Introduction
Pathological cardiac hypertrophy is an independent
risk factor for arrhythmia, myocardial infarction, sudden death and
heart failure. Concentric ventricular hypertrophy results in
systolic or diastolic dysfunction and can be caused by chronic
hypertension, aortic stenosis, myocardial infarction, hereditary
cardiomyopathy, obesity and diabetes (1-3).
Pathological cardiac hypertrophy is characterized by numerous
decompensations, such as cardiomyocyte death, fibrosis,
Ca2+-regulated protein dysregulation, mitochondrial
dysfunction, metabolic reprogramming, reactivation of fetal gene
expression, altered sarcomere structure and insufficient
angiogenesis leading to microvascular sparseness (4-8).
Calcineurin is a cytoplasmic
Ca2+/calmodulin-dependent protein phosphatase that
contributes to pathological cardiac hypertrophy. The
calcineurin/nuclear factor-activated T-cell (NFATc) pathway affects
cardiac structure under pathological conditions and serves a role
in cardiac hypertrophy (9,10). In the adult heart, NFATc activity
is continuously upregulated during pathological cardiac hypertrophy
caused by pressure overload or hypertension and is more pronounced
after myocardial infarction-induced heart failure (11,12).
NFATc4 is required for calcineurin-mediated cardiomyocyte
hypertrophy (13,14). Angiotensin II (Ang II) or
phenylephrine can activate calcineurin/NFATc4 to promote
cardiomyocyte hypertrophy (15).
The tricarboxylic acid (TAC) cycle in the
mitochondrial matrix is considered a core process in cellular
metabolism and energy balance (16-18).
Succinate is an intermediate of this cycle and its concentrations
are significantly increased in hypertensive, obese and diabetic
animal models (19). It is also
the only metabolite whose levels are significantly increased in the
coronary sinus of patients with ST-segment elevation myocardial
infarction (STEMI) (20).
Succinate produces broad pathophysiological effects by binding to
the G-protein-coupled receptor 91 (GPR91)/Succinate receptor 1
(SUCNR1). SUCNR1 is expressed in the kidney, liver, heart, retinal
cells and other tissues and is involved in regulating blood
pressure, inhibiting lipolysis of white fat, forming retinal blood
vessels, promoting cardiac hypertrophy and activating hepatic
stellate cells (21,22). In a pulmonary arterial hypertension
model of pressure overload-induced right ventricular hypertrophy in
Sprague-Dawley rats, succinate treatment further aggravated right
ventricular hypertrophy, upregulated the right ventricular
hypertrophy-related gene ANP and activated PI3K/Akt axis signaling
(23). Succinate was found to
cause cardiac hypertrophy in SUCNR1-null mice in a SUCNR1-dependent
manner. Activation of SUCNR1 triggers signals of cardiac
hypertrophy such as phosphorylation of extracellular
signal-regulated kinases 1/2 (ERK1/2) and expression of
calmodulin-dependent protein kinase II delta (CaMKIIδ) (24).
Fructus Psoraleae (the dried fruits of Psoralea
corylifolia L.) is a common herb in traditional Chinese
medicine (TCM) that has been included in Chinese Pharmacopoeia
records (25). According to the
theory of TCM, Fructus Psoraleae has the effect of warming and
tonifying the kidney-Yang. It is widely used in the treatment of
coronary artery disease, osteoporosis, vitiligo and psoriasis.
Psoralea mainly contains coumarin, terpene phenol and
prenylflavonoids, which are the material basis of drug treatment.
Our team has summarized the pharmacological effects of five major
psoralea prenylflavonoids, which have anti-inflammatory,
cardiovascular protective, neuroprotective and antiosteoporosis
effects (26).
4'-O-methylbavachalcone (MeBavaC) (Fig. 1B) is a prenylflavonoid that is
abundantly present in Fructus Psoraleae (27). Few pharmacological studies have
been conducted on MeBavaC; the only known report on the subject
indicated that MeBavaC inhibits SARS-CoV papain-like protease PLpro
(28). The present study
investigated whether MeBavaC can ameliorate succinate-induced
cardiomyocyte hypertrophy by inhibiting the calcineurin/NFATc4
pathway. The results revealed that succinate activated the
calcineurin/NFATc4 and ERK1/2 pathways to promote cardiomyocyte
hypertrophy and that MeBavaC treatment inhibited succinate-induced
cardiomyocyte hypertrophy and related signaling; In addition,
molecular docking analysis indicated that MeBavaC interacted with
SUCNR1 to form a relatively stable binding and produced a certain
inhibitory effect.
Materials and methods
Reagents and antibodies
Sodium succinate and Dulbecco's modified Eagle's
medium (High Glucose) were purchased from MilliporeSigma. Fetal
bovine serum was obtained from Gibco; Thermo Fisher Scientific,
Inc. Penicillin-streptomycin solution, trypsin cell digestion
solution (containing 0.25% trypsin and phenol red), cDNA
first-strand synthesis kit (cat. no. D7178L), RIPA lysis solution
(cat. no. P0013B) and BCA protein concentration assay kit (cat. no.
P0012) were purchased from Beyotime Institute of Biotechnology.
Dimethyl sulfoxide, Triton X-100, paraformaldehyde and anhydrous
ethanol were purchased from Shanghai Titan Scientific Co., Ltd.
Trichloromethane and isopropanol were purchased from Sinopharm
Chemical Reagent Co., Ltd. Tetramethylrhodamine (TRITC)-phalloidin
(cat. no. MX4405-300T) was purchased from Shanghai Maokang
Biotechnology Co., Ltd. Power SYBR Green PCR master mix (cat. no.
4367659) was purchased from Thermo Fisher Scientific, Inc.
Anti-GAPDH (cat. no. 2118), anti-phospho-p38 MAPK (cat. no. 4511),
anti-phospho-SAPK/JNK (cat. no. 4668), anti-SAPK/JNK (cat. no.
9252), anti-phospho-p44/42 MAPK (cat. no. 4370), anti-p44/42 MAPK
(cat. no. 4695), anti-NFAT3/NFATc4 (23E6) rabbit mAb (cat. no.
2183) and antimouse IgG (cat. no. 7076) were purchased from Cell
Signaling Technology, Inc. Anti-p38α/β (sc-7149) was obtained from
Santa Cruz Biotechnology, Inc. Donkey anti-rabbit IgG H&L
(Alexa Fluor 488; cat. no. ab150073) was obtained from Abcam.
Antirabbit IgG (cat. no. MR-R100) was purchased from Shanghai
Mingrui Biotech Co., Ltd. PD98059 (cat. no. S1177), SP600125 (cat.
no. S1640) and SB203580 (cat. no. S1076) were purchased from
Selleck Chemicals. VIVIT (cat. no. HY-P1026) was obtained from
MedChemExpress. Cyclosporin A (cat. no. A600352-0001) was purchased
from Sangon Biotech Co., Ltd.
Cell culture
H9c2 cardiomyocytes (American Type Culture
Collection) were cultured in DMEM containing 10% FBS supplemented
with antibiotics (100 U/ml penicillin G and 100 µg/ml streptomycin
sulfate) at 37˚C in a humidified atmosphere of 5%
CO2.
MTT assay
H9c2 cells were seeded into a 96-well plate (5,000
cells/well) and cultured overnight in a 37˚C and 5% CO2
cell incubator. On the second day, sodium succinate or MeBavaC with
different final concentrations (diluted with serum- and
antibiotic-free H-DMEM medium) was added, whereas for the control
group, the same amount of H-DMEM medium was added. Then, six
parallel wells were set up for each group. After 48 h, 10 µl of MTT
solution [5 mg/ml in phosphate-buffered saline (PBS)] was added to
each well and incubated for 4 h at 37˚C and 5% CO2.
Next, 150 µl of DMSO solution was mixed with the cell-MTT
suspension and the formazan crystals were fully dissolved by
shaking on a constant temperature microtiter plate with a fast
shaker for 10 min. The OD value of each well was detected using a
Varioskan Flash microplate spectrophotometer (Thermo Fisher
Scientific, Inc.) at 570 nm.
Cell size analysis
The cell surface area was determined by staining the
cells with TRITC-phalloidin. Briefly, the H9C2 cells were seeded at
a density of 10,000 cells/plate in a 12-well plate. On the basis of
the principle that phalloidin can combine with actin (29), when the confluence of H9c2 cells
reached 50%, TRITC-phalloidin staining was performed to observe
cardiomyocyte hypertrophy induced by succinate. Phalloidin staining
of F-actin as a marker for measuring cell surface area has been
used in numerous cell experiments (30,31).
The cells were incubated with or without MeBavaC and then treated
with 1 mM sodium succinate for 0-48 h. They were then washed with
PBS and fixed with 4% formaldehyde solution for 10 min at room
temperature, followed by washing twice with PBS. The cells were
then treated with 0.5% Triton X-100 at room temperature for 5 min
to increase permeability then washed twice with PBS. Finally, the
cells were stained with 100 nM TRITC-Phallodin staining solution
for 30 min in the dark at room temperature with gently shaking. The
cells were washed twice with PBS, which was followed by staining
with DAPI for ~30 sec at room temperature. After washing,
fluorescence microscopy images were recorded separately at
excitation/emission wavelengths (Ex/Em=546/575 nm for TRITC and
Ex/Em=364/454 nm for DAPI). A total of five images were taken from
each plate of cells from different areas (each group n=3; counting
50 cells). Under an Olympus IX71 microscope (Olympus Corporation),
the ‘Count and Measure’ facility in the imaging software CellSens
1.14 (Olympus Corporation) was used, the cell regions of interest
were selected and defined, automatic image size measurement was
implemented and the area test parameters were derived for
statistical analysis.
