Introduction
Hepatitis B virus (HBV) infection is a major public
health concern. The World Health Organization estimated in 2015
that >257 million individuals were chronically infected with HBV
(1). Long-term HBV infection
causes acute and chronic hepatitis B (CHB) and the development of
complications, including cirrhosis and liver cancer (2). T helper type 1 (Th1) reactions and
antigen-specific cytotoxic T-lymphocytes (CTLs) appear to be
crucial in the clearance of chronic HBV infection (3). The elimination of HBV is mainly
dependent on effective and diverse T-cell immune responses
(4). However, as the body is
unable to destroy affected hepatocytes, chronic HBV infection can
persist for an extended period of time (5). Antiviral medications inhibit the
replication of HBV; however, they have minimal effects on the
ability of the body to restore the function of Th cells or on the
role of CTLs (6). Therefore,
boosting HBV-specific T-cell reactions may be a potential treatment
strategy for patients with CHB.
HBV core antigen (HBcAg) displays distinct
immunological characteristics. Patients in which the virus is
completely eradicated often have strong CTL reactions that are
specific to HBcAg (7). Ubiquitin
is a very small protein comprising 76 amino acids that is very well
conserved. In the proteolytic process, ubiquitin acts as a signal
for the target protein to be identified and broken down in the
proteasome (8). Previous studies
have indicated that a lentivector encoding the ubiquitinated form
of HBcAg (LV-Ub-HBcAg) produced HBV-specific CTLs, promoted
dendritic cell (DC) maturation and promoted lymphocyte growth
(9-11).
DC-based immunotherapy is a very promising
therapeutic strategy; however, the separation and transduction of
DCs from patients to generate specific autologous DC vaccines is a
costly and time-consuming process, and DCs have a short survival
time and stringent preparation requirements (12,13).
Exosomes derived from DCs are known as dexosomes (Dexs) and are
capable of triggering and increasing antigen-specific T-cell
reactions in vivo. Dexs express major histocompatibility
complex (MHC) class I/II and costimulatory molecules (14). The in vitro production
process of exosomes utilizing DCs is simple (14). Phase I and II clinical trials in
which patients with malignant melanoma and non-small cell lung
cancer were treated with Dexs demonstrated the feasibility of using
Dexs as an antitumor vaccination (15-17);
these trials demonstrated the safety and immunotherapeutic effects
of Dex-based vaccines. In the present study, high-purity Dexs were
generated from murine DCs loaded with LV-Ub-HBcAg. Splenic
T-lymphocytes were then stimulated with Dexs to investigate the
HBV-specific T-cell immune reaction.
Materials and methods
Mice
A total of 20 mice of the C57BL/6 (H-2b) strain,
aged between 6 and 8 weeks (weight, 20-24 g) and with an equal
number of males and females, were obtained from the Jiangsu
University Experimental Animal Center (Zhenjiang, China). All mice
were bred in an environment that was free of all pathogens
(22-26˚C; humidity 50-55%; 12-h light/dark cycle) and allowed
access to food and water ad libitum. Animals were euthanized
by the intraperitoneal injection of an overdose of sodium
pentobarbital (200 mg/kg; cat. no. 69020181; Sinopharm Chemical
Reagent Co., Ltd.). Death of the mice was verified by the absence
of heartbeat, breathing or respiration for ≥5 min. The Laboratory
Animal Ethics Committee of Jiangsu University approved all the
experimental methods (ref. no. K-20180031-Y).
Reagents and cells
Abcam provided the anti-HBcAg antibody (cat. no.
ab8637) that was used in the present study. R&D Systems, Inc.
supplied the enzyme-linked immunosorbent assay (ELISA) kits used to
measure IFN-γ (cat. no. MIF00), IL-2 (cat. no. M2000), IL-4 (cat.
no. M4000B) and IL-10 (cat. no. M1000B) levels. The P815/c cell
line, which comprises H-2b mastocytoma cells expressing the HBV
core antigen, was preserved in the authors' laboratory (11). Briefly, P815 mouse mastocytoma
cells (https://www.cellosaurus.org/CVCL_2154) were
transfected with recombinant lentiviruses carrying HBcAg and a
puromycin resistance gene. After 48 h of transfection, 2 µg/ml
puromycin was applied for screening for 10 days. The surviving
cells were P815/c cells, which were resistant to puromycin and
carried the HBcAg gene. The cells were cultured at 37˚C in a
humidified environment containing 5% CO2 in Dulbecco's
modified Eagle's medium (Invitrogen; Thermo Fisher Scientific,
Inc.) containing 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml streptomycin.
The recombinant lentiviral vectors (LV-Ub-HBcAg and LV) were
constructed as previously described (10).
DC isolation and LV-Ub-HBcAg
transfection
The generation of murine DCs was carried out
according to the methodology outlined in the study by Chen et
al (18). Briefly, bone marrow
cells were obtained from the tibiae and femurs of C57BL/6 mice and
erythrocytes were lysed. The bone marrow cells were cultured at
37˚C at a concentration of 2x106 cells/ml in complete
RPMI-1640 culture medium (Gibco; Thermo Fisher Scientific, Inc.)
containing 20 ng/ml murine granulocyte-macrophage
colony-stimulating factor (mGM-CSF; PeproTech, Inc.) and 10 ng/ml
murine IL-4 (mIL-4; PeproTech, Inc.). On day 3, after establishing
the initial culture, non-adherent single cells were discarded and
fresh RPMI-1640 containing mGM-CSF and mIL-4 was added. The
transfection of DCs with LVs was performed as previously described
(10). In brief, on day 5, the
immature DCs (imDCs) and their progenitors were seeded in a 24-well
plate containing 0.5 ml complete RPMI-1640 enriched with mIL-4,
mGM-CSF and polybrene (2 µg/ml). The cells were then transfected
with LV-Ub-HBcAg to produce Dexs-Ub-HBcAg, or with LV to produce
control Dexs (Dexs-Con). Subsequently, the infected cells were
cultured at 37˚C for 24 h. The supernatant was then removed and
replaced with fresh medium, and the cells were treated with 1 mg/ml
lipopolysaccharide (LPS; MilliporeSigma) for 24 h. Untransfected
imDCs were stimulated with LPS for 24 h to produce blank Dexs as
the blank control (Dexs-Blank). At 5 days post-transduction, green
fluorescent protein (GFP) expression was measured to determine the
transduction efficiency in the DCs using a fluorescence microscope
(Nikon Eclipse TE2000-U; Nikon Corporation) by Image J software
(National Institutes of Health). CD11c is a specific DC marker
(19), therefore,
CD11c+GFP+ cells were sorted for enrichment
using a MoFLo® High-Performance Cell Sorter (code S2500;
Beckman Coulter, Inc.). Trypan blue (MilliporeSigma) labeling was
utilized to ascertain whether the DCs were viable, and only those
with >85% viability were employed.
