Introduction
Hepatic ischemia-reperfusion (IR) injury is a
biphasic condition defined by a transient interruption in the blood
supply to the liver followed by rapid reperfusion (1). Despite developments in liver surgery
protocols, IR injury is a concern in hepatic surgery that has an
impact on postoperative morbidity and mortality (1). Therefore, it is a concern in
hepatobiliary surgery, particularly liver resection and
transplantation surgery, in which graft dysfunction is still a
problem (1). The best course of
action remains a matter of debate.
Due to its intensive metabolic functions, the liver
is highly sensitive to redox disturbance. Increased reactive oxygen
species (ROS) have been related to a series of molecular events
that result in hepatocellular injury (2). Therefore, antioxidants are being used
in current research on the treatment of hepatic IR injury to avoid
the formation of excessive ROS (3-5).
Cerium oxide (Co) is one of several potent ROS
scavengers and its antioxidant effects have piqued attention in the
medical industry (6,7). Consequently, Co has been considered a
therapeutic agent not only in hepatic IR (8) but also to treat stroke (9), ovarian cancer (10), cardiomyopathy (11), sepsis (12), obesity (13), lower extremity (14), intestinal (15) and lung IR (16).
Volatile agents are an essential part of
perioperative medicine and are present in almost every patient
undergoing general anesthesia (17). Several anesthetic agents (such as
sevoflurane, desflurane, isoflurane, halothane, enflurane and
xanthine oxidase) have been shown in various organs of animal
models to decrease oxidative damage and inflammation, as well as
protect against IR injury (18-20).
Sevoflurane is a halogenated volatile anesthetic
that is one of the most commonly used for the induction and
maintenance of general anesthesia in all age groups due to its ease
of administration, versatility and stable hemodynamic profile
(17). Since isoflurane has a
recovery time longer than that of sevoflurane and desflurane is
more irritating to the airway, sevoflurane induction and recovery
tend to be smoother and are associated with fewer complications
compared with isoflurane and desflurane (17,21,22).
To the best of our knowledge, although the precise
mechanism remains unclear, volatile anesthetics are hypothesized to
lessen IR injury and oxidative stress to the liver by lowering
inflammatory cytokine levels (23). Sevoflurane inhibited cytokines more
effectively compared with other volatiles (23). However, if Co nanoparticles prevent
hepatic injury caused by IR under sevoflurane remains unclear. The
present study aimed to investigate the combined effects of
sevoflurane and Co on IR-injured liver tissue using biochemical and
histological examination.
Materials and methods
Animals
Procedures were approved by the Gazi University
Ethical Committee of Experimental Animals (approval no.
G.Ü.ET-21.064) and performed at the Gazi University Animal
Laboratory following the Guidelines for the Care and Use of
Laboratory Animals, Ankara, Turkey (24). In the present study, a total of 36
female Wistar albino rats (age, 5 months; weight, 250-350 g), which
were supplied by Gazi University Experimental Animals Research
Center, were used. Animals were housed under identical
environmental conditions and kept in a temperature/humidity
controlled room (20-21˚C, 45-55%) under a 12/12-hlight/dark cycle.
Food and water were available ad libitum.
Experimental groups
A total of 36 rats were randomly assigned and
equally (n=6) divided into six groups (Control, Co, IR, IRS, Co +
IR and Co + IRS). All surgical procedures were performed under
general anesthesia. An intramuscular injection of 50 mg/kg ketamine
hydrochloride (500 mg/10 ml; Ketalar®vial; Parke-Davis;
Pfizer, Inc.) +10 mg/kg xylazine hydrochloride
(Alfazyne® vial 2%; Ege Vet, Ltd.) was administered for
anesthesia. After 30 min, the procedure was performed under a
warming lamp with the rats in the supine position. In the surgical
groups, after skin asepsis was achieved, a midline abdominal
incision was applied to the rats and the porta hepatis was
explored. In the IR groups, an atraumatic micro clamp was placed on
the porta hepatis for 120 min, then the clamp was withdrawn, and
the liver was re-perfused for another 120 min. In Co groups 0.5
mg/kg Co was administered intraperitoneally. In all groups, liver
tissue of the rats was excised after having been sacrificed under
anesthesia and the experiment lasted 270 min in total.
In the control group, rats were anesthetized 30 min
before laparotomy. A midline laparotomy was the sole surgical
procedure without any additional intervention. Blood samples (5-10
ml) were taken after 4 h follow-up. The liver tissue of rats was
excised after having been sacrificed under anesthesia.
In the Co group, 0.5 mg/kg Co (Co aqueous
nanoparticle dispersion, 100 ml; Sigma-Aldrich; Merck KGaA) was
administered intraperitoneally 30 min before laparotomy. Laparotomy
was the sole surgical procedure without hepatic ischemia
intervention. At 4 h after the procedure, liver tissue of the rats
was excised after having been sacrificed under anesthesia.
In the IR group, hepatic IR was performed following
laparotomy. Subsequently, the rats were sacrificed and liver tissue
was excised.
In the IRS group, the anesthesia procedure was
conducted on the rats in a transparent plastic box. Hepatic IR
procedure was performed following laparotomy. During the ischemia
period, anesthetic gas vaporizers were calibrated and set at a
minimum alveolar concentration of 2.3% sevofluranein oxygen
(Sevorane Likid; 250 ml; AbbVie Tıbbi İlaçlar San. ve Tic. Ltd.
Şti.).
In the Co + IR group, following laparotomy, Co was
administered (0.5 mg/kg) 30 min before the ischemia period and the
liver was re-perfused.
In the Co + IRS group, following laparotomy, Co was
administered (0.5 mg/kg) intraperitoneally 30 min before ischemia.
During the ischemia period, sevoflurane was applied with the 2.3%
inspiratory concentration in a transparent plastic box and the
liver was re-perfused.
Anesthesia was maintained in the control, Co, IR and
Co +IR groups, which did not receive sevoflurane, with injections
of 20 mg/kg ketamine with 5 mg/kg xylazine if a positive reaction
to surgical stress or intermittent tail pinch was observed.
