Introduction
Ischemic heart disease is the most common
cardiovascular disease (1). With
the ageing of the population, the incidence of ischemic heart
disease is increasing every year in China (2). During 2007-2008 in an urban community
of Romania, it was estimated that the incidence of ischemic heart
disease was considerably higher in elderly women than in men, with
an increase from 55.7 to 65.1% in women and from 47.4 to 60.8% in
men (3). Intravenous thrombolysis,
percutaneous coronary intervention and coronary artery bypass
grafting have improved the long-term prognosis of patients with
acute myocardial infarction. However, myocardial
ischemia-reperfusion (I/R) injury (MIRI) caused by the
aforementioned treatments is a serious complication (4). MIRI refers to the pathological
process of progressive aggravation of myocardial tissue damage
following reperfusion of interrupted coronary blood flow, which can
lead to severe arrhythmia and sudden death (5). However, to the best of our knowledge,
there is no effective MIRI prevention strategy in clinical
practice.
Tranilast, with the chemical formula
N-(3',4'-dimethoxycinnamoyl) anthranilic acid, is an analogue of a
metabolite of tryptophan (Fig.
1A), which is used to treat allergic disease, such as bronchial
asthma, allergic rhinitis, dermatitis and conjunctivitis (6). A previous study demonstrated that
long-term use of tranilast could inhibit allergic reactions in
patients with bronchial asthma (7). The anticancer effects of tranilast
via different mechanisms have been recently demonstrated (8). Additionally, a study suggested that
tranilast could exert a significant therapeutic effect on heart
disease (9). Moreover, recent
studies showed that tranilast prevents doxorubicin-induced
myocardial hypertrophy (10) and
decreases myocardial fibrosis in diabetic rats via the TGF-β/SMAD
signalling pathway (11).
Furthermore, tranilast attenuates cerebral IR injury in rats via
NF-κB and peroxisome proliferator-activated receptor-mediated
inflammatory responses (12) and
improves myocardial infarction in mice by inhibiting nucleotide
binding oligomerization domain-like receptor family pyrin domain
containing-3 inflammasome and affecting the phenotype of
macrophages (13). However, to the
best of our knowledge, the effect of tranilast on MIRI remains
unclear.
Tranilast is a selective transient receptor
potential vanilloid type 2 (TRPV2) inhibitor. A study demonstrated
that TRPV2 downregulation protects against MIRI (14), while TRPV2 knockdown partially
reverses the effect of H2O2 on nuclear factor
erythroid 2-related factor 2 (Nrf2) inhibition in hepatocellular
carcinoma (15). In addition,
activation of the Nrf2/heme oxygenase-1 (HO-1)/NF-κB signalling
pathway protects the heart during MIRI (16).
It was hypothesized that tranilast decreases
I/R-induced cardiomyocyte injury via Nrf2/HO-1/NF-κB signalling.
Therefore, the present study aimed to investigate if tranilast
attenuates I/R-induced cardiomyocyte injury by using a
hypoxia/reoxygenation (H/R) model in H9c2 cardiomyocytes to trigger
I/R-stimulated cardiomyocyte injury.
Materials and methods
Cell culture and establishment of the
in vitro H/R model
H9c2 cardiomyocytes were obtained from the American
Type Culture Collection (cat. no. CRL-1446) and cultured in DMEM
supplemented with 10% FBS (Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin solution at 37˚C in an incubator with 5%
CO2. Cells were treated with of tranilast (25, 50 and
100 µM; cat. no. S1439; Selleck Chemicals) for 24 h, as previously
described (9,17). To establish the H/R in vitro
model, 3 ml H9c2 cell suspension in 10% FBS-DMEM at a density of
1x106 cells/ml was plated onto a 60-mm dish and cells
were cultured in an incubator with 5% CO2 at 37˚C. When
90% confluency was reached, cells were exposed to H/R. Briefly,
H9c2 cardiomyocytes were incubated in serum- and glucose-free DMEM
(Thermo Fisher Scientific, Inc.) at 37˚C in a hypoxic incubator
with 5% CO2 and 95% N2 for 2 h to induce
hypoxia. For reoxygenation, cells were transferred to normal
culture conditions with routine culture medium in a normal oxygen
environment (74% N2, 5% CO2 and 21%
O2) at 37˚C for 24 h (15). H9c2 cells cultured in complete
culture medium were referred to the Control group. H9c2 cells were
pre-treated with 25, 50 or 100 µM tranilast for 24 h at 37˚C prior
to being subjected to hypoxia for 2 h and reoxygenation for 4 h.
Furthermore, to verify if tranilast exerts its effect on I/R injury
via the Nrf2/HO-1/NF-κB signalling pathway, H9c2 cells were
pre-treated with 20 µM ML-385, an Nrf2 inhibitor, for 1 h at 37˚C
prior to treatment with tranilast for 24 h and exposure to hypoxic
conditions.
Cell Counting Kit-8 (CCK-8) assay
H9c2 cells were seeded into a 96-well plate at a
density of 5x103 cells/well and incubated for 12 h at
37˚C. Cells were treated with 25, 50 or 100 µM tranilast with the
absence or presence of H/R exposure and a CCK-8 assay (Beyotime
Institute of Biotechnology) was then used to assess cell viability.
Briefly, each well of the 96-well plate was supplemented with 10 µl
CCK-8 reagent, followed by incubation in a humidified incubator
with 5% CO2 at 37˚C for 2 h. Finally, absorbance of each
well was detected at a wavelength of 450 nm using the Model 680
Microplate Reader (Bio-Rad Laboratories, Inc.).
