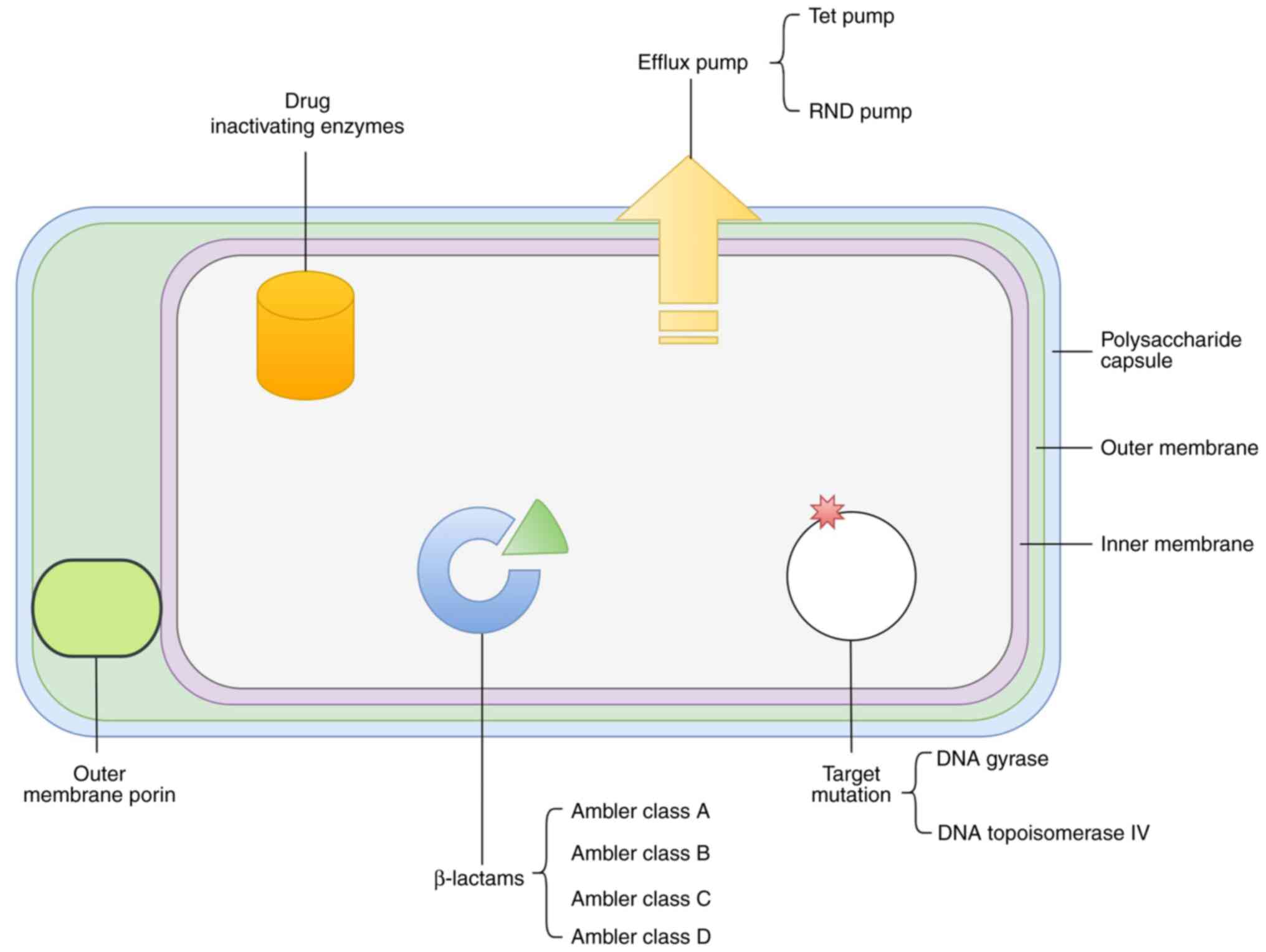
1. Introduction
The problem of bacterial resistance is ever
increasingly becoming a serious threat to humans, and superbugs now
account for >540,000 infections and nearly 14,000 deaths each
year in the United States alone (1). The discovery of penicillin and the
synthesis and application of antibacterial sulfonamides in the 20th
century have greatly eased the suffering of patients, but the
uncontrolled abuse of antibiotics in the past 50 years has made
‘ESKAPE’ (Enterococcus faecium, Staphylococcus
aureus, Klebsiella pneumoniae, Acinetobacter
baumannii, Pseudomonas aeruginosa and Enterobacter
species) increasingly resistant and treatment of these
bacterial infections has become increasingly difficult (2). Amongst these superbugs,
drug-resistant Acinetobacter baumannii infections are
notably serious with the increasing number of its infections
(3). The purpose of this review is
to highlight the molecular mechanisms underlying drug resistance in
Acinetobacter baumannii and to summarize novel ideas for
solving the problem of drug resistance.
2. Epidemiology of Acinetobacter
baumannii
The history of Acinetobacter can be traced back to
1991 when the Danish microbiologist Martinus Willem Beijerinck
discovered Micrococcus calcoaceticus (4). The first identification analysis of
Acinetobacter species was based on their biochemical
characterization, while the use of molecular methods, in particular
DNA-DNA hybridization, identified at least 33 genetically distinct
species of Acinetobacter (5). In the majority of laboratories,
Acinetobacter baumannii, Acinetobacter pittii and
Acinetobacter nosocomialis are difficult to distinguish
(6), as they possess closely
related microbiological characteristics. Hence, this review will
use Acinetobacter baumannii in the comprehensive sense to
refer to these three species collectively. The identification of
Acinetobacter baumannii can be distinguished by multilocus
sequence typing, as it utilizes 16s ribosomal RNA as well as
conserved regions of seven housekeeping genes: gltA, gyrB, gdhB,
recA, cpn60, gpi and rpoD (7).
Acinetobacter baumannii, once even considered
benign, is now considered a global threat in healthcare settings,
and it is gaining resistance at an unforeseen rate (8). In early 2019, the World Health
Organization stated that Acinetobacter baumannii is
considered the most dangerous multidrug-resistant bacteria
(9). Until the early 1970s,
Acinetobacter strains showed susceptibility to most
antibiotics (10). Extensive
resistance to carbapenem antibiotics is considered to be a sign of
extensively drug-resistant bacteria, and carbapenem-resistant
Acinetobacter baumannii is now causing serious problems in
Asia and the Americas. In Southern Europe, Middle East and Asia and
North Africa ~90% of clinical isolates of Acinetobacter
baumannii are resistant to carbapenems (11). Globally, ~45% of Acinetobacter
baumannii isolates are multi-drug resistant, with >70% of
isolates in Latin America and the Middle East exhibiting multi-drug
resistance (12).
3. Features of Acinetobacter
baumannii
Acinetobacter baumannii was considered a
low-virulence bacterium in the past, and its severity was not taken
seriously until the mid-1990s (13). In recent years, following the
continual increase in its virulence and the difficulty in treating
infections due to drug resistance has resulted in increased
attention from public health bodies (14). Acinetobacter baumannii is a
Gram-negative bacterium that is a strictly aerobic,
catalase-positive, oxidase-negative and non-lactose-fermentative
opportunistic pathogen (6).
Acinetobacter baumannii is almost everywhere such as
waterbodies, soil, mines, crude oil, sewage, sludge, solid
surfaces, human skin and wild animals (11), it is not only difficult to treat
but also difficult to eliminate. This is due to its excellent
anti-starvation (15),
anti-desiccating (16), seasonal
adaptation and high-temperature resistance properties (17), in addition to reduced sensitivity
to disinfectants (18) and biofilm
protection (19). Drug-resistant
Acinetobacter baumannii is one of the most common pathogens
of nosocomial infections, especially in immunocompromised patients
and in ICU wards (20). In
addition, prolonged use of antibiotics, major surgery, severe burns
and immunosuppression increase the risk of Acinetobacter
baumannii infections (21).
