Introduction
Mast cells, located in the skin, lungs and mucosal
surfaces, are crucial effector cells of both innate and adaptive
immune defense against bacterial infection and various toxins
(1,2). Activated mast cells release a broad
range of immune mediators such as histamine, hexosaminidase,
cytokines, lipid compounds and vasoactive amines to defend host
(3). In addition to their positive
effects of mast cells in host defense, abnormally activated mast
cells are also reported to have detrimental effect in various
allergic conditions including bronchial asthma, eczema, hay fever
and food allergies (4,5). In allergic diseases, the excessive
release of various mediators such as TNF-α from abnormally
activated mast cells can initiate immediate hypersensitivity
response associated with allergy. Therefore, pharmacological
intervention of the proliferation and migration of mast cells could
be a valuable approach for the attenuation of allergic conditions
(6).
MAPKs have been reported to play a crucial role in
the cytokine production of activated mast cells and subsequent
proliferation and differentiation of mast cells (7). MAPKs consist of extracellular
signal-regulated kinase1/2 (ERK1/2), c-Jun N-terminal kinase (JNK),
and p38 mitogen-activated protein kinase (p38) (8). Phosphorylation of ERK has been
reported to regulate various mast cell responses such as
proliferation, migration and differentiation in allergic conditions
(9). JNK has been also reported to
be involved in an inflammatory response of activated mast cells
(10). Activated JNK results in
the expression of transcription factor AP1, which subsequently
leads to the expression of many inflammatory mediators (10). P38 has been reported to be involved
in the production of pro-inflammatory cytokines in activated mast
cells by increasing nuclear translocation of pro-inflammatory
transcription factor, NF-κB (11).
Anti-inflammatory property of caffeic acid
derivatives were reported in multiple studies (12-15).
3,4,5-Trihydroxycinnamic acid (THC), a derivative of caffeic acid,
was originally reported in Rooibos tea (16) and has been demonstrated to possess
a variety of pharmacological actions such as anti-inflammatory and
neuroprotection actions (17-19).
Previously, we reported that THC significantly suppressed
LPS-induced expression of pro-inflammatory mediators through the
suppression of NF-κB activation in BV2 microglial cells (20). We also demonstrated that THC
suppressed LPS-induced inflammation via the upregulation of HO-1
through Nrf2 activation in RAW264.7 macrophage cells and increased
the survival of animal in an LPS-induced endotoxemia mouse model
(21,22). Caffeic acid phenethyl ester (CAPE),
an ester derivative of hydroxycinnamic acid, was reported to
inhibit cytokine-induced NF-κB signaling in macrophage cells
(23) and plays an important role
in the regulation of the host immune response (24). Recently, CAPE has been demonstrated
to exert anti-allergic effects by inhibiting MAPK signaling and
NF-κB activation abnormally activated HMC-1 human mast cells
(6). Given that THC exerts wide
range of anti-inflammatory actions and its derivative, CAPE
possesses anti-allergic property, THC might also exhibit
anti-allergic action in mast cells. Therefore, the objective of the
current study was to examine the anti-allergic action of THC and
its underlying mechanism in PMA/A23187-challenged RBL-2H3 mast
cells in order to provide an useful therapeutic agent that could
suppress various allergic conditions.
Materials and methods
Reagents and cell culture
Phorbol 12-myristate 13-acetate (PMA) and A23187
were purchased from Sigma-Aldrich; Merck KGaA).
3,4,5-Trihydroxycinnamic acid (THC) was purchased from AApin
Chemicals Limited. Rat basophilic leukemia (RBL-2H3) cells were
purchased from the Korea cell line bank (KCLB), KCLB cat #22256.
RBL-2H3 cells were maintained in medium RPMI 1640 (RPMI 1640;
Hyclon Laboratories) containing 10% heat-inactivated fetal bovine
serum and 100 U/ml penicillin-streptomycin (Gibco) at 37˚C, 5%
CO2. Cells were incubated in the presence of the
indicated concentrations of THC and then stimulated with 50 nM of
PMA and 1 µM of A23187 for the indicated times.
TNF-α release assays
RBL-2H3 cells were incubated with THC (10~100 µM)
for 1 h and then incubated in the presence or absence of PMA/A23187
for 30 min. TNF-α released into the medium of RBL-2H3 cultures was
detected using enzyme-linked immunosorbent (ELISA) kits (R&D
System) according to the manufacturer's instructions.
β-Hexosaminidase and histamine release
assay
To examine the effect of THC on degranulation, the
concentrations of β-hexosaminidase and histamine release were
quantitatively measured. These enzymes are restricted within
granules in mast cells and has been utilized as granule markers
(25). RBL-2H3 cells were cultured
in 12-well plates for 24 h. Then the supernatant was removed and
the cells were further incubated with various concentrations of THC
diluted in PIPES buffer for 1 h at 37˚C. After pretreatment, cells
were washed twice with PIPES buffer, then stimulated with
PMA/A23187 for 30 min at 37˚C. And 20 µl of supernatant was used to
react with 80 µl of substrate buffer (2 mM
4-p-nitrophenyl-N-acetyl-β-D-glucosaminide
in 0.05 M sodium citrate buffer, pH 4.5) 30 min at 37˚C. The
reaction was terminated with the addition of 200 µl of stop buffer
(0.1 M NaHCO3, pH 10). The absorbance was determined at
405 nm using microplate spectrophotometer (SpectraMax M5, Molecular
Devices). The amount of histamine was detected by
o-phthalaldehyde (OPT) spectroflurometric procedure. To 0.5
ml of media from each well, 0.1 ml of 1 M NaOH and 25 µl of OPT (1%
(w/v) in methanol) were added. The supernatant was incubated for 4
min at room temperature. The reaction stopped by the addition of 50
µl of 3 M HCl. The absorbance was measured at excitation and
emission wavelengths of 360 and 450 nm, respectively, using
microplate spectrophotometer (SpectraMax M5, Molecular
Devices).
