Introduction
Juvenile polyposis syndrome (JPS) is an autosomal
dominant genetic disease with multiple hamartoma polyps in the
gastrointestinal tract and the estimated incidence of the syndrome
ranges between 1 in 16,000 and 1 in 100,000 worldwide (1). JPS is closely related to the risk of
gastric cancer and colorectal cancer (2). JPS is clinically diagnosed in an
individual with any of the following: i) ≥5 Colorectal juvenile
polyps; ii) juvenile polyps in other parts of the gastrointestinal
tract; or iii) any number of juvenile polyps and ≥1 affected family
member (1). A total of ≤60% of
individuals with clinically defined JPS carry variants in SMAD4 or
bone morphogenetic protein receptor type 1A genes, and ~20-50% of
JPS cases have no family history (3-5).
The pathological mechanism underlying JPS frequently involves the
epithelium of the polyps becoming ulcerated, which leads to the
infiltration of inflammatory cells-this is the first step in a
series of sequential events (2).
As the glands and crypts of the juvenile polyp begin to fill with
mucus, the polyp becomes inflamed and enlarged, progressing to the
classic hamartomatous juvenile polyp (4,5).
Finding an early, effective method of identifying JPS is becoming
an urgent clinical issue. Patients with JPS are usually
asymptomatic, but in the case of symptoms, rectal bleeding with
concurrent anemia is most common, followed by abdominal pain,
diarrhea and eventually intussusception at the site of the largest
polyps (6). Colonoscopy should
start at the age of 15 years (or sooner if symptomatic) and then
repeated at a 1-3-yearly interval, and genotype-specific
recommendations may be required (6). The majority of cases may be managed
with therapeutic endoscopy with polypectomy; however, surgery is
necessary for patients who develop cancer or where endoscopic
management is not possible (7).
By contrast, solitary JPS is frequently less
predictive of a malignant tumor tendency (8). Juvenile polyps of the colorectum are
the most common type of polyp in children but are rare in adults
(9,10). The present case report provides
details on the diagnosis and treatment process of an adult solitary
juvenile rectal polyp with elevated fecal calprotectin (FCP).
Case report
Chief complaints
A 57-year-old female was admitted to The Affiliated
Hospital of Qingdao University (Qingdao, China) for treatment,
presenting with intermittent stool with mucus and blood on December
30, 2021.
History of present illness
The patient complained of intermittent excretion of
mucus in the past month, covered with a small amount of dark red
blood stains, and the initial time of appearance was unknown. The
patient did not have any symptoms of abdominal pain or diarrhea,
and colonoscopy indicated that the colonic mucosa was smooth, the
submucosal vascular network was clear and the colonic folds were
regular. The colonoscopy result excluded a diagnosis of
enteritis.
History of past illness
The patient had a history of hypertension and had
not been given standardized treatment.
Family history
The patient had no family history of colorectal
polyps or cancer.
Physical examination upon
admission
The physical examination revealed no positive signs
of the heart, lungs and abdomen.
Laboratory examinations
Blood analysis indicated red blood cell, white blood
cell and platelet counts within the normal ranges. Prothrombin
time, partial thromboplastin time and D-dimer level were normal. A
fecal occult blood test had a positive ‘+’ result and FCP was
elevated to 805 mg/kg (normal range, 0-49 mg/kg). Indicators of
thyroid function, rheumatism-related indices, serum tumor markers,
urinalysis and electrocardiogram were all normal.
Imaging examinations
The abdominal ultrasound indicated mild fatty liver
(data not shown). Echocardiography was normal. Chest CT revealed
multiple small nodules in both lungs and regular follow-up was
recommended. The scan parameters were as follows: Tube voltage, 100
kV; tube current modulation, 200-480 mAsec; spiral pitch factor,
0.98; collimation width, 0.625; and gantry rotation time, 0.6
sec.
