Introduction
Improving the survival prognosis of patients with
end-stage renal disease (ESRD) is the ultimate goal of renal
replacement therapy, and it is also an important criterion for
determining the clinical efficacy of dialysis mode. Although
haemodialysis treatment has made great progress, the survival
prognosis of maintenance haemodialysis patients is still not
optimistic. Previous studies reported that the annual mortality
rate of haemodialysis patients in Japan is 9.8-10.2% (1), and the annual mortality rate of
dialysis patients is 23.6% in the United States (2). The mortality rate of dialysis
patients in China is 20% (3).
Cardiovascular complications are the leading cause of death in
end-stage renal disease. With the continuous deterioration of renal
function, the incidence of cardiovascular complications in patients
with end-stage renal disease is markedly increased. Deaths caused
by cerebrovascular disease (CVD) account for 50% of the total
mortality of end-stage renal disease (4). Infective endocarditis (IE) is the
main complication of ESRD-related CVD.
IE with acute injury of the heart valve or
ventricular wall lining is caused by invasion of the endocardium by
bacteria, fungi, and other pathogenic microorganisms. Haemodialysis
patients are more likely to develop IE than nonhaemodialysis
patients. The incidence of haemodialysis complicated with IE is
2-5% (5), and the mortality rate
of haemodialysis patients with IE is extremely high.
The aim of the present retrospective study was to
explore the risk factors and survival prognosis of haemodialysis
patients with IE to further improve the quality of treatment and
reduce mortality.
Patients and methods
Patients
A total of 101 IE patients admitted to Affiliated
Hangzhou First People's Hospital, Zhejiang University School of
Medicine (Hangzhou, China), from January 1, 2012, to April 1, 2022,
were retrospectively included (50 males and 51 females; their age
was 57.9±18.1 years old). The present study was performed according
to the Declaration of Helsinki and approved by the local ethics
board of Affiliated Hangzhou First People's Hospital, Zhejiang
University School of Medicine. Individual patient consents were
waived on the condition that all patients were deidentified before
analysis since this study was a retrospective analysis.
The inclusion criteria were as follows: i) The age
of the patients was ≥18 years old; ii) the echocardiography of the
heart showed the formation of valvular vegetations; and iii) the
diagnosis met the modified Duke diagnostic criteria (6).
The exclusion criteria were as follows: i) Patients
with mental disorders who could not cooperate and ii) patients who
were identified as having an infection in other parts of the
body.
Groups
In the IE with haemodialysis (HD-IE) group, 15 IE
patients were diagnosed with end-stage renal disease and had been
on haemodialysis for >3 months. Among them, the primary disease
was chronic glomerulonephritis in seven cases, diabetic nephropathy
in six cases, hypertensive nephropathy in one case, and amyloid
nephropathy in one case. Vascular access was used in eight patients
with autologous arteriovenous fistula. A total of seven patients
had long-term indwelling subcutaneous tunnel polyester sleeve
catheters, and the frequency of dialysis was three times a week.
The IE without haemodialysis (NHD-IE) group consisted of 86 IE
patients with normal renal function.
Clinical data
The demographics, primary disease, and clinical
indicators of the two groups were collected and compared. The
clinical indicators included blood leukocytes, haemoglobin, blood
albumin, high-sensitivity C-reactive protein (hs-CRP),
triglycerides, high-density lipoprotein cholesterol (HDL-C), total
cholesterol, blood leukocytes, helper T cells CD4, procalcitonin
(PCT), and echocardiography.
Follow-up
The follow-up time was from January 1, 2012 to April
1, 2022. Patient survival was defined as the end event of death,
and time was defined as the number of months from the diagnosis of
IE to the death of the patient.
Statistical analysis
SPSS 20.0 software (IBM Corp.) was used for
statistical analysis. The measurement data of continuous variables
conforming to a normal distribution are expressed as the mean ± SD,
and the comparison between groups was performed using one-way ANOVA
followed by Tukey's post hoc test. Continuous variables with
non-normal distribution data are expressed as M (1/4, 3/4). The
independent samples t-test was used for normally distributed
variables, and the χ2 test was used for categorical
variables; the nonparametric rank sum test was used to compare
variables that did not conform to the normal distribution.
Prognostic survival analysis was performed using the Kaplan-Meier
method. A Kaplan-Meier survival analysis was used to evaluate the
survival rate of the HD-IE group and NHD-IE group using the Breslow
test. A Cox regression model was used to analyse the independent
risk factors for IE, and the relative risk was described by hazard
ratios (HRs) and 95% confidence intervals (CIs). P<0.05 was
considered to indicate a statistically significant difference.
