Introduction
Pulmonary fibrosis (PF) is induced by multiple
triggers, including apoptosis of alveolar epithelial cells (AECs),
activation of fibroblasts and deposition of large amounts of
collagen (1). Although
understanding of IPF pathogenesis, diagnostic evaluation and
treatment approaches has evolved (2-3), the
typical survival time of patients diagnosed with PF is 3-5 years,
as highlighted by a previous literature review (4). Currently, pirfenidone and nintedanib
are the most common commercially available antifibrotic drugs
(5). However, these drugs only
slow disease progression and do not restore damaged lung tissue and
function (6,7). Additionally, both pirfenidone and
nintedanib have strong gastrointestinal adverse effects (6,7).
Currently, lung transplantation is the most efficacious therapy for
PF (8). However, owing to factors
such as the need for donor matching, high cost of surgery, and use
of immunosuppressant drugs, the rate of lung transplantation is
low, with nearly 4500 performed in 2017, including 32.4% for PF
(9). Additionally, compared with
patients requiring transplantation for other reasons, patients with
PF have a high waiting list mortality rate of 18-67% (8). Therefore, affordable treatments or
drugs with low toxicity are needed.
The imbalance between fibrogenic and antifibrogenic
molecules in the lung may induce apoptosis and prevent normal
re-epithelialization of AECs, as well as promote fibroblastic
proliferation and alveolar epithelial-mesenchymal transition, all
of which are involved in the development of PF (10,11).
Ultrastructural studies have revealed that AEC death is notable in
the initial phase of PF (12,13).
The pulmonary histopathology of patients with PF and
bleomycin-induced PF animal models have revealed fibroblastic
proliferation in the area of apoptosis of AECs (14,15).
Apoptosis of AECs has also been observed in near-normal lung areas
(16,17).
During apoptosis, apoptotic signals [such as
transforming growth factor β (TGF-β)] induce intracellular cytokine
changes, leading to a loss of cellular structural integrity and
degradation of DNA and phagocytic clearance. Apoptosis is generally
classified into two pathways based on the signaling pathway
involved: Endogenous (mitochondrial-mediated) and exogenous (death
receptor-mediated) pathways (16).
Mitochondria are the control center of cell activity; they provide
energy via oxidative phosphorylation and mediate endogenous
apoptotic signaling pathways (18). The alteration of mitochondrial
transmembrane potential and membrane permeability promotes the
activation of downstream apoptosis-associated factors (such as Bax
and Bcl-2), leading to apoptosis. BH3-interacting domain death
agonist, a Bcl-2 family member, induces intrinsic apoptosis of AECs
by suppressing Bcl-2 and upregulating Bax/Bak (19).
The phosphoinositide 3-kinase (PI3K)/protein kinase
B (AKT) pathway is a signaling pathway composed of intracellular
signaling enzymes (20). PI3K,
when activated by extracellular signals, upregulates production of
the intracellular secondary messenger
phosphatidylinositol-3,4,5-trisphosphate (PIP3) via phosphorylation
of phosphatidylinositol-bisphosphate. PIP3 binds to specific
docking sites in the pleckstrin homology structural domain of AKT1
and induces conformational changes in AKT1(21). Activated AKT1 proteins translocate
to cellular compartments, such as the cytoplasm and nucleus, where
they phosphorylate various substrate proteins, thereby regulating
cellular functions such as those associated with proliferative
metabolism, migration and survival. Activation of PI3K/AKT has been
reported in PF tissue (20,22).
Activated AKT can directly regulate the expression of Bcl-2 and
other downstream factors, thereby affecting cellular apoptosis
(23).
Traditional Chinese medicine (TCM) has been utilized
in China for >2,000 years and is an important part of clinical
practice. Baicalein, quercetin, and safflower yellow pigment, all
extracted from Chinese herbs, effectively alleviate symptoms of
fibrosis in experimental animal models (24-26).
Furthermore, new dosages of antifibrotic drugs used in TCM have
been developed in the form of inhalants, which are convenient to
use, increase the concentration of drug in the lungs and exhibit a
good effect in the treatment of PF (27,28).
Therefore, research on the active components of Chinese herbal
medicines may contribute to the development of anti-PF drugs.
Oxymatrine (OMT), a monomer alkaloid isolated from
Sophora flavescens, has a tetracyclic quinoline structure
and is considered to be the primary component responsible for the
pharmacological effects of Sophora flavescens. These
pharmacological effects include anti-oxidative stress (29), anti-inflammatory (30), anti-tissue fibrotic (31), and tumor apoptosis-inducing effects
(32). OMT is commonly used in
tumor radiotherapy regimes; it is used to treat leukopenia caused
by chemotherapy and other factors (33,34).
In addition, OMT has been confirmed to regulate apoptosis (35,36).
Moreover, it regulates the PI3K/AKT signaling pathway in colitis
and hypoxic-ischemic brain damage (37,38).
Additionally, OMT significantly alleviates bleomycin-induced PF by
inhibiting the expression of inducible nitric oxide synthase
(39). However, to the best of our
knowledge, the mechanisms of action and role of OMT in the
regulation of PF remain unclear.
TGF-β is a key factor that regulates fibrosis and
tumorigenesis. TGF-β is a pleiotropic cytokine that has three
isoforms: TGF-β1, TGF-β2 and TGF-β3. These isoforms regulate
biological processes, including embryogenesis, cell
differentiation, organ development and immune response (40). TGF-β1 is involved in PF as a strong
pro-fibrotic mediator that promotes epithelial-mesenchymal
transition, apoptosis of epithelial cells, migration, production of
other pro-fibrotic mediators, recruitment of circulating
fibroblasts and activation, and proliferation and transformation of
fibroblasts into myofibroblasts (41). TGF-β1 was the first cellular factor
found to induce interstitial-epithelial transformation in the
alveolar epithelium (41).
TGF-β1-induced A549 cell line is an established model of PF
(42). Therefore, the present
study aimed to determine whether OMT decreases TGF-β1-induced PF by
inhibiting apoptosis and whether this mechanism is associated with
the PI3K/AKT pathway.
Materials and methods
Materials
The human lung AEC line A549 was purchased from
Beijing Solarbio Science & Technology Co., Ltd. Cellular short
tandem repeat typing of DNA from the A549 cell line showed no human
cell crossover contamination and the DNA matched the DNA of the
A549 strain found in the cell bank by 100%.
TGF-β1 was purchased from Sizhe Technology (cat. no.
CHE0029, http://www.sagene.com.cn/ms/). OMT
was purchased from Macklin (cat. no. 16837-52-8). LY294002 was
purchased from MedChemExpress (cat. no. 154447-36-6). JC-1
Mitochondrial Membrane Potential (MMP) Assay kit was purchased from
Beyotime Institute of Biotechnology (cat. no. C2006). Annexin
V-FITC/PI Fluorescence Double Staining Apoptosis Detection kit was
purchased from Elabscience Biotechnology, Inc. (cat. no.
E-CK-A211). Antibodies against β-actin (rabbit; cat. no. AF7018),
Bcl-2 (mouse; cat. no. BF9103) and phosphorylated (p)-PI3K (rabbit;
cat. no. AF3241) were obtained from Affinity Biosciences, Ltd.
Antibodies against AKT (rabbit; cat. no. YT0185) and p-AKT (rabbit;
cat. no. YP0006) were purchased from Ruiying Biotechnology.
Antibody against PI3K (mouse; cat. no. Bsm33219m) was purchased
from Bioss, Inc.; that against BAX (rabbit; cat. no. GB11690) was
purchased from Wuhan Seville Biotechnology Co., Ltd. The 5X
All-In-One MasterMix (with AccuRT Genomic DNA Removal kit) and
EvaGreen Express 2X qPCR MasterMix-No Dye were purchased from
Applied Biological Materials, Inc. (cat. no. G492;
MasterMix-ES).
The automatic medical PCR analysis system was from
Shanghai Hongshi Medical Technology Co., Ltd. (cat. no. SLAN-96S).
The electrophoresis apparatus (PowerPac Basic) was purchased from
Bio-Rad Laboratories, Inc. The chemiluminescence imaging system
(ChemiScope 6100), image acquisition software (ChemiScope Capture)
and analysis software (ChemiScope analysis) were purchased from
Shanghai Qingxiang Science Instrument Co., Ltd. The flow cytometer
(CytoFLEX) was purchased from Beckman Coulter, Inc. YOUPU series
ultrapure water heater was procured from Sichuan YOUPU Ultrapure
Technology Co., Ltd. (cat. no. upr-ii-10t). A low-speed centrifuge
was purchased from Scilogex, LLC (cat. no. dm0412s). A transmission
electron microscope (TEM) was purchased from Hitachi, Ltd. (cat.
no. HT7700).