Preparation of cytoplasmic and nuclear
fractions
RBL-2H3 cells were treated with 10, 50, and 100 µM
concentrations of THC for 1 h prior to PMA/A23187 treatment. Cells
were washed with ice-cold PBS, and harvested, and centrifuged at
15,000 x g for 10 min at 4˚C. Cytoplasmic and nuclear extracts were
prepared as described previously (26). Briefly, cells were resuspended in
40 µl of an ice-cold hypotonic buffer (10 mM HEPES/KOH, 2 mM
MgCl2, 0.1 mM EDTA, 10 mM KCl, 1 mM DTT, and 0.5 mM
PMSF, pH 7.9). The cells were left on ice for 10 min after which
cells were lysed gently with 2.5 µl of 10% Nonidet P (NP)-40. The
cell lysate was centrifuged at 15,000 x g for 3 min at 4˚C. The
supernatant was carefully collected and marked as the cytoplasmic
fraction. The nuclear pellets were gently resuspended in 40 µl of
ice-cold saline buffer (50 mM HEPES/KOH, 50 mM KCl, 300 mM NaCl,
0.1 mM EDTA, 10% glycerol, 1 mM DTT, and 0.5 mM PMSF, pH 7.9) and
left 20 min on ice. After centrifuge at 15,000 x g for 15 min at
4˚C, the supernatant was collected and marked as the nuclear
fraction.
Western blot analysis
RBL-2H3 cells were incubated with THC for 1 h or 2 h
prior to PMA/A23187 treatment. Cells were washed with ice-cold PBS
and lysed in PRO-PREP lysis buffer (iNtRON Biotechnology). Same
quantity of protein were separated on 10% SDS-polyacrylamide gel.
Then, proteins were transferred to Hypond PVDF membrane (Amersham
Biosciences) and blocked in 5% skim milk in TBST for 1 h at room
temperature. Specific antibodies against COX-2 (Cell signaling
Technology (CST), #12282), p38 (CST, #9212), p-p38 (CST, #9211),
ERK1/2 (CST, #9102), p-ERK1/2 ((CST, #9101), JNK (CST, #9252),
p-JNK (CST, #9251), NF-kB (p65, CST, #8242), lamin B1 (CST, #13435)
(1:1,000), and β-actin (1:2,500; Sigma, #A2228) were diluted in 5%
skim milk. After stringent washing with TBST, horseradish
peroxidase-conjugated secondary antibodies were incubated. The
blots were then developed by the enhanced chemiluminescence
detection (Amersham Biosciences).
Statistical analysis
All values shown in the figures were obtained from
at least three independent experiments and expressed as mean ± SD
and analyzed using SPSS 20.0 (IBM Corp.). A one-way analysis of
variance (ANOVA) followed by Dunnett's multiple comparison test was
used to analyze differences between multiple groups. Data with
values of P<0.05 were interpreted as statistically significant.
Single */# and double **/## represent statistical significance in
P<0.05 and P<0.01, respectively.
Results
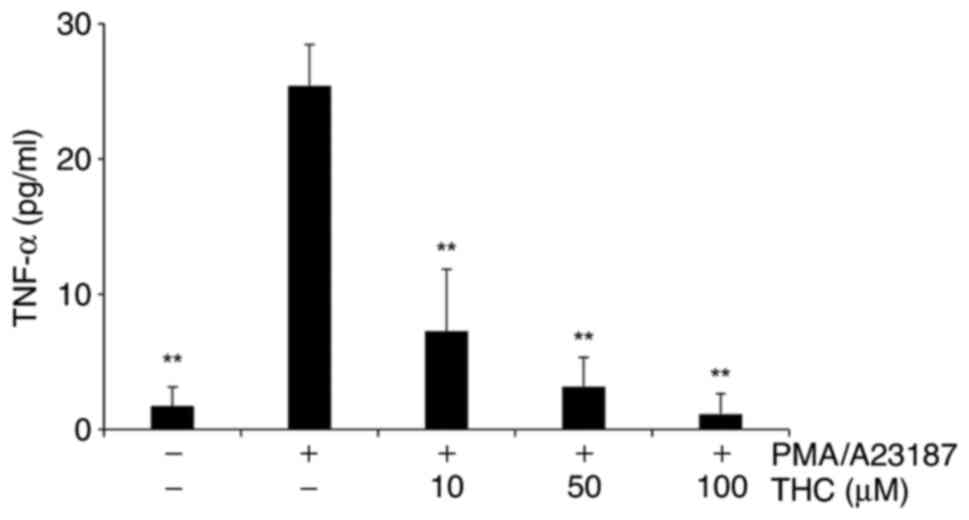
THC significantly suppressed
PMA/A23187-induced TNF-α secretion
Pro-inflammatory cytokines such as TNF-α have been
extensively reported to play an essential role in the inflammation
progression (27,28). The effects of THC on the
extracellular release of TNF-α in PMA and A23187-challenged RBL-2H3
mast cells were measured. Cells were incubated with THC for 1h
prior to PMA/A23187 treatment. PMA/A231897 treatment clearly
increased the secretion of TNF-α in RBL-2H3 mast cells and THE
significantly suppressed PMA/A23187-induced TNA-α release in a
concentration-dependent manner (Fig.
1). Noticeable cytotoxicity was not observed with THC treatment
in the concentrations applied in the study (data not shown).