Further diagnostic work-up
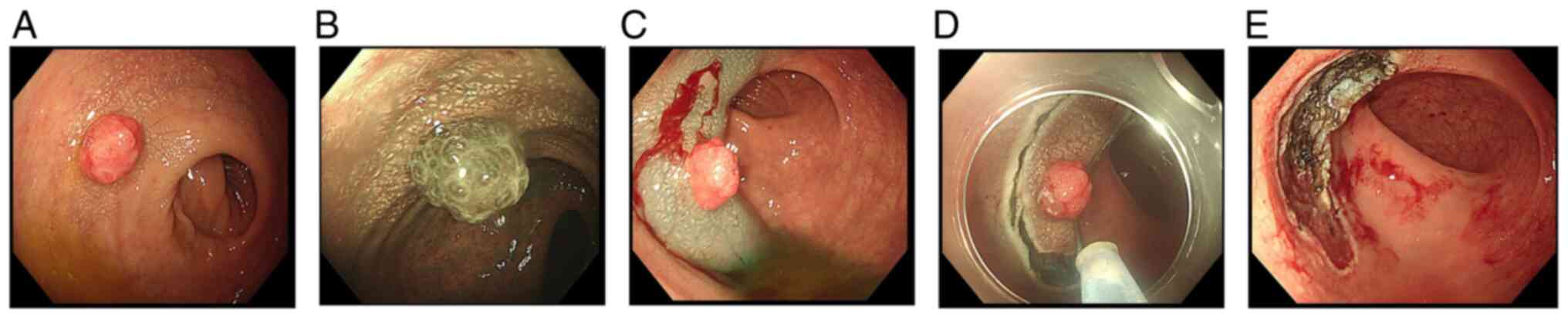
Colonoscopy revealed a solitary polyp in the rectum,
~2.0 cm in diameter, with a short and wide pedicle, hyperemia and
edema of the mucosa on the surface of the polyp, plus mild
lobulation (Fig. 1). Observations
using narrow-band imaging (NBI) indicated irregular expansion of
glands on the lesion surface, and blood vessels were unclear
(Fig. 1).
Treatment
Polyps were successfully removed by endoscopic
submucosal dissection (ESD; Fig.
1).
Hematoxylin and eosin (H&E)
staining
A 5-mm section was prepared from the
paraffin-embedded polyp and H&E staining was performed for
histological analysis. In brief, fresh tissue was fixed with 4%
paraformaldehyde for 24 h at room temperature, then dehydrated with
a graded alcohol series (75% alcohol for 4 h, 85% alcohol for 2 h,
90% alcohol for 2 h and 95% alcohol for 1 h), absolute ethanol II
for 30 min, alcohol benzene for 5-10 min, xylene for 5-10 min and
wax for 3 h. The wax-soaked tissue was embedded and stored at
-20˚C. Following the solidification of the wax, the tissue was
paraffin-embedded and sliced. At room temperature, the sliced
tissue was stained the hematoxylin for 5 min, then washed with
water for 10-30 sec, and with 0.5% eosin added for 1-3 min, then
washed with water again for 1-2 sec, followed by absolute ethanol
II and xylene washed for 2 min. Finally, the slide was sealed with
neutral gum at room temperature. The H&E-stained sections were
imaged with a light microscope (model, BX51; Olympus
Corporation).
Outcome and follow-up
Histopathological examination revealed that the
polyp was an inflammatory hyperplastic polyp with surface erosion
and granulation tissue hyperplasia, surface ulceration (arrow 1),
slightly cystic expansion of glands in polyps (arrow 2), abundant
polyp stroma (arrow 3), a large number of inflammatory cells (arrow
4) and proliferation of capillaries (arrow 5), and it was diagnosed
as a juvenile polyp (Fig. 2A and
B). The histological appearance of
chicken skin-like changes surrounded this juvenile polyp, including
densely deposited foamy macrophages located in the lamina propria
(arrow 1), interstitial edema (arrow 2), telangiectasia (arrow 3),
glandular papillary hyperplasia (arrow 4) and massive inflammatory
cell infiltration [e.g., eosinophils (arrow 5), neutrophils (arrow
6), lymphocytes (arrow 7) and plasma cells (arrow 8)] (Fig. 2C and D). Following the Chinese ESD guidelines
(11), endoscopic polyp removal
was deemed appropriate, since the patient was middle-aged, had no
other comorbidities and exhibited only one polyp. ESD is
recommended because it takes a short time (~10 min), does not
damage the muscular layer and there is no risk of perforation. The
patient did not use any other drugs, such as antibiotics, to alter
the number of intestinal neutrophils or intestinal flora after
polypectomy. Following polypectomy, food intake was forbidden for
12 h, followed by a liquid diet, and the patient was discharged
without any discomfort. At the clinical review 2 weeks later, the
patient was no longer experiencing the above symptoms and the FCP
level had returned to 36.2 mg/kg. Repeat colonoscopy 6 months later
indicated that the local mucosa was well-healed after rectal ESD.
Repeat abdominal CT indicated mild fatty liver.