Cell culture and treatment
A549 cells were cultured in RPMI-1640 medium
(Solebro; cat. no. 31800) supplemented with 10% fetal bovine serum
(Merck KGaA; cat. no. F8687) in a 5% CO2 incubator at
37˚C. The medium was changed once daily, digested and passaged
using 0.25% trypsin (Merck KGaA; cat. no. 9002). The cells were
passaged at 80% confluency. Cells of the same passage were used in
each experiment and the experiments were repeated using different
batches of cells. A cell model of PF was induced using 5 ng/ml
TGF-β1, as previously described (34). The experimental groups were as
follows: Control (cells were cultured in complete medium without
any treatment); TGF-β1 (cells were treated with 5 ng/ml TGF-β1);
OMT (cells were treated with 5 ng/ml TGF-β1 and 0.25, 0.50 or 1.00
mg/ml OMT); and OMT + LY294002 (cells were treated with 5 ng/ml
TGF-β1, 1 mg/ml OMT, and 25 µmol/l LY294002). The OMT + LY294002
group was pretreated with LY294002 for 2 h, and then TGF-β1 and OMT
were administered simultaneously. OMT 0.25, 0.5, and 1.0 mg/ml
concentrations were selected based on a previous study in which OMT
attenuated fibroblast proliferation (43). All treatments were performed for 48
h at 26˚C.
Cell resuspension
The cryopreserved cells (stored in liquid
nitrogen/-80˚C freezer) were placed in a water bath (37˚C) for ~1
min to allow liquid from cells to thaw. Subsequently, 1 ml cell
suspension was pipetted into a 15-ml centrifuge tube on an
ultraclean stage and 10 ml complete medium [50 ml fetal bovine
serum into 500 ml RPMI 1640 medium (including 1%
penicillin/streptomycin antibody)] was added. After centrifugation
at 200 x g for 6 min in an ordinary centrifuge at room temperature
(~26˚C), the supernatant was discarded and 2 ml complete medium was
added to the cell pellet, which was suspended in the medium using a
pipette to mix. The cell suspension was seeded on a 10-cm cell
culture dish at a 1:3 ratio of inoculation. Thereafter, the cells
were mixed in the dish and incubated at 37˚C under 5%
CO2 for culture.
Cell passages
Cell passaging was performed when the cells reached
~90% confluence. The complete medium, pancreatin and sterile 1X
phosphate-buffered saline (PBS) were pre-warmed to 37˚C. The old
culture medium was discarded, and the cells in the dish were washed
twice with PBS, after which 1 ml 0.25% pancreatin EDTA (0.01%)
digestion solution was added to cover the bottom of the dish. The
digestion was performed at 26˚C until the bottom of the dish showed
a frosted appearance. The dish was placed under an inverted light
microscope to observe whether the intercellular space had increased
and whether the cells had shrunk and were free-floating at the
bottom of the dish, indicating that digestion was complete. The
digestion was terminated by addition of complete medium (3 ml), and
cells were suspended by pipetting gently against the bottom of the
dish. On an ultraclean table, the suspension was pipetted into a
15-ml centrifuge tube and centrifuged at 26˚C at 200 x g for 6 min.
The supernatant was discarded, complete medium was added, and the
cell pellet was resuspended using a pipette. Subsequently, cells
were seeded on a 10-cm cell culture dish at a 1:3 ratio, followed
by mixing the cells in the dish and incubation at 37˚C under 5%
CO2 for culture.
Cell cryopreservation
The cells at the top of the culture were collected,
and cell freezing was performed when the cells reached ~90%
confluence. The digested cells were centrifuged to pellet the cells
at 200 x g for 5 min. The supernatant was discarded, and 1 ml
pre-chilled cell cryoprotectant solution (formulated by adding 1 ml
dimethyl sulfoxide to 9 ml fetal bovine serum; the serum was
configured as a mixture and stored at -20˚C after mixing) was added
to the cell pellet. Cells were resuspended using a pipette and
aliquoted into cryovials. Following gradient freezing (4˚C for 30
min, -20˚C for 1 h and -80˚C for 24 h), the cryopreserved cells
were transferred to a liquid nitrogen tank for storage at 26˚C.
Morphological changes
The cells were digested with trypsin for ~1 min at
26˚C. Subsequently, an inverted light microscope was used to check
whether the cells were round and bright and whether the
intercellular gap had increased, after which, 500 µl complete
medium was added immediately to stop digestion. The cell suspension
was transferred to a 15-ml bullet head centrifuge tube and
centrifuged at 1,000 x g for 5 min at 25˚C. After discarding
supernatant, 200 µl 2.5% glutaraldehyde was added and the cells
were fixed overnight at 4˚C. Subsequently, the cells were washed
three times with PBS, fixed with 1% osmium for 2 h at 26˚C and
washed three times with PBS. The cells were subjected to ethanol
and acetone gradient ascending dehydration at 26˚C (50% ethanol and
dehydrated for 10 min → 70% ethanol for 10 min → 90% ethanol for 10
min → 90% acetone for 10 min → 100% acetone three times for 10 min
each), epoxy resin infiltration, and embedding for 2 h. Samples
(50-70-nm thick) were obtained using ultramicrotome sectioning; the
sections were subjected to double staining with uranyl acetate and
lead citrate (cells were stained with 2% uranyl acetate for 20 min
and lead dye solution for 5 min at 26˚C and observed and imaged
under a TEM at x1,500 magnification).
MMP analysis
Cells (1x106) were centrifuged at 300 x g
for 5 min at 26˚C. The supernatant was discarded and cells were
resuspended in 500 µl JC-1 working solution and incubated for 20
min at 37˚C. Following centrifugation (300 x g, 5 min) at 26˚C, the
cells were washed once with pre-cooled 1X JC-1 assay buffer.
Subsequently, cells were centrifuged (300 x g, 5 min) at 26˚C and
supernatant was discarded. The cells were resuspended in 500 µl 1X
JC-1 assay buffer. Flow cytometry (CytoFLEX, Beckman Coulter, Inc.)
using JC-1 MMP Assay kit was used to analyze MMP. The data were
analyzed using CytExpert 2.3 (Beckman Coulter, Inc.). A 488-nm
laser was selected, and the FITC and phycoerythrin (PE) channels
were used.
Cell apoptosis assay
Following aforementioned drug treatment, the cells
were digested with 0.25% trypsin (without EDTA) for 6 min at 26˚C
and collected. The cells were washed twice with pre-cooled PBS and
resuspended in 100 µl 1X binding buffer. Annexin V-FITC (5 µl) was
added and the cells were incubated at 2-8˚C for 15 min in the dark.
Subsequently, 10 µl PI was added and cells were incubated at 2-8˚C
under dark conditions for 5 min. Thereafter, apoptosis was analyzed
using flow cytometry.
Western blotting
RIPA lysis solution (Beyotime Institute of
Biotechnology; cat. no. P0013B) was used to extract total protein
from A549 cells. The total protein concentration was determined
using the bicinchoninic acid method and bovine serum albumin
(PA115, Tiangen Biochemical Technology Co., Ltd.) as a standard.
Proteins (1 ml) were separated using SDS-PAGE on a 10% gel. After
transferring proteins onto PVDF membranes, 5% skimmed milk powder
in 5% PBS-Tween was used to block the membranes for 1 h at 26˚C.
Primary antibodies against Bcl-2, BAX, PI3K, p-PI3K, AKT, p-AKT and
β-actin (all 1:1,000) were added, and the membranes were incubated
overnight at 4˚C. The corresponding secondary antibody (1:3,000,
Wuhan Saiwei Biotechnology Co., Ltd., GB23303 GB23301) was added,
and the membranes were incubated at 26˚C for 2 h. Subsequently, a
Pro-light horseradish peroxidase chemiluminescence substrate (Merck
KGaA; cat no. WBKLS) was added and signals of the immunoreactive
protein bands were captured on a film (ChemiScope analysis 1.0,
Shanghai Qingxiang Science Instrument Co., Ltd.), which was
developed in a dark room. The expression of the protein of interest
was normalized to the expression of β-actin, an internal reference
protein.
Reverse transcription-quantitative
(RT-q)PCR
A549 cell suspension (2x105 cells/ml) was
treated with RNase and subjected to total cellular RNA extraction
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's instructions.