TUNEL assay
H9c2 cardiomyocytes were fixed in xylene for 15 min
at room temperature, blocked with 5% goat serum (Thermo Fisher
Scientific, Inc.) for 30 min at room temperature and incubated with
antibody provided with the TUNEL kit (cat no. KA4159; Abnova GmbH)
at 4˚C overnight. Slides were incubated with a TUNEL reaction
mixture for 1 h at 37˚C in the dark followed by treatment with
0.05% DAPI for 10 min in the dark at room temperature. Apoptotic
cells were observed under a fluorescence microscope (magnification,
x200; Olympus Corporation) from five random fields of views and
were then quantified using the Image-Pro Plus 6.0 software (Media
Cybernetics).
Western blot analysis
H9c2 cardiomyocytes from each group were collected
and total protein was extracted after cell lysis in RIPA buffer
(Protech Technology Enterprise Co., Ltd.) at 4˚C for 20 min,
followed by centrifugation at 8,798 x g for 10 min at 4˚C. Protein
concentration was measured using a BCA kit (cat. no. A045-4-2;
Nanjing Jiancheng Bioengineering Institute). Total protein extract
from each group (20 µg/lane) was separated by SDS-PAGE on a 10% gel
and transferred onto PVDF membranes (EMD Millipore). Following
blocking with 5% skimmed milk for 2 h at room temperature,
membranes were incubated with the following primary antibodies at
4˚C overnight: Anti-B-cell lymphoma 2 (Bcl-2; cat. no. ab196495;
1:1,000); anti-Bcl-2-associated X protein (Bax; cat. no. ab32503;
1:1,000); anti-poly(ADP-ribose) polymerase (PARP; cat. no.
ab191217; 1:1,000); anti-cleaved PARP (cat. no. ab32064; 1:1,000);
anti-Nrf2 (cat. no. ab92946; 1:1,000) and anti-HO-1 (cat. no.
ab189491; 1:2,000; all Abcam); anti-phosphorylated (p)-NF-κB (cat.
no. 3033; 1:1,000) and anti-NF-κB (cat. no. 8242; 1:1,000; both
Cell Signaling Technology, Inc.) and β-actin (cat. no. ab8227;
1:1,000; Abcam). The membranes were incubated with corresponding
horseradish peroxidase-conjugated secondary antibody (cat. no.
ab6721; 1:2,000; Abcam) for 1 h at room temperature. The protein
bands were visualized using ECL Western Blotting Substrate (Pierce;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
instructions. Densitometry analysis was performed using ImageJ
1.52a software (National Institutes of Health) with β-actin as the
loading control.
Detection of oxidative stress
indicators
Following treatment with 25, 50 or 100 µM tranilast
prior to H/R exposure, H9c2 cardiomyocytes were cultured in the
presence of 10 µM dichloro-dihydro-fluorescein diacetate solution
at 37˚C for 20 min in the dark, according to the manufacturer's
instructions [reactive oxygen species (ROS) assay kit; cat. no.
S0033S; Beyotime Institute of Biotechnology]. The production of
intracellular ROS was detected using a fluorescence microscope
(magnification, x400; Olympus Corporation) and results were
analyzed using ImageJ 1.8.0 software (National Institutes of
Health). Lysates were obtained following centrifugation at 10,000 x
g at 4˚C for 12 min. The levels of malondialdehyde (MDA) and
activity of superoxide dismutase (SOD) and glutathione peroxidase
(GSH-Px) were detected using corresponding kits according to the
manufacturer's instructions (cat. nos. A003-1-2, A001-3-2 and
A006-2-1, respectively; Nanjing Jiancheng Bioengineering Institute)
at wavelengths of 532, 450 and 405 nm, respectively.
ELISA
The cell culture supernatant from each group of H9c2
cardiomyocytes was collected via centrifugation at 1,000 x g for 5
min at 4˚C. The secretion of TNF-α, IL-6 and IL-8 was detected
using the TNF-α (cat. no. PT516; Beyotime Institute of
Biotechnology), IL-6 (cat. no. PI328; Beyotime Institute of
Biotechnology) and IL-8 (cat. no. SEKR-0071; Beijing Solarbio
Science & Technology Co., Ltd.) ELISA kits according to the
manufacturer's instructions, respectively.
Statistical analysis
All statistical analysis was performed using
GraphPad Prism 8 (GraphPad Software, Inc.; Dotmatics). The
differences between multiple groups were assessed using one-way
ANOVA followed by Tukey's post hoc test. All data are expressed as
the mean ± standard deviation of three independent experimental
repeats and exhibited normal distribution. P<0.05 was considered
to indicate a statistically significant difference.
