Introduction
As a deadly hyperplastic disease, acute myeloid
leukemia (AML) results from clonal expansion as well as loss of
differentiation of hematopoietic stem cells in the bone marrow.
This disease is associated with high morbidity and mortality,
notably in children (1). AML is a
common type of leukemia accounting for 80% of acute leukemia
(2). Previous studies have
reported that the occurrence of AML is closely associated with gene
mutations, signal pathway abnormalities, epigenetic regulation,
leukemia microenvironment and immune imbalance (3-5).
In recent years, the standard of treatment for AML
has been the induction chemotherapy that is based on 7 days of
cytosine and 3 days of anthracycline treatment (6). Complete remission can be achieved in
60-80% of young adults. However, the disease relapses following
remission in nearly 50% of patients and the 5-year overall survival
rate has been estimated to be only 30% (7,8).
Conventional high-intensity induction regimens can cure a
considerable number of newly diagnosed patients. However, several
patients exist who are resistant to chemotherapy or relapse
following treatment (9).
Therefore, it is important to develop effective therapeutic methods
and identify therapeutic targets for patients with AML.
Acyl-CoA medium-chain synthetase-3 (ACSM3) is a
member of the acyl-CoA synthase gene family, located in the
mitochondrial matrix (10). ACSM3
is present in abundance in the kidney, bone marrow, liver, stomach,
gallbladder, anterior gland, fetal heart and in the central nervous
system (11). Previous studies
have shown that the ACSM3 gene is associated with the development
of multiple types of diseases, such as hypertriglyceridemia,
obesity, hypertension and insulin resistance (12,13).
Moreover, the expression level of ACSM3 is downregulated in
patients with high-grade serous ovarian carcinoma (14). In addition, ACSM3 has also been
revealed to be reduced in hepatocellular carcinoma tissues
(15). Yan et al (16) supported the notion that ACSM3
overexpression suppresses the proliferative, migratory and invasive
ability of ovarian cancer cells by inactivating the integrin β1/AKT
signaling pathway. In addition, Ruan et al (17) demonstrated that ACSM3 expression is
downregulated in hepatocellular carcinoma (HCC) tissues and its low
expression is associated with poor prognosis in patients with HCC.
Overexpression of ACSM3 represses migration and invasion of HCC
cells in vitro and in vivo by inactivation of the
phosphorylation of WNK lysine deficient protein kinase 1 and AKT
(17). However, the biological
role of ACSM3 in AML and the understanding of the potential
mechanism are still incomplete.
The insulin-like growth factor 2 mRNA-binding
protein 2 (IGF2BP2) has been reported to be a notable marker for
m6A modification and participates in the progression of a variety
of malignancies (18). For
example, IGF2BP2 facilitates the advancement of colorectal cancer
(19). Moreover, Xu et al
(20) revealed that IGF2BP2 plays
an oncogenic role in pancreatic cancer carcinogenesis. Notably,
IGF2BP2 has been revealed to be overexpressed in patients with AML
(21).
Collectively, the present study was implemented to
investigate the biological role of ACSM3 in AML as well as to
discuss its association with IGF2BP2.
Materials and methods
Bioinformatics analysis
The mRNA expression levels of ACSM3 and IGF2
mRNA-binding protein (IGF2BP) 2 in AML and the association between
ACSM3 expression and the overall survival were analyzed with the
Gene Expression Profiling Interactive Analysis (GEPIA) database
(http://gepia.cancer-pku.cn).
Cell culture and treatment
The human AML cell lines Kasumi-1, ME-1, MOLM-14,
HL-60 and the human bone marrow stromal cell line HS-5 were
provided by the Institute of Basic Medical Sciences, Chinese
Academy of Medical Sciences. RPMI-1640 medium (cat, no. 11875093;
Gibco, Thermo Fisher Scientific, Inc.) was used for the maintenance
of the AML cell lines, while HS-5 cells were cultured in DMEM,
which was mixed with 10% FBS (Gibco; Thermo Fisher Scientific,
Inc.) and 1% penicillin-streptomycin (Gibco; Thermo Fisher
Scientific, Inc.) at 37˚C with 5% CO2.
Cell transfection
To overexpress ACSM3 and IGF2BP2, pc-DNA3.1 vectors
containing the complete sequence of ACSM3 (Ov-ACSM3; accession no.
NM_202000.3), IGF2BP2 (Ov-IGF2BP2; accession no. NM_001291873.3)
and the corresponding empty vector (Ov-NC) were synthesized by
Genepharm, Inc. Lipofectamine® 2000 reagent (Shanghai
Aiyan Biotechnology Co., Ltd.) was employed for the transfection of
100 nM of these recombinants into HL-60 cells. Following 48 h of
culture at 37˚C after transfection, the cells were collected for
subsequent experiments.
Cell Counting Kit-8 assay
HL-60 cells were cultured in RPMI-1640 medium with
10% FBS at 37˚C for 24, 48 and 72 h. A total of 10 µl of WST-8
(Beyotime Institute of Biotechnology) was added per well and the
cells were incubated for 2 h. A microplate reader was adopted for
the estimation of the optical density at 450 nm.
5-ethynyl-2'-deoxyuridine (EdU) cell
proliferation assay
HL-60 cells were seeded in six-well plates at a
density of 4x105/well and were incubated overnight at
room temperature. Subsequently, the cells were fixed with 4%
polyformaldehyde (Shanghai Lingfeng Chemical Reagent Co., Ltd.) at
room temperature for 15 min and incubated with 0.5% Triton X-100
(Beijing Solarbio Science & Technology Co., Ltd.) at room
temperature for 15 min. Finally, the cells were stained with
Cell-Light™ EdU Cell Proliferation Detection Assay (LifeSpan
BioSciences, Inc.) and counterstained with
4',6-diamidino-2-phenylindole (DAPI) at room temperature for 30
min. The images of the positive cells were captured using a
fluorescent microscope and number of positive cells was calculated
utilizing ImageJ 1.8.0 software (National Institutes of Health).
The calculation of the cellular proliferative ratio was achieved by
estimating the ratio of EdU positive cells to DAPI positive
cells.
TUNEL assay
The number of apoptotic cells was assessed using an
apoptosis detection kit (Roche Diagnostics) in accordance with the
manufacturer's protocols. Briefly, the cells were fixed in 4%
paraformaldehyde at 37˚C for 15 min and incubated with proteinase K
(Beyotime Institute of Biotechnology) at room temperature for 15
min. Subsequently, the cells were placed in 3%
H2O2 at room temperature for 15 min,
cultivated with TUNEL regent (Beyotime Institute of Biotechnology)
at 37˚C for 1 h and counterstained with DAPI for 5 min at room
temperature. The number of positive cells was mounted with
fluorescent mounting media (Beijing Solarbio Science &
Technology Co., Ltd.) and analyzed using ImageJ 1.8.0 software
(National Institutes of Health). Overall, >10 fields of
view/section for each sample were assessed. A fluorescence
microscope was used for the visualization of the positive
cells.