Results
General information comparison
The general data of the two groups are compared in
Table I. There were significant
differences in the levels of haemoglobin, red blood cells, CRP,
PCT, and serum albumin between the two groups at admission
(P<0.05). The levels of haemoglobin, red blood cells, and serum
albumin in the HD-IE group were generally lower than those in the
NHD-IE group, while CRP and PCT were significantly higher than
those in the NHD-IE group. The differences in the positive rates of
bacterial culture, diabetes, and invasive procedures were
significant (P<0.05), while the differences in the levels of
triglycerides, HDL-C, triglyceride (TG)/HDL-C and total cholesterol
were not (P>0.05).
 | Table IComparison of baseline clinical data
of patients with NHD-IE and HD-IE. |
Table I
Comparison of baseline clinical data
of patients with NHD-IE and HD-IE.
| Variables | NHD-IE | HD-IE | P-value |
|---|
| Sex (M/F) | 43/43 | 7/8 | 0.812 |
| Age (years) | 61.5 (39.75,
71.00) | 70 (53.00,
75.00) | 0.145 |
| WBC
(x109/l) | 8.25 (6.00,
12.27) | 9.56 (6.40,
15.39) | 0.420 |
| RBC (g/l) | 108.73±21.05 | 77.27±17.86 | <0.001 |
| Hb (g/l) | 3.76 (3.28,
4.10) | 2.45 (2.17,
3.18) | <0.001 |
| hs-CRP (mg/l) | 58.35 (21.25,
94.00) | 132.60 (26.00,
160.00) | 0.009 |
| PCT (ng/ml) | 0.19 (0.08,
1.01) | 3.31 (0.78,
13.72) | <0.001 |
| Alb (g/l) | 33.25±4.84 | 27.89±3.70 | <0.001 |
| TG (mmol/l) | 1.11 (0.87,
1.49) | 1.31 (1.10,
1.84) | 0.162 |
| TC (mmol/l) | 3.59 (3.04,
4.22) | 3.31 (2.89,
3.84) | 0.207 |
| HDL-C (mmol/l) | 0.92 (0.70,
1.18) | 0.89 (0.75,
0.99) | 0.625 |
| TG/HDL-C
(mmol/l) | 1.29 (0.88,
1.85) | 1.57 (1.17,
2.06) | 0.139 |
| Combined hypotension
(yes/no) | 13/73 | 4/11 | 0.466 |
| Combined diabetes
(yes/no) | 10/76 | 7/8 | 0.003 |
| Invasive surgery
(yes/no) | 26/60 | 10/5 | 0.007 |
| Paravalvular
complications | | | |
|
Perforation
(yes/no) | 15/71 | 1/14 | 0.502 |
|
Abscess
(yes/no) | 2/84 | 2/13 | 0.194 |
|
New moderate
or severe regurgitation | 64/22 | 11/3 | 0.741 |
|
Calcification
(yes/no) | 15/71 | 7/8 | 0.028 |
|
Concomitant
heart disease (yes/no) | 42/44 | 7/8 | 0.877 |
|
Vegetation
size (mm) | 0.88 (0.50,
1.20) | 1.20 (0.55,
1.55) | 0.203 |
|
EF | 64.43±7349 | 57.99±9.408 | 0.018 |
|
Positive
blood culture | 21/65 | 9/6 | 0.013 |
|
Cardiac
surgery performed (yes/no) | 21/65 | 1/14 | 0.231 |
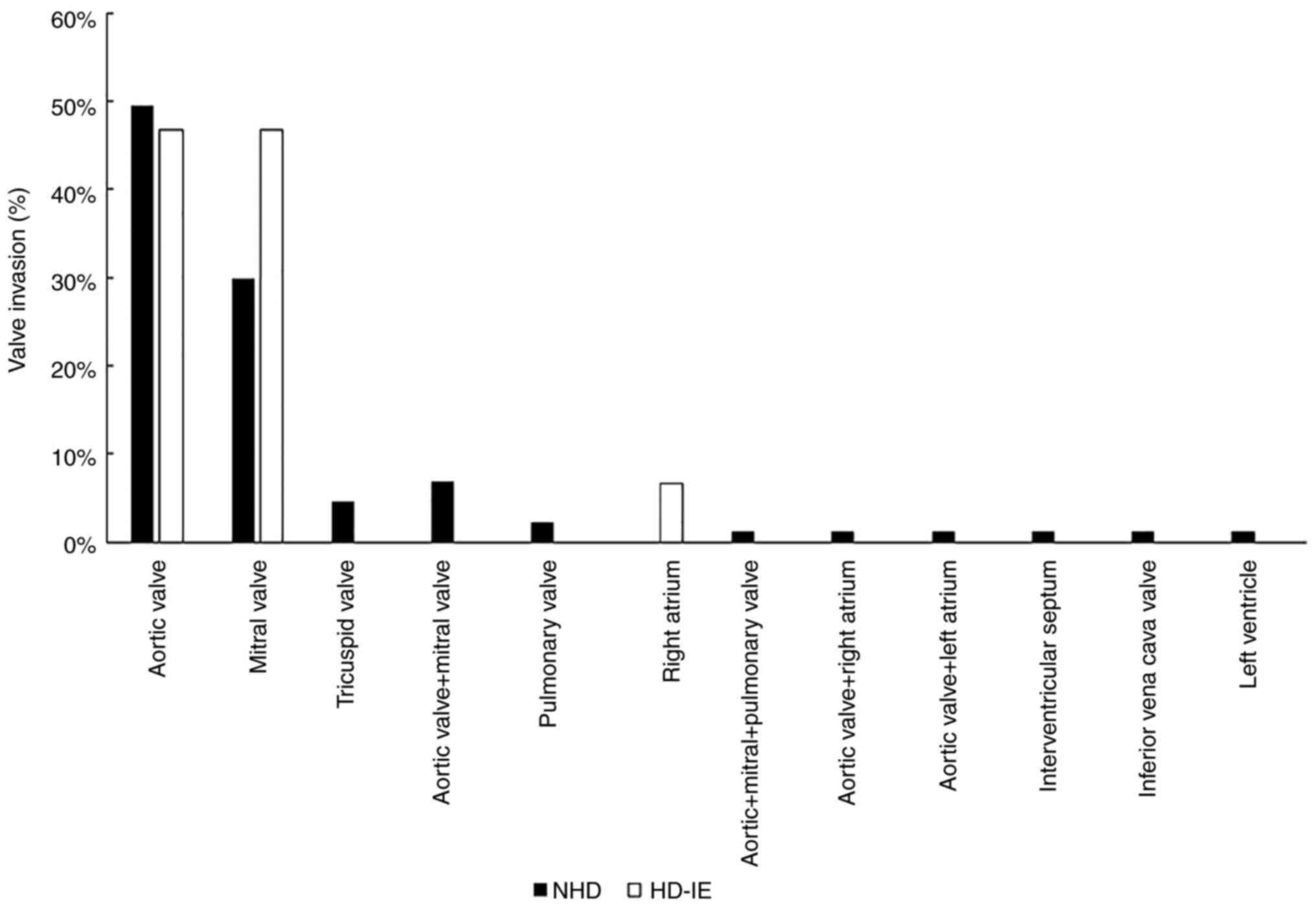
As revealed in Fig.