Dex isolation and
characterization
Dexs were isolated by differential velocity
centrifugation, as previously described (20,21).
The culture supernatants of DCs transfected with LV-Ub-HBcAg or LV
were retrieved and centrifuged at 300 x g for 10 min at 37˚C. The
supernatant was collected and centrifuged at 3,000 x g for 15 min
at 4˚C followed by 10,000 x g for 30 min at 4˚C. The supernatant
was then filtered using a PVDF membrane (MilliporeSigma) with a
pore size of 0.22 µm and transferred to an ultracentrifuge tube. In
some previous studies, the supernatant was centrifuged at 100,000 x
g for 2 h at 4˚C (22,23). However, in the present study, it
was considered more appropriate to centrifuge the supernatant at
110,000 x g for 90 min at 4˚C according to another previously
described protocol as this method was shorter in time while
maintaining a similar extraction efficiency (21). The supernatant was then discarded,
and the residual Dex pellets were resuspended in 100 µl
phosphate-buffered saline (PBS; Gibco; Thermo Fisher Scientific,
Inc.). The Dexs were stored at -80˚C for use in subsequent
experiments.
The total protein content in the Dexs was measured
using a Pierce bicinchoninic acid (BCA) assay (Thermo Fisher
Scientific, Inc.). Transmission electron microscopy (TEM) using a
JEM-2100 instrument (JEOL, Ltd.) was utilized to visualize the
Dexs. The Dexs were processed for TEM following standard
experimental methods (24). The
size of the Dexs was measured using the ZetaVIEW®
nanoparticle tracking analysis system (Particle Metrix GmbH)
according to the manufacturer's guidelines. Furthermore, western
blot analysis was utilized to evaluate the expression levels of Dex
marker proteins. RIPA lysis buffer containing a protease inhibiter
mixture (Beyotime Institute of Biotechnology) was employed to lyse
the Dexs. Protein concentration levels were measured using a BCA
protein assay kit. After separation by 12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis, 30 µl protein
lysate/lane was subsequently transferred onto a PVDF membrane
(MilliporeSigma). Subsequently, the membrane was blocked with 5%
non-fat milk at room temperature for 1 h. The primary antibodies
used were rabbit anti-mouse CD63 (1:500; cat. no. ab216130; Abcam),
anti-CD9 (1:500; cat. no. ab92726; Abcam) and tumor susceptibility
gene 101 (TSG101; 1:500; cat. no. 102286-T38; SinoBiological)
monoclonal antibodies. Rabbit anti-human HBcAg antibody (1:1,000;
cat. no. ab115992; Abcam) was also used as the primary antibody.
All the primary antibodies were employed at 4˚C overnight. The
secondary antibody, a horseradish peroxidase-conjugated anti-rabbit
immunoglobulin-G antibody (1:5,000; cat. no. 7074; Cell Signaling
Technology, Inc.) was employed at room temperature for 1.5 h.
Enhanced chemiluminescence (BeyoECL Plus; cat. no. P0018M; Beyotime
Institute of Biotechnology) was used to visualize the protein
bands.
T-lymphocyte generation
T-lymphocytes were isolated from splenocytes using
nylon wool columns (FUJIFILM Wako Pure Chemical Corporation).
Single-cell suspensions of the lymphocytes at a concentration of
2x106 cells/ml were plated on six-well plates with
RPMI-1640 containing 10% FBS and allowed to develop at 37˚C for 24
h. After labeling with CD3 monoclonal antibody (17A2),
phycoerythrin-Cyanine5 (anti-CD3-PE-Cy5; cat. no. 15-0032-82;
eBioscience), the extracted T-cells were analyzed using CytoFLEX
flow cytometry (Beckman Coulter, Inc.) to measure their purity and
only cells with >80% purity were employed.
Cytokine release assay
T-lymphocytes were plated at a concentration of
2x106 cells/ml in RPMI-1640 culture medium with 10 µg/ml
Dexs-Ub-HBcAg, Dexs-Con, Dexs-Blank or PBS in 24-well plates at
37˚C for 72 h. The supernatants were retrieved and the quantities
of IFN-γ, IL-2, IL-4 and IL-10 were determined using ELISA kits in
compliance with the manufacturer's protocol. The results are
presented in units of pg/ml.
T-lymphocyte proliferation assay
On day 5 of DC isolation, DCs were co-cultured with
10 µg/ml Dexs-Ub-HBcAg, Dexs-Con, Dexs-Blank or PBS for 72 h.
Subsequently, the DCs were pre-treated with mitomycin C (25 µg/ml;
MilliporeSigma) for 30 min. Separately, T-lymphocytes
(2x106 cells/ml) were plated in six-well plates coated
with anti-CD3 (cat. no. 14-0032-82; eBioscience) at a concentration
of 0.5 µg/ml overnight at 4˚C. The plates were then maintained at
37˚C and supplied with RPMI-1640 culture medium. Subsequently,
anti-CD28 (0.5 µg/ml; cat. no. 14-0281-82; eBioscience) was added
to activate the T-cells for 24 h at 37˚C. The activated
T-lymphocytes were then used as responder cells in a co-culture
with the DCs using a responder/stimulator (T-cell/DC) ratio of
20:1. The cells were cultured in a final volume of 100 µl for 96 h
at 37˚C, during which 10 µl Cell Counting Kit-8 solution (Dojindo
Laboratories, Inc.) was added for the final 4 h. The absorbance of
the cultures was measured at 450 nm using a Multiskan Ascent
microplate reader (Thermo Fisher Scientific, Inc.).
CTL assay
T-cells were activated in a humidified environment
with 5% CO2 at 37˚C for 72 h with Dexs-Ub-HBcAg (10
µg/ml), Dexs-Con (10 µg/ml), Dexs-Blank (10 µg/ml) or PBS. P815/c
cells were plated as the target cells, and previously activated
T-lymphocytes were used as the effector cells. The T-lymphocytes
were co-cultured with the P815/c cells for 4 h at 37˚C in a
humidified environment containing 5% CO2.