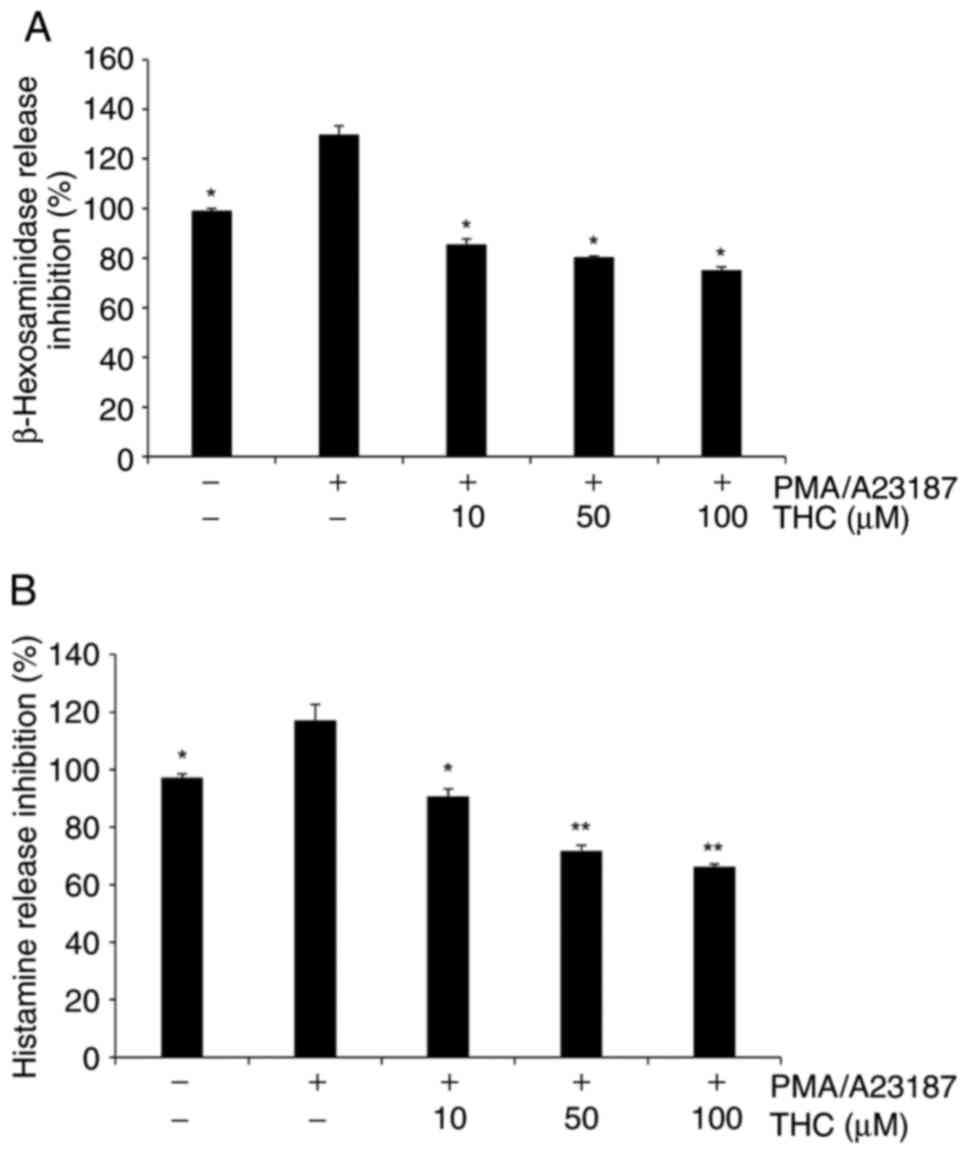
THC significantly suppressed
PMA/A23187-induced β-hexosaminidase and histamine release
The determination of the secretion of
β-hexosaminidase has been widely reported to measure the level of
mast cell degranulation (29). In
the current study, PMA/A23187 treatment resulted in the increased
the secretion of β-hexosaminidase and THC treatment significantly
suppressed PMA/A23187-induced β-hexosaminidase in RBL-2H3 cells
(Fig. 2A). In addition, PMA/A23187
treatment showed increased secretion of histamine and THC treatment
significantly attenuated PMA/A23187-induced histamine extracellular
secretion in RBL-2H3 cells in a positive association with the
concentration of THC (Fig.
2B).
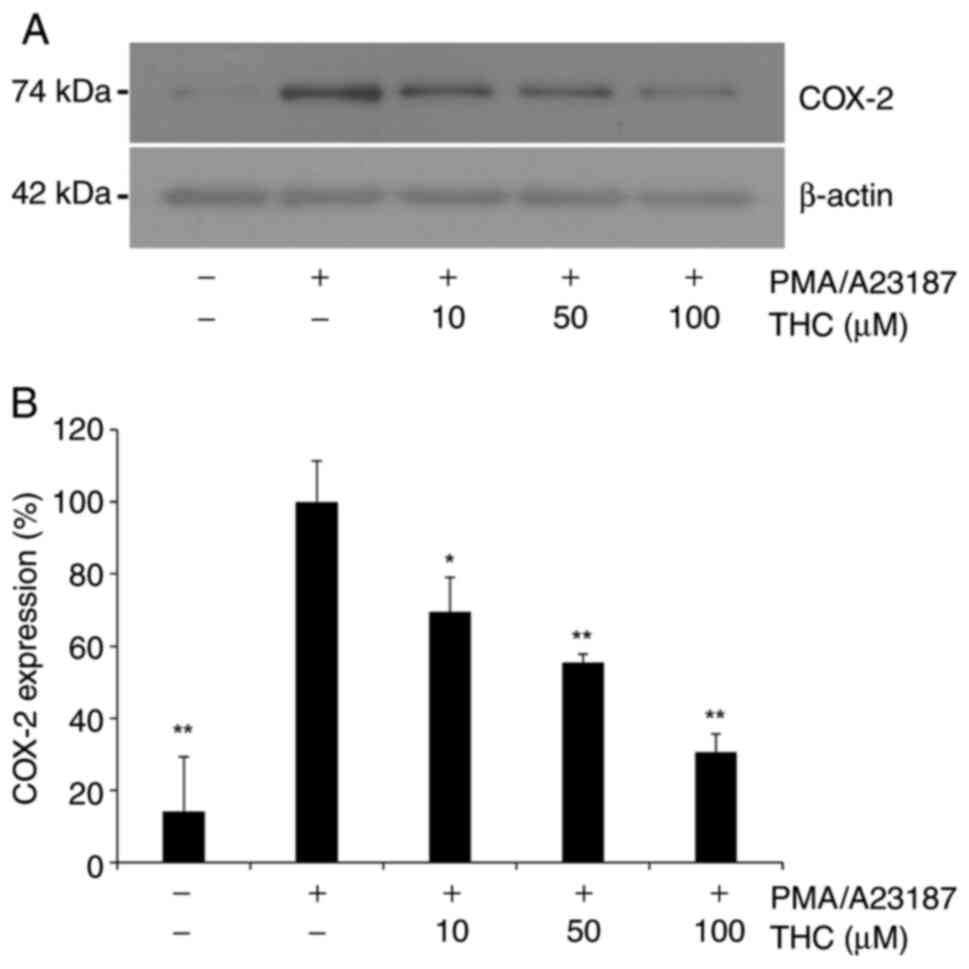
THC significantly attenuated
PMA/A23187-induced COX-2 expression
Given the previous report that elevated levels of
COX-2 is associated with mast cell activation (30), COX-2 expression was examined in
PMA/A23187-challenged RBL-2H3 cells in the present study.