Total RNA concentration was quantified using a nucleic acid
analyzer (Kaiao Technology Co., Ltd.; cat. no. k2900). RT was
performed to obtain cDNA, which was used for PCR amplification
using the primer pairs listed in Table
I. For qPCR, a 20 µl reaction mixture containing 2 µl cDNA, 1.2
µl 7.5 µM primer mix of forward and reverse primers, 10 µl 2X qPCR
master mix, and 6.8 µl double-distilled water was used. The
thermocycling conditions were as follows: Initial denaturation at
95˚C for 10 min, followed by 40 cycles of denaturation at 95˚C for
10 sec and annealing and extension at 60˚C for 30 sec. The
amplification and dissolution curves were obtained, and the data
were analyzed using the 2-∆∆Cq method (44). β-actin was used as the housekeeping
gene.
 | Table IPrimer pairs for reverse
transcription-quantitative PCR. |
Table I
Primer pairs for reverse
transcription-quantitative PCR.
| Gene | NCBI gene ID | NCBI search
no. | Forward primer,
5'→3' | Reverse primer,
5'→3' | Product length,
bp | Melting
temperature, ˚C |
|---|
| β-actin | 60 | NM_001101 |
AATCTGGCACCACACCTTCTACAA |
GGATAGCACAGCCTGGATAGCAA | 172 | 60 |
| PI3K | 5290 | NM_006218 |
CGGTGACTGTGTGGGACTTATTGAG |
TGTAGTGTGTGGCTGTTGAACTGC | 111 | 60 |
| AKT | 207 | NM_001014431 |
TCCTCCTCAAGAATGATGGCA |
GTGCGTTCGATGACAGTGGT | 181 | 60 |
| Bax | 581 | NM_001291428 |
CGAACTGGACAGTAACATGGAG |
CAGTTTGCTGGCAAAGTAGAAA | 157 | 60 |
| Bcl-2 | 596 | NM_000633 |
GACTTCGCCGAGATGTCCAG |
GAACTCAAAGAAGGCCACAATC | 129 | 60 |
| Caspase-3 | 836 | NM_001354777 |
CCAAAGATCATACATGGAAGCG |
CTGAATGTTTCCCTGAGGTTTG | 185 | 60 |
Statistical analysis
The experimental data were analyzed using GraphPad
Prism 9 (GraphPad Software, Inc.). Data that followed a normal
distribution are expressed as the mean ± standard deviation (n=3).
One-way ANOVA followed by Tukey's post hoc test was used for
comparison between ≥3 groups. P<0.05 was considered to indicate
a statistically significant difference.
Results
OMT attenuates TGF-β1-induced
morphology damage
The ultrastructure of cells in each group was
observed under a TEM (Fig. 1). In
the control group, A549 cells showed clear nucleoli in small clumps
with uniform nucleoplasm and cytoplasm without swelling, no
discernible swelling of the mitochondria, homogeneous matrix,
orderly cristae and no discernible dilatation of the rough
endoplasmic reticulum (Fig. 1A).
Additionally, the structure of each organelle was clear. In the
TGF-β1 group, A549 cells showed loss of structural integrity,
nuclear pyknosis, increased cytoplasmic electron density, blebbing,
unclear organelle structure and vacuolated intra-organelle
autophagosomes (Fig. 1B). In the
0.25 mg/ml OMT group, nuclear pyknotic electron density was
increased, chromatin was homogenized, cytoplasmic electron density
was increased, and a notable number of vacuoles in organelles and
small number of autophagosomes were present in A549 cells (Fig. 1C). Additionally, pyknosis of
mitochondria was observed. In the 0.5 mg/ml OMT group, A549 cells
exhibited indented nuclear membranes and relatively uniform
nucleoplasm. Additionally, the cytoplasm was uniform without
discernible swelling and few vacuoles in organelles and
autophagosomes in cytoplasm were observed (Fig. 1D). Notable mitochondrial pyknosis
was observed. In the 1.0 mg/ml OMT group, A549 cells exhibited
indented nuclear membranes and a relatively uniform nucleoplasm and
cytoplasm without discernible swelling. A small number of vacuoles
in organelles and a few lipid droplets were present in the cells.
Furthermore, the structure of the mitochondria and endoplasmic
reticulum was unremarkable (Fig.
1E). The images of the cell culture are shown in Fig. S1.
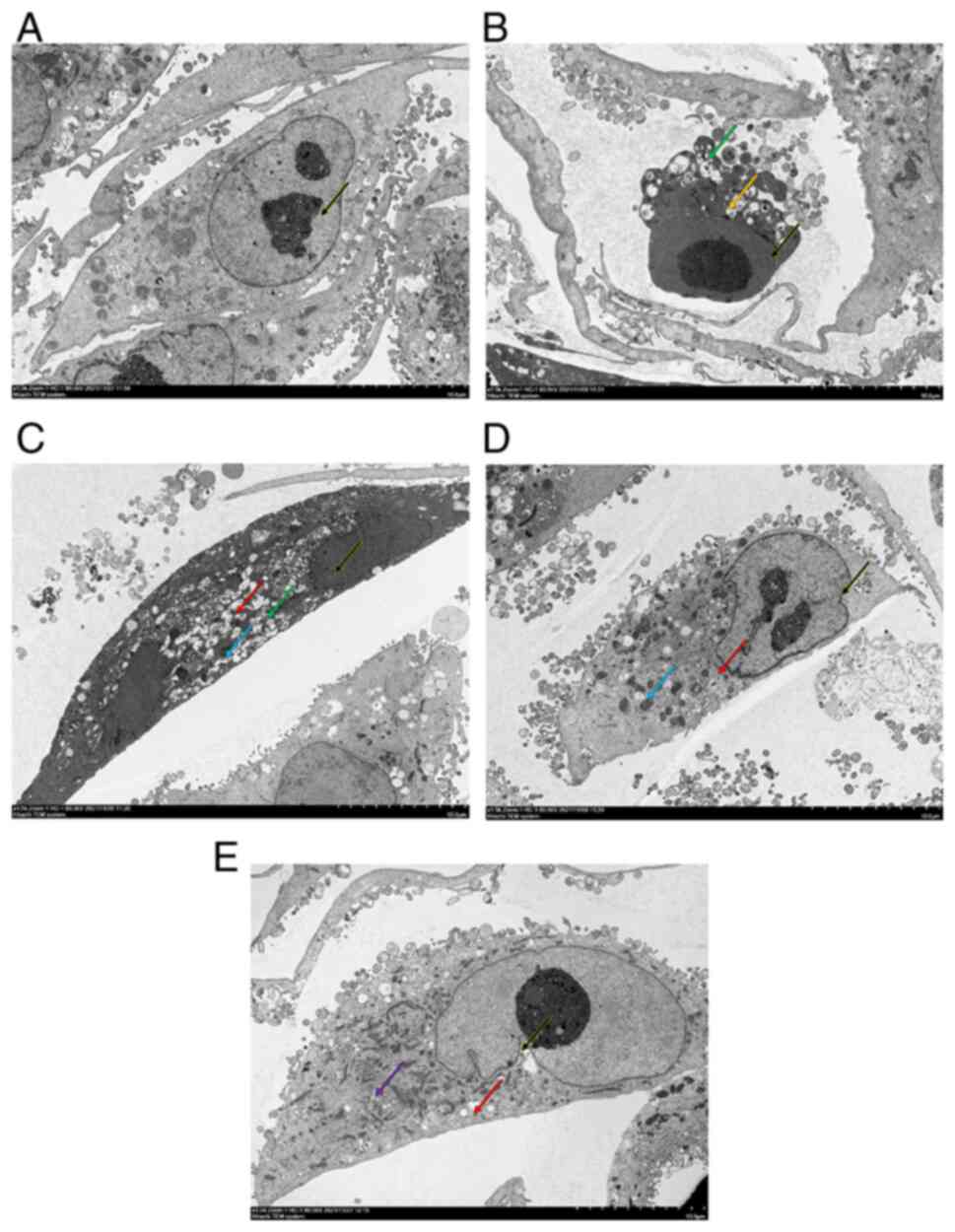 | Figure 1OMT inhibits TGF-β1-induced
ultrastructural changes in A549 cells. (A) Control (black arrow,
clear nucleoli). (B) TGF-β1 (black arrow, nuclear pyknosis; green
arrow, autophagosomes; yellow arrow, cell membrane blebbing). (C)
0.2 (black arrow, nuclear pyknosis; green arrow, autophagosomes;
blue arrows, mitochondrial pyknosis; and red arrow, organelle
vacuole), (D) 0.5 (black arrow, nuclear pyknosis; blue arrow,
mitochondrial pyknosis; and red arrow, organelle vacuolization),
and (E) 1.0 mg/ml (Black arrows, pyknosis; purple arrows, lipid
droplets; red arrows, organelle vacuole) OMT. Magnification,
x1,500. OMT, oxymatrine. |
OMT prevents TGF-β1-induced apoptosis
of A549 cells
Annexin V-FITC/PI assay indicated that the
percentage of apoptotic cells considerably increased following
TGF-β1 treatment; however, following OMT treatment, the fraction of
apoptotic cells decreased considerably. This suggested that OMT
treatment can reduce the TGF-β1-induced apoptosis of A549 cells
(Figs. 2 and S2).
