Introduction
Iron is an essential trace element for a variety of
physiological functions in the human body, including oxygen
transportation, metabolism, DNA synthesis and damage repair,
neurotransmitter production, and transcriptional regulation
(1,2). Iron deficiency is a widespread
nutritional deficiency worldwide (3,4).
Severe iron deficiency can lead to iron deficiency anemia, heart
failure, and ischemic heart disease (5-7).
However, iron deficiency in blood donors who are at high risk of
iron deficiency will cause more serious iron homeostasis disorder,
lower hemoglobin level, hypochromic anemia, and high delayed blood
donation rate than non-donors, and even threaten the health of
blood donors (8-10).
Iron monitoring is therefore critical for human health, especially
blood donors. Appropriate monitoring of ferritin and transferrin
levels in blood donors has been shown to be beneficial for the
regulation of national blood donation policies, measures to prevent
iron deficiency and anemia in donors, adjustment of donation
intervals, donor health, and blood supply (11).
Monitoring of iron in humans is usually reflected by
the level of serum ferritin (12).
Transferrin, the circulating iron-binding protein, is another key
marker of iron status because its levels increase with iron
deficiency (13). Other studies
have shown that ferritin and transferrin levels may have a
significant impact on blood donation and donor health (14). The current universally accepted
cut-off value for the diagnosis of iron insufficiency is serum
ferritin <15 ng/ml (excluding infection or inflammation)
(15). However, the RIs and
influencing factors for the levels of ferritin and transferrin in
the diagnosis of iron deficiency in blood donors are not well
established in China yet, although an iron deficiency in whole
blood donors has been found to be much more common than expected,
and regular platelet donation can also reduce ferritin levels and
lead to iron deficiency in some developed countries (16-20).
There are only limited reports on the levels of
ferritin and transferrin and the factors affecting iron metabolism
in Chinese blood donors, and there is a lack of large sample
studies from mainland China. No study has established ferritin and
transferrin RIs following the protocols of the Clinical and
Laboratory Standards Institute (CLSI) and International Federation
of Clinical Chemistry and Laboratory Medicine, Committee on RIs and
Decision Limits (IFCC/C-RIDL) (21-23).
Therefore, In the present study, the serum ferritin
and transferrin level of whole blood and platelet donors from two
blood centers was examined, and a 95% normal range as established
for ferritin and transferrin of Chinese blood donors. The
distribution pattern of ferritin and transferrin levels in Chinese
blood donors of different ages and genders was explored. It was
found that the ferritin and transferrin RIs of blood donors were
associated with age. In addition, the type of donation influenced
the level of transferrin, and the ferritin level was negatively
correlated with the transferrin level. These findings can be used
to establish more reasonable blood donation recruitment and
screening strategies in China to protect the health of blood donors
and promote voluntary non-remunerated blood donation.
Materials and methods
Donor and consent
Volunteer blood donors from Xi'an (North West China)
and Guangzhou (South China) that met the Blood Donation Law of the
People's Republic of China, were recruited in the present study.
Requirements for blood donors: 18-55 years old; The weight of male
donors should be at least 50 kg and that of female donors should be
at least 45 kg. Donors should be free of colds and acute
gastroenteritis for a week, without medication, and without alcohol
for 24 h; The interval between two blood donations should be more
than 6 months. Individuals who have a minor surgery within half a
month, a general surgery within three months, and major surgery
within six months, are not allowed to donation. Women should not
donate blood during and 3 days before and after menstruation;
Platelets should not be donated again before three months of whole
blood donation; After 28 days of platelet donation, whole blood can
be donated again. Donors were categorized into several groups
according to age: the 18-22, 23-27, 28-32, 33-37, 38-42, 43-47,
48-52 and >52-year age groups. Donors who donated 3 times at
intervals of <12 months were considered regular donors.
According to the standards set by the World Health Organization,
smokers are defined as those who have smoked continuously or
cumulated for six months or more during their lifetime, and
drinkers are defined as those who have used alcohol (beer, liquor,
etc.) more than once in the past year (24,25).
The study protocol was approved by the ethics committee of the
Guangzhou Blood Center [approval no. Guangzhou Blood Center Lun
(2020) No. 1]. Written informed consent was obtained from all
recruited donors.
Inclusion and exclusion criteria
Donors who had lived in Xi'an or Guangzhou for >2
years were included in the present study. Donors who had been
diagnosed with hemopathy or a metabolic disease were excluded.