Results
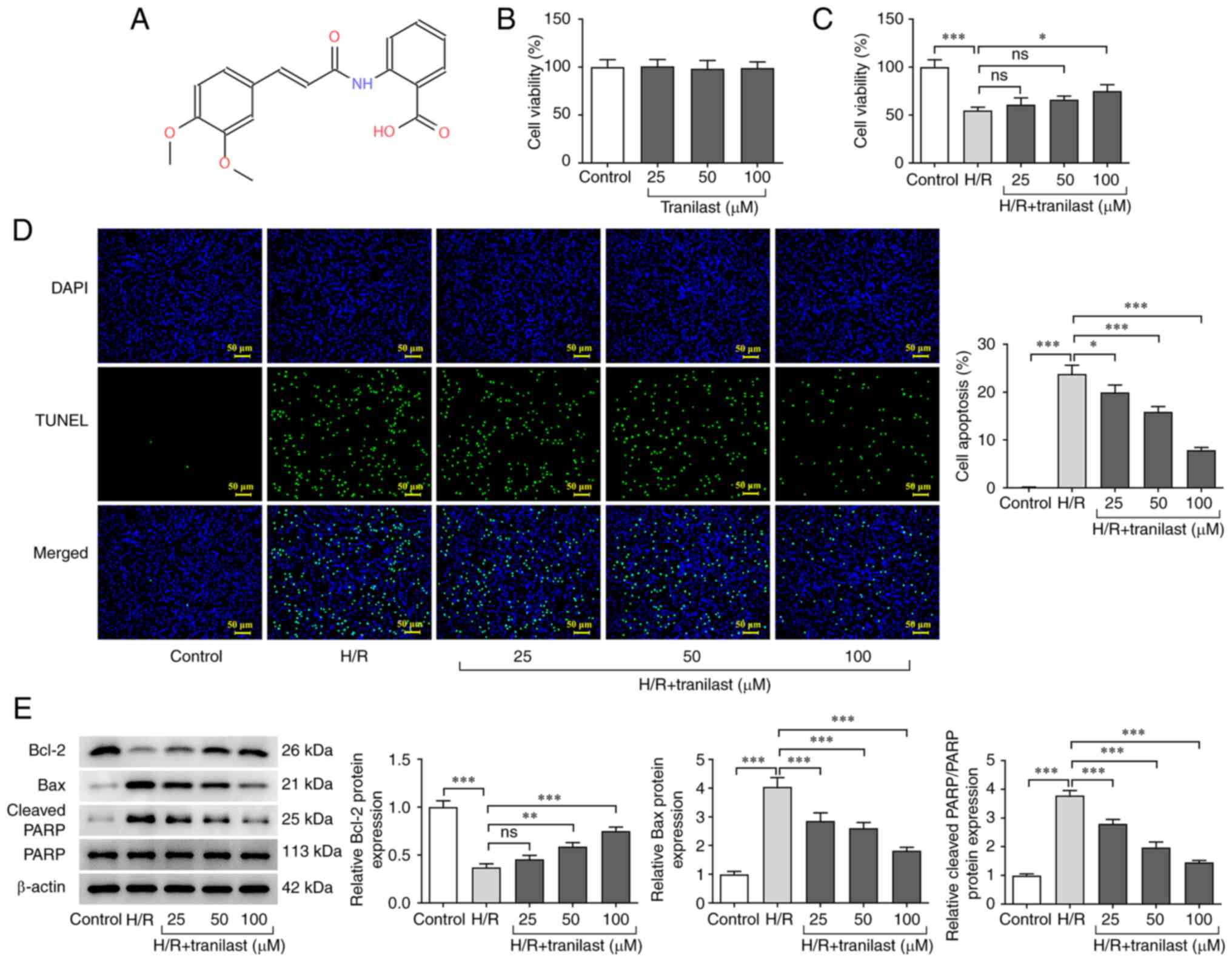
Tranilast promotes H/R-induced H9c2
cell viability
To investigate if different concentrations of
tranilast affect viability of H9c2 cells or H/R-induced H9c2 cells,
a CCK-8 assay was performed. In addition, the apoptosis rate of
H/R-induced H9c2 cells in the presence or absence of tranilast was
determined using TUNEL assay and western blot analysis. Following
exposure of H9c2 cells to tranilast, viability was not
significantly affected (Fig. 1B).
However, cell viability (Fig. 1C)
was decreased and apoptosis (Fig.
1D) was increased in H9c2 cells subjected to H/R. The
aforementioned effects were reversed following treatment with
tranilast and this effect was significant only at 100 µM of
tranilast. The expression levels of apoptosis-related proteins
Bcl-2 were decreased and Bax/cleaved PARP were increased in
H/R-induced H9c2 cells; these effects were also reversed by
tranilast treatment (Fig. 1E).
These findings indicated that tranilast increased cell viability
and decreased the apoptotic rate in H/R-induced H9c2 cells.
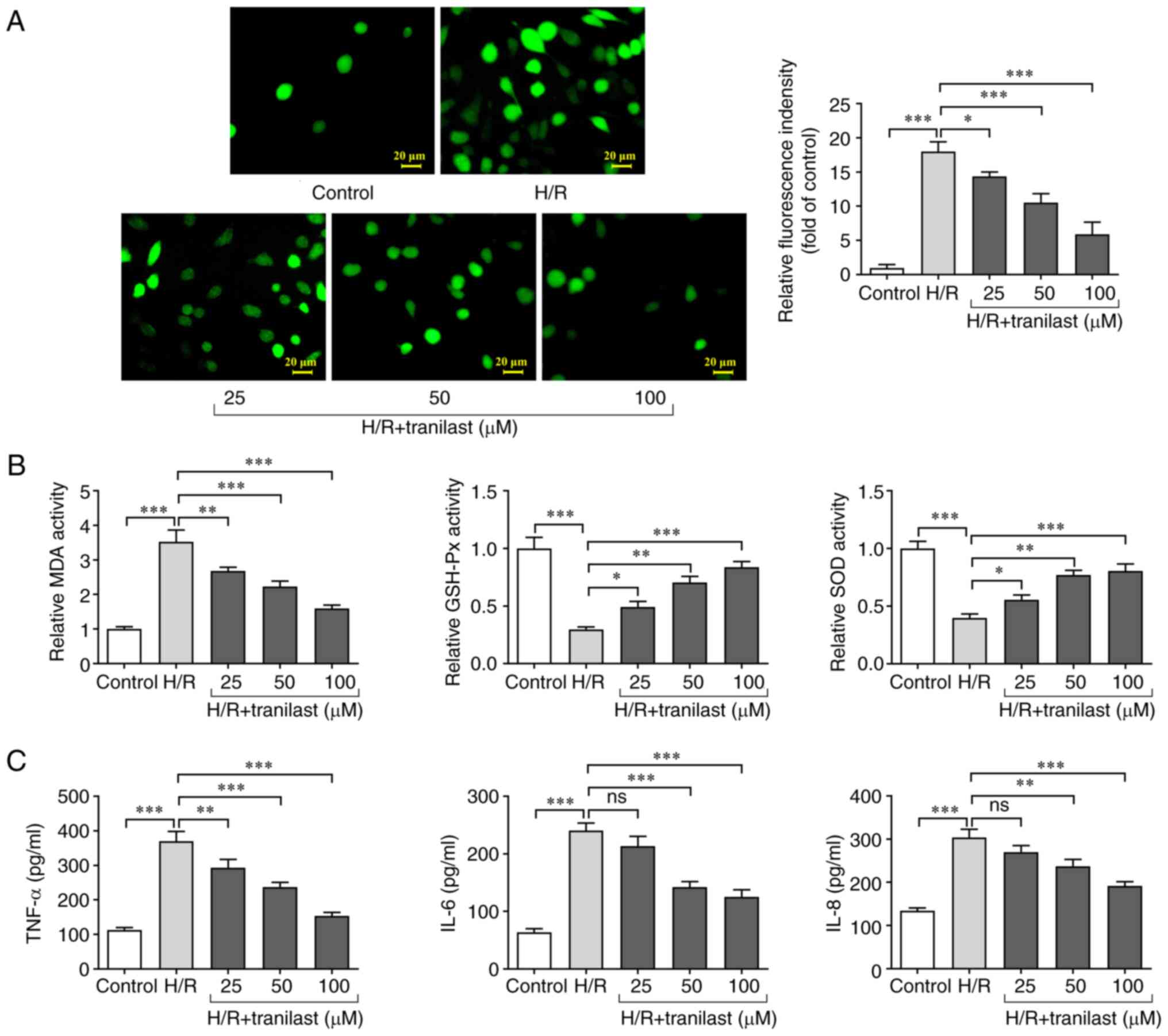
Tranilast attenuates oxidative stress
and inflammatory response in H/R-induced H9c2 cells
Oxidative stress and inflammatory response in
H/R-induced H9c2 cells in the presence or absence of tranilast were