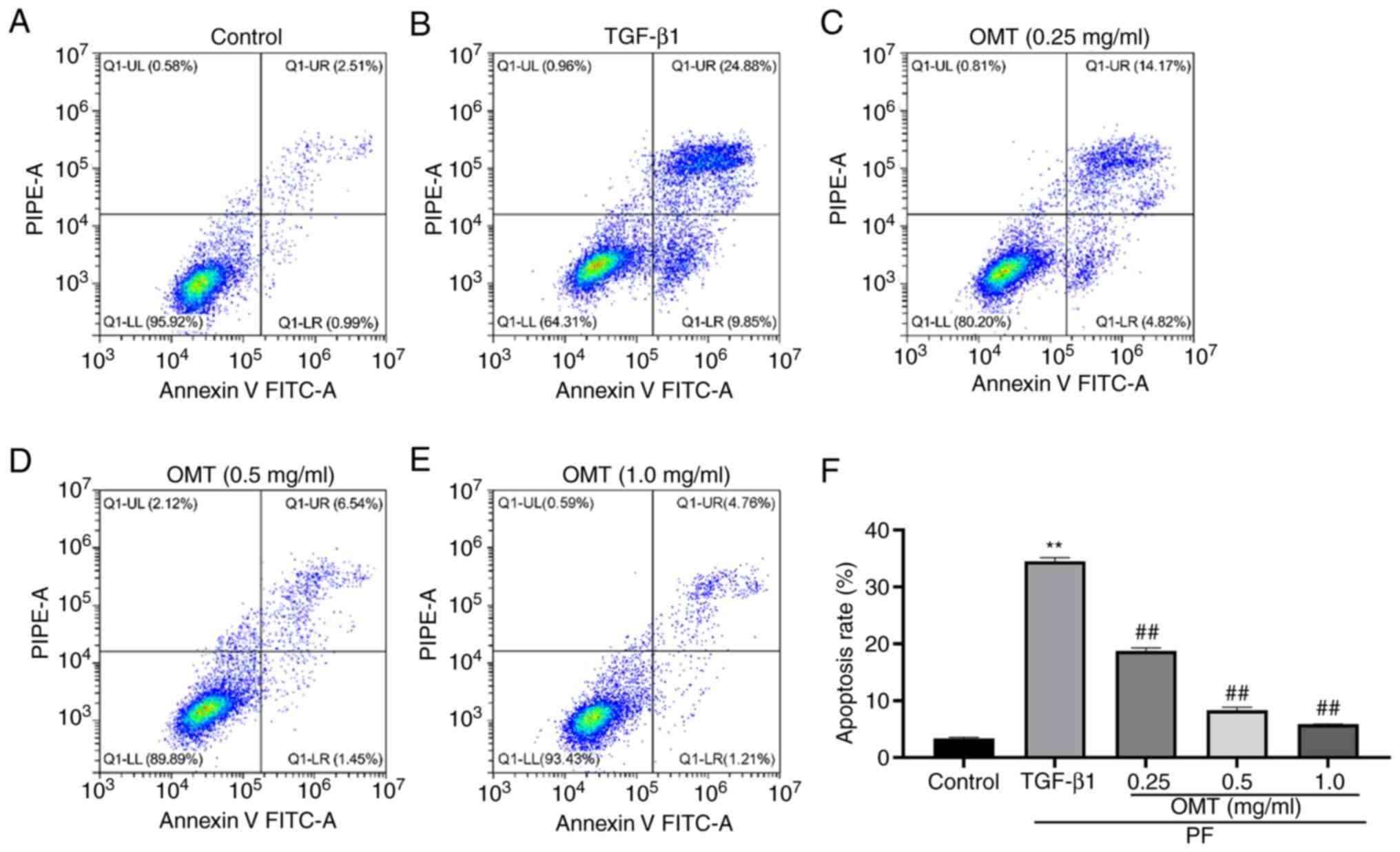 | Figure 2OMT decreases TGF-β1-induced
apoptosis of A549 cells. Number of apoptotic A549 cells in (A)
control, (B) TGF-β1 and (C) 0.2, (D) 0.5 and (E) 1.0 mg/ml OMT
groups. (F) Cell apoptosis rate. **P<0.01 vs.
Control; ##P<0.01 vs. TGF-β1. n=3. Q1-LL, Annexin-V
negative and PI-negative (live cells); Q1-LR, Annexin V-positive
and PI-negative (early apoptosis); Q1-UR, Annexin V-positive,
PI-positive cells (late apoptosis); Q1-UL, Annexin V-negative and
PI-positive (necrotic cells); OMT, oxymatrine; PF, pulmonary
fibrosis; PIPE-A, phycoerythrin channel detecting PI. |
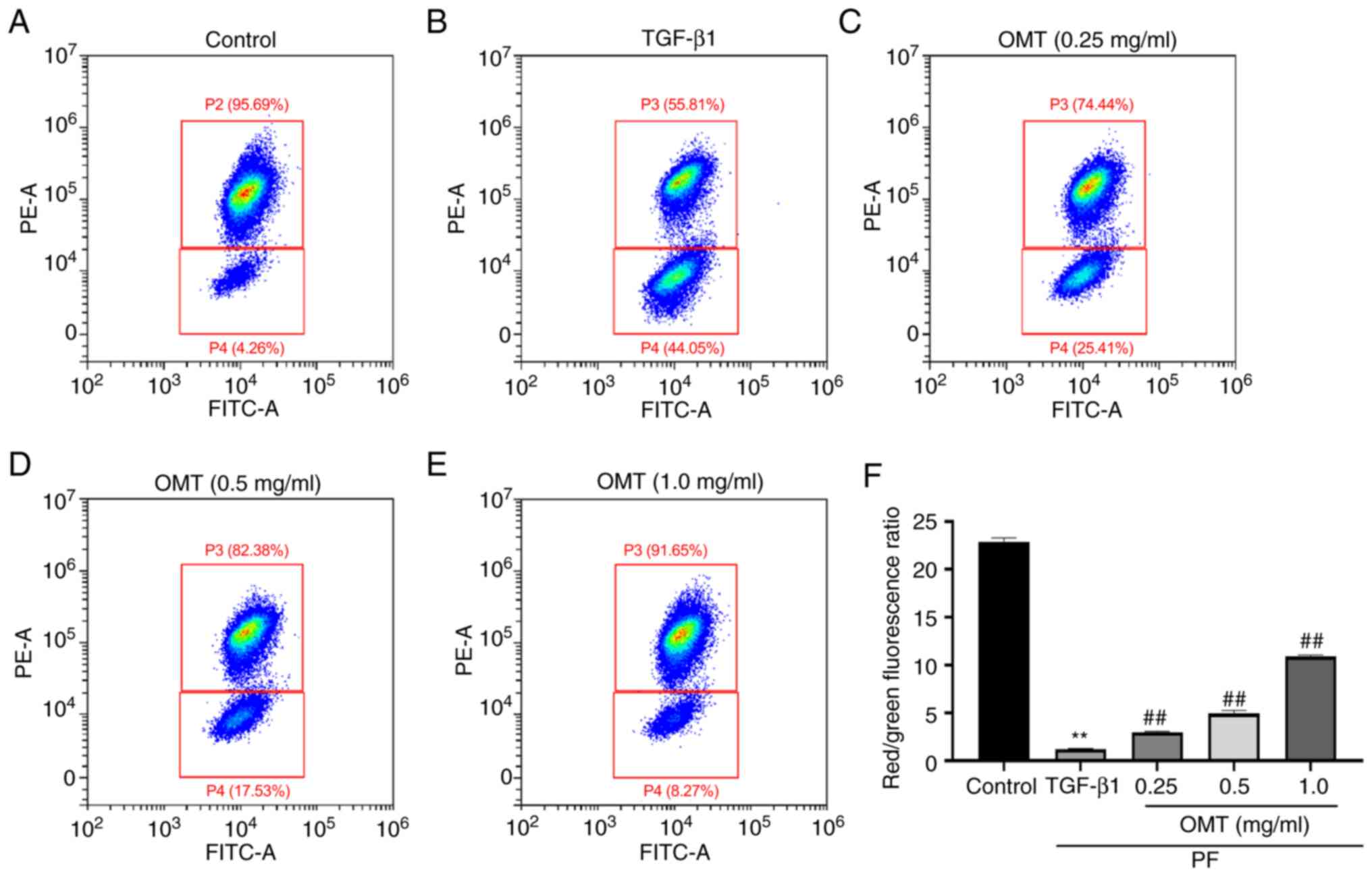
OMT attenuates TGF-β1-induced
dissipation of MMP
TGF-β1-treated A549 cells showed strong
intracellular green fluorescence and weak red fluorescence,
indicating that the MMP decreased following TGF-β1-induced damage
of AECs. Following OMT treatment, cells showed increased red and
decreased green fluorescence, suggesting that OMT alleviated
abnormal MMP induced by TGF-β1 (Fig.