1, aortic valve injury was most common in IE patients, followed
by mitral valve, tricuspid valve, and pulmonary valve injury. The
involvement rates of the aortic valve, mitral valve and right
atrium in HD-IE were 46.7, 46.7 and 6.6%, respectively. The aortic
and mitral valve involvement rates in NHD-IE were 47.7 and 30.2%,
respectively. As shown in Table I,
there were no significant differences in the diameter of the
longest vegetation of the heart valve, the degree of heart valve
regurgitation, whether the heart valve was complicated by abscess
perforation, whether it was complicated by hypotension, or whether
it was complicated by underlying cardiovascular disease between the
two groups (P>0.05). The proportion of heart valve calcification
and the level of left ventricular ejection fraction were
significant (P<0.05). The proportion of heart valve
calcification in the HD-IE group was significantly higher than that
in the NHD-IE group, and the level of left ventricular ejection
fraction was lower than that in the NHD-IE group.
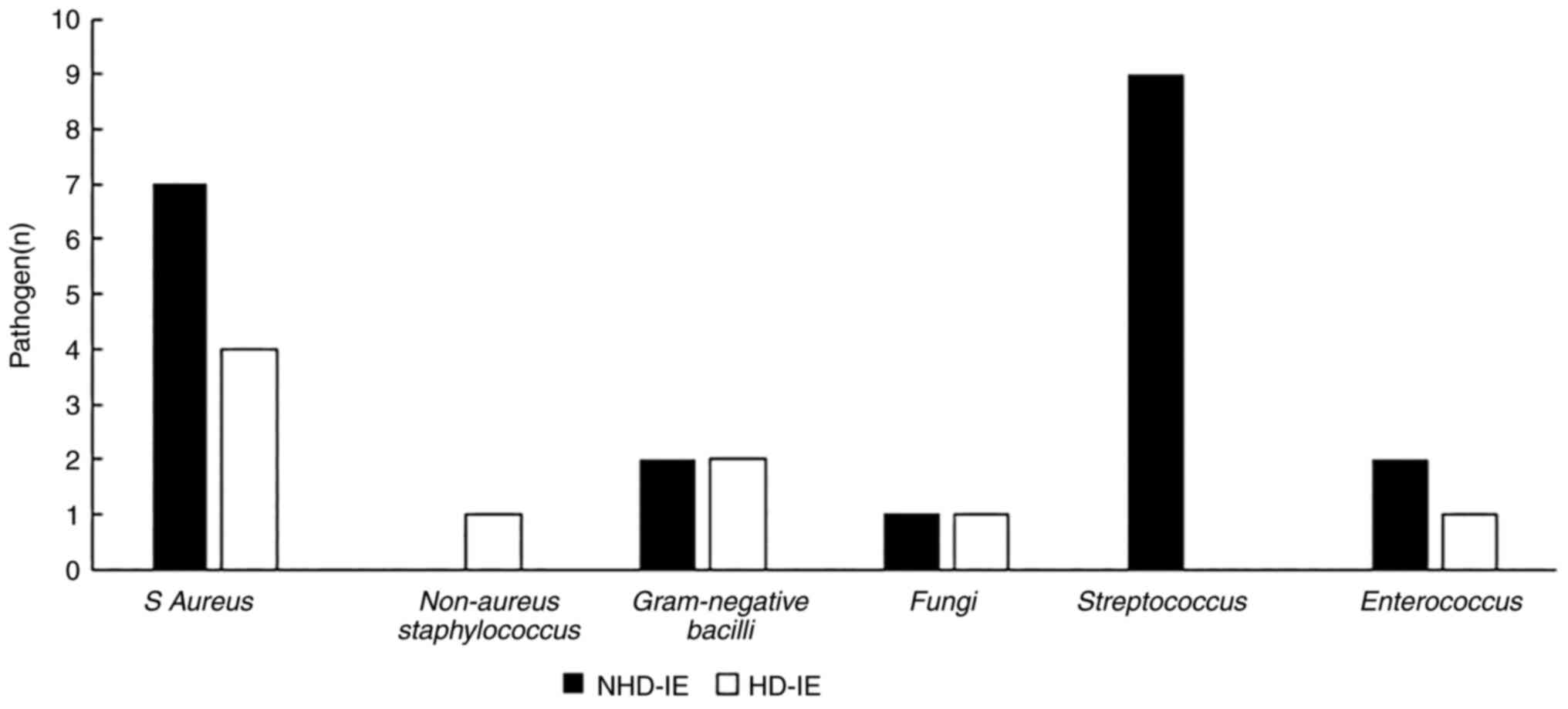
Aetiology
As revealed in Table
I, the positive rate of bacterial culture in the NHD-IE group
was 24.4%, and that in the HD-IE group was 60%. Staphylococcus
aureus (S. aureus) is relatively common in the general
population (7); however, the
common pathogen in the NHD-IE group was Streptococcus, while
the common pathogen in the HD-IE group was S. aureus
(Fig. 2).