Flow cytometry analysis
HL-60 cells were seeded into 96-well plates and
transfected with Ov-ACSM3 in the presence or absence of Ov-IGF2BP2.
At 48 h post transfection, the collected cells were fixed with 70%
ethanol at 4˚C overnight. Subsequently, the cells were stained with
the staining solution (20 µg/ml propidium iodide and 200 µg/ml
RNase; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocols. The analysis of the cell cycle was
conducted by flow cytometry (NovoCyte 2060R; ACEA Biosciences,
Inc.) with Software NovoExpress 1.4.0 (ACEA Biosciences, Inc.).
RNA stability assay
Following transfection, HL-60 cells were cultured in
six-well plates overnight with 5 µg/ml actinomycin D
(MedChemExpress) at 4˚C to inhibit gene transcription for 0-12 h.
Subsequently, reverse transcription-quantitative PCR (RT-qPCR)
analysis was applied for the isolation and determination of RNA.
ACSM3 mRNA expression was calculated in the indicated groups at
different time periods and normalized to those of GAPDH.
RNA extraction and RT-qPCR
The concentration of RNA, which was isolated from
the sample cells using TRIzol® reagent (Invitrogen;
Thermo Fisher Scientific, Inc.), was determined using NanoDrop 2000
(Thermo Fisher Scientific, Inc.) at 260 and 280 nm according to the
manufacturer's protocol. Subsequently, the synthesis of RNA into
cDNA was implemented using PrimeScript RT Master Mix (Takara Bio,
Inc.) according to the manufacturer's protocol. The primer
sequences for PCR are presented as below: ACSM3, forward
5'-AAGGTTCAGGGCTGCTCTTC-3', and reverse 5'-AGCATCTTCCTGGTGACACG-3';
IGF2BP2, forward 5'-GGAACAAGTCAACACAGACACA-3', and reverse
5'-CGCAGCGGGAAATCAATCTG-3'; GAPDH, forward
5'-GGGAAACTGTGGCGTGAT-3', and reverse 5'-GAGTGGGTGTCGCTGTTGA-3'.
RT-qPCR was performed using SYBR Premix Ex Taq™ II kit (Takara Bio,
Inc.). The relative quantification (2-ΔΔCq) method was
applied for the calculation of the relative gene expression
(22).
RNA immunoprecipitation (RIP)
assay
The RIP assay was performed to explore the
interaction between ACSM3 and IGF2BP utilizing a Magna RIP RNA
Binding Protein Immunoprecipitation kit (EMD Millipore) according
to the manufacturer's protocol. HL-60 cells were lysed on ice for 5
min by means of RIP buffer (Beyotime Institute of Biotechnology).
The cultivation with anti-IGF2BP2 antibody (cat. no. 11601-1-AP;
ProteinTech Group, Inc.) and anti-IgG (cat. no. 30000-0-AP;
ProteinTech Group, Inc.) were performed at 37˚C overnight. The
protein A/G beads (40 µl; cat. no. 20422; Thermo Fisher Scientific,
Inc.) were coated with 2 µg anti-IGF2BP2 2 or 2 µg anti-IgG
antibodies at 4˚C for 6 h. Afterwards, the captured protein-RNA
complex was digested with 0.5 mg/ml proteinase K containing 0.1%
SDS to extract RNA. To remove non-specific adsorption as much as
possible, the magnetic beads were repeatedly washed with RIP
washing buffer. RT-qPCR was applied for the analysis of resultant
RNA levels.
Western blotting
The quantification of protein, isolated from HL-60
cells using RIPA lysis buffer (NanJing SunShine Biotech Co., Ltd.),
was carried out using a BCA Assay kit (Beijing Dingguo Changsheng
Biotechnology Co., Ltd.) according to the manufacturer's protocols.
Following SDS-PAGE (10% gels), the proteins (60 µg/lane) were
transferred to polyvinylidene membranes. The membranes were blocked
with 5% non-fat milk for 2 h at room temperature. Overnight
incubation of the membranes was carried out with primary antibodies
against ACSM3 (1:500; cat. no. 10168-2-AP; ProteinTech Group,
Inc.), B-cell lymphoma-2 (Bcl-2; 1:1,000; cat. no. ab32124),
Bcl2-Associated X (Bax; 1:1,000; cat. no. ab32503), cleaved caspase
3 (1:500; cat. no. ab32042), cyclin dependent kinase (CDK) 4
(1:1,000; cat. no. ab108357), CDK6 (1:50,000; cat. no. ab124821),
cyclin D1 (1:200; cat. no. ab16663), IGF2BP2 (1:2,000; cat. no.
ab124930) and GAPDH (1:2,500; cat. no. ab9485) (Abcam) at 4˚C. The
following morning, the membranes were incubated for 2 h with
anti-rabbit HRP-conjugated secondary (1:5,000; cat. no. ab6759;
Abcam) at room temperature. Protein signals were visualized using
enhanced chemiluminescence reagent (Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. Densitometry analysis
was performed using ImageJ (version 1.49; National Institutes of
Health). The ratio of the target to GAPDH light density was
regarded as the relative expression of the protein.
Statistical analysis
Data, displayed in the format of mean ± standard
deviation (unless otherwise shown), were analyzed using SPSS 17.0
(SPSS Inc.). The significant differences between the two groups
were assessed with the unpaired Student's t-test, whereas the
comparisons among multiple groups were examined utilizing one-way
ANOVA with Bonferroni's post hoc multiple comparison test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
ACSM3 expression is downregulated in
AML and is associated with poor prognosis
To explore the role of ACSM3 in AML, bioinformatical
analysis was performed. As presented in Fig. 1A, a signification elevation in the
expression level of ACSM3 was noted in AML compared with other
types of cancer. The GEPIA database further indicated that ACSM3
expression was low in patients with AML compared with healthy
controls (Fig. 1B). In addition,
it was noted that patients with AML and high ACSM3 expression had
higher overall survival compared with patients with low ACSM3
expression (Fig. 1C). As presented
in Fig. 1D and E, ACSM3 expression significantly declined
in AML cell lines compared with that of HS-5 cells. Among these AML
cell lines, the HL-60 cell line exhibited the lowest ACSM3
expression and was consequently selected for subsequent assays.