3, Fig. S3).
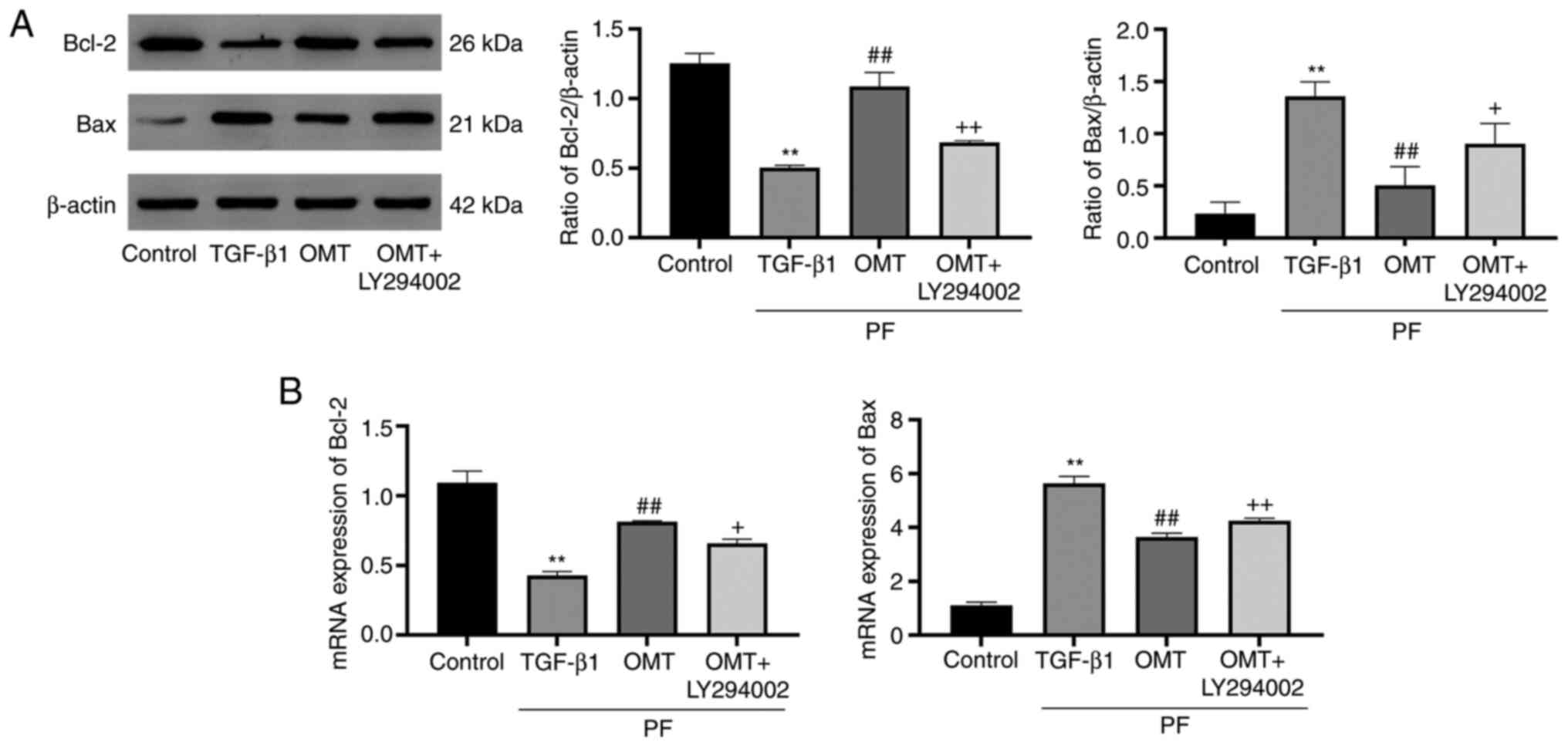
OMT inhibits cell apoptosis by
activating mitochondrial apoptotic signaling
The expression of apoptosis-associated proteins
downstream of the PI3K pathway was investigated (Fig. 4). The expression of Bax
(pro-apoptotic protein) was upregulated following TGF-β1 treatment
compared with that in the control group; however, treatment with
OMT inhibited upregulation of Bax expression. Conversely,
expression of Bcl-2 was inhibited by TGF-β1 treatment but increased
by OMT treatment. These results suggested that OMT inhibited
TGF-β1-mediated activation of mitochondrial apoptotic signaling. In
the OMT + LY294002 group, expression of Bax was notably inhibited
and that of Bcl-2 was enhanced compared with those in the OMT group
(Fig. 5A). PCR analysis indicated
that OMT could enhance mRNA expression of Bcl-2 and inhibit Bax
compared with the control group (Fig.
5B). These findings suggested that OMT inhibited
TGF-β1-mediated activation of mitochondrial apoptotic
signaling.
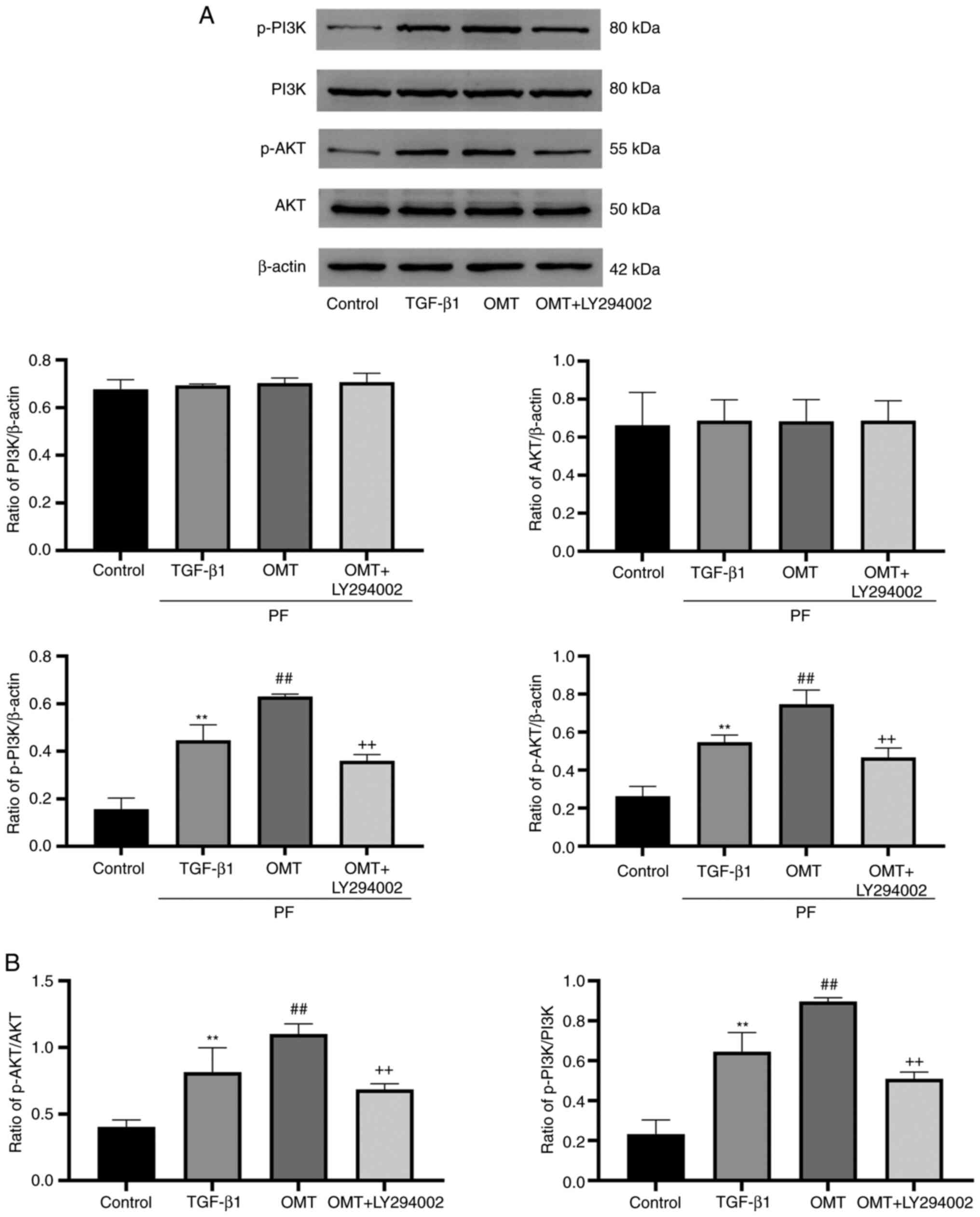
OMT inhibits cell apoptosis by
activating PI3K pathway
The expression of proteins associated with the
PI3K/AKT signaling pathway was examined (Fig. 5). The total protein expression of
PI3K and AKT in the experimental groups was not significantly
different. TGF-β1-treated cells showed upregulation of p-AKT and
p-PI3K expression compared with the control group. Additional OMT
treatment augmented these effects. Moreover, upregulation of p-AKT
and p-PI3K was reversed following OMT + LY294002 treatment
(Fig. 5A). Collectively, these
results suggest that OMT activated the PI3K/AKT pathway.