assessed. The results showed that the levels of ROS and MDA were
increased, while activity of GSH-Px and SOD decreased following H/R
injury. However, treatment of H/R-induced H9c2 cells with tranilast
decreased ROS and MDA levels (Fig.
2A) whereas it increased activity of GSH-Px and SOD (Fig. 2B). Furthermore, ELISA revealed that
the secretion of TNF-α, IL-6 and IL-8 increased in H/R-induced H9c2
cells while these effects were reversed by tranilast pre-treatment
and no significant changes were observed in IL-6 and IL-8 levels at
25 µM of tranilast (Fig. 2C).
These findings demonstrated that the H/R-mediated increase in
oxidative stress and inflammatory response in H9c2 cells was
suppressed by tranilast.
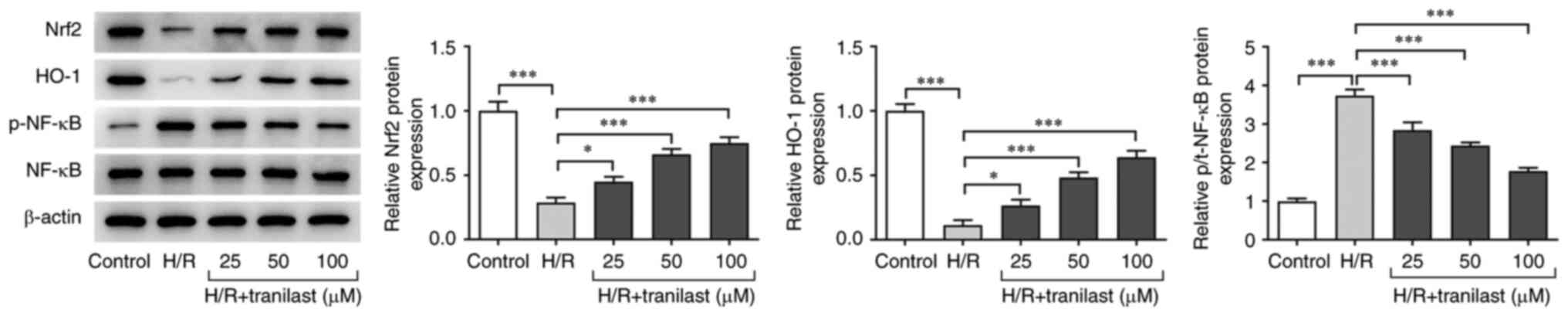
Tranilast activates Nrf2/HO-1/NF-κB
signalling in H/R-induced H9c2 cells
Western blot analysis was performed to investigate
the role of Nrf2/HO-1/NF-κB signalling in the protective effects of
tranilast on H/R-induced H9c2 cells. Nrf2 and HO-1 were upregulated
while p-NF-κB was downregulated in H/R-induced H9c2 cells. However,
the effect of H/R on Nrf2/HO-1/NF-κB signalling was reversed in a
dose-dependent manner following pre-treatment with 25-100 µM
tranilast (Fig. 3). These results
indicated that tranilast treatment reversed the effects of H/R
exposure on Nrf2/HO-1/NF-κB signalling in H9c2 cells.