Following the end of the reperfusion period, all rats were
anesthetized with ketamine (50 mg/kg) and xylazine (10 mg/kg) and
sacrificed by collecting blood (5-10 ml) from their abdominal
aorta. After heartbeat and respiration ceased, rats were monitored
for a further 2 min to confirm death. Liver tissue specimens were
excised for subsequent biochemical and histopathological
analysis.
Histopathological evaluation
Histopathological assessment was performed at the
Department of Histology at Kirikkale University. After tissues were
fixed in 10% formalin for 48 h at room temperature specimens were
prepared with paraffin blocks. Tissue sections of 5-µm stained with
hematoxylin for 10 min and then in eosin for 5 min at room
temperature. The histopathological assessment and scoring were
performed under light microscopy (magnification x100; Nikon
Corporation). The same pathologist performed the histological
evaluations in a blinded manner.
Each preparation was examined for hepatocyte
degeneration, sinusoidal dilatation, pre-necrotic cell and
mononuclear cellular infiltration in the parenchyma. Histological
testing semiquantitative evaluation technique used by Abdel-Wahhab
et al (25) was applied for
interpreting the structural changes in hepatic tissues of control
and treatment groups. According to this, a negative point (0)
represents no structural changes; one positive point (1,+)
indicates mild changes; two positive points (2,++) represent medium
structural changes and three positive points (3,+++) indicate
severe structural changes.
Biochemical evaluation
The biochemical examination was performed at the
Department of Medical Biochemistry at Gazi University. Oxidative
stress and lipid peroxidation in liver tissue was evaluated by
measuring thiobarbituric acid (TBA) reactive substance (TBARS)
levels and catalase (CAT) and glutathione-S-transferase (GST)
enzyme activity.
TBARS assay was performed to measure lipid
peroxidation as previously described (26). TBARS assay (CAS Number:122-31-6,
Sigma Aldrich, Lot: MKBH2096V) is based on the reaction of
malondialdehyde with TBA, which forms a pink pigment with an
absorption maximum at 532 nm in acidic pH and
1,1,3,3-tetraethoxypropane was used as a standard MDA solution
freshly in 0.1 M pH 7 TTRIS-HCl buffer solution from concentrated
TEP.
CAT activity is based on the measurement of