Acinetobacter baumannii infections can lead to
ventilator-associated pneumonia, bacteremia, urinary tract
infection and meningitis (22).
The overall prevalence of multidrug-resistant strains of
Acinetobacter baumannii in patients with hospital-acquired
pneumonia and ventilator-associated pneumonia is estimated at
79.9%, with an overall mortality rate that can be as high as 56.2%
(23).
4. Mechanisms of drug resistance and their
clinical implications
With improvements in research equipment and methods
in the fields of modern medicine and microorganisms, the mechanisms
underlying Acinetobacter baumannii drug resistance have
become increasingly understood. The known mechanisms of
Acinetobacter baumannii drug resistance and potential
developmental directions are summarized in Table I and Fig. 1, with treatment options being
listed in Table II. Below, an
in-depth summary of the known body of knowledge on Acinetobacter
baumannii drug resistance is provided.
 | Table IMechanisms of resistance employed by
Acinetobacter baumannii. |
Table I
Mechanisms of resistance employed by
Acinetobacter baumannii.
| Antibiotic | Resistance
mechanism | Enzyme or
target | Key point | (Refs.) |
|---|
| β-lactams | β-lactamases | Ambler class A | TEM, SHV, CTX-M,
KPC | (26-28) |
| | | Ambler class B | NDM, VIM, SIM,
IMP | (30,31) |
| | | Ambler class C | AmpC, ADC | (27,36,37) |
| | | Ambler class D | OXA | (7,39-41,43,44) |
| | Permeability
lesions | Outer membrane
porin | CarO | (46) |
| | | | OmpA | (46-48) |
| | Efflux pump
overactivity | RND pump | AdeABC | (49,50) |
| Tetracyclines | Efflux pump
overactivity | RND pump | AdeABC, AdeIJK | (79,80) |
| | | Tet pump | TetA, TetG | (81) |
| Quinolones | Target
mutation | DNA gyrase | GyrA | (88) |
| | | DNA topoisomerase
IV | ParC | (88) |
| | Efflux pump
overactivity | RND pump | AdeABC | (89) |
|
Aminoglycosides | Drug inactivating
enzymes | Aminoglycoside
modifying enzymes | aadB, apa6, aadA,
aacc1 | (92) |
| | Target
mutation | 16s RNA methylase
genes | armA | (93) |
| | Efflux pump
overactivity | RND pumps | AdeABC | (94) |
| Polymyxins | Target
mutation | Abnormalities of
lipid A and LPS | PmrC, PmrB, lpx
gene | (68-71) |
 | Table IITreatment options for drug-resistant
Acinetobacter baumannii infections. |
Table II
Treatment options for drug-resistant
Acinetobacter baumannii infections.
| Resistance to
antibiotics | Treatment (for
reference only) | (Refs.) |
|---|
| β-lactams | Cefiderocol | (53-55) |
| | CCCP and
imipenem/cefepime | (56) |
| | Quercetin and
imipenem | (57) |
| | DBOs and
sulbactam | (59) |
| | QPX7728 and
meropenem/ceflorazone/piperacillin/cefepime | (60) |
| | Ampicillin and
sulbactam | (61) |
| | Berberine
hydrochloride and sulbactam | (63) |
| | Piper betle and
antibiotics | (65) |
| | Vaccines
(preventative) | (58,62) |
| | Iron control | (52) |
| | Cilantro oil
combined with piperacillin or cefoperazone | (64) |
| Tetracyclines | KBP-7072 and
omadacycline | (83) |
| | Omadacycline and
sulbactam | (84) |
| | Tetracycline and
D-LANA-14 | (85) |
| Quinolones |
Ciprofloxacin/imipenem and Mentha
longifolia/Menthol | (90) |
| | Ciprofloxacin and
Na-3DH-DCA/Na-3DH-CDCA | (91) |
|
Aminoglycosides | Tobramycin and
colistin | (97) |
| | Aminoglycosides and
L-lysine | (96) |
| | Colistin and Silver
nanoparticles | (74) |
| | Macolacin | (75) |
| | Polymyxin B and
rifampicin/imipenem/meropenem/tigecycline | (76) |
| | Scutellaria
barbata | (77) |
| Biofilm | Myrtenol and
antibiotics | (102) |
| | Polymyxin B/E and
azithromycin | (103) |
| | Illicium verum
Hook | (104) |
| | Phage | (105) |
| | Antimicrobial
photodynamic therapy | (106) |
| | Antimicrobial
peptides | (107) |
β-lactams
Since the first β-lactam antibiotic was discovered
(penicillin), they have become incorporated as a core part of
clinical practice as treatments for various bacterial infections;
β-lactam antibiotics are chosen as the antibacterial drug of choice
(16). β-lactam antibiotics act on
the peptidoglycan in the cell walls of fungi and bacteria, and they
work by suppressing bacterial cell division or inducing bacterial
rupture (24). However, bacteria
can produce β-lactamase to enzymatically break down β-lactam
antibiotics, which is the most prevalent mechanism of drug
resistance. In the Ambler classification, β-lactamases can be
grouped into one of four classes (A-D) according to the sequences
of the amino acids that make up the enzyme (25).
Ambler class A enzymes. The serine
β-lactamases of molecular class A are the most important enzymatic
source of both natural and acquired resistance to β-lactams,
particularly in Acinetobacter baumannii (26). TEM, SHV, CTX-M and KPC are
the primary Ambler class A enzymes (27). TEM, CTX-M and KPC can hydrolyze
penicillin, cephalosporin and carbapenem. Additionally, the use of
antibiotics allows these enzymes to evolve and develop stronger
drug resistance (28).
Ambler class B enzymes. Zinc-dependent
metallo-β-lactamases (MBLs) are typically associated with gene
cassettes of integrons and thus spread easily amongst bacteria
(29). MBLs are classified into 3
subclasses. B1 and B3 are catalytically inactivated by two
Zn2+ ions, and B2 is catalytically inactivated by one
Zn2+ ion (14). NDM,
VIM, SPM and IMP are the primary Ambler class B enzymes. The
presence of the plasmid enables the rapid spread of the MBL gene,
and the spread of NDM-1 is closely associated with drug resistance
in Acinetobacter baumannii (30,31).
Since the discovery of NDM-1 in India, over 24 NDM variants have
been identified (32). NDM
enzymes, composed of 270 amino acids, hydrolyze most β-lactams
(including carbapenems) but not monobactams. However, NDM enzymes
cannot be countered by clinically available β-lactamase inhibitors,
including avibactam, clavulanate, sulbactam and tazobactam
(33). Studies have shown that the
percentage of NDM-1-positive isolates tends to be the highest, and
Acinetobacter baumannii with the NDM gene show resistance to
ampicillin (34). The acquisition
of the NDM-1 gene is likely facilitated by the action of
Tn125(35).
Ambler class C enzymes. Acinetobacter-derived
cephalosporinases (ADCs) are responsible for resistance to
cephalosporin antibiotics. ADC is the primary Ambler class C enzyme
(27). ADC-mediated drug
resistance is achieved through overexpression of ADC, and this
overexpression itself is achieved through an ISAba1 insertion
sequence, which is located in close proximity to the genes which
confer resistance (36). The
production of AmpC β-lactamases may be either chromosomally
mediated or plasmid-mediated. AmpC β-lactamases are not inhibited
by clavulanic acid, but are inhibited by cloxacillin or boronic
acid (37). Acinetobacter
baumannii can rapidly develop drug resistance due to the
chemical similarity of the molecules between β-lactamase inhibitors
and β-lactams, thus β-lactamase inhibitors, such as sulbactam and
clavulanic acid, eventually become ineffective against
Acinetobacter baumannii (38).