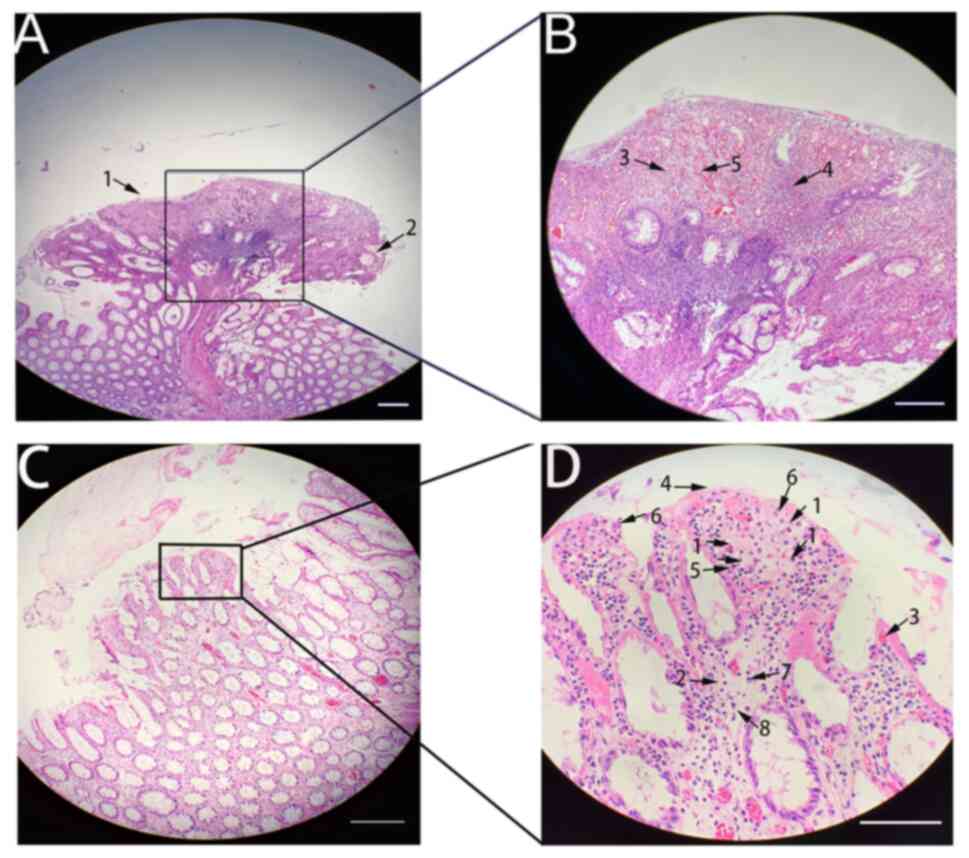 | Figure 2Histological images of the polyp and
the surrounding area. (A) H&E staining of the juvenile polyp
showed an inflammatory hyperplastic polyp with surface erosion and
granulation tissue hyperplasia, surface ulceration (arrow 1),
slightly cystic expansion of glands in polyps (arrow 2)
(magnification, x10). (B) In the magnified window from A, abundant
polyp stroma (arrow 3), a large number of inflammatory cells (arrow
4) and the proliferation of capillaries (arrow 5) were observed
(magnification, x40). (C) Histological appearance of chicken
skin-like changes surrounding juvenile polyps (magnification, x40).
(D) The magnified window indicates densely deposited foamy
macrophages located in the lamina propria (arrow 1), interstitial
oporotic edema (arrow 2), telangiectasia (arrow 3), glandular
papillary hyperplasia (arrow 4) and massive inflammatory cell
infiltration [e.g., eosinophils (arrow 5), neutrophils (arrow 6),
lymphocytes (arrow 7) and plasma cells (arrow 8)] (magnification,
x200; scale bars, 50 µm). |
Discussion
JPS is a relatively rare autosomal dominant genetic
disease characterized by scattered juvenile polyps in the
gastrointestinal tract and is closely related to the risk of
gastrointestinal tumors (2). By
contrast, colorectal sporadic juvenile polyps are not associated
with an increased risk of tumors (12). Sporadic juvenile polyps may also
undergo dysplastic changes (13,14).
Previous research indicated that juvenile polyps occur in 2% of
children and adolescents, accounting for the majority of childhood
polyps (12,15). The most common types of polyps in
adults are hyperplastic polyps and adenomas. Most sporadic juvenile
polyps occur in the sigmoid colon, followed by the rectum (14). Patients with juvenile polyps have
symptoms, such as rectal bleeding and abdominal pain, which are
similar to those of other types of polyp (14,16).
