Introduction
According to global cancer statistics, lung cancer
is the leading cause of cancer-related deaths worldwide (1). As a new category of non-small cell
lung cancer (NSCLC) in 2015 according to World Health Organization
classification, invasive mucinous adenocarcinoma (IMA) accounts for
2-5% of lung adenocarcinomas (2,3).
Based on image diagnosis, IMA usually has abundant
intracellular/extracellular mucus and invasive adenocarcinoma
patterns. The typical computed tomography (CT) of IMA, incudes
consolidation, ground-glass opacity and nodules (2). Clinically, IMA is easily misdiagnosed
as pneumonia (4,5).
The present study presented an IMA case, which
manifested as diffuse, patchy and blurry density shadows throughout
both sides. It emphasized that bronchioloalveolar carcinoma should
be suspected when radiological manifestations show diffuse, patchy
and blurry density shadows throughout all lobes. The present study
was approved by the ethics committee review board of the First
Affiliated Hospital of Chengdu Medical College (approval no.
2019CYFYIRB-BA-Jun13). The patient provided signed informed consent
and authorized the publication of the images.
Case presentation
A 45-year-old male complained of a cough with
production of sputum without obvious cause for a year. He denied
chest pain, fever, chest tightness, shortness of breath,
hemoptysis, night sweats, palpitations, eyelid edema and extremity
edema. He was treated at another hospital and diagnosed with
‘pneumonia’. He was discharged from the hospital with symptom
improvement after anti-infection treatment. Following discharge, he
repeatedly coughed, with sputum production. For every episode, his
symptoms were relieved after anti-infection treatment. However, the
symptoms recurred and became worse after 2 days of treatment. Chest
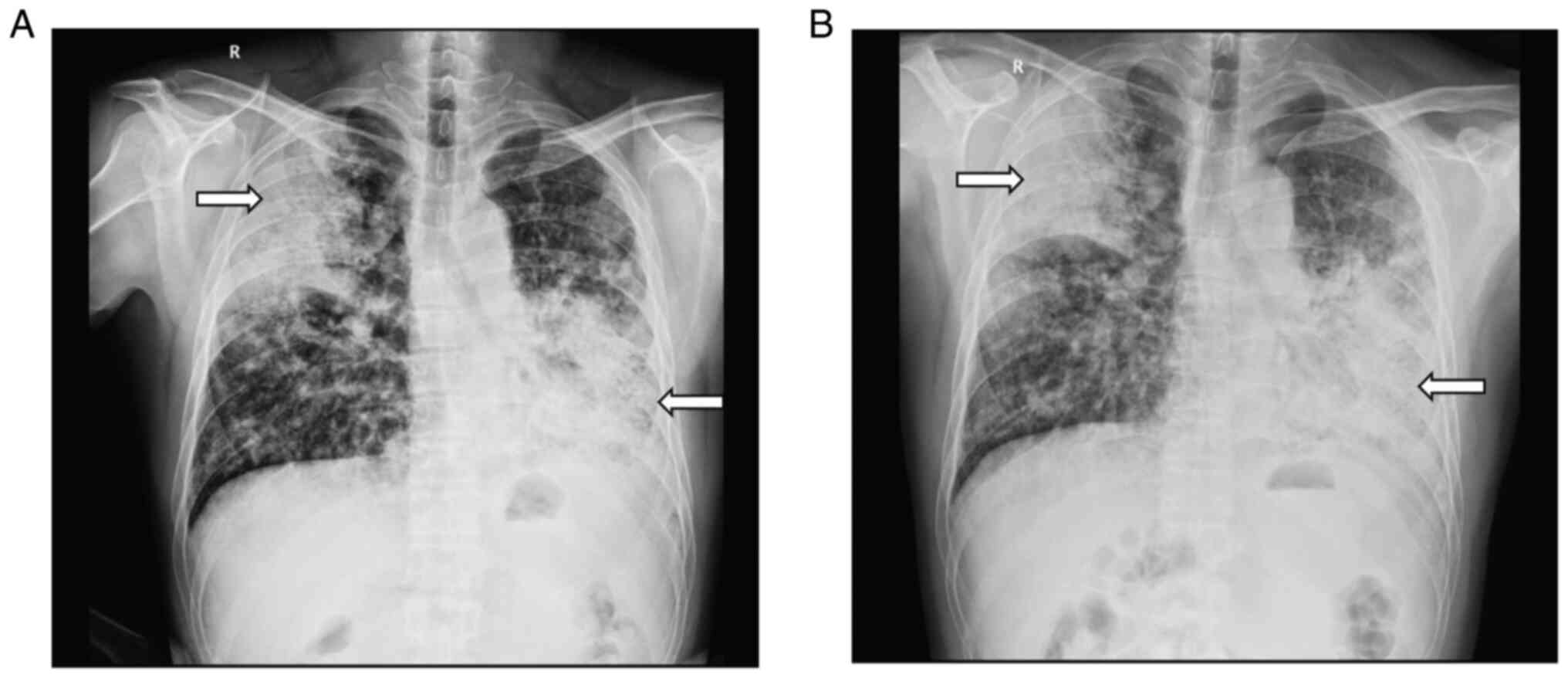
digital radiography (DR) showed diffuse, patchy and blurry density
shadows throughout both lungs (Fig.