Ambler class D enzymes. Amongst the D-type
β-lactamases, oxacillinase (OXA) is associated with resistance to
carbapenems (39). The primary
reason for carbapenem resistance is the presence of oxacillinase,
which belongs to class D Ambler β-lactamases. To date, >400 OXA
enzymes encoded by chromosomal or plasmid-localized genes have been
characterized (40). The
hydrolytic activity of OXA-type groups is more potent for oxacillin
than benzylpenicillin; however, OXA-type enzymes are not considered
extended-spectrum β-lactamases (ESBLs) as they do not hydrolyze
broad-spectrum cephalosporins (7).
The OXA-23 enzyme is encoded by a chromosomal gene or located on a
plasmid, and it confers resistance to several antibiotics including
ticarcillin, meropenem, amoxicillin and imipenem. The OXA-40 enzyme
can hydrolyze penicillin; however, its ability to hydrolyze
cephalosporins and carbapenems is weak, and it is resistant to
inhibitors such as tazobactam, sulbactam, clavulanic acid and NaCl.
The OXA-51 gene is generally non-transferable, encoded by
chromosomal DNA. Clavulanic acid, tazobactam, or NaCl effectively
blocks the activity of OXA-51. OXA-58 is found on a
non-transferable 30k plasmid. When this plasmid is incorporated
into the gene chain of Acinetobacter baumannii, carbapenem
susceptibility is reduced (7).
Because of certain insertion sequences, such as ISAbaI, ISAba125
and ISAba825, the overproduction ADC and OXA-51 confer high-level
resistance to third- and fourth-generation cephalosporins (41). Carbapenem antibiotics, as the most
commonly used antibiotics for nosocomial infections in the world,
have successfully led to the enhancement of drug resistance in
microorganisms such as Acinetobacter baumannii (16). The prevalence of
carbapenem-resistant Acinetobacter Baumannii (CRAB) is
increasing rapidly in many countries and regions, and this has
complicated treatment choices (42). Carbapenem resistance is primarily
mediated by B-type and D-type. The most common OXA-type
carbapenemases include OXA-23, OXA-24, OXA-48, OXA-51 and OXA-58.
Among them, OXA-23, OXA-24, OXA-48 and OXA-58 are acquired
carbapenemases, whereas OXA-51 is intrinsic to Acinetobacter
baumannii (43). The genes
encoding these enzymes are regulated by upstream insertion
sequences (IS), specifically ISAba1, ISAba2, ISAba3, ISAba9 and
IS18. They lead to increased resistance to carbapenems through the
expression of the blaOXA gene. In addition to OXA carbapenemases,
the transferable MBL family, including VIM, IMP, GIM, SIM and NDM
enzymes are also associated with the drug-resistant phenotype of
Acinetobacter baumannii (44).
Other aspects aside from enzymes. Outer
membrane proteins (OMPs) in general are β-barrel-shaped monomeric
or trimeric porins that allow the diffusion of small molecules into
and out of the periplasmic space of Gram-negative bacteria
(45). The outer membrane of
Acinetobacter baumannii contains several OMPs, including
OmpA, CarO, OprD-like OMPs, Omp 33-36 kDa, AbuO, TolB, DcaP,
Oma87/BamA, NmRmpM, CadF and OprF, amongst others. OMP has multiple
functions, which confers bacterial resistance to threats such as
harsh environments and antibiotics (46). OmpA is the most abundant outer
membrane porin in Acinetobacter baumannii, and it functions
by binding to efflux pumps and expelling antimicrobial compounds
from the periplasm (47). OmpA
increases the sensitivity of Acinetobacter baumannii to
nalidixic acid, chloramphenicol, aztreonam, imipenem and meropenem,
this feature is inseparable from its C-terminal region and
Acinetobacter baumannii peptidoglycan (PG) coupling
regulates outer membrane vesicle (OMV) stability (48). In addition, OmpA also actively
siphons extracellular drugs to mediate antibiotic resistance and
isogenic mutants, which in turn leads to a loss of cell wall
integrity that sensitizes bacteria to colistin and also confers
virulence (46). The outer
membrane protein CarO is a carbapenem drug resistance-related OMP
encoded by the CarO gene. CarO is divided into two subgroups,
namely CarOa and CarOb. Facing different environments and hosts,
the rapid adaptation of Acinetobacter baumannii results in
alterations of the CarO gene (46). The resistance-nodulation-cell
division (RND) efflux pump system is also associated with
resistance in Acinetobacter baumannii. The efflux pump can
extrude a variety of antibacterial agents, reducing the
accumulation of antibiotics (49).
The overexpression of adeABC plays an important role in acquired
resistance to antibiotics. Cefepime, cefpirome and cefotaxime are
the β-lactams most affected by the adeABC efflux system (50). Moreover, penicillin-binding protein
7/8 increases susceptibility to complement and contributes either
directly or indirectly to serum resistance (51).
Novel options for resistance against
β-lactam-based antibiotics. In recent years, researchers have
found that the amino acid sequence of OmpA is highly conserved
(>89%) in various clinical isolates, and OmpA mediates the
adhesion and invasion of Acinetobacter baumannii to
epithelial cells. OmpA can stimulate the innate immune response and
induce biofilm formation, thus OmpA is a potential therapeutic
target, although it has been shown that OmpA is not necessary for
bacterial survival (22). It has
been shown that increased iron content enhances OmpA protein
expression in Acinetobacter baumannii, and strains with high
OmpA protein expression are more aggressive, thus iron control
strategies can be used in the management of Acinetobacter
baumannii to reduce drug resistance (52). Cefiderocol, a member of the
β-lactam antibiotics family, inhibits the synthesis of
Gram-negative bacterial cell walls by binding to penicillin-binding
proteins. However, due to its siderophore-like properties, it can
enter the periplasmic space in bacteria and exhibits high stability
to various β-lactamases such as AmpC and ESBLs (53,54).
In an in vitro study, Cefiderocol was shown to be effective
against OXA-23, OXA-40 and OXA-58. as well as NDM and IMP-producing
Acinetobacter baumannii isolates (55). Efflux pumps are an important part
of drug resistance. The efflux pump inhibitor carbonyl cyanide
m-chlorophenylhydrazone can enhance the susceptibility of
Acinetobacter baumannii to imipenem and cefepime (56). Equally effective efflux pump
inhibitors include Quercetin, particularly when combined with
imipenem, and it has a significant inhibitory effect on NDM and
mexB/adeB (57). Acinetobacter
baumannii vaccine studies has shown that the most effective
vaccines tend to be multiplexed (consisting of outer membrane
vesicles, bacterial ghosts, or multi-subunits) and are usually
composed of antigens from OmpA, OmpW, OmpK and Omp22(58). For resistance to carbapenem
antibiotics, the development of β-lactamase inhibitors has shown
favorable results. β-lactamase inhibitor diazabicyclooctanes
combined with sulbactam restored the sensitivity of sulbactam to
carbapenem-resistant Acinetobacter (59). QPX7728 is a boric acid-lactamase
inhibitor, which was shown to inhibit class A ESBLs, class B
carbapenemases (NDM, VIM and IMP), class C and class D (OXA-23),
and it enhanced its action against carbapenem-resistant
Acinetobacter baumannii when combined with meropenem,
ceflorazone, piperacillin and cefepime (60). Additionally, the combination of
ampicillin and sulbactam (18 g per day) is an effective regimen for
reducing the mortality of patients with CRAB (61). In addition, in terms of vaccine
development, vaccines against BauA and OmpA that are vital
virulence factors in pathogenicity of Acinetobacter
baumannii play a certain role and combination of these antigens
that can bind BauA and OmpA enhanced clearance of bacteria in liver
and spleen (62). TCM ingredients
can also be used to treat drug-resistant Acinetobacter
baumannii. Possibly due to the synergistic action with
antibiotics on efflux pump AdeB, Berberine hydrochloride combined
with sulbactam can improve the antibacterial efficiency against
Acinetobacter baumannii (63). Cilantro oil combined with
piperacillin or cefoperazone can enhance the efficacy of the latter
(64); however, the mechanism
underlying the improved efficacy when combined needs to be
determined. Other TCMs such as Piper betle combined with
antibiotics are also worthy of research (65).