The size of juvenile polyps ranges from mm for sessile nodules to
cm. Larger polyps frequently appear leafy, and even a small amount
may be observed and is frequently accompanied by erosion and
granulation tissue hyperplasia (16). In the present case, chicken
skin-like mucosal changes were found to be distributed around the
juvenile rectal polyp; however, chicken skin-like changes most
often appear in advanced colorectal adenomas (17,18).
Chicken skin-like changes with adenocarcinoma or high-grade
dysplasia exhibit much higher infiltration of lipid-laden
macrophages (17,18). It is assumed that lipid-laden
macrophages move toward the tumor and infiltrate the area around
it. This phenomenon may explain the extent of inflammatory activity
and carcinogenetic progression of the tumor (18). In the present case report, the
juvenile polyp was a benign polyp, and a large number of
inflammatory cells, including macrophages, were observed in the
pathological tissue. Therefore, it was hypothesized that its
appearance may have been caused by repeated long-term inflammatory
activity of the juvenile polyp. A juvenile polyp was diagnosed
mainly by histopathology. In the present case, the types of
macrophage, lymphocyte and neutrophil cells in the pathology
specimen was able to be separated under the microscope and
immunohistochemistry was to be considered if a combination of
lymphatic hematopoietic system diseases was indicated by
microscopy; however, the cell distribution of this specimen was
loose and diverse under the microscope and did not support
lymphatic hematopoietic system diseases. The mucosal epithelium and
glands of the specimen were not found to be dysplastic under the
microscope, and thus, performing immunohistochemistry to detect
potential markers of colorectal cancer was not deemed
necessary.
In the present case report, under white light
endoscopy, a subpedicle-like polyp with a size of ~2.0 cm was
observed in the rectum, with hyperemia, nodules and unevenness on
the surface and chicken skin-like changes in the surrounding
mucosa. Similarly, observations using NBI indicated irregular
expansion of glands on the lesion surface, and blood vessels were
unclear. Based on the aforementioned considerations and Kudo
classification as suspicious Vi type (19), it may be hypothesized that these
appearances provide most doctors with the initial impression of
potential early rectal cancer, whereas the final pathology results
suggest juvenile polyps. Therefore, an important finding of the
present case report is the indication that for adult colorectal
lesions, in addition to the common adenomatous polyps, there is
also a possibility for diagnosis of the relatively rare lesion that
is a hamartomatous polyp. A key feature of juvenile polyps is that
the surface may have congestion, swelling and nodular changes. The
glandular dilatation indicated by NBI is inflammatory dilation
rather than tumor glandular dilatation, and the unclear display of
blood vessels may be due to severe swelling of the inflammatory
blood vessels and not the disappearance of tumor blood vessels.
Calprotectin is a nonglycosylated human protein with
a molecular weight of 36.5 kDa found in high concentrations in the
cytosol of neutrophil granulocytes (20,21).
Currently, FCP is recommended as an aid in distinguishing between
inflammatory bowel disease (IBD), such as Crohn's disease or
ulcerative colitis, and non-IBD, such as irritable bowel syndrome
(20). The present case study
suggests that elevated FCP levels may be related to the presence of
juvenile polyps in adults, since after endoscopic resection, FCP
was found to be within the normal range. This is consistent with a
previous report by Hodgson-Parnell et al (21). In the present case, elevated FCP
was considered to be associated with repeated inflammatory stimuli
of juvenile polyps, where neutrophils aggregated and were released.
However, to the best of our knowledge, reports on the relationship
between FCP and adult juvenile polyps are currently scarce
(21), and further research is
required to determine this relationship and the underlying
mechanism.
In conclusion, the case of an adult patient with a
solitary juvenile polyp of the rectum was reported, with chicken
skin-like changes in the surrounding mucosa and a high FCP level.
The polyp was ~2.0 cm in diameter and had a short and wide pedicle.
This polyp was successfully removed by ESD without any
complications and the patient will be thoroughly followed up after
one year. The correlation between juvenile polyps and FCP requires
further study.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XS and TS designed and supervised the study. XS, YH
and XS performed the data collection and analysis. XS, MS and XS
conducted data analysis and interpretation. All authors confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The study procedures were performed in accordance
with protocols approved by the Medical Ethics Committee of The
Affiliated Hospital of Qingdao University (Qingdao, China).