absorbance decrease due to H2O2
(Sigma-Aldrich H1009, CAS Number 7722-84-1) consumption at 240 nm
as described by Aebi (27).
GST enzyme activity was measured as described by
Habig et al (28). GST
activity method is based on the measurement of absorbance increase
at 340 nm due to the monitoring the absorbance increase of the
GSH-CDNB complex, which is the product of the GSH (L-Glutathione
reduced Sigma-Aldrich G4251, CAS Number 70-18-8) and CDNB
(1-Chloro-2,4-dinitrobenzene Sigma-Aldrich 138630 CAS Number
97-00-7) reaction. The results were expressed in IU/mg protein for
CAT and GST, nmol/mg protein for TBARS.
Statistical analysis
SPSS 20.0(IBM Corp.) was used for statistical
analysis. Data were analyzed using Kruskal-Wallis test followed by
Dunn's test or one-way ANOVA followed by post hoc Tukey's test.
P<0.05 was considered to indicate a statistically significant
difference. Data are expressed as the mean ± standard error.
Results
Histopathological results
Hydropic degeneration, sinusoidal dilation, necrosis
and parenchymal mononuclear cell infiltration were significantly
different between the groups (hydropic degeneration; P=0.003,
sinusoidal dilation; P=0.013, necrosis; P=0.006 and parenchymal
mononuclear cell infiltration; P=0.011; Table I).
 | Table IHistopathological data of liver
tissue. |
Table I
Histopathological data of liver
tissue.
| Variable | Control (n=6) | Co (n=6) | IR (n=6) | IRS (n=6) | Co + IR (n=6) | Co + IRS (n=6) | Kruskal Wallis
P-value | Comparison | P-value |
|---|
| Hydropic
degeneration |
0.17±0.17a |
0.33±0.21a | 1.67±0.33 |
0.83±0.31a |
0.50±0.22a |
0.50±0.22a | 0.003 | Control vs. Co | 0.642 |
| | | | | | | | | Control vs. IR | <0.0001 |
| | | | | | | | | IR vs. Co | 0.001 |
| | | | | | | | | IR vs. IRS | 0.026 |
| | | | | | | | | IR vs. Co +IR | 0.003 |
| | | | | | | | | IR vs. Co +IRS | 0.003 |
| Sinusoidal
dilation |
0.33±0.21a |
0.50±0.22a | 1.67±0.33 |
0.83±0.17a |
0.50±0.22a |
0.67±0.21a | 0.013 | Control vs. Co | 0.649 |
| | | | | | | | | Control vs. IR | 0.001 |
| | | | | | | | | IR vs. Co | 0.003 |
| | | | | | | | | IR vs. IRS | 0.029 |
| | | | | | | | | IR vs. Co +IR | 0.003 |
| | | | | | | | | IR vs. Co +
IRS | 0.010 |
| Pycnotic
nuclei | 0.17±0.17 | 0.33±0.21 | 1.00±0.26 | 0.67±0.21 | 0.33±0.21 | 0.50±0.22 | 0.120 | Control vs. Co | 1.000 |