PMA/A23187 treatment resulted in increased expression of COX-2
(Fig. 3), and THC treatment
significantly attenuated PMA/A23187-induced expression of COX-2
(Fig. 3A). Quantitative analysis
of COX-2 expression showed significant suppression of COX-2
expression in a positive association of the concentration of
(Fig. 3B), suggesting that THC
suppresses mast cell activation through the inhibition of COX-2
expression in RBL-2H3 cells.
THC significantly suppressed
PMA/A23187-induced MAPKs phosphorylation
Signaling pathways of MAPKs have been extensively
demonstrated to be involved in the degranulation of activated mast
cells (30,31). In the present study, the
phosphorylation of MAPKs was examined in the presence of PMA/A23187
to determine the effect of mast cell activation on MAPK
phosphorylation and then the role of THC was measured on the
PMA/A23187-induced MAPKs phosphorylation in RBL-2H3 cells.
PMA/A23187 challenge showed an increased phosphorylation of all
three MAPKs in RBL-2H3 cells (Fig.
4). THC treatment significantly suppressed the
PMA/A23187-induced phosphorylation of MAPKs (Fig. 4). Quantitative analyses of p-p38
and p-JNK immunoblots showed a concentration-dependent inhibition
(Fig. 4B and D) whereas phosphorylation level of
p-ERK1/2 was significantly attenuated in low concentration of THC
and maintained through the tested concentrations (Fig. 4C).
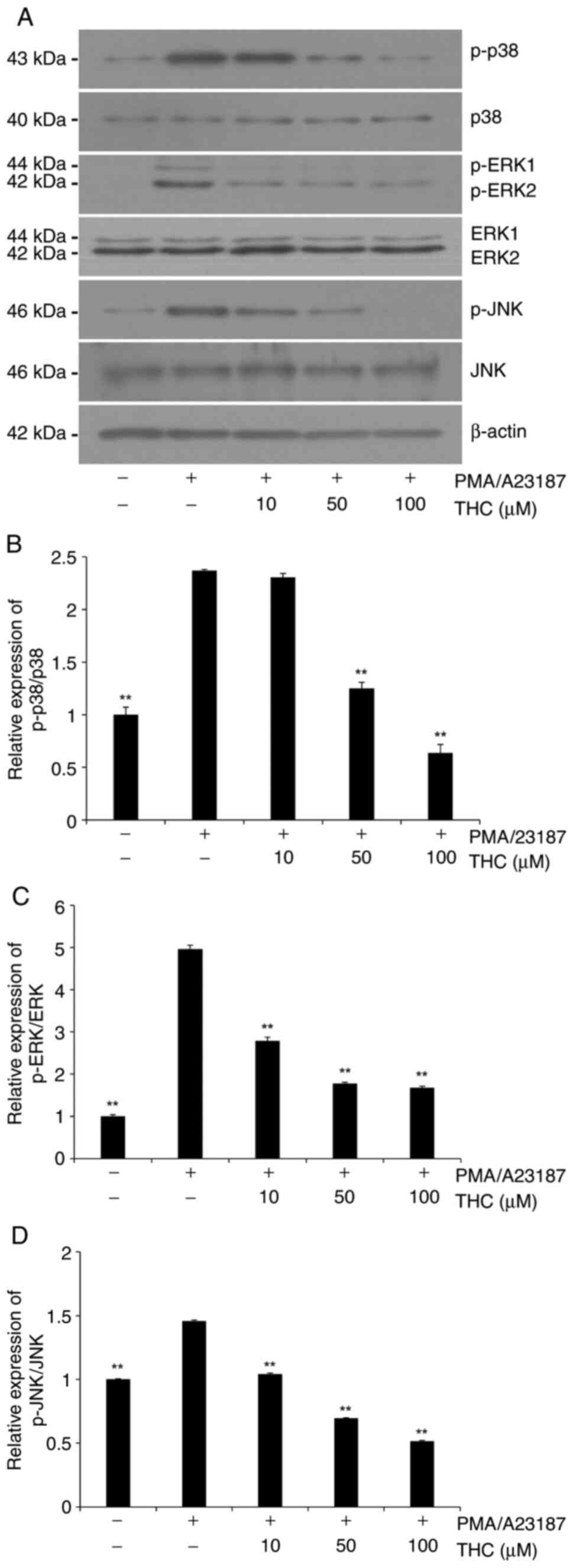 | Figure 4Effect of THC on PMA/A23187-induced
phosphorylation of MAPKs in RBL 2H3 cells. Cells were pretreated
with THC (10, 50 and 100 µM) for 1 h and then stimulated with
PMA/A23187 for 30 min. (A) Representative immunoblots of MAPKs. (B)
Quantitative analyses of immunoblots of p-p38. (C) Quantitative
analyses of immunoblots of p-ERK1/2. (D) Quantitative analyses of
immunoblots of p-JNK. THC exhibited a significant suppression of
PMA/A23187-induced MAPKs activation. p-p38 and p-JNK showed
significant suppression in a positive association with the
concentration of THC whereas p-ERK1/2 showed a significant
suppression with all tested concentration of THC. The data are
presented as means ± SD from three independent experiments.