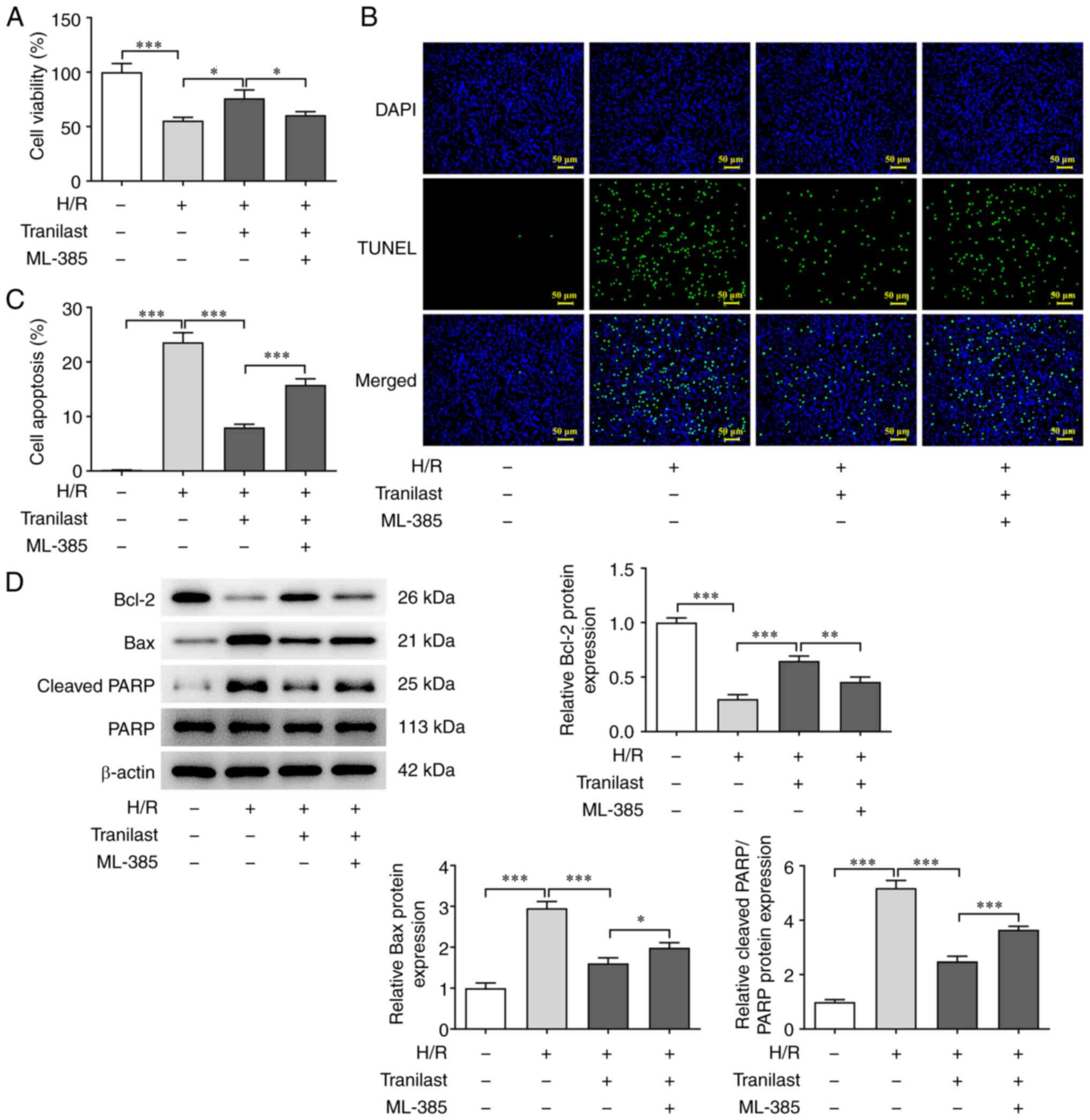
Tranilast enhances viability of
H/R-induced H9c2 cells via Nrf2/HO-1/NF-κB signalling
To investigate the role of Nrf2/HO-1/NF-κB
signalling in the promoting effect of tranilast on H/R-induced H9c2
cell viability, cells were co-treated with ML-385, an Nrf2
inhibitor. Viability and apoptosis of H/R-induced H9c2 cells
co-treated with tranilast and ML-385 were assessed by CCK-8 and
TUNEL assay and western blot analysis. To investigate the effect of
tranilast on H/R-induced H9c2 cells, a concentration of 100 µM
tranilast was selected for subsequent experiments as 100 µM
tranilast exhibited the greatest effect. Pre-treatment with ML-385
of tranilast-treated H/R-induced H9c2 cells decreased viability
(Fig. 4A) and increased apoptosis
(Fig. 4B and C). Pre-treatment with ML-385
downregulated Bcl-2 and upregulated both Bax and cleaved PARP in
tranilast-treated H/R-exposed H9c2 cells (Fig. 4D). Overall, ML-385 suppressed Nrf2
expression, thus suggesting that Nrf2 interference inhibited the
promotive effect of tranilast on H/R-induced H9c2 cell
viability.