Effector/target ratios of 5:1, 10:1 and 20:1 were used. The
HBcAg-specific CTL activity was evaluated utilizing a lactate
dehydrogenase (LDH) release assay (CytoTox 96®
Non-Radioactive Cytotoxicity Assay kit; Promega Corporation), in
accordance with the manufacturer's guidelines. The absorbance was
measured at 490 nm using a Multiskan Ascent microplate reader. The
percentage of cytotoxicity was determined using the following
formula: [(Experimental release-effector spontaneous release-target
spontaneous release)/(target maximum release-target spontaneous
release)] x100 (18,25).
Statistical analysis
Data are presented as the mean ± SD of at least
three separate experiments. To detect statistically significant
differences, the data were analyzed using one-way analysis of
variance with Tukey's post hoc test. SPSS 20.0 software (IBM Corp.)
was used to analyze the data. P<0.05 was considered to indicate
a statistically significant difference.
Results
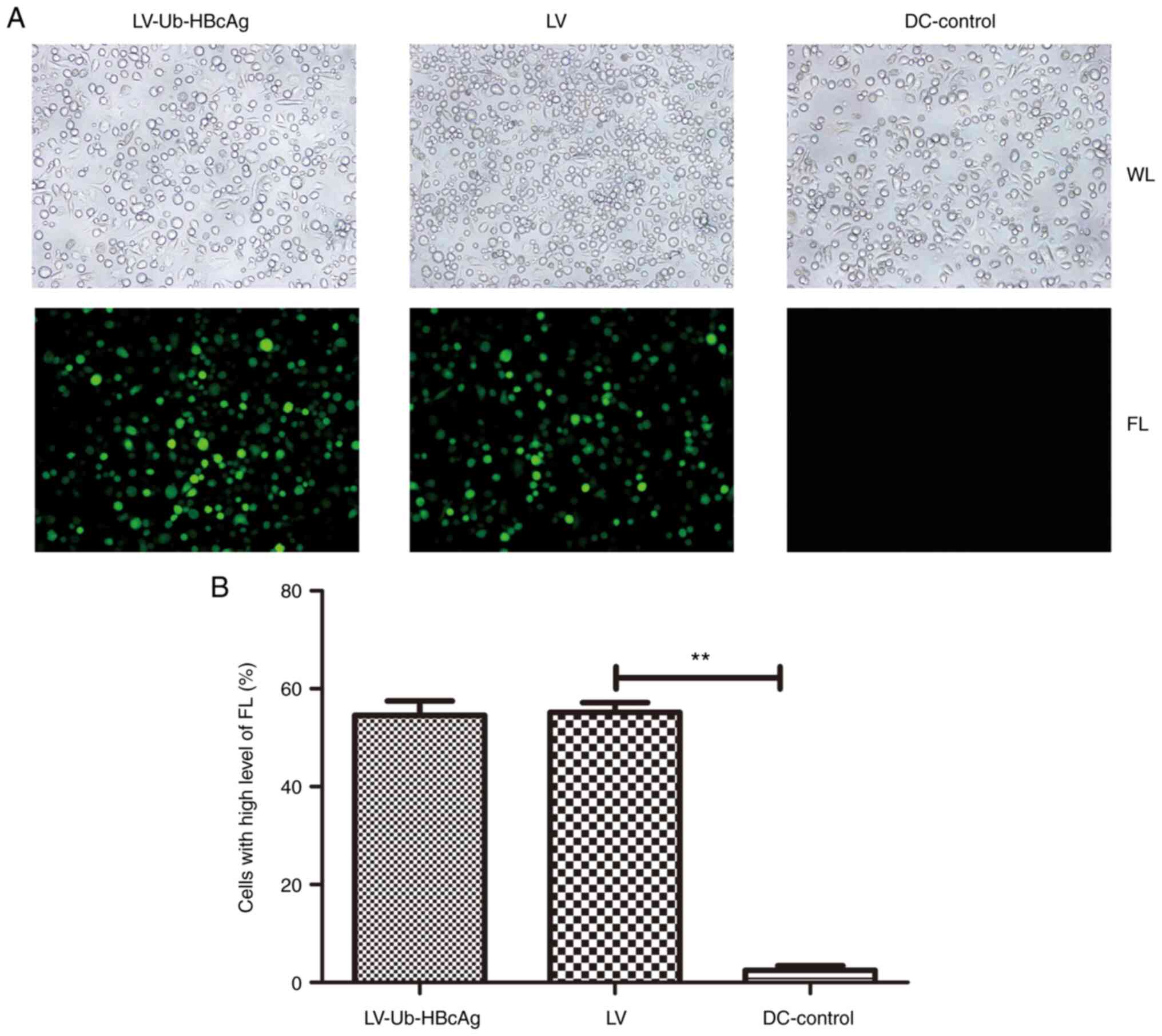
Lentiviral transduction of DCs
Bone marrow-derived DCs were cultured in RPMI-1640
medium supplemented with mIL-4 and mGM-CSF. On day 5 post-culture,
the isolated DCs were transduced with LV-Ub-HBcAg or LV. GFP
expression was measured using a fluorescence microscope to evaluate
the transduction efficiency in the DCs. A positivity rate of 50-60%
was reached, with higher levels of fluorescence observed in the
LV-Ub-HBcAg and LV group compared with DC control group (P<0.01;
Fig. 1).