**P<0.01 vs. PMA/A23187 alone. THC,
3,4,5-trihydroxycinnamic acid; PMA,
phorbol-12-myristate-13-acetate; MAPKs, mitogen-activated protein
kinases; ERK1/2, extracellular signal-regulated kinase 1/2; JNK,
c-Jun N-terminal kinase; p38, p38 mitogen-activated protein kinase;
p-, phosphorylated. |
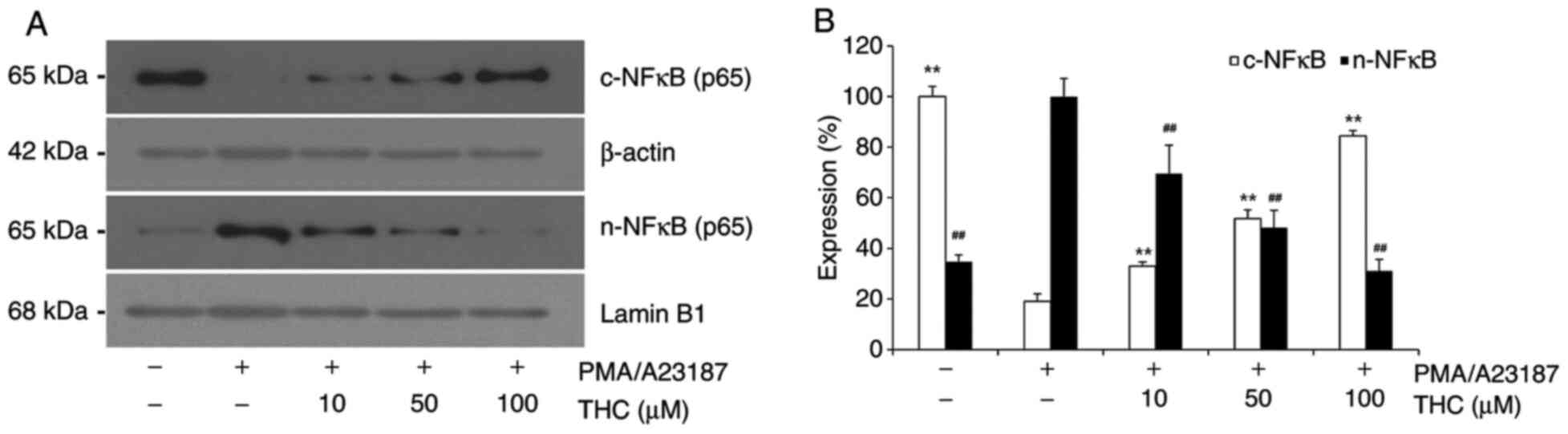
THC significantly attenuated
PMA/A23187-induced nuclear translocation of NF-κB
In the present study, the effect of THC on
PMA/A23187 induced nuclear translocation of NF-κB was examined in
RBL-2H3 cells as NF-κB is a major transcription factor of
pro-inflammatory genes in mast cells (28). PMA/A23187 challenge showed
significantly increased nuclear translocation of NF-κB in RBL-2H3
cells (Fig. 5). Representative
immunoblot showed almost complete translocation of NF-κB upon
PMA/A23187 treatment. However, THC treatment significantly
attenuated PMA/A23187-induced nuclear translocation of NF-κB in a
concentration-dependent manner (Fig.
5). With THC treatment, the level of nuclear NF-κB was
reversely associated with the level of cytosolic NF-κB (Fig. 5).
Discussion
The present results clearly demonstrate that THC
significantly inhibits PMA/A23187-induced allergic responses in
RBL-2H3 mast cells. THC significantly suppressed PMA/A23187-induced
secretion of TNF-α, hexosaminidase, and histamine in
PMA/A23187-challenged RBL-2H3 cells. THC significantly attenuated
PMA/A23187-induced COX-2 expression, MAPKs phosphorylation, and
nuclear translocation of NF-κB in RBL-2H3 cells.
Aberrant mast cell activation plays a detrimental
role in the allergic response in a variety of allergic diseases
including asthma, anaphylaxis and autoimmune disorders (4,5).
Allergic response is initiated by a variety of inflammatory
mediators such as histamine, proteases and various cytokines from
aberrantly activated mast cells. Released inflammatory mediators
consequently cause, vasodilation, increased vascular permeability,
leukocyte recruitment and bronchoconstriction and severe symptoms
such as asthmatic attack and anaphylactic shock might happen
(32,33). Especially, cytokines such as TNF-α
play a key role in leukocyte recruitment (34), The secretion of TNF-α was examined
in PMA/A23187-challenged RBL-2H3 cells in the present study. THC
significantly suppressed PMA/A23187-induced TNF-α secretion in a
concentration-dependent manner. The degranulation response of mast
cells has been reported to be quantitatively determined by
measuring the level of released β-hexosaminidase (35) and histamine is the most well
characterized and most potent vasoactive mediator in activated mast
cells (6). Secretion of
β-hexosaminidase and histamine was quantitatively determined in the
present study. PMA/A23187-challenged RBL-2H3 cells resulted in the
increased release of β-hexosaminidase and histamine and THC
significantly attenuated PMA/A23187-induced secretion of
β-hexosaminidase and histamine in RBL-2H3 cells. The increased
expression of COX-2 in activated mast cells results in the
production of prostaglandin E2, which causes increased vascular
permeability contributing to the aggravation of inflammation
(36). THC significantly
attenuated PMA/A23187-induced increased COX-2 expression in
PMA/A23187-challenged RBL-2H3 cells.