Tranilast decreases H/R-induced
oxidative stress and inflammatory response in H9c2 cells via
Nrf2/HO-1 signalling
To investigate the role of Nrf2/HO-1/NF-κB
signalling in the inhibitory effect of tranilast on oxidative
stress and inflammatory response, H/R-induced H9c2 cells were
pre-treated with ML-385. Oxidative stress and inflammatory response
were evaluated using assay kits. The levels of ROS and MDA
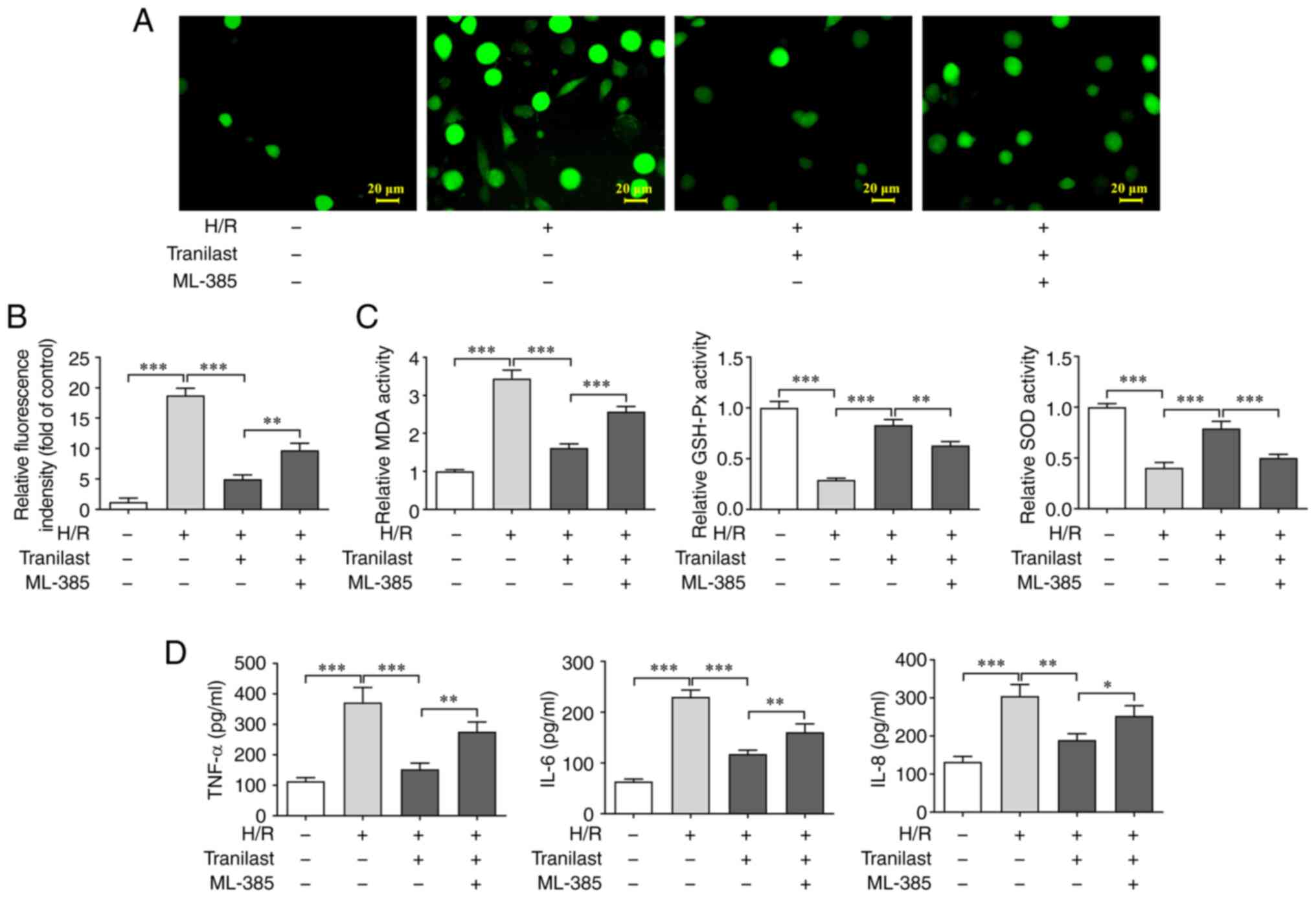
(Fig. 5A and B) were increased, while activity of
GSH-Px and SOD (Fig. 5C) was
decreased in H/R-induced H9c2 cells pre-treated with ML-385 prior
to tranilast treatment. Additionally, the secretion of TNF-α, IL-6
and IL-8 were increased in H/R-induced H9c2 cells pre-treated with
ML-385 prior to tranilast treatment (Fig. 5D). These findings demonstrated that
tranilast exerted the inhibitory effect on oxidative stress and
inflammatory response in H/R-induced H9c2 cells via activating
Nrf2/HO-1 signalling.
Discussion
Tranilast is a fatty cell membrane stabilizer,
commonly used in clinical trials and practice as an antiallergic
agent (6,7). Tranilast exhibits antifibrotic
effects in the myocardium (18)
and inhibits inflammatory responses (19-21)
and oxidative stress (22). The
results of the present study indicated that tranilast decreased
secretion levels of IL-6, TNF-α and IL-8 in H/R-induced H9c2 cells.
Inflammatory response serve a critical role in MIRI (23). In addition to the release of
cytokines, such as TNF-α and IL-6, inflammation is associated with
neutrophilic granulocyte-activating cytokines and adhesion
molecules, as well as vascular endothelial cell injury (24). Here, treatment of H/R-induced H9c2
cells with tranilast increased activity of GSH-Px and SOD while
decreasing levels of MDA. Oxidative stress is also associated with
MIRI by promoting ROS production and exacerbating myocardial injury
(24). Excessive ROS react with
lipids to produce MDA (25). In
addition, ROS overproduction is eliminated by endogenous
antioxidants, leading to depletion of cell storage and cell
structure damage (26). SOD is an
important oxygen-free scavenger in the human body based on
superoxide anion-free radical (O2-). SOD
catalyzes its disproportionation into H2O2,
which is reduced to water through a reaction with glutathione under
the catalysis of GSH-Px (27).
When cells are exposed to high levels of ROS, they trigger
inflammatory cascades and expression of adhesion molecules, thus
promoting pro-inflammatory responses characterized by increased
levels of TNF-α, IL-1β, IL-6 and IL-8(28). Additionally, adhesion molecules,
such as vascular cell adhesion molecule-1 and intercellular
adhesion molecule-1, are upregulated, thus inducing oxidative
stress (29). The present study
demonstrated that inflammation and oxidative stress were aggravated
in H/R-induced H9c2 cells, accompanied by increased levels of
inflammatory factors TNF-α, IL-6 and IL-8, enhanced levels of ROS
and MDA, and decreased activities of GSH-Px and SOD.