Demographic information of recruited
donors
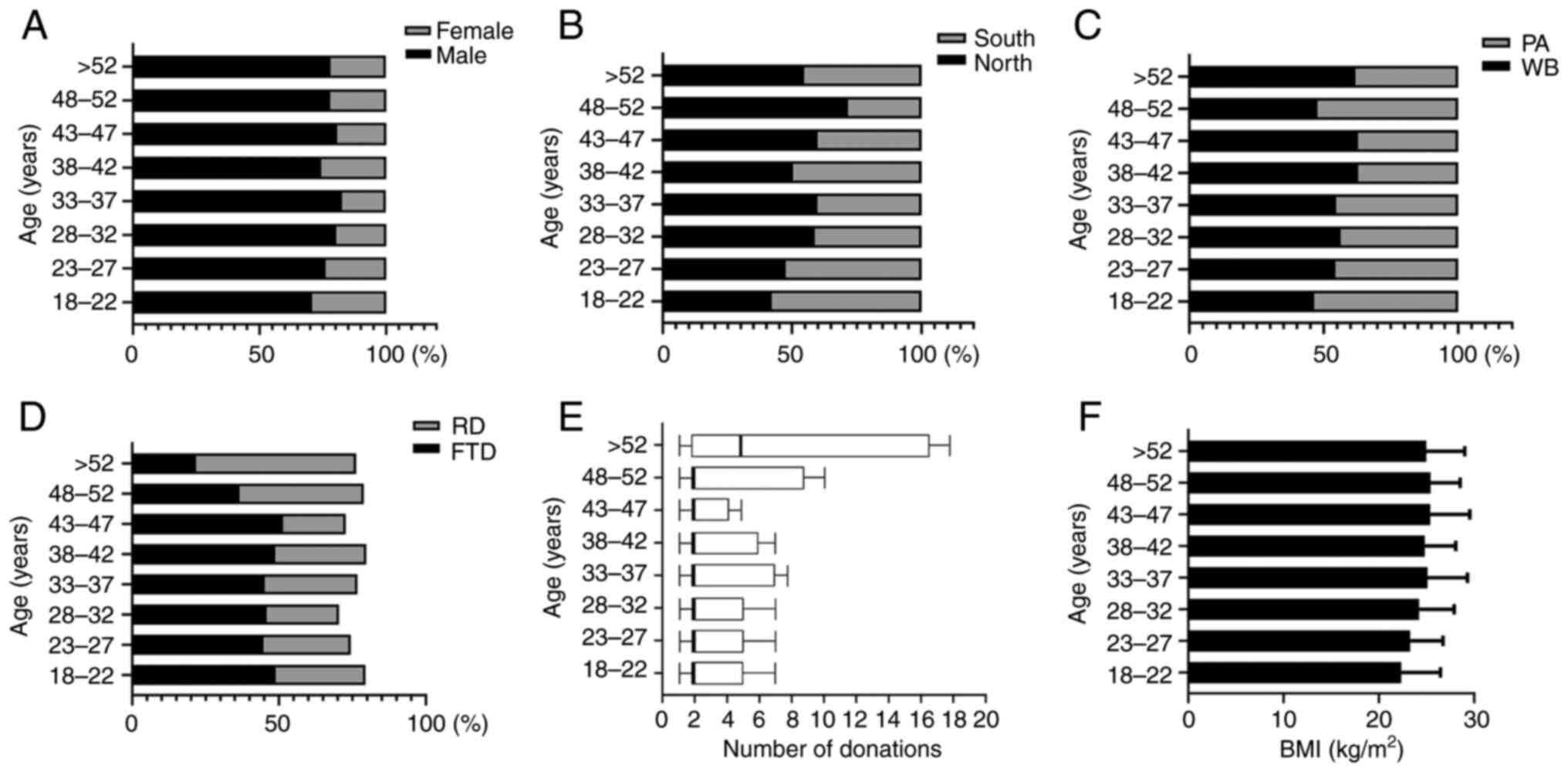
In the present study, a total of 1,817 blood donor
samples were used (Table I;
Fig. 1), including 977 from Xi'an
and 840 from Guangzhou. The study population comprised 1,410 males
and 407 females, with a male-to-female ratio of 3.46:1. A total of
997 individuals donated whole blood and 820 donated apheresis
platelets. Of those, 827 were first-time donors and 548 were
regular blood donors who donated more than three times in
<12-month intervals. The donation times increased with age.