Immunofluorescence
Following treatment, the H9c2 cells were fixed in 4%
paraformaldehyde for 10 min at room temperature and then
permeabilized with 1% Triton X-100 for 10 min at room temperature.
After blocking with 5% bovine serum albumin (MilliporeSigma), the
H9c2 cells were incubated with the anti-NFATc4 overnight at 4˚C in
a humidified chamber. This was followed by incubation with donkey
antirabbit IgG H&L (Alexa Fluor® 488) for 2 h at
37˚C. After washing, images were taken with an immunofluorescence
microscope (Olympus IX71; Olympus Corporation).
Reverse transcription-quantitative
(RT-q) PCR
According to the manufacturer's user manuals, total
RNA was extracted from ~5x106 cultured cardiomyocytes
per sample with NucleoSpin RNA Plus kit (Takara Bio, Inc.) and cDNA
was synthesized using the BeyoR III cDNA Synthesis kit (Beyotime
Institute of Biotechnology). Gene expression analysis was performed
on an ABI 7500 Fast real-time PCR system using the Power SYBR Green
PCR master mix according to the manufacturer's instructions (Thermo
Fisher Scientific, Inc.). The primer sequences (synthesized by
Sangon Biotech Co., Ltd.) were: rat GAPDH: forward primer
5'-GATCCCGCTAACATCAAATG-3', reverse primer
5'-GAGGGAGTTGTCATATTTCTC-3'; rat α-actinin: forward primer
5'-AGAAGAGATCGTGGATGGCAATGC-3', reverse forward primer
5'-GATGTCTTGGATGGCGAACCTGAG-3'; rat GATA4: forward primer
5'-CCTCCTTCTCTACCTGCCTGTCC-3'; and reverse primer
5'-TGTCTTGAAGCCTCGGTCCCTAC-3'. Gene expression was normalized to
the reference gene GAPDH. The reaction conditions were:
Denaturation (95˚C for 10 min), annealing (60˚C for 1 min) and
extension (95˚C for 15 sec) for 40 cycles. Three independent
experiments were conducted. PCR results were calculated using the
2-ΔΔCq method and statistically analyzed (32).
Western blotting analysis
The cultured cells were washed twice with cold PBS
containing PMSF (1 mM), NaF (2 mM) and Na3VO4
(2 mM) and then lysed with 100 µl of RIPA lysis buffer containing
PMSF, NaF and Na3VO4 at the same final
concentration. The cells at the bottom of the dish were collected
with a scraper into a 1.5 ml microcentrifuge tubes, lysed on ice
for 30 min and then centrifuged at 13,400 x g in a microcentrifuge
for 15 min at 4˚C. The supernatant was taken into new prechilled
1.5 ml microcentrifuge tubes and employed for measuring the protein
concentration at 562 nm by using a bicinchoninic acid protein assay
kit. The protein samples were adjusted to a uniform concentration
with SDS-PAGE protein loading buffer (5X) and PBS and then
denatured in a water bath at 95˚C for 10 min. Protein (30 µg) was
separated by performing 10% SDS-PAGE and transferred to
nitrocellulose membranes (Pall Filter Beijing Co. Ltd.). The
membranes were blocked at room temperature for 2 h with 5% non-fat
dried milk in a buffer containing 140 mmol/l NaCl, 20 mmol/l
Tris-HCl (pH 7.5) and 0.1% Tween-20 and incubated overnight at 4˚C
with the primary antibodies (dilution ratio: 1:1,000). Finally, the
membranes were incubated for 2 h with horseradish
peroxidase-conjugated mouse monoclonal antibody (1:2,000) at room
temperature with gentle shaking. The PVDF membrane was immersed in
ECL working solution (Sangon Biotech Co., Ltd.) and incubated in
the dark for 1 min. The membrane was placed in a chemiluminescence
developer (Tanon Science and Technology Co., Ltd.) for gradient
exposure and the images was captured with the Tanon-5200 Multi
chemiluminescent imaging system (Tanon Science and Technology Co.,
Ltd.), which uses the Sony ICX694 sensor CCD chip. Quantitative
analysis of band density was performed using Quantity One (version
25.0) software from Bio-Rad Laboratories, Inc.
Molecular docking simulations
Molecular docking simulations were performed to
analyze the inhibitory mechanism of compounds against SUCNR1. Due
to the absence of a crystal structure of human SUCNR1, the
structure of a rat SUCNR1 (PDB Code: 6IBB) was acquired from the
Protein Data Bank (https://www.rcsb.org) and was used as a receptor
(33,34). Graphical user interface AutoDock
(https://ccsb.scripps.edu/) Tools 1.5.6
software was used to format coordinate files of SUCNR1 and
compounds by adding polar hydrogens, deriving Kollman charges and
setting AutoDock 4 type of atoms. The SUCNR1 docking site was
placed at the orthosteric site described in previous studies
(34,35). The grid box was settled to encircle
the orthosteric site and AutoDock Vina (1.1.2) was used as the
docking and scoring program. Further analyses of the
SUCNR1-inhibitor interactions based on the docking and scoring
results were aided by BIOVIA Discovery Studio Visualizer (Dassault
Systemes).
Statistical analysis
GraphPad Prism 7 software (Dotmatics) was used for
data analysis and statistics and the data were expressed as means ±
standard deviations. Comparisons between groups were analyzed using
one-way analysis of variance followed by Dunnett's multiple
comparison test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Succinate induces cardiomyocyte
hypertrophy and activates nuclear translocation of NFATc4
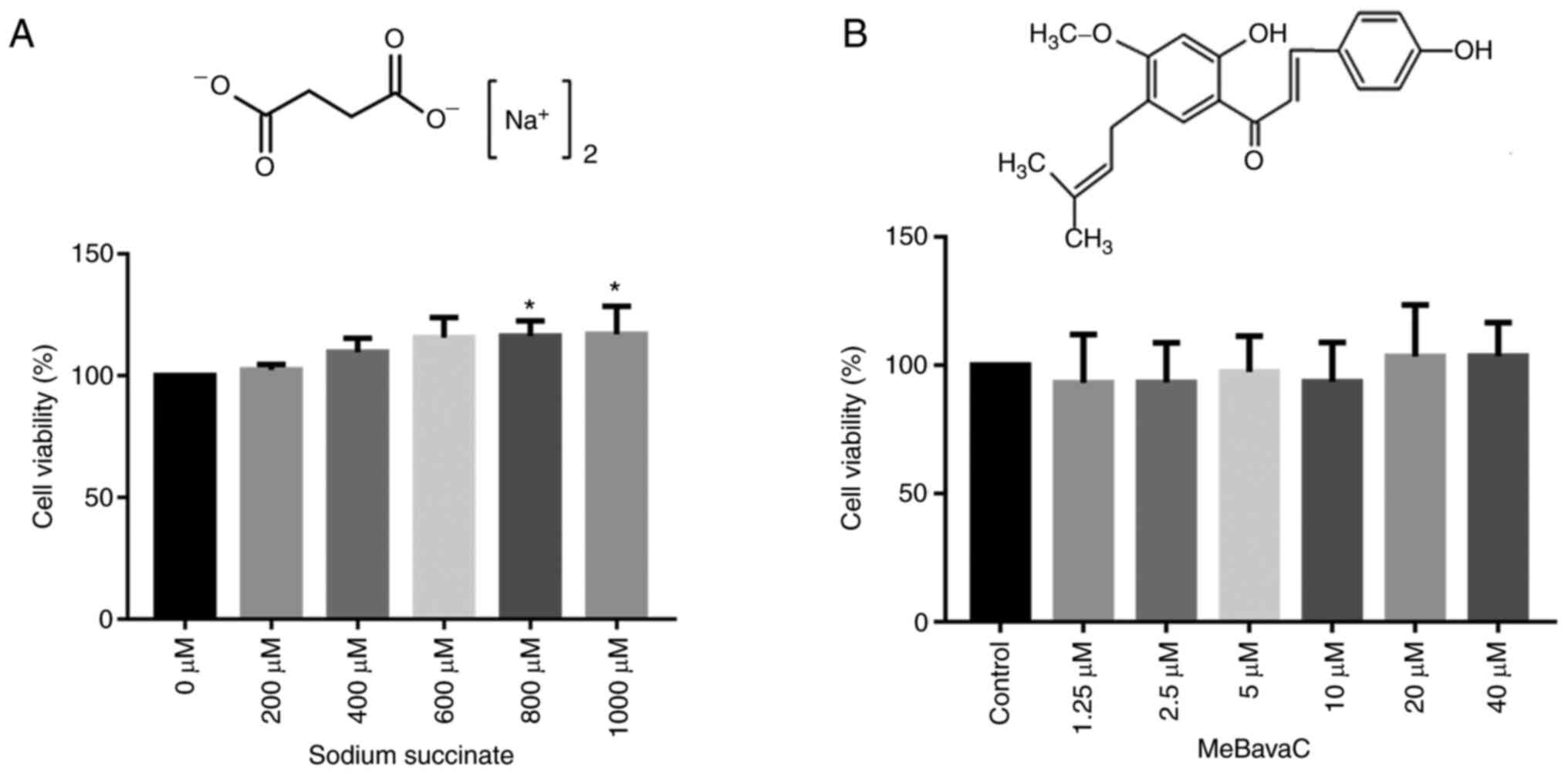
First, MTT assay was used to evaluate the effects of
sodium succinate and MeBavaC on the survival of H9C2
cardiomyocytes. Sodium succinate was dissolved in DMEM medium and
MeBavaC was dissolved in DMSO as a stock solution and diluted with
the medium to the final concentration. The final concentration of
DMSO was <0.1%, which ensured it was safe for the cells. As
presented in Fig. 1A, no
cytotoxicity was detected in the H9C2 cells, indicating that the
concentrations of sodium succinate within 1,000 µM and stimulation
for 48 h were safe (six wells per test, n=3 tests). In addition,
the effects of 1, 2, 5, 10, 25 and 50 mM sodium succinate on the
H9C2 cardiomyocytes was measured and no toxicity found (data not
shown). When the cardiomyocytes were treated with 800 and 1,000 µM
of sodium succinate, a slight increase in MTT was observed
(116.4±5.5 and 117.1±5.5, respectively). The effect of MeBavaC
within 40 µM on H9C2 cells was also tested and no cytotoxicity was
discovered (six wells per test, n=3 tests, Fig. 1B). Given that no cytotoxicity was
noted with 1 mM sodium succinate (Fig.