| | | | | | | | | Control vs. IR | 0.154 |
| | | | | | | | | IR vs. Co | 0.545 |
| | | | | | | | | IR vs. IRS | 1.000 |
| | | | | | | | | IR vs. Co+ IR | 1.000 |
| | | | | | | | | IR vs. Co +IRS | 1.000 |
| Necrosis |
0.17±0.17a |
0.17±0.17a | 1.33±0.21 | 0.83±0.21 |
0.50±0.22a |
0.67±0.21a | 0.006 | Control vs. Co | 1.000 |
| | | | | | | | | Control vs. IR | 0.001 |
| | | | | | | | | IR vs. Co | 0.001 |
| | | | | | | | | IR vs. IRS | 0.118 |
| | | | | | | | | IR vs. Co + IR | 0.012 |
| | | | | | | | | IR vs. Co +IRS | 0.040 |
| Parenchymal
mononuclear cell infiltration |
0.33±0.21a |
0.50±0.22a | 1.50±0.22 | 1.00±0.26 |
0.50±0.22a |
0.67±0.21a | 0.011 | Control vs. Co | 0.605 |
| | | | | | | | | Control vs. IR | 0.001 |
| | | | | | | | | IR vs. Co | 0.004 |
| | | | | | | | | IR vs. IRS | 0.128 |
| | | | | | | | | IR vs. Co +IR | 0.004 |
| | | | | | | | | IR vs. Co +IRS | 0.014 |
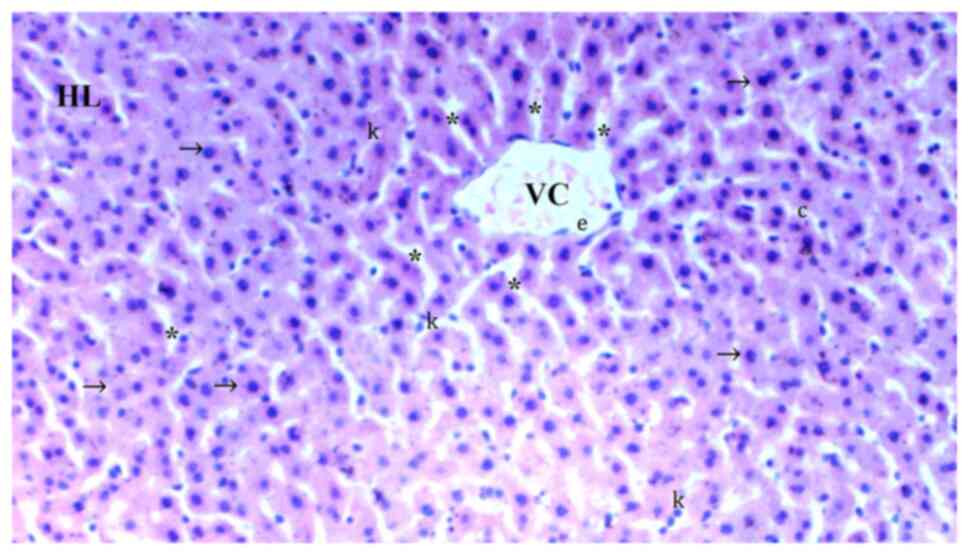
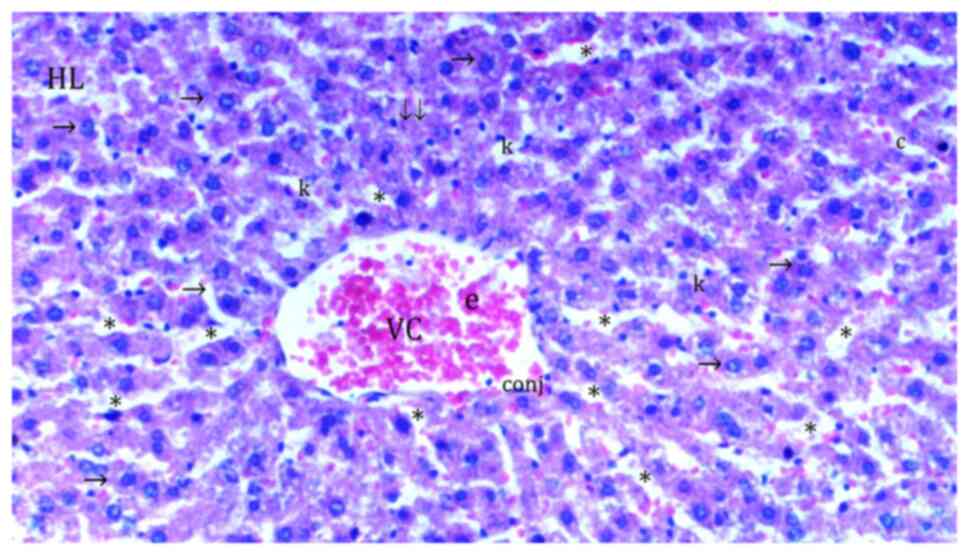
In comparison with the control group (Fig. 1), hydropic degeneration was more
common in IR (P<0.0001; Fig.
2). Hydropic degeneration was found to be significantly lower
in Co (Fig. 3), IRS (Fig. 4), Co + IR (Fig. 5) and Co + IRS (Fig. 6) groups compared with those in the
IR group (P=0.001, P=0.026, P=0.003 and P=0.003, respectively;
Table I).
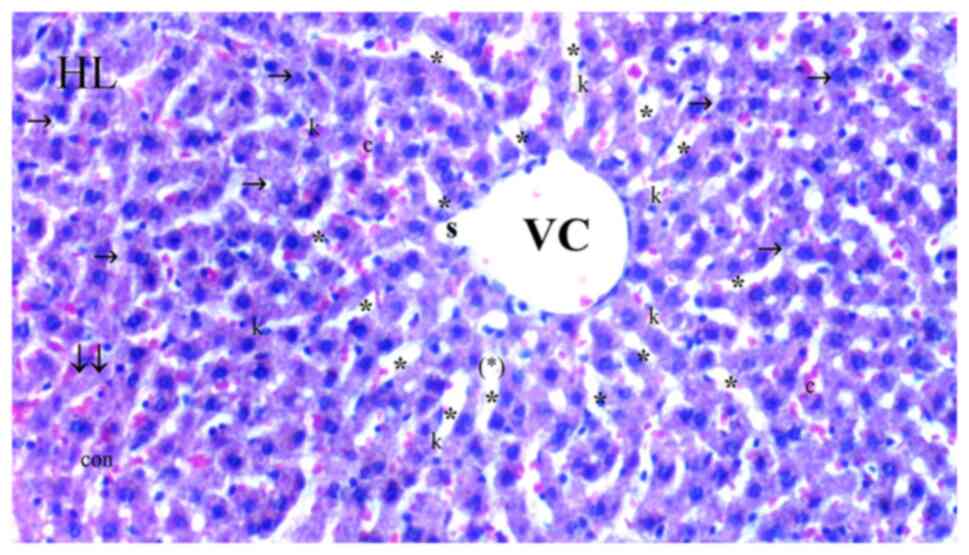
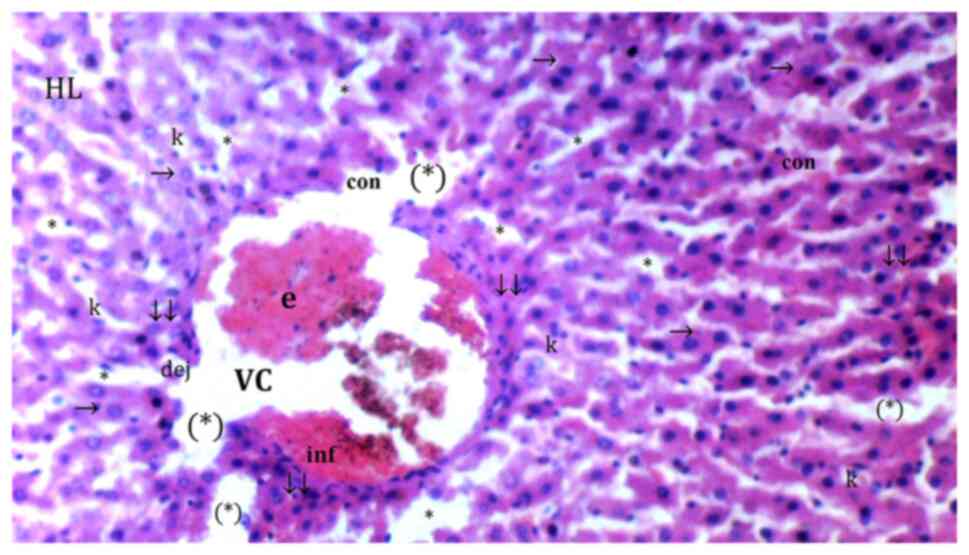 | Figure 3Representative light microscopy of
hepatic tissue from cerium oxide group. HL, hepatic lobules; VC,
vena centralis; e, erythrocyte; con, congestion; *, sinusoid
dilatation; →, hepatocyte; c, dikaryotic hepatocytes; (*), necrotic
and apoptotic hepatocyte; ↓↓, infiltration; dej, hydrophilic
degeneration; inf, inflammation; k, Kupffer cell hyperplasia.
H&Ex100. |
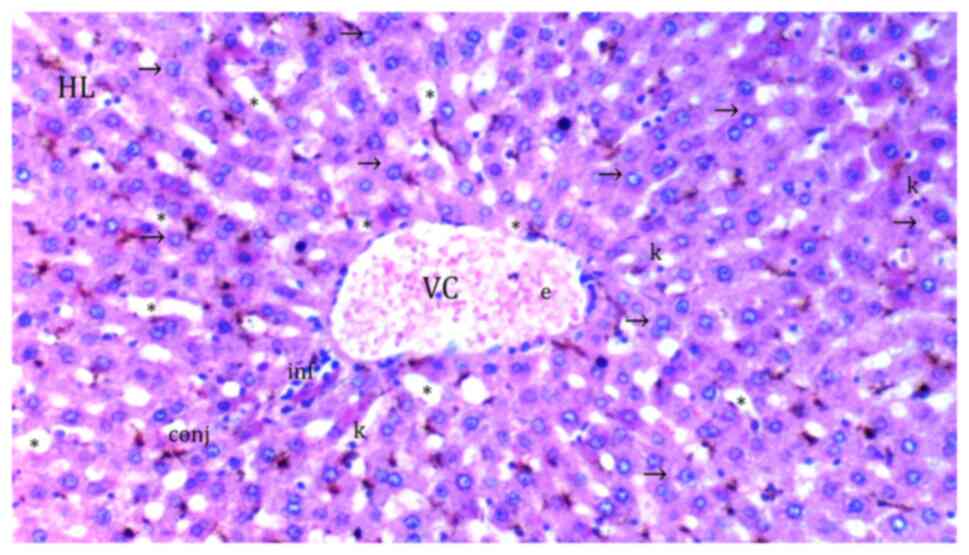
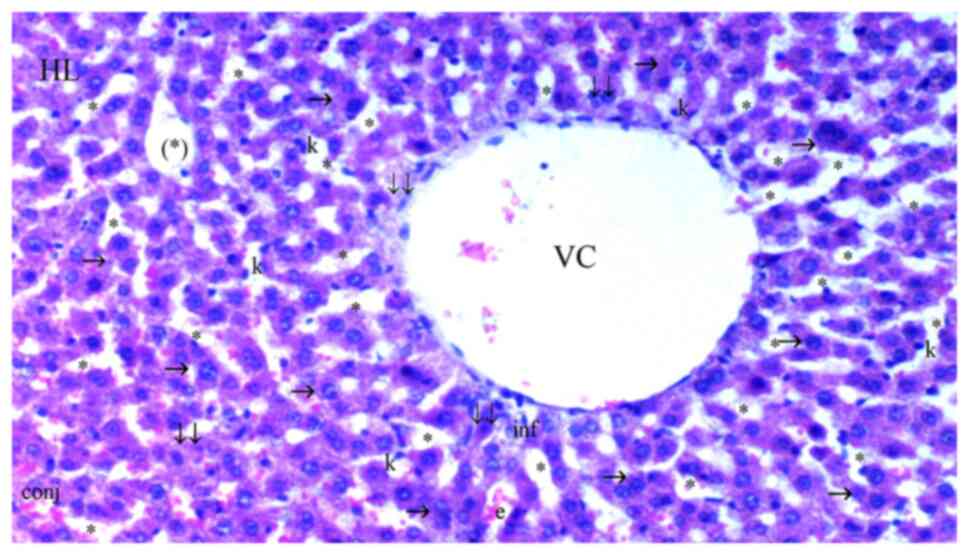 | Figure 5Representative light microscopy of
hepatic tissue from cerium oxide-ischemia reperfusion group. HL,
hepatic lobule; VC, vena centralis; con, congestion; *, sinusoid
dilatation; ↓↓, infiltration; →, hepatocyte; c, dikaryotic
hepatocyte; k, Kupffer cell hyperplasia; inf, inflammation; conj,
congestion; (*), necrotic and apoptotic hepatocyte.
H&Ex100. |
Sinusoidal dilatation was more common in the IR than
in the control group (P=0.001). Sinusoidal dilatation was
significantly lower in the Co (Fig.