There was no significant difference in the average BMI among the
age groups (P=0.248).
 | Table IDemographic information stratified by
age. |
Table I
Demographic information stratified by
age.
| Variable | 18-22 years
(n=292) | 23-27 years
(n=383) | 28-32 years
(n=332) | 33-37 years
(n=318) | 38-42 years
(n=188) | 43-47 years
(n=140) | 48-52 years
(n=109) | >52 years
(n=55) |
|---|
| Male (%) | 207 (70.9) | 292 (76.2) | 267 (80.4) | 263 (82.7) | 140 (74.5) | 113 (80.7) | 85 (78.0) | 43 (78.2) |
| Female (%) | 85 (29.1) | 91 (23.8) | 65 (19.6) | 55 (17.3) | 48 (25.5) | 27 (19.3) | 24 (22.0) | 12 (21.8) |
| North (%) | 123 (42.1) | 182 (47.5) | 195 (58.7) | 190 (59.7) | 95 (50.5) | 84 (60.0) | 78 (71.6) | 30 (54.5) |
| South (%) | 169 (57.9) | 201 (52.5) | 137 (41.3) | 128 (40.3) | 93 (49.5) | 56 (40.0) | 31 (28.4) | 25 (45.5) |
| WB (%) | 136 (46.6) | 208 (54.3) | 187 (56.3) | 174 (54.7) | 118 (62.8) | 88 (62.9) | 52 (47.7) | 34 (61.8) |
| PA (%) | 156 (53.4) | 175 (45.7) | 145 (43.7) | 144 (45.3) | 70 (37.2) | 52 (37.1) | 57 (52.3) | 21 (38.2) |
| FTD (%) | 143 (49.0) | 172 (44.9) | 152 (45.8) | 144 (45.3) | 92 (48.9) | 72 (51.4) | 40 (36.7) | 12 (21.8) |
| RD (%) | 89 (30.5) | 113 (29.5) | 82 (24.7) | 100 (31.4) | 58 (30.9) | 30 (21.4) | 46 (42.2) | 30 (54.5) |
| BMI
(kg/m2)a | 22.36±4.13 | 23.30±3.45 | 24.25±3.68 | 25.13±4.20 | 24.85±3.24 | 25.41±4.16 | 25.43±3.12 | 24.98±4.06 |
| Number of
donationsb | 2 (2-5) | 2 (2-5) | 2 (2-5) | 2 (2-7) | 2 (2-6) | 2 (2-4) | 2 (2-9) | 5 (2-17) |
Blood sample preparation
All peripheral blood samples were obtained following
the donation. Samples were collected in the dry tubes without
anti-coagulant. The serum was then prepared by centrifugation.
Serum was stored at -20˚C prior to testing.
Ferritin and transferrin test
According to current recommendations, the level of
ferritin in the prepared serum was measured with a chemiluminescent
microparticle immunoassay (Abbott i2000, Abbott Laboratories, USA).
The transferrin concentration was determined by a turbidimetric
inhibition immunoassay (ROCHE e701; Roche Diagnostics GmbH).
Statistical analysis
According to the data distribution, abnormal values
were excluded by the Dixon method or Tukey's fence, as appropriate.
An improved Box-Cox formula was utilized to convert the
non-normally distributed data into normally distributed data.
Multiple-regression analysis (MRA) and two-way nested ANOVA, based
on Ichihara's method, was used to analyze age, gender and region
difference (26). Two-way nested
ANOVA was used to determine the necessity of partitioning reference
values based on age, gender or region. Briefly, the standard
deviation ratio (SDR) which represents the ratio of
between-subgroup SD to between-individual SD was computed by
two-way nested ANOVA. Based on the IFCC/C-RIDL protocol for
establishing a RI, an SDR of >0.3 was viewed as a guide to
consider stratifying reference by gender, age or region.
Furthermore, a secondary exclusion procedure, latent abnormal
values exclusion (LAVE), was used to eliminate potential iron
metabolism abnormality (27).
Briefly, the donors with levels of ferritin and transferrin
simultaneously exceeding the established RIs were excluded from the
calculation of the new reference ranges. A secondary round of
exclusion was performed by excluding the enrolled donors with
potential iron metabolism abnormalities to establish the new RIs
based on data from the remaining participants. After six rounds of
exclusion, the reference values were nearly stable. Blood type,
body mass index (BMI), donation interval, donation times, drinking
and smoking were also considered in the RI analysis. SPSS 19.0 (IBM
Corp.) and GraphPad 8.0 (GraphPad Software Inc.) were used in the
present study. P<0.05 was considered to indicate a statistically
significant difference.