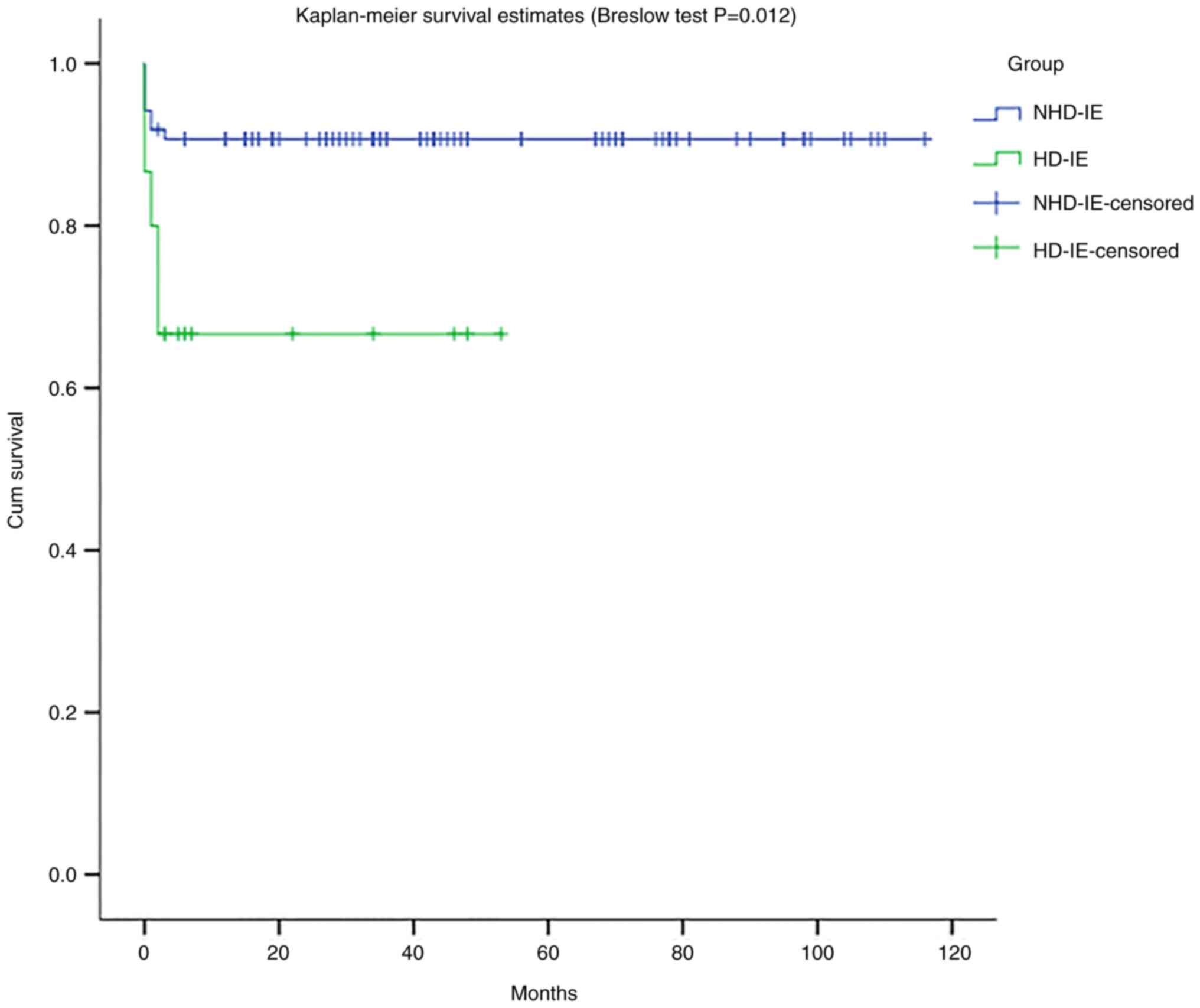
Prognosis analysis
Patients were followed up until death or until the
end of observation on April 1, 2022. The survival rate of the two
groups was assessed by a Kaplan-Meier survival analysis. The
results of the Breslow test showed that the difference in the
survival rate between the two groups was significant
(χ2=6.323, P=0.012). The results revealed that NHD-IE
had a longer survival time than HD-IE, and HD-IE had a higher early
mortality rate, as shown in Fig.