1A) and that this concentration has also been used in a number
of studies (24,36), 1 mM sodium succinate was used in
all subsequent experiments.
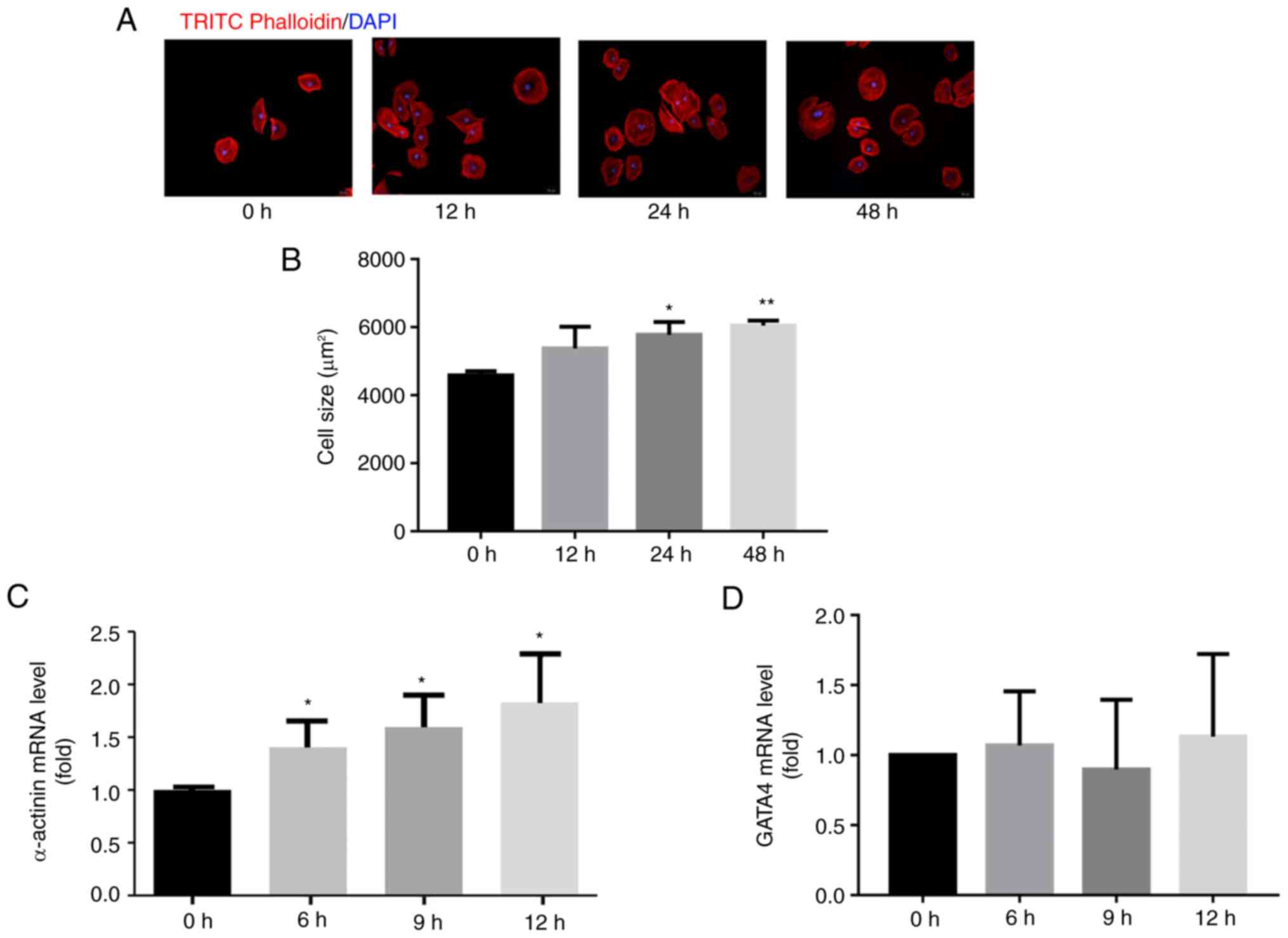
To explore the molecular mechanisms of
MeBavaC-mediated protection against cardiac hypertrophy, a model of
cardiomyocyte hypertrophy was first established by exposing H9C2
cells to sodium succinate (1 mM) for 12, 24 and 48 h, respectively.
TRITC-phalloidin staining revealed a time-dependent increase in
cardiomyocyte size in the group that underwent sodium succinate
treatment compared with the nontreatment group. In addition, sodium
succinate treatment increased the relative cell cross-sectional
area from 4,601±124 µm2 at 0 h to 6,049±120
µm2 at 48 h (n=3, counting 50 cells, Fig. 2A and B). Stimulation with sodium succinate for
6 h also promoted a nearly three-fold increase in
cardiomyopathy-associated α-actinin mRNA expression without
altering GATA4 expression (n=3, Fig.
2C and D). In addition, H9c2
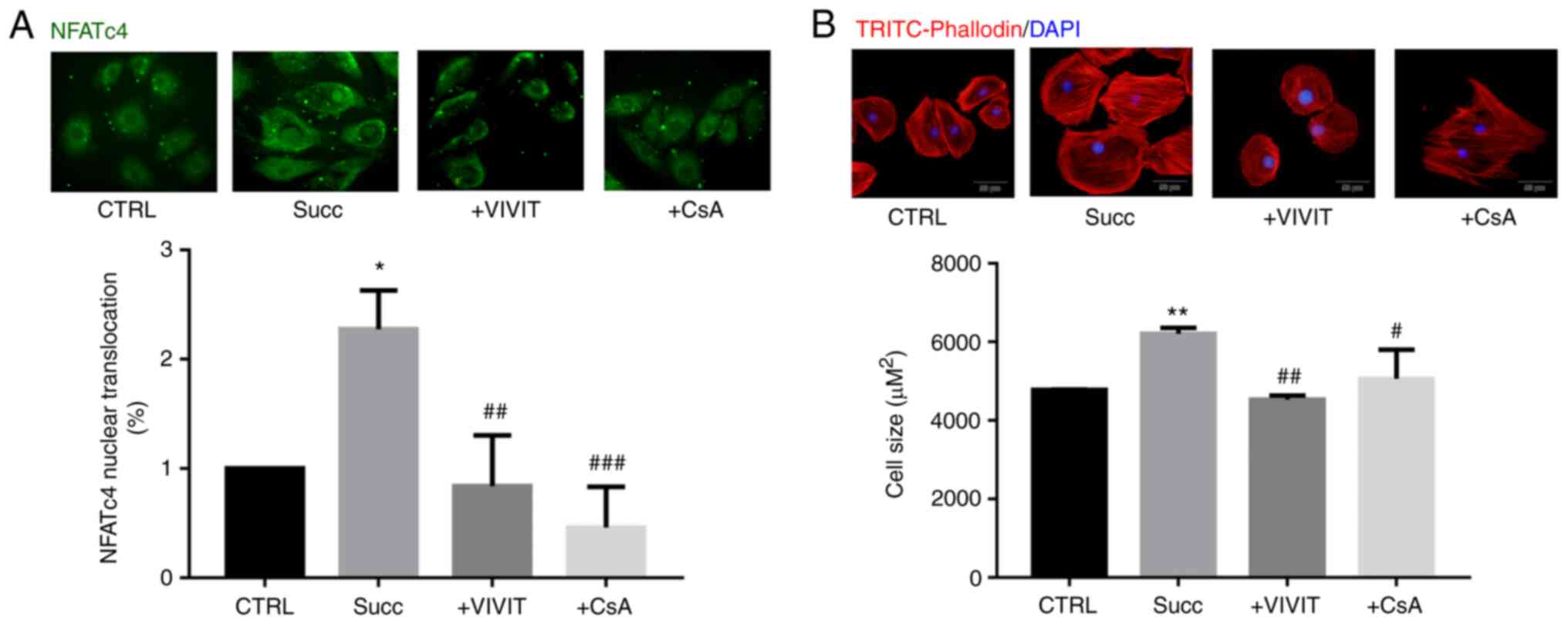
cardiomyocytes were treated with sodium succinate for 60 min and
immunofluorescence staining indicated a significant greater
two-fold increase in NFATc4 content related to its nuclear
translocation (n=3; counting 50 cells; Fig. 3A). However, treatment with the NFAT
inhibitor VIVIT (2 µM) or the calcineurin inhibitor cyclosporine A
(CsA, 5 nM) for 30 min prior to sodium succinate significantly
blocked the nuclear translocation of NFATc4 induced by sodium
succinate (n=3; counting 50 cells; Fig
3B), indicating that succinate activates the calcineurin-NFATc4
pathway in cardiomyocytes. Additionally, the induction of
cardiomyocyte hypertrophy for 48 h by sodium succinate was blocked
by VIVIT or CsA, with the relative cell cross-sectional area
decreasing from 6,211±122 µm2 in the
succinate-stimulated model group to 4,529±90 µm2 in the
VIVIT-treated group or to 5,064±605 µm2 in the
CsA-treated group (n=3; counting 50 cells; Fig. 3B). The results demonstrated that
succinate induced cardiomyocyte hypertrophy and activated NFATc4
nuclear translocation.