Results
Box-Cox transformation and SDR
analysis
According to the Kolmogorov-Smirnov normality test,
the ferritin (ng/ml) and transferrin (g/l) values were not normally
distributed. Following Box-Cox transformation, the data of
transferrin and ferritin were transformed to a Gaussian
distribution. To analyze variations according to age, gender and
geography, the SDR was calculated using two-way nested ANOVA. The
criterion for considering partitioning reference values was set to
an SDR of >0.3 following the IFCC/C-RIDL protocol. In the
present study, all SDRs were >0.3, which indicated that it was
essential to establish RIs based on different ages, genders and
regions (Table II).
 | Table IITwo-way nested ANOVA for comparison
of the results stratified by age, gender and region. |
Table II
Two-way nested ANOVA for comparison
of the results stratified by age, gender and region.
| | Nested ANOVA |
|---|
| Variable | Unit | Box-Cox (λ) |
SDR_Age |
SDR_Gen |
SDR_Reg |
|---|
| TRF | g/l | Yes (-0.5) | 0.60 | 1.35 | 1.21 |
| Fer | ng/ml | Yes (0.0) | 0.77 | 6.59 | 5.69 |
RIs were determined by age, gender and
region
Considering the inconvenience and misjudgment
resulting from the excessive reference ranges for different age
groups (8 groups in the present study) based on the value of SDR, a
Z-test was used to confirm the necessity of stratifying the RIs and
determine the cut-off age for partitioning. The Z-test results
showed that there was no significant difference in the transferrin
levels between different age groups. Therefore, it was not
essential to establish 8 RIs based on age groups (as shown in
Table I). As for the ferritin
value, there was a significant difference between the older group
(aged >52 years) and the younger groups (aged <52 years),
while there was no significant difference between the older group
(aged >52 years) and the 43-52 years old groups. Therefore, 42
years of age was used as the cut-off age to calculate the RIs of
ferritin levels. Both parametric and nonparametric methods were
used to calculate RIs, and the results showed that the RIs obtained
using the parametric method were wider than those obtained using
the nonparametric method (Table
III). After performing LAVE for secondary exclusion, 12
participants with potential iron metabolism disorders were
excluded. Data from 1,805 individuals were used to establish the
RIs (Table IV). The LAVE
procedures generated narrower RIs for transferrin and ferritin
level in the subgroups.
 | Table IIIReference intervals derived by
parametric and non-parametric methods for different ages, genders
or regions, respectively. |
Table III
Reference intervals derived by
parametric and non-parametric methods for different ages, genders
or regions, respectively.
| | North | South |
|---|
| | Male (n=813) | Female (n=164) | Male (n=597) | Female (n=243) |
|---|
| Variable | Unit | Age, years | Para (LL-UL) | Nonpara
(LL-UL) | Para (LL-UL) | Nonpara
(LL-UL) | Para (LL-UL) | Nonpara
(LL-UL) | Para (LL-UL) | Nonpara
(LL-UL) |
|---|
| TRF | g/l | >18 | 1.85-3.53 | 1.79-3.57 | 1.79-3.66 | 1.75-3.54 | 1.80-3.47 | 1.78-3.43 | 1.84-3.28 | 1.78-3.16 |
| Fer | ng/ml | 18-42 | 9.24-443.68 | 5.48-263.61 | 2.76-127.51 | 2.48-105.36 | 6.01-569.98 | 3.67-380.33 | 4.05-743.82 | 2.70-373.55 |
| | | >42 | 7.74-364.27 | 5.90-245.86 | 2.76-158.14 | 3.02-113.32 | 4.20-557.97 | 3.00-307.69 | 6.17-383.45 | 8.76-385.63 |
 | Table IVReference intervals calculated of
data from the latent abnormal values exclusion method. |
Table IV
Reference intervals calculated of
data from the latent abnormal values exclusion method.
| | North | South |
|---|
| | Male (n=810) | Female (n=162) | Male (n=592) | Female (n=241) |
|---|
| Variable | Unit | Age, years | Para (LL-UL) | Nonpara
(LL-UL) | Para (LL-UL) | Nonpara
(LL-UL) | Para (LL-UL) | Nonpara
(LL-UL) | Para (LL-UL) | Nonpara
(LL-UL) |
|---|
| TRF | g/l | >18 | 1.85-3.51 | 1.80-3.55 | 1.80-3.61 | 1.75-3.53 | 1.81-3.45 | 1.81-3.38 | 1.85-3.27 | 1.83-3.16 |
| Fer | ng/ml | 18-42 | 9.69-429.66 | 5.74-260.78 | 2.93-126.86 | 2.48-105.36 | 6.24-553.96 | 4.46-379.29 | 4.01-773.77 | 2.70-380.50 |
| | | >42 | 7.74-364.27 | 5.90-245.86 | 2.76-158.14 | 3.02-113.32 | 4.20-557.97 | 3.00-307.69 | 6.64-296.19 | 8.76-385.63 |
MRA
Blood type, BMI, whole blood or platelet apheresis
donation, donation times, donation intervals, alcohol consumption
and smoking were used as dependent variables to calculate the
correlation coefficient using the MRA method. The ferritin level
was negatively correlated with the transferrin level. As compared
with whole blood donation, platelet apheresis donation was more
positively correlated with transferrin, with a positive effect on
serum transferrin levels. Notable changes in ferritin and
transferrin levels were observed but not associated with blood
donor blood type, BMI, donation times, donation interval, alcohol
consumption and smoking (Table
V).