MAPK cascade is a crucial signaling pathway involved
in various immune response (37,38).
Previous reports have demonstrated that the production of
inflammatory cytokines and mediators during the mast cell
activation is associated with MAPKs signaling (7,30,31).
The addition of PMA and A21387 to mast cells results in the
phosphorylation of MAPK cascade including p38, ERK1/2, and JNK
pathways and subsequent expression of cytokines (39,40).
It has been reported that coumarin derivative attenuated
PMA/A21378-induced allergic response by suppressing ERK1/2
signaling pathway in RBL-2H3 cells (41) and CAME, a caffeic acid derivate,
has been reported to suppress allergic response by inhibiting JNK
activation in HMC-1 human mast cells (6). In addition, bisdemmethoxycoumarin has
been reported to inhibit all three MAPKs and suppress allergic
response in PMA/A21378-induced HMC-1 human mast cells (7). These reports strongly suggest that
MAPKs are involved in the propagation of allergic response during
the mast cell activation. In the present study, PMA/A21378
treatment caused clear activation of all three MAPKs and THC
treatment resulted in the significant suppression of MAPKs
phosphorylation. Especially, activation of p38 and JNK was
significantly attenuated in a concentration-dependent manner.
However, phosphorylation of ERK1/2 was significantly inhibited with
low concentration of THC.
NF-κB is the major transcription factor for the
inflammatory responses (28).
NF-κB is involved in the production of pro-inflammatory mediators
such as iNOS, interleukins, and TNF-α and the upregulation of
adhesion molecules (42,43). Inflammatory stimuli such as
lipopolysaccharide cause nuclear translocation of NF-κB in various
immune cells (21,22). In the present study, nuclear
translocation of NF-κB was observed with PMA/A21387 treatment in
RBL-2H3 cells. THC treatment significantly suppressed
PMA/A21387-induced nuclear translocation of NF-κB. With THC
treatment, NF-κB was retained in the cytosol in a
concentration-dependent manner. Nuclear translocation of NF-κB has
been demonstrated to be regulated by MAPKs, which regulate the
degradation of IκB, NF-κB inhibitory protein (44).
Caffeic acid and its derivatives have been reported
to possess a variety of biological actions including anti-tumor,
anti-inflammatory, immunosuppressive, antibiotic and
neuroprotection actions (17-19).
THC showed anti-inflammatory action via the inhibition of NF-κB
activation in LPS-stimulated BV2 microglial cells (28). We demonstrated that THC exhibited
the significant attenuation of LPS-induced inflammatory response
via the activation of cytoprotective Nrf2/HO-1 signaling in
RAW264.7 macrophage cells (45).
THC inhibited LPS-induced macrophage infiltration to kidney and
showed improved survival of animal in LPS-induced endotoxemia mouse
model (45,46). Recently, THC has been also reported
to possess anti-inflammatory action on atopic dermatitis model in
human keratinocyte cell line, HaCat cells (47).
In conclusion, in addition to our previous studies
that THC exerts anti-inflammatory response in microglial,
macrophage, and keratinocyte cells (45-47),
and endotoxemia animal model (45,46),
the present study clearly demonstrates that THC significantly
inhibits PMA/A23187-induced mast cell activation through the
suppression of MAPKs and NF-κB signaling pathways in BRL-2H3 cells,
suggesting that THC might be an important therapeutic agent in the
treatment of allergy-related various disorders. However, to clearly
evaluate anti-allergic effect of THC, further examinations might be
necessary in various study models including animal models.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by the National Research
Foundation of Korea grant funded by the Korean government (grant
no. 2021-R1A4A1031574).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JYP was involved in the experimental operation and
data analysis. HJL, ETH, JHH and WSP were involved in statistical
analysis and data interpretation and in the study methodology. YSK
was involved in the design and result discussion of the study. WC
was involved in the conceptualization and the writing, reviewing
and editing of the manuscript. YSK and WC confirm the authenticity
of all the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Marshall JS: Mast-cell responses to
pathogens. Nat Rev Immunol. 4:787–799. 2004.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
Johnzon CF, Ronnberg E and Pejler G: The
role of mast cells in bacterial infection. Am J Pathol. 186:4–14.
2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wernersson S and Pejler G: Mast cell
secretory granules: Armed for battle. Nat Rev Immunol. 14:478–494.
2014.PubMed/NCBI View
Article : Google Scholar
|
|
4
|
Voehringer D: Protective and pathological
roles of mast cells and basophils. Nat Rev Immunol. 13:362–375.
2013.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
Galli SJ, Grimbaldeston M and Tsai M:
Immunomodulatory mast cells: Negative, as well as positive,
regulators of immunity. Nat Rev Immunol. 8:478–486. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
6
|
Cho MS, Park WS, Jung WK, Qian ZJ, Lee DS,
Choi JS, Lee DY, Park SG, Seo SK, Kim HJ, et al: Caffeic acid
phenethyl ester promotes anti-inflammatory effects by inhibiting
MAPK and NF-κB signaling in activated HMC-1 human mast cells. Pharm
Biol. 52:926–932. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kong R, Kang OH, Seo YS, Zhou T, Kim SA,
Shin DW and Kwon DY: MAPKs and NFkappaB pathway inhibitory effect
of bisdemethoxycurcumin on phorbol 12-myristate13-acetate and
A23187-induced inflammation in human mast cells. Mol Med Rep.