4'-O-methylbavachalcone prevents
succinate-induced cardiomyocyte hypertrophy via NFATc4
signaling
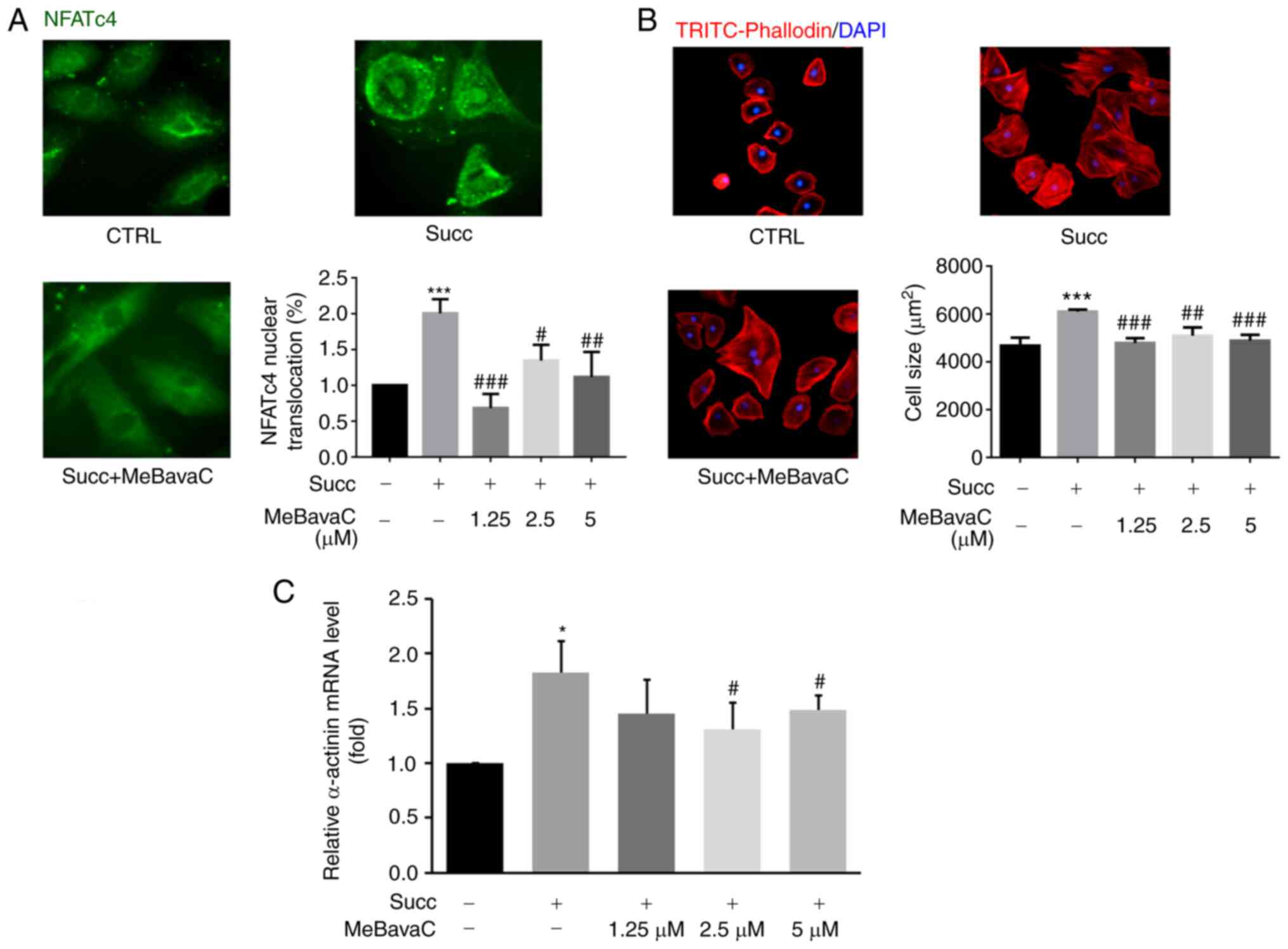
To determine whether MeBavaC prevent cardiomyocyte
hypertrophy, the present study investigated the effect of MeBavaC
on succinate-induced cardiomyocyte hypertrophy. H9c2 cardiomyocytes
were pretreated with MeBavaC at the concentrations of 1.25, 2.5 and
5 µM for 30 min. MeBavaC was found to inhibit NFATc4 nuclear
translocation in a concentration-dependent manner. This inhibition
was induced by sodium succinate for 60 min. NFATc4 nuclear
translocation nearly returned to the prestimulation levels after 5
µM MeBavaC treatment (n=3; counting 50 cells; Fig. 4A). The inhibitory effect of MeBavaC
on cardiomyocyte hypertrophy was determined by TRITC-phalloidin
staining. Pretreatment with MeBavaC for 30 min effectively
prevented cardiomyocyte hypertrophy induced by sodium succinate for
48 h, with an inhibition of up to 20% compared with the model group
(n=3; counting 50 cells; Fig. 4B).
RT-qPCR revealed that MeBavaC restored the gene expression of
α-actinin induced by sodium succinate for 6 h, which is a
hypertrophic marker (n=3; Fig.
4C).
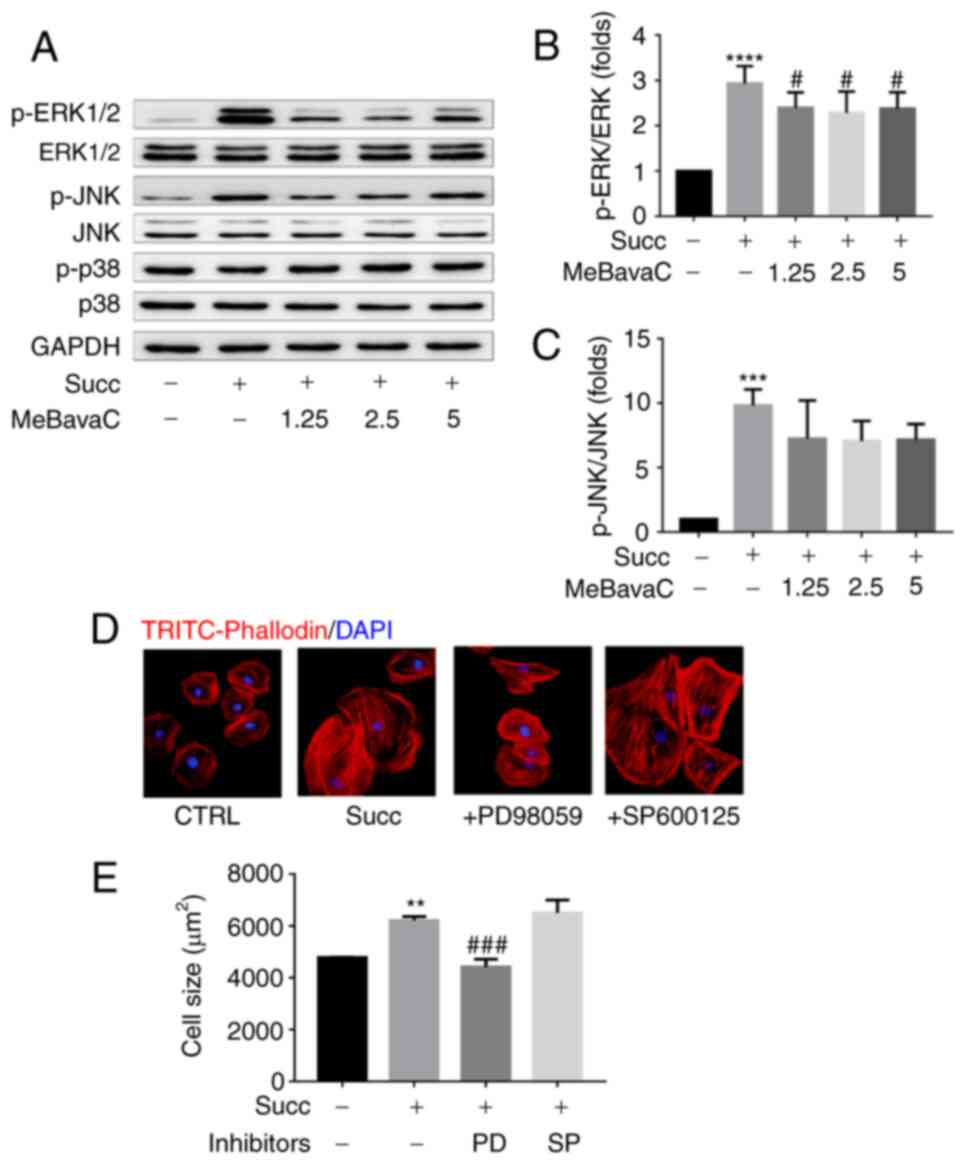
Role of MAPK on
4'-O-methylbavachalcone in improving cardiomyocyte hypertrophy
The phosphorylation activities of ERK1/2 and JNK
kinases, two essential members of MAPK signaling, were activated by
sodium succinate for 15 min as detected using western blotting, but
not by p38 kinase (n=3; Fig.