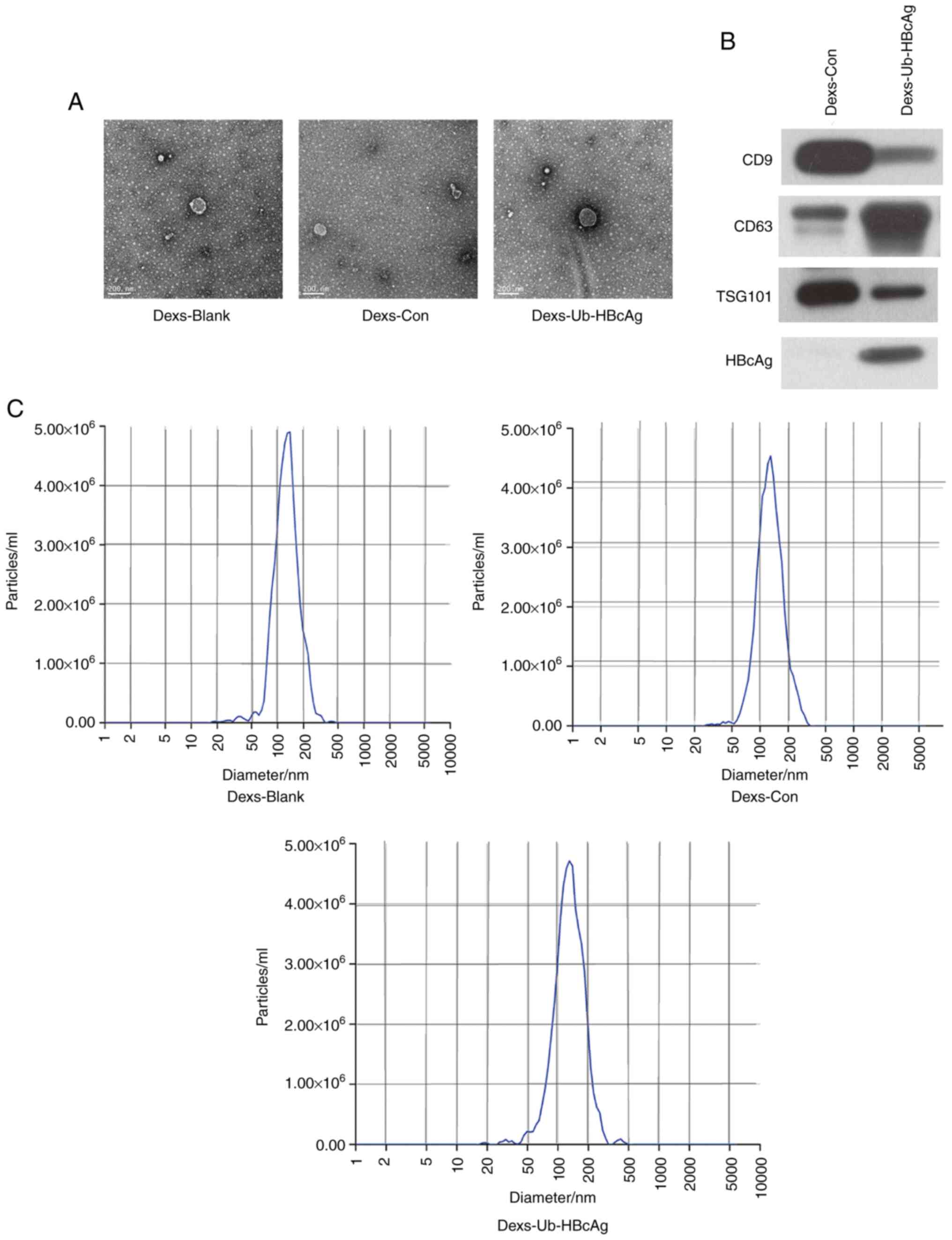
Dex morphology, size and marker
protein expression
Exosomes were extracted and purified from the
culture supernatants via ultracentrifugation and ultrafiltration.
TEM images revealed that the Dexs were spherical or ovoid in shape
with an envelope-like structure (Fig.
2A). The expression of the exosomal protein markers CD9, CD63
and TSG101 was then measured in the Dexs-Ub-HBcAg and Dexs-Con
groups. A protein band for HBcAg was observed in the Dexs-Ub-HBcAg
group, indicating that the isolated exosomes expressed HBcAg
(Fig. 2B). However, the expression
levels of these proteins were not measured in the Dexs-Blank group,
which is a limitation of the study. The analysis of the size of the
exosomes using nanoparticle tracking analysis revealed a scattered
or clustered distribution with a mean particle diameter of 123.8 nm
(Fig. 2C).