 | Table VStandardized partial regression
coefficient in MRA for blood type, job type, BMI, donation type,
donation times, donation interval, alcohol consumption, smoking,
drugs as explanatory variables. |
Table V
Standardized partial regression
coefficient in MRA for blood type, job type, BMI, donation type,
donation times, donation interval, alcohol consumption, smoking,
drugs as explanatory variables.
| Variable | Blood type | BMI | Donation (WB or
PA) | Donation times | Donation
interval | Ethyl alcohol | Smoking | TRF | Fer |
|---|
| TRF | 0.024 | 0.067 | 0.241a | 0.127 | -0.089 | 0.049 | 0.032 | \ | -0.252a |
| Fer | -0.015 | 0.046 | -0.007 | 0.052 | 0.128 | 0.036 | 0.079 | -0.283a | \ |
RIs were determined by blood component
donation and blood donation frequency
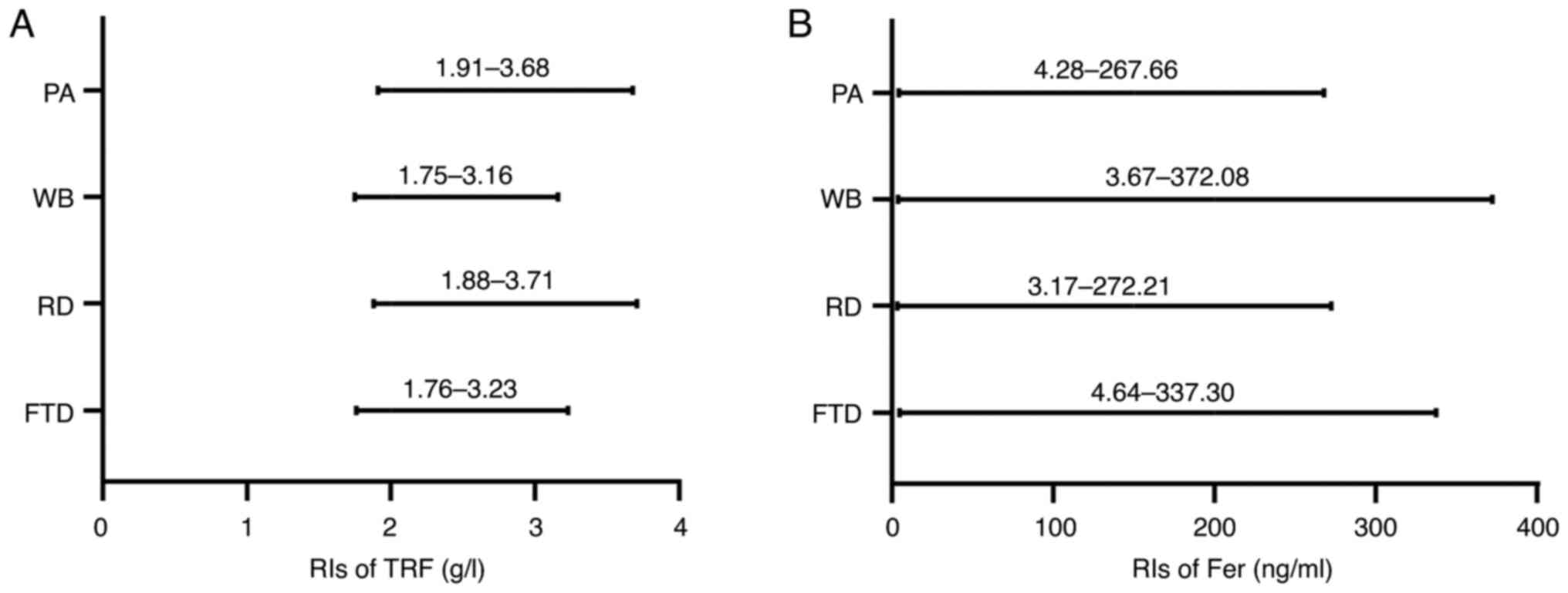
Next, RIs were established based on the donation
frequency and components using the nonparametric method. As shown
in Table VI and Fig. 2, regular donors had higher RIs for
transferrin than first-time donors, but regular donors had a lower
RI for ferritin than first-time donors. With regards to component
analysis, it was observed that the transferrin RIs in whole blood
donors was 1.75-3.16 g/l, while in platelet donors they were
1.91-3.68 g/l. The ferritin RIs in whole blood donors were higher
than those in apheresis donors. In combination, these data
suggested that the ferritin and transferrin RIs differed between
first-time and regular donors, as were those between whole blood
and platelet donors.
