Introduction
Myocardial ischemia is a pathological condition
characterized by interruption of blood supply to myocardium.
Reperfusion has become a standard therapy but may lead to
irreversible damage to heart tissue, known as myocardial
ischemia/reperfusion (MI/R) injury (1), which is a leading cause of mortality
worldwide (2). MI/R injury
contributes to a range of cardiovascular outcomes, including
systolic myocardial dysfunction, cardiac electrophysiology disorder
and myocardial death (3,4). Therefore, it is key to determine the
underlying mechanisms and develop novel agents to protect
myocardial cells during MI/R injury.
Previous studies have identified that microRNAs
(miRNAs or miRs) play essential roles in MI/R injury (5-7).
miRNAs are small non-coding RNAs 19-22 nucleotides in length. They
regulate target mRNAs by binding the sequences in the
3'untranslated regions (3'-UTRs) to facilitate degradation or
suppress translation (8). MI/R
injury is aggravated by dysregulation of miRNAs. Hinkel et
al (9) reported that miR-92a
is significantly upregulated in pigs subjected to percutaneous I/R.
With the utilization of locked nucleic acid-modified antisense
miR-92a treatment, infarct size was significantly decreased
(9). Similarly, miR-15 family,
including miR-15a, miR-15b, miR-16-1, miR-16-2, miR-195 and
miR-497, is upregulated in the infarcted region of the heart in
response to I/R injury in mice and pigs (10). On the other hand, miRNAs serve as
an antagonist in cardiovascular disease. For example, miR-103/107
repairs I/R injury by antagonizing receptor-interacting
serine/threonine-protein kinase 1 and 3-dependent necrosis and
binding to Fas-associated protein with death domain (11). Overexpression of miR-494 activates
the Akt/mitochondrial signaling pathway, resulting in
cardioprotective effects against I/R-induced injury in a mouse
model (12). Despite many studies
(13-15)
demonstrating that MI/R injury is associated with microRNAs, the
mechanisms are unknown because of the complexity of cellular
events. Therefore, it is key to understand the role of miRNAs in
MI/R injury.
SRY-related high mobility group-Box gene 9 (SOX9) is
a transcription factor of the SRY family, which is involved in cell
processes, including oxidation and tumor growth (16-18).
SOX9 is expressed in multiple types of tissue, including lung,
brain, and cardiomyocytes (19).
SOX9 involved in hepatic (20) and
cerebral (21) I/R injury.
Suppressing SOX9 markedly decreases hepatic I/R and concurrent
injury by inhibiting TGF-β1 activation (20). Moreover, SOX9 plays a vital role in
myocardial disease. For example, the loss or inactivation of SOX9
may reduce the initiation of cardiac hypertrophy, fibrosis and
inflammation. SOX9 may act as a regulator of myocardial infarction
and is involved in crosstalk between myocytes and fibroblasts
(22,23). For example, in patients with
ischemic heart disease, the loss of SOX9 may delay the cardiac
fibrotic response (24). SOX9
exacerbates hypoxia-induced cardiomyocyte apoptosis by promoting
miR-223-3p expression (19). SOX9
suppression by miR-30e elevation protects cardiac function and
decreases ventricular remodeling (25). The aforementioned data indicate
that SOX9 may be involved in the onset of MI/R injury. However, the
underlying molecular mechanism remains unknown. Therefore, the
present study aimed to determine the role of miRNAs in regulating
MI/R injury and the underlying mechanisms.
Materials and methods
Animal model
Animal experiments were approved [approval no.
KLLY(A)-2019-008] and performed in accordance with animal welfare
regulations (26) of Zunyi Medical
University Committee (Zunyi, Guizhou, China). All experimental
procedures were fully compliant with the guidelines for animal
research from the National Institutes of Health (27). A total of 84 adult male
Sprague-Dawley rats (age, 3 months; weight, ~200 g) that were
purchased from Laboratory Animal Center of Third Military Medical
University (Chongqing, China) were used in the present study.
Animals were housed at 18-22˚C, relative humidity of 50-70% and a
12/12-h light/dark cycle. Food and water were available ad
libitum. At the end of experiments, all animals were euthanized
by intraperitoneal injection of pentobarbital (150 mg/kg).
To establish the MI/R model, rats were divided into
I/R or Sham group, n=15 in each group, or I/R + control (n=5), I/R
+ miR-30c mimics (n=5), I/R + miR-30c inhibitor (n=5). Rats were
anesthetized with 45 mg/kg sodium pentobarbital. The rats undergone
endotracheal intubation and ventilation (tidal volume, 7 ml; ratio
of exhalation/inhalation time, 1.25; breathing rate, 80/min). The
thorax was opened by left thoracotomy to expose the heart. A 6.0
prolene suture was used to induce myocardial ischemia by ligating
the proximal left anterior descending coronary artery (LAD) with a
slipknot. The slipknot was removed after 30 min to allow
reperfusion for 30 min. The sham group underwent the same procedure
but was not subjected to ligation. Heart samples were obtained for
biological and molecular analyses.
Hematoxylin and eosin (H&E)
staining
Myocardial tissue samples were collected, fixed with
4% polyformaldehyde at room temperature overnight, dehydrated and
then embedded in paraffin. Then the tissues were cut into 5-µm
sections. The sections were stained using H&E staining kit
(C0105S, Beyotime Institute of Biotechnology) according to the
manufacturer's instructions. Images of stained sections were
obtained by light microscope at 400x (Nikon Corporation 80i).
ELISA
Serum samples of rats after I/R injury or sham
surgery, or cell culture supernatant of isolated RPM cells was
collected and centrifugated at 200 x g for 5 min at 4˚C. The serum
levels of lactate dehydrogenase (LDH; cat. no. A020-2-2), cardiac
troponin I (cTnI; cat. no. H149-2) and creatine kinase isoenzymes
(CK; cat. no. A032-1-1) were detected using ELISA kits (all Nanjing
Jiancheng Bioengineering Institute) according to the manufacturer's
instructions. Levels of IL-18 (cat. no. H015) and IL-1β (cat. no.
H002-1-1) in cell culture supernatant and serum samples were
determined with commercial kits (both Nanjing Jiancheng
Bioengineering Institute) according to the manufacturer's
instructions.
Cell culture
293T cells have high transfection efficiency and are
easy to obtain and culture in vitro (28). 293T cells were obtained from
Nanjing Synthgene Medical Technology, Co., Ltd. Cells were cultured
using DMEM (HyClone; Cytiva) supplemented with 10% FBS (Gibco;
Thermo Fisher Scientific, Inc.), 1 mM sodium pyruvate and 1%
antibiotic-antimycotic (15240-112, Invitrogen, Gibco) at 37˚C in a
humidified incubator (Thermo Fisher Scientific, Inc.) with 95%
atmosphere and 5% CO2.
Isolation of rat primary myocardial
(RPM) cells and hypoxia/reoxygenation (H/R) treatment
The ventricular tissue of 39 rats heart was excised
immediately at the end of reperfusion and cut into small pieces,
followed by 5 min digestion with 0.25% pancreatin at 37˚C three
times. After centrifugation at room temperature at 1,000 x g for 5
min, RPM cells were collected and cultured according as previously
described (29,30). To simulate MI/R injury,
FBS/glucose-free DMEM was used and cells were cultured at 37˚C in
hypoxic condition (5% CO2, 94% N2 and 1%
O2) for 4 h. Then, cells were maintained in normoxic
conditions at 37˚C (5% CO2 and 95% air) for 2 h, as
previously described (31). Cells
under normoxic conditions at 37˚C for 2 h served as the control
group.
Western blotting
RPM cells or myocardium tissue were lysed in RIPA
lysis buffer (Beyotime Institute of Biotechnology) supplemented
with 1% PMSF (cat. no. 329-98-6, Roche Applied Science) and 1%
phosphatase inhibitor (Thermo Fisher Scientific, Inc.). The
bicinchoninic acid protein assay kit was used to determine the
total protein concentration. Equal amounts of protein (20 mg) from
each sample were resolved on 10% SDS-PAGE (Bio-Rad Laboratories,
Inc.) and transferred onto polyvinylidene fluoride membrane
(Bio-Rad Laboratories, Inc.). Following blocking with 5% milk for 2
h at room temperature, the membranes were incubated with primary
antibodies against SOX9 (cat. no. ab185966; 1:1,000), NF-κB p65
(cat. no. ab239882; 1:1,000), NLRP3 (cat. no. ab263899; 1:2,000),
apoptosis-associated speck-like protein containing a caspase
recruitment domain (ASC; cat. no. ab180799; 1:5,000), caspase-1
(cat. no. ab179515; 1:1,000) and GAPDH (cat. no. ab181602;
1:10,000) overnight at 4˚C. PVDF membranes were incubated at room
temperature with horseradish peroxidase-conjugated secondary
antibody (goat anti-rabbit IgG H&L; cat. no. ab6721; 1:10,000)
for 1 h, then Amersham ECL Western Blotting Detection Reagent
(Cytiva, Amersham). The antibodies were all from Abcam. The blots
were scanned with a ChemiDoc MP system (Bio-Rad Laboratories,
Inc.). The density of each specific band was measured using ImageJ
software V1.52a (National Institutes of Health).
miRNA sequencing
The total RNA was extracted from the heart of MI/R
model and sham group rats with TRIzol reagent (Invitrogen, USA).
After construction of small RNA libraries using the NEB Next
Multiplex Small RNA Library Prep Set for Illumina (catalog #
E7330L, New England Biolabs, Inc.), index codes were added to
attribute sequences for each sample. Agilent 2100 Bioanalyzer and
DNA 1000 chip kit (Agilent, part # 5067-1504) were used to
determine the quality of the sequencing library. The length of the
library is ~135-155 bp. After PCR products were purified, 140-160
bp DNA fragments were recovered. The concentration of the desired
products was determined from the Qubit Fluorometer (Thermo Fisher
Scientific, Inc.) and used to prepare an equimolar pool of 7 pM
solution for cluster generation on an Illumina flow cell. Finally,
single-end reads were sequenced using NovaSeq 6000 SP reagent kit
(100 cycles; cat. no. 2002746; Illumina Inc.) with Illumina MiSeq
(Illumina, Inc.), as previously described (32). miRDeep2 software (github.com/rajewsky-lab/mirdeep2) was
used to predict the novel miRNAs. Then, the trimmed reads were
aligned to merged pre-miRNA databases (known pre-miRNA from miRBase
v21 plus the newly predicted pre-miRNAs) using Novoalign software
(v2.07.11) with at most one mismatch.
Analysis of differentially expressed
miRNAs
The expression of miRNAs was determined by the
number of reads per million clean tags. Differentially expressed
miRNAs were analyzed using DEGseq software v.3.16 (bioconductor.org/packages/release/bioc/html/DESeq.html).
For data analysis, differentially expressed miRNA profiles between
sham group and I/R group were compared with unpaired t-test. The
miRNAs with fold-change >1.5 and P-value <0.05(33) were defined as differentially
expressed miRNAs. Then, hierarchical clustering was performed.
miRNAs were selected to verify expression by reverse
transcription-quantitative (RT-q)PCR.
RT-qPCR
Total RNA was extracted from RPM cells or heart
tissue using TRIzol (Invitrogen, USA) according to the
manufacturer's instructions for the analysis of miRNA expression.
cDNA was synthesized using a TaqMan™ Advanced miRNA cDNA Synthesis
kit (Thermo Fisher Scientific, Inc.) following the manufacturer's
protocol. RT-qPCR was performed using TaqMan™ Fast Advanced Master
Mix (Thermo Fisher Scientific, Inc.). The thermocycling conditions
were as follows: Initial denaturation for 10 min at 95˚C, followed
by 40 cycles of 2 sec (95˚C), 20 sec (60˚C) and 10 sec (70˚C), as
previously described (34).
Quantitative measurements were determined via the 2-ΔΔCq
method (31). β-actin and U6 snRNA
were used as internal controls for RNA and miRNA, respectively. The
primers were designed with the miRNA Design V1.01 (Vazyme Biotech
Co., Ltd.) with a universal reverse primer sequence. Primer
sequences were as follows: miR-328a-3p: Forward (F),
5'-GCTGGCCCTCTCTGCCC-3' and reverse (R),
5'-AGTGCAGGGTCCGAGGTATT-3'; miR-128-3p: F,
5'-CGCGTCACAGTGAACCGGT-3' and R, 5'-AGTGCAGGGTCCGAGGTATT-3';
miR-148a-3p: F, 5'-GCGCGTCAGTGCACTACAGA-3' and R,
5'-AGTGCAGGGTCCGAGGTATT-3'; miR-676: F, 5'-CGCCGTCCTGAGCTTGTC-3'
and R, 5'-AGTGCAGGGTCCGAGGTATT-3'; miR-30d-5p, F:
5'-TGTAAACATCCCCGACTGGA-3' and R, 5'-GAACATGTCTGCGTATCTC-3';
miR-30c-5p: F, 5'-GCGCGTGTAAACATCCTACACT-3' and R,
5'-AGTGCAGGGTCCGAGGTATT-3'; SOX9, F: 5'-GTCGGTGAAGAATGGGCAAG-3' and
R, 5'-GACCCTGAGATTGCCCGGA-3'; β-actin: F,
5'-CGCGAGTACAACCTTCTTGC-3' and R, 5'-CGTCATCCATGGCGAACTGG-3' and
U6: F, 5'-CTCGCTTCGGCAGCACATATACT-3' and R,
5'-ACGCTTCACGAATTTGCGTGTC-3'.
Luciferase assay
For reporter assay, pMIR-SOX9-3'-UTR-wild-type (WT)
and pMIR-SOX9-3'-UTR-mutant (MUT) luciferase reporter plasmids
(Nanjing Synthgene Medical Technology, Co., Ltd.) were transfected
into 293T cells using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). The transfected cells were treated with miR-30c
mimics (3'-UCACAUCCUACAAAUGU-5', 100 pmol) or miR-NC (sequence:
5'-CUAACGCAUGCACAGUCGUACG-3'), both from Nanjing Synthgene Medical
Technology, Co., Ltd. Luciferase activity was measured 48 h after
miR-30c mimics transfection using a Dual-Luciferase Reporter Assay
(Promega Corporation). Data were normalized to Renilla
luciferase activity.
Cell transfection
For in vitro transfection, 100 nM miR-30c-5p
mimic (sequence: 5'-UGUAAACAUCCUACACUCUCAGC-3'), miR-30c-5p
inhibitor (sequence: 5'-GCUGAGAGUGUAGGAUGUUUACU-3'), miR-30c-5p
mimics control (sequence: 5'-CUAACGCAUGCACAGUCGUACG-3') (35), miR-30c-5p inhibitor control
(sequence: 5'-CAGUACUUUUGUGUAGUACAA-3') and, which were from
Nanjing Synthgene Medical Technology Co., Ltd, were transfected
into RPM cells using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) for 48 h at 37˚C according to the manufacturer's
instructions. miR-30c mimic was co-transfected with 5 µg plasmid
pcDNA3.1-SOX9-Flag (Nanjing Synthgene Medical Technology, Co.,
Ltd.). A total of 5 µg plasmid vector was transferred. For in
vivo transfection, 5 µg miR-30c mimic, miR-30c inhibitor or
controls was transferred into the left ventricle anterior wall of
rat myocardium by 8 µl Entranster (Nanjing Synthgene Medical
Technology Co., Ltd.). After injection, the chest was closed and
the rat was permitted to recover. I/R was performed 48 h later.
Cell viability assay
RPM cells were harvested at 48 h after transfection.
Then, 200 µl 0.5 mg/ml MTT solution (Nanjing Synthgene Medical
Technology Co., Ltd.) was added to each group and incubated for 4 h
at 37˚C. DMSO was used to dissolve purple formazan. Absorbance was
detected at a wavelength of 450 nm, as previously described
(30).
Statistical analysis
Graphpad Prism 8.0 (GraphPad Software, Inc.) and
SPSS 19.0 (IBM Corp.) were used for statistical analysis. Data are
presented as the mean ± SD of three independent experiments.
Two-tailed unpaired Student's t-test was used to compare two
groups. One-way ANOVA followed by Tukey's post hoc test was used to
compare >2 groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
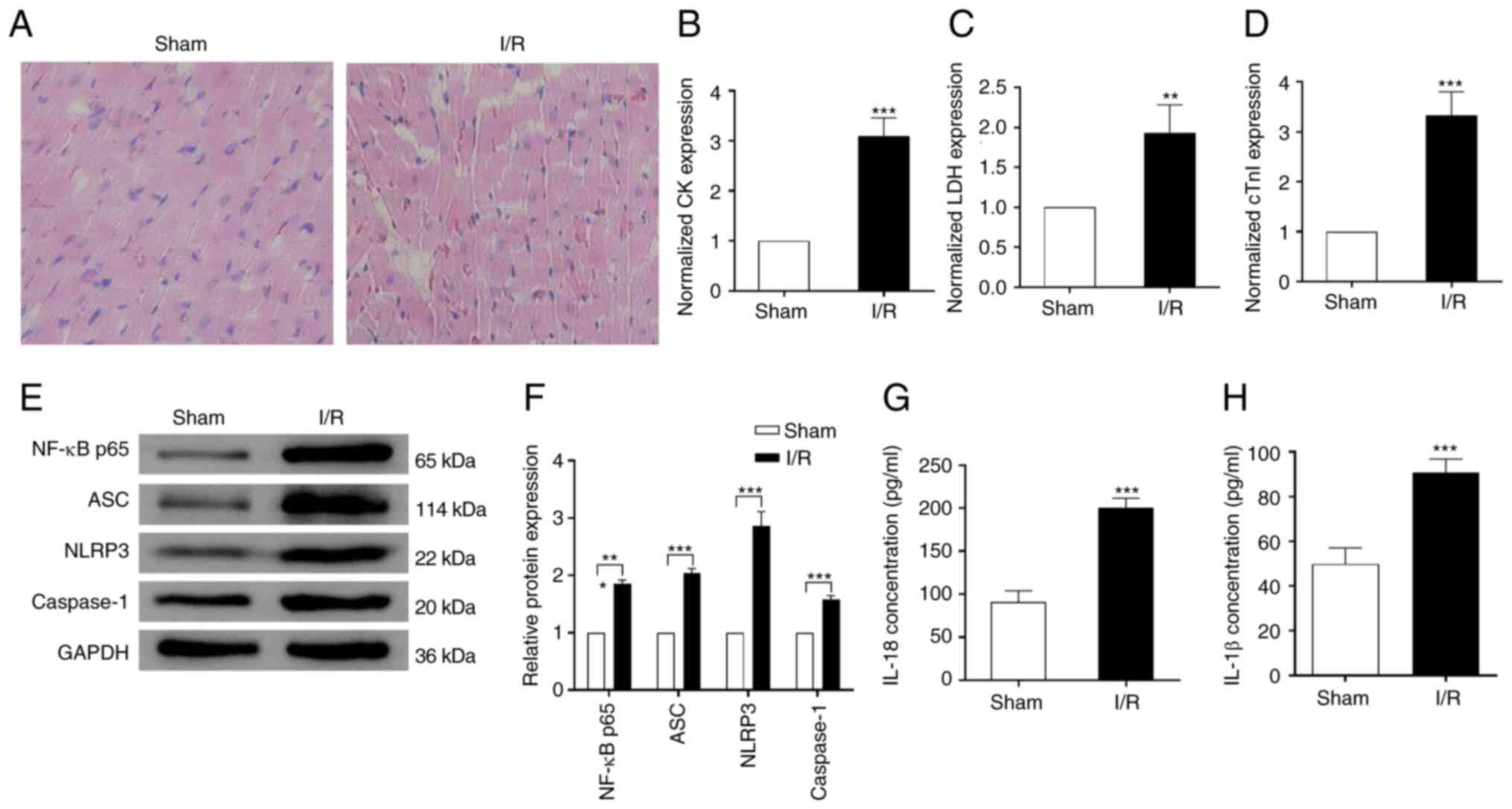
MI/R injury induces pyroptosis and
inflammation in vivo
To investigate the effect of MI/R injury on
myocardium pyroptosis and inflammation, a rat model of MI/R injury
was established. Infiltrating inflammatory cells, myocardial cell
swelling, degeneration, cardiac necrosis and loss of transverse
striations were observed in the heart subjected to I/R (Fig. 1A). Compared with the sham group,
the concentrations of serum CK, LDH, and cTnI were significantly
increased in the I/R group (Fig.
1B-D), which suggested successful establishment of the MI/R
injury model. Western blotting showed that the expression of
pyroptosis-associated protein markers, including ASC, NLRP3 and
caspase-1 were significantly upregulated in the rats subjected to
I/R injury compared with the sham group. Moreover, the levels of
inflammatory marker NF-κB p65 also increased almost 2-fold in the
I/R compared with the sham group (Fig.
1E-F). Consistently, following I/R treatment, the expression
levels of IL-18 and IL-1β were significantly increased ~2-fold
compared with the sham group (Fig.
1G-H). These results indicated that MI/R injury induced
myocardium pyroptosis and inflammation in vivo.
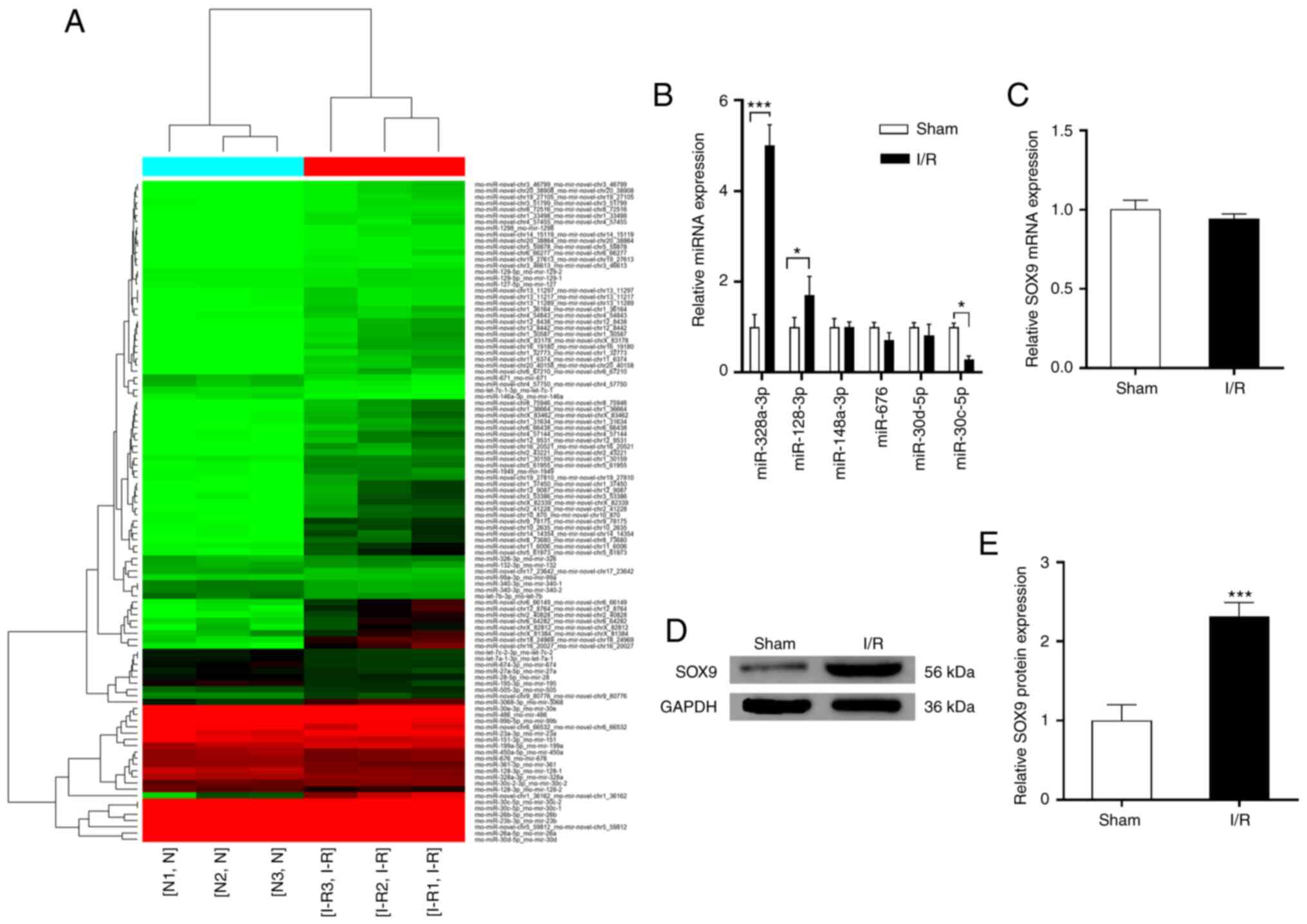
miR-30c is downregulated and SOX9 is
upregulated after I/R treatment in vivo
MI/R induced significant changes in expression of
candidate miRNAs, including miR-328a-3p, miR-128-3p, miR-148-3p,
miR-676, miR-30d-5p and miR-30c-5p (Fig. 2A-B). Expression levels of
miR-328a-3p and miR-128-3p were significantly increased, while
expression of miR-30c-5p significantly decreased following MI/R
injury (Fig. 2B). In the present
study, RNA sequencing showed that fold-change of expression of
miR-30c-5p was 1.52 (P=0.0176). The reasons for choosing miR-30c-5p
are its high expression following I/R (TagCount, 7,078 vs. 10,736).
miRNA-30c-5p has two precursors, MI0000866 and MI0000871, which are
slightly different judging from the tags detected in miRNA
sequencing. Therefore, two expression data are given. Moreover, the
expression levels calculated with the two precursors is almost the
same, therefore, the two miRNAs are both miR-30c-5p (Table SI). Expression of SOX9 in rats was
detected by western blotting and RT-qPCR. There was significant
upregulation of SOX9 expression in the MI/R group at the protein
level but no significant change at the mRNA level (Fig. 2C and D).
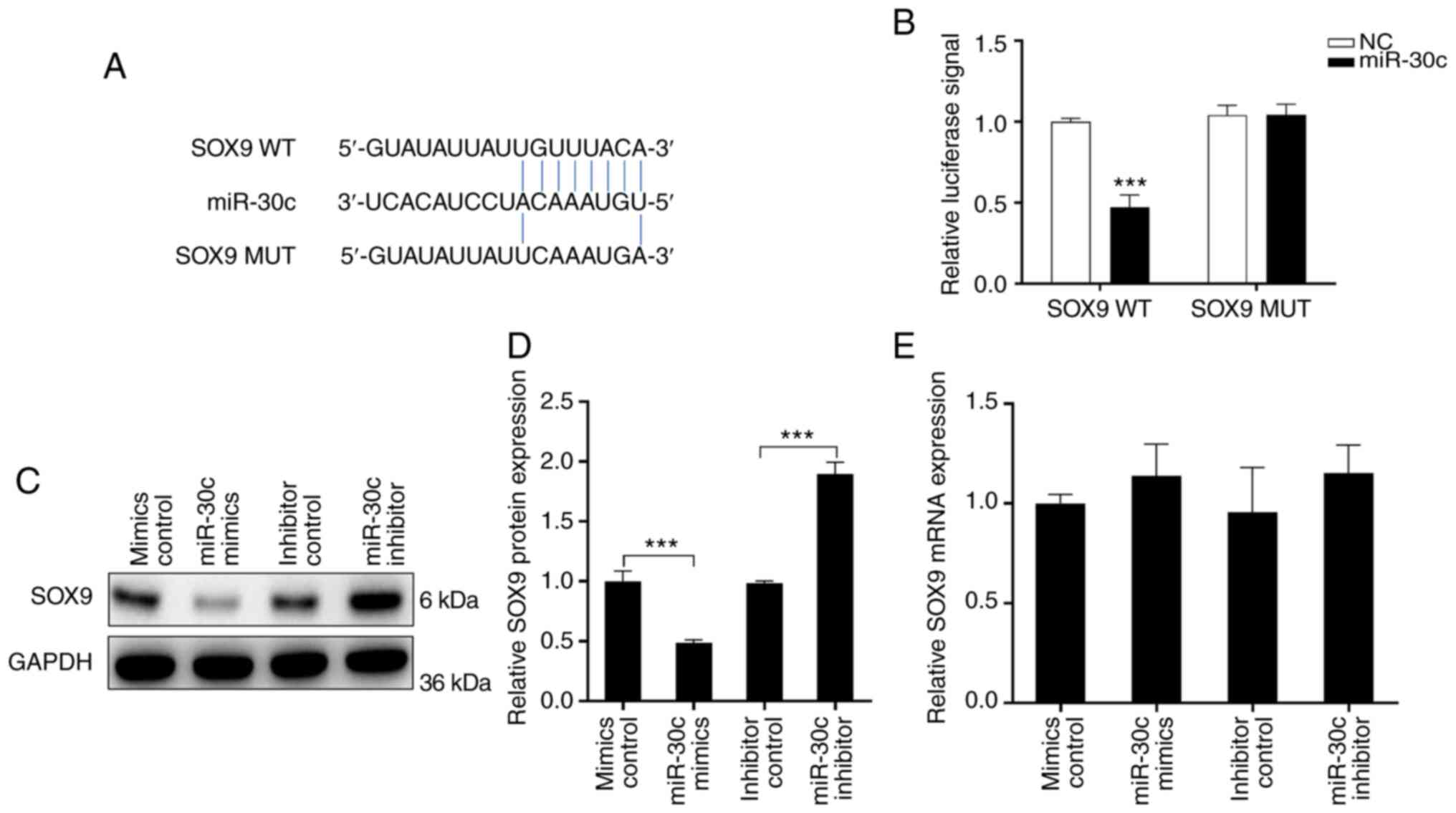
SOX9 is a target of miR-30c
To confirm the association between miR-30c and SOX9,
bioinformatics analysis was performed, which predicted a binding
site for miR-30c in the 3'-UTR of WT SOX9 (Fig. 3A). Luciferase activity was
significantly reduced by miR-30c transfection in with SOX9 WT, but
not MUT UTR (Fig. 3B).
Overexpression of miR-30c in RPM cells significantly downregulated
the protein expression of SOX9. Conversely, following treatment
with miR-30c inhibitor, the protein expression of SOX9 was
increased ~2-fold. However, compared with the control, the mRNA
expression of SOX9 was not significantly altered following miR-30c
mimics or inhibitor transfection (Fig.
3C and D). These data
suggested that SOX9 was a direct target of miR-30c.
miR-30c inhibits cell pyroptosis in
vitro
To investigate the function of miR-30c, H/R exposed
cells were transfected with miR-30c mimics or inhibitor. Expression
of miR-30c was notably decreased following H/R (Fig. 4A) but increased ~200-fold in RPM
cells following transfection with miR-30c mimics. Conversely,
expression decreased following transfection with miR-30c inhibitor.
After H/R treatment, the protein levels of NF-κB p65, ASC, NLRP3
and caspase-1 in the RPM cells were significantly upregulated.
Compared with the H/R group, miR-30c mimics treatment significantly
inhibited expression of these proteins. However, inhibition of
miR-30c restored the expression of these proteins (Fig. 4B-C). Effects of miR-30c on
expression of pro-inflammatory factors IL-18 and IL-1β secreted by
RPM cells following H/R exposure was determined using ELISA.
Overexpression of miR-30c markedly decreased IL-18 and IL-1β levels
in the H/R exposed cells, while these levels were further increased
after miR-30c inhibitor transfection (Fig. 4D-E). H/R significantly inhibited
primary myocardial cell proliferation. miR-30c mimics treatment
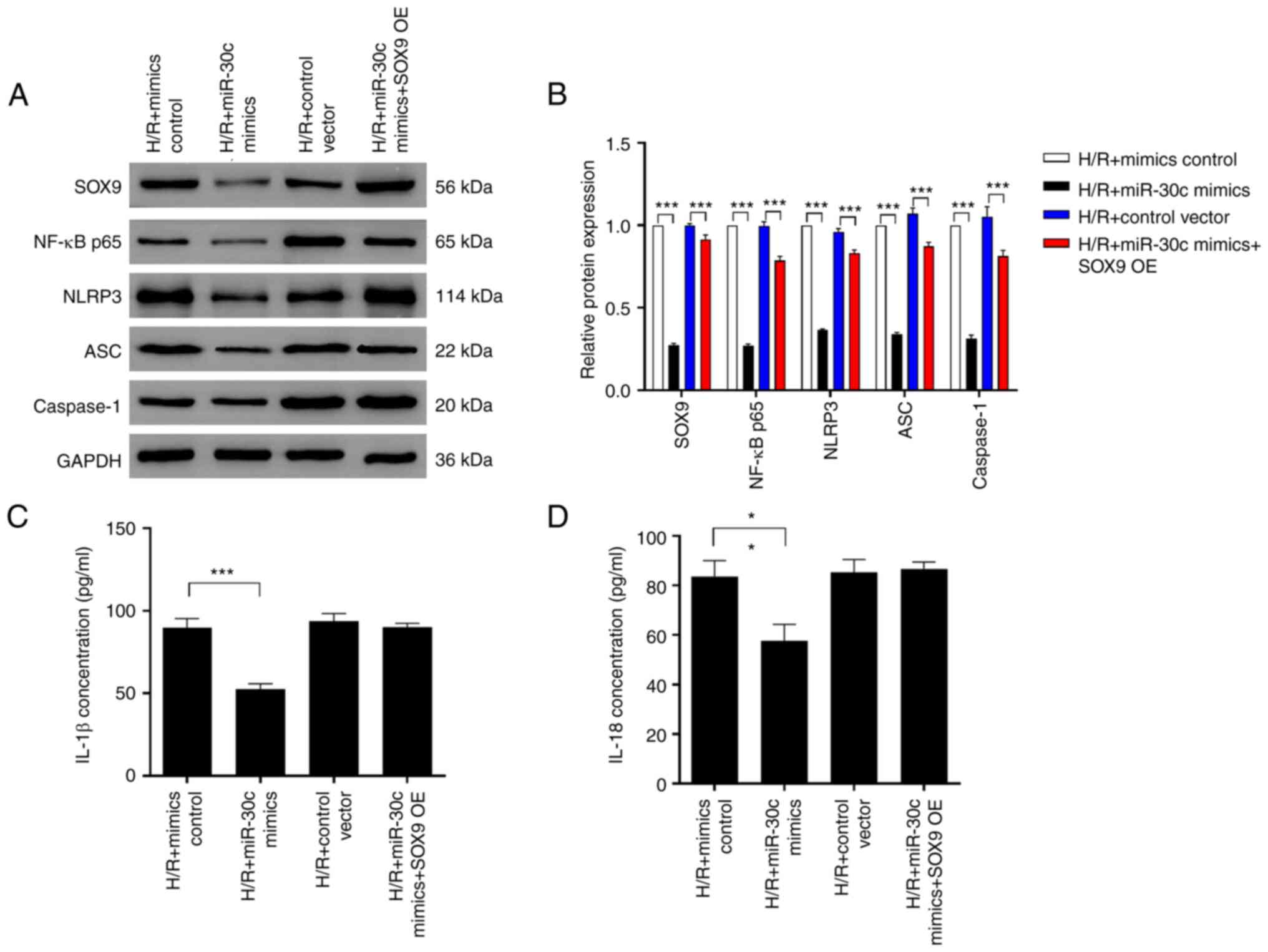
reversed the effect of H/R on cell viability (Fig. 4F). Overexpression of miR-30c
restored downregulation of SOX9 caused by H/R (Fig. 5A-B). Moreover, overexpression of
SOX9 before miR-30c mimics transfection could abolish effects of
SOX9. Consistent with these results, the pyroptosis protein markers
showed a similar trend (Fig.
5A-D). Expression of IL-1β and IL-18 showed no difference
between the H/R + mimics control and H/R + miR-30c mimics + SOX9
overexpression group (Fig.
5B).
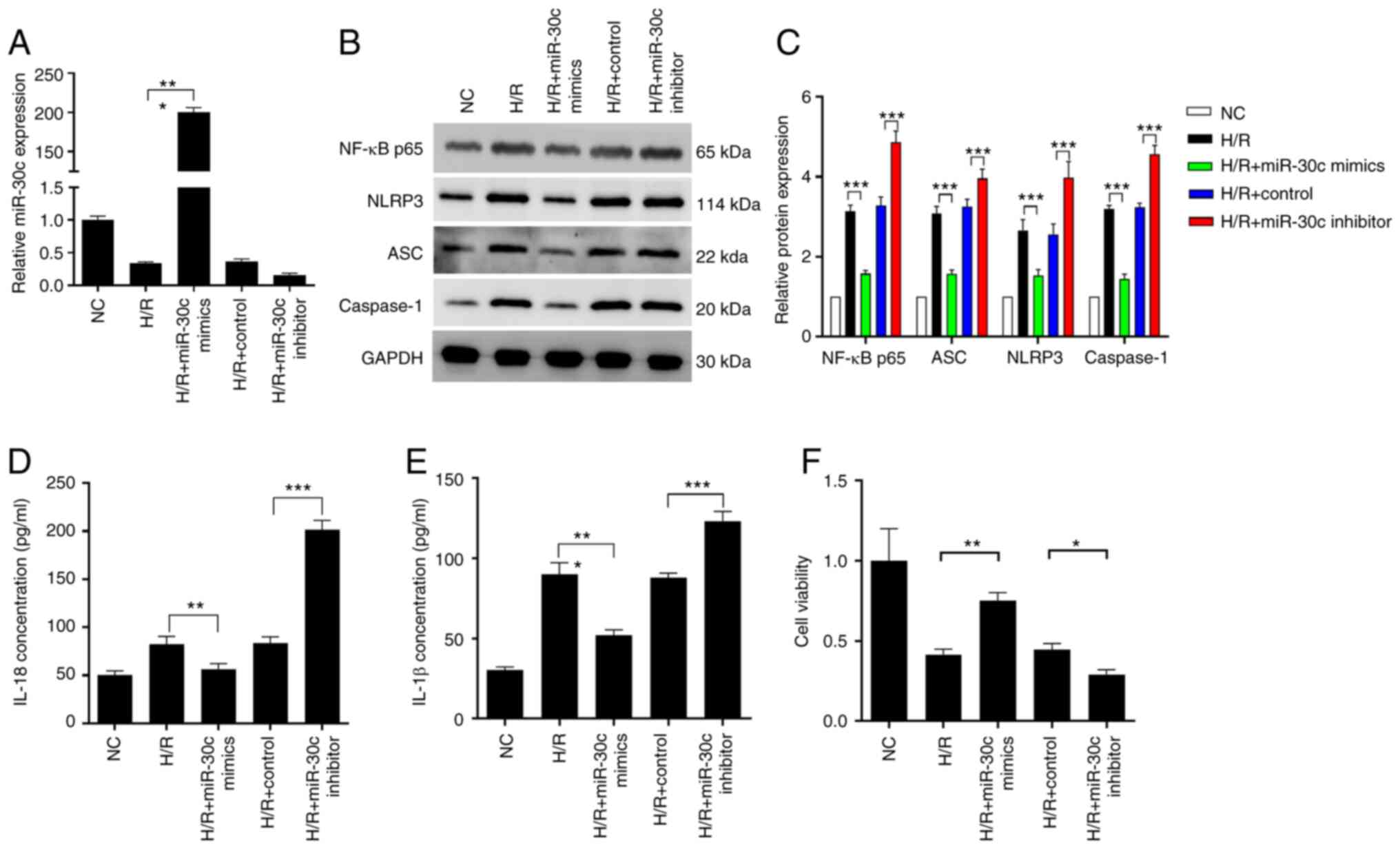 | Figure 4miR-30c inhibits cardiomyocyte
pyroptosis under H/R in vitro. (A) Expression of miR-30c was
assessed by reverse transcription-quantitative PCR following
miR-30c mimics, miR-30c inhibitor and control transfection under
H/R exposure. (B) Protein expression of (C) NF-κB p65, ASC, NLRP3
and caspase-1 was analyzed by western blotting. Levels of (D) IL-8
and (E) IL-1β were assessed by ELISA after miR-30c mimics, miR-30c
inhibitor and control transfection under H/R exposure. (F)
Viability of H/R-exposed cells after transfection with miR-30c
mimics, miR-30c inhibitor and control. Data are expressed as the
mean ± SD (n=3). *P<0.05, **P<0.01,
***P<0.001. NC, negative control; miR, microRNA; H/R,
hypoxia/reoxygenation; ASC, apoptosis-associated speck-like protein
containing a caspase recruitment domain. |
SOX9/miR-30c axis regulates MI/R
injury in vivo
To confirm the effect of the SOX9/miR-30c axis in
vivo, MI/R injury rats were treated with miR-30c mimics and
miR-30c inhibitor. Compared with the sham group, more infiltrating
inflammatory cells, myocardial cell swelling, degeneration, cardiac
necrosis and loss of transverse striations were observed in the I/R
and I/R + miR-30 inhibitor group. Conversely, it was observed in
the present study that the I/R injured heart showed obvious
integrity of the myocardial membrane, a normal myofibrillar
structure with striations, branched appearance and continuity with
adjacent myofibrils after miR-30c mimics treatment (Fig. 6A) (36). According to the results of western
blotting, there was notable upregulation in expression of NF-κB
p65, ASC, NLRP3, SOX9 and caspase-1 when the rats were subjected to
I/R. Elevated expression levels of these proteins were further
upregulated by the miR-30c inhibitor transfection. However, the
upregulated expression levels of these proteins in the heart of I/R
injury rats were restored by miR-30c mimics treatment. (Fig. 6B). Consistently, the levels of
cTnI, CK and LDH, key diagnostic markers of MI/R injury (30), were increased in I/R-treated rats
compared with the sham group. There was a significant reduction in
all these markers following miR-30c mimics transfection. However,
following miR-30c inhibitor treatment, the levels of these markers
were not significantly decreased (Fig.
6D-F). Similarly, miR-30c mimics decreased serum levels of
IL-18 and IL-1β, while miR-30c inhibitor did not significantly
affect production of IL-18 and IL-1β (Fig. 6G and H).
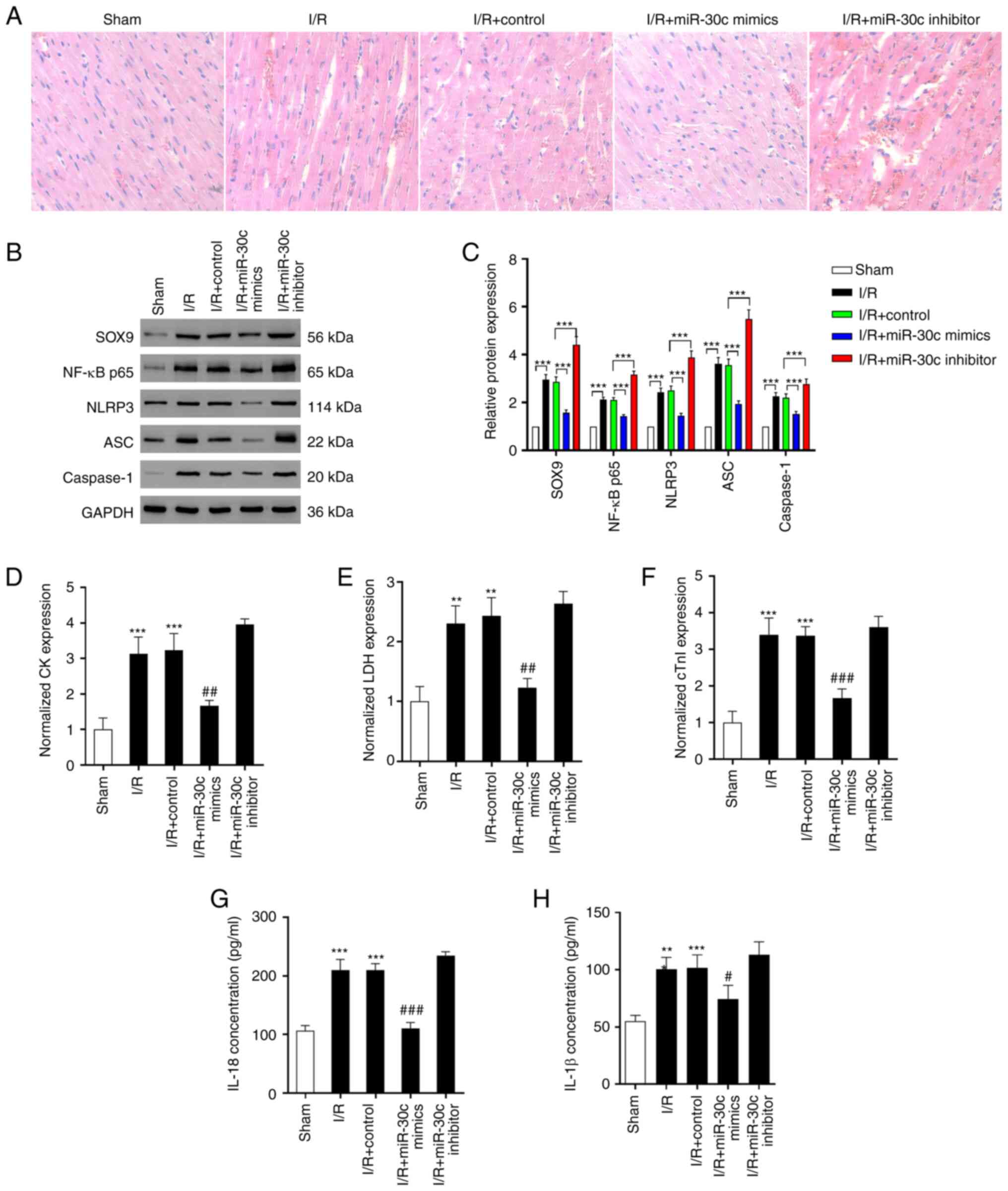 | Figure 6SOX9/miR-30c axis regulates cardiac
I/R injury in vivo. (A) Hematoxylin and eosin staining of
rat tissue (400x magnification). (B) Protein expression of (C)
NF-κB p65, ASC, NLRP3, caspase-1 and SOX9 in rat heart tissue.
Serum levels of (D) CK, (E) LDH and (F) cTnI were assessed by
ELISA. Expression levels of (G) IL-8 and (H) IL-1β were assessed by
ELISA. Data are expressed as the mean ± SD (n=3).
**P<0.01, ***P<0.001 vs. Sham;
#P<0.05, ##P<0.01,
###P<0.001 vs. I/R + control. CK, creatine kinase;
LDH, lactate dehydrogenase; cTn, cardiac troponin; ASC,
apoptosis-associated speck-like protein containing a caspase
recruitment domain; SOX9, SRY-related high mobility group-box gene
9; I/R, ischemia/reperfusion. |
Discussion
The present study aimed to investigate the function
and the underlying mechanisms of miRNAs on I/R injury-induced
pyroptosis. miR-30c was downregulated, while the expression of SOX9
was upregulated in the MI/R rat model. Overexpression of miR-30c
inhibited pyroptosis both in vivo and in vitro.
Furthermore, miR-30c negatively regulated SOX9 expression by
binding its 3'UTR.
In the present study, NLRP3 was activated following
MI/R injury, which initiates adaptor protein (ASC) cleavage of
caspase-1 and IL-1β and IL-18 generation (37). There was a high level of pyroptosis
in rats subjected to MI/R or cells treated with H/R. I/R causes
cell death, including apoptosis, autophagy, necrosis and
pyroptosis. A number of studies have demonstrated that pyroptosis
is triggered by NLRP3, AIM2-like receptor proteins and tripartite
motif-containing protein and other inflammasomes (38,39).
Following the stimulation of these inflammasomes, downstream
inflammatory caspase-1 or caspase-11 is cleaved, leading to
secretion of pro-inflammatory cytokines IL-1β and IL-18(40). This process is involved in various
types of heart disease, including MI/R injury, myocardial
infarction and heart failure (41). For examples, inflammasomes
activated by I/R lead to IL-1β production in the heart (42). The accumulation of inflammasomes
ASC, cryopyrin and caspase-1 has been observed in an acute
myocardial infarction mouse model: Following cryopyrin inhibitor
treatment, the formation of inflammasome and infarct size are
limited (43). Sandanger et
al (44) found that compared
with WT hearts, a marked improvement of cardiac function and
decreased hypoxic damage were present in the hearts of
NLRP3-deficient mice subjected to I/R. Therefore, targeting
components of pyroptosis may decrease cardiac I/R injury.
miRNAs modulate target genes by binding the 3'UTR of
specific mRNAs and may serve a role in the regulation of
cardiovascular disease (45). In a
rat in vivo I/R model, upregulating miR-30c-5p decreases
myocardial injury, histopathological changes and apoptosis
(46). The reasons for
investigating miR-30c-5p are its high expression following I/R and
its target gene SOX9 is a transcription factor of the SRY family,
which is involved in various cell processes, including oxidation
and tumor growth (47). Studies
(19,24) indicate that SOX9 may be involved in
the onset of MI/R injury, although the underlying molecular
mechanism remains unknown.
Consistent with the present results, Sun et
al (48) found that in a rat
MI/R model with LAD ligation, miR-30c-5p expression is
downregulated. In addition, overexpression of miR-30c inhibits
apoptosis, oxidative stress and inflammation both in vivo
and in vitro (48). In
addition, overexpression of miR-30c-5p-induced protective effects
are associated with its target gene Bach1 and subsequent activation
of Nrf2(48). However, another
study found that miR-30c expression is increased in a rat model of
MI/R injury and overexpression of miR-30c-5p promotes MI/R injury
by activating NF-κB pathway and targeting sirtuin 1(49). In the aforementioned study
(49), LAD was ligated for 30 min
before reperfusion for 2 h, while in the present study, following
30 min LAD ligation, hearts were reperfused for 30 min before
sample collection. Therefore, both the reperfusion and the sample
collection timepoint may be key for miRNA expression profile.
miR-30c-5p can target different signaling pathways during MI/R
injury (48,49). The present study reported the
association between miR-30c, MI/R injury and pyroptosis. The
present study demonstrated that under MI/R injury, SOX9 expression
was significantly increased and miR-30c was notably downregulated
in vivo. miR-30c directly targeted SOX9 by binding the 3'UTR
of SOX9. There was no significant difference in mRNA expression of
SOX9 however, the protein expression of SOX9 was significantly
increased by I/R. It was hypothesized that miR-30c-5p in
cardiomyocytes bound to SOX9 mRNA and inhibited SOX9 protein
expression but did not directly degrade SOX9 mRNA. This
translational suppression mechanism has been discussed by
Huntzinger and Izaurralde (50).