1A). He was transferred to the Departments of Pulmonary and
Critical Care Medicine, The First Affiliated Hospital of Chengdu
Medical College, for further treatment.
His vital signs were within normal limits. Physical
examination revealed coarse breath sounds and scattered wet rales
in the bilateral lungs. Laboratory tests at admission included
white blood cell count (0.42x109/l), percentage of
lymphocytes (12.1%), and percentage of neutrophils (80.1%). His
C-reactive protein level was 5.4 mg/l, and his erythrocyte
sedimentation rate was 20 mm/h. T-SPOT for tuberculosis infection
was positive, with 84 spots in panel A and 18 spots in panel B. A
tumor marker test showed the following: Carcinoembryonic antigen,
48.15 ng/ml; carbohydrate antigen 19-9, >400.00 U/ml;
carbohydrate antigen 153, 75.49 U/ml; carbohydrate antigen 242,
>200.00 U/ml; cytokeratin 19-fragment, 5.19 ng/ml; and
carbohydrate antigen 72-4, 70.33 U/ml. Renal function, electrolyte
level, and blood coagulation function were in the normal ranges.
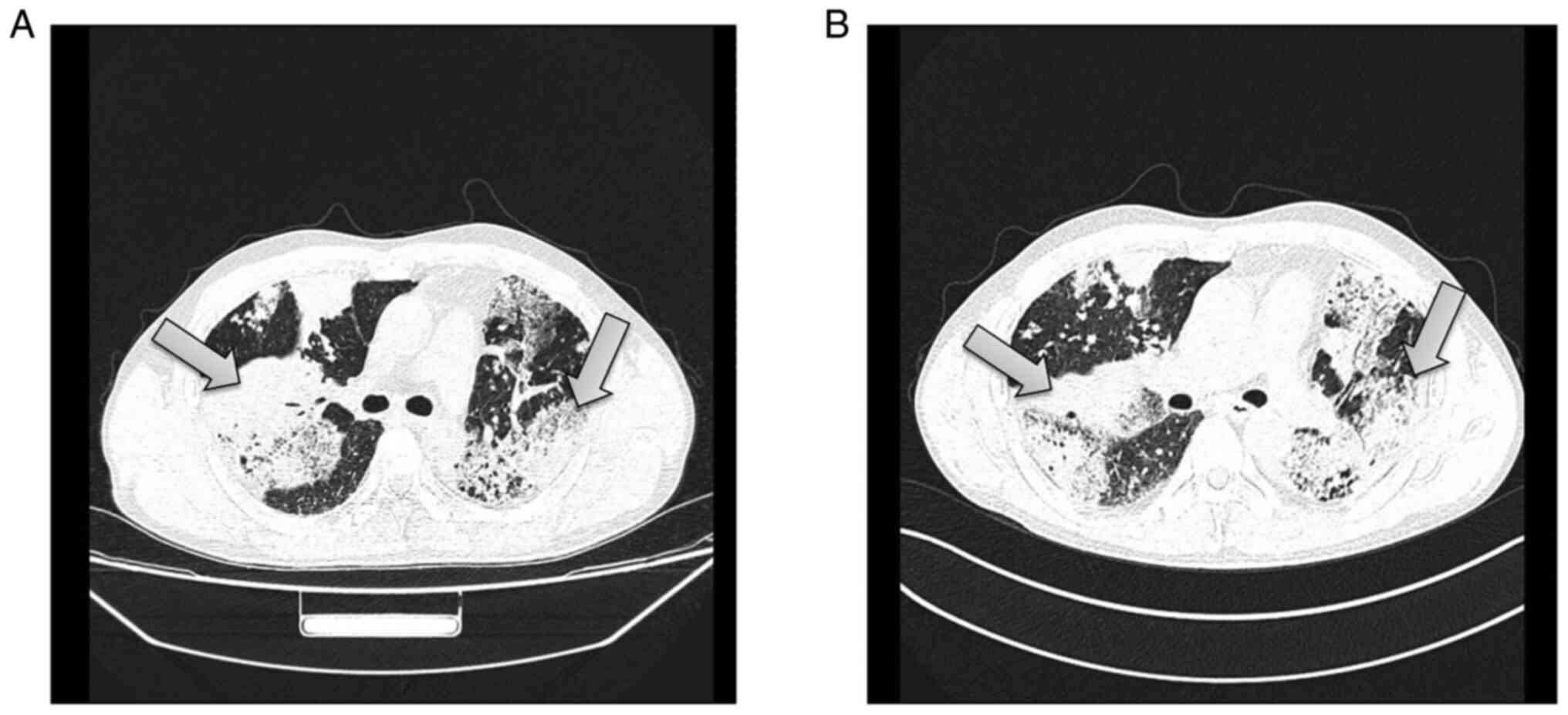
Chest CT showed the following: i) Diffuse, patchy and blurry
hyperdense shadows, nodular shadows and patchy consolidation, which
were more obvious in the right upper lobe and left lung; the image
findings suggested a suspected infectious lesion, but other lesions
could not be ruled out; ii) multiple lymph nodes with partial