 | Table VIRIs determined by donation frequency
and components donation. |
Table VI
RIs determined by donation frequency
and components donation.
| Variable | Unit | Methods | FTD (LL-UL) | RD (LL-UL) | WB (LL-UL) | PA (LL-UL) |
|---|
| TRF | g/l | Nonpara | 1.76-3.23 | 1.88-3.71 | 1.75-3.16 | 1.91-3.68 |
| Fer | ng/ml | Nonpara | 4.64-337.30 | 3.17-272.21 | 3.67-372.08 | 4.28-267.66 |
Discussion
Iron level is a vital indicator for human health
(1). However, iron deficiency, a
common nutritional deficiency worldwide, is a serious issue for
blood donors (3,15). Thus, blood centers around the world
investigated the transferrin and ferritin levels in blood donors
(16-20,28).
Although a large amount of data has been collected in different
countries, the transferrin and ferritin levels of donors in China
has not yet been fully examined. One important reason is that the
RIs in China remain to be determined. Therefore, an initial
objective of the project was to determine the RIs for ferritin and
transferrin of Chinese blood donors. In the present study, both
transferrin and ferritin level data were collected in two blood
centers in China, and an RI analysis was conducted. It was also
observed that apheresis platelet donation was positively correlated
with serum transferrin level, as compared with whole blood
donation, and that the ferritin level was negatively correlated
with the transferrin level. We believe these findings are
significant for the following reasons. First, the RIs of
transferrin and ferritin were determined by multiple factor
analysis in Chinese donors. Secondly, the collected data will help
refine donor recruitment strategies in China.
Without available RIs in China, references originate
from studies conducted in other countries or regions. However, due
to the differences among individuals from different parts of the
world, using the RIs from other countries or regions in China may
not be appropriate. In fact, it was found that the RIs of
transferrin and ferritin in the present study differ from those
from previous studies (14,20,29).
In addition, it was observed herein that iron deficiency in China
is not infrequent, according to the RIs established in the present
study. This study may be the most comprehensive and systematic
study with the largest sample size in China that was based on the
standard protocols of the CLSI and IFCC/C-RIDL. Two-way nested
ANOVA and MRA were used to explore the sources of variation in the
present study. In addition to the inclusion and exclusion criteria
used for enrolment, the application of the LAVE method further
excludes individuals with latent diseases, which is considered the
appropriate approach to eliminate the effect of potential factors
(26,30).
Compared with a report from Finland, the transferrin
level is 1.15-2.75 mg/l in healthy adults (31). This value is lower compared with
that in the present study. The reason may be due to the use of
different test methods. The previous study used sandwich
immunoenzymometric assay, while the present study used the
turbidimetric inhibition immunoassay. With regards to the ferritin
level, Chinese donors exhibited lower values (14,20).
We hypothesized that ethnic/racial groups, eating habits, and/or
the regional environment distinction accounted for this
difference.
Donation frequency and times were considered the
main factors influencing transferrin and ferritin levels (16,32).
However, in the present study, it was not confirmed more frequent
donations resulted in lower transferrin and ferritin levels
(9,16,32).
This inconsistency may be due to the difference in sample size,
sample populations and/or donation policies. It should be pointed
out that the minimum interval for whole blood donation was 6 months
in China. Such a long interval might be a reason for the
discrepancy in the results. Previous studies from other countries
have suggested that prolonging the blood donation interval from 4
to 6 months can alleviate iron deficiency (7,14,33).
However, the whole blood donation interval was already 180 days in
China. If further extended, the shortage in blood supplies will be
increased.
It was found that individuals aged >42 years
exhibited lower transferrin and ferritin levels than younger donors
when nonparametric methods were used. This finding was inconsistent
with that of previous studies, which have suggested that adolescent
donors had lower transferrin and ferritin levels (34,35).
A possible reason for this is the use of different analysis
methods. In addition, the elderly donors were in a lower metabolic
state, with a reduced turnover of transferrin and ferritin.
Of note, it was found that apheresis platelet
donation was positively correlated with serum transferrin levels
compared with whole blood donation. Previous reports have often
focused on serum ferritin in both types of donations (8). It was also confirmed that the
ferritin level was negatively correlated with the transferrin level
in blood donors, since the latter is a vital marker of iron status
and its level rises with iron deficiency. It therefore seems that
donation type is a key factor influencing transferrin levels.
