L, Curello S, Ferrari R, Knight R and Latchman D:
Apoptosis of endothelial cells precedes myocyte cell apoptosis in
ischemia/reperfusion injury. Circulation. 104:253–256.
2001.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chen X, Li X, Zhang W, He J, Xu B, Lei B,
Wang Z, Cates C, Rousselle T and Li J: Activation of AMPK inhibits
inflammatory response during hypoxia and reoxygenation through
modulating JNK-mediated NF-κB pathway. Metabolism. 83:256–270.
2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Neri M, Fineschi V, Di Paolo M, Pomara C,
Riezzo I, Turillazzi E and Cerretani D: Cardiac oxidative stress
and inflammatory cytokines response after myocardial infarction.
Curr Vasc Pharmacol. 13:26–36. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Arslan F, Keogh B, McGuirk P and Parker
AE: TLR2 and TLR4 in ischemia reperfusion injury. Mediators
Inflamm. 2010(704202)2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ebrahimi H, Badalzadeh R, Mohammadi M and
Yousefi B: Diosgenin attenuates inflammatory response induced by
myocardial reperfusion injury: Role of mitochondrial ATP-sensitive
potassium channels. J Physiol Biochem. 70:425–432. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Civril F, Deimling T, de Oliveira Mann CC,
Ablasser A, Moldt M, Witte G, Witte G, Hornung V and Hopfner KP:
Structural mechanism of cytosolic DNA sensing by cGAS. Nature.
498:332–337. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gulen MF, Koch U, Haag SM, Schuler F,
Apetoh L, Villunger A, Radtke F and Ablasser A: Signalling strength
determines proapoptotic functions of STING. Nat Commun.
8(427)2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Heidegger S, Haas T and Poeck H: Cutting
edge in IFN regulation: Inflammatory caspases cleave cGAS.
Immunity. 46:333–335. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Liao Y, Cheng J, Kong X, Li S, Li X, Zhang
M, Zhang H, Yang T, Dong Y, Li J, et al: HDAC3 inhibition
ameliorates ischemia/reperfusion-induced brain injury by regulating
the microglial cGAS-STING pathway. Theranostics. 10:9644–9662.
2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Shi X, Chen G and Liu X, Qiu Y, Yang S,
Zhang Y, Fang X, Zhang C and Liu X: Scutellarein inhibits cancer
cell metastasis in vitro and attenuates the development of
fibrosarcoma in vivo. Int J Mol Med. 35:31–38.
2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hong H and Liu GQ: Protection against
hydrogen peroxide-induced cytotoxicity in PC12 cells by
scutellarin. Life Sci. 74:2959–2973. 2004.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yang N, Zhao Y, Wang Z, Liu Y and Zhang Y:
Scutellarin suppresses growth and causes apoptosis of human
colorectal cancer cells by regulating the p53 pathway. Mol Med Rep.
15:929–935. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Guo H, Hu LM, Wang SX, Wang YL, Shi F, Li
H, Liu Y, Kang LY and Gao XM: Neuroprotective effects of
scutellarin against hypoxic-ischemic-induced cerebral injury via
augmentation of antioxidant defense capacity. Chin J Physiol.
54:399–405. 2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhang HF, Hu XM, Wang LX, Xu SQ and Zeng
FD: Protective effects of scutellarin against cerebral ischemia in
rats: Evidence for inhibition of the apoptosis-inducing factor
pathway. Planta Med. 75:121–126. 2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wang Z, Yu J, Wu J, Qi F, Wang H, Wang Z
and Xu Z: Scutellarin protects cardiomyocyte ischemia-reperfusion
injury by reducing apoptosis and oxidative stress. Life Sci.
157:200–207. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Xu LJ, Chen RC, Ma XY, Zhu Y, Sun GB and
Sun XB: Scutellarin protects against myocardial
ischemia-reperfusion injury by suppressing NLRP3 inflammasome
activation. Phytomedicine. 68(153169)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hu XM, Zhou MM, Hu XM and Zeng FD:
Neuroprotective effects of scutellarin on rat neuronal damage
induced by cerebral ischemia/reperfusion. Acta Pharmacol Sin.
26:1454–1459. 2005.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Gao J, Chen G, He H, Liu C, Xiong X, Li J
and Wang J: Therapeutic effects of breviscapine in cardiovascular
diseases: A review. Front Pharmacol. 8(289)2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Li Y, Lu Y, Hu J, Gong Z, Yang W, Wang A,
Zheng J, Liu T, Chen T, Hu J, et al: Pharmacokinetic comparison of
scutellarin and paeoniflorin in sham-operated and middle cerebral
artery occlusion ischemia and reperfusion injury rats after
intravenous administration of Xin-Shao formula. Molecules.
21(1191)2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wu H and Jia L: Scutellarin attenuates
hypoxia/reoxygenation injury in hepatocytes by inhibiting apoptosis
and oxidative stress through regulating Keap1/Nrf2/ARE signaling.
Biosci Rep. 39(BSR20192501)2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lin LL, Liu AJ, Liu JG, Yu XH, Qin LP and
Su DF: Protective effects of scutellarin and breviscapine on brain
and heart ischemia in rats. J Cardiovasc Pharmacol. 50:327–332.