Polymyxins
The resistance of Acinetobacter baumannii to
Polymyxins include: i) modification of the lipid A structure, ii)
complete loss of Lipopolysaccharide (LPS) via mutations in the
genes that synthesize lipid A, iii) reduction in the expression of
cofactors involved in LPS synthesis, and iv) downregulation of
proteins that participate in the export and/or stabilization of
outer membrane precursors (66).
LPS is part of the outer membrane of bacteria. Polymyxins inhibit
bacterial membranes after binding to LPS, interact with lipid A of
the bacterial outer membrane, and cause cell permeability and death
by destroying membrane phospholipids. However, polymyxins
antibiotics take a long time to work, and the use of colistin may
increase the probability of nephrotoxic and neurotoxic
complications (67). Colistin
resistance in Acinetobacter baumannii is primarily caused by
mutations in the PmrBTCS sensor kinase resulting in overexpression
of PmrC. It has been shown that by knocking out the colistin PmrA
mutant, its MICs is reduced by 64 to 1,024-fold, thereby restoring
sensitivity to polymyxins (68).
In Acinetobacter baumannii, various mutations and small
fragments in the PmrB region are the primary cause of colistin
resistance, and the most common PmrB mutation is A138T (69). The mutation of PmrA and PmrB of
Acinetobacter baumannii can lead to resistance to
polymyxins, and its virulence and fitness are also reduced. In
addition, impaired virulence and fitness are also related to the
lpx gene (70). Mutations in lpxA,
lpxC and lpx affect lipid A synthesis. These spontaneous mutations
include single-base changes, large deletions, and insertions of IS
elements, all of which contribute to the high resistance exhibited
by Acinetobacter baumannii (71). In addition, the induction of
endogenous production of reactive oxygen species (ROS) by
polymyxins, thus leading to oxidative killing of bacteria via
hydroxyl radicals. Acinetobacter baumannii via inhibiting
the formation of hydroxyl radicals attenuates polymyxin killing
(72).
In the face of increasing drug resistance, it is a
novel direction to identify new targets for use in combination with
multiple drugs, such as the development of inhibitors against the
targets of the modified bacterial outer membrane LPS two-component
signal transduction system (73).
Solving the problem of drug resistance should not only rely on
antibiotics, instead, it may be favorable to combine current
therapeutics with silver nanoparticles. Silver nanoparticles can
penetrate microbial cell walls and alter cell membrane structure;
this may reduce the MIC by 8-32X when used in combination with
colistin (74). In addition,
macolacin, a chemically synthesized substance that targets the
plasmid-borne polymyxin resistance gene mcr-1, is also effective
for Gram-negative pathogens expressing mcr-1 including
Acinetobacter baumannii (75). The combination of polymyxin B with
imipenem, meropenem, tigecycline and rifampicin in the treatment of
Acinetobacter baumannii was superior to any of these alone,
and the combination with rifampicin had the best effect (76). In terms of TCMs, the extract of
Scutellaria barbata was shown to exhibit a good inhibitory
effect on Acinetobacter baumannii, and the mechanism may be
related to ROS (77), and the
combination of Scutellaria barbata and polymyxin may have
unexpected effects.
Tetracyclines
Tigecycline, a unique semi-synthetic antibacterial
agent of the glycylcycline class, is derived from tetracycline and
designed to overcome common resistance mechanisms to tetracycline
(78). Its mechanism of action is
to inhibit bacterial growth by binding to the bacterial 30S
ribosome and blocking the entry of tRNA, ultimately preventing
protein synthesis. Although tigecycline circumvents resistance
mechanisms of tetracycline, Acinetobacter baumannii can
acquire tigecycline resistance through overexpression of efflux
pumps, particularly AdeABC, and modification of the
tigecycline-binding site in the ribosome through rpsJ mutations
(79). Likewise, the adeIJK of the
RND efflux pump confers Acinetobacter baumannii resistance
against tetracycline antibiotics (80). It has been shown that
Acinetobacter baumannii expressing tetracycline transporter
gene (tet)A have significantly increased MICs for tetracycline and
tigecycline. Acinetobacter baumannii that express tetG also
show resistance to these tetracyclines in addition to drug
resistance to tigecycline (81).
Although there are also genetic studies showing that the increased
resistance of strains induced by tigecycline can be recovered, this
also indicates that the use of tigecycline therapy may increase the
risk of multidrug-resistant gaining additional resistance (82).
It has been shown that third-generation
tetracyclines (aminomethylcycline) KBP-7072 and omadacycline
overcome efflux and ribosomal protection resistance mechanisms
observed during tetracycline resistance, highlighting a novel
direction for the development of tetracycline-based antibiotics
(83). In addition, Omadacycline
in combination with sulbactam was shown to be synergistic and
bactericidal against 80% of isolates (84). A study showed that D-lysine
conjugated aliphatic norspermidine analogue bearing tetradecanoyl
chain (also known as D-LANA-14) increased the permeability of cell
membranes. When D-LANA-14 was combined with tetracycline and other
inactive antibiotics, it exhibited synergistic activity against
Acinetobacter baumannii (85).
Quinolones
Through gene knockout studies, it has been shown
that the transporter AbaQ is primarily involved in the extrusion of
quinolones from Acinetobacter baumannii (86). Resistance to quinolones has also
been attributed to spontaneous mutations in genes, including DNA
gyrase and topoisomerase IV. This leads to high levels of
resistance to quinolones in Acinetobacter baumannii
(87). Changes in antibiotic
target sites are an important mechanism of bacterial resistance,
that manifests through random point mutations with a minimal impact
on bacterial cell homeostasis. In Acinetobacter baumannii,
the most common mechanism of resistance is fluoroquinolone
resistance, which is acquired by spontaneous mutations in the gyrA,
gyrB and parC genes which encode gyrase and topoisomerase IV
(88). The existence of the efflux
pump adeABC is still an important cause of drug resistance in
Acinetobacter baumannii (89).
A newer study shows that Mentha longifolia
and Menthol can facilitate the entry of material into the cell
membrane of bacteria and mitochondria, thereby facilitating the
inhibition of the adeABC efflux pump in Acinetobacter
baumannii. When Mentha longifolia and Menthol are combined with
ciprofloxacin and imipenem, it can significantly reduce the MIC for
Acinetobacter baumannii (90). Bile salt derivatives, Na-3DH-DCA
and Na-3DH-CDCA, have synergistic effects on certain strains of
Acinetobacter baumannii when used in combination with
ciprofloxacin, highlighting a potential future direction (91).
Aminoglycosides
The most common aminoglycoside resistance gene in
Acinetobacter baumannii is aadB (42%), followed by apa6
(26%), while aadA1 (18%), with aacc1 (12%) being rare (92). The armA gene is an effective factor
for the resistance of Acinetobacter baumannii to
aminoglycosides; the gene encodes 16S rRNA methylase, which leads
to the limited access of aminoglycosides into the bacterial
ribosomes, and furthermore leads to high-level aminoglycoside
resistance (HLAR) to gentamicin, bruomycin, amikacin and kanamycin
(93). In addition, AdeABC has a
restrictive effect in reducing the susceptibility of
Acinetobacter baumannii to aminoglycoside antibiotics
(94).
It has been shown that strains with a single
well-defined resistance mechanism lack cross-resistance to
gentamicin, amikacin, tobramycin and prazomycin (95). Thus, the cross-use of
aminoglycosides is a temporary solution. Additionally, L-lysine
combats drug-resistant Acinetobacter baumannii by increasing
the transmembrane DpH difference which in-turn increases the
bacterial proton motive force and stimulates the uptake of
aminoglycoside antibiotics (96).
The combination of antibiotics is another method of treatment.
Tobramycin and colistin can be used to treat or eradicate
Acinetobacter baumannii by reducing the expression of the
universal stress protein (uspA) (97).