calcification in the mediastinum and the lungs; and iii) minor
effusion in the left thoracic cavity and bilateral pleural
thickening (Fig. 2A). Enhanced
chest CT showed: i) diffuse, patchy and blurry hyperdense shadows
and nodular shadows that were more obvious in the right upper lobe
and left lung; enhancement of CT was not clear, the image findings
suggested a suspected infectious lesion, but other lesions could
not be ruled out; ii) multiple lymph nodes with partial
calcification in the mediastinum and the lungs; and iii) minor
effusion in the left thoracic cavity and bilateral pleural
thickening (Fig. 2B).
The patient received empiric antimicrobial therapy
following admission. Combined cefuroxime sodium (0.75 g/8 h,
intravenous injection) and moxifloxacin hydrochloride (0.4 g/day,
intravenous injection) were administered for anti-infection
treatment. According to the results of chest CT and enhanced chest
CT, it was possible to consider infectious lesion. At one week
following anti-infective treatment, chest DR examination showed
that the lesions were more advanced than before (Fig. 1B). The T-SPOT test was positive but
with a small number of spots. The possibility of tuberculosis was
low. The patient's tumor marker levels were significantly elevated.
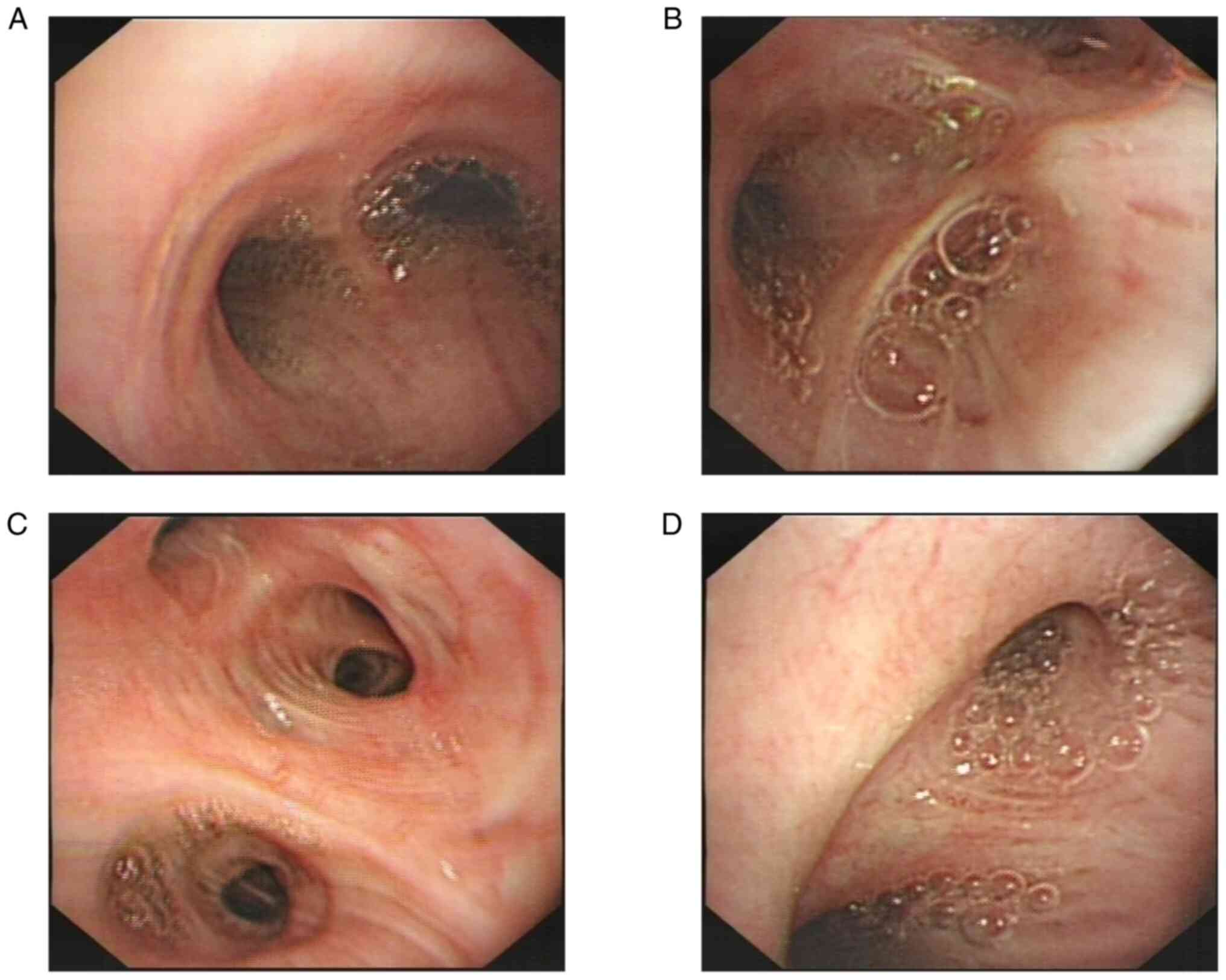