3. The Cox proportional hazards model was used to explore the
factors influencing survival prognosis. The univariate Cox
regression analysis showed that age, haemoglobin, red blood cells,
blood albumin, left ventricular ejection fraction, longest
vegetation diameter and comorbid diabetes were the factors
influencing mortality. Furthermore, multivariate Cox regression
showed that age (HR=1.187, P=0.015), ejection fraction (HR=0.921,
P=0.025), and the longest vegetation diameter (HR=9.191, P=0.004)
were the main independent risk factors affecting the survival of
patients, as revealed in Table
II.
 | Table IIIndependent risk factors for
mortality in NHD-IE and HD-IE patients (Cox regression
analysis). |
Table II
Independent risk factors for
mortality in NHD-IE and HD-IE patients (Cox regression
analysis).
| | Univariate
analysis | Multivariate
analysis |
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Sex (M/F) | 1.139 | (0.383, 3.390) | 0.815 | | | |
| Age (years) | 1.039 | (0.998, 1.081) | 0.06 | 1.187 | (1.034, 1.363) | 0.015 |
| WBC
(x109/l) | 0.994 | (0.953, 1.037) | 0.78 | | | |
| Hb (g/l) | 0.97 | (0.947, 0.995) | 0.017 | 0.994 | (0.994,
1.0466) | 0.818 |
| RBC (g/l) | 0.479 | (0.248, 0.925) | 0.028 | 1.054 | (0.884, 1.258) | 0.557 |
| hs-CRP (mg/l) | 1.006 | (0.997, 1.014) | 0.183 | | | |
| PCT (ng/ml) | 1.027 | (0.984, 1.073) | 0.225 | | | |
| Alb (g/l) | 0.864 | (0.772, 0.967) | 0.011 | 0.892 | (0.665, 1.196) | 0.444 |
| TG/HDL-C
(mmol/l) | 1.268 | (0.762, 2.110) | 0.36 | | | |
| EF | 0.969 | (0.947, 0.992) | 0.008 | 0.921 | (0.856, 0990) | 0.025 |
| Vegetation size
(mm) | 2.083 | (1.315, 3.299) | 0.002 | 9.191 | (1.966,
43.322) | 0.004 |
| Combined diabetes
(yes/no) | 3.246 | (1.061, 9.927) | 0.039 | 0.04 | (0.001, 2.13) | 0.112 |
| Invasive surgery
(yes/no) | 1.128 | (0.369, 3.447) | 0.883 | | | |
| Perforation
(yes/no) | 0.969 | (0.215, 4.37) | 0.967 | | | |
| Abscess
(yes/no) | 2.113 | (0.274,
16.279) | 0.473 | | | |
| New moderate or
severe regurgitation | 1.380 | (0.425, 4.481) | 0.592 | | | |
| Calcification
(yes/no) | 1.598 | (0.492, 5.191) | 0.435 | | | |
| Cardiac surgery
performed (yes/no) | 30.032 | (0.117,
7737.065) | 0.23 | | | |
Discussion
Cardiovascular disease is the main complication and
the leading cause of mortality in patients with end-stage renal
disease. Among them, IE complicated with haemodialysis exhibits
higher mortality than IE without haemodialysis. Patients with
end-stage renal disease have a higher risk of IE than the general
population due to their low immunity, multiorgan failure and a
variety of underlying diseases, poor resistance to viruses or
bacteria, and weakened stress responses. There is decreased
erythropoietin production, a shortened erythropoiesis cycle, and
insufficient clearance of accumulated toxins in the body to
suppress bone marrow, resulting in different degrees of renal
anaemia (8). At the same time,
combined with insufficient protein intake, digestive tract
malabsorption and disease consumption, it is very easy to develop
hypoproteinemia, which leads to further reduction of immune
function and aggravates the incidence of infection (9,10).
In the present study, the levels of haemoglobin, red blood cells,
and albumin in the HD-IE group were lower than those in the NHD-IE
group. Therefore, it is necessary to consider the malnutrition
status of haemodialysis patients, prevent renal anaemia, and
increase the intake of high-quality protein to improve immune
function and prevent infection. Fever is the first symptom of IE,
but echocardiography remains the main method for the imaging
diagnosis of IE. Some patients need multiple echocardiography and
transesophageal echocardiography to diagnose IE. In particular,
experienced echocardiologists are particularly important for the
diagnosis of IE. The present study did not analyse the differences
in initial symptoms between the two groups, such as whether there
was a difference in the type of fever in the two groups. This will
be the direction of our future research.
Diabetic nephropathy accounts for a high proportion
of end-stage renal disease in China. Hyperglycaemia causes changes
in plasma osmotic pressure, inhibits the phagocytic ability of
immune cells, and inhibits leukocyte mobilization during ketosis; a
high-glucose environment is conducive to the growth and
reproduction of pathogens such as bacteria and fungi (10-12).