Biofilm
Several pathogens, including Acinetobacter
Baumannii, produce biofilms in response to dry conditions,
nutrient shortages, resistance to antibiotics, and other challenges
(98). The formation of
Acinetobacter Baumannii is associated with the Quorum
sensing pathway, two-component system signal transduction pathway,
cyclic-di-GMP signaling and the capsular polysaccharide synthesis
pathway. Biofilm-associated proteins such as Bap in
Acinetobacter Baumannii also serve a vital role in biofilm
(99). Several studies have shown
that csuE, pgaB, epsA, ptk, bfmS and the ompA biofilm-related genes
are involved in biofilm formation (99,100). However, resistance due to
biofilms is specific and these genes are not direct factors for the
resistance of Acinetobacter Baumannii (101). Thus, additional research is
required to clarify the specific mechanisms involved.
Myrtenol is an important dicyclic monoterpene
alcohol, which inhibits the growth of biofilms by affecting the
adhesion factors associated with biofilms and improves the
sensitivity of certain antibiotics to Acinetobacter
baumannii. Myrtenol has the potential to be used in combination
with antibiotics (102). The
combination of polymyxin B or E with azithromycin can inhibit
biofilm formation (103). These
studies suggest that the combination of antibiotics is still a
valuable method for the treatment of multiple drug resistant
infections. The extract of star anise (Illicium verum Hook.)
has a significant inhibitory effect on biofilm, which does not
affect the growth of cells. The underlying mechanism may involve
the disruption of the cell membrane of bacteria due to the
lipophilic nature of the extract (104). In addition, phage (105), antimicrobial photodynamic therapy
(106) and antimicrobial peptides
(107) are seen as non-antibiotic
therapies with significant potential for the future.
5. Conclusions and future perspectives
Bacterial infections are the cause of several
diseases and can aggravate already present diseases as well. The
development of drug resistance caused by its unique physiological
characteristics makes infections caused by drug-resistant
Acinetobacter baumannii considerably more difficult to
treat. Therefore, a deeper understanding on the drug resistance
mechanisms is required to improve our armamentarium against said
infections. At present, differing combinations of antibiotics is
the easiest and most effective way to manage infections. However,
novel therapeutics will likely be required going forward as drug
resistance increases. Thus, robust clinical trials will also be
required for any novel therapeutics. That is, to manage the
ever-increasing drug resistance, improved drugs, newer treatment
technologies and alternative treatment methods are required.
Acknowledgements
The authors would like to thank Dr Chen-Xia Lu
(Department of Hepatology, Hubei Key Laboratory of the Theory and
application research of liver and kidney in traditional Chinese
medicine, Hubei Provincial Hospital of Traditional Chinese
Medicine, Affiliated Hospital of Hubei University of Chinese
Medicine, Hubei Province Academy of Traditional Chinese Medicine,
Wuhan, China) and Dr Hui Zhu (Department of Clinical College of
Traditional Chinese Medicine, Hubei University of Chinese Medicine,
Wuhan, China) for their assistance in the development of this
review.
Funding
Funding: The preparation of this manuscript was funded by the
Hubei Province Traditional Chinese Medicine Infectious Disease
Discipline Construction Project and the Inheritance and Development
Project of Traditional Chinese medicines (grant no.
Z155080000004).
Availability of data and materials
Not applicable.
Authors' contributions
HJW and ZGX designed the subject of review. HFL
revised the article. HJW, ZGX, XJL, HTH, CYH, CL, YX and HFL
participated in writing and reviewing the manuscript. All authors
have read and approved the final manuscript. Data authentication is
not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mojica MF, Rossi MA, Vila AJ and Bonomo
RA: The urgent need for metallo-β-lactamase inhibitors: An
unattended global threat. Lancet Infect Dis. 22:e28–e34.
2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Khan SN and Khan AU: Breaking the spell:
Combating multidrug resistant ‘superbugs.’. Front Microbiol.
7(174)2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Boral B, Unaldi Ö, Ergin A, Durmaz R and
Eser ÖK: Acinetobacter Study Group. A prospective multicenter study
on the evaluation of antimicrobial resistance and molecular
epidemiology of multidrug-resistant Acinetobacter baumanni
infections in intensive care units with clinical and environmental
features. Ann Clin Microbiol Antimicrob. 18(19)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Torres HA, Vázquez EG, Yagüe G and Gómez
JG: Multidrug resistant Acinetobacter baumanii: Clinical
update and new highlights. Rev Esp Quimioter. 23:12–19.
2010.PubMed/NCBI(In Spanish).
|
|
5
|
Vázquez-López R, Solano-Gálvez SG, Juárez
Vignon-Whaley JJ, Abello Vaamonde JA, Padró Alonzo LA, Rivera
Reséndiz A, Muleiro Álvarez M, Vega López EN, Franyuti-Kelly G,
Álvarez-Hernández DA, et al: Acinetobacter baumannii
resistance: A real challenge for clinicians. Antibiotics (Basel).
9(205)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ayoub Moubareck C and Hammoudi Halat D:
Insights into Acinetobacter baumannii: A review of
microbiological, virulence, and resistance traits in a threatening
nosocomial pathogen. Antibiotics (Basel). 9(119)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ibrahim S, Al-Saryi N, Al-Kadmy IMS and
Aziz SN: Multidrug-resistant Acinetobacter baumannii as an
emerging concern in hospitals. Mol Biol Rep. 48:6987–6998.
2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Harding CM, Hennon SW and Feldman MF:
Uncovering the mechanisms of Acinetobacter baumannii
virulence. Nat Rev Microbiol. 16:91–102. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Bagińska N, Pichlak A, Górski A and
Jończyk-Matysiak E: Specific and selective bacteriophages in the
fight against multidrug-resistant Acinetobacter baumannii.
Virol Sin. 34:347–357. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Roy S, Chowdhury G, Mukhopadhyay AK, Dutta
S and Basu S: Convergence of biofilm formation and antibiotic
resistance in Acinetobacter baumannii infection. Front Med
(Lausanne). 9(793615)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ma C and McClean S: Mapping global
prevalence of Acinetobacter baumannii and recent vaccine
development to tackle it. Vaccines (Basel). 9(570)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Colquhoun JM and Rather PN: Insights into
mechanisms of biofilm formation in Acinetobacter baumannii
and implications for uropathogenesis. Front Cell Infect Microbiol.
10(253)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Bergogne-Bérézin E and Towner KJ:
Acinetobacter spp. as nosocomial pathogens: Microbiological,
clinical, and epidemiological features. Clin Microbiol Rev.
9:148–165. 1996.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ramirez MS, Bonomo RA and Tolmasky ME:
Carbapenemases: Transforming Acinetobacter baumannii into a
yet more dangerous menace. Biomolecules. 10(720)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chapartegui-González I, Lázaro-Díez M,
Bravo Z, Navas J, Icardo JM and Ramos-Vivas J: Acinetobacter
baumannii maintains its virulence after long-time starvation.
PLoS One. 13(e0201961)2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Nguyen M and Joshi SG: Carbapenem
resistance in Acinetobacter baumannii, and their importance
in hospital-acquired infections: a scientific review. J Appl
Microbiol. 131:2715–2738. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kim YA, Kim JJ, Won DJ and Lee K: Seasonal
and temperature-associated increase in community-onset
Acinetobacter baumannii complex colonization or infection.
Ann Lab Med. 38:266–270. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Guo J and Li C: Molecular epidemiology and
decreased susceptibility to disinfectants in carbapenem-resistant
Acinetobacter baumannii isolated from intensive care unit
patients in central China. J Infect Public Health. 12:890–896.
2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Weinberg SE, Villedieu A, Bagdasarian N,
Karah N, Teare L and Elamin WF: Control and management of multidrug
resistant Acinetobacter baumannii: A review of the evidence
and proposal of novel approaches. Infect Prev Pract.
2(100077)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Oh DH, Kim YC, Kim EJ, Jung IY, Jeong SJ,
Kim SY, Park MS, Kim A, Lee JG and Paik HC: Multidrug-resistant
Acinetobacter baumannii infection in lung transplant
recipients: Risk factors and prognosis. Infect Dis (Lond).