17:630–635. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Li L, Zhang XH, Liu GR, Liu C and Dong YM:
Isoquercitrin suppresses the expression of histamine and
pro-inflammatory cytokines by inhibiting the activation of MAP
Kinases and NF-κB in human KU812 cells. Chin J Nat Med. 14:407–412.
2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Cagnol S and Chambard JC: ERK and cell
death: Mechanisms of ERK-induced cell death-apoptosis, autophagy
and senescence. FEBS J. 277:2–21. 2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhao J, Wang L, Dong X, Hu X, Zhou L, Liu
Q, Song B, Wu Q and Li L: The c-Jun N-terminal kinase (JNK) pathway
is activated in human interstitial cystitis (IC) and rat protamine
sulfate induced cystitis. Sci Rep. 6(19670)2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhou Y, Yang Q, Xu H, Zhang J, Deng H, Gao
H, Yang J, Zhao D and Liu F: miRNA-221-3p enhances the secretion of
interleukin-4 in mast cells through the phosphatase and tensin
Homolog/p38/Nuclear Factor-kappaB pathway. PLoS One.
11(e0148821)2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bose JS, Gangan V, Jain SK and Manna SK:
Novel caffeic acid ester derivative induces apoptosis by expressing
FasL and downregulating NF-KappaB: Potentiation of cell death
mediated by chemotherapeutic agents. J Cell Physiol. 218:653–662.
2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Murtaza G, Karim S, Akram MR, Khan SA,
Azhar S, Mumtaz A and Bin Asad MH: Caffeic acid phenethyl ester and
therapeutic potentials. Biomed Res Int. 2014(145342)2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Armutcu F, Akyol S, Ustunsoy S and Turan
FF: Therapeutic potential of caffeic acid phenethyl ester and its
anti-inflammatory and immunomodulatory effects (Review). Exp Ther
Med. 9:1582–1588. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Choi HG, Tran PT, Lee JH, Min BS and Kim
JA: Anti-inflammatory activity of caffeic acid derivatives isolated
from the roots of Salvia miltiorrhiza Bunge. Arch Pharm Res.
41:64–70. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Rabe C, Steenkamp AJ, Joubert E, Burger
JFW and Ferreira D: Phenolic metabolites from rooibos tea.
Phytochemistry. 35:1559–1565. 1994.
|
|
17
|
Nagasaka R, Chotimarkorn C, Shafiqul IM,
Hori M, Ozaki H and Ushio H: Anti-inflammatory effects of
hydroxycinnamic acid derivatives. Biochem Biophys Res Commun.
358:615–619. 2007.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kim YC: Neuroprotective phenolics in
medicinal plants. Arch Pharm Res. 33:1611–1632. 2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lee JW, Cheong IY, Kim HS, Lee JJ, Lee YS,
Kwon YS, Kim MJ, Lee HJ, Kim SS and Chun W: Anti-inflammatory
activity of 1-docosanoyl cafferate isolated from Rhus verniciflua
in LPS-stimulated BV2 microglial cells. Korean J Physiol Pharmacol.
15:9–15. 2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Itharat A and Hiransai P: Dioscoreanone
suppresses LPS-induced nitric oxide production and inflammatory
cytokine expression in RAW 264.7 macrophages by NF-κB and ERK1/2
signaling transduction. J Cell Biochem. 113:3427–3435.
2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kim YJ, Shin Y, Lee KH and Kim TJ: Anethum
graveloens flower extracts inhibited a lipopolysaccharide-induced
inflammatory response by blocking iNOS expression and NF-κB
activity in macrophages. Biosci Biotechnol Biochem. 76:1122–1127.
2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Rehman MU, Yoshihisa Y, Miyamoto Y and
Shimizu T: The anti-inflammatory effects of platinum nanoparticles
on the lipopolysaccharide-induced inflammatory response in RAW
264.7 macrophages. Inflamm Res. 61:1177–1185. 2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lee Y, Shin DH, Kim JH, Hong S, Choi D,
Kim YJ, Kwak MK and Jung Y: Caffeic acid phenethyl ester-mediated
Nrf2 activation and IkappaB kinase inhibition are involved in
NFkappaB inhibitory effect: Structural analysis for NFkappaB
inhibition. Eur J Pharmacol. 643:21–28. 2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Fidan H, Sahin O, Yavuz Y, Kilbas A,
Cetinkaya Z, Ela Y, Ozen OA and Altuntas I: Caffeic acid phenethyl
ester reduces mortality and sepsis-induced lung injury in rats.
Crit Care Med. 35:2822–2829. 2007.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Schwartz LB, Lewis RA, Seldin D and Austen
KF: Acid hydrolases and tryptase from secretory granules of
dispersed human lung mast cells. J Immunol. 126:1290–1294.
1981.PubMed/NCBI
|
|
26
|
Schoonbroodt S, Legrand-Poels S,
Best-Belpomme M and Piette J: Activation of the NF-kappaB
transcription factor in a T-lymphocytic cell line by hypochlorous
acid. Biochem J. 321:777–785. 1997.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ock J, Kim S and Suk K: Anti-inflammatory
effects of a fluorovinyloxyacetamide compound KT-15087 in microglia
cells. Pharmacol Res. 59:414–422. 2009.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lee JW, Bae CJ, Choi YJ, Kim SI, Kim NH,
Lee HJ, Kim SS, Kwon YS and Chun W: 3,4,5-trihydroxycinnamic acid
inhibits LPS-Induced iNOS expression by suppressing NF-κB
Activation in BV2 microglial cells. Korean J Physiol Pharmacol.