IMA could not be ruled out. Thus, fiberoptic bronchoscopy was
performed and found no obvious morphological change (Fig. 3). The pathological examination
suggested that the mucosal epithelium was undergoing chronic
inflammatory changes with mucosal epithelial cell proliferation
(data not shown). A case discussion in Departments of Pulmonary and
Critical Care Medicine, The First Affiliated Hospital of Chengdu
Medical College considered a tumor diagnosis according to the tumor
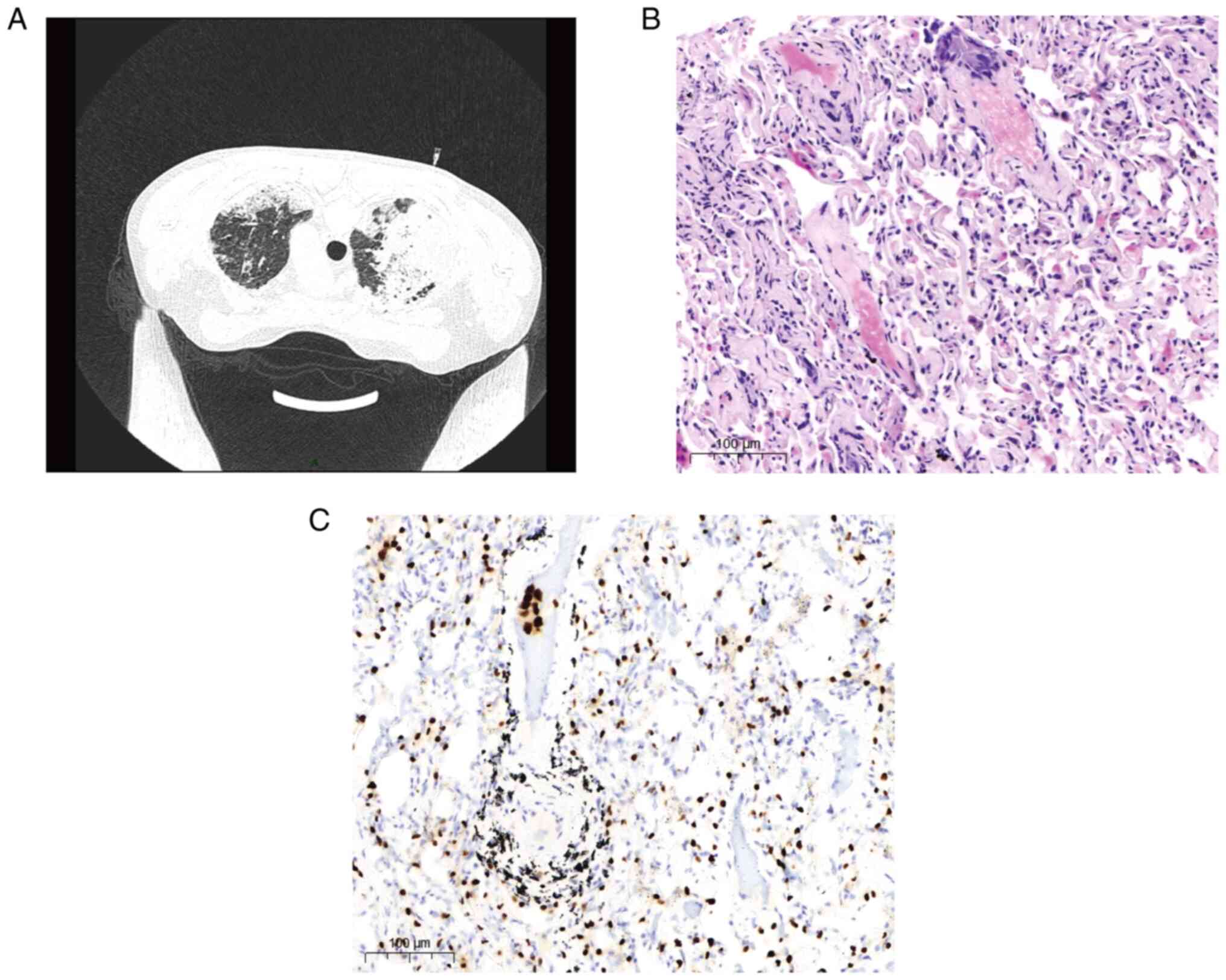
marker and pathology results. A percutaneous lung puncture biopsy
was performed (Fig. 4A). The
collected samples were fixed in 10% neutral buffered formalin 24 h
at room temperature and embedded in paraffin. Sections were cut at
5 µm and hematoxylin-eosin and thyroid transcription factor 1
(TTF-1) immunohistochemical (IHC) staining (6) were performed following the
manufacturer's protocols. The adenocarcinoma was diagnosed based on
the pathological staining which were evaluated independently by two
pathologists at the First Affiliated Hospital of Chengdu Medical
College in a double-blinded manner (Fig. 4B and C). Pathological specimens were almost
negative for all driver genes, except KRAS (Table I). However, there was no KRAS G12C
molecular targeted drug in 2019 and the patients and family refused
treatment, including chemotherapy, radiotherapy and interventional