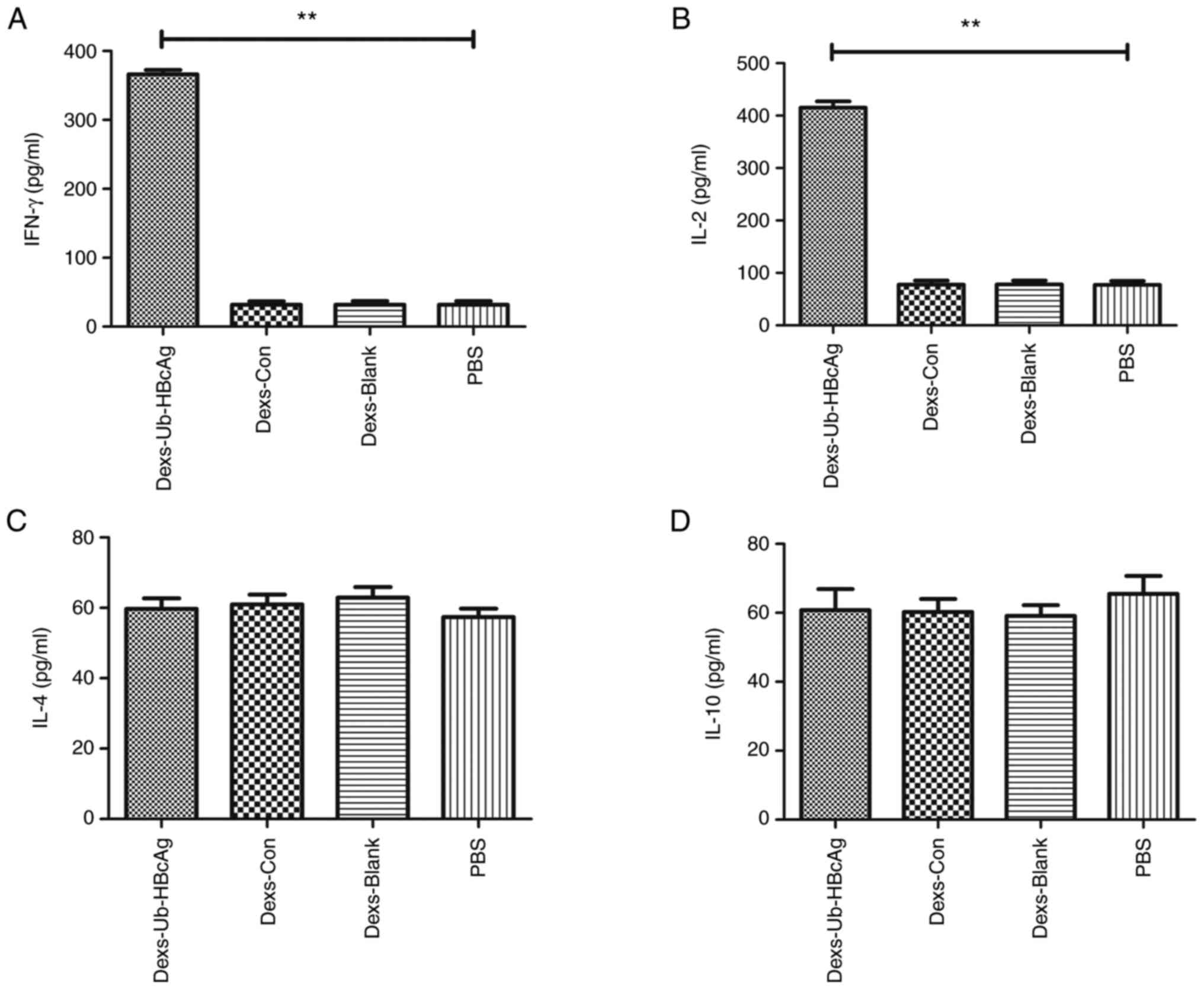
Dexs-Ub-HBcAg stimulates the secretion
of cytokines
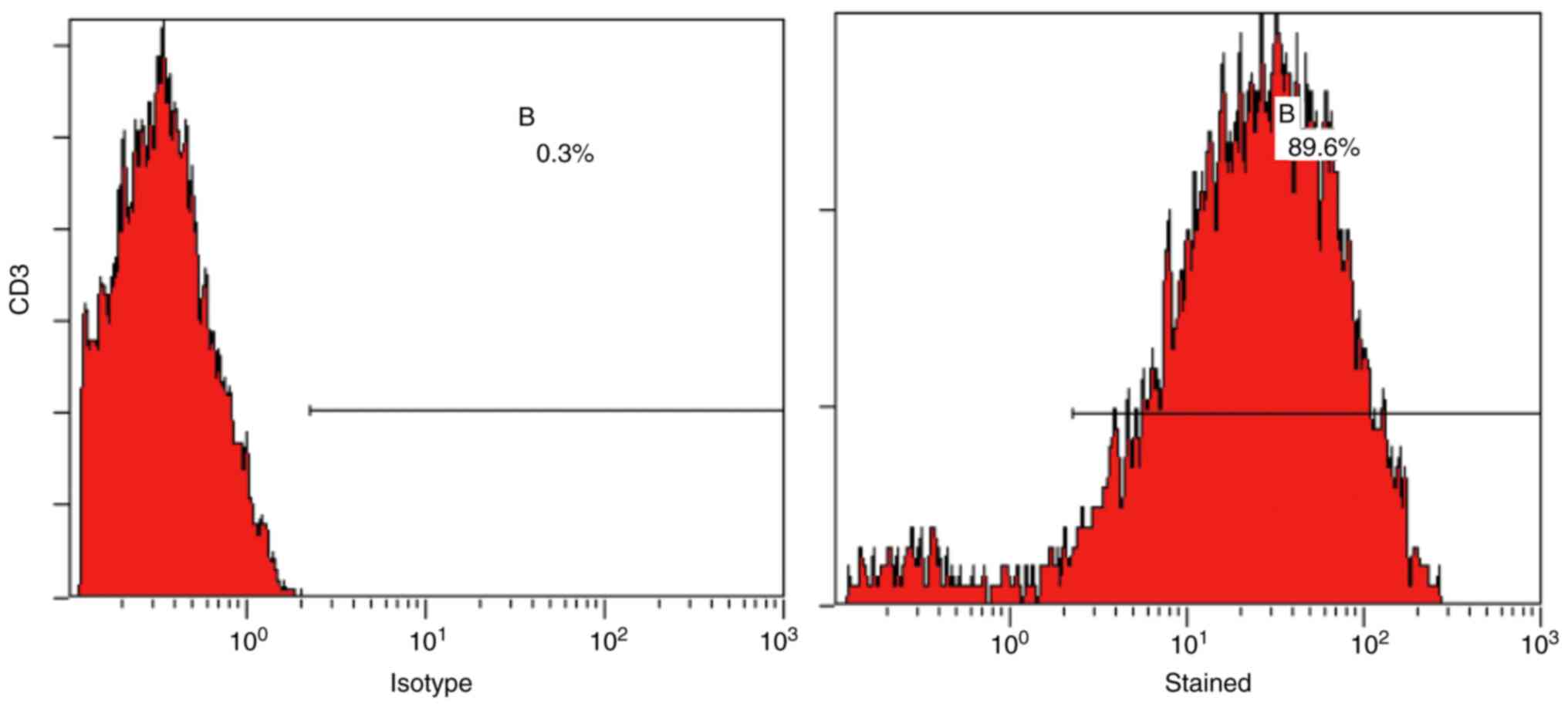
T-lymphocytes were isolated from mouse splenocytes
and analyzed using flow cytometry, which confirmed that they were
of adequate purity (>80%) (Fig.
3). The production of the cytokines IFN-γ, IL-2, IL-4 and IL-10
by the T-lymphocytes was examined in the presence of Dexs-Ub-HBcAg
(10 µg/ml), Dexs-Con (10 µg/ml), Dexs-Blank (10 µg/ml) or PBS. As
shown in Fig. 4A and B, T-lymphocytes from the Dexs-Ub-HBcAg
group released larger amounts of IFN-γ and IL-2, a Th1-like
cytokine, compared with those from the other groups (P<0.01).
However, the secretion of IL-4 and IL-10, a Th2-like cytokine, did
not exhibit any significant differences among the groups (Fig. 4C and D). These results indicate that the Th1
immune response was preferentially primed.