3), IRS (Fig. 4), Co + IR
(Fig. 5) and Co + IRS (Fig. 6) groups compared with the IR group
(P=0.003, P=0.010, P=0.003 and P=0.029, respectively; Table I).
Necrosis was more common in the IR group than in the
control group (P=0.001; Table I).
Necrosis was significantly lower in Co (Fig. 3), Co + IR (Fig. 5) and Co + IRS (Fig. 6) groups than in the IR group
(P=0.001, P=0.012, P=0.040, respectively; Table I) while it was similar between IR
and IRS groups (Fig. 6; P=0.118;
Table I).
Parenchymal mononuclear cell infiltration was
significantly decreased in the Co, Co + IR and Co + IRS groups
compared with that in the IR group (P=0.004, P=0.004 and P=0.014,
respectively) while it was found similar between IR and IRS groups
(P=0.128; Table I).
The number of pyknotic nuclei was similar between
all groups (P=0.120; Table I).
Biochemical results. TBARS levels
A significant difference was found in levels of
TBARS in the liver tissue between groups (P<0.0001; Table II). In the IR group, TBARS levels
were higher than in the control group (P=0.001). TBARS levels were
significantly lower in Co, IRS, Co + IR and Co + IRS groups
compared with in the IR group (P<0.0001 for all; Table II).
 | Table IIBiochemical data of liver tissue. |
Table II
Biochemical data of liver tissue.
| Variable | Control (n=6) | Co (n=6) | IR (n=6) | IRS (n=6) | Co + IR (n=6) | Co + IRS (n=6) | ANOVA P-Value | Comparison | P-value |
|---|
| Thiobarbituric
acid, nmol/ml |
0.36±0.04a |
0.45±0.05a | 1.22±0.24 |
0.55±0.07a |
0.44±0.05a |
0.47±0.04a | 0.0001 | Control vs. Co | 0.566 |
| | | | | | | | | Control vs. IR | <0.0001 |
| | | | | | | | | IR vs. Co | <0.0001 |
| | | | | | | | | IR vs. IRS | <0.0001 |
| | | | | | | | | IR vs. Co + IR | <0.0001 |
| | | | | | | | | IR vs. Co +
IRS | <0.0001 |
| Catalase,
IU/mgpro |
2.69±0.37a |
2.51±0.40a | 0.93±0.23 | 1.67±0.36 |
2.60±0.50a |
1.93±0.35a | 0.016 | Control vs. Co | 0.737 |
| | | | | | | | | Control vs. IR | 0.002 |
| | | | | | | | | IR vs. Co | 0.006 |
| | | | | | | | | IR vs. IRS | 0.172 |
| | | | | | | | | IR vs. Co +IR | 0.004 |
| | | | | | | | | IR vs. Co +
IRS | 0.043 |
|
Glutathione-S-transferase, IU/mgpro |
0.62±0.20a |
0.49±0.15a | 0.05±0.01 | 0.26±0.11 |
0.53±0.17a | 0.42±0.12 | 0.049 | Control vs. Co | 0.523 |
| | | | | | | | | Control vs. IR | 0.007 |
| | | | | | | | | IR vs. Co | 0.030 |
| | | | | | | | | IR vs. IRS | 0.296 |
| | | | | | | | | IR vs. Co +IR | 0.019 |
| | | | | | | | | IR vs. Co +IRS | 0.068 |
CAT enzyme activity
CAT enzyme activity in liver tissue was
significantly different between all the groups (P=0.016; Table II). CAT enzyme activity was found
to be significantly decreased in the IR group compared with that in
the control group (P=0.002; Table
II). CAT enzyme activity in Co, Co + IR and Co + IRS groups was
significantly increased compared with that in the IR group
(P=0.006, P=0.004, P=0.043, respectively; Table II). There was no difference
between IR and IRS groups (P=0.172; Table II).
GST enzyme activity. GST enzyme activity in
liver tissue was significantly different between the groups
(P=0.049; Table II). In the IR
group, GST enzyme activity was significantly lower than that in the
control group (P=0.007) and increased compared with that in the Co
and Co + IR groups (P=0.030 and P=0.019, respectively; Table II). There was no difference
between IR and IRS and Co + IRS groups (P=0.296 and P=0.068,
respectively; Table II).
Discussion
Hepatic IR is a severe issue that impairs graft
function, particularly in liver transplantation (1,29).
It involves a short halt in blood flow to all or part of the liver,
followed by rapid reperfusion, disrupting normal homeostatic
systems and generating free radicals (1). Hepatocellular injury and mortality
are associated with high levels of ROS and the consequent
activation of an inflammatory cascade (2). Certain approaches, such as Co and
inhalation anesthetics, may decrease the severity of IR-induced
harm (8,16). Co proven to be beneficial in fatty
liver, fibrosis and drug-induced hepatocidal toxicity, such as
doxorubicin and paracetamol, in addition to IR models (6,30-32).
The present study investigated the protective effect of Co in a rat
model of experimental hepatic IR damage. To the best of our
knowledge, this is the first study to combine Co with sevoflurane
in a liver IR model.
Several enzymes protect cells from IR-induced
oxidative damage by acting as intracellular antioxidants. While the
present study did not directly measure ROS levels, it investigated
TBARS levels and CAT and GST enzyme activities, as well as
histological examination using H&E staining to see if Co had a
therapeutic impact. The TBARS assay, which detects MDA, is a common
laboratory test for determining the degree of harm caused by free
radicals generated by IR (33).
Cellular antioxidant defense functions are supported by CAT and
GST. These enzymes degrade superoxide anions and hydrogen peroxide
while also preventing the formation of free radicals. Antioxidant
efficacy is shown by high levels of CAT and GST in the blood
(34). In the present study,
increased TBARS levels were present in the IR group and CAT and GST
enzyme activities revealed the protective effect of Co on liver IR.
In the hepatic tissue of rats that received Co before hepatic IR,
there was a considerable decrease in TBARS levels, as well as a
significant rise in CAT and GST enzyme activity compared with those
in the IR group; this finding was consistent with earlier
investigations (6,8).