This study had several limitations. First, it had a
limited sample size; a total of 1,817 blood donor samples were
collected. Secondly, the donors' transferrin and ferritin levels
were not followed up after initial testing, which may have affected
the adjustment of donation intervals and early detection of iron
deficiency. Therefore, further large-scale data collection and
longer follow-up are needed for a more comprehensive and extensive
study.
In conclusion, the present results will help
establish the RIs for ferritin and transferrin in Chinese blood
donors. In addition, factors that influence transferrin and
ferritin levels in Chinese donors were identified. Finally, these
findings should help formulate improved blood donation strategies
in China, protecting the health of blood donors, and contributing
to the development of blood donation.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contribution
XFZ, XM and YFW performed most of the experiments
and analysis. SJL, QL, ZGS, JTH, JYS, LWW and HYC were responsible
for sample collection and analyzed data. XBH and XR were the
research sponsors, designed the study and drafted the manuscript.
XFZ and XM confirm the authenticity of all the raw data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Guangzhou Blood Center [Guangzhou, China; approval no.
Guangzhou Blood Center Lun (2020) No. 1]. All patients gave written
informed consent before participation in this study.
Patient consent for publication
All patients gave written informed consent for
publication in this study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hentze MW, Muckenthaler MU and Andrews NC:
Balancing acts: Molecular control of mammalian iron metabolism.
Cell. 117:285–297. 2004.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wang J and Pantopoulos K: Regulation of
cellular iron metabolism. Biochem J. 434:365–381. 2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Camaschella C: Iron deficiency. Blood.
133:30–39. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Miller JL: Iron deficiency anemia: a
common and curable disease. Cold Spring Harb Perspect Med.
3(a011866)2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Miller JL: Iron deficiency anemia: A
common and curable disease. Cold Spring Harb Perspect Med.
3(a011866)2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Dijkstra A, van den Hurk K, Bilo HJG,
Slingerland RJ and Vos MJ: Repeat whole blood donors with a
ferritin level of 30 ug/L or less show functional iron depletion.
Transfusion. 59:21–25. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Schotten N, Pasker-de Jong PC, Moretti D,
Zimmermann MB, Geurts-Moespot AJ, Swinkels DW and van Kraaij MG:
The donation interval of 56 days requires extension to 180 days for
whole blood donors to recover from changes in iron metabolism.
Blood. 128:2185–2188. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Duggan F, O'Sullivan K, Power JP, Healy M
and Murphy WG: Serum ferritin in plateletpheresis and whole blood
donors. Transfus Apher Sci. 55:159–163. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Rigas AS, Pedersen OB, Magnussen K,
Erikstrup C and Ullum H: Iron deficiency among blood donors:
Experience from the Danish Blood Donor Study and from the
Copenhagen ferritin monitoring scheme. Transfus Med. 29 (Suppl
1):S23–S27. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Di Angelantonio E, Thompson SG, Kaptoge S,
Moore C, Walker M, Armitage J, Ouwehand WH, Roberts DJ and Danesh
J: INTERVAL Trial Group. Efficiency and safety of varying the
frequency of whole blood donation (INTERVAL): A randomised trial of
45 000 donors. Lancet. 390:2360–2371. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lee SJ, Min HK, Jang JS, Lee S, Chung Y
and Kim MJ: Donor protection: Iron supplementation for frequent
blood donors in Korea. Transfus Apher Sci.
59(102611)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Garcia-Casal MN, Pasricha SR, Martinez RX,
Lopez-Perez L and Peña-Rosas JP: Serum or plasma ferritin
concentration as an index of iron deficiency and overload. Cochrane
Database Syst Rev. 5(CD011817)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Podmore C, Meidtner K, Schulze MB, Scott
RA, Ramond A, Butterworth AS, Di Angelantonio E, Danesh J, Arriola
L, Barricarte A, et al: Association of multiple biomarkers of iron
metabolism and type 2 diabetes: The EPIC-InterAct Study. Diabetes
Care. 39:572–581. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Vinkenoog M, van den Hurk K, van Kraaij M,
van Leeuwen M and Janssen MP: First results of a ferritin-based
blood donor deferral policy in the Netherlands. Transfusion.
60:1785–1792. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
World Health Organization (WHO): Iron
deficiency anaemia assessment, revention, and Control. WHO, Geneva,
2001.
|
|
16
|
Cable RG, Glynn SA, Kiss JE, Mast AE,
Steele WR, Murphy EL, Wright DJ, Sacher RA, Gottschall JL, Tobler
LH, et al: Iron deficiency in blood donors: The REDS-II Donor Iron
Status Evaluation (RISE) study. Transfusion. 52:702–711.