Discussion
Although lung transplantation and medicines
(pirfenidone and nintedanib) can be used to treat PF, these
therapies are associated with adverse effects. Gastrointestinal and
skin-associated adverse events are reported in pirfenidone
treatment (45). By contrast,
antifibrotic therapy based on natural chemicals may lower the risk
of adverse effects while acting on various targets (46). The lipid disorders of lung fibrosis
can be corrected upon baicalin treatment. In addition, treatment
with dexamethasone does not improve pf but leads to the
accumulation of fat in the liver (47). Consequently, the use of natural
substances in the treatment of PF is becoming increasingly popular
(24-26).
The present study investigated if OMT prevents apoptosis in PF and
inhibits processes involved in apoptosis.
Apoptosis is a cell death mechanism triggered by
activation of certain signals and subsequent gene regulation
(48). It is key for clearing
cells that have the potential to develop abnormally and preserving
homeostasis. The alveolus is the origin of PF and early lesions of
PF include alveolar epithelial injury and alveolitis (49). Additionally, apoptosis of AECs is
key in the development of PF (50). Studies have demonstrated that OMT
exerts its protective effect by inhibiting apoptosis (37,38).
Therefore, the present study tested the hypothesis that OMT
alleviates PF by inhibiting apoptosis and investigated the
potential underlying mechanisms.
AECs are classified into types I and II, of which
type I cells are less abundant, accounting for ~15% of alveolar
cells. However, the area occupied by type I cells accounts for 95%
of the entire alveolar area and their primary function is to
maintain alveolar structure and perform gas exchange (51). By contrast, type II cells are
abundant, accounting for 85% of alveolar cells, but only 5% of
alveolar area. Type II cells proliferate to replace type I cells
that are cleared following aging-related cellular damage and
essentially function as stem cells; additionally, they synthesize
alveolar surfactant, participate in immune regulation, and adjust
the fluid balance of the alveolus (52,53).
However, type II cells, as alveolar epithelial stem cells, cannot
divide sufficiently to replenish damaged type I epithelial cells
(11). The injury is repaired by
fibroblasts, which proliferate and induce deposition of a large
amount of extracellular matrix, leading to PF (54). A549 cells, which are human alveolar
basal epithelial cells that exhibit characteristics of type II
AECs, are widely used in the study of PF (55,56).
These cells were therefore selected for use in the present study to
establish a cell model of PF.
A decrease in MMP occurs during apoptosis in all
types of cells. Once the MMP decreases, the cell enters the
irreversible stage of apoptosis (53). In the present study, MMP of
TGF-β1-treated A549 cells increased significantly following OMT
treatment. Concurrently, morphological changes and the number of
apoptotic cells were also ameliorated by OMT treatment. Bcl-2
family members participate in mitochondria-mediated apoptosis by
regulating the release of pro-apoptotic factors via mitochondrial
membrane pore formation proteins (57). Bcl-2 localizes to the nuclear and
mitochondrial membranes and is widely distributed in hematopoietic
and epithelial cells, lymphocytes, and nerve cells (58). By contrast, pro-apoptotic protein
Bax primarily exists in the cytoplasm under normal conditions
(59,60). Upon induction of apoptosis, Bcl-2
family proteins are localized to the mitochondrial membrane by
various mechanisms, such as isomerization and phosphorylation,
causing changes in mitochondrial membrane permeability and
initiation of the apoptotic response (18). In the present study, following OMT
treatment, expression of the pro-apoptotic gene Bax significantly
decreased, whereas that of the apoptotic-inhibitory gene Bcl-2
significantly increased. These results support the hypothesis that
OMT exerts an anti-apoptotic effect through the mitochondrial
apoptosis pathway.
The PI3K/AKT pathway is involved in various
physiological processes, including apoptosis, proliferation, and
metabolism (61). Following
phosphorylation, PI3K-activated AKT regulates downstream molecules,
including Bad, caspase-9, glycogen synthase kinase-3- and p21,
facilitating cell proliferation triggered by insulin and growth
factors and encouraging cell survival. Hence, AKT is a key
anti-apoptotic regulator (62).
The PI3K/AKT pathway has been reported to be abnormally active in
PF (63). OMT regulates the
PI3K/AKT pathway in hypoxic-ischemic brain damage and colitis
(31,38). It was hypothesized that PI3K/AKT
signaling is implicated in pathogenesis of PF and that OMT protects
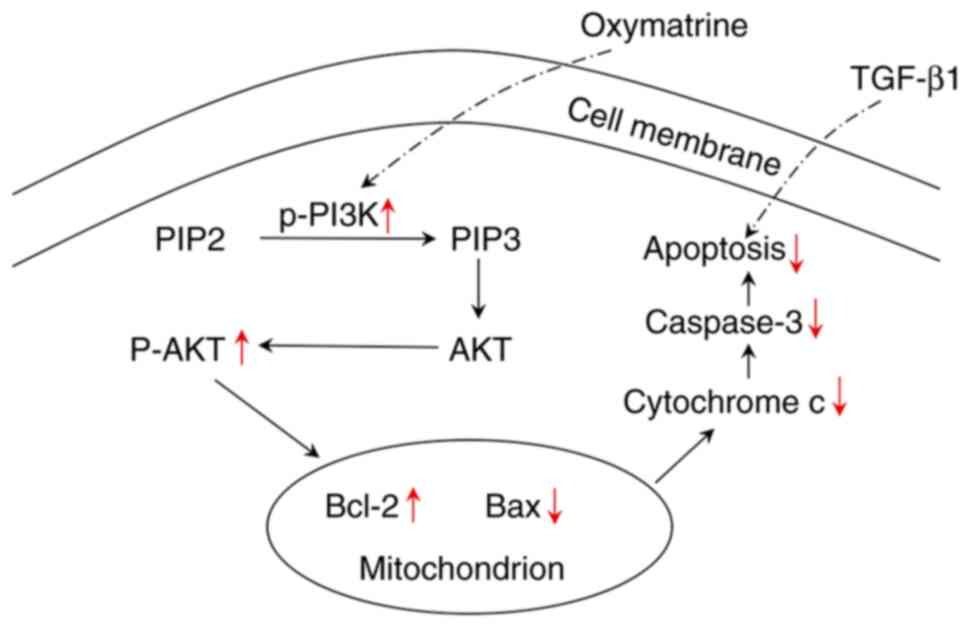
against PF partially via the PI3K/AKT pathway. In the present
study, OMT exhibited the potential to reverse PF by activating the
PI3K/AKT pathway (Fig. 6).
However, a previous review suggested that PI3K/AKT inhibitors are
beneficial in PF treatment (1).
Baicalin alleviates PF by decreasing p-AKT levels in lung
fibroblasts (22). Ligustrazine
attenuates paraquat-induced PF by blocking PI3K/AKT/mTOR signaling
in mouse lung tissue (64). The
differences in the findings of these studies could be attributed to
the different research subjects. PF is characterized by excessive
apoptosis of fine bronchial and AECs, destroying alveolar
structural integrity and inhibiting pulmonary fibroblast apoptosis
(13,65). During fibrosis, AECs undergo
injury, apoptosis, fibroblast proliferation, and fibroblast to
myofibroblast conversion; at this point, the main cells present in
the fibrotic tissue of the lung are fibroblasts and myofibroblasts.
Thus, inhibition of PI3K/AKT signaling, which promotes fibroblast
and myofibroblast apoptosis, abrogates fibroblast proliferation and
collagen production in response to bleomycin (66). In the present study, OMT
upregulated PI3K/AKT signaling and inhibited AEC apoptosis, thus
inhibiting PF.
The present study had some limitations. The present
study primarily investigated the inhibition of the PI3K/AKT
pathway. Further research on PI3K/AKT pathway activators is needed
to corroborate the present findings. Additionally, the PI3K/AKT
signaling pathway involves multiple cytokines and these downstream
cytokines may be regulated by factors other than PI3K. Thus, more
research on the effects of these downstream cytokines on the
antifibrotic activity of OMT is required. Furthermore, only in
vitro cell-based experiments were performed and further in
vivo research using animal models is needed to validate the
anti-PF properties of OMT. The lack of OMT-alone treatment group
for comparison was also a limitation of the present study.
In summary, the present study showed that OMT
attenuates TGF-β1-mediated mitochondrial apoptosis signaling in
AECs by activating the PI3K/AKT signaling pathway. Based on these
findings, OMT may be a potential therapeutic agent for PF.