Patient consent for publication
The patient provided written informed consent for
publication of this case report and all the accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Leonard NB and Bronner MP: Giant gastric
folds in juvenile polyposis. Case Rep Gastroenterol. 15:985–993.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Brosens LA, van Hattem A, Hylind LM,
Iacobuzio-Donahue C, Romans KE, Axilbund J, Cruz-Correa M,
Tersmette AC, Offerhaus GJ and Giardiello FM: Risk of colorectal
cancer in juvenile polyposis. Gut. 56:965–967. 2007.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Calva-Cerqueira D, Chinnathambi S, Pechman
B, Bair J, Larsen-Haidle J and Howe JR: The rate of germline
mutations and large deletions of SMAD4 and BMPR1A in juvenile
polyposis. Clin Genet. 75:79–85. 2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gao XH, Li J, Zhao ZY, Xu XD, Du YQ, Yan
HL, Liu LJ, Bai CG and Zhang W: Juvenile polyposis syndrome might
be misdiagnosed as familial adenomatous polyposis: A case report
and literature review. BMC Gastroenterol. 20(167)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Aretz S, Stienen D, Uhlhaas S, Stolte M,
Entius MM, Loff S, Back W, Kaufmann A, Keller KM, Blaas SH, et al:
High proportion of large genomic deletions and a genotype phenotype
update in 80 unrelated families with juvenile polyposis syndrome. J
Med Genet. 44:702–709. 2007.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Latchford AR, Neale K, Phillips RK and
Clark SK: Juvenile polyposis syndrome: A study of genotype,
phenotype, and long-term outcome. Dis Colon Rectum. 55:1038–1043.
2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Patel R and Hyer W: Practical management
of polyposis syndromes. Frontline Gastroenterol. 10:379–387.
2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gammon A, Jasperson K, Kohlmann W and Burt
RW: Hamartomatous polyposis syndromes. Best Pract Res Clin
Gastroenterol. 23:219–231. 2009.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Liu S, Ma Y, You W, Li J, Li JN and Qian
JM: Hamartomatous polyposis syndrome associated malignancies: Risk,
pathogenesis and endoscopic surveillance. J Dig Dis. 22:444–451.
2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chen YW, Tu JF, Shen WJ, Chen WY and Dong
J: Diagnosis and management of a solitary colorectal juvenile polyp
in an adult during follow-up for ulcerative colitis: A case report.
World J Gastroenterol. 26:877–882. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zheng Z, Yin J, Li Z, Ye Y, Wei B, Wang X,
Tian Y, Li M, Zhang Q, Zeng N, et al: Protocol for expanded
indications of endoscopic submucosal dissection for early gastric
cancer in China: A multicenter, ambispective, observational,
open-cohort study. BMC Cancer. 20(801)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zbuk KM and Eng C: Hamartomatous polyposis
syndromes. Nat Clin Pract Gastroenterol Hepatol. 4:492–502.
2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Dickey W, Alderdice J and McConnell B:
Dysplastic change in the solitary juvenile polyp. Endoscopy.
28(641)1996.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Jelsig AM, Ousager LB, Brusgaard K and
Qvist N: Juvenile polyps in Denmark from 1995 to 2014. Dis Colon
Rectum. 59:751–757. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chow E and Macrae F: A review of juvenile
polyposis syndrome. J Gastroenterol Hepatol. 20:1634–1640.
2005.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Brosens LA, Langeveld D, Van Hattem WA,
Giardiello FM and Offerhaus GJ: Juvenile polyposis syndrome. World
J Gastroenterol. 17:4839–4844. 2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hirotani A, Sakai E, Nakajima A, Kawana K
and Nagase H: Endoscopic findings of atypical juvenile colonic
polyps. Gastrointest Endosc. 83:476–477. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chung EJ, Lee JY, Choe J, Chang HS, Kim J,
Yang DH, Ye BD, Byeon JS, Kim KJ, Yang SK, et al: Colonic chicken
skin mucosa is an independent endoscopic predictor of advanced
colorectal adenoma. Intest Res. 13:318–325. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Adamiec C, Folwarski M, Dubowik M, Adrych
K, Kaźmierczak-Siedlecka K and Makarewicz W: Kudo's pit pattern
classification for in vivo optical diagnosis and discrimination of
advanced colorectal polyps. Eur Rev Med Pharmacol Sci.
26:2832–2839. 2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hijos-Mallada G, Velamazán R, Marti R,
Chueca E, Arechavaleta S, Lué A, Gomollón F, Lanas A and Sostres C:
A patient self-made point-of-care fecal test improves diagnostic
accuracy compared with fecal calprotectin alone in inflammatory
bowel disease patients. Diagnostics (Basel).
11(2323)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hodgson-Parnell L, Spence O and Chapple K:
Solitary juvenile polyp as a cause of elevated faecal calprotectin
in an adult. BMJ Case Rep. 2018(bcr2018224770)2018.PubMed/NCBI View Article : Google Scholar
|