The hepatoprotective effects of Co were confirmed by
histological findings. The IR damage was linked with notable
hepatocyte degradation, sinusoidal dilatation, parenchymal
mononuclear infiltration and several regions of necrosis, according
to histological examination. Treatment with CO2 h before
hepatic IR prevented these alterations and protected hepatocellular
architecture. These modifications showed that Co can reduce
ROS-induced cell death and thereby protect hepatocytes from
IR-induced damage. This may be attributed to excess caspase 3 and
inflammatory cytokine levels as well as reduced macrophage
infiltration in presence of Co (32).
Although most antioxidants used to treat liver
disease have difficulty targeting hepatocytes, necessitating
repeated administration at high concentrations (35), in the present study Co was
administered once before ischemia. Yokel et al (36) revealed that Co nanoparticles exist
in the circulation for a short time on intravenous administration
(half-life, 7.5 min). Even if the remaining intravenous time is
limited, nanoparticles translocate to the liver and other organs
(37). Nanoceria, nanoparticles of
cerium oxide, in particular, has been demonstrated to be taken up
by Kupffer cells in the liver, in which nanoceria partially
dissolves to generate second-generation nanoceria clouds, which are
smaller and may be more effective at reducing free radicals
(38). Manne et al
(8) demonstrated that Co
nanoparticles protect against hepatic IR injury by infusing 0.5
mg/kg of 10-30 nm spherical Co nanoparticles intravenously into
Sprague-Dawley rats 1 h before inducing hepatic ischemia in the
left lateral and median lobes. The present study administered 0.5
mg/kg Co intraperitoneally for 2 h before the ischemia. It was
speculated that a single intraperitoneal dose of Co may preserve
liver tissue in IR models in rats without the requirement for
numerous intravenous administrations due to in vivo
distribution and cellular uptake of nanoceria and intrinsic
autocatalytic activity.
Sevoflurane, desflurane and isoflurane, all
extensively used volatile anesthetics in clinical practice, may be
viable options for reducing IR damage. Through the control of
inflammatory cytokines, oxidative stress and complement, they
protect against hepatic IR damage (19,23,39).
However, compared with isoflurane, sevoflurane has a stronger
effect in reversing liver function, inhibiting inflammatory
cytokines and decreasing oxidative stress (23). There is interest in the
non-anesthetic effects of sevoflurane. Its principal mechanisms are
lowering oxygen free radical and excess calcium level, suppressing
inflammatory responses and enhancing liver cell energy consumption
(40). Both experimental and
clinical investigations imply that the mechanism of sevoflurane
conditioning in decreasing hepatic IR damage is similar to that of
ischemia preconditioning (39,41,42).
Although it remains unclear how sevoflurane reduces
hepatic IR, some mechanisms have been discussed (43). Sevoflurane attenuates aggregation
of macrophages and neutrophils in the liver sinusoid. Furthermore,
it preserves the endothelial glycocalyx, reduces apoptosis and
exerts antioxidant effects by regulating the nuclear factor
erythroid 2-related factor 2 (Nrf2) pathway, thereby decreasing
liver IR injury (44). The
transcription factor Nrf2 is a pivotal agent in protection against
oxidative stress. It is involved in the regulation of the
expression of antioxidants, such as GST (45). In the absence of Nrf2, liver
regeneration is significantly delayed and hepatocyte death is also
increased (46). It has been
demonstrated that Co and sevoflurane both downregulate Nrf2
(44,46).
The protective effect of sevoflurane in liver IR
injury was revealed by the present investigation. When compared
with the IR group, the IRS group had lower levels of TBARS,
hepatocyte degeneration and sinusoidal dilatation. The combination
of sevoflurane and Co also decreased IR damage in the liver. The
significant decrease in TBARS levels shows that damage was
associated with lipid peroxidation. Co and sevoflurane
administration may decrease IR damage by altering lipid
peroxidation. Histopathological findings supported the biochemical
findings, demonstrating that co-administration of Co with
sevoflurane may be more effective in avoiding liver damage than
sevoflurane alone. Compared with the IR group, the Co + IR group
exhibited notably decreased hepatocyte deterioration, sinusoidal
dilatation, parenchymal mononuclear infiltration and necrosis.
The primary limitation of the present study was the
absence of aspartate transaminase (AST) and alanine transaminase
(ALT) level measurements. ALT and AST are standard biomarkers of
choice for detecting liver injury (47). However, considering ALT and AST are
not liver-specific and limited blood volume in rats, CAT, TBARS and
GST were selected. ALT levels should be reported in future studies
to improve the understanding of the effect of Co on liver tissue in
the IR model.
In summary, the present findings revealed that Co
therapy decreased oxidative stress generated by IR, as evidenced by
a decrease in TBARS levels and increased activity of CAT and GST.
The biochemical and histological findings in rats reveal a decrease
in liver damage in Co + IR and Co +IRS groups compared with IR
group. These findings support the hepatoprotective effects of Co.
Taken together, the findings imply that intraperitoneal 0.5 mg/kg
prophylactic Co administration may be a potential therapeutic
method for treatment of hepatic IR damage. These effects should be
confirmed at different concentrations and dosing regimens.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
MA and AK designed the study and analyzed and
interpreted data. HG, CO and EK performed the experiments. SE and
MA analyzed and interpreted data. MA, SE and HG confirm the
authenticity of all the raw data. XX, AK, MA and MK provided
scientific and technical assistance and critically revised the
article for important intellectual content. CO and MA collected
samples. TM and MK performed cellular and molecular experiments.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Ethical approval for the study was obtained from
Gazi University Experimental Animals Ethics Committee (Ankara,
Turkey; approval no:G.Ü.ET-21.064).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Peralta C, Jiménez-Castro MB and
Gracia-Sancho J: Hepatic ischemia and reperfusion injury: Effects
on the liver sinusoidal milieu. J Hepatol. 59:1094–1106.
2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bhogal RH, Curbishley SM, Weston CJ, Adams
DH and Afford SC: Reactive oxygen species mediate human hepatocyte
injury during hypoxia/reoxygenation. Liver Transpl. 16:1303–1313.