Supplementary Material
Micrographs of cells (40x
magnification). (A) Control cells grown in a complete medium
without any treatment. (B) TGF-β1 group treated with 5 ng/ml
TGF-β1. (C) OMT cells treated with 5 ng/ml TGF-β1 and 1.0 mg/ml
OMT. (D) OMT + LY294002 cells treated with 5 ng/ml TGF-β1, 1.0
mg/ml OMT and 25 μmol/l LY294002. OMT, oxymatrine.
Cell apoptosis analysis (A) Physical
parameter plot. (B) Plot excluding adherent cells. (C) Plot
reporting the signal from the FITC channel detecting Annexin V and
PE channel detecting PI. Q1-LL, Annexin-V negative and PI-negative
(live cells); Q1-LR, Annexin V-positive and PI-negative (early
apoptosis); Q1-UR, Annexin V-positive, PI-positive cells (late
apoptosis); Q1-UL, Annexin V-negative and PI-positive (necrotic
cells); SSC-A, side scatter area; SSC-H, side scatter height;
FSC-A, forward scatter area; PIPE-A, phycoerythrin channel
detecting PI.
Mitochondrial membrane potential
analysis. (A) Physical parameter plot. (B) Plot excluding adherent
cells. (C) Plot reporting the signal from the FITC channel
detecting JC-1 monomers and signal from the PE channel detecting
JC-1 polymer. SSC-A, side scatter area; FSC-H, forward scatter
height; PE, phycoerythrin.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Key Laboratory
of Geriatrics Respiratory Disease Education Department of Sichuan
[grant no. (2015) 603] and Research Fund of Chengdu Medical College
(grant no: CYFY2017GLPXH08 CYFY2018GLPXH01).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TF designed the study. PZ, QL and JQ performed
experiments. RD and WL analyzed the data. TF and RD wrote the
manuscript. TF and RD confirm the authenticity of all the raw data.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wang J, Hu K, Cai X, Yang B, He Q, Wang J
and Weng Q: Targeting PI3K/AKT signaling for treatment of
idiopathic pulmonary fibrosis. Acta Pharm Sin B. 12:18–32.
2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Yanagihara T, Sato S, Upagupta C and Kolb
M: What have we learned from basic science studies on idiopathic
pulmonary fibrosis? Eur Respir Rev. 28(190029)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Otoupalova E, Smith S, Cheng G and
Thannickal VJ: Oxidative stress in pulmonary fibrosis. Compr
Physiol. 10:509–547. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Richeldi L, Collard HR and Jones MG:
Idiopathic pulmonary fibrosis. Lancet. 389:1941–1952.
2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Somogyi V, Chaudhuri N, Torrisi SE, Kahn
N, Müller V and Kreuter M: The therapy of idiopathic pulmonary
fibrosis: What is next? Eur Respir Rev. 28(190021)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kropski JA and Blackwell TS: Progress in
understanding and treating idiopathic pulmonary fibrosis. Annu Rev
Med. 70:211–224. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tepede A and Yogaratnam D: Nintedanib for
idiopathic pulmonary fibrosis. J Pharm Pract. 32:199–206.
2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
George PM, Patterson CM, Reed AK and
Thillai M: Lung transplantation for idiopathic pulmonary fibrosis.
Lancet Respir Med. 7:271–282. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chambers DC, Cherikh WS, Harhay MO, Hayes
D Jr, Hsich E, Khush KK, Meiser B, Potena L, Rossano JW, Toll AE,
et al: The international thoracic organ transplant registry of the
international society for heart and lung transplantation:
Thirty-sixth adult lung and heart-lung transplantation Report-2019;
Focus theme: Donor and recipient size match. J Heart Lung
Transplant. 38:1042–1055. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Fattman CL: Apoptosis in pulmonary
fibrosis: Too much or not enough? Antioxid Redox Signal.
10:379–385. 2008.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Olajuyin AM, Zhang X and Ji HL: Alveolar
type 2 progenitor cells for lung injury repair. Cell Death Discov.
5(63)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Corrin B, Dewar A, Rodriguez-Roisin R and
Turner-Warwick M: Fine structural changes in cryptogenic fibrosing
alveolitis and asbestosis. J Pathol. 147:107–119. 1985.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Myers JL and Katzenstein AL: Epithelial
necrosis and alveolar collapse in the pathogenesis of usual
interstitial pneumonia. Chest. 94:1309–1311. 1988.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Li XP, Shu RJ, Filippatos G and Uhal BD:
Apoptosis in lung injury and remodeling. J Appl Physiol (1985).
97:1535–1542. 2004.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Uhal BD, Joshi I, Hughes WF, Ramos C,
Pardo A and Selman M: Alveolar epithelial cell death adjacent to
underlying myofibroblasts in advanced fibrotic human lung. Am J
Physiol. 275:L1192–L1199. 1998.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Barbas-Filho JV, Ferreira MA, Sesso A,
Kairalla RA, Carvalho CR and Capelozzi VL: Evidence of type II
pneumocyte apoptosis in the pathogenesis of idiopathic pulmonary
fibrosis (IFP)/usual interstitial pneumonia (UIP). J Clin Pathol.
54:132–138. 2001.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chen F, Gong L, Zhang L, Wang H, Qi X, Wu
X, Xiao Y, Cai Y, Liu L, Li X and Ren J: Short courses of low dose
dexamethasone delay bleomycin-induced lung fibrosis in rats. Eur J
Pharmacol. 536:287–295. 2006.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bock FJ and Tait SWG: Mitochondria as
multifaceted regulators of cell death. Nat Rev Mol Cell Biol.
21:85–100. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Budinger GR, Mutlu GM, Eisenbart J, Fuller
AC, Bellmeyer AA, Baker CM, Wilson M, Ridge K, Barrett TA, Lee VY
and Chandel NS: Proapoptotic Bid is required for pulmonary
fibrosis. Proc Natl Acad Sci USA. 103:4604–4609. 2006.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hu X, Xu Q, Wan H, Hu Y, Xing S, Yang H,
Gao Y and He Z: PI3K-AKT-mTOR/PFKFB3 pathway mediated lung
fibroblast aerobic glycolysis and collagen synthesis in
lipopolysaccharide-induced pulmonary fibrosis. Lab Invest.
100:801–811. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Engelman JA, Luo J and Cantley LC: The
evolution of phosphatidylinositol 3-kinases as regulators of growth
and metabolism. Nat Rev Genet. 7:606–619. 2006.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Zhao H, Li C, Li L, Liu J, Gao Y, Mu K,
Chen D, Lu A, Ren Y and Li Z: Baicalin alleviates bleomycin-induced
pulmonary fibrosis and fibroblast proliferation in rats via the
PI3K/AKT signaling pathway. Mol Med Rep. 21:2321–2334.
2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Schmitt-Ney M: The FOXO's advantages of
being a family: Considerations on function and evolution. Cells.
9(787)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Li H, Kan B, Song L, Liu Y and Jian X:
Role of the Hippo signaling pathway in safflower yellow pigment
treatment of paraquat-induced pulmonary fibrosis. J Int Med Res.
48(300060520905425)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Takano M, Deguchi J, Senoo S, Izumi M,
Kawami M and Yumoto R: Suppressive effect of quercetin against
bleomycin-induced epithelial-mesenchymal transition in alveolar
epithelial cells. Drug Metab Pharmacokinet. 35:522–526.
2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Sun X, Cui X, Chen X and Jiang X:
Baicalein alleviated TGF β1-induced type I collagen production in
lung fibroblasts via downregulation of connective tissue growth
factor. Biomed Pharmacother. 131(110744)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhou Y, Zhu WP, Cai XJ and Chen M:
Atomized paclitaxel liposome inhalation treatment of
bleomycin-induced pulmonary fibrosis in rats. Genet Mol Res.
15(10)2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Hu Y, Li M, Zhang M and Jin Y: Inhalation
treatment of idiopathic pulmonary fibrosis with curcumin large
porous microparticles. Int J Pharm. 551:212–222. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhang YY, Yi M and Huang YP: Oxymatrine
ameliorates doxorubicin-induced cardiotoxicity in rats. Cell
Physiol Biochem. 43:626–635. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Xiang X, Tu C, Li Q, Wang W, Huang X, Zhao
Z, Xiong H and Mei Z: Oxymatrine ameliorates imiquimod-induced
psoriasis pruritus and inflammation through inhibiting heat shock
protein 90 and heat shock protein 60 expression in keratinocytes.