chemotherapy and the patient was discharged from the hospital.
 | Table IDetection of genes mutations. |
Table I
Detection of genes mutations.
| Gene | Mutation | Conclusion |
|---|
| EGFR | NO | KRAS G12C is the
‘driver’ gene in this case. Sotorasib and Adagrasib are recommended
for treatment. |
| HER2 | NO | |
| KRAS | G12C | |
| ALK | NO | |
| BRAF | NO | |
| PI3KA | NO | |
| L861Q | NO | |
| TRIM4 | NO | |
| VAMP2 | NO | |
| NRG1 | NO | |
| CD74 | NO | |
Discussion
IMA, formerly known as mucinous bronchioloalveolar
carcinoma, is a special type lung adenocarcinoma. It is more common
in women and not associated with smoking. Its incidence has
increased in recent decades (7).
Its etiology and mechanism remain unclear, but the occurrence of
IMA may be related to a variety of risk factors such as EGFR, KRAS
and/or HER2 genes mutation (8-10).
Its occurrence is associated with genetic factors, environmental
factor and chronic inflammation. The pathological basis may be the
invasive growth of cancer tissue derived from bronchioles or
alveoli. When disseminated in the airway, the cancer cells cover
the surface of the alveolar wall and grow along the alveolar wall
(8). Recently, Kimura et al
(11) reported a esophageal
metastasis of IMA and combined with emerging data (12), suggests that IMA can progress into
a more aggressive status.