2010.PubMed/NCBI View
Article : Google Scholar
|
|
3
|
Bonatsos V, Kappas I, Birbas K,
Vlachodimitropoulos D, Toutouzas K, Karampela E, Syrmos N, Bonatsos
G and Papalois AE: Effects of U-74389G (21-lazaroid) and ascorbic
acid on liver recovery after acute ischemia and reperfusion in
rats. In Vivo. 29:585–594. 2015.PubMed/NCBI
|
|
4
|
Liu A, Huang L, Fan H, Fang H, Yang Y, Liu
S, Hu J, Hu Q, Dirsch O and Dahmen U: Baicalein pretreatment
protects against liver ischemia/reperfusion injury via inhibition
of NF-κB pathway in mice. Int Immunopharmacol. 24:72–79.
2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ramalho LN, Pasta ÂA, Terra VA, Augusto M,
Sanches SC, Souza-Neto FP, Cecchini R, Gulin F and Ramalho FS:
Rosmarinic acid attenuates hepatic ischemia and reperfusion injury
in rats. Food and Chem Toxicol. 74:270–278. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Córdoba-Jover B, Arce-Cerezo A, Ribera J,
Pauta M, Oró D, Casals G, Fernández-Varo G, Casals E, Puntes V,
Jiménez W and Morales-Ruiz M: Cerium oxide nanoparticles improve
liver regeneration after acetaminophen-induced liver injury and
partial hepatectomy in rats. J Nanobiotechnology.
17(112)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Colon J, Herrera L, Smith J, Patil S,
Komanski C, Kupelian P, Seal S, Jenkins DW and Baker CH: Protection
from radiation-induced pneumonitis using cerium oxide
nanoparticles. Nanomedicine. 5:225–231. 2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Manne NDPK, Arvapalli R, Graffeo VA,
Bandarupalli VVK, Shokuhfar T, Patel S, Rice KM, Ginjupalli GK and
Blough ER: Prophylactic treatment with cerium oxide nanoparticles
attenuate hepatic ischemia reperfusion injury in sprague dawley
rats. Cell Physiol Biochem. 42:1837–1846. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Estevez AY, Pritchard S, Harper K, Aston
JW, Lynch A, Lucky JJ, Ludington JS, Chatani P, Mosenthal WP,
Leiter JC, et al: Neuroprotective mechanisms of cerium oxide
nanoparticles in a mouse hippocampal brain slice model of ischemia.
Free Radic Biol Med. 51:1155–1163. 2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Giri S, Karakoti A, Graham RP, Maguire JL,
Reilly CM, Seal S, Rattan R and Shridhar V: Nanoceria: A rare-earth
nanoparticle as a novel anti-angiogenic therapeutic agent in
ovarian cancer. PLoS One. 8(e54578)2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Niu J, Azfer A, Rogers LM, Wang X and
Kolattukudy PE: Cardioprotective effects of cerium oxide
nanoparticles in a transgenic murine model of cardiomyopathy.
Cardiovasc Res. 73:549–559. 2007.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Manne ND, Arvapalli R, Nepal N, Shokuhfar
T, Rice KM, Asano S and Blough ER: Cerium oxide nanoparticles
attenuate acute kidney injury induced by intra-abdominal infection
in Sprague-Dawley rats. J Nanobiotechnology. 13(75)2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Rocca A, Moscato S, Ronca F, Nitti S,
Mattoli V, Giorgi M and Ciofani G: Pilot in vivo investigation of
cerium oxide nanoparticles as a novel anti-obesity pharmaceutical
formulation. Nanomedicine. 11:1725–1734. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tatar T, Polat Y, Çomu FM, Kartal H,
Arslan M and Küçük A: Effect of cerium oxide on erythrocyte
deformability in rat lower extremity ischemia reperfusion injury.
Bratisl Lek Listy. 119:441–443. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gubernatorova EO, Liu X, Othman A, Muraoka
WT, Koroleva EP, Andreescu S and Tumanov AV: Europium-Doped cerium
oxide nanoparticles limit reactive oxygen species formation and
ameliorate intestinal ischemia-reperfusion injury. Adv Healthc
Mater 6: May 8, 2017 (Epub ahead of print).
|
|
16
|
Tuncay A, Sivgin V, Ozdemirkan A, Sezen
SC, Boyunaga H, Kucuk A, Gunes I and Arslan M: The effect of cerium
oxide on lung tissue in lower extremity ischemia reperfusion injury
in sevoflurane administered rats. Int J Nanomedicine. 15:7481–7489.
2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Perouansky M, Pearce RA and Hemmings HC:
Inhaled Anesthetics: Mechanism of Action. In: Miller Anesthesia
Eight Edition. Miller RD (ed.). Elsevier Saunders Inc., Philadephia
PA, pp614-635, 2015.
|
|
18
|
Bedirli N, Ofluoglu E, Kerem M, Utebey G,
Alper M, Yilmazer D, Bedirli A, Ozlu O and Pasaoglu H: Hepatic
energy metabolism and the differential protective effects of
sevoflurane and isoflurane anesthesia in a rat hepatic
ischemia-reperfusion injury model. Anesth Analg. 106:830–837, table
of contents. 2008.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Nielsen VG, Tan S, Kirk KA, Baird MS,
McCammon AT, Samuelson PN and Parks DA: Halothane and xanthine
oxidase increase hepatocellular enzyme release and circulating
lactate after ischemia-reperfusion in rabbits. Anesthesiology.
87:908–917. 1997.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhong M, Che L, Du M, Liu K and Wang D:
Desflurane protects against liver ischemia/reperfusion injury via
regulating miR-135b-5p. J Chin Med Assoc. 84:38–45. 2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sloan MH, Conard PF, Karsunky PK and Gross
JB: . Sevoflurane versus isoflurane: Induction and recovery
characteristics with single-breath inhaled inductions of
anesthesia. Anesth Analg. 82:528–532. 1996.PubMed/NCBI View Article : Google Scholar
|
|
22
|
TerRiet MF, DeSouza GJ, Jacobs JS, Young
D, Lewis MC, Herrington C and Gold MI: Which is most pungent:
Isoflurane, sevoflurane or desflurane? Br J Anaest. 85:305–307.
2000.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yang P, Du Y, Zeng H, Xing H, Tian C and
Zou X: Comparison of inflammatory markers between the sevoflurane
and isoflurane anesthesia in a rat model of liver
ischemia/reperfusion injury. Transplant Proc. 51:2017–2075.