Nrf2/HO-1 signalling is involved in antioxidant and
anti-inflammatory processes, decreased mitochondrial damage and
regulation of Ca2+ influx and cell death, ultimately
affecting the outcome of several diseases, such as lung diseases,
cardiovascular disease and neurological disorders and so on
(30). A previous study
demonstrated that the Nrf2/HO-1 axis is involved in
resveratrol-induced inflammation and oxidative stress in rats with
MIRI via downregulating the inflammatory factor myeloperoxidase,
decreasing MDA content and enhancing SOD and GSH-Px levels
(31). Additionally, Konrad et
al (32) revealed that HO-1
activation attenuates cytoskeletal actin remodelling and the
migration of chemokine C-X-C motif ligand 1-related
polymorphonuclear leukocytes into the alveolar septa and decreases
microvascular endothelial permeability in a conditional HO-1
knockout [flanked by loxP (FLOX/FLOX)] pneumonia mouse model
compared with wild-type mice. The same study also showed that HO-1
knockout stabilizes lung barrier function, thus exerting
anti-inflammatory effects. In terms of oxidative stress, a study
showed that the levels of TNF-α and IL-1β in hippocampus tissues
are significantly lower in the rats that received Nrf2 activator
sulforaphane compared with the rats that received treatment with
advanced glycation end products, while activity of SOD, GSH-Px and
catalase was enhanced (33). The
aforementioned studies demonstrated that the Nrf2/HO-1 axis
exhibits both anti-inflammatory and antioxidant activity. A
previous study also demonstrated that MIRI activates NF-κB to
promote its translocation into the nucleus, where it serves as a
specific DNA target sequence and transcriptionally regulates
release of inflammatory factors, such as IL-1, IL-6, TNF-α and
IL-8, thus promoting inflammatory responses and aggravating
myocardial tissue injury (34). In
the present study, Nrf2/HO-1 signalling was suppressed and NF-κB
was activated in H/R-induced H9c2 cells. Furthermore,
Nrf2/HO-1/NF-κB signalling inactivation decreased viability and
promoted apoptosis, inflammation and oxidative stress of
H/R-induced H9c2 cells treated with tranilast.
The present study has limitations. Firstly,
experiments were only performed on a single cell line. Therefore,
further experiments on more cell lines and animal models should be
performed in future. Secondly, the effect of tranilast on treating
I/R-induced cardiomyocyte injury in clinical practice was not
investigated.
In conclusion, the present study demonstrated that
tranilast improved viability and alleviated apoptosis, inflammation
and oxidative stress of H/R-induced H9c2 cells by activating
Nrf2/HO-1/NF-κB signaling. The aforementioned effects were
suppressed following treatment with the Nrf2 inhibitor ML-385.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WW and QS designed the study, performed the
experiments and revised the manuscript. WW analyzed the data. QS
interpreted the data and drafted the manuscript. All authors have
read and approved the final manuscript. WW and QS confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kang IS and Kwon K: Potential application
of biomimetic exosomes in cardiovascular disease: Focused on
ischemic heart disease. BMB Rep. 55:30–38. 2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Chang J, Li B, Li J and Sun Y: The effects
of age, period, and cohort on mortality from ischemic heart disease
in China. Int J Environ Res Public Health. 14(50)2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Pop D, Dădârlat A, Zdrenghea M, Zdrenghea
DT and Sitar-Tăut AV: Evolution of cardiovascular risk factors and
ischemic heart disease in an elderly urban Romanian population over
the course of 1 year. Clin Interv Aging. 8:1497–1503.
2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Al-Herz W and Babiker F: Acute intravenous
infusion of immunoglobulins protects against myocardial
ischemia-reperfusion injury through inhibition of caspase-3. Cell
Physiol Biochem. 42:2295–2306. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wu XY, Miao L, Zheng R and Fan GW:
Research progress of myocardial ischemia-reperfusion injury. J Clin
Pharmacol. 32(1043)2016.(In Chinese).
|
|
6
|
Isaji M, Aruga N, Naito J and Miyata H:
Inhibition by tranilast of collagen accumulation in hypersensitive
granulomatous inflammation in vivo and of morphological changes and
functions of fibroblasts in vitro. Life Sci. 55:Pl287–P1292.
1994.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sun M and Hou M: Research progress of
clinical application of Trinistast. Chin J Dermatovenereology
Integr Trad Western Med. 17:87–90. 2018.(In Chinese).
|
|
8
|
Osman S, Raza A, Al-Zaidan L, Inchakalody
VP, Merhi M, Prabhu KS, Abdelaziz N, Hydrose S, Uddin S and Dermime
S: Anti-cancer effects of Tranilast: An update. Biomed
Pharmacother. 141(111844)2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Pfab T and Hocher B: Tranilast and
hypertensive heart disease: Further insights into mechanisms of an
anti-inflammatory and anti-fibrotic drug. J Hypertens. 22:883–886.
2004.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhan C, Bai N, Zheng M, Wang Y, Wang Y,
Zhang L, Li J, Li G, Zhao H, Liu G, et al: Tranilast prevents
doxorubicin-induced myocardial hypertrophy and angiotensin II
synthesis in rats. Life Sci. 267(118984)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Li X, Liu J and Shang X: Effect of
tranilast on myocardial fibrosis in diabetes rats through
TGF-β/Smad pathway. Minerva Med. 112:153–154. 2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhuo Y and Zhuo J: Tranilast treatment
attenuates cerebral ischemia-reperfusion injury in rats through the
inhibition of inflammatory responses mediated by NF-κB and PPARs.
Clin Transl Sci. 12:196–202. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Qu D, Guo H and Xu Y: Effects of tranilast
on inflammasome and macrophage phenotype in a mouse model of
myocardial infarction. J Interferon Cytokine Res. 41:102–110.
2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Li Y, Li Q, Zhang O, Guan X, Xue Y, Li S,
Zhuang X, Zhou B and Miao G: miR-202-5p protects rat against
myocardial ischemia reperfusion injury by downregulating the
expression of Trpv2 to attenuate the Ca2+ overload in
cardiomyocytes. J Cell Biochem. 120:13680–13693. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ma W, Li C, Yin S, Liu J, Gao C, Lin Z,
Huang R, Huang J and Li Z: Novel role of TRPV2 in promoting the
cytotoxicity of H2O2-mediated oxidative stress in human hepatoma
cells. Free Radic Biol Med. 89:1003–1013. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Liu K, Wang F, Wang S, Li WN and Ye Q:
Mangiferin attenuates myocardial ischemia-reperfusion injury via
MAPK/Nrf-2/HO-1/NF-κB in vitro and in vivo. Oxid Med Cell Longev.