Some patients with IMA are asymptomatic, while some
have nonspecific respiratory symptoms, including coughing with
sputum production, blood-tinged sputum, and dyspnea, and systemic
symptoms including fever, fatigue and weight loss. Physical
examination is usually unremarkable (4).
The diagnosis of IMA is challenging. Imaging
findings can vary and be nonspecific, including consolidation of
the lung parenchyma, nodules, honeycomb signs and ground-glass
changes (13). However, common
findings including irregular masses and absence of lung cancer
signs. Mucus changes may manifest as consolidation that is
difficult to differentiate from infectious pneumonia. It is easily
misdiagnosed as pneumonia tuberculosis or pulmonary actinomycosis
(14). IMA is difficult to
diagnose not only because of its nonspecific clinical
manifestations but also because of inflammation signs in imaging
studies. The affected alveoli and normal alveoli are arranged in a
mixed manner, which in this case manifested in the imaging study as
diffuse and vague patchy shadows throughout both lungs. These
findings are consistent with pneumonia-like changes; therefore, IMA
can easily be misdiagnosed as pneumonia (4,15,16).
The diffuse, patchy, and blurry shadows throughout both lungs are
nonspecific and can be noted in various pulmonary infectious
diseases such as pneumonia and tuberculosis. Differentiating IMA
from other lung diseases depends mainly on pathological results.
According to the literature, the case reported is relatively rare
because diffuse and patchy shadows are unusual in patients with
IMA. Most IMA cases manifest as patch consolidation in the lungs
rather than diffuse and patchy shadows in the bilateral lungs. It
was learned from this case that diffuse patchy shadows may be signs
of IMA.
As a subtype of lung adenocarcinoma, IMA has similar
epidemiology with other subtypes. However, IMA has unique imaging
characters, such as consolidation, ground-glass opacity and nodules
(2). IMA is divided into two types
by the shape of image, pneumonic and solid types. These types have
a great difference in clinical outcome. Compared with pneumonia
IMA, the isolated type usually has lower pathological stage with
more satisfying outcome. The difference is probably due to the
prevalence of the pulmonary type IMA (17,18).
Reports have also found the disease free survival of pneumonic type
patients is significantly worse and this type of patient was more
prone to have cancer recurrence and/or metastasis after resection
(17,19). In the present study, the IMA case
had typical pneumonic characters. It predicted unfavorable survival
of this patient, although the family refused to disclose the status
of patient when this case was followed up.
According to previous reports, KRAS mutation is the
most common ‘driver’ mutation in IMA, its incidence in IMA is
significantly higher compared with other lung adenocarcinomas
(20,21). By contrast, other targeted ‘driver’
mutations are rare in IMA patients, such as EGFR mutation, ALK gene
rearrangement and BRAF V600E mutation (20,22,23).
In addition, rare gene mutations, such as HER2, BRAF and PI3KA
mutations and rare gene fusion, such as TRIM4-BRAF, VAMP2-NRG1 and
CD74-NRG1 fusion, are observed in IMA patients with
alteration-negative K-RAS (24).
In the present study, the sequencing data showed the present case
has KRAS gene alteration without EGFR, ALK and BRAF mutation, as
well as rare genes, such as, TRIM4, VAMP2, NRG1 and CD74. In
2021-2022, Sotorasib (25) and
Adagrasib (26) were approved for
KRAS G12C-mutated non-small cell lung cancer (NSCLC). Thus, there
are more molecular therapeutic choices for this type of IMA.
Immune checkpoint inhibitors (ICI) are widely used
to treat patients with NSCLC (27). Nakagomi et al (28) reported that expression of PD-L1 in
≥1% of cells is observed in only 6.1% of IMAs, but in 59.7% of
conventional adenocarcinomas. In agreement, Xu et al
(29) found only 9.7% (3/31) of
patients with IMA revealed positive PD-L1 expression. The
aforementioned evidence suggests that ICI treatment rarely benefits
IMA patients.
Compared with untreated IMA patients, the overall
survival rate of IMA patients receiving conventional chemotherapy
does not improve (14). Early IMA
patients can benefit from surgery and postoperative chemotherapy.
At present, there are no effective drugs for the treatment of
advanced pulmonary IMA (6,14).