51:493–501. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Martín-Aspas A, Guerrero-Sánchez FM,
García-Colchero F, Rodríguez-Roca S and Girón-González JA:
Differential characteristics of Acinetobacter baumannii
colonization and infection: Risk factors, clinical picture, and
mortality. Infect Drug Resist. 11:861–872. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Nie D, Hu Y, Chen Z, Li M, Hou Z, Luo X,
Mao X and Xue X: Outer membrane protein A (OmpA) as a potential
therapeutic target for Acinetobacter baumannii infection. J
Biomed Sci. 27(26)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kyriakidis I, Vasileiou E, Pana ZD and
Tragiannidis A: Acinetobacter baumannii antibiotic
resistance mechanisms. Pathogens. 10(373)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sawa T, Kooguchi K and Moriyama K:
Molecular diversity of extended-spectrum β-lactamases and
carbapenemases, and antimicrobial resistance. J Intensive Care.
8(13)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Tsivkovski R, Totrov M and Lomovskaya O:
Biochemical characterization of QPX7728, a new ultrabroad-spectrum
beta-lactamase inhibitor of serine and metallo-beta-lactamases.
Antimicrob Agents Chemother. 64:e00130–20. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Philippon A, Jacquier H, Ruppé E and Labia
R: Structure-based classification of class A beta-lactamases, an
update. Curr Res Transl Med. 67:115–122. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Tooke CL, Hinchliffe P, Bragginton EC,
Colenso CK, Hirvonen VHA, Takebayashi Y and Spencer J: β-Lactamases
and β-lactamase inhibitors in the 21st century. J Mol Biol.
431:3472–3500. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Palzkill T: Structural and mechanistic
basis for extended-spectrum drug-resistance mutations in altering
the specificity of TEM, CTX-M, and KPC β-lactamases. Front Mol
Biosci. 5(16)2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Nikibakhsh M, Firoozeh F, Badmasti F,
Kabir K and Zibaei M: Molecular study of metallo-β-lactamases and
integrons in Acinetobacter baumannii isolates from burn
patients. BMC Infect Dis. 21(782)2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Amin M, Navidifar T, Saleh Shooshtari F
and Goodarzi H: Association of the genes encoding
metallo-β-lactamase with the presence of integrons among
multidrug-resistant clinical isolates of Acinetobacter
baumannii. Infect Drug Resist. 12:1171–1180. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
López C, Ayala JA, Bonomo RA, González LJ
and Vila AJ: Protein determinants of dissemination and host
specificity of metallo-β-lactamases. Nat Commun.
10(3617)2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ejaz H, Alzahrani B, Hamad MFS, Abosalif
KOA, Junaid K, Abdalla AE, Elamir MYM, Aljaber NJ, Hamam SSM and
Younas S: Molecular analysis of the antibiotic resistant NDM-1 gene
in clinical isolates of enterobacteriaceae. Clin Lab.
66:2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wu W, Feng Y, Tang G, Qiao F, McNally A
and Zong Z: NDM metallo-β-lactamases and their bacterial producers
in health care settings. Clin Microbiol Rev. 32:e00115–18.
2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Junaid K: Molecular diversity of NDM-1,
NDM-5, NDM-6, and NDM-7 variants of new delhi metallo-β-lactamases
and their impact on drug resistance. Clin Lab. 67:2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Jiang L, Yu Y, Zeng W, Guo J, Lv F, Wang
X, Liu X and Zhao Z: Whole-genome analysis of New Delhi
metallo-beta-lactamase-1-producing Acinetobacter haemolyticus from
China. J Glob Antimicrob Resist. 20:204–208. 2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ingti B, Upadhyay S, Hazarika M, Khyriem
AB, Paul D, Bhattacharya P, Joshi SR, Bora D, Dhar D and
Bhattacharjee A: Distribution of carbapenem resistant
Acinetobacter baumannii with blaADC-30 and
induction of ADC-30 in response to beta-lactam antibiotics. Res
Microbiol. 171:128–133. 2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Said HS, Benmahmod AB and Ibrahim RH:
Co-production of AmpC and extended spectrum beta-lactamases in
cephalosporin-resistant Acinetobacter baumannii in Egypt.
World J Microbiol Biotechnol. 34(189)2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Bouza AA, Swanson HC, Smolen KA, VanDine
AL, Taracila MA, Romagnoli C, Caselli E, Prati F, Bonomo RA, Powers
RA and Wallar BJ: Structure-based analysis of boronic acids as
inhibitors of acinetobacter-derived cephalosporinase-7, a unique
class C β-lactamase. ACS Infect Dis. 4:325–336. 2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Porbaran M, Tahmasebi H and Arabestani M:
A comprehensive study of the relationship between the production of
β-lactamase enzymes and iron/siderophore uptake regulatory genes in
clinical isolates of Acinetobacter baumannii. Int J
Microbiol. 2021(5565537)2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Monem S, Furmanek-Blaszk B, Łupkowska A,
Kuczyńska-Wiśnik D, Stojowska-Swędrzyńska K and Laskowska E:
Mechanisms protecting Acinetobacter baumannii against
multiple stresses triggered by the host immune response,
antibiotics and outside-host environment. Int J Mol Sci.
21(5498)2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Lupo A, Haenni M and Madec JY:
Antimicrobial Resistance in Acinetobacter spp. and Pseudomonas spp.
Microbiol Spectr. 16:2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Hamidian M and Nigro SJ: Emergence,
molecular mechanisms and global spread of carbapenem-resistant
Acinetobacter baumannii. Microb Genom.
5(e000306)2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Shi X, Wang H, Wang X, Jing H, Duan R, Qin
S, Lv D, Fan Y, Huang Z, Stirling K, et al: Molecular
characterization and antibiotic resistance of Acinetobacter
baumannii in cerebrospinal fluid and blood. PLoS One.
16(e0247418)2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Ibrahim ME: Prevalence of Acinetobacter
baumannii in Saudi Arabia: Risk factors, antimicrobial
resistance patterns and mechanisms of carbapenem resistance. Ann
Clin Microbiol Antimicrob. 18(1)2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Slusky JSG and Dunbrack RL Jr: Charge
asymmetry in the proteins of the outer membrane. Bioinformatics.
29:2122–2128. 2013.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Uppalapati SR, Sett A and Pathania R: The
outer membrane proteins OmpA, CarO, and OprD of Acinetobacter
baumannii confer a two-pronged defense in facilitating its
success as a potent human pathogen. Front Microbiol.
11(589234)2020.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Tsai YK, Liou CH, Lin JC, Fung CP, Chang
FY and Siu LK: Effects of different resistance mechanisms on
antimicrobial resistance in Acinetobacter baumannii: A
strategic system for screening and activity testing of new
antibiotics. Int J Antimicrob Agents. 55(105918)2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Skerniškytė J, Karazijaitė E, Lučiūnaitė A
and Sužiedėlienė E: OmpA protein-deficient Acinetobacter
baumannii outer membrane vesicles trigger reduced inflammatory
response. Pathogens. 10(407)2021.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Zhang Y, Fan B, Luo Y, Tao Z, Nie Y, Wang
Y, Ding F, Li Y and Gu D: Comparative analysis of carbapenemases,
RND family efflux pumps and biofilm formation potential among
Acinetobacter baumannii strains with different carbapenem
susceptibility. BMC Infect Dis. 21(841)2021.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Kim CM, Park G, Ko YJ, Kang SH and Jang
SJ: Relationships between relative expression of RND efflux pump
genes, H33342 efflux activity, biofilm-forming activity, and
antimicrobial resistance in Acinetobacter baumannii clinical
isolates. Jpn J Infect Dis. 74:499–506. 2021.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Badmasti F, Habibi M, Firoozeh F,
Fereshteh S, Bolourchi N and Goodarzi NN: The combination of CipA
and PBP-7/8 proteins contribute to the survival of C57BL/6 mice
from sepsis of Acinetobacter baumannii. Microb Pathog.