16:107–112. 2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kunder CA, St John AL and Abraham SN: Mast
cell modulation of the vascular and lymphatic endothelium. Blood.
118:5383–5393. 2011.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hundley TR, Prasad AR and Beaven MA:
Elevated levels of cyclooxygenase-2 in antigen-stimulated mast
cells is associated with minimal activation of p38
mitogen-activated protein kinase. J Immunol. 167:1629–1636.
2001.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Hu Frisk JM, Kjellen L, Melo FR, Ohrvik H
and Pejler G: Mitogen-Activated protein kinase signaling regulates
proteoglycan composition of mast cell secretory granules. Front
Immunol. 9(1670)2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kim DH, Jung WS, Kim ME, Lee HW, Youn HY,
Seon JK, Lee HN and Lee JS: Genistein inhibits proinflammatory
cytokines in human mast cell activation through the inhibition of
the ERK pathway. Int J Mol Med. 34:1669–1674. 2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Su-Jin Kim SJ, Kim YJ, Lee JH, Oh SR, Park
CI, Jeong JW, Um JY, Hong SH and Ahn EM:
Genistein-4'-O-α-L-rhamnopyranosyl-(1→2)-β-D-glucopyranoside from
Sophora japonica (Leguminosae) ameliorates mast cell-mediated
allergic inflammation in vivo and in vitro. Orient Pharm Exp Med.
11:207–213. 2011.
|
|
34
|
Woolley DE and Tetlow LC: Mast cell
activation and its relation to proinflammatory cytokine production
in the rheumatoid lesion. Arthritis Res. 2:65–74. 2000.PubMed/NCBI View
Article : Google Scholar
|
|
35
|
Chen BH, Hung MH, Chen JYF, Chang HW, Yu
ML, Wan L, Tsai FJ, Wang TP, Fu TF and Chiu CC: Anti-allergic
activity of grapeseed extract (GSE) on RBL-2H3 mast cells. Food
Chem. 132:968–974. 2012.
|
|
36
|
Tang T, Scambler TE, Smallie T, Cunliffe
HE, Ross EA, Rosner DR, O'Neil JD and Clark AR: Macrophage
responses to lipopolysaccharide are modulated by a feedback loop
involving prostaglandin E2, dual specificity phosphatase 1 and
tristetraprolin. Sci Rep. 7(4350)2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Johnson GL and Lapadat R:
Mitogen-activated protein kinase pathways mediated by ERK, JNK, and
p38 protein kinases. Science. 298:1911–1912. 2002.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Kaminska B: MAPK signalling pathways as
molecular targets for anti-inflammatory therapy-from molecular
mechanisms to therapeutic benefits. Biochim Biophys Acta.
1754:253–262. 2005.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Oliver JM, Kepley CL, Ortega E and Wilson
BS: Immunologically mediated signaling in basophils and mast cells:
Finding therapeutic targets for allergic diseases in the human
FcvarepsilonR1 signaling pathway. Immunopharmacology. 48:269–281.
2000.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kang OH, Jang HJ, Chae HS, Oh YC, Choi JG,
Lee YS, Kim JH, Kim YC, Sohn DH, Park H and Kwon DY:
Anti-inflammatory mechanisms of resveratrol in activated HMC-1
cells: Pivotal roles of NF-kappaB and MAPK. Pharmacol Res.
59:330–337. 2009.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Yoo G, Lee K and Lee DC: Inhibitory
effects of 2-oxo-2H-chromen-4-yl 4-methylbenzenesulfonate on
allergic inflammatory responses in rat basophilic leukemia cells.
Int Immunopharmacol. 48:196–202. 2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Wan M, Liu J and Ouyang X:
Nucleotide-binding oligomerization domain 1 regulates Porphyromonas
gingivalis-induced vascular cell adhesion molecule 1 and
intercellular adhesion molecule 1 expression in endothelial cells
through NF-κB pathway. J Periodontal Res. 50:189–196.
2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Wang L, Xu Y, Yu Q, Sun Q, Xu Y, Gu Q and
Xu X: H-RN, a novel antiangiogenic peptide derived from hepatocyte
growth factor inhibits inflammation in vitro and in vivo through
PI3K/AKT/IKK/NF-κB signal pathway. Biochem Pharmacol. 89:255–265.
2014.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Blackwell TS, Blackwell TR and Christman
JW: Impaired activation of nuclear factor-kappaB in
endotoxin-tolerant rats is associated with down-regulation of
chemokine gene expression and inhibition of neutrophilic lung
inflammation. J Immunol. 158:5934–5940. 1997.PubMed/NCBI
|
|
45
|
Lee JW, Bae CJ, Choi YJ, Kim SI, Kwon YS,
Lee HJ, Kim SS and Chun W: 3,4,5-trihydroxycinnamic acid inhibits
lipopolysaccharide (LPS)-induced inflammation by Nrf2 activation in
vitro and improves survival of mice in LPS-induced endotoxemia
model in vivo. Mol Cell Biochem. 390:143–153. 2014.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Lee JW, Kwon JH, Lim MS, Lee HJ, Kim SS,
Lim SY and Chun W: 3,4,5-Trihydroxycinnamic acid increases
heme-oxygenase-1 (HO-1) and decreases macrophage infiltration in
LPS-induced septic kidney. Mol Cell Biochem. 397:109–116.
2014.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Park JW, Oh JH, Hwang D, Kim SM, Min JH,
Seo JY, Chun W, Lee HJ, Oh SR, Lee JW and Ahn KS:
3,4,5Trihydroxycinnamic acid exerts antiinflammatory effects on
TNF-α/IFN-ү-stimulated HaCaT cells. Mol Med Rep.
24(509)2021.PubMed/NCBI View Article : