Introduction
The incidence of tendinopathy is >30% in patients
with musculoskeletal disorders (1). The tendon lesions are multifactorial
and could be split into two main categories: Tendinitis,
characterized by inflammation, and tendinosis, characterized by
degenerative modifications of tendon structures. The extrinsic
factors, such as physical activity, are usually linked with tendon
lesions. A series of predisposing factors, such as age, sex,
diabetes, rheumatoid arthritis and hereditary factors could also be
responsible (2).
Tendon lesions have great effect on the quality of
life and medical spending. Thus is important to investigate the
mechanisms responsible for tendon healing and to identify new
treatment options.
Data from the literature indicates the fact that
after tendon injuries, a strong inflammatory response takes place
(2-4).
During this process, one of the main mechanisms responsible is
based on Reactive Oxygen Species (ROS) (3). These appear in the inflammation area
and have an essential role during the inflammatory stage. Studies
demonstrate that these hyperreactive molecules decrease the
synthesis and polymerization of collagen (4). This effect also slows the
regeneration of soft tissues and may be linked to the development
of tendinopathies (4,5). Thus the regulation of oxidative
stress is important. Some studies demonstrated that vitamin C,
vitamin D or quercetin are beneficial for tendon healing,
regeneration of its structure and reducing the risk of tendon
adhesion (6-8).
The importance of selenium (Se) as an essential
element is well established in the literature and it is considered
an important element of antioxidant enzymes such as glutathione
peroxidase (GPx), thioredoxin reductase (TrxR) or deiodinase
iodothyronine (IDD) (9,10). The deficit of this element has
significant negative effects, such as Keshan disease, Kashin-Beck
disease, hypothyroidism, recurrent miscarriage or cognitive
impairment (11,12). It is well known the fact that
Selenium deficiency slows the development and growth of bone and
cartilage (13,14).
It should be noted that selenium is a microelement
that, administered in high dosages, can be toxic. Vinceti et
al mention that human intake of ≥260 µg/day for organic
selenium and 16 µg/day for inorganic selenium is toxic. An intake
of <13-19 µg/day of inorganic selenium seems to be a risk factor
for Keshan disease or cardiomyopathy (15). Thus, every attempt in order to use
selenium for a therapeutic purpose should take into account its
toxic potential.
Another parameter that has been noted in the
literature is bone turnover. It is well known that the bone
turnover markers are lower following Se administration. However, it
seems that this effect is noticeable only in short-term (16).
By taking into account the aforementioned data, the
aim of the present study was to evaluate the effect of selenium as
an essential promoter of antioxidant effects on the healing
processes of injured tendons.
Materials and methods
Biologic material
A total of 20 Wistar male rats (200-250 g), aged
between 6 and 12 months, brought from the Experimental Medicine
Center of the Faculty of Veterinary Medicine of Bucharest were
used. The animals were held in polypropylene cages with a
temperature of 22˚C, 40% humidity and a 12-h light/dark cycle. All
rats received a commercial standard chow (18% protein; Global 2018;
Harlam Tekland). The food and water was administered ad
libitum. All the animals were examined and treated according to
the national legislation regarding animal caretaking and all the
ethical norms were taken into account. All the experimental
procedures were approved by the ethics committee of USAMV
Cluj-Napoca, Romania (approval no. 24692/2021).
Experimental protocol
The animal models were split into two groups with
two different treatment methods. The first group received a normal
food administration, while the second group received 1.2 mg/kg/food
of Na2SeO3 (MilliporeSigma). According to
Woth et al (17), inorganic
selenium possesses a stronger antioxidant effect. Thus, this form
of the element was used in the experimental protocol. By taking
into account the potential toxicity of
Na2SeO3, a certain dosage was used in order
to not harm the rats (18,19). The dosage was calculated according
to Jacevic et al (20). The
experiment had a duration of 28 days. On the eighth day, all
animals underwent surgical experimental Achilles tendon lesions.
The animals were monitored for 21 days following the surgery for
their recovery. Their behavior was analyzed and also a short
clinical exam was performed. None of the animals succumbed during
the experiment. All were sacrificed at the end of the
experiment.
Surgical procedure
In order to minimize the suffering induced by the
experiment, the animals were anesthetized during the surgical
procedure using ketamine (80 mg/kg) and xylazine (12 mg/kg) as an
anesthetic. A 0.5 cm longitudinal incision was developed right over
the Achilles tendon. Proximal to the calcaneal insertion (5 mm) a
tendon section was created and a Kessler tendon suture was
developed. After that, the wound was closed using a 4.0
monofilament separate suture. The whole surgical procedure
respected asepsis measures. After the treatment, the animals were
kept in the cages aforementioned. No movement restrictions or
immobilization were applied. The animals were supervised for 2 h
after the surgery by checking their vital signs.
Preparation and histological
examination of tendon tissue
After 3 weeks, the animals were sacrificed according
to approved experimental protocol and legal procedures. It
consisted of a rich CO2 atmosphere exposure of the rat
cage. The death of animals following sacrifice was verified by
checking the vital signs, pupil dilation and also by checking the
pupilar reflex. The Achilles tendon was extracted for histological
evaluation in order to do a comparison according to the Movin scale
(modified by Bonar) (7). The
tendons were dissected and then immersed in paraformaldehyde 4% at
room temperature for 12 h. Then, the tendons were washed with
phosphate-buffered saline 0.1 M, pH 7.4 and cryoprotected by using
sequential immersion in different concentrations of sucrose (10, 20
and 30%). Longitudinal sections (20 µm) were stained using
hematoxylin-eosin technique (HE) which consisted of 5 min of
hematoxylin staining and minutes of eosin staining at room
temperature. Images were captured using a light microscope at 10x
and 20x magnification.
Histologic evaluation
The histological analysis consisted of 5
histological examinations of each sample evaluated using the Movin
scale (modified by Bonar) measures the following (7): i) The form, alignment and orientation
of collagen fibers; ii) the cellular aspect and concentration; iii)
the number of glycosaminoglycans and iv) vascularization. These
assessments are recorded on a scale between 0 and 4(7).
The average result tends to be towards 0 while total
points rarely exceed 1.9, according to Maffulli et al
(21) study on rotator cuff
diseases. This tendon health evaluation method was used because of
its wide usage in the literature despite the fact that some authors
suggest a revision of this scale (22). According to Fearon et al
(22), the tendon may present a
disorganized area that may not be secondary to trauma. The present
study compared the results between two homogenous experimental
groups which followed the same treatment. Thus, the presence of
nontraumatic disorganized collagen fibers may be identified in both
groups in equal proportions which may not influence the assessment
and results of the present study.
Statistical analysis
The data were analyzed using SPSS 22.0 (IBM Corp.).
The mean and standard deviations were calculated. Unpaired Student
t-test was used in order to evaluate the statistical significance
between groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
There were no infections or mortality during the
experiment. The following histological criteria were taken into
account to evaluate the tendon healing process: The morphological
aspects of the tenocytes, the shape and direction of the collagen
fibers, the amount of angiogenetic processes and intracellular
matrix composition.
At three weeks after the experimental tenotomy, the
histological evaluation revealed an even orientation of the
collagen fibers in the case of the experimental group (Se) compared
with the second group. The Bonar score was 1.62 for the Se group,
while the control group had a Bonar score of 1.98. Those
differences were significant in the proximal area but in the distal
portion of the tendons as well. The healing process was faster at
the muscle-tendon junction, compared with the midportion or distal
portion of the tendon.
In the Se group, primary collagen fibers synthesis
was identified, which consists of longitudinal and undulated
fibers. By contrast, in the control group, the collagen fibers were
much less organized, with abnormal orientation and departed from
the natural orientation.
The average number of tenocytes in the Se group was
lower which is demonstrated by a lower Bonar score (1.22), compared
with the second group (Bonar Score 1.85).
In case of the Se group, the microscopy sections of
the tenotomy site, demonstrated advanced healing after 3 weeks with
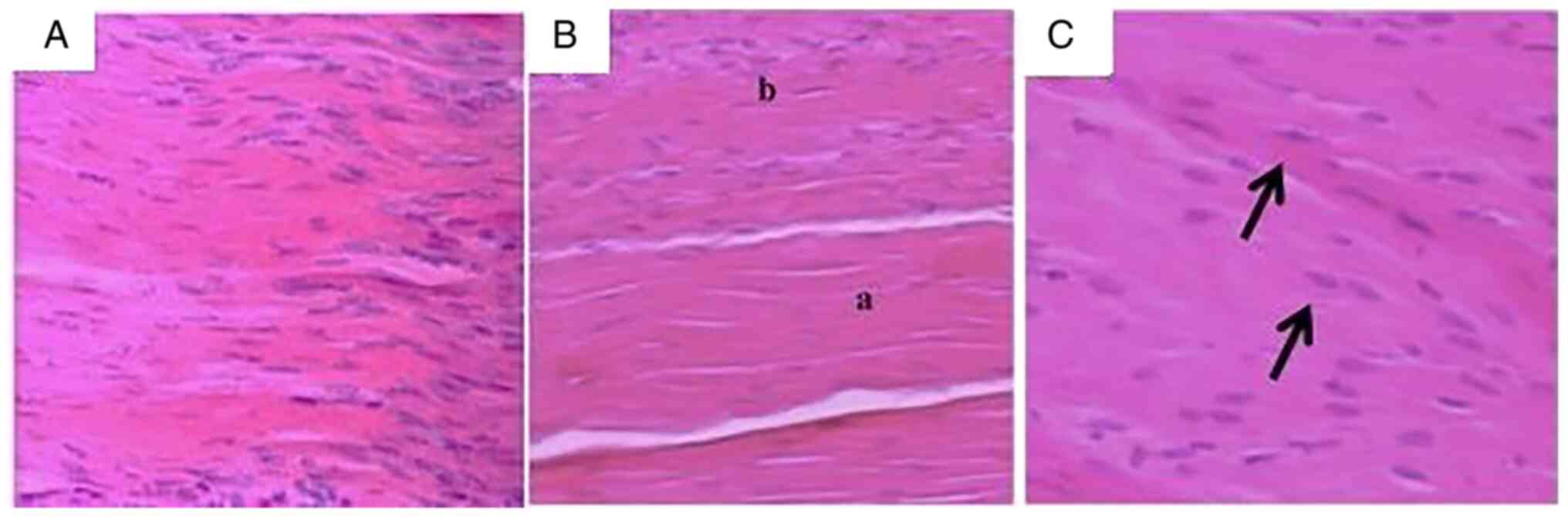
longitudinal orientation of collagen fibers (Fig. 1A). A slightly higher number of
tenocytes compared with the intact tendon areas was also noticed
(Fig. 1B). Those cells were
oval-shaped and with an increased size of the nucleus. (Fig. 1C). Cells were correctly positioned
and followed the collagen fiber's direction. The tenocyte cytoplasm
was not visible under the microscope. The tendinous regions had a
similar aspect to the healthy tendons. Those regions presented
sporadic, oblong and darker nuclei.
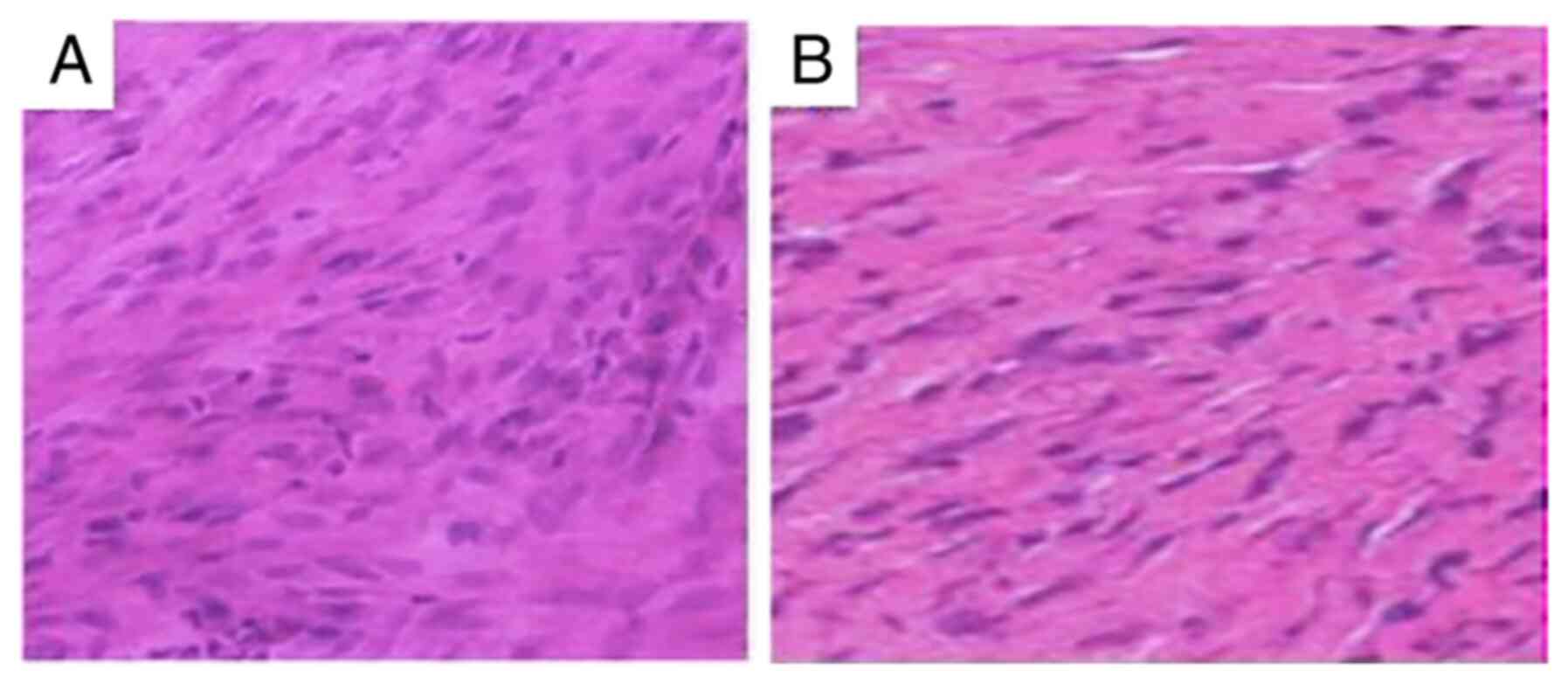
The control group possessed a significantly greater
amount of cells (Fig. 2A and
B). The fibroblasts showed bigger,
oval or round-shaped nuclei (Fig.
2A). Those cells were unevenly distributed under the
microscopic field and occasionally the cytoplasm was
noticeable.
In regard to vascularization, there was a decreased
number of blood vessels in the experimental group (Se; Bonar Score
1.70), compared with the control group (Bonar score 1.96). In the
case of Se group, a decreased amount of blood vessels was
identified, especially at the tenotomy level, compared with the
control group, which showed a significantly increased amount of
blood vessels. The Bonar score regarding bone marrow evaluation was
significantly lower in the Se group compared with the control
group. (1.45 and 2.15 respectively).
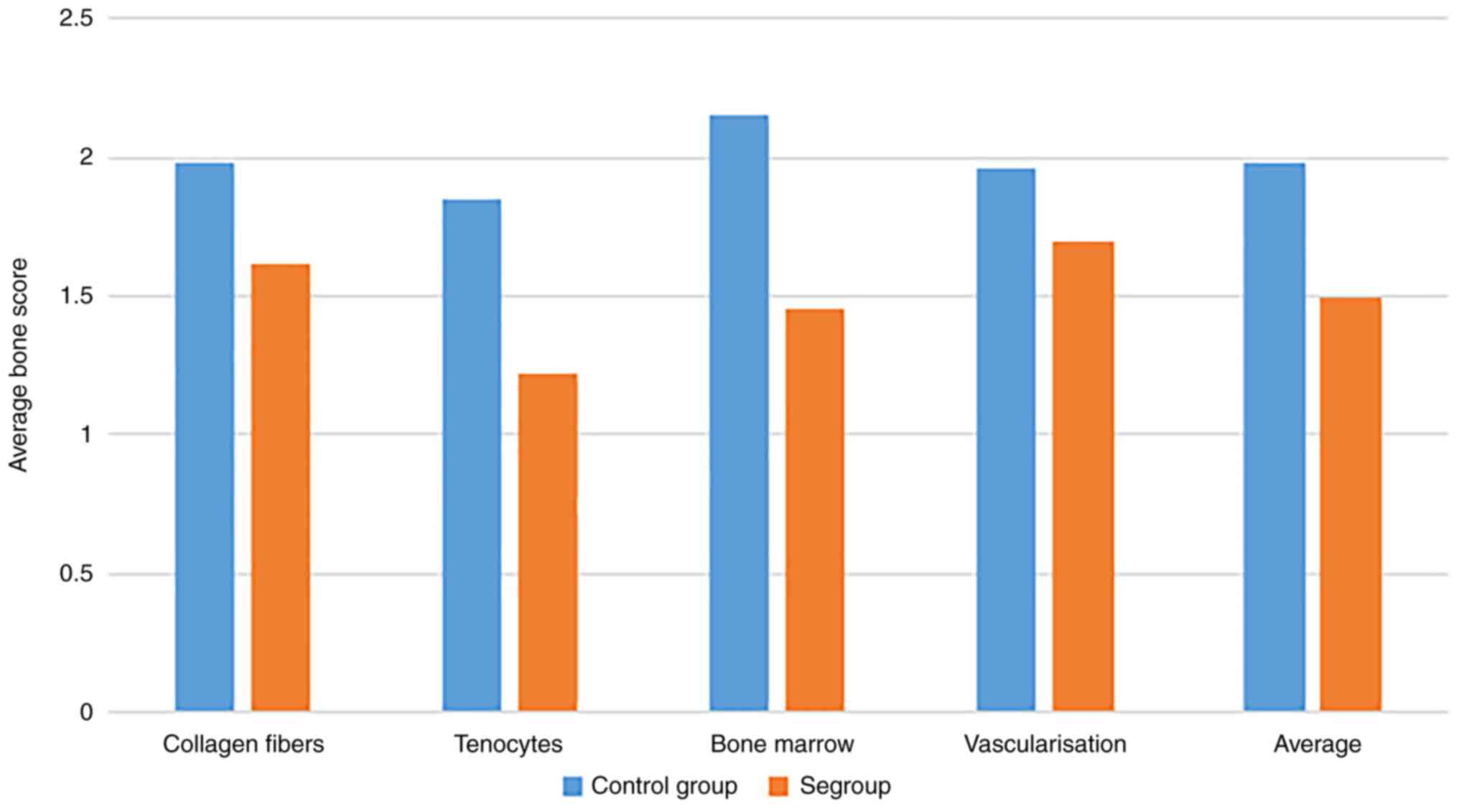
The differences between those two groups are shown
in Table I and Fig. 3. The average Bonar score, for all
parameters, was 1.985 for the control group and 1.4975 for the Se
group. The difference in average Bonar score between groups was
0.488. Thus, the score for the control group was 24.5% higher than
the Se group which was statistically significant. Moreover, the
total points for the control group were 7.94±0.94, but 5.99±0.61
(P<0.01) for the Se group. The maximum differences were recorded
regarding the tenocytes, while the vascularization showed the
lowest differences.
 | Table IAverage and standard deviation of
Bonar score, detailed on all four parameters included in the
present study. |
Table I
Average and standard deviation of
Bonar score, detailed on all four parameters included in the
present study.
| | Average Bonar
score |
|---|
| | Collagen fibers | Tenocytes | Bone marrow | Vascularization | Average |
|---|
| Control group
(n=10) | 1.98±0.1 | 1.85±0.2 | 2.15±0.12 | 1.96±0.13 | 1.985±0.11 |
| Se group (n=10) | 1.62a±0.15 | 1.22a±1.75 | 1.45a±0.11 | 1.70a±0.21 | 1.4975a±0.13 |
The experiment demonstrated histological
modifications which were more significant after three weeks in the
control group, while the Se group demonstrated a higher healing
rate. This result is supported by the lower Bonar score for the Se
group, especially regarding the number of tenocytes and the
mucopolysaccharides in the extracellular matrix.
The present study also evaluated the toxicity of
sodium selenite treatment. The heart, liver, lung and kidneys were
investigated using HE staining. No significant changes were
found.
Discussion
After injury, the tendon healing process begins with
an acute inflammatory reaction, which is followed by proliferation
and tissue remodeling (23). These
separate histological events represent the main reason for using
murine models to evaluate tendon tissue regeneration.
The inflammatory reaction aforementioned is
modulated by cytokines which regulate the processes following this
healing phase (23,24). During this period, an
overproduction of reactive oxygen species (ROS) and cellular
phagocytosis takes place, which eventually leads to a reduction of
the inflammatory process. ROS are partly reduced oxygen metabolites
that have a high oxidative potential. They have a high oxidative
effect on cellular protein and lipids, but also on DNA. Through
this mechanism, they also inhibit the synthesis and polymerization
of tendons that suffer lesions (6,24,25).
According to these data, oxidative stress could be
harmful to the tendon healing process by increasing the amount of
extracellular matrix and proliferation of interstitial fibroblasts.
It also seems to be linked with the development of pathological
fibrosis (26).
The present study gathered tendon samples to
evaluate collagen fibers, tenocytes, bone marrow and
vascularization according to the Bonar score. All four parameters
demonstrated significantly improved results following Se
administration. This result could be linked to decrease of
oxidative stress and this hypothesis is supported by Murrell
(27) and Moraes et al
(28), who demonstrated that
decreased oxidative stress helps to provide a much more potent
tissue regeneration. Thus, the present study complimented the
aforementioned data. The Se group demonstrated a slightly higher
number of tenocytes but cells were correctly positioned and
followed the direction of collagen fibers. On the other hand, the
control group had fibroblasts with bigger, oval, or round-shaped
nuclei. Those cells were unevenly distributed without following the
fiber direction. This could be caused by an increased oxidative
stress which eventually increases the extracellular matrix
components and proliferates interstitial fibroblasts. Some studies
also note that, among the markers of oxidative stress,
malondialdehyde is strongly linked with pathological fibrosis
(26,29).
Referring to the selenium mechanism, it is well
known the fact the effects of selenium are achieved mainly through
selenoproteins. These enzymes, such as GPx and TrxR are responsible
for protection against oxidative stress. The literature underlines
the role of selenoproteins against ROS (30,31).
Due to the fact that the mitochondrial electron
transport is also a source of ROS, the loss of mitochondrial
integrity could be a source of oxygenation and inflammation which
may eventually lead to cellular apoptosis Kaushal et al
(32). Selenium has a protective
potential that directly targets mitochondria and upregulates
mitochondrial transcription factors (33).
Moreover, the relations between cellular redox
status and cyclooxygenase (COX) and lipoxygenase activation are
well known. Those enzymes are involved in a process responsible for
the synthetization of prostaglandins (PG), thromboxanes and
prostacyclins (PGI2), which are inflammatory biomarkers that are
released as a response to potential pathogen events (such as
stress, free radicals and infections). It was discovered that a
selenium deficit leads to a lower GPx activity, which may reduce
the control of COX and LOC (33-36).
Thus, selenium is highly efficient in suppressing the
aforementioned elements (32).
Selenium deficit is also proved to be linked with increased
production of reactive nitrogen species and C reactive proteins
(32,34,35).
Selenium administration proved to be beneficial for
tendon healing. The present study showed the availability and the
efficiency of studying tendon healing processes in murine models.
No significant adverse reactions were noted regarding wound
healing. The results indicated the positive effects on tendon
healing, although the results provided by other randomized
controlled trials demonstrate that in certain conditions selenium
has no effect on musculoskeletal health and bone turnover (16,37).
According to Perri et al (16), these results could be due to the
fact that the test participants had a physiological level of
selenium at the moment of evaluation, concluding that the study
would need to be extended to populations with selenium deficit.
The present study also investigated the potential
toxicity of low-dosage selenium and histological analysis
demonstrated no adverse reactions on major organs.
The results of the present study underlined the
necessity of additional research to clarify the mechanisms
responsible for the tendon healing process under selenium treatment
and the long-term results, which is also the main weakness of the
present study. Additional research is required regarding the effect
of treatment on major organs.
The present study demonstrated that selenium
administration on murine models could be beneficial for tendon
healing. Further clinical research is required in order to warrant
recommendation.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
DC planned the clinical study and contributed to the
conception and design of the study, as well as the acquisition,
analysis, and interpretation of the data. SD contributed to the
conception and design of the study, the translation and critical
revision for important intellectual content. AI planned the
clinical study and contributed to the conception and design of the
study. GZ contributed to the analysis and data interpretation. AD
contributed to the analysis and interpretation of the data and the
critical revision for important intellectual content. All authors
read and approved the final manuscript. DC and AD confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
All procedures performed in studies involving animal
participants were in accordance with the national ethical
standards. The present study was approved by the Ethics Committee
of the University of Agricultural Sciences and Veterinary Medicine,
011464, Bucharest, Romania (approval no. 1153/2021; date of
approval 10 March 2021).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Astrom M and Rausing A: Chronic Achilles
tendinopathy: A survey of surgical and histopathologic findings.
Clin Orthop. 316:151–164. 1995.PubMed/NCBI
|
|
2
|
Maffulli N, Wong J and Almekinders LC:
Types and epidemiology of tendinopathy. Clin Sports Med.
22:675–692. 2003.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ghita M, Cotor G, Viţălaru AB and Braslasu
D: Comparative study on the effect of prednisone and dexamethasone
on leukocytes, in rabbit. J Biotechnol. 208(S92)2015.
|
|
4
|
Longo UG, Oliva F, Denaro V and Maffulli
N: Oxygen species and overuse tendinopathy in athletes. Disabil
Rehabil. 30:1563–1571. 2008.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Cotor G, Birtoiu IA, Ionita L, Tanase A
and Vitalaru BA: The anti-inflammatory effect of a Cannabis sativa
oil supplement in experimental acute inflammation in rats. J
Biotechnol. 185(S45)2014.
|
|
6
|
DePhillipo NN, Aman ZS, Kennedy MI, Begley
JP, Moatshe G and LaPrade RF: Efficacy of Vitamin C supplementation
on collagen synthesis and oxidative stress after musculoskeletal
injuries: A systematic review. Orthop J Sports Med.
6(2325967118804544)2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hung LK, Fu SC, Lee YW, Mok TY and Chan
KM: Local vitamin-C injection reduced tendon adhesion in a chicken
model of flexor digitorum profundus tendon injury. J Bone Joint
Surg Am. 95(e41)2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gajaila G, Gajaila I, Cotor G and Ionita
L: Testing the killing ability of pig neutrophils after stimulation
with an ethanolamine derivative. J Biotechnol. 231(S63)2016.
|
|
9
|
Stadtman TC: Selenium biochemistry.
Mammalian selenoenzymes. Ann N Y Acad Sci. 899:399–402.
2000.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Rayman MP: The importance of selenium to
human health. Lancet. 356:233–241. 2000.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Toxicological Profile for Selenium. Agency
for Toxic Substances and Disease Registry. U.S. Department of
Health and Human Services, Atlanta, GA, 2003.
|
|
12
|
Moreno-Reyes R, Suetens C, Mathieu F,
Begaux F, Zhu D, Rivera MT, Boelaert M, Nève J, Perlmutter N and
Vanderpas J: Kashin-Beck osteoarthropathy in rural Tibet in
relation to selenium and iodine status. N Engl J Med.
339:1112–1120. 1998.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Thompson KM, Haibach H and Sunde RA:
Growth and plasma triiodothyronine concentrations are modified by
selenium deficiency and repletion in second-generation
selenium-deficient rats. J Nutr. 125:864–873. 1995.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yang C, Wolf E, Roser K, Delling G and
Muller PK: Selenium deficiency and fulvic acid supplementation
induces fibrosis of cartilage and disturbs subchondral ossification
in knee joints of mice: An animal model study of Kashin-Beck
disease. Virchows Arch A Pathol Anat Histopathol. 423:483–491.
1993.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Vinceti M, Filippini T, Cilloni S,
Bargellini A, Vergoni AV, Tsatsakis A and Ferrante M: Health risk
assessment of environmental selenium: Emerging evidence and
challenges (Review). Mol Med Rep. 15:3323–3335. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Perri G, Hill TR, Mathers JC, Walsh JS,
Gossiel F, Winther K, Frölich J, Folkestad L, Cold S and Eastell R:
Long-term selenium-yeast supplementation does not affect bone
turnover markers: A randomized placebo-controlled trial. J Bone
Miner Res. 37:2165–2173. 2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Woth G, Nagy B, Mérei Á, Ernyey B, Vincze
R, Kaurics Z, Lantos J, Bogár L and Mühl D: The effect of
Na-selenite treatment on the oxidative stress-antioxidants balance
of multiple organ failure. J Crit Care. 29:883.e7–11.
2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Vinceti M, Chiari A, Eichmüller M, Rothman
KJ, Filippini T, Malagoli C, Weuve J, Tondelli M, Zamboni G,
Nichelli PF and Michalke B: A selenium species in cerebrospinal
fluid predicts conversion to Alzheimer's dementia in persons with
mild cognitive impairment. Alzheimers Res Ther.
9(100)2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Mandrioli J, Michalke B, Solovyev N, Grill
P, Violi F, Lunetta C, Conte A, Sansone VA, Sabatelli M and Vinceti
M: Elevated levels of selenium species in cerebrospinal fluid of
amyotrophic lateral sclerosis patients with disease-associated gene
mutations. Neurodegener Dis. 17:171–180. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Jacevic V, Jokic G, Dragojevic-Simic V,
Bokonjic D, Vucinic S and Vuksa M: Acute toxicity of sodium
selenite in rodents: Pathomorphological study. Mil Med Sci Lett
(Voj Zdrav Listy). 80:90–96. 2011.
|
|
21
|
Maffulli N, Longo UG, Franceschi F,
Rabitti C and Denaro V: Movin and Bonar scores assess the same
characteristics of tendon histology. Clin Orthop Relat Res.
466:1605–1611. 2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Fearon A, Dahlstrom JE, Twin J, Cook J and
Scott A: The Bonar score revisited: region of evaluation
significantly influences the standardized assessment of tendon
degeneration. J Sci Med Sport. 17:346–350. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Leadbetter WB: Cell-matrix response in
tendon injury. Clin Sports Med. 11:533–578. 1992.PubMed/NCBI
|
|
24
|
Longo UG, Franceschi F, Ruzzini L, Rabitti
C, Morini S, Maffulli N and Denaro V: Histopathology of the
supraspinatus tendon in rotator cuff tears. Am J Sports Med.
36:533–538. 2008.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Murrell GA, Szabo C, Hannafin JA, Jang D,
Dolan MM, Deng XH, Murrell DF and Warren RF: Modulation of tendon
healing by nitric oxide. Inflamm Res. 46:19–27. 1997.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Sun J, Wu Y, Long C, He P, Gu J, Yang L,
Liang Y and Wang Y: Anthocyanins isolated from blueberry
ameliorates CCl4 induced liver fibrosis by modulation of oxidative
stress, inflammation and stellate cell activation in mice. Food
Chem Toxicol. 120:491–499. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Murrell GA: Oxygen free radicals and
tendon healing. J Shoulder Elbow Surg. 16 (5 Suppl):S208–S214.
2007.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Moraes SA, Oliveira KR, Crespo-López ME,
Picanço-Diniz DL and Herculano AM: Local NO synthase inhibition
produces histological and functional recovery in Achilles tendon of
rats after tenotomy: Tendon repair and local NOS inhibition. Cell
Tissue Res. 353:457–463. 2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhao Q, Yang F, Meng L, Chen D, Wang M, Lu
X, Chen D, Jiang Y and Xing N: Lycopene attenuates chronic
prostatitis/chronic pelvic pain syndrome by inhibiting oxidative
stress and inflammation via the interaction of NF-κB, MAPKs, and
Nrf2 signaling pathways in rats. Andrology. 8:747–755.
2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Roman M, Jitaru P and Barbante C: Selenium
biochemistry and its role for human health. Metallomics. 6:25–54.
2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Flohé L and Brigelius-Flohé R:
Selenoproteins of the Glutathione Peroxidase Family. In: Selenium.
Hatfield D, Berry M and Gladyshev V (eds). Springer, New York, NY,
2011.
|
|
32
|
Kaushal N, Gandhi UH, Nelson SM, Narayan V
and Prabhu KS: Selenium and Inflammation. In: Selenium. Hatfield D,
Berry M and Gladyshev V (eds). Springer, New York, NY, 2011.
|
|
33
|
Tirosh O, Levy E and Reifen R: . High
selenium diet protects against TNBS-induced acute inflammation,
mitochondrial dysfunction and secondary necrosis in rat colon.
Nutrition. 23:878–886. 2007.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Maehira F, Luyo GA, Miyagi I, Oshiro M,
Yamane N, Kuba M and Nakazato Y: Alterations of serum selenium
concentrations in the acute phase of pathological conditions. Clin
Chim Acta. 316:137–146. 2002.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Sakr Y, Reinhart K, Bloos F, Marx G,
Russwurm S, Bauer M and Brunkhorst F: Time course and relationship
between plasma selenium concentrations, systemic inflammatory
response, sepsis, and multiorgan failure. Br J Anaesth. 98:775–784.
2007.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Dragosloveanu Ş, Cotor DC, Dragosloveanu
CDM, Stoian C and Stoica CI: Preclinical study analysis of massive
magnesium alloy graft for calcaneal fractures. Exp Ther Med.
22(731)2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Walsh JS, Jacques RM, Schomburg L, Hill
TR, Mathers JC, Williams GR and Eastell R: Effect of selenium
supplementation on musculoskeletal health in older women: A
randomised, double-blind, placebo-controlled trial. Lancet Healthy
Longev. 2:e212–e221. 2021.PubMed/NCBI View Article : Google Scholar
|