158(105063)2021.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Liu H, Cao CY, Qiu FL, Huang HN, Xie H,
Dong R, Shi YZ and Hu XN: Iron-rich conditions induce OmpA and
virulence changes of Acinetobacter baumannii. Front
Microbiol. 12(725194)2021.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Abdul-Mutakabbir JC, Alosaimy S,
Morrisette T, Kebriaei R and Rybak MJ: Cefiderocol: A novel
siderophore cephalosporin against multidrug-resistant gram-negative
pathogens. Pharmacotherapy. 40:1228–1247. 2020.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Zhanel GG, Golden AR, Zelenitsky S, Wiebe
K, Lawrence CK, Adam HJ, Idowu T, Domalaon R, Schweizer F, Zhanel
MA, et al: Cefiderocol: A siderophore cephalosporin with activity
against carbapenem-resistant and multidrug-resistant gram-negative
bacilli. Drugs. 79:271–289. 2019.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Isler B, Doi Y, Bonomo RA and Paterson DL:
New treatment options against carbapenem-resistant Acinetobacter
baumannii infections. Antimicrob Agents Chemother.
63:e01110–18. 2018.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Sanchez-Carbonel A, Mondragón B,
López-Chegne N, Peña-Tuesta I, Huayan-Dávila G, Blitchtein D,
Carrillo-Ng H, Silva-Caso W, Aguilar-Luis MA and Del Valle-Mendoza
J: The effect of the efflux pump inhibitor carbonyl cyanide
m-chlorophenylhydrazone (CCCP) on the susceptibility to imipenem
and cefepime in clinical strains of Acinetobacter baumannii.
PLoS One. 16(e0259915)2021.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Pal A and Tripathi A: Quercetin
potentiates meropenem activity among pathogenic
carbapenem-resistant Pseudomonas aeruginosa and
Acinetobacter baumannii. J Appl Microbiol. 127:1038–1047.
2019.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Gellings PS, Wilkins AA and Morici LA:
Recent advances in the pursuit of an effective Acinetobacter
baumannii vaccine. Pathogens. 9(1066)2020.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Pasteran F, Cedano J, Baez M, Albornoz E,
Rapoport M, Osteria J, Montaña S, Le C, Ra G, Bonomo RA, et al: A
new twist: The combination of sulbactam/avibactam enhances
sulbactam activity against carbapenem-resistant Acinetobacter
baumannii (CRAB) isolates. Antibiotics (Basel).
10(577)2021.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Yahav D, Giske CG, Grāmatniece A, Abodakpi
H, Tam VH and Leibovici L: New β-lactam-β-lactamase inhibitor
combinations. Clin Microbiol Rev. 34:e00115–20. 2020.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Bartal C, Rolston KVI and Nesher L:
Carbapenem-resistant Acinetobacter baumannii: Colonization,
infection and current treatment options. Infect Dis Ther.
11:683–694. 2022.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Tamehri M, Rasooli I, Pishgahi M,
Jahangiri A, Ramezanalizadeh F and Banisaeed Langroodi SR:
Combination of BauA and OmpA elicit immunoprotection against
Acinetobacter baumannii in a murine sepsis model. Microb
Pathog. 173(105874)2022.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Li X, Song Y, Wang L, Kang G, Wang P, Yin
H and Huang H: A potential combination therapy of berberine
hydrochloride with antibiotics against multidrug-resistant
Acinetobacter baumannii. Front Cell Infect Microbiol.
11(660431)2021.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Duarte A, Ferreira S, Silva F and
Domingues FC: Synergistic activity of coriander oil and
conventional antibiotics against Acinetobacter baumannii.
Phytomedicine. 19:236–238. 2012.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Herman A and Herman AP: Herbal products
and their active constituents used alone and in combination with
antibiotics against multidrug-resistant bacteria. Planta Med.
89:168–182. 2023.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Lima WG, Alves MC, Cruz WS and Paiva MC:
Chromosomally encoded and plasmid-mediated polymyxins resistance in
Acinetobacter baumannii: A huge public health threat. Eur J
Clin Microbiol Infect Dis. 37:1009–1019. 2018.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Nasr P: Genetics, epidemiology, and
clinical manifestations of multidrug-resistant Acinetobacter
baumannii. J Hosp Infect. 104:4–11. 2020.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Trebosc V, Gartenmann S, Tötzl M, Lucchini
V, Schellhorn B, Pieren M, Lociuro S, Gitzinger M, Tigges M, Bumann
D and Kemmer C: Dissecting colistin resistance mechanisms in
extensively drug-resistant Acinetobacter baumannii clinical
isolates. mBio. 10:e01083–19. 2019.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Nurtop E, Bayındır Bilman F, Menekse S,
Kurt Azap O, Gönen M, Ergonul O and Can F: Promoters of Colistin
Resistance in Acinetobacter baumannii Infections. Microb
Drug Resist. 25:997–1002. 2019.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Wang Y, Luo Q, Xiao T, Zhu Y and Xiao Y:
Impact of polymyxin resistance on virulence and fitness among
clinically important gram-negative bacteria. Engineering.
13:178–185. 2022.
|
|
71
|
Moffatt JH, Harper M and Boyce JD:
Mechanisms of polymyxin resistance. Adv Exp Med Biol. 1145:55–71.
2019.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Nang SC, Azad MAK, Velkov T, Zhou Q and Li
J: Rescuing the last-line polymyxins: Achievements and challenges.
Pharmacol Rev. 73:679–728. 2021.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Mohapatra SS, Dwibedy SK and Padhy I:
Polymyxins, the last-resort antibiotics: Mode of action, resistance
emergence, and potential solutions. J Biosci. 46(85)2021.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Khaled JM, Alharbi NS, Siddiqi MZ,
Alobaidi AS, Nauman K, Alahmedi S, Almazyed AO, Almosallam MA and
Al Jurayyan AN: A synergic action of colistin, imipenem, and silver
nanoparticles against pandrug-resistant Acinetobacter
baumannii isolated from patients. J Infect Public Health.
14:1679–1685. 2021.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Wang Z, Koirala B, Hernandez Y, Zimmerman
M, Park S, Perlin DS and Brady SF: A naturally inspired antibiotic
to target multidrug-resistant pathogens. Nature. 601:606–611.
2022.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Zhang H, Zhu Y, Yang N, Kong Q, Zheng Y,
Lv N, Chen H, Yue C, Liu Y, Li J and Ye Y: In vitro and in vivo
Activity of combinations of polymyxin B with other antimicrobials
against carbapenem-resistant Acinetobacter baumannii. Infect
Drug Resist. 14:4657–4666. 2021.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Tsai CC, Lin CS, Hsu CR, Chang CM, Chang
IW, Lin LW, Hung CH and Wang JL: Using the Chinese herb
Scutellaria barbata against extensively drug-resistant
Acinetobacter baumannii infections: In vitro and in vivo
studies. BMC Complement Altern Med. 18(96)2018.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Yaghoubi S, Zekiy AO, Krutova M, Gholami
M, Kouhsari E, Sholeh M, Ghafouri Z and Maleki F: Tigecycline
antibacterial activity, clinical effectiveness, and mechanisms and
epidemiology of resistance: Narrative review. Eur J Clin Microbiol
Infect Dis. 41:1003–1022. 2022.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Jo J and Ko KS: Tigecycline
heteroresistance and resistance mechanism in clinical isolates of
Acinetobacter baumannii. Microbiol Spectr.
9(e0101021)2021.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Zhang Z, Morgan CE, Bonomo RA and Yu EW:
Cryo-EM determination of eravacycline-bound structures of the
ribosome and the multidrug efflux pump AdeJ of Acinetobacter
baumannii. mBio. 12(e0103121)2021.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Foong WE, Wilhelm J, Tam H-K and Pos KM:
Tigecycline efflux in Acinetobacter baumannii is mediated by
TetA in synergy with RND-type efflux transporters. J Antimicrob
Chemother. 75:1135–1139. 2020.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Cheng J, Kesavan DK, Vasudevan A, Cai W,
Wang H, Su Z, Wang S and Xu H: Genome and transcriptome analysis of
A. baumannii's ‘Transient’ increase in drug resistance under
tigecycline pressure. J Glob Antimicrob Resist. 22:219–225.