2019(7285434)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhuang T, Li S, Yi X, Guo S, Wang Y, Chen
J, Liu L, Jian Z, Gao T, Kang P and Li C: Tranilast directly
targets NLRP3 to protect melanocytes from keratinocyte-derived
IL-1β under oxidative stress. Front Cell Dev Biol.
8(588)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wen C, Xie G, Zeng P, Huang LF and Chen
CY: Tranilast inhibits myocardial fibrosis in mice with viral
myocarditis. Zhongguo Dang Dai Er Ke Za Zhi. 18:446–454.
2016.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
19
|
Pae HO, Jeong SO, Koo BS, Ha HY, Lee KM
and Chung HT: Tranilast, an orally active anti-allergic drug,
up-regulates the anti-inflammatory heme oxygenase-1 expression but
down-regulates the pro-inflammatory cyclooxygenase-2 and inducible
nitric oxide synthase expression in RAW264.7 macrophages. Biochem
Biophys Res Commun. 371:361–365. 2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chen S, Wang Y, Pan Y, Liu Y, Zheng S,
Ding K, Mu K, Yuan Y, Li Z, Song H, et al: Novel role for tranilast
in regulating NLRP3 ubiquitination, vascular inflammation, and
atherosclerosis. J Am Heart Assoc. 9(e015513)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Shiota N, Kovanen PT, Eklund KK, Shibata
N, Shimoura K, Niibayashi T, Shimbori C and Okunishi H: The
anti-allergic compound tranilast attenuates inflammation and
inhibits bone destruction in collagen-induced arthritis in mice. Br
J Pharmacol. 159:626–635. 2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tan SM, Zhang Y, Cox AJ, Kelly DJ and Qi
W: Tranilast attenuates the up-regulation of
thioredoxin-interacting protein and oxidative stress in an
experimental model of diabetic nephropathy. Nephrol Dial
Transplant. 26:100–110. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Jian J, Xuan F, Qin F and Huang R: The
antioxidant, anti-inflammatory and anti-apoptotic activities of the
bauhinia championii flavone are connected with protection against
myocardial ischemia/reperfusion injury. Cell Physiol Biochem.
38:1365–1375. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hu R, Chen ZF, Yan J, Li QF, Huang Y, Xu
H, Zhang XP and Jiang H: Endoplasmic reticulum stress of
neutrophils is required for ischemia/reperfusion-induced acute lung
injury. J Immunol. 195:4802–4809. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sani M, Ghanem-Boughanmi N, Gadacha W,
Sebai H, Boughattas NA, Reinberg A and Ben-Attia M: Malondialdehyde
content and circadian variations in brain, kidney, liver, and
plasma of mice. Chronobiol Int. 24:671–685. 2007.PubMed/NCBI View Article : Google Scholar
|
|
26
|
da Cunha MJ, da Cunha AA, Loureiro SO,
Machado FR, Schmitz F, Kolling J, Marques EP and Wyse ATS:
Experimental lung injury promotes changes in oxidative/nitrative
status and inflammatory markers in cerebral cortex of rats. Mol
Neurobiol. 52:1590–1600. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Treviño S, Aguilar-Alonso P, Flores
Hernandez JA, Brambila E, Guevara J, Flores G, Lopez-Lopez G,
Muñoz-Arenas G, Morales-Medina JC, Toxqui V, et al: A high calorie
diet causes memory loss, metabolic syndrome and oxidative stress
into hippocampus and temporal cortex of rats. Synapse. 69:421–433.
2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wang H, Zhou H, Duan X, Jotwani R,
Vuddaraju H, Liang S, Scott DA and Lamont RJ: Porphyromonas
gingivalis-induced reactive oxygen species activate JAK2 and
regulate production of inflammatory cytokines through c-Jun. Infect
Immun. 82:4118–4126. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Sundd P, Gladwin MT and Novelli EM:
Pathophysiology of sickle cell disease. Annu Rev Pathol.
14:263–292. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wang T, Chen C, Yang L, Zeng Z and Zhao M:
Role of Nrf2/HO-1 signal axis in the mechanisms for oxidative
stress-relevant diseases. Zhong Nan Da Xue Xue Bao Yi Xue Ban.
44:74–80. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Cheng L, Jin Z, Zhao R, Ren K, Deng C and
Yu S: Resveratrol attenuates inflammation and oxidative stress
induced by myocardial ischemia-reperfusion injury: Role of Nrf2/ARE
pathway. Int J Clin Exp Med. 8:10420–10428. 2015.PubMed/NCBI
|
|
32
|
Konrad FM, Knausberg U, Höne R, Ngamsri KC
and Reutershan J: Tissue heme oxygenase-1 exerts anti-inflammatory
effects on LPS-induced pulmonary inflammation. Mucosal Immunol.
9:98–111. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Dong S, Xu S, Hou X, Luo D, Chen J and Liu
X: Anti-inflammatory and anti-oxidative protective effects of Nrf2
activation on AGEs-induced hippocampal damage in rats. Stroke Nerv
Dis. 24:388–392. 2017.
|
|
34
|
Kono H, Nakagawa K, Morita S, Shinoda K,
Mizuno R, Kikuchi E, Miyajima A, Umezawa K and Oya M: Effect of a
novel nuclear factor-κB activation inhibitor on renal
ischemia-reperfusion injury. Transplantation. 96:863–870.
2013.PubMed/NCBI View Article : Google Scholar
|