2007.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Deng F, Zhao BC, Yang X, Lin ZB, Sun QS,
Wang YF, Yan ZZ, Liu WF, Li C, Hu JJ and Liu KX: The gut microbiota
metabolite capsiate promotes Gpx4 expression by activating TRPV1 to
inhibit intestinal ischemia reperfusion-induced ferroptosis. Gut
Microbes. 13:1–21. 2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Huang KY, Que JQ, Hu ZS, Yu YW, Zhou YY,
Wang L, Xue YJ, Ji KT and Zhang XM: Metformin suppresses
inflammation and apoptosis of myocardiocytes by inhibiting
autophagy in a model of ischemia-reperfusion injury. Int J Biol
Sci. 16:2559–2579. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Thompson CB: Apoptosis in the pathogenesis
and treatment of disease. Science. 267:1456–1462. 1995.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Gross A, McDonnell JM and Korsmeyer SJ:
BCL-2 family members and the mitochondria in apoptosis. Genes Dev.
13:1899–1911. 1999.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Youle RJ and Strasser A: The BCL-2 protein
family: Opposing activities that mediate cell death. Nat Rev Mol
Cell Bio. 9:47–59. 2008.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Vaux DL, Cory S and Adams JM: Bcl-2 gene
promotes haemopoietic cell survival and cooperates with c-myc to
immortalize pre-B cells. Nature. 335:440–442. 1988.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Lee R, Kim DW, Lee WY and Park HJ:
Zearalenone induces apoptosis and autophagy in a spermatogonia cell
line. Toxins (Basel). 14(148)2022.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Siddiqui WA, Ahad A and Ahsan H: The
mystery of BCL2 family: Bcl-2 proteins and apoptosis: An update.
Arch Toxicol. 89:289–317. 2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Liu LS, Bai XQ, Gao Y, Wu Q, Ren Z, Li Q,
Pan LH, He NY, Peng J and Tang ZH: PCSK9 promotes oxLDL-induced
PC12 cell apoptosis through the Bcl-2/Bax-caspase 9/3 signaling
pathway. J Alzheimers Dis. 57:723–734. 2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Yao L, Chen H, Wu Q and Xie K:
Hydrogen-rich saline alleviates inflammation and apoptosis in
myocardial I/R injury via PINK-mediated autophagy. Int J Mol Med.
44:1048–1062. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Wu J, Sun L, Chen X, Du F, Shi H, Chen C
and Chen ZJ: Cyclic GMP-AMP is an endogenous second messenger in
innate immune signaling by cytosolic DNA. Science. 339:826–830.
2013.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Ishikawa H and Barber GN: STING is an
endoplasmic reticulum adaptor that facilitates innate immune
signalling. Nature. 455:674. 2008.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ma R, Ortiz Serrano TP, Davis J, Prigge AD
and Ridge KM: The cGAS-STING pathway: The role of self-DNA sensing
in inflammatory lung disease. FASEB J. 34:13156–13170.
2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Hao F: An overview of the crosstalk
between YAP and cGAS-STING signaling in non-small cell lung cancer:
It takes two to tango. Clin Transl Oncol. 24:1661–1672.
2022.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Xu D, Tian Y, Xia Q and Ke B: The
cGAS-STING pathway: Novel perspectives in liver diseases. Front
Immunol. 12(682736)2021.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Wan DS, Jiang W and Hao JW: Research
advances in how the cGAS-STING pathway controls the cellular
inflammatory response. Front Immunol. 11(615)2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Cao DJ, Schiattarella GG, Villalobos E,
Jiang N, May HI, Li T, Chen ZJ, Gillette TG and Hill JA: Cytosolic
DNA sensing promotes macrophage transformation and governs
myocardial ischemic injury. Circulation. 137:2613–2634.
2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
King KR, Aguirre AD, Ye YX, Sun Y, Roh JD,
Ng RP Jr, Kohler RH, Arlauckas SP, Iwamoto Y, Savol A, et al: IRF3
and type I interferons fuel a fatal response to myocardial
infarction. Nat Med. 23:1481–1487. 2017.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Chen HL, Jia WJ, Li HE, Han H, Li F, Zhang
XLN, Li JJ, Yuan Y and Wu CY: Scutellarin exerts anti-inflammatory
effects in activated microglia/brain macrophage in cerebral
ischemia and in activated BV-2 microglia through regulation of
MAPKs signaling pathway. Neuromolecular Med. 22:264–277.
2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Yuan Y, Zha H, Rangarajan P, Ling EA and
Wu C: Anti-inflammatory effects of edaravone and scutellarin in
activated microglia in experimentally induced ischemia injury in
rats and in BV-2 microglia. BMC Neurosci. 15(125)2014.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Deng M, Sun J, Peng L, Huang Y, Jiang W,
Wu S, Zhou L, Chung S and Cheng X: Scutellarin acts on the AR-NOX
axis to remediate oxidative stress injury in a mouse model of
cerebral ischemia/reperfusion injury. Phytomedicine.
103(154214)2022.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Zhang Y, Zhang Z, Wang J, Zhang X, Zhao J,
Bai N, Vijayalakshmi A and Huo Q: Scutellarin alleviates cerebral
ischemia/reperfusion by suppressing oxidative stress and
inflammatory responses via MAPK/NF-κB pathways in rats. Environ
Toxicol. 37:2889–2896. 2022.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Hwang I, Uchida H, Dai Z, Li F, Sanchez T,
Locasale JW, Cantley LC, Zheng H and Paik J: Cellular stress
signaling activates type-I IFN response through FOXO3-regulated
lamin posttranslational modification. Nat Commun.
12(640)2021.PubMed/NCBI View Article : Google Scholar
|



