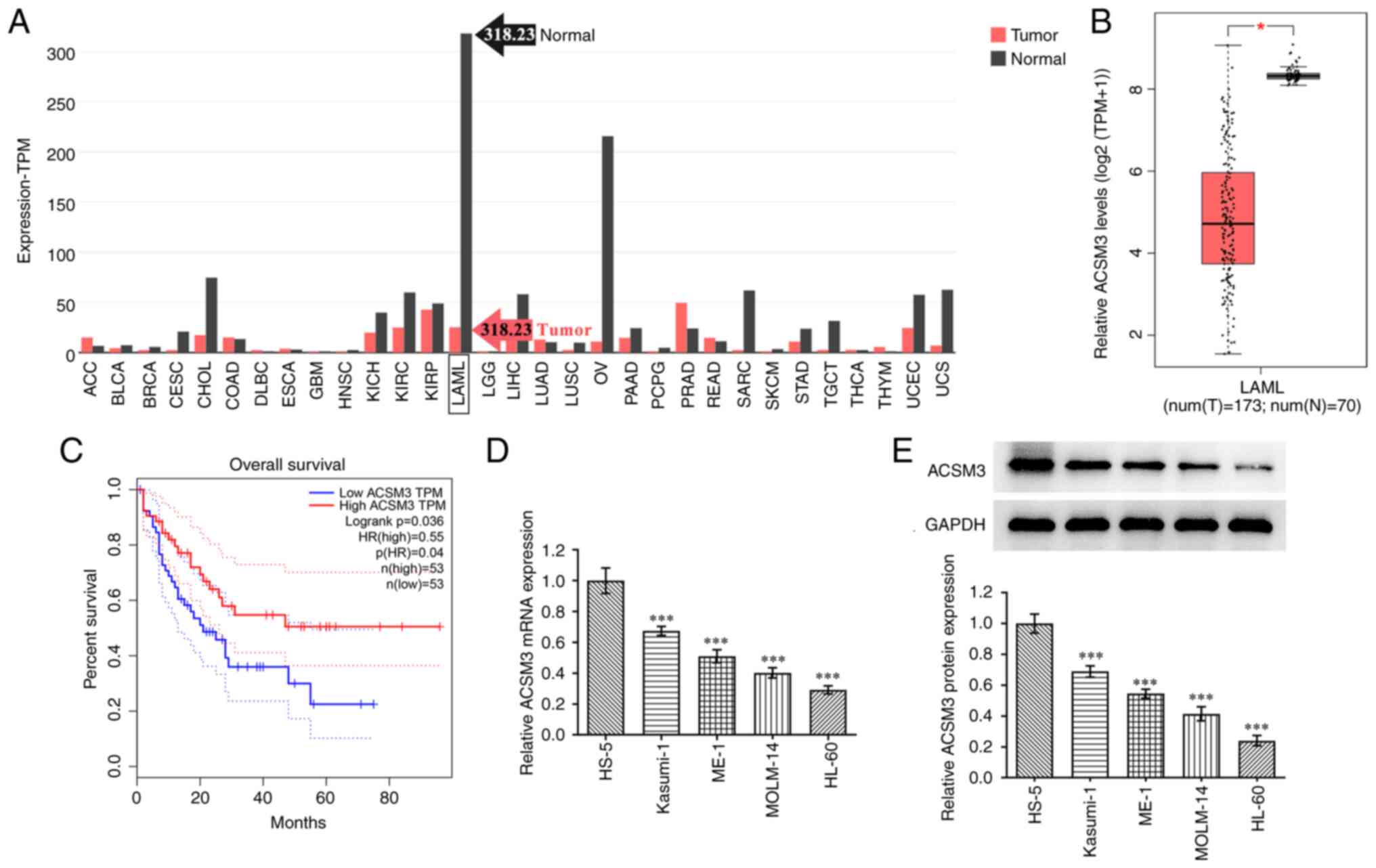 | Figure 1ACSM3 is downregulated in AML and is
associated with poor prognosis. (A) Analysis of ACSM3 expression in
multiple types of cancer by GEPIA database. (B) Analysis of ACSM3
expression in patients with AML and healthy controls by GEPIA
database. (C) Association of ACSM3 and overall survival in patients
with AML, by GEPIA database. (D) mRNA expression and (E) protein
expression of ACSM31 in AML cells detected using reverse
transcription-quantitative PCR and western blotting, respectively.
*P<0.05. ***P<0.001 vs. HS-5. ACSM3,
acyl-CoA medium-chain synthetase-3; GEPIA, gene expression
profiling interactive analysis; ACC, adrenocortical carcinoma;
BLCA, bladder urothelial carcinoma; BRCA, breast invasive
carcinoma; CESC, cervical squamous cell carcinoma and endocervical
adenocarcinoma; CHOL, cholangiocarcinoma; COAD, colon
adenocarcinoma; DLBC, lymphoid neoplasm diffuse large B-cell
lymphoma; ESCA, esophageal carcinoma; GBM, glioblastoma multiforme;
HNSC, head and neck squamous cell carcinoma; KICH, kidney
chromophobe; KIRC, kidney renal clear cell carcinoma; KIRP, kidney
renal papillary cell carcinoma; AML/LAML, acute myeloid leukemia;
LGG, brain lower grade glioma; LIHC, liver hepatocellular
carcinoma; LUAD, lung adenocarcinoma; LUSC, lung squamous cell
carcinoma; OV, ovarian serous cystadenocarcinoma; PAAD, pancreatic
adenocarcinoma; PCPG, pheochromocytoma and paraganglioma; PRAD,
prostate adenocarcinoma; READ, rectum adenocarcinoma; SARC,
sarcoma; SKCM, skin cutaneous melanoma; STAD, stomach
adenocarcinoma; TGCT, testicular germ cell tumors; THCA, thyroid
carcinoma; THYM, thymoma; UCEC, uterine corpus endometrial