2020.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Huband MD, Mendes RE, Pfaller MA, Lindley
JM, Strand GJ, Benn VJ, Zhang J, Li L, Zhang M, Tan X, et al: In
vitro activity of KBP-7072, a novel third-generation tetracycline,
against 531 recent geographically diverse and molecularly
characterized Acinetobacter baumannii species complex
isolates. Antimicrob Agents Chemother. 64:e02375–19.
2020.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Abbey T, Vialichka A, Jurkovic M, Biagi M
and Wenzler E: Activity of omadacycline alone and in combination
against carbapenem-nonsusceptible Acinetobacter baumannii
with varying minocycline susceptibility. Microbiol Spectr.
10:e00542–22. 2022.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Konai MM and Haldar J: Lysine-based small
molecule sensitizes rifampicin and tetracycline against
multidrug-resistant Acinetobacter baumannii and
Pseudomonas aeruginosa. ACS Infect Dis. 6:91–99.
2020.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Pérez-Varela M, Corral J, Aranda J and
Barbé J: Functional characterization of AbaQ, a novel efflux pump
mediating quinolone resistance in Acinetobacter baumannii.
Antimicrob Agents Chemother. 62:e00906–18. 2018.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Mohammed MA, Salim MTA, Anwer BE,
Aboshanab KM and Aboulwafa MM: Impact of target site mutations and
plasmid associated resistance genes acquisition on resistance of
Acinetobacter baumannii to fluoroquinolones. Sci Rep.
11(20136)2021.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Vrancianu CO, Gheorghe I, Czobor IB and
Chifiriuc MC: Antibiotic resistance profiles, molecular mechanisms
and innovative treatment strategies of Acinetobacter
baumannii. Microorganisms. 8(935)2020.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Nogbou ND, Nkawane GM, Ntshane K, Wairuri
CK, Phofa DT, Mokgokong KK, Ramashia M, Nchabeleng M, Obi LC and
Musyoki AMz: Efflux pump activity and mutations driving multidrug
resistance in Acinetobacter baumannii at a Tertiary Hospital
in Pretoria, South Africa. Int J Microbiol.
2021(9923816)2021.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Mahmoudi H, Shokoohizadeh L, Zare Fahim N,
Mohamadi Bardebari A, Moradkhani S and Alikhani MY: Detection of
adeABC efllux pump encoding genes and antimicrobial effect of
Mentha longifolia and Menthol on MICs of imipenem and
ciprofloxacin in clinical isolates of Acinetobacter
baumannii. BMC Complement Med Ther. 20(92)2020.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Aleksić Sabo V, Škorić D, Jovanović-Šanta
S, Nikolić I, János C and Knežević P: Synergistic activity of bile
salts and their derivatives in combination with conventional
antimicrobial agents against Acinetobacter baumannii. J
Ethnopharmacol. 264(113266)2021.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Rizk M and Abou El-Khier N: Aminoglycoside
resistance genes in Acinetobacter baumannii clinical
isolates. Clin Lab. 65:2019.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Jouybari MA, Ahanjan M, Mirzaei B and Goli
HR: Role of aminoglycoside-modifying enzymes and 16S rRNA methylase
(ArmA) in resistance of Acinetobacter baumannii clinical
isolates against aminoglycosides. Rev Soc Bras Med Trop.
54(e05992020)2021.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Sheikhalizadeh V, Hasani A, Ahangarzadeh
Rezaee M, Rahmati-Yamchi M, Hasani A, Ghotaslou R and Goli HR:
Comprehensive study to investigate the role of various
aminoglycoside resistance mechanisms in clinical isolates of
Acinetobacter baumannii. J Infect Chemother. 23:74–79.
2017.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Juhas M, Widlake E, Teo J, Huseby DL,
Tyrrell JM, Polikanov YS, Ercan O, Petersson A, Cao S, Aboklaish
AF, et al: In vitro activity of apramycin against multidrug-,
carbapenem- and aminoglycoside-resistant Enterobacteriaceae and
Acinetobacter baumannii. J Antimicrob Chemother. 74:944–952.
2019.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Deng W, Fu T, Zhang Z, Jiang X, Xie J, Sun
H, Hu P, Ren H, Zhou P, Liu Q and Long Q: L-lysine potentiates
aminoglycosides against Acinetobacter baumannii via
regulation of proton motive force and antibiotics uptake. Emerg
Microbes Infect. 9:639–650. 2020.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Kashyap S, Kaur S, Sharma P and Capalash
N: Combination of colistin and tobramycin inhibits persistence of
Acinetobacter baumannii by membrane hyperpolarization and
down-regulation of efflux pumps. Microbes Infect.
23(104795)2021.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Law SKK and Tan HS: The role of quorum
sensing, biofilm formation, and iron acquisition as key virulence
mechanisms in Acinetobacter baumannii and the corresponding
anti-virulence strategies. Microbiol Res.
260(127032)2022.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Kaushik V, Tiwari M, Joshi R and Tiwari V:
Therapeutic strategies against potential antibiofilm targets of
multidrug-resistant Acinetobacter baumannii. J Cell Physiol.
237:2045–2063. 2022.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Donadu MG, Mazzarello V, Cappuccinelli P,
Zanetti S, Madléna M, Nagy ÁL, Stájer A, Burián K and Gajdács M:
Relationship between the biofilm-forming capacity and antimicrobial
resistance in clinical Acinetobacter baumannii isolates:
Results from a laboratory-based in vitro study. Microorganisms.
9(2384)2021.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Shenkutie AM, Yao MZ, Siu GKH, Wong BKC
and Leung PHM: Biofilm-induced antibiotic resistance in clinical
Acinetobacter baumannii Isolates. Antibiotics (Basel).
9(817)2020.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Selvaraj A, Valliammai A, Sivasankar C,
Suba M, Sakthivel G and Pandian SK: Antibiofilm and antivirulence
efficacy of myrtenol enhances the antibiotic susceptibility of
Acinetobacter baumannii. Sci Rep. 10(21975)2020.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Meng Q, Lin F and Ling B: In vitro
activity of peptide antibiotics in combination with other
antimicrobials on extensively drug-resistant Acinetobacter
baumannii in the planktonic and biofilm cell. Front Pharmacol.
13(890955)2022.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Salem MA, El-Shiekh RA, Hashem RA and
Hassan M: In vivo antibacterial activity of star anise (Illicium
verum Hook.) extract using murine MRSA skin infection model in
relation to its metabolite profile. Infect Drug Resist. 14:33–48.
2021.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Shahed-Al-Mahmud M, Roy R, Sugiokto FG,
Islam MN, Lin MD, Lin LC and Lin NT: Phage φAB6-borne depolymerase
combats Acinetobacter baumannii biofilm formation and
infection. Antibiotics (Basel). 10(279)2021.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Figueiredo-Godoi LMA, Garcia MT, Pinto JG,
Ferreira-Strixino J, Faustino EG, Pedroso LLC and Junqueira JC:
Antimicrobial photodynamic therapy mediated by fotenticine and
methylene blue on planktonic growth, biofilms, and burn infections
of Acinetobacter baumannii. Antibiotics (Basel).
11(619)2022.PubMed/NCBI View Article : Google Scholar
|
|
107
|
das Neves RC, Mortari MR, Schwartz EF,
Kipnis A and Junqueira-Kipnis AP: Antimicrobial and antibiofilm
effects of peptides from venom of social wasp and scorpion on
multidrug-resistant Acinetobacter baumannii. Toxins (Basel).
11(216)2019.PubMed/NCBI View Article : Google Scholar
|