5A-C). Pretreatment with MeBavaC for 30 min significantly
inhibited ERK1/2 and JNK phosphorylation with a maximum inhibition
of ~15 and 40%, respectively (n=3; Fig. 5A-C). However, sodium
succinate-induced cardiomyocyte hypertrophy was blocked by 30 min
of pretreatment of the ERK1/2 pathway inhibitor PD98059 (20 µM),
but not the JNK pathway inhibitor SP600125 (20 µM), as indicated by
a decreased cardiomyocytes size (~30%; n=3; counting 50 cells;
Fig. 5D and E). These results indicated that ERK1/2
signaling partly serves a role in mediating succinate-induced
cardiomyocyte hypertrophy.
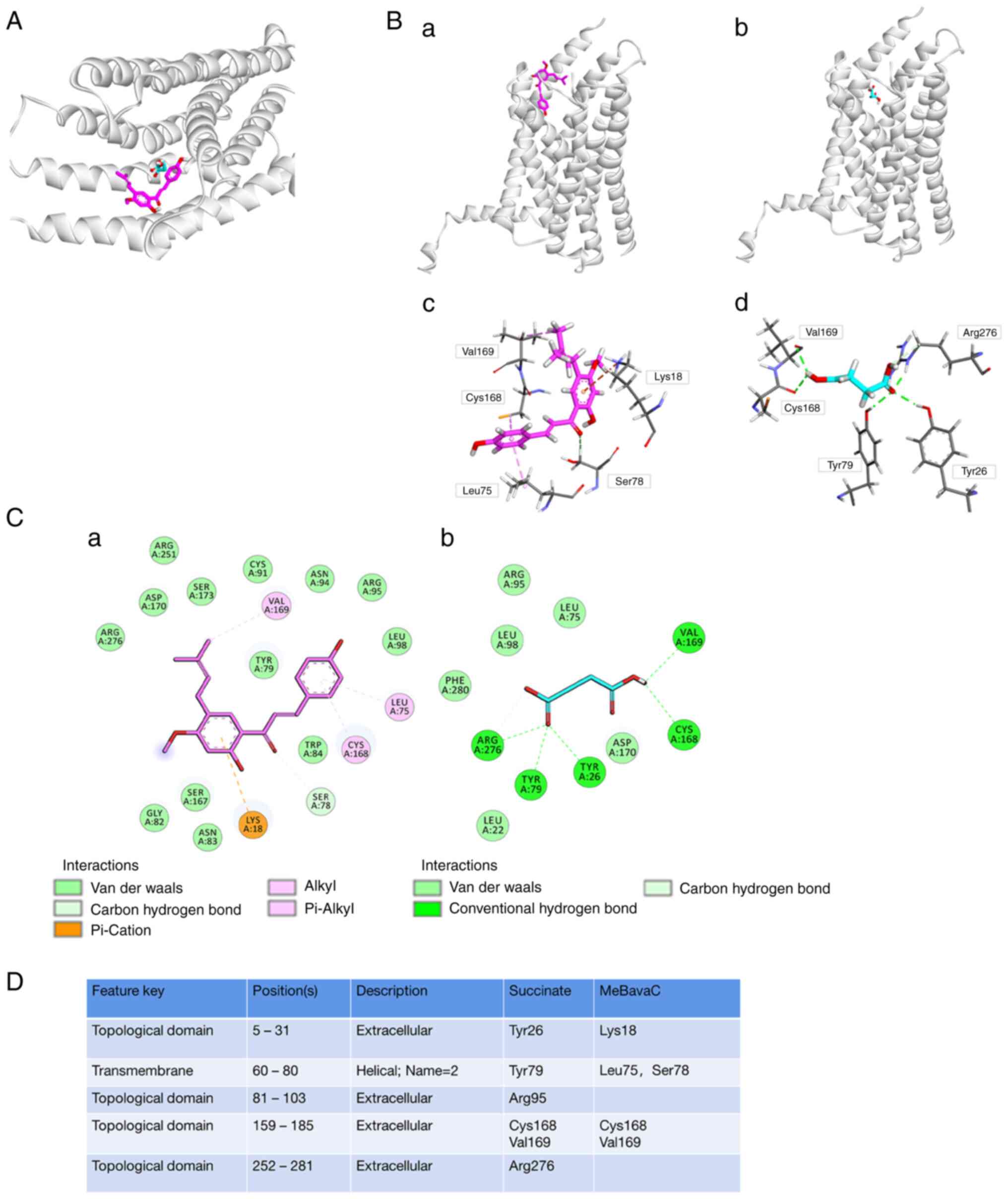
Molecular docking analysis of
4'-O-methylbavachalcone and the SUCNR1 receptor
To elucidate the binding mechanism of MeBavaC on
SUCNR1 in detail, a docking simulation analysis was performed. As
presented in Fig. 6A, MeBavaC and
succinate bound SUCNR1 at extremely close position. Binding to both
the Cys168 and Val169 groups of the SUCNR1 receptor, succinate was
bound via hydrogen bonds, whereas MeBavaC was bound via the alkyl
and Pi-alkyl groups (Fig. 6B and
C). Markedly, MeBavaC was
predicted to be more compatible with the orthosteric site of SUCNR1
than was succinate, with the two having a docking score of -8.1 and
-4.7 kcal/mol, respectively. The carbonyl of succinate is
integrally docked to a pseudooxyanion whole (Fig. 6C and D), which is generally a proteinaceous
substruction preferring multiform oxygen atoms and consists of
Tyr26, Tyr29 and Arg276. Nevertheless, MeBavaC formed stable
Pi-cation with Lys18 as well as Pi-Alkyl with Leu75. Taken
together, these results indicate that MeBavaC inhibited SUCNR1
activity.
Discussion
Succinate is an intermediate product of
mitochondrial metabolism and mitochondrial damage results in
succinate leakage. A number of cardiovascular events, such as
myocardial infarction, atrial fibrillation and heart failure,
promote succinate leakage from cardiomyocytes (20,37-39).
Succinate is the only metabolite whose levels are significantly
increased in the coronary sinus compared with in the venous blood
in patients with STEMI, suggesting that it is released in the
heart. Succinate concentrations in arterial, coronary sinus and
peripheral venous blood are higher in patients with STEMI than in
patients without STEMI or with stable angina pectoris (20). The mean succinate concentration in
the coronary sinus blood of patients with STEMI is reported to be
2.73 µM, which is ~ twice that of patients with angina pectoris and
without STEMI (20). In addition,
enhanced levels of succinate are closely associated with an
increased risk of atrial fibrillation due to the extreme quartiles
of TAC metabolites in patients with atrial fibrillation (37). Elevated myocardial energy
expenditure has been associated with reduced left ventricular
ejection fraction and an independent predictor of cardiovascular
mortality. In patients with heart failure, serum succinate
concentrations also significantly increased with increasing
myocardial energy expenditure elevation (38). Succinate accumulates during
ischemia and two-thirds of ischemic succinate accumulation is
extracellular (40). In addition,
succinate produced by gut microbiota is also directly related to
the development of cardiovascular disease (41,42).