In the present study, the proportion of patients with diabetes
mellitus in the HD-IE group was higher than that in the NHD-IE
group, resulting in a higher risk of IE. In the HD-IE group, eight
patients had autologous arteriovenous fistula for vascular access,
and 7 patients had long-term indwelling subcutaneous tunnel
polyester sleeve catheters. Previous reports have noted that
subcutaneous tunnel polyester sheath catheters are a risk factor
for HD-IE because patients with long-term indwelling subcutaneous
tunnel polyester sheath catheters have long-term contact with the
wall of the haemodialysis catheter during dialysis, forming a layer
of plasma protein membrane analogues. Known as plasma protein
biofilms, plasma protein biofilms provide nutrients for bacterial
aggregation, adhesion, colonization, and proliferation (13,14).
Considering the increased risk of infection/bacteremia,
infection-related hospitalizations and adverse consequences with
long-term indwelling subcutaneous tunnel polyester sleeve
catheters, experts suggest that most HD patients starting dialysis
with long-term indwelling subcutaneous tunnel polyester sleeve
catheters should convert to an arteriovenous fistula (15). However, it has been revealed that
IE can also occur in patients with arteriovenous fistula, which is
inconsistent with another previous report (16). The possible reasons for this could
be that with the popularity of arteriovenous fistula, the
utilization rate of arteriovenous fistula is increasing, and
repeated skin puncture will increase the risk of bacterial
infection. In addition, gram-positive bacteria (such as the S.
aureus commonly present on the surface of medical staff and
patients) more easily colonize and multiply on the skin surface to
form infections (17). This is
consistent with the findings of the present study, which revealed
that S. aureus was a common pathogen in the HD-IE group.
This suggests that the practice of handwashing, compliance with
aseptic surgery procedures, publicity of surgery norms, and
haemodialysis surgery training for medical staff before surgery,
should be strengthened to reduce the risk of infection. As the
sample size of present study was small, further study with a large
multi-center sample size should be performed.
Compared with the general population, haemodialysis
patients are more likely to develop vascular and valve
calcification. Calcification is common in haemodialysis patients,
and calcification-stimulating factors such as advanced age, high
blood calcium, high intact PTH, inflammatory factors, oxidative
stress, uraemic toxins, and glycation end products increase;
meanwhile, calcification-inhibiting factors decrease, and
calcification-stimulating factors and calcification inhibitors
become imbalanced. Thus, supersaturated calcium and phosphorus in
serum are deposited in blood vessels and valves. Mature vascular
smooth muscle cells, mesangial progenitor cells in heart valves and
cells with calcification tendency in blood circulation undergo
apoptosis or transdifferentiation to become osteoblast-like cells,
which in turn produce valve calcification (18,19).
In the present study, the proportion of valve calcification in
HD-IE patients was higher than that in NHD-IE patients. Therefore,
for maintenance haemodialysis patients, vascular and valve
calcification should be verified as soon as possible in order for
effective intervention measures to be taken in time. In addition,
the present study determined that the most common IE in
haemodialysis patients is the left heart system and not the right
heart system. There is no definite conclusion as to why IE is more
likely to involve the left heart system. It may be related to
intracardiac pressure (20).
In summary, compared with the general population,
the possible causes of IE in haemodialysis patients include low
immunity, malnutrition, diabetes and valve calcification. However,
due to the limited sample size, the present study did not conduct
in-depth analysis on etiology. In future studies, the sample size
will be expanded to further analyse whether, for example, long-term
indwelling subcutaneous tunnel polyester sleeve catheters will
increase the risk of infection, especially since femoral vein
catheterization is close to the perineum region. The influence of
different types of vascular access on IE or the influence of
catheter indentation time on IE will also be further explored.
Haemodialysis patients complicated with IE have a
high mortality rate, and this is considered to be related to
patients with end-stage renal disease complicated by multiple
diseases, haemodynamic changes, autoimmune dysfunction, and a
microinflammatory state (16,21).
Raza et al (22) reported
that the in-hospital mortality of HD-IE patients was 2.6 times that
of ordinary patients. Ramos-Martinez et al (23) reported that the in-hospital
mortality rate of HD-IE patients was 41%, and the 1-year mortality
rate was as high as 56%. The present study suggests that HD-IE
patients have a lower survival rate and shorter survival time than
NHD-IE patients, which is consistent with a previous report
(24). In terms of treatment,
antibiotic therapy is the basic means of IE, followed by surgical
treatment. HD-IE patients are considered to be a high-risk
population due to the combination of multiple diseases and low
autoimmunity (5). Regarding the
surgical rate, only one HD-IE patient in the present study received
surgical treatment, while the rest of the patients selected
conservative treatment. According to the IE management guidelines
of the European Society of Cardiology Annual Meeting in 2015, the
necessity of antibiotics and early surgical treatment of IE was
emphasized (25). After the
diagnosis of IE, a multidisciplinary treatment team should be
quickly formed. In the present study, all cases received
anti-infective therapy according to IE management guidelines of the
Annual Meeting of the European Society of Cardiology, and the
course of treatment was 4-6 weeks. Antibiotics according to drug
sensitivity were selected; for staphylococcal endocarditis, in
which pathogen drug sensitivity shows methicillin-sensitive
Staphylococcus, benzoxicillin is the first choice; for
streptococcal endocarditis, penicillin is preferred for sensitive
strains; for Enterococcus faecium endocarditis, penicillin
in combination with amoxicillin or ampicillin in combination with
aminoglycoside antibiotics are preferred; for aerobic gram-negative
bacterial endocarditis, piperacillin combined with gentamicin or
tobramycin, or ceftazidime combined with aminoglycosides should be
used (25). Long-term intravenous
antibacterial therapy is a necessary means for IE treatment, and a
reasonable course of antibiotics can improve the prognosis of
patients to some extent (26).