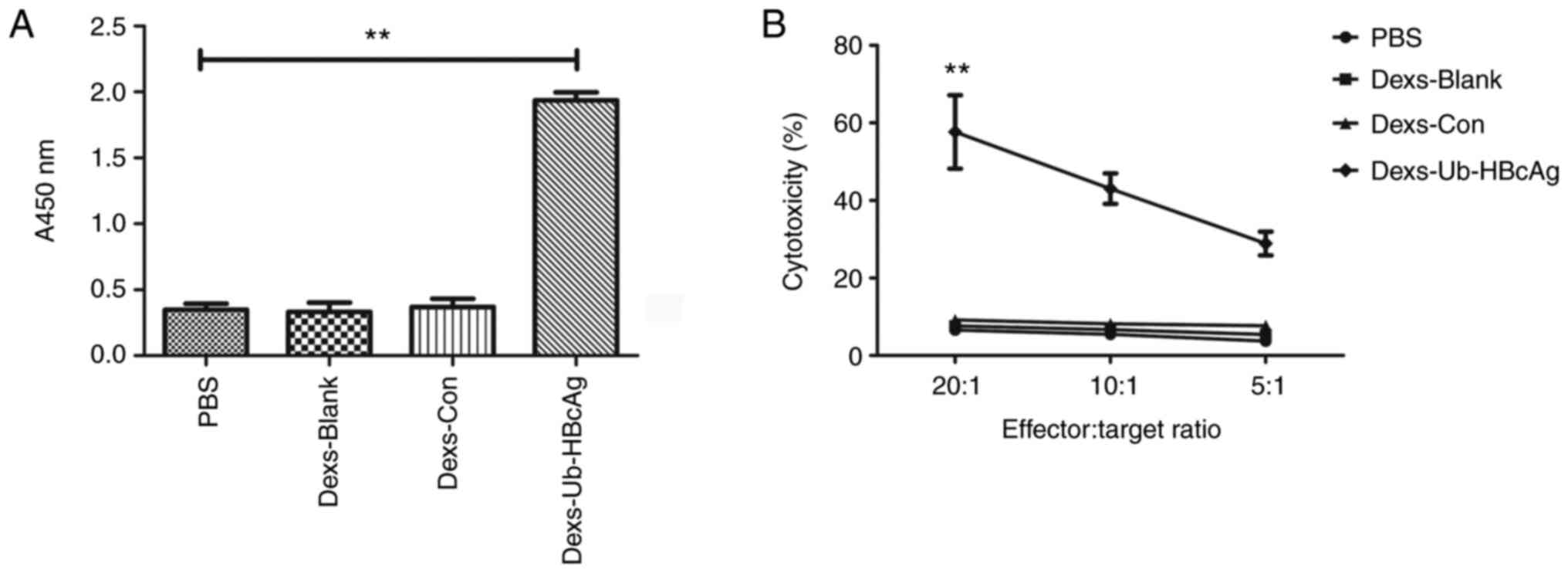
Dexs-Ub-HBcAg enhances T-cell
proliferation
The growth of T-lymphocytes in the different groups
was then evaluated. The Dexs-Ub-HBcAg group exhibited a
significantly greater T-lymphocyte proliferative capacity compared
with the other groups (P<0.01), as illustrated in Fig. 5A. This result indicates the
markedly higher T-cell proliferation in the Dexs-Ub-HBcAg
group.
Dexs-Ub-HBcAg enhances CTL
activity
The HBcAg-specific CTL activity towards P815/c cells
was evaluated using an LDH release assay. As demonstrated in
Fig. 5B, the proportions of
specific cytolysis in the Dexs-Ub-HBcAg group were 57.66±9.48,
43.04±3.94 and 28.89±3.07%, respectively, at effector:target ratios
of 20:1, 10:1 and 5:1. The Dexs-Ub-HBcAg group generated a
significantly greater proportion of specific cytolysis compared
with the other groups (P<0.01). These results indicate that the
HBV-specific CTL activity was enhanced in the Dexs-Ub-HBcAg
group.
Discussion
Patients who have persistent HBV infection have a
significant possibility of developing cirrhosis of the liver and
hepatocellular carcinoma (26). A
weak Th1 immunity combined with the inefficient activation of
CD8+ CTLs leads to therapeutic failure in patients with
CHB (25). Host anti-HBV immune
responses have been identified as the major determinants during
viral replication and clearance (27,28).
Several therapeutic vaccination strategies for HBV have recently
been developed to enhance the immune response and eliminate the
virus (29). DCs are potent
antigen-presenting cells with a notable capacity to interact with
naive T-cells and trigger immunological reactions (27). The viability of activating Th1
immunity and CTL reactions to remove chronic HBV infection using
DC-based therapeutic immunotherapy has already been demonstrated.
Specifically, in previous research, it was demonstrated that DCs
transduced with LV-Ub-HBcAg preferentially initiated anti-HBcAg Th1
immunity and induced specific CTL activity (10,11).
Nonetheless, DC-based vaccines are challenging to prepare and use
on a large scale in clinical settings. The implementation of
DC-based therapeutic immunotherapy in large populations is costly
and dependent on professional competence. Additionally, the
long-term storage of DCs and maintenance of their efficacy is
difficult (30). Dexs possess the
crucial immunostimulatory capacity of DCs. In addition, they may be
stored in a frozen state for ≥6 months due to the stability of the
exosomal membranes (31). Dexs
have been recommended as a potential solution to a number of
technical challenges involved in DC-based immunotherapy (15). As biological agents, Dexs are also
more suitable than DCs for preparation using a highly supervised
and monitored process. In addition, they do not carry the risks
associated with viable cellular or viral therapies, such as in
vivo replication (15). To
date, exosomes have been utilized as medication carriers,
vaccination and immunotherapy tools, as well as biomarker
transporters (32,33).
Exosome-bound antigens may produce higher
antigen-specific anticancer or antiviral immune reactions than
those produced by soluble antigens (34,35).
In the present study, it was discovered that antigen-modified Dexs
stimulated T-lymphocyte growth, cytokine release and CTL
development in vitro. Exosomes derived from mature DCs have
higher surface expression levels of intercellular adhesion
molecule-1, MHC and CD86 molecules than imDCs, which may increase
their uptake by DCs and thereby promote T-cell activation (36-38).
In contrast to exosomes released from imDCs, these exosomes have a
stronger ability to activate T cells (36-39).
In the present study, DCs derived from mouse bone marrow cells were
loaded with LV-Ub-HBcAg and then stimulated with mIL-4, mGM-CSF and
LPS. Following differential velocity centrifugation, very pure
exosomes were isolated from mature DCs, which were termed
Dexs-Ub-HBcAg. These exosomes were 50-150 nm in diameter with
potent immunostimulatory properties.
Previous studies have demonstrated that the
responsiveness of patients with CHB to antiviral medication is
associated with the predominance of the Th1 immune reaction and
elevated CTL function, suggesting that Th1 immunity may be a
crucial modulator in the treatment of patients with CHB (27,40).
Th1 cells release substantial quantities of type 1 cytokines,
including IFN-γ and IL-2. By contrast, Th2 cells release
substantial amounts of type 2 cytokines, such as IL-4 and
IL-10(6). In the present study,
the Dexs-Ub-HBcAg group clearly produced the largest amounts of the
Th1-like cytokines IFN-γ and IL-2. Furthermore, no significant
variations in the IL-4 and IL-10 levels were detected among the
groups. These results indicate that anti-HBcAg Th1 immunity was
preferentially primed. IL-2 plays a key role in the growth,
differentiation and maturation of T-cells, as well as in the growth
of Th cells (41). IFN-γ is
required for the development of Th1 cells, and CTL activity is
associated with the stimulation of Th1 immunity. The findings of
the present study suggest that the CTL activity of HBV-specific
CD8+ T-cells was increased in the Dexs-Ub-HBcAg group
due to the stimulatory effect of cytokines secreted by Th1-type
cells. In the present study, Dexs-Ub-HBcAg were found to induce
greater CTL cytotoxicity and higher killing potency against P815/c
cells compared with the controls. These findings demonstrate that
Dexs-Ub-HBcAg enhanced T-cell growth, cytokine production and
differentiation into CTLs in vitro.