Compared with healthy animals, the blood succinate concentration of
spontaneously hypertensive rats, ob/ob mice, fa/fa rats and db/db
mice was significantly increased. Therefore, ultra-high succinate
concentrations in the blood may be involved in the occurrence of
numerous cardiovascular events. In the present study, 1 mM of
sodium succinate incubated with H9C2 cardiomyocytes for 48 h
promoted cardiomyocyte hypertrophy and increased the expression of
cardiomyopathy-associated gene α-actinin (Fig. 2), which is partly consistent with
the finding of Aguiar et al (24). Their findings indicate that
intravenous injection of 0.066 mg/kg of sodium succinate salt into
8-week-old Wistar rats with cardiac ischemia once daily for 5 days
can lead to cardiac hypertrophy. They further stimulated
cardiomyocytes from neonatal (3-5 days old) Wistar rats with 1 mM
of sodium succinate, which triggered hypertrophy of cardiac
cardiomyocytes by activating the SUCNR1 receptor and through
phosphorylation of ERK1/2 and expression of CaMKIIδ (24). In vivo experiments in rats
have also indicated that succinate enhanced pressure
overload-induced right ventricular hypertrophy. Yang et al
(43) selected three succinate
concentrations of 30, 50 and 100 mg/kg/day in the pre-experiment.
As rats given a dose of 100 mg/kg/day succumbed before the end of
the experiment, 50 mg/kg/day was chosen as the follow-up
experimental dose. Stimulation with 50 mg/kg/day succinate resulted
in right ventricular hypertrophy in rats and enhanced ANP gene
expression and Akt signaling (43). High sodium succinate concentrations
(1 mM) also promote platelet aggregation and cross-cellular
biosynthesis of leukotriene C4 by activating
SUCNR1(36).
Succinate is primarily sensed by the
G-protein-coupled receptor SUCNR1, which serves a role in
extracellular metabolic stress signaling. SUCNR1 is widely
expressed in various tissues, including the kidney, liver, heart
and retinal, resulting in a wide range of physiological and
pathological effects. The increase in blood succinate concentration
in hypertension, obesity and diabetes model animals is consistent
with SUCNR1 activation (19).
SUCNR1 serves specific proinflammatory and antiinflammation roles
in macrophages. When SUCNR1-expressing macrophages are activated by
inflammatory signals, the accumulated succinate is released into
the extracellular milieu. SUCNR1 activation binding to autocrine or
paracrine succinate can perpetuate inflammation by enhancing IL-1β
production in macrophages (44).
Several cardiac hypertrophy-related signals, including
phosphorylation of ERK1/2, expression of CaMKIIδ and translocation
of histone deacetylase 5 into cytoplasm, are triggered by SUCNR1
activation and serve critical roles in the hypertrophic remodeling
of the myocardium (24),
indicating that cardiac hypertrophy is induced in a
SUCNR1-dependent manner. SUCNR1 stimulation activates Gαi and
initiates Gαq activity (45,46),
which is required to trigger intracellular Ca2+ flux.
SUCNR1 requires PLCβ activation to increase intracellular
Ca2+ through an inositol phosphate-dependent mechanism
(47). The canonical
Gi-Gβγ-Ca2+ signaling also requires the participation of
the Gαq subunit in living cells (48). Ca2+ flux is the upstream
signal of the CaMKIIδ and calcineurin-NFAT pathways and abnormal
Ca2+ flux is one of the molecular bases of cardiac
hypertrophy (12,49,50).
The results of the present study also revealed that succinate
treatment promoted cardiomyocyte size enlargement and increased
α-actinin expression (Figs. 2 and
4). The gene α-actinin is
associated with dilated cardiomyopathy, impaired ventricular
diastolic function and cardiac hypertrophy (51,52)
and α-actinin-1 promotes the activity of the L-type Ca2+
channel Cav 1.2 (53,54), which regulates cardiac hypertrophy
(55,56). Together, these findings indicate
the presence of at least two pathways by which stimulation of
SUCNR1 increase intracellular Ca2+ flux to contribute to
cardiac hypertrophy; by triggering Gαq activity and PLCβ and
activating the L-type Ca2+ channel Cav 1.2 opening.
Calcineurin-NFAT signaling is an important
regulatory pathway of cardiac hypertrophy. However, whether
succinate can activate calcineurin-NFAT signaling has not been
determined. To this end, the nuclear translocation of NFATc4 was
observed in cardiomyocytes after they were treated with succinate.
The results suggested that succinate-induced cardiomyocyte
hypertrophy is mediated by the calcineurin-NFATc4 signaling
(Fig. 3). Indeed, four NFAT
members (NFATc1-c4) are expressed in cardiomyocytes; they trigger
nuclear translocation upon calcineurin activation and yet promote
cardiomyocyte hypertrophy (14,57).
The data from the present study also indicated that succinate
activated ERK1/2 and JNK signaling and that inhibition of ERK1/2
signaling, but not of JNK signaling, attenuated cardiomyocyte
hypertrophy (Fig. 5). ERK1/2
stimulation induces cardiac hypertrophy, whereas JNK activation in
cardiomyocytes directly antagonizes activated NFAT signaling
(15,58). In cultured cardiomyocytes, ERK1/2
signaling inhibition attenuates hypertrophy induced by activated
calcineurin. Nevertheless, the targeted inhibition of ERK1/2
signaling does not directly affect calcineurin-NFAT activation
(58).
Fructus Psoraleae mainly contains coumarins, terpene
phenols and prenylflavonoids, which are the material basis of drug
therapy. MeBavaC is an active component of prenylflavonoids. The
authors previously reviewed the mechanisms of five major psoralea
prenylflavonoids and their multiple effects including
anti-inflammation, cardiovascular protection, neuroprotection,
osteoporosis improvement and intervention on diabetes and obesity
(26). No cardiovascular
pharmacological study of MeBavaC has been conducted and the present
study revealed its mechanism in preventing cardiac hypertrophy. The
results demonstrated that MeBavaC blocks both calcineurin-NFAT and
ERK1/2 signaling and inhibits succinate-induced cardiomyocyte
hypertrophy (Figs. 4 and 5). A number of prenylflavonoids, such as
xanthohumol and icariin, have multiple cardiovascular protection
functions. Xanthohumol inhibits abnormal ryanodine receptor
Ca2+ release, possesses antiarrhythmic properties,
attenuates isoproterenol-induced cardiac hypertrophy and fibrosis
and protects rat myocardia from ischemia/reperfusion injury-induced
ferroptosis (59-61).
Icariin inhibits isoproterenol-induced cardiomyocyte hypertrophic
injury and severe heart failure, protects cardiomyocytes from
ischemia/reperfusion injury and attenuates Ang II-induced H9c2
cardiomyocyte hypertrophy and apoptosis (62-65).
In Fructus Psoraleae, only bakuchiol, a terpene phenol, was
reported to block aortic banding-induced cardiac hypertrophy by
blocking the NF-κB signaling pathway and to attenuate pressure
overload-induced fibrosis and inflammation (66).
In 2011 year, compounds numbered 2C, 4C and 5G were
identified as potent and selective antagonists for human
SUCNR1(67). Another group
recently discovered an optimized SUCNR1 antagonist scaffold to
improve oral exposure by designing Zwitterionic Derivatives with
salt bridges (68). Using a
humanized rat construct, the X-ray structure of optimized compound
20 binding to SUCNR1 was determined (67). On this published humanized rat
SUCNR1 construct, the team also confirmed the structure of another
antagonist, NF-56-EJ40, in complex with its receptor,
SUCNR1(34). In the present study,
molecular docking analysis indicated that MeBavaC binds to the
orthosteric site of succinate receptor SUCNR1 through alkyl and
Pi-alkyl, which are more compatible than succinate with a higher
docking score (Fig. 6). When
binding to the pseudo-oxyanionic protein subunit group of SUCNR1,
MeBavaC formed stable Pi-cation complexes with Lys18, as well as
Pi-alkyl bonds with Leu75 (Fig.
6). The results indicated that MeBavaC inhibited SUCNR1
activity.
The results of the present study revealed that
succinate stimulated cardiomyocyte hypertrophy through the
calcineurin-NFATc4 and ERK1/2 pathways. MeBavaC ameliorated
succinate-induced cardiomyocyte hypertrophy mediated by
calcineurin-NFATc4 and ERK1/2 pathways. Molecular docking analysis
indicated that MeBavaC had a certain affinity for the succinate
receptor SUCNR1 and the ability to inhibit SUCNR1 binding with
succinate. Therefore, MeBavaC has fully demonstrated the potential
ability to improve myocardial hypertrophy and heart failure,
associated with elevated myocardial energy expenditure and an
increased extra serum succinate concentration.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by grants from the
Specialized Research Fund for the National Natural Science
Foundation of China (grant no. 81973511).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HS implemented most of the experiments, data
collation and statistics and wrote the first draft of the
manuscript. GZ implemented the molecular docking research, data
collation and statistical analyses and wrote the first draft of the
molecular docking section of the manuscript. SL participated in
part of the experimental design, gave technical guidance on
cardiomyocyte culture, conducted financial management and assisted
in the revision of the manuscript. JL provided experimental design
for the Western blot, validated some of the real-time PCR
experiments, provided technical guidance on microscopy and
monitored the progress of the experiments, and assisted in the
revision of the manuscript. JWX proposed the research idea,
obtained financial support and revised and finalized the
manuscript. HS, GZ and JL confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests
References
|
1
|
Gould KL, Lipscomb K, Hamilton GW and
Kennedy JW: Left ventricular hypertrophy in coronary artery
disease. A cardiomyopathy syndrome following myocardial infarction.