However, unfortunately, statistical analysis on the duration of
antibacterial drug use in the two groups was not conducted, which
warrants further research and analysis. Research has shown that
early surgical treatment of IE can effectively reduce the risk of
systemic embolism, improve heart function, control infection and
significantly reduce overall mortality (27). Furthermore, HD-IE patients have a
low surgical rate and poor postoperative prognosis (24). However, in the present study, there
was no significant difference between the surgical treatment of IE
patients and the survival prognosis of patients, which may be due
to the small sample size. Therefore, whether there is a significant
difference in the long-term survival rate of these patients with
early surgical intervention and simple medical conservative
treatment remains to be further studied.
Haemodialysis is an independent risk factor
affecting the survival of patients with IE. Antibiotic treatment or
surgical treatment should be strongly recommended for active and
effective treatment. Larger sample size studies are required to
help improve the survival of haemodialysis patients with IE.
Acknowledgements
Not applicable.
Funding
Funding: The present research was supported by The Construction
Fund of Key Medical Disciplines of Hangzhou (grant no. 0020200479),
the Zhejiang Provincial Medical and Health Technology Project
(grant no. 2021ZB223), and the Science and Technology Development
Project of Hangzhou (grant no. A20220658).
Availability of data and materials
All data generated and/or analysed during this study
are included in this published article.
Authors' contributions
YJH and CSY designed and performed the study
together, and they are responsible for data analysis, writing and
revising the manuscript. YJH, CSY, KYX, KLW, XMW, MYL, YW, QSY and
LLY are responsible for data collection and detection of samples.
XY, MW and SJQ were responsible for data analysis, interpretation
of the data, obtaining ethics approval and confirm the authenticity
of all the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was performed according to the
Declaration of Helsinki and approved by the local ethics board of
Affiliated Hangzhou First People's Hospital, Zhejiang University
School of Medicine (Hangzhou, China). Individual patient consents
were waived on the condition that all patients were deidentified
before analysis since this study was a retrospective analysis.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Masakane I, Nakai S, Ogata S, Kimata N,
Hanafusa N, Hamano T, Wakai K, Wada A and Nitta K: An overview of
regular dialysis treatment in Japan (As of 31 December 2013). Ther
Apher Dial. 19:540–574. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Collins AJ, Foley RN, Chavers B,
Gilbertson D, Herzog CA, Ishani A, Johansen K, Kasiske BL, Kutner
N, Liu J, et al: 2013 USRDS annual data report: Atlas of chronic
kidney disease and end-stage renal disease in the United States. Am
J Kidney Dis. 63:e1–e478. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wang L, Wu H and Zhang H: Epidemiological
characteristics of hemodialysis death patients from 2019 to 2021 in
a single center. Chi J Nephrol Dial Transplant. 31:519–524.
2022.
|
|
4
|
Kanbay M, Afsar B, Goldsmith D and Covic
A: Sudden death in hemodialysis: An update. Blood Purif.
30:135–145. 2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bhatia N, Agrawal S, Garg A, Mohananey D,
Sharma A, Agarwal M, Garg L, Agrawal N, Singh A, Nanda S and
Shirani J: Trends and outcomes of infective endocarditis in
patients on dialysis. Clin Cardiol. 40:423–429. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Li JS, Sexton DJ, Mick N, Nettles R,
Fowler VG Jr, Ryan T, Bashore T and Corey GR: Proposed
modifications to the Duke criteria for the diagnosis of infective
endocarditis. Clin Infect Dis. 30:633–638. 2000.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Murdoch DR, Corey GR, Hoen B, Miró JM,
Fowler VG Jr, Bayer AS, Karchmer AW, Olaison L, Pappas PA,
Moreillon P, et al: Clinical presentation, etiology, and outcome of
infective endocarditis in the 21st century: The International
Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern
Med. 169:463–473. 2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Paul DiMondi V, Townsend ML, Johnson M and
Durkin M: Antifungal catheter lock therapy for the management of a
persistent Candida albicans bloodstream infection in an adult
receiving hemodialysis. Pharmacotherapy. 34:e120–e127.
2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Aslam S, Vaida F, Ritter M and Mehta RL:
Systematic review and meta-analysis on management of hemodialysis
catheter-related bacteremia. J Am Soc Nephrol. 25:2927–2941.