In conclusion, the present study demonstrated that
Dexs, a cell-free vaccine that includes ubiquitinated HBcAg and is
antigen-presenting, may efficiently promote T-cell growth and
activation to develop antigen-specific CTLs that display
HBcAg-specific CTL immune reactions in vitro. Based on their
unique combination of DCs and cell-free vectors, Dexs have great
potential as a replacement for DCs in therapeutic vaccines.
Additionally, in mice carrying the hepatitis delta virus,
antigen-modified Dexs have exhibited beneficial effects on the
antiviral immune response (42).
This suggests that treatment with Dexs-Ub-HBcAg may provide an
effective therapeutic option for the elimination of HBV.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the grants from
Jiangsu Provincial Natural Science Foundation (grant no.
BK20181225), the Jiangsu Provincial Commission of Health and Family
Planning (grant no. H2018020) and the Zhenjiang Social Development
Project (grant no. SH2020033).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SD conceived and designed the study. YY, KL and WZ
performed the experiments. YY wrote the manuscript. SD, KL, WZ and
YY revised and edited the manuscript. YY, KL, WZ and SD confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The Laboratory Animal Ethics Committee of Jiangsu
University approved all the experimental methods (approval no.
K-20180031-Y).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Smith S, Harmanci H, Hutin Y, Hess S,
Bulterys M, Peck R, Rewari B, Mozalevskis A, Shibeshi M, Mumba M,
et al: Global progress on the elimination of viral hepatitis as a
major public health threat: An analysis of WHO member state
responses 2017. JHEP Rep. 1:81–89. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Chen S, Hu Q, Chen H, Zhang FF, Duan L,
Wang B, Li DD, Zhang J and Chen WX: Identification of serum CMTM2
as a potential biomarker for HBV-related disorders. Dis Markers.
2020(2032056)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Boni C, Fisicaro P, Valdatta C, Amadei B,
Di Vincenzo P, Giuberti T, Laccabue D, Zerbini A, Cavalli A,
Missale G, et al: Characterization of hepatitis B virus
(HBV)-specific T-cell dysfunction in chronic HBV infection. J
Virol. 81:4215–4225. 2007.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Maini MK, Boni C, Lee CK, Larrubia JR,
Reignat S, Ogg GS, King AS, Herberg J, Gilson R, Alisa A, et al:
The role of virus-specific CD8(+) cells in liver damage and viral
control during persistent hepatitis B virus infection. J Exp Med.
191:1269–1280. 2000.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chyuan IT, Tsai HF, Tzeng HT, Sung CC, Wu
CS, Chen PJ and Hsu PN: Tumor necrosis factor-alpha blockage
therapy impairs hepatitis B viral clearance and enhances T-cell
exhaustion in a mouse model. Cell Mol Immunol. 12:317–325.
2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chen W, Shi M, Shi F, Mao Y, Tang Z, Zhang
B, Zhang H, Chen L, Chen L, Xin S and Wang FS: HBcAg-pulsed
dendritic cell vaccine induces Th1 polarization and production of
hepatitis B virus-specific cytotoxic T lymphocytes. Hepatol Res.
39:355–365. 2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tang TJ, de Man RA, Kusters JG, Kwekkeboom
J, Hop WCJ, van der Molen RG, Schalm SW and Janssen HLA:
Intrahepatic CD8 T-lymphocytes and HBV core expression in relation
to response to antiviral therapy for chronic hepatitis B patients.
J Med Virol. 72:215–222. 2004.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gao G and Luo H: The ubiquitin-proteasome
pathway in viral infections. Can J Physiol Pharmacol. 84:5–14.
2006.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Dai S, Chen X, Yu Y, Zang G and Tang Z:
Immunization with lentiviral vector-modified dendritic cells
encoding ubiquitinated hepatitis B core antigen promotes Th1
differentiation and antiviral immunity by enhancing p38 MAPK and
JNK activation in HBV transgenic mice. Mol Med Rep. 18:4691–4699.
2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Dai S, Zhuo M, Song L, Chen X, Yu Y, Tang
Z and Zang G: Dendritic cell-based vaccination with lentiviral
vectors encoding ubiquitinated hepatitis B core antigen enhances
hepatitis B virus-specific immune responses in vivo. Acta Biochim
Biophys Sin (Shanghai). 47:870–879. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Dai S, Zhuo M, Song L, Chen X, Yu Y, Zang
G and Tang Z: Lentiviral vector encoding ubiquitinated hepatitis B
core antigen induces potent cellular immune responses and
therapeutic immunity in HBV transgenic mice. Immunobiology.
221:813–821. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chuma M, Terashita K and Sakamoto N: New
molecularly targeted therapies against advanced hepatocellular
carcinoma: From molecular pathogenesis to clinical trials and
future directions. Hepatol Res. 45:E1–E11. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Rao Q, Zuo B, Lu Z, Gao X, You A, Wu C, Du
Z and Yin H: Tumor-derived exosomes elicit tumor suppression in
murine hepatocellular carcinoma models and humans in vitro.
Hepatology. 64:456–472. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Taïeb J, Chaput N and Zitvogel L:
Dendritic cell-derived exosomes as cell-free peptide-based
vaccines. Crit Rev Immunol. 25:215–223. 2005.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Besse B, Charrier M, Lapierre V, Dansin E,
Lantz O, Planchard D, Le Chevalier T, Livartoski A, Barlesi F,
Laplanche A, et al: Dendritic cell-derived exosomes as maintenance
immunotherapy after first line chemotherapy in NSCLC.
Oncoimmunology. 5(e1071008)2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Escudier B, Dorval T, Chaput N, André F,
Caby MP, Novault S, Flament C, Leboulaire C, Borg C, Amigorena S,
et al: Vaccination of metastatic melanoma patients with autologous
dendritic cell (DC) derived-exosomes: results of thefirst phase I
clinical trial. J Transl Med. 3(10)2005.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Morse MA, Garst J, Osada T, Khan S,
Hobeika A, Clay TM, Valente N, Shreeniwas R, Sutton MA, Delcayre A,
et al: A phase I study of dexosome immunotherapy in patients with
advanced non-small cell lung cancer. J Transl Med.