Toxicol Appl Pharmacol. 405(115209)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wang D, Lou XQ, Jiang XM, Yang C, Liu XL
and Zhang N: Oxymatrine protects against the effects of
cardiopulmonary resuscitation via modulation of the TGF-β1/Smad3
signaling pathway. Mol Med Rep. 17:4747–4752. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ying XJ, Jin B, Chen XW, Xie J, Xu HM and
Dong P: Oxymatrine downregulates HPV16E7 expression and inhibits
cell proliferation in laryngeal squamous cell carcinoma Hep-2 cells
in vitro. Biomed Res Int. 2015(150390)2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Liu Y, Xu Y, Ji W, Li X, Sun B, Gao Q and
Su C: Anti-tumor activities of matrine and oxymatrine: Literature
review. Tumour Biol. 35:5111–5119. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Song WJ, Luo J, Wu T and Yao SK: Oral
oxymatrine preparation for chronic hepatitis B: A systematic review
of randomized controlled trials. Chin J Integr Med. 22:141–149.
2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Shi HJ, Zhou H, Ma AL, Wang L, Gao Q,
Zhang N, Song HB, Bo KP and Ma W: Oxymatrine therapy inhibited
epidermal cell proliferation and apoptosis in severe plaque
psoriasis. Br J Dermatol. 181:1028–1037. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Huang Y, Li X, Zhang X and Tang J:
Oxymatrine ameliorates memory impairment in diabetic rats by
regulating oxidative stress and apoptosis: Involvement of
NOX2/NOX4. Oxid Med Cell Longev. 2020(3912173)2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Chen Q, Duan X, Fan H, Xu M, Tang Q, Zhang
L, Shou Z, Liu X, Zuo D, Yang J, et al: Oxymatrine protects against
DSS-induced colitis via inhibiting the PI3K/AKT signaling pathway.
Int Immunopharmacol. 53:149–157. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Liu Y, Wang H, Liu N, Du J, Lan X, Qi X,
Zhuang C, Sun T, Li Y and Yu J: Oxymatrine protects neonatal rat
against hypoxic-ischemic brain damage via PI3K/AKT/GSK3β pathway.
Life Sci. 254(116444)2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Liu L, Lu W, Ma Z and Li Z: Oxymatrine
attenuates bleomycin-induced pulmonary fibrosis in mice via the
inhibition of inducible nitric oxide synthase expression and the
TGF-β/Smad signaling pathway. Int J Mol Med. 29:815–822.
2012.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Morikawa M, Derynck R and Miyazono K:
TGF-β and the TGF-β family: Context-dependent roles in cell and
tissue physiology. Cold Spring Harb Perspect Biol.
8(a021873)2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Kim KK, Sheppard D and Chapman HA: TGF-β1
signaling and tissue fibrosis. Cold Spring Harb Perspect Biol.
10(34)2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Zhang C, Zhu X, Hua Y, Zhao Q, Wang K,
Zhen L, Wang G, Lü J, Luo A, Cho WC, et al: YY1 mediates
TGF-β1-induced EMT and pro-fibrogenesis in alveolar epithelial
cells. Respir Res. 20(249)2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Chen X, Sun R, Hu J, Mo Z, Yang Z, Liao D
and Zhong N: Attenuation of bleomycin-induced lung fibrosis by OMT
is associated with regulation of fibroblast proliferation and
collagen production in primary culture. Basic Clin Pharmacol
Toxicol. 103:278–286. 2008.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Ruwanpura SM, Thomas BJ and Bardin PG:
Pirfenidone: Molecular mechanisms and potential clinical
applications in lung disease. Am J Respir Cell Mol Biol.
62:413–422. 2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Hosseini SA, Zahedipour F, Sathyapalan T,
Jamialahmadi T and Sahebkar A: Pulmonary fibrosis: Therapeutic and
mechanistic insights into the role of phytochemicals. Biofactors.
47:250–269. 2021.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Hu C, Wang Y, Fan Y, Li H, Wang C, Zhang
J, Zhang S, Han X and Wen C: Lipidomics revealed idiopathic
pulmonary fibrosis-induced hepatic lipid disorders corrected with
treatment of baicalin in a murine model. AAPS J. 17:711–722.
2015.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Kumar RK: Idiopathic pulmonary fibrosis:
An epithelial/fibroblasticcross-talk disorder. Respir Res.
3(29)2002.
|
|
50
|
Snijder J, Peraza J, Padilla M, Capaccione
K and Salvatore MM: Pulmonary fibrosis: A disease of alveolar
collapse and collagen deposition. Expert Rev Respir Med.
13:615–619. 2019.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Guillot L, Nathan N, Tabary O, Thouvenin
G, Rouzic PL, Corvol H, Amselem S and Clement A: Alveolar
epithelial cells: Master regulators of lung homeostasis. Int J
Biochem Cell Biol. 45:2568–2573. 2013.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Serrano-Mollar A: Alveolar epithelial cell
injury as an etiopathogenic factor in pulmonary fibrosis. Arch
Bronconeumol. 48:2–6. 2012.PubMed/NCBI View Article : Google Scholar : (In Spanish).
|
|
53
|
Lu J, Wu L, Wang X, Zhu J, Du J and Shen
B: Detection of mitochondria membrane potential to study CLIC4
knockdown-induced HN4 cell apoptosis in vitro. J Vis Exp.
137(56137)2018.PubMed/NCBI View
Article : Google Scholar
|
|
54
|
Hill C, Jones MG, Davies DE and Wang Y:
Epithelial-mesenchymal transition contributes to pulmonary fibrosis
via aberrant epithelial/fibroblastic cross-talk. J Lung Health Dis.
3:31–35. 2019.PubMed/NCBI
|
|
55
|
Wang YC, Liu JS, Tang HK, Nie J, Zhu JX,
Wen LL and Guo QL: miR 221 targets HMGA2 to inhibit bleomycin
induced pulmonary fibrosis by regulating TGF β1/Smad3-induced EMT.
Int J Mol Med. 38:1208–1216. 2016.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Liu Y, Li Y, Xu Q, Yao W, Wu Q, Yuan J,
Yan W, Xu T, Ji X and Ni C: Long non-coding RNA-ATB promotes EMT
during silica-induced pulmonary fibrosis by competitively binding
miR-200c. Biochim Biophys Acta Mol Basis Dis. 1864:420–431.
2018.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Braun F, de Carné Trécesson S,
Bertin-Ciftci J and Juin P: Protect and serve: Bcl-2 proteins as
guardians and rulers of cancer cell survival. Cell Cycle.
12:2937–2947. 2013.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Willis S, Day CL, Hinds MG and Huang DC:
The Bcl-2-regulated apoptotic pathway. J Cell Sci. 15:4053–4056.
2003.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Kuwano K, Hagimoto N, Tanaka T, Kawasaki
M, Kunitake R, Miyazaki H, Kaneko Y, Matsuba T, Maeyama T and Hara
N: Expression of apoptosis-regulatory genes in epithelial cells in
pulmonary fibrosis in mice. J Pathol. 190:221–229. 2000.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Youle RJ and Strasser A: The BCL-2 protein
family: Opposing activities that mediate cell death. Nat Rev Mol
Cell Biol. 9:47–59. 2008.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Franke TF, Hornik CP, Segev L, Shostak GA
and Sugimoto C: PI3K/AKT and apoptosis: Size matters. Oncogene.
22:8983–8998. 2003.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Liu R, Chen Y, Liu G, Li C, Song Y, Cao Z,
Li W, Hu J, Lu C and Liu Y: PI3K/AKT pathway as a key link
modulates the multidrug resistance of cancers. Cell Death Dis.
11(797)2020.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Sun Y, Zhang Y and Chi P: Pirfenidone
suppresses TGF-β1-induced human intestinal fibroblasts activities
by regulating proliferation and apoptosis via the inhibition of the
Smad and PI3K/AKT signaling pathway. Mol Med Rep. 18:3907–3913.
2018.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Liu MW, Su MX, Tang DY, Hao L, Xun XH and
Huang YQ: Ligustrazin increases lung cell autophagy and ameliorates
paraquat-induced pulmonary fibrosis by inhibiting PI3K/AKT/mTOR and
hedgehog signalling via increasing miR-193a expression. BMC Pulm
Med. 19(35)2019.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Moodley YP, Caterina P, Scaffidi AK, Misso
NL, Papadimitriou JM, McAnulty RJ, Laurent GJ, Thompson PJ and
Knight DA: Comparison of the morphological and biochemical changes
in normal human lung fibroblasts and fibroblasts derived from lungs
of patients with idiopathic pulmonary fibrosis during FasL-induced
apoptosis. J Pathol. 202:486–495. 2004.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Lu Y, Azad N, Wang L, Iyer AK, Castranova
V, Jiang BH and Rojanasakul Y: Phosphatidylinositol-3-kinase/AKT
regulates bleomycin-induced fibroblast proliferation and collagen
production. Am J Respir Cell Mol Biol. 42:432–441. 2010.PubMed/NCBI View Article : Google Scholar
|