2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Salvin HE, Pasricha SR, Marks DC and
Speedy J: Iron deficiency in blood donors: A national
cross-sectional study. Transfusion. 54:2434–2444. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Rigas AS, Sørensen CJ, Pedersen OB,
Petersen MS, Thørner LW, Kotzé S, Sørensen E, Magnussen K,
Rostgaard K, Erikstrup C and Ullum H: Predictors of iron levels in
14,737 Danish blood donors: Results from the Danish Blood Donor
Study. Transfusion. 54 (3 Pt 2):789–796. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Baart AM, van Noord PA, Vergouwe Y, Moons
KG, Swinkels DW, Wiegerinck ET, de Kort WL and Atsma F: High
prevalence of subclinical iron deficiency in whole blood donors not
deferred for low hemoglobin. Transfusion. 53:1670–1677.
2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Goldman M, Uzicanin S, Osmond L, Scalia V
and O'Brien SF: A large national study of ferritin testing in
Canadian blood donors. Transfusion. 57:564–570. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Horowitz G: Defining, Establishing, and
Verifying Reference Intervals in the Clinical Laboratory; Approved
Guideline. 3rd edition. Clinical and Laboratory Standards
Institute, Wayne, PA, 2008.
|
|
22
|
Ozarda Y, Ichihara K, Barth JH and Klee G:
Committee on Reference Intervals and Decision Limits (C-RIDL),
International Federation for Clinical Chemistry and Laboratory
Medicine. Protocol and standard operating procedures for common use
in a worldwide multicenter study on reference values. Clin Chem Lab
Med. 51:1027–1040. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ichihara K: Statistical considerations for
harmonization of the global multicenter study on reference values.
Clin Chim Acta. 432:108–118. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Molarius A, Seidell JC, Kuulasmaa K,
Dobson AJ and Sans S: Smoking and relative body weight: An
international perspective from the WHO MONICA Project. J Epidemiol
Community Health. 51:252–260. 1997.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wood AM, Kaptoge S, Butterworth AS,
Willeit P, Warnakula S, Bolton T, Paige E, Paul DS, Sweeting M,
Burgess S, et al: Risk thresholds for alcohol consumption: Combined
analysis of individual-participant data for 599 912 current
drinkers in 83 prospective studies. Lancet. 391:1513–1523.
2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ichihara K and Boyd JC: IFCC Committee on
Reference Intervals and Decision Limits (C-RIDL). An appraisal of
statistical procedures used in derivation of reference intervals.
Clin Chem Lab Med. 48:1537–1551. 2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zeng X, Fang L, Peng Y, Zhang Y, Li X,
Wang Z, Zhang B, Cao Q and Hu X: A multicenter reference interval
study of thromboelastography in the Chinese adult population.
Thromb Res. 195:180–186. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
van den Berg K, Swanevelder R, Ingram C,
Lawrie D, Glencross DK, Hilton C and Nieuwoudt M: The iron status
of South African blood donors: Balancing donor safety and blood
demand. Transfusion. 59:232–241. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
World Health Organization (WHO): WHO
guideline on use of ferritin concentrations to assess iron status
in individuals and populations. WHO, Geneva, 2020.
|
|
30
|
Ichihara K, Ceriotti F, Tam TH, Sueyoshi
S, Poon PM, Thong ML, Higashiuesato Y, Wang X, Kataoka H, Matsubara
A, et al: The Asian project for collaborative derivation of
reference intervals: (1) strategy and major results of standardized
analytes. Clin Chem Lab Med. 51:1429–1442. 2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Suominen P, Punnonen K, Rajamäki A and
Irjala K: Serum transferrin receptor and transferrin
receptor-ferritin index identify healthy subjects with subclinical
iron deficits. Blood. 92:2934–2939. 1998.PubMed/NCBI
|
|
32
|
Mittal R, Marwaha N, Basu S, Mohan H and
Ravi Kumar A: Evaluation of iron stores in blood donors by serum
ferritin. Indian J Med Res. 124:641–646. 2006.PubMed/NCBI
|
|
33
|
Spekman MLC, Ramondt S and Sweegers MG:
Whole blood donor behavior and availability after deferral:
Consequences of a new ferritin monitoring policy. Transfusion.
61:1112–1121. 2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Patel EU, White JL, Bloch EM, Grabowski
MK, Gehrie EA, Lokhandwala PM, Brunker PAR, Goel R, Shaz BH, Ness
PM and Tobian AAR: Association of blood donation with iron
deficiency among adolescent and adult females in the United States:
A nationally representative study. Transfusion. 59:1723–1733.
2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Vassallo RR, Hilton JF, Bravo MD,
Vittinghoff E, Custer B and Kamel H: Recovery of iron stores after
adolescents donate blood. Pediatrics. 146(e20193316)2020.PubMed/NCBI View Article : Google Scholar
|