Clinically, the clinical manifestations of IMA are
atypical, and the imaging findings vary. The diagnosis is often
missed, and misdiagnosis is common. These issues may delay
treatment. The diagnosis of IMA can be confirmed by a pathological
examination. Therefore, in clinical practice, when patients with
large lung consolidation do not respond to regular anti-infection
treatment and the lesion progresses, IMA should be considered.
Early pathological examination should be performed to rule out IMA,
so as not to delay disease treatment. For early staged IMA, surgery
and postoperative chemotherapy is recommended. Selected
molecular-targeted is a superior choice base on the result of
sequencing.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Natural Science
Foundation of Sichuan Province (grant no. 2022NSFSC0725).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YY and RH performed data curation. LX and JZ
utilised software. Hematoxylin-eosin and immunohistochemical
staining was performed by RH. WZ and NH conceived and designed the
study. WZ provided supervision. NH and WZ wrote the original draft.
NH analyzed and interpretated data. WZ and NH confirm the
authenticity of all the raw data. All authors revised the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Clinical data collection was approved by the Medical
Ethics Committee of The First Affiliated Hospital of Chengdu
Medical College (approval no. 2019CYFYIRB-BA-Jun13). Informed
written consent for participation in the study or use of the
medical data was obtained from the patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sheng A, Zhou P, Ye Y, Sun K and Yang Z:
Diagnostic efficacy of CT radiomic features in pulmonary invasive
mucinous adenocarcinoma. Scanning. 2022(5314225)2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Xu X, Shen W, Wang D, Li N, Huang Z, Sheng
J, Rucker AJ, Mao W, Xu H and Cheng G: . Clinical features and
prognosis of resectable pulmonary primary invasive mucinous
adenocarcinoma. Transl Lung Cancer Res. 11:420–431. 2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Butt YM and Allen TC: The demise of the
term bronchioloalveolar carcinoma. Arch Pathol Lab Med.
139:981–983. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Read WL, Page NC, Tierney RM, Piccirillo
JF and Govindan R: The epidemiology of bronchioloalveolar carcinoma
over the past two decades: Analysis of the SEER database. Lung
Cancer. 45:137–142. 2004.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Oktay E, Oflazoglu U, Varol Y, Tanriverdi
O, Mermur N, Arda HU, Demir L, Keskin O, Ahmadli T, Somali I, et
al: The prognostic role of thyroid transcription factor-1 in lung
adenocarcinoma. J Cancer Res Ther. 16:737–744. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Gardiner N, Jogai S and Wallis A: The
revised lung adenocarcinoma classification-an imaging guide. J
Thorac Dis. 6:537–546. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Moser L, Kegler K, Precht C and Zanolari
P: Bronchioalveolar carcinoma in an adult alpaca (Vicugna pacos).
BMC Vet Res. 15(139)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
He Y, Huang H, Xu M, Fu Z, Zhang X, Chen X
and Guo W: The effect of afatinib in a pretreated patient with
invasive mucinous adenocarcinoma of the lung harboring HER2 YVMA
insertion: A case report. Transl Cancer Res. 11:1819–1823.
2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chi K, Sun W, Yang X, Wu J, Wang H, Liu X,
Mao L, Zhou L, Huang X and Lin D: A prognostic classification based
on the International Association for the Study of Lung Cancer
histologic grading and immunoscore in KRAS-mutant invasive
non-mucinous adenocarcinoma. Thorac Cancer. 13:1050–1058.
2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kimura S, Onishi I and Kobayashi M: A rare
case of esophageal metastasis of invasive mucinous adenocarcinoma
of the lung. ACG Case Rep J. 9(e00857)2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chen X, Zhao Y, Wang D, Lin Y, Hou J, Xu
X, Wu J, Zhong L, Zhou Y, Shen J, et al: The
HNF4α-BC200-FMR1-positive feedback loop promotes growth and
metastasis in invasive mucinous lung adenocarcinoma. Cancer Res.
81:5904–5918. 2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhang X, Qiao W, Kang Z, Pan C, Chen Y, Li
K, Shen W and Zhang L: CT features of stage IA invasive mucinous
adenocarcinoma of the lung and establishment of a prediction model.
Int J Gen Med. 15:5455–5463. 2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhu D, Zhang Q, Rui Z and Xu S: Pulmonary
invasive mucinous adenocarcinoma mimicking pulmonary actinomycosis.