2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hayvan Deneyleri Merkezi Etik Kurulu.
Mevzuat. Available from: https://hadmek.tarimorman.gov.tr/Sayfa/Detay/644.
Accessed November 22, 2022.
|
|
25
|
Abdel-Wahhab MA, Nada SA and Arbid MS:
Ochratoxicosis: Prevention of developmental toxicity by
L-methionine in rats. J Appl Toxicol. 19:7–12. 1999.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hodges DM, DeLong JM, Forney CF and Prange
RK: Improving the thiobarbituric acid-reactive-substances assay for
estimating lipid peroxidation in plant tissues containing
anthocyanin and other interfering compounds. Planta. 207:604–611.
1999.
|
|
27
|
Aebi H: Catalase. In: Methods of Enzymatic
Analysis. Bergmeyer H (ed). Academic Press, New York, London,
pp673-677, 1974.
|
|
28
|
Habig WH, Pabst MJ and Jakoby WB:
Glutathione S-transferases. The first enzymatic step in mercapturic
acid formation. J Biol Chem. 249:7130–7139. 1974.PubMed/NCBI
|
|
29
|
Li J, Wang F, Xia Y, Dai W, Chen K, Li S,
Liu T, Zheng Y, Wang J, Lu W, et al: Astaxanthin pretreatment
attenuates hepatic ischemia reperfusion-induced apoptosis and
autophagy via the ROS/MAPK pathway in mice. Mar Drugs.
13:3368–3387. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ibrahim HG, Attia N, Hashem FEZA and El
Heneidy MAR: Cerium oxide nanoparticles: In pursuit of liver
protection against doxorubicin-induced injury in rats. Biomed
Pharmacother. 103:773–781. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kobyliak N, Abenavoli L, Falalyeyeva T,
Virchenko O, Natalia B, Beregova T, Bodnar P and Spivak M:
Prevention of NAFLD development in rats with obesity via the
improvement of pro/antioxidant state by cerium dioxide
nanoparticles. Clujul Med. 89:229–235. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Oró D, Yudina T, Fernández-Varo G, Casals
E, Reichenbach V, Casals G, González de la Presa B, Sandalinas S,
Carvajal S, Puntes V and Jiménez W: Cerium oxide nanoparticles
reduce steatosis, portal hypertension and display anti-inflammatory
properties in rats with liver fibrosis. J Hepatol. 64:691–698.
2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Granger DN and Korthuis RJ: Physiologic
mechanisms of postischemic tissue injury. Annu Rev Physiol.
57:311–332. 1995.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Matés JM, Pérez-Gómez C and Núñez de
Castro I: Antioxidant enzymes and human diseases. Clin Biochem.
32:595–603. 1999.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Hochhauser E, Lahat E, Sultan M, Pappo O,
Waldman M, Sarne Y, Shainberg A, Gutman M, Safran M and Ben Ari Z:
Ultra low dose delta 9-tetrahydrocannabinol protects mouse liver
from ischemia reperfusion injury. Cell Physiol Biochem.
36:1971–1981. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Yokel RA, Hussain S, Garantziotis S,
Demokritou P, Castranova V and Cassee FR: The yin: An adverse
health perspective of nanoceria: Uptake, distribution,
accumulation, and mechanisms of its toxicity. Environ Sci Nano.
1:406–428. 2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Manne ND, Arvapalli R, Nepal N, Thulluri
S, Selvaraj V, Shokuhfar T, He K, Rice KM, Asano S, Maheshwari M
and Blough ER: Therapeutic potential of cerium oxide nanoparticles
for the treatment of peritonitis induced by polymicrobial insult in
Sprague-Dawley rats. Crit Care Med. 43:e477–e489. 2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Graham UM, Tseng MT, Jasinski JB, Yokel
RA, Unrine JM, Davis BH, Dozier AK, Hardas SS, Sultana R, Grulke EA
and Butterfield DA: In vivo processing of ceria nanoparticles
inside liver: Impact on free-radical scavenging activity and
oxidative stress. Chempluschem. 79:1083–1088. 2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Figueira ERR and Filho JAR: Is there a
place for sevoflurane to prevent liver ischemia-reperfusion injury
during liver transplantation? Edorium J Anesth. 1:1–5. 2015.
|
|
40
|
Kan C, Ungelenk L, Lupp A, Dirsch O and
Dahmen U: Ischemia-reperfusion injury in aged livers-the energy
metabolism, inflammatory response, and autophagy. Transplantation.
102:368–377. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Jeong JS, Kim D, Kim KY, Ryu S, Han S,
Shin BS, Kim GS, Gwak MS and Ko JS: Ischemic preconditioning
produces comparable protection against hepatic ischemia/reperfusion
injury under isoflurane and sevoflurane anesthesia in rats.
Transplant Proc. 49:2188–2193. 2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Jaiswal AK: Nrf2 signaling in coordinated
activation of antioxidant gene expression. Free Radic Biol Med.
36:1199–1207. 2004.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Lee HT, Ota-Setlik A, Fu Y, Nasr SH and
Emala CW: Differential protective effects of volatile anesthetics
against renal ischemia-reperfusion injury in vivo. Anesthesiology.
101:1313–1324. 2004.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Wu Y, Gu C and Huang X: Sevoflurane
protects against hepatic ischemia/reperfusion injury by modulating
microRNA-200c regulation in mice. Biomed Pharmacother.
84:1126–1136. 2016.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Ma H, Yang B, Yu L, Gao Y, Ye X, Liu Y, Li
Z, Li H and Li E: Sevoflurane protects the liver from
ischemia-reperfusion injury by regulating Nrf2/HO-1 pathway. Eur J
Pharmacol. 898(173932)2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Bashandy PhD MM, Saeed HE, Ahmed WMS,
Ibrahim MA and Shehata O: Cerium oxide nanoparticles attenuate the
renal injury induced by cadmium chloride via improvement of the NBN
and Nrf2 gene expressions in rats. Toxicol Res (Camb). 11:339–347.
2022.PubMed/NCBI View Article : Google Scholar
|
|
47
|
McGill MR: The past and present of serum
aminotransferases and the future of liver injury biomarkers. EXCLI
J. 15:817–828. 2016.PubMed/NCBI View Article : Google Scholar
|