carcinoma; UCS, uterine carcinosarcoma. |
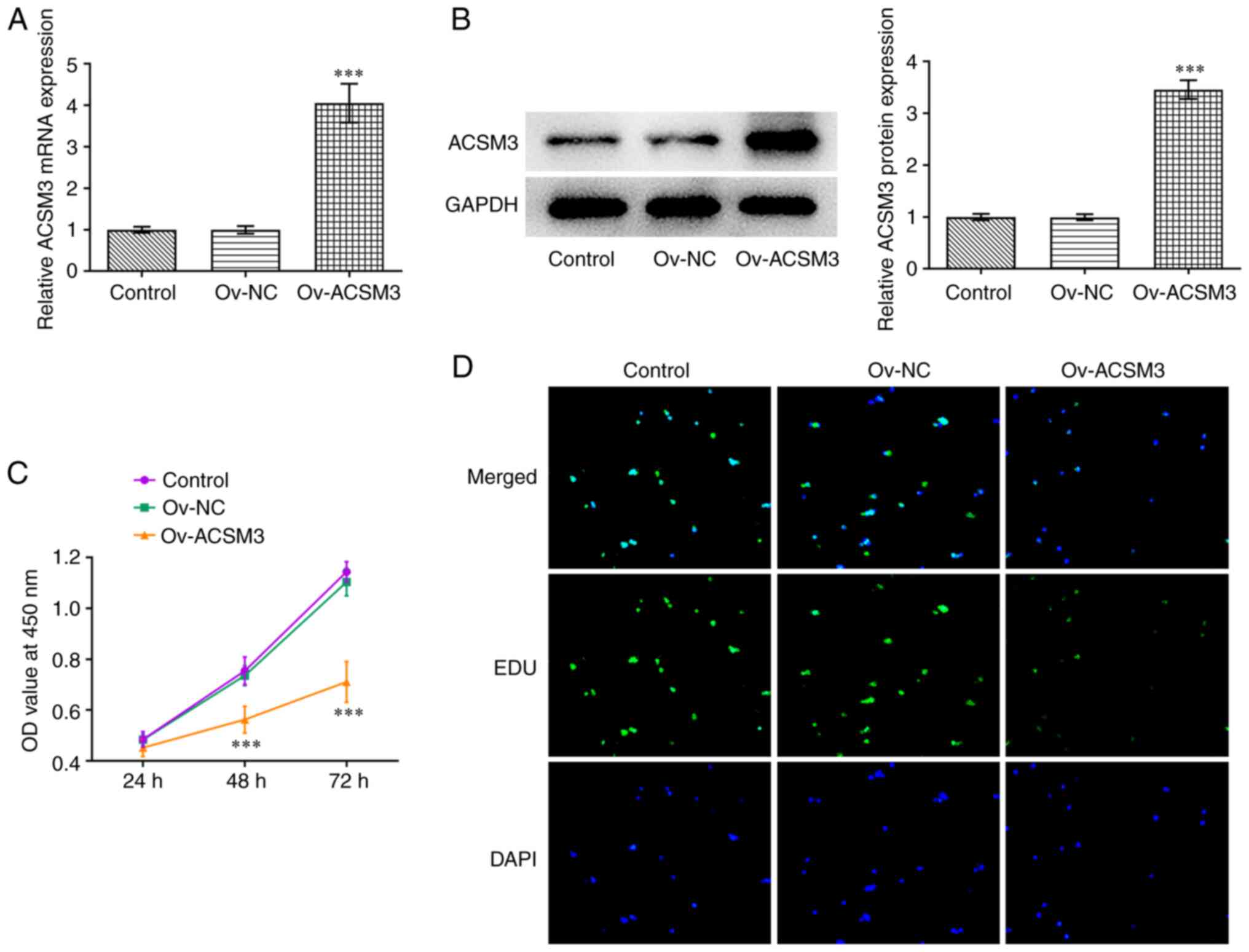
Overexpression of ACSM3 inhibits HL-60
cell proliferation
To investigate the biological roles of ACSM3 in
HL-60 cells, Ov-ACSM3 was transfected into HL-60 cells. As
demonstrated in Fig. 2A and
B, Ov-ACSM3 significantly
upregulated the mRNA and protein expression levels of ACSM3
compared with Ov-NC. The CCK-8 assay indicated that ACSM3
overexpression markedly repressed the proliferation of HL-60 cells
compared with the negative control group (Fig. 2C). Moreover, EdU staining indicated
a reduction in the number of positive-green cells following
transfection with Ov-ACSM3 compared with transfection with Ov-NC,
demonstrating the inhibition in cell proliferation following ACSM3
overexpression (Fig. 2D).
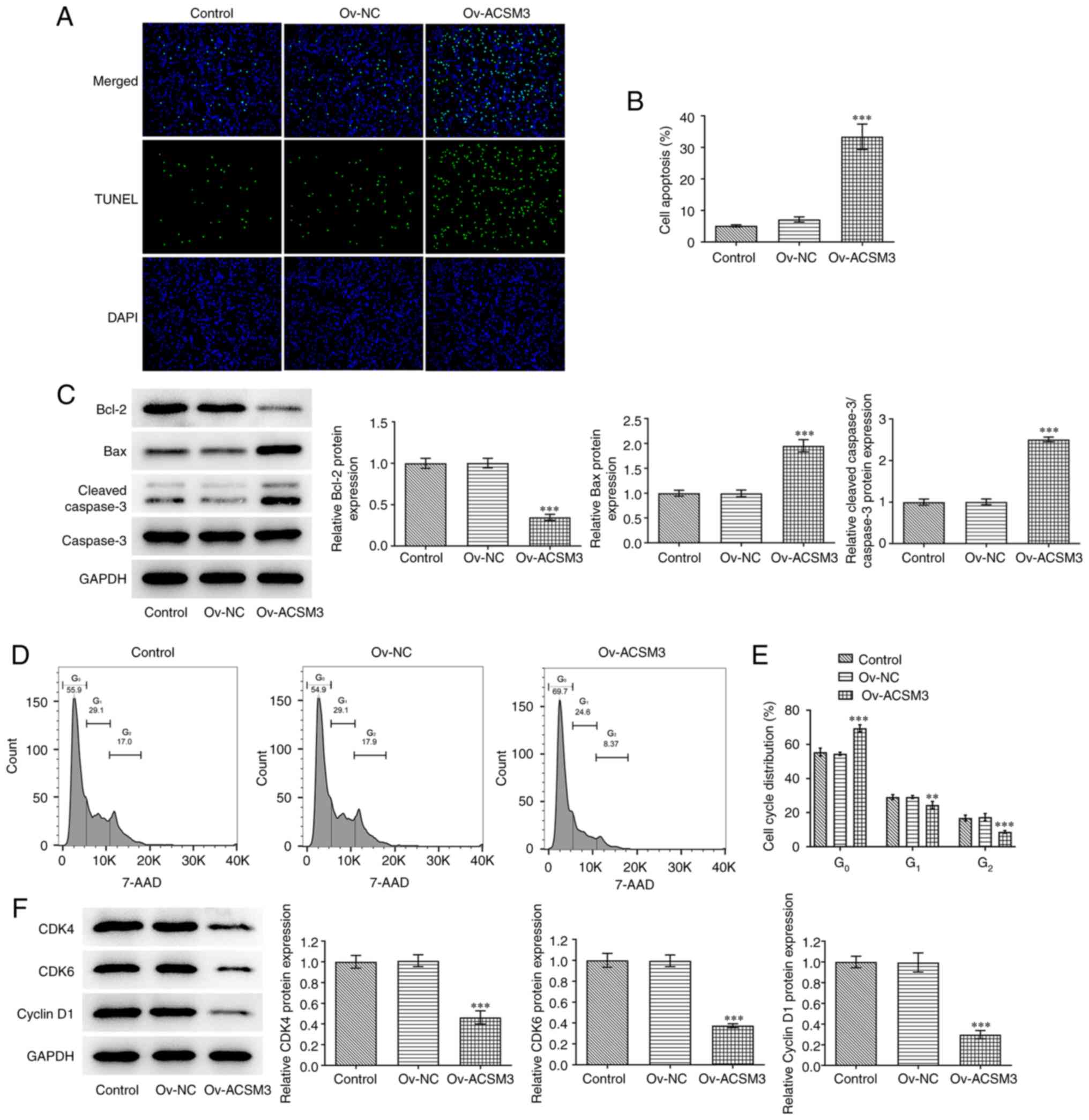
Upregulation of ACSM3 expression
induces apoptosis and cycle arrest of HL-60 cells
As shown in Fig. 3A
and B, an significant increase in
the apoptotic rate was observed following ACSM3 overexpression
compared with that of the negative control cells. Moreover, ACSM3
overexpression led to significantly decreased Bcl-2 expression
levels and significantly increased levels of Bax and cleaved
caspase 3 in HL-60 cells compared with the Ov-NC (Fig. 3C). Furthermore, it was also
discovered that the percentage of cells in the
G0/G1 phase was significantly increased,
while the G2 phase population was significantly
decreased following overexpression of ACSM3 (Fig. 3D and E). In addition, overexpression of ACSM3
significantly reduced the protein levels of CDK4, CDK6 and cyclin
D1 compared with those of the negative control (Fig. 3F).