2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sun J, Xu Y, Dai Z and Sun Y: Intermittent
high glucose stimulate MCP-l, IL-18, and PAI-1, but inhibit
adiponectin expression and secretion in adipocytes dependent of
ROS. Cell Biochem Biophys. 55:173–180. 2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Trevelin SC, Carlos D, Beretta M, da Silva
JS and Cunha FQ: Diabetes mellitus and sepsis: A challenging
association. Shock. 47:276–287. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Koh GC, Peacock SJ, van der Poll T and
Wiersinga WJ: The impact of diabetes on the pathogenesis of sepsis.
Eur J Clin Microbiol Infect Dis. 31:379–388. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Bouza E, Rojas L, Guembe M, Marín M, Anaya
F, Luño J, López JM and Muñoz P: COCADI Study Group. Predictive
value of superficial cultures to anticipate tunneled hemodialysis
catheter-related bloodstream infection. Diagn Microbiol Infect Dis.
78:316–319. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Mohamed H, Ali A, Browne LD, O'Connell NH,
Casserly L, Stack AG and Hussein WF: Determinants and outcomes of
access-related blood-stream infections among Irish haemodialysis
patients; a cohort study. BMC Nephrol. 20(68)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lok CE, Huber TS, Lee T, Shenoy S, Yevzlin
AS, Abreo K, Allon M, Asif A, Astor BC, Glickman MH, et al: KDOQI
clinical practice guideline for vascular access: 2019 update. Am J
Kidney Dis. 75 (Suppl 2):S1–S164. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Rekik S, Trabelsi I, Hentati M, Hammami A,
Jemaa MB, Hachicha J and Kammoun S: Infective endocarditis in
hemodialysis patients: Clinical features, echocardiographic data
and outcome: A 10-year descriptive analysis. Clin Exp Nephrol.
13:350–354. 2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhang HH, Cortes-Penfield NW, Mandayam S,
Niu J, Atmar RL, Wu E, Chen D, Zamani R and Shah MK: Dialysis
catheter-related bloodstream infections in patients receiving
hemodialysis on an emergency-only basis: A retrospective cohort
analysis. Clin Infect Dis. 68:1011–1016. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yamada S and Giachelli CM: Vascular
calcification in CKD-MBD: Roles for phosphate, FGF23, and Klotho.
Bone. 100:87–93. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Cianciolo G, Capelli I, Cappuccilli M,
Schillaci R, Cozzolino M and La Manna G: Calcifying circulating
cells: An uncharted area in the setting of vascular calcification
in CKD patients. Clin Kidney J. 9:280–286. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Bentata Y, Haddiya I, Ismailli N, El Ouafi
N, Benzirar A, El Mahi O and Azzouzi A: Infective endocarditis in
chronic hemodialysis: A transition from left heart to right heart.
Saudi J Kidney Dis Transpl. 27:1200–1206. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Omoto T, Aoki A, Maruta K and Masuda T:
Surgical outcome in hemodialysis patients with active-phase
infective endocarditis. Ann Thorac Cardiovasc Surg. 22:181–185.
2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Raza S, Hussain ST, Rajeswaran J, Ansari
A, Trezzi M, Arafat A, Witten J, Ravichandren K, Riaz H,
Javadikasgari H, et al: Value of surgery for infective endocarditis
in dialysis patients. J Thorac Cardiovasc Surg. 154:61–70.e6.
2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ramos-Martinez A, Roque F, Farinas MC,
Muñoz P, Verde E, Cuerpo GP, de Alarcón A, Lepe JA, Miró JM, Plata
A, et al: Prognostic factors of infective endocarditis in patients
on hemodialysis: A case series from a National Multicenter
Registry. Int J Cardiol. 241:295–301. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Pericas JM, Llopis J, Jimenez-Exposito MJ,
Kourany WM, Almirante B, Carosi G, Durante-Mangoni E, Fortes CQ,
Giannitsioti E, Lerakis S, et al: Infective endocarditis in
patients on chronic hemodialysis. J Am Coll Cardiol. 77:1629–1640.
2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Habib G, Lancellotti P, Antunes MJ,
Bongiorni MG, Casalta JP, Del Zotti F, Dulgheru R, El Khoury G,
Erba PA, Iung B, et al: 2015 ESC Guidelines for the management of
infective endocarditis: The Task Force for the Management of
Infective Endocarditis of the European Society of Cardiology (ESC).
Endorsed by: European Association for Cardio-Thoracic Surgery
(EACTS), the European Association of Nuclear Medicine (EANM). Eur
Heart J. 36:3075–3128. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
AATS Surgical Treatment of Infective
Endocarditis Consensus Guidelines Writing Committee Chairs.
Pettersson GB and Coselli JS: Writing Committee. Pettersson GB,
Coselli JS, Hussain ST, Griffin B, Blackstone EH, Gordon SM, et al:
2016 The American Association for Thoracic Surgery (AATS) consensus
guidelines: Surgical treatment of infective endocarditis: Executive
summary. J Thorac Cardiovasc Surg. 153:1241–1258.e29.
2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kang DH, Kim YJ, Kim SH, Sun BJ, Kim DH,
Yun SC, Song JM, Choo SJ, Chung CH, Song JK, et al: Early surgery
versus conventional treatment for infective endocarditis. N Engl J
Med. 366:1241–1258. 2012.PubMed/NCBI View Article : Google Scholar
|