Overexpression of miR-30c in vitro suppressed H/R-induced
pyroptosis. Conversely, miR-30c inhibitor treatment promoted the
effect of H/R treatment. In addition, overexpression of SOX9
abolished the effect of miR-30c on pyroptosis. Following
overexpression of miR-30c in vivo, pyroptosis was suppressed
and MI/R injury was alleviated. This suggested that regulation of
miR-30c expression may contribute to pyroptosis and may serve as a
therapeutic target to decrease MI/R injury.
miR-30c decreases the production of reactive oxygen
species (ROS) (51,52), which are key for tissue damage and
myocardial protection. Furthermore, it is widely reported that
overactivation of ROS pathway signaling increases pyroptosis
(53,54). During MI/R injury, excessive
production ROS and increased pyroptosis of cardiomyocytes may be
induced by decreased expression of miR-30c.
In summary, miR-30c was notably downregulated in
rats subjected to I/R and directly targeted SOX9. Moreover, miR-30c
may serve as a suppressor of cell pyroptosis to decrease
I/R-induced heart disease. In the future, gene therapy targeting
miR-30c-5p overexpression and SOX9 knockdown may serve a role in
MI/R injury clinically.
Supplementary Material
miRNA expression for I-R_vs_N (based
on TagCount_ALL_Isoform data).
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National Natural
Science Foundation of China (grant no. 81860062) and Guizhou
Science and Technology Planning Project [grant no.
QianKeHeJiChu(2018)1195].
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available in the Gene Expression Omnibus
repository: ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE221780.
Authors' contributions
JN, WZ, SY and SC performed the experiments, wrote
the manuscript and constructed figures. HW and TY conceived and
designed the experiments and provided reagents. JN and HW confirm
the authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Animal experiments were approved [approval no.
KLLY(A)-2019-008] by Zunyi Medical University Committee (Guizhou,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hausenloy DJ and Yellon DM: Ischaemic
conditioning and reperfusion injury. Nat Rev Cardiol. 13:193–209.
2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ibanez B, Heusch G, Ovize M and Van de
Werf F: Evolving therapies for myocardial ischemia/reperfusion
injury. J Am Coll Cardiol. 65:1454–1471. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Eltzschig HK and Eckle T: Ischemia and
reperfusion-from mechanism to translation. Nat Med. 17:1391–1401.
2011.PubMed/NCBI View
Article : Google Scholar
|
|
4
|
Han J, Wang D, Ye L, Li P, Hao W, Chen X,
Ma J, Wang B, Shang J, Li D and Zheng Q: Rosmarinic acid protects
against inflammation and cardiomyocyte apoptosis during myocardial
ischemia/reperfusion injury by activating peroxisome
proliferator-activated receptor gamma. Front Pharmacol.
8(456)2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Makkos A, Ágg B, Petrovich B, Varga ZV,
Görbe A and Ferdinandy P: Systematic review and network analysis of
microRNAs involved in cardioprotection against myocardial
ischemia/reperfusion injury and infarction: Involvement of redox
signalling. Free Radic Biol Med. 172:237–251. 2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Jayawardena E, Medzikovic L, Ruffenach G
and Eghbali M: Role of miRNA-1 and miRNA-21 in acute myocardial
ischemia-reperfusion injury and their potential as therapeutic
strategy. Int J Mol Sci. 23(1512)2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Marinescu MC, Lazar AL, Marta MM, Cozma A
and Catana CS: Non-coding RNAs: Prevention, diagnosis, and
treatment in myocardial ischemia-reperfusion injury. Int J Mol Sci.
23(2728)2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lorenzen JM, Batkai S and Thum T:
Regulation of cardiac and renal ischemia-reperfusion injury by
microRNAs. Free Radic Biol Med. 64:78–84. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hinkel R, Penzkofer D, Zuhlke S, Fischer
A, Husada W, Xu QF, Baloch E, van Rooij E, Zeiher AM, Kupatt C and
Dimmeler S: Inhibition of microRNA-92a protects against
ischemia/reperfusion injury in a large-animal model. Circulation.
128:1066–1075. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hullinger TG, Montgomery RL, Seto AG,
Dickinson BA, Semus HM, Lynch JM, Dalby CM, Robinson K, Stack C,
Latimer PA, et al: Inhibition of miR-15 protects against cardiac
ischemic injury. Circ Res. 110:71–81. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wang JX, Zhang XJ, Li Q, Wang K, Wang Y,
Jiao JQ, Feng C, Teng S, Zhou LY, Gong Y, et al: MicroRNA-103/107
regulate programmed necrosis and myocardial ischemia/reperfusion
injury through targeting FADD. Circ Res. 117:352–363.
2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wang X, Zhang X, Ren XP, Chen J, Liu H,
Yang J, Medvedovic M, Hu Z and Fan GC: MicroRNA-494 targeting both
proapoptotic and antiapoptotic proteins protects against
ischemia/reperfusion-induced cardiac injury. Circulation.
122:1308–1318. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
He Y, Cai Y, Sun T, Zhang L, Irwin MG, Xu
A and Xia Z: MicroRNA-503 exacerbates myocardial
ischemia/reperfusion injury via inhibiting PI3K/Akt- and
STAT3-Dependent prosurvival signaling pathways. Oxid Med Cell
Longev. 2022(3449739)2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lee TL, Lai TC, Lin SR, Lin SW, Chen YC,
Pu CM, Lee IT, Tsai JS, Lee CW and Chen YL: Conditioned medium from
adipose-derived stem cells attenuates ischemia/reperfusion-induced
cardiac injury through the microRNA-221/222/PUMA/ETS-1 pathway.
Theranostics. 11:3131–3149. 2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Song R, Dasgupta C, Mulder C and Zhang L:
MicroRNA-210 controls mitochondrial metabolism and protects heart
function in myocardial infarction. Circulation. 145:1140–1153.
2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Shah S, Esdaille CJ, Bhattacharjee M, Kan
HM and Laurencin CT: The synthetic artificial stem cell (SASC):
Shifting the paradigm of cell therapy in regenerative engineering.
Proc Natl Acad Sci USA. 119:2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kubo Y, Beckmann R, Fragoulis A, Conrads
C, Pavanram P, Nebelung S, Wolf M, Wruck CJ, Jahr H and Pufe T:
Nrf2/ARE signaling directly regulates SOX9 to potentially alter
age-dependent cartilage degeneration. Antioxidants (Basel).
11(263)2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Gong X, Li Y, He Y and Zhou F:
USP7-SOX9-miR-96-5p-NLRP3 network regulates myocardial injury and
cardiomyocyte pyroptosis in sepsis. Hum Gene Ther. 33:1073–1090.
2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Rui L, Liu R, Jiang H and Liu K: Sox9
Promotes cardiomyocyte apoptosis after acute myocardial infarction
by promoting miR-223-3p and inhibiting MEF2C. Mol Biotechnol.
64:902–913. 2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Fan XD, Zheng HB, Fan XS and Lu S:
Increase of SOX9 promotes hepatic ischemia/reperfusion (IR) injury
by activating TGF-β1. Biochem Biophys Res Commun. 503:215–221.
2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yang B, Nie Y, Wang L and Xiong W:
Flurbiprofen axetil protects against cerebral ischemia/reperfusion
injury via regulating miR-30c-5p and SOX9. Chem Biol Drug Des.
99:197–205. 2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Scharf GM, Kilian K, Cordero J, Wang Y,
Grund A, Hofmann M, Froese N, Wang X, Kispert A, Kist R, et al:
Inactivation of Sox9 in fibroblasts reduces cardiac fibrosis and
inflammation. JCI Insight. 5(e126721)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Schauer A, Adams V, Poitz DM, Barthel P,
Joachim D, Friedrich J, Linke A and Augstein A: Loss of Sox9 in
cardiomyocytes delays the onset of cardiac hypertrophy and
fibrosis. Int J Cardiol. 282:68–75. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lacraz GPA, Junker JP, Gladka MM, Molenaar
B, Scholman KT, Vigil-Garcia M, Versteeg D, de Ruiter H, Vermunt
MW, Creyghton MP, et al: Tomo-Seq Identifies SOX9 as a key
regulator of cardiac fibrosis during ischemic injury. Circulation.