IGF2BP2 downregulates ACSM3 expression
by reducing the stability of ACSM3 mRNA
Subsequently, the function of ACSM3 was investigated
in AML. As shown in Fig. 4A,
IGF2BP2 exhibited significantly increased expression in the tissues
of patients suffering from AML compared with healthy controls. The
results from RT-qPCR and western blotting indicated that IGF2BP2
mRNA and protein expression were significantly increased in HL-60
cells compared with those noted in HS-5 cells (Fig. 4B and C). RIP assay confirmed the binding of the
IGF2BP2 protein with the ACSM3 mRNA (Fig. 4D). As presented in Fig. 4E and F, the mRNA and protein expression levels
of IGF2BP2 were significantly increased in the Ov-IGF2BP2 group
compared with Ov-NC group. The RNA stability assay indicated that
IGF2BP2 overexpression significantly decreased the stability of
ACSM3 mRNA compared with the Ov-NC group (Fig. 4G). Overexpression of IGF2BP2
significantly decreased mRNA and protein expression of ACSM3 as
determined by RT-qPCR and western blotting (Fig. 4H and I).
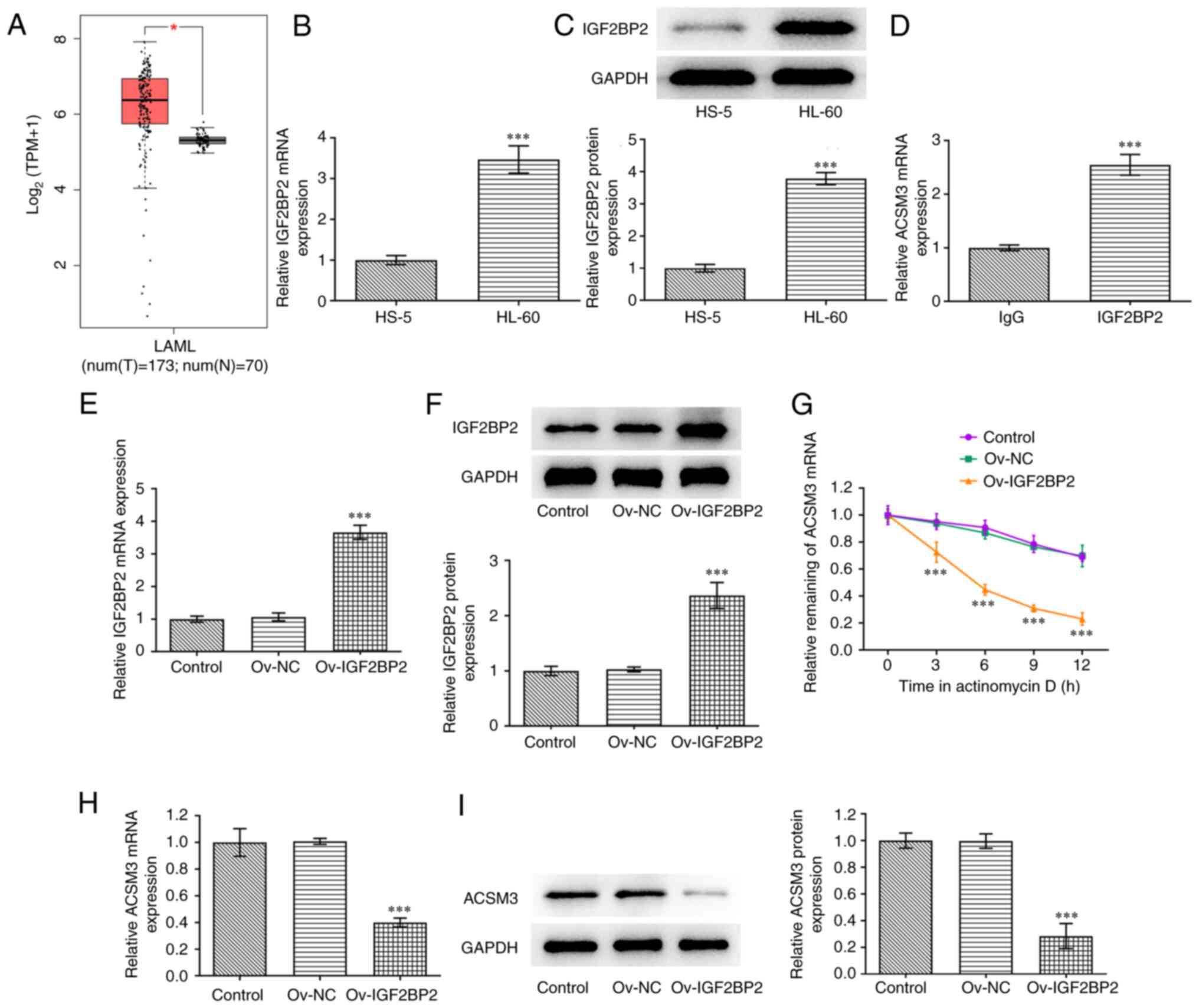 | Figure 4IGF2BP2 reduces the stability of
ACSM3 mRNA and downregulates ACSM3 expression. (A) Analysis of
IGF2BP2 expression in patients with AML and healthy controls by
GEPIA database. (B) mRNA and (C) protein expression levels of
IGF2BP2 in HS-5 and HL-60 cells were detected by RT-qPCR and
western blotting, respectively. (D) RNA immunoprecipitation assay
confirmed the binding of IGF2BP2 and ACSM3 mRNA. (E) mRNA and (F)
protein expression levels of IGF2BP2 in HL-60 cells transfected
with Ov-IGF2BP2 were detected by RT-qPCR and western blotting,
respectively. (G) RNA stability assay was performed to assess the
stability of ACSM3 mRNA. (H) mRNA and (I) protein expression levels
of ACSM3 in HL-60 cells transfected with Ov-IGF2BP2 were detected
using RT-qPCR and western blotting, respectively.