Am J Med. 55:595–601. 1973.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Momiyama Y, Suzuki Y, Ohsuzu F, Atsumi Y,
Matsuoka K and Kimura M: Left ventricular hypertrophy and diastolic
dysfunction in mitochondrial diabetes. Diabetes Care. 24:604–605.
2001.PubMed/NCBI View Article : Google Scholar
|
|
3
|
De Simone G, Devereux RB, Roman MJ,
Alderman MH and Laragh JH: Relation of obesity and gender to left
ventricular hypertrophy in normotensive and hypertensive adults.
Hypertension. 23:600–606. 1994.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Li H, He C, Feng J, Zhang Y, Tang Q, Bian
Z, Bai X, Zhou H, Jiang H, Heximer SP, et al: Regulator of G
protein signaling 5 protects against cardiac hypertrophy and
fibrosis during biomechanical stress of pressure overload. Proc
Natl Acad Sci USA. 107:13818–13823. 1994.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Cibi DM, Bi-Lin KW, Shekeran SG,
Sandireddy R, Tee N, Singh A, Wu Y, Srinivasan DK, Kovalik JP,
Ghosh S, et al: Prdm16 deficiency leads to age-dependent cardiac
hypertrophy, adverse remodeling, mitochondrial dysfunction, and
heart failure. Cell Rep. 33(108288)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ritterhoff J, Young S, Villet O, Shao D,
Neto FC, Bettcher LF, Hsu YA, Kolwicz SC Jr, Raftery D and Tian R:
Metabolic remodeling promotes cardiac hypertrophy by directing
glucose to aspartate biosynthesis. Circ Res. 126:182–196.
2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhang T and Brown JH: Role of
Ca2+/calmodulin-dependent protein kinase II in cardiac
hypertrophy and heart failure. Cardiovasc Res. 63:476–486.
2004.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yang L, Cao J, Ma J, Li M and Mu Y:
Differences in the microcirculation disturbance in the right and
left ventricles of neonatal rats with hypoxic pulmonary
hypertension. Microvasc Res. 135(104129)2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Liu HB, Yang BF and Dong DL: Calcineurin
and electrical remodeling in pathologic cardiac hypertrophy. Trends
Cardiovasc Med. 20:148–153. 2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Molkentin JD: Calcineurin and beyond:
Cardiac hypertrophic signaling. Circ Res. 87:731–738.
2000.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Crabtree GR: Generic signals and specific
outcomes: Signaling through Ca2+, calcineurin, and
NF-AT. Cell. 96:611–614. 1999.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wilkins BJ, Dai YS, Bueno OF, Parsons SA,
Xu J, Plank DM, Jones F, Kimball TR and Molkentin JD:
Calcineurin/NFAT coupling participates in pathological, but not
physiological, cardiac hypertrophy. Circ Res. 94:110–118.
2004.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Mathew S, Mascareno E and Siddiqui MA: A
ternary complex of transcription factors, Nished and NFATc4, and
co-activator p300 bound to an intronic sequence, intronic
regulatory element, is pivotal for the up-regulation of myosin
light chain-2v gene in cardiac hypertrophy. J Biol Chem.
279:41018–41027. 2004.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Van Rooij E, Doevendans PA, de Theije CC,
Babiker FA, Molkentin JD and de Windt LJ: Requirement of nuclear
factor of activated T-cells in calcineurin-mediated cardiomyocyte
hypertrophy. J Biol Chem. 277:48617–48626. 2002.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Molkentin JD: Calcineurin-NFAT signaling
regulates the cardiac hypertrophic response in coordination with
the MAPKs. Cardiovasc Res. 63:467–475. 2004.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Pin F, Barreto R, Couch ME, Bonetto A and
O'Connell TM: Cachexia induced by cancer and chemotherapy yield
distinct perturbations to energy metabolism. J Cachexia Sarcopenia
Muscle. 10:140–154. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lehwald N, Tao GZ, Jang KY, Papandreou I,
Liu B, Liu B, Pysz MA, Willmann JK, Knoefel WT, Denko NC and
Sylvester KG: β-Catenin regulates hepatic mitochondrial function
and energy balance in mice. Gastroenterology. 143:754–764.
2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Björntorp P: The oxidation of fatty acids
combined with albumin by isolated rat liver mitochondria. J Biol
Chem. 241:1537–1543. 1966.PubMed/NCBI
|
|
19
|
Sadagopan N, Li W, Roberds SL, Major T,
Preston GM, Yu Y and Tones MA: Circulating succinate is elevated in
rodent models of hypertension and metabolic disease. Am J
Hypertens. 20:209–1215. 2007.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kohlhauer M, Dawkins S, Costa ASH, Lee R,
Young T, Pell VR, Choudhury RP, Banning AP and Kharbanda RK: Oxford
Acute Myocardial Infarction (OxAMI) Study et al. Metabolomic
profiling in acute ST-segment-elevation myocardial infarction
identifies succinate as an early marker of human
ischemia-reperfusion injury. J Am Heart Assoc.
7(e007546)2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
de Castro Fonseca M, Aguiar CJ, da Rocha
Franco JA, Gingold RN and Leite MF: GPR91: Expanding the frontiers
of Krebs cycle intermediates. Cell Commun Signal.
14(3)2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Li X, Xie L, Qu X, Zhao B, Fu W, Wu B and
Wu J: GPR91, a critical signaling mechanism in modulating
pathophysiologic processes in chronic illnesses. FASEB J.
34:13091–13105. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yang L, Yu D, Mo R, Zhang J, Hua H, Hu L,
Feng Y, Wang S, Zhang WY, Yin N and Mo XM: The succinate receptor
GPR91 is involved in pressure overload-induced ventricular
hypertrophy. PLoS One. 11(e0147597)2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Aguiar CJ, Rocha-Franco JA, Sousa PA,
Santos AK, Ladeira M, Rocha-Resende C, Ladeira LO, Resende RR,
Botoni FA, Melo MB, et al: Succinate causes pathological
cardiomyocyte hypertrophy through GPR91 activation. Cell Commun
Signal. 12(78)2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Chinese Pharmacopoeia Commission, 2020.
Psoraleae. Fructus. In: Pharmacopoeia of the People's Republic of
China. China Medical Science Press, Beijing. pp 195.
|
|
26
|
Zhou YT, Zhu L, Yuan Y, Ling S and Xu JW:
Effects and mechanisms of five psoralea prenylflavonoids on
aging-related diseases. Oxid Med Cell Longev.
2020(2128513)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Yan C, Wu Y, Weng Z, Gao Q, Yang G, Chen
Z, Cai B and Li W: Development of an HPLC method for absolute
quantification and QAMS of flavonoids components in Psoralea
corylifolia L. J Anal Methods Chem. 2015(792637)2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kim DW, Seo KH, Curtis-Long MJ, Oh KY, Oh
JW, Cho JK, Lee KH and Park KH: Phenolic phytochemical displaying
SARS-CoV papain-like protease inhibition from the seeds of Psoralea
corylifolia. J Enzyme Inhib Med Chem. 29:59–63. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Cooper JA: Effects of cytochalasin and
phalloidin on actin. J Cell Biol. 105:1473–1478. 1987.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Jia G, Liang C, Li W and Dai H: MiR-410-3p
facilitates angiotensin II-induced cardiac hypertrophy by targeting
Smad7. Bioengineered. 13:119–127. 2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Sugawara Y, Kamioka H, Honjo T, Tezuka K
and Takano-Yamamoto T: Three-dimensional reconstruction of chick
calvarial osteocytes and their cell processes using confocal
microscopy. Bone. 36:877–883. 2005.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Burley SK, Berman HM, Kleywegt GJ, Markley
JL, Nakamura H and Velankar S: Protein data bank (PDB): The single
global macromolecular structure archive. Methods Mol Biol.
1607:627–641. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Haffke M, Fehlmann D, Rummel G, Boivineau
J, Duckely M, Gommermann N, Cotesta S, Sirockin F, Freuler F,
Littlewood-Evans A, et al: Structural basis of species-selective
antagonist binding to the succinate receptor. Nature. 574:581–585.
2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Trauelsen M, Ulven ER, Hjorth SA, Brvar M,
Monaco C, Frimurer TM and Schwartz TW: Receptor structure-based
discovery of non-metabolite agonists for the succinate receptor
GPR91. Mol Metab. 6:1585–1596. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Tang X, Fuchs D, Tan S, Trauelsen M,
Schwartz TW, Wheelock CE, Li N and Haeggström JZ: Activation of
metabolite receptor GPR91 promotes platelet aggregation and
transcellular biosynthesis of leukotriene C4. J Thromb Haemost.
18:976–984. 2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Bulló M, Papandreou C, García-Gavilán J,
Ruiz-Canela M, Li J, Guasch-Ferré M, Toledo E, Clish C, Corella D,
Estruch R, et al: Tricarboxylic acid cycle related-metabolites and
risk of atrial fibrillation and heart failure. Metabolism.
125(154915)2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Du Z, Shen A, Huang Y, Su L, Lai W, Wang
P, Xie Z, Xie Z, Zeng Q, Ren H and Xu D: 1H-NMR-based metabolic
analysis of human serum reveals novel markers of myocardial energy
expenditure in heart failure patients. PLoS One.