136:1396–1409. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Cheng N, Li L, Wu Y, Wang M, Yang M, Wei S
and Wang R: microRNA-30e up-regulation alleviates myocardial
ischemia-reperfusion injury and promotes ventricular remodeling via
SOX9 repression. Mol Immunol. 130:96–103. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Li J, Wei Y, Zhou J, Zou H, Ma L, Liu C,
Xiao Z, Liu X, Tan X, Yu T and Cao S: Activation of locus
coeruleus-spinal cord noradrenergic neurons alleviates neuropathic
pain in mice via reducing neuroinflammation from astrocytes and
microglia in spinal dorsal horn. J Neuroinflammation.
19(123)2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Health NIo: Guide for the care and use of
laboratory animals. National Academies, 1985.
|
|
28
|
Douglas KL, Piccirillo CA and Tabrizian M:
Cell line-dependent internalization pathways and intracellular
trafficking determine transfection efficiency of nanoparticle
vectors. Eur J Pharm Biopharm. 68:676–687. 2008.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wang K, Liu CY, Zhou LY, Wang JX, Wang M,
Zhao B, Zhao WK, Xu SJ, Fan LH, Zhang XJ, et al: APF lncRNA
regulates autophagy and myocardial infarction by targeting
miR-188-3p. Nat Commun. 6(6779)2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Cao S, Liu Y, Sun W, Zhao L, Zhang L, Liu
X and Yu T: Genome-Wide expression profiling of
anoxia/reoxygenation in rat cardiomyocytes uncovers the role of
MitoKATP in energy homeostasis. Oxid Med Cell Longev.
2015(756576)2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Liu W, Huang L, Liu X, Zhu L, Gu Y, Tian
W, Zhang L, Deng S and Yu T: Urocortin I protects against
myocardial ischemia/reperfusion injury by sustaining respiratory
function and cardiolipin content via mitochondrial ATP-Sensitive
potassium channel opening. Oxid Med Cell Longev.
2022(7929784)2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wang QL, Li TT, Fang CL and Zhang BL:
Bioinformatics analysis of the wheel treadmill test on motor
function recovery after spinal cord injury. Ibrain. 7:265–277.
2021.
|
|
33
|
Cao S, Zhang D, Yuan J, Liu C, Zhou W,
Zhang L, Yu S, Qin B, Li Y and Deng W: MicroRNA And circular RNA
expression in affected skin of patients with postherpetic
neuralgia. J Pain Res. 12:2905–2913. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Deng W, Wang Y, Long X, Zhao R, Wang Z,
Liu Z, Cao S and Shi B: miR-21 Reduces Hydrogen Peroxide-Induced
Apoptosis in c-kit+ Cardiac stem cells in vitro through
PTEN/PI3K/Akt signaling. Oxid Med Cell Longev.
2016(5389181)2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Cui L, Yu M and Cui X: MiR-30c-5p/ROCK2
axis regulates cell proliferation, apoptosis and EMT via the
PI3K/AKT signaling pathway in HG-induced HK-2 cells. Open Life Sci.
15:959–970. 2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Chang X, Zhang K, Zhou R, Luo F, Zhu L,
Gao J, He H, Wei T, Yan T and Ma C: Cardioprotective effects of
salidroside on myocardial ischemia-reperfusion injury in coronary
artery occlusion-induced rats and Langendorff-perfused rat hearts.
Int J Cardiol. 215:532–544. 2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Yajima N, Takahashi M, Morimoto H, Shiba
Y, Takahashi Y, Masumoto J, Ise H, Sagara J, Nakayama J, Taniguchi
S and Ikeda U: Critical role of bone marrow apoptosis-associated
speck-like protein, an inflammasome adaptor molecule, in neointimal
formation after vascular injury in mice. Circulation.
117:3079–3087. 2008.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Xiao L, Magupalli VG and Wu H: Cryo-EM
structures of the active NLRP3 inflammasome disc. Nature.
613:595–600. 2023.PubMed/NCBI View Article : Google Scholar
|
|
39
|
McKee CM and Coll RC: NLRP3 inflammasome
priming: A riddle wrapped in a mystery inside an enigma. J Leukoc
Biol. 108:937–952. 2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Jorgensen I, Rayamajhi M and Miao EA:
Programmed cell death as a defence against infection. Nat Rev
Immunol. 17:151–164. 2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Yu Y, Shi H, Yu Y, Liu M, Li M, Liu X,
Wang Y and Chen R: Inhibition of calpain alleviates coxsackievirus
B3-induced myocarditis through suppressing the canonical NLRP3
inflammasome/caspase-1-mediated and noncanonical
caspase-11-mediated pyroptosis pathways. Am J Transl Res.
12:1954–1964. 2020.PubMed/NCBI
|
|
42
|
Kawaguchi M, Takahashi M, Hata T, Kashima
Y, Usui F, Morimoto H, Izawa A, Takahashi Y, Masumoto J, Koyama J,
et al: Inflammasome activation of cardiac fibroblasts is essential
for myocardial ischemia/reperfusion injury. Circulation.
123:594–604. 2011.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Mezzaroma E, Toldo S, Farkas D, Seropian
IM, Van Tassell BW, Salloum FN, Kannan HR, Menna AC, Voelkel NF and
Abbate A: The inflammasome promotes adverse cardiac remodeling
following acute myocardial infarction in the mouse. Proc Natl Acad
Sci USA. 108:19725–19730. 2011.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Sandanger O, Ranheim T, Vinge LE, Bliksøen
M, Alfsnes K, Finsen AV, Dahl CP, Askevold ET, Florholmen G,
Christensen G, et al: The NLRP3 inflammasome is up-regulated in
cardiac fibroblasts and mediates myocardial ischaemia-reperfusion
injury. Cardiovasc Res. 99:164–174. 2013.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Dong XJ, Chen JJ, Xue LL and Al-hawwas M:
Treadmill training improves cognitive function by increasing IGF2
targeted downregulation of miRNA-483. Ibrain. 8:264–275. 2022.
|
|
46
|
Meng S, Hu Y, Zhu J, Feng T and Quan X:
miR-30c-5p acts as a therapeutic target for ameliorating myocardial
ischemia-reperfusion injury. Am J Transl Res. 13:2198–2212.
2021.PubMed/NCBI
|
|
47
|
Luanpitpong S, Li J, Manke A, Brundage K,
Ellis E, McLaughlin SL, Angsutararux P, Chanthra N, Voronkova M,
Chen YC, et al: SLUG is required for SOX9 stabilization and
functions to promote cancer stem cells and metastasis in human lung
carcinoma. Oncogene. 35:2824–2833. 2016.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Sun M, Guo M, Ma G, Zhang N, Pan F, Fan X
and Wang R: MicroRNA-30c-5p protects against myocardial
ischemia/reperfusion injury via regulation of Bach1/Nrf2. Toxicol
Appl Pharmacol. 426(115637)2021.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Chen J, Zhang M, Zhang S, Wu J and Xue S:
Rno-microRNA-30c-5p promotes myocardial ischemia reperfusion injury
in rats through activating NF-κB pathway and targeting SIRT1. BMC
Cardiovasc Disord. 20(240)2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Huntzinger E and Izaurralde E: Gene
silencing by microRNAs: Contributions of translational repression
and mRNA decay. Nat Rev Genet. 12:99–110. 2011.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Jung YD, Park SK, Kang D, Hwang S, Kang
MH, Hong SW, Moon JH, Shin JS, Jin DH, You D, et al: Epigenetic
regulation of miR-29a/miR-30c/DNMT3A axis controls SOD2 and
mitochondrial oxidative stress in human mesenchymal stem cells.
Redox Biol. 37(101716)2020.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Wang X, Zhang Y, Han S, Chen H, Chen C, Ji
L and Gao B: Overexpression of miR-30c-5p reduces cellular
cytotoxicity and inhibits the formation of kidney stones through
ATG5. Int J Mol Med. 45:375–384. 2020.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Zheng D, Liu J, Piao H, Zhu Z, Wei R and
Liu K: ROS-triggered endothelial cell death mechanisms: Focus on
pyroptosis, parthanatos, and ferroptosis. Front Immunol.
13(1039241)2022.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Abais JM, Xia M, Zhang Y, Boini KM and Li
PL: Redox regulation of NLRP3 inflammasomes: ROS as trigger or
effector? Antioxid Redox Signal. 22:1111–1129. 2015.PubMed/NCBI View Article : Google Scholar
|