*P<0.05 compared to healthy controls.
***P<0.001 vs. HS-5, IgG and Ov-NC. ACSM3, acyl-CoA
medium-chain synthetase-3; AML/LAML, acute myeloid leukemia;
IGFBP2, IGF2 binding protein 2; NC, negative control; Ov,
overexpressing; RT-qPCR, reverse transcription-quantitative
PCR. |
ACSM3 affects the proliferation,
apoptosis and cell cycle of HL-60 cells by regulating the
expression levels of IGF2BP2
As presented in Fig.
5A, IGF2BP2 overexpression significantly reversed the decreased
proliferative ability induced by ACSM3 overexpression. EdU staining
indicated that the proliferation of HL-60 cells was increased
following transfection with Ov-IGF2BP2, which was consistent with
the previous findings (Fig. 5B).
In addition, the apoptotic rate of the cells co-transfected with
Ov-ACSM3 and Ov-IGF2BP2 was significantly decreased compared with
cells transfected with only Ov-ACSM3 (Fig. 5C and D). This was in line with western blotting
results that showed Bcl-2 expression was significantly increased,
whereas the expression of Bax and cleaved caspase 3 were
significantly decreased by IGF2BP2 overexpression (Fig. 5E). Moreover, IGF2BP2 overexpression
significantly reduced the percentage cell population in the
G0/G1 phase and increased the percentage of
cells in the S phase cells compared with the Ov-ACSM3 + Ov-NC group
(Fig. 5F and G). Western blotting revealed that IGF2BP2
overexpression elevated the expression levels of CDK4, CDK6 and
cyclin D1 in ACSM3-overexpressed HL-60 cells compared with the
Ov-ACSM3 + Ov-NC group (Fig.
5H).
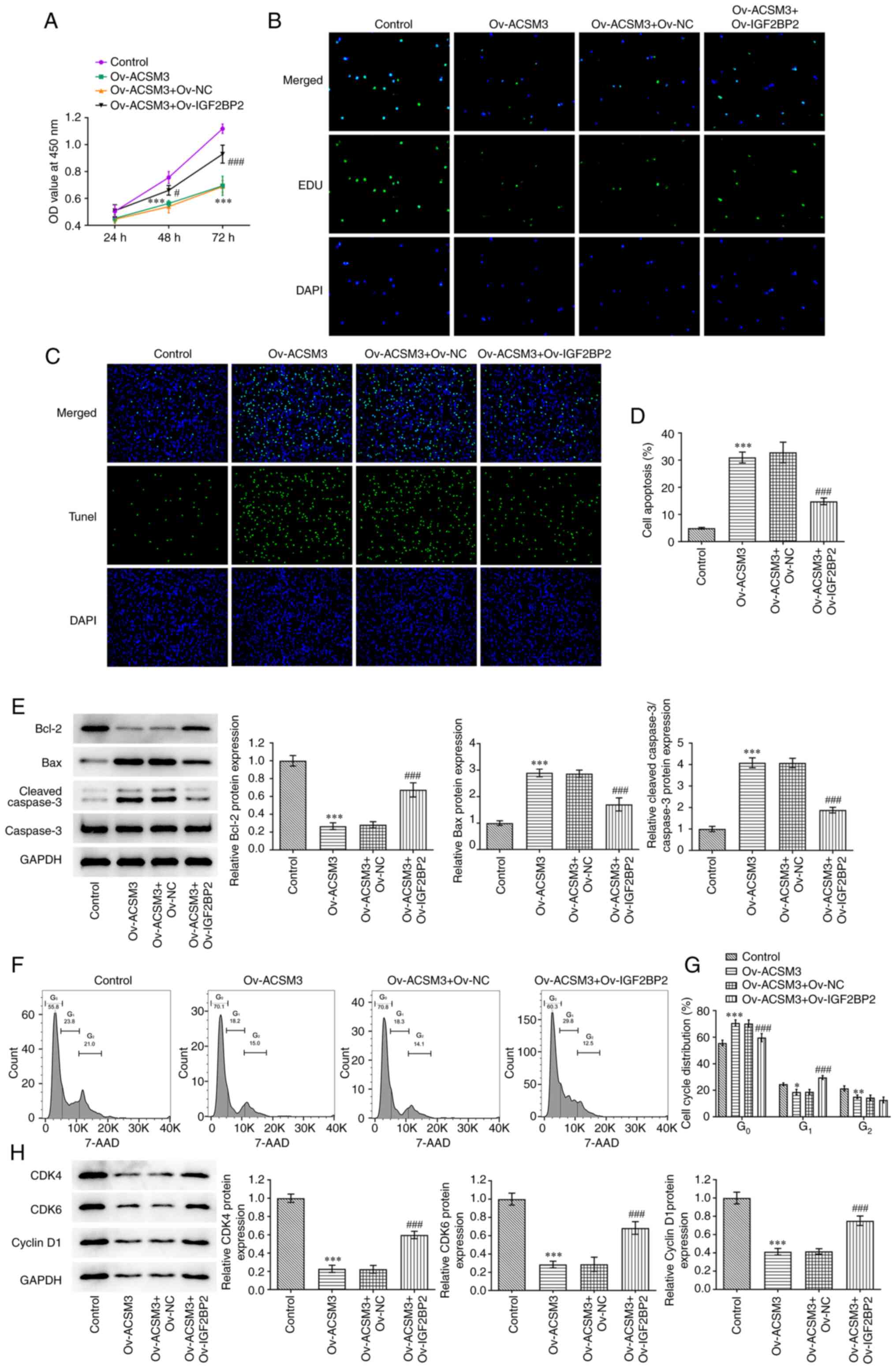 | Figure 5Effects of ACSM3 on the
proliferation, apoptosis and cell cycle of HL-60 cells is
associated with regulation of IGF2BP2. Cell proliferation was
evaluated using (A) CCK-8 assay and (B) EdU staining. (C) Apoptosis
was detected and (D) quantified using TUNEL assay. (E) Western
blotting was used to assess the protein levels of Bcl-2, Bax and
cleaved caspase 3/caspase 3. (F) Cell cycle was detected and (G)
quantified using flow cytometry analysis. (H) Western blotting was
used to evaluate the protein levels of CDK4, CDK6 and Cyclin D1.