BMC Pulm Med. 22(181)2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Mir E, Sareen R, Kulshreshtha R and Shah
A: Bronchioloalveolar cell carcinoma presenting as a ‘non-resolving
consolidation’ for two years. Pneumonol Alergol Pol. 83:208–211.
2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Narahari NK, Uppin SG, Kapoor A, Stalin BJ
and Paramjyothi GK: Invasive mucinous adenocarcinoma of the lung in
a 19-year-old female. Asian Cardiovasc Thorac Ann. 26:635–639.
2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Nie K, Nie W, Zhang YX and Yu H: Comparing
clinicopathological features and prognosis of primary pulmonary
invasive mucinous adenocarcinoma based on computed tomography
findings. Cancer Imaging. 19(47)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kim DH, Bae SY, Na KJ, Park S, Park IK,
Kang CH and Kim YT: Radiological and clinical features of
screening-detected pulmonary invasive mucinous adenocarcinoma.
Interact Cardiovasc Thorac Surg. 34:229–235. 2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wang T, Yang Y, Liu X, Deng J, Wu J, Hou
L, Wu C, She Y, Sun X, Xie D and Chen C: Primary invasive mucinous
adenocarcinoma of the lung: prognostic value of CT imaging features
combined with clinical factors. Korean J Radiol. 22:652–662.
2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Horiguchi T, Yanagi S, Tomita M, Maeda R,
Uto K, Shigekusa T, Tsubouchi H, Matsumoto N and Nakazato M: A case
of bilateral invasive mucinous adenocarcinoma of the lung with
severe productive cough and dyspnea successfully treated with
palliative lung lobectomy. Respir Med Case Rep.
32(101368)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kadota K, Yeh YC, D'Angelo SP, Moreira AL,
Kuk D, Sima CS, Riely GJ, Arcila ME, Kris MG, Rusch VW, et al:
Associations between mutations and histologic patterns of mucin in
lung adenocarcinoma: Invasive mucinous pattern and extracellular
mucin are associated with KRAS mutation. Am J Surg Pathol.
38:1118–1127. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Boland JM, Maleszewski JJ, Wampfler JA,
Voss JS, Kipp BR, Yang P and Yi ES: Pulmonary invasive mucinous
adenocarcinoma and mixed invasive mucinous/nonmucinous
adenocarcinoma-a clinicopathological and molecular genetic study
with survival analysis. Hum Pathol. 71:8–19. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Cha YJ, Kim HR, Lee HJ, Cho BC and Shim
HS: Clinical course of stage IV invasive mucinous adenocarcinoma of
the lung. Lung Cancer. 102:82–88. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Shim HS, Kenudson M, Zheng Z, Liebers M,
Cha YJ, Hoang Ho Q, Onozato M, Phi Le L, Heist RS and Iafrate AJ:
Unique genetic and survival characteristics of invasive mucinous
adenocarcinoma of the lung. J Thorac Oncol. 10:1156–1162.
2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Nakajima EC, Drezner N, Li X,
Mishra-Kalyani PS, Liu Y, Zhao H, Bi Y, Liu J, Rahman A, Wearne E,
et al: FDA approval summary: Sotorasib for KRAS G12C-mutated
metastatic NSCLC. Clin Cancer Res. 28:1482–1486. 2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Frontline promise for
Adagrasib-Pembrolizumab combination. Cancer Discov.
13(OF2)2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Mazieres J, Drilon A, Lusque A, Mhanna L,
Cortot AB, Mezquita L, Thai AA, Mascaux C, Couraud S, Veillon R, et
al: Immune checkpoint inhibitors for patients with advanced lung
cancer and oncogenic driver alterations: results from the
IMMUNOTARGET registry. Ann Oncol. 30:1321–1328. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Nakagomi T, Goto T, Hirotsu Y, Shikata D,
Yokoyama Y, Higuchi R, Otake S, Amemiya K, Oyama T, Mochizuki H and
Omata M: Genomic characteristics of invasive mucinous
adenocarcinomas of the lung and potential therapeutic targets of
B7-H3. Cancers. 10:2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Xu X, Li N, Wang D, Chen W and Fan Y:
Clinical relevance of PD-L1 expression and CD8+ T cells'
infiltration in patients with lung invasive mucinous
adenocarcinoma. Front Oncol. 11(683432)2021.PubMed/NCBI View Article : Google Scholar
|