3(9)2005.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chen JH, Yu YS, Chen XH, Liu HH, Zang GQ
and Tang ZH: Enhancement of CTLs induced by DCs loaded with
ubiquitinated hepatitis B virus core antigen. World J
Gastroenterol. 18:1319–1327. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Gardner A and Ruffell B: Dendritic cells
and cancer immunity. Trends Immunol. 37:855–865. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Li J, Huang S, Zhou Z, Lin W, Chen S, Chen
M and Ye Y: Exosomes derived from rAAV/AFP-transfected dendritic
cells elicit specific T cell-mediated immune responses against
hepatocellular carcinoma. Cancer Manag Res. 10:4945–4957.
2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Nigro A, Finardi A, Ferraro MM, Manno DE,
Quattrini A, Furlan R and Romano A: Selective loss of microvesicles
is a major issue of the differential centrifugation isolation
protocols. Sci Rep. 11(3589)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Li H, Yang C, Shi Y and Zhao L: Exosomes
derived from siRNA against GRP78 modified bone-marrow-derived
mesenchymal stem cells suppress Sorafenib resistance in
hepatocellular carcinoma. J Nanobiotechnology.
16(103)2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Gao X, Wan Z, Wei M, Dong Y, Zhao Y, Chen
X, Li Z, Qin W, Yang G and Liu L: Chronic myelogenous leukemia
cells remodel the bone marrow niche via exosome-mediated transfer
of miR-320. Theranostics. 9:5642–5656. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Tatu RF, Anuşca DN, Groza SŞ, Marusciac L,
Bojin FM, Tatu C, Hurmuz M and Păunescu V: Morphological and
functional characterization of femoral head drilling-derived
mesenchymal stem cells. Rom J Morphol Embryol. 55:1415–1422.
2014.PubMed/NCBI
|
|
25
|
Chen X, Liu H, Tang Z, Yu Y and Zang G:
The modification of Tapasin enhances cytotoxic T lymphocyte
activity of intracellularly delivered CTL epitopes via cytoplasmic
transduction peptide. Acta Biochim Biophys Sin (Shanghai).
45:203–212. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ganem D and Prince AM: Hepatitis B virus
infection-natural history and clinical consequences. N Engl J Med.
350:1118–1129. 2004.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Tsai SL, Sheen IS, Chien RN, Chu CM, Huang
HC, Chuang YL, Lee TH, Liao SK, Lin CL, Kuo GC, et al: Activation
of Th1 immunity is a common immune mechanism for the successful
treatment of hepatitis B and C: Tetramer assay and therapeutic
implications. J Biomed Sci. 10:120–135. 2003.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Phillips S, Chokshi S, Riva A, Evans A,
Williams R and Naoumov NV: CD8(+) T cell control of hepatitis B
virus replication: direct comparison between cytolytic and
noncytolytic functions. J Immunol. 184:287–295. 2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Cargill T and Barnes E: Therapeutic
vaccination for treatment of chronic hepatitis B. Clin Exp Immunol.
205:106–118. 2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Pitt JM, Charrier M, Viaud S, André F,
Besse B, Chaput N and Zitvogel L: Dendritic cell-derived exosomes
as immunotherapies in the fight against cancer. J Immunol.
193:1006–1011. 2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Andre F, Escudier B, Angevin E, Tursz T
and Zitvogel L: Exosomes for cancer immunotherapy. Ann Oncol. 15
(Suppl 4):iv141–iv144. 2004.PubMed/NCBI View Article : Google Scholar
|
|
32
|
EL Andaloussi S, Mäger I, Breakefield XO
and Wood MJ: Extracellular vesicles: Biology and emerging
therapeutic opportunities. Nat Rev Drug Discov. 12:347–357.
2013.PubMed/NCBI View
Article : Google Scholar
|
|
33
|
Alvarez-Erviti L, Seow Y, Yin H, Betts C,
Lakhal S and Wood MJ: Delivery of siRNA to the mouse brain by
systemic injection of targeted exosomes. Nat Biotechnol.
29:341–345. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
34
|
Lindenbergh MFS, Wubbolts R, Borg EGF, van
't Veld EM, Boes M and Stoorvogel W: Dendritic cells release
exosomes together with phagocytosed pathogen; potential
implications for the role of exosomes in antigen presentation. J
Extracell Vesicles. 9(1798606)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ramos-Zayas Y, Franco-Molina MA,
Hernádez-Granados AJ, Zárate-Triviño DG, Coronado-Cerda EE,
Mendoza-Gamboa E, Zapata-Benavides P, Ramírez-Romero R,
Santana-Krymskaya SE, Tamez-Guerra R and Rodríguez-Padilla C:
Immunotherapy for the treatment of canine transmissible venereal
tumor based in dendritic cells pulsed with tumoral exosomes.
Immunopharmacol Immunotoxicol. 41:48–54. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Viaud S, Ploix S, Lapierre V, Théry C,
Commere PH, Tramalloni D, Gorrichon K, Virault-Rocroy P, Tursz T,
Lantz O, et al: Updated technology to produce highly immunogenic
dendritic cell-derived exosomes of clinical grade: A critical role
of interferon-γ. J Immunother. 34:65–75. 2011.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Segura E, Amigorena S and Théry C: Mature
dendritic cells secrete exosomes with strong ability to induce
antigen-specific effector immune responses. Blood Cells Mol Dis.
35:89–93. 2005.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Segura E, Nicco C, Lombard B, Véron P,
Raposo G, Batteux F, Amigorena S and Théry C: ICAM-1 on exosomes
from mature dendritic cells is critical for efficient naive T-cell
priming. Blood. 106:216–223. 2005.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Utsugi-Kobukai S, Fujimaki H, Hotta C,
Nakazawa M and Minami M: MHC class I-mediated exogenous antigen
presentation by exosomes secreted from immature and mature bone
marrow derived dendritic cells. Immunol Lett. 89:125–131.
2003.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Boni C, Bertoletti A, Penna A, Cavalli A,
Pilli M, Urbani S, Scognamiglio P, Boehme R, Panebianco R,
Fiaccadori F and Ferrari C: Lamivudine treatment can restore T cell
responsiveness in chronic hepatitis B. J Clin Invest. 102:968–975.
1998.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Yu X, Wang Z, Chen H, Niu X, Dou Y, Yang
J, Tang Y and Diao Y: Serological and pathogenic analyses of fowl
adenovirus serotype 4 (FAdV-4) strain in muscovy ducks. Front
Microbiol. 9(1163)2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Yao T, Lv M, Ma S, Chen J, Zhang Y, Yu Y,
Zang G and Chen X: Ubiquitinated hepatitis d antigen-loaded
microvesicles induce a potent specific cellular immune response to
inhibit HDV replication in vivo. Microbiol Spectr.
9(e0102421)2021.PubMed/NCBI View Article : Google Scholar
|