Results are displayed as the mean ± SD. *P<0.05,
**P<0.01 and ***P<0.001 vs. Control.
#P<0.05 and ###P<0.001 vs. Ov-ACSM3 +
Ov-NC. ACSM3, acyl-CoA medium-chain synthetase-3; EdU,
5-ethynyl-2'-deoxyuridine; Ov, overexpressing; NC, negative
control; OD, optical density. Bcl-2, B-cell lymphoma-2; Bax,
Bcl2-Associated X; CDK4, cyclin dependent kinase 4; CDK6, cyclin
dependent kinase 6. |
Discussion
AML is a malignant hyperplastic disease caused by
clonal expansion and loss of differentiation of hematopoietic stem
cells in the bone marrow. It is imperative to improve the survival
rate of patients with AML and investigate the pathogenesis of this
disease so as to develop therapeutic drugs for the treatment of
this disease (23). Nevertheless,
the mechanism of action of AML is obscure. In the present study, it
was discovered that ACSM3 expression was reduced in AML and that
this was closely associated with poor prognosis in patients
suffering from AML. ACSM3 overexpression suppressed cell
proliferative ability and facilitated the induction of apoptosis
and cell cycle arrest in AML. In addition, IGF2BP2 reduced the
stability of ACSM3 mRNA and its overexpression reversed the
influence of ACSM3 upregulation on AML cell proliferation,
apoptosis and cycle arrest.
Previous studies have shown that dysregulation of
ACSM3 expression is involved in the progression of various types of
cancer (24,25). A previous study that used The
Cancer Genome Atlas-MM, Gene Expression Omnibus, Genomics of Drug
Sensitivity in Cancer datasets and the human protein atlas,
indicated that ACSM3 expression is significantly downregulated in
malignant melanoma (MM) (26). The
results of this study also showed that lower ACSM3 expression
results in worse prognosis for patients with MM, which is common
among Asian patients. Knockdown of ACSM3 expression and
overexpression of this protein significantly increases and
decreases MM cell proliferative, invasive and colony formation
abilities, respectively (26). In
addition, An et al (27)
demonstrated that higher ACSM3 expression is associated with
improved prognosis for patients with ovarian cancer and
upregulation of ACSM3 can promote the chemosensitivity of ovarian
cancer cells by inhibiting the PI3K/AKT signaling pathway.
Nevertheless, the functional role of ACSM3 in AML has not been
fully clarified.
In the current study, a bioinformatic software was
used to analyze the expression levels of ACSM3 in AML and the data
indicated that ACSM3 expression was significantly altered in AML
compared with the corresponding levels of this protein in other
types of cancer. Moreover, GEPIA analysis revealed the low
expression of ACSM3 in AML and its association with the poor
overall survival rate of patients suffering from AML. Subsequently,
in vitro experiments also indicated that ACSM3 expression
was significantly downregulated in AML cells compared with that of
the control cells. Following overexpression of ACSM3 in AML cells,
proliferation was inhibited and the induction of apoptosis and
cycle arrest was noted. The data indicated that ACSM3
overexpression played a suppressive role in AML cell growth.
IGF2BP2 is an RNA-binding protein, which plays an
important role in regulating proliferation, myogenesis, muscle cell
motility, differentiation potentials and metabolic energy levels
(28-30).
IGF2BP2 has been shown to be involved in the progression of
numerous types of cancer. For example, upregulation of IGF2BP2 is
associated with poor survival in esophageal adenocarcinoma and
patients with basal-like breast cancer (31,32).
Moreover, a previous study indicated that overexpression of IGF2BP2
is associated with a low survival rate in patients with AML
(21). Huang et al
(33) revealed that Linc01305
aggravates proliferation and metastasis of esophageal squamous cell
carcinoma by stabilizing 5-hydroxytryptamine receptor 3A mRNA via
its interaction with IGF2BP2 and IGF2BP3. In addition, Shen et
al (34) reported that
Linc01559 recruits IGF2BP2 to stabilize Zinc finger E-box-binding
homeobox 1 mRNA to facilitate cell proliferation and migratory
abilities as well as epithelial-mesenchymal transition process in
gastric cancer. In line with these results, the present study
demonstrated that IGF2BP2 destabilized ACSM3 by reducing the
stability of its mRNA, which contributed to the reversal of the
proliferation, apoptosis and cell cycle arrest that were regulated
by ACSM3 overexpression. Herein are several limitations of the
present study. Investigations mainly focused on the effect of
changes in the ACSM3 expression in AML but the effect of
localization of ACSM3 in the cells was not considered, which will
be explored in the future studies. In addition, results were only
supported by in vitro experiments and previous studies were
unable to confirm experimental outcomes. Thus, further experiments
using animal and clinical studies are required to confirm these
findings.
In summary, the present study indicated the
inhibitory role of ACSM3 in AML and revealed the functions of
IGF2BP2 in ACSM3-modulated cell proliferation, apoptosis and cycle
arrest by regulating the stability of ACSM3 mRNA. Collectively, the
data implied that the IGF2BP2/ACSM3 axis could be a prospective
therapeutic strategy for AML.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XZ and BH designed the study and drafted and revised
the manuscript. JW and LS analyzed the data and searched the
literature. XZ and BH confirm the authenticity of all the raw data.
XZ, JW, LS and BH performed experiments. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cammarata-Scalisi F, Girardi K, Strocchio
L, Merli P, Garret-Bernardin A, Galeotti A, Magliarditi F, Inserra
A and Callea M: Oral manifestations and complications in childhood
acute myeloid leukemia. Cancers (Basel). 12(1634)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Tomizawa D, Miyamura T, Imamura T,
Watanabe T, Moriya Saito A, Ogawa A, Takahashi Y, Hirayama M, Taki
T, Deguchi T, et al: A risk-stratified therapy for infants with
acute lymphoblastic leukemia: A report from the JPLSG MLL-10 trial.
Blood. 136:1813–1823. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Prada-Arismendy J, Arroyave JC and
Röthlisberger S: Molecular biomarkers in acute myeloid leukemia.
Blood Rev. 31:63–76. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Medinger M, Heim D, Halter JP, Lengerke C
and Passweg JR: Diagnosis and therapy of acute myeloid leukemia.
Ther Umsch. 76:481–486. 2019.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
5
|
Chopra M and Bohlander SK: The cell of
origin and the leukemia stem cell in acute myeloid leukemia. Genes
Chromosomes Cancer. 58:850–858. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Medeiros BC, Chan SM, Daver NG, Jonas BA
and Pollyea DA: Optimizing survival outcomes with post-remission
therapy in acute myeloid leukemia. Am J Hematol. 94:803–811.
2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tallman MS, Wang ES, Altman JK, Appelbaum
FR, Bhatt VR, Bixby D, Coutre SE, De Lima M, Fathi AT, Fiorella M,
et al: Acute myeloid leukemia, version 3.2019, NCCN clinical
practice guidelines in oncology. J Natl Compr Canc Netw.