9(e88102)2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Yao H, Shi P, Zhang L, Fan X, Shao Q and
Cheng Y: Untargeted metabolic profiling reveals potential
biomarkers in myocardial infarction and its application. Mol
Biosyst. 6:1061–1070. 2010.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Zhang J, Wang YT, Miller JH, Day MM,
Munger JC and Brookes PS: Accumulation of succinate in cardiac
ischemia primarily occurs via canonical Krebs cycle activity. Cell
Rep. 23:2617–2628. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Galla S, Chakraborty S, Cheng X, Yeo JY,
Mell B, Chiu N, Wenceslau CF, Vijay-Kumar M and Joe B: Exposure to
amoxicillin in early life is associated with changes in gut
microbiota and reduction in blood pressure: Findings from a study
on rat dams and offspring. J Am Heart Assoc.
9(e014373)2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Serena C, Ceperuelo-Mallafré V, Keiran N,
Queipo-Ortuño MI, Bernal R, Gomez-Huelgas R, Urpi-Sarda M, Sabater
M, Pérez-Brocal V, Andrés-Lacueva C, et al: Elevated circulating
levels of succinate in human obesity are linked to specific gut
microbiota. ISME J. 12:1642–1657. 2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Yang L, Yu D, Fan HH, Feng Y, Hu L, Zhang
WY, Zhou K and Mo XM: Triggering the succinate receptor GPR91
enhances pressure overload-induced right ventricular hypertrophy.
Int J Clin Exp Pathol. 7:5415–5428. 2014.PubMed/NCBI
|
|
44
|
Littlewood-Evans A, Sarret S, Apfel V,
Loesle P, Dawson J, Zhang J, Muller A, Tigani B, Kneuer R, Patel S,
et al: GPR91 senses extracellular succinate released from
inflammatory macrophages and exacerbates rheumatoid arthritis. J
Exp Med. 213:1655–1662. 2016.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Ko SH, Choi GE, Oh JY, Lee HJ, Kim JS,
Chae CW, Choi D and Han HJ: Succinate promotes stem cell migration
through the GPR91-dependent regulation of DRP1-mediated
mitochondrial fission. Sci Rep. 7(12582)2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Trauelsen M, Hiron TK, Lin D, Petersen JE,
Breton B, Husted AS, Hjorth SA, Inoue A, Frimurer TM, Bouvier M, et
al: Extracellular succinate hyperpolarizes M2 macrophages through
SUCNR1/GPR91-mediated Gq signaling. Cell Rep.
35(109246)2021.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Sundström L, Greasley PJ, Engberg S,
Wallander M and Ryberg E: Succinate receptor GPR91, a Gα(i) coupled
receptor that increases intracellular calcium concentrations
through PLCβ. FEBS Lett. 587:2399–2404. 2013.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Pfeil EM, Brands J, Merten N, Vögtle T,
Vescovo M, Rick U, Albrecht IM, Heycke N, Kawakami K, Ono Y, et al:
Heterotrimeric G protein subunit Gαq is a master switch for
Gβγ-mediated calcium mobilization by Gi-coupled GPCRs. Mol Cell.
80:940–954.e6. 2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Backs J, Backs T, Neef S, Kreusser MM,
Lehmann LH, Patrick DM, Grueter CE, Qi X, Richardson JA, Hill JA,
et al: The delta isoform of CaM kinase II is required for
pathological cardiac hypertrophy and remodeling after pressure
overload. Proc Natl Acad Sci USA. 106:2342–2347. 2009.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Pieske B, Kretschmann B, Meyer M,
Holubarsch C, Weirich J, Posival H, Minami K, Just H and Hasenfuss
G: Alterations in intracellular calcium handling associated with
the inverse force-frequency relation in human dilated
cardiomyopathy. Circulation. 92:1169–1178. 1995.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Prondzynski M, Lemoine MD, Zech AT,
Horváth A, Di Mauro V, Koivumäki JT, Kresin N, Busch J, Krause T,
Krämer E, et al: Disease modeling of a mutation in α-actinin 2
guides clinical therapy in hypertrophic cardiomyopathy. EMBO Mol
Med. 11(e11115)2019.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Sheng JJ, Feng HZ, Pinto JR, Wei H and Jin
JP: Increases of desmin and α-actinin in mouse cardiac myofibrils
as a response to diastolic dysfunction. J Mol Cell Cardiol.
99:218–229. 2016.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Hall DD, Dai S, Tseng PY, Malik Z, Nguyen
M, Matt L, Schnizler K, Shephard A, Mohapatra DP, Tsuruta F, et al:
Competition between α-actinin and Ca²+-calmodulin
controls surface retention of the L-type Ca²+ channel
Ca(V)1.2. Neuron. 78:483–497. 2013.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Turner M, Anderson DE, Bartels P,
Nieves-Cintron M, Coleman AM, Henderson PB, Man KNM, Tseng PY,
Yarov-Yarovoy V, Bers DM, et al: α-Actinin-1 promotes activity of
the L-type Ca2+ channel Cav 1.2. EMBO J. 39(e102622)2020.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Han JW, Kang C, Kim Y, Lee MG and Kim JY:
Isoproterenol-induced hypertrophy of neonatal cardiac myocytes and
H9c2 cell is dependent on TRPC3-regulated CaV1.2 expression. Cell
Calcium. 92(102305)2020.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Hu Z, Wang JW, Yu D, Soon JL, de Kleijn
DP, Foo R, Liao P, Colecraft HM and Soong TW: Aberrant splicing
promotes proteasomal degradation of L-type Cav1.2 calcium channels
by competitive binding for Cavβ subunits in cardiac hypertrophy.
Sci Rep. 6(35247)2016.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Molkentin JD, Lu JR, Antos CL, Markham B,
Richardson J, Robbins J, Grant SR and Olson EN: A
calcineurin-dependent transcriptional pathway for cardiac
hypertrophy. Cell. 93:215–228. 1998.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Sanna B, Bueno OF, Dai YS, Wilkins BJ and
Molkentin JD: Direct and indirect interactions between
calcineurin-NFAT and MEK1-extracellular signal-regulated kinase 1/2
signaling pathways regulate cardiac gene expression and cellular
growth. Mol Cell Biol. 25:865–878. 2005.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Arnaiz-Cot JJ, Cleemann L and Morad M:
Xanthohumol modulates calcium signaling in rat ventricular
myocytes: Possible antiarrhythmic properties. J Pharmacol Exp Ther.
360:239–248. 2017.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Lin JH, Yang KT, Lee WS, Ting PC, Luo YP,
Lin DJ, Wang YS and Chang JC: Xanthohumol protects the rat
myocardium against ischemia/reperfusion injury-induced ferroptosis.
Oxid Med Cell Longev. 2022(9523491)2022.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Sun TL, Li WQ, Tong XL, Liu XY and Zhou
WH: Xanthohumol attenuates isoprenaline-induced cardiac hypertrophy
and fibrosis through regulating PTEN/AKT/mTOR pathway. Eur J
Pharmacol. 891(173690)2021.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Hu L, Wang Z, Li H, Wei J, Tang F, Wang Q,
Wang J, Zhang X and Zhang Q: Icariin inhibits isoproterenol-induced
cardiomyocyte hypertropic injury through activating autophagy via
the AMPK/mTOR signaling pathway. Biochem Biophys Res Commun.
593:65–72. 2022.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Song YH, Cai H, Gu N, Qian CF, Cao SP and
Zhao ZM: Icariin attenuates cardiac remodelling through
down-regulating myocardial apoptosis and matrix metalloproteinase
activity in rats with congestive heart failure. J Pharm Pharmacol.
63:541–549. 2011.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Wu B, Feng JY, Yu LM, Wang YC, Chen YQ,
Wei Y, Han JS, Feng X, Zhang Y, Di SY, et al: Icariin protects
cardiomyocytes against ischaemia/reperfusion injury by attenuating
sirtuin 1-dependent mitochondrial oxidative damage. Br J Pharmacol.
175:4137–4153. 2018.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Zhou H, Yuan Y, Liu Y, Deng W, Zong J,
Bian ZY, Dai J and Tang QZ: Icariin attenuates angiotensin
II-induced hypertrophy and apoptosis in H9c2 cardiomyocytes by
inhibiting reactive oxygen species-dependent JNK and p38 pathways.
Exp Ther Med. 7:1116–1122. 2014.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Wang Z, Gao L, Xiao L, Kong L, Shi H, Tian
X and Zhao L: Bakuchiol protects against pathological cardiac
hypertrophy by blocking NF-κB signaling pathway. Biosci Rep.
38(BSR20181043)2018.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Bhuniya D, Umrani D, Dave B, Salunke D,
Kukreja G, Gundu J, Naykodi M, Shaikh NS, Shitole P, Kurhade S, et
al: Discovery of a potent and selective small molecule hGPR91
antagonist. Bioorg Med Chem Lett. 21:3596–3602. 2011.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Velcicky J, Wilcken R, Cotesta S, Janser
P, Schlapbach A, Wagner T, Piechon P, Villard F, Bouhelal R, Piller
F, et al: Discovery and optimization of novel SUCNR1 inhibitors:
Design of zwitterionic derivatives with a salt bridge for the
improvement of oral exposure. J Med Chem. 63:9856–9875.
2020.PubMed/NCBI View Article : Google Scholar
|