17:721–749. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kantarjian H: Acute myeloid leukemia-major
progress over four decades and glimpses into the future. Am J
Hematol. 91:131–145. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ganzel C, Sun Z, Cripe LD, Fernandez HF,
Douer D, Rowe JM, Paietta EM, Ketterling R, O'Connell MJ, Wiernik
PH, et al: Very poor long-term survival in past and more recent
studies for relapsed AML patients: The ECOG-ACRIN experience. Am J
Hematol. 93:1074–1081. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhang L, Sun W, Ren W, Zhang J and Xu G:
Predicting panel of metabolism and immune-related genes for the
prognosis of human ovarian cancer. Front Cell Dev Biol.
9(690542)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Junková K, Mirchi LF, Chylíková B, Janků
M, Šilhavý J, Hüttl M, Marková I, Miklánková D, Včelák J, Malínská
H, et al: Hepatic transcriptome profiling reveals lack of Acsm3
expression in polydactylous rats with high-fat diet-induced
hypertriglyceridemia and visceral fat accumulation. Nutrients.
13(1462)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bi WL, Abedalthagafi M, Horowitz P,
Agarwalla PK, Mei Y, Aizer AA, Brewster R, Dunn GP, Al-Mefty O,
Alexander BM, et al: Genomic landscape of intracranial meningiomas.
J Neurosurg. 125:525–535. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wang D, Liu CD, Li HF, Tian ML, Pan JQ,
Shu G, Jiang QY, Yin YL and Zhang L: LSD1 mediates microbial
metabolite butyrate-induced thermogenesis in brown and white
adipose tissue. Metabolism. 102(154011)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yang X, Wu G, Zhang Q, Chen X, Li J, Han
Q, Yang L, Wang C, Huang M, Li Y, et al: ACSM3 suppresses the
pathogenesis of high-grade serous ovarian carcinoma via promoting
AMPK activity. Cell Oncol (Dordr). 45:151–161. 2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gopal R, Selvarasu K, Pandian PP and
Ganesan K: Integrative transcriptome analysis of liver cancer
profiles identifies upstream regulators and clinical significance
of ACSM3 gene expression. Cell Oncol (Dordr). 40:219–233.
2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yan L, He Z, Li W, Liu N and Gao S: The
Overexpression of Acyl-CoA Medium-Chain Synthetase-3 (ACSM3)
Suppresses the Ovarian Cancer Progression via the Inhibition of
Integrin β1/AKT Signaling Pathway. Front Oncol.
11(644840)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ruan HY, Yang C, Tao XM, He J, Wang T,
Wang H, Wang C, Jin GZ, Jin HJ and Qin WX: Downregulation of ACSM3
promotes metastasis and predicts poor prognosis in hepatocellular
carcinoma. Am J Cancer Res. 7:543–553. 2017.PubMed/NCBI
|
|
18
|
Hu X, Peng WX, Zhou H, Jiang J, Zhou X,
Huang D, Mo YY and Yang L: IGF2BP2 regulates DANCR by serving as an
N6-methyladenosine reader. Cell Death Differ. 27:1782–1794.
2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Cui J, Tian J, Wang W, He T, Li X, Gu C,
Wang L, Wu J and Shang A: IGF2BP2 promotes the progression of
colorectal cancer through a YAP-dependent mechanism. Cancer Sci.
112:4087–4099. 2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Xu X, Yu Y, Zong K, Lv P and Gu Y:
Up-regulation of IGF2BP2 by multiple mechanisms in pancreatic
cancer promotes cancer proliferation by activating the PI3K/Akt
signaling pathway. J Exp Clin Cancer Res. 38(497)2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
He X, Li W, Liang X, Zhu X, Zhang L, Huang
Y, Yu T, Li S and Chen Z: IGF2BP2 overexpression indicates poor
survival in patients with acute myelocytic leukemia. Cell Physiol
Biochem. 51:1945–1956. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Swaminathan M and Wang ES: Novel therapies
for AML: A round-up for clinicians. Expert Rev Clin Pharmacol.
13:1389–1400. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhao Z, Zhan Y, Jing L and Zhai H: KLF10
upregulates ACSM3 via the PI3K/Akt signaling pathway to inhibit the
malignant progression of melanoma. Oncol Lett.
23(175)2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Shu C, Zheng X, Wuhafu A, Cicka D, Doyle
S, Niu Q, Fan D, Qian K, Ivanov AA, Du Y, et al: Acquisition of
taxane resistance by p53 inactivation in ovarian cancer cells. Acta
Pharmacol Sin. 43:2419–2428. 2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhu Z, Wang D and Shen Y: Loss of ACSM3
confers worsened prognosis and immune exclusion to cutaneous
melanoma. J Cancer. 11:6582–6590. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
An Y and Duan H: Acyl-CoA medium-chain
synthetase-3 promotes chemosensitivity of ovarian cancer through
the inhibition of PI3K/AKT signaling pathway. Research Square.
2021.
|
|
28
|
Fujii Y, Kishi Y and Gotoh Y: IMP2
regulates differentiation potentials of mouse neocortical neural
precursor cells. Genes Cells. 18:79–89. 2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Li Z, Gilbert JA, Zhang Y, Zhang M, Qiu Q,
Ramanujan K, Shavlakadze T, Eash JK, Scaramozza A, Goddeeris MM, et
al: An HMGA2-IGF2BP2 axis regulates myoblast proliferation and
myogenesis. Dev Cell. 23:1176–1188. 2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Boudoukha S, Cuvellier S and Polesskaya A:
Role of the RNA-binding protein IMP-2 in muscle cell motility. Mol
Cell Biol. 30:5710–5725. 2010.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Barghash A, Helms V and Kessler SM:
Overexpression of IGF2 mRNA-binding protein 2 (IMP2/p62) as a
feature of basal-like breast cancer correlates with short survival.
Scand J Immunol. 82:142–143. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Barghash A, Golob-Schwarzl N, Helms V,
Haybaeck J and Kessler SM: Elevated expression of the IGF2 mRNA
binding protein 2 (IGF2BP2/IMP2) is linked to short survival and
metastasis in esophageal adenocarcinoma. Oncotarget. 7:49743–49750.
2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Huang GW, Chen QQ, Ma CC, Xie LH and Gu J:
linc01305 promotes metastasis and proliferation of esophageal
squamous cell carcinoma through interacting with IGF2BP2 and
IGF2BP3 to stabilize HTR3A mRNA. Int J Biochem Cell Biol.
136(106015)2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Shen H, Zhu H, Chen Y, Shen Z, Qiu W, Qian
C and Zhang J: ZEB1-induced LINC01559 expedites cell proliferation,
migration and EMT process in gastric cancer through recruiting
IGF2BP2 to stabilize ZEB1 expression. Cell Death Dis.
12(349)2021.PubMed/NCBI View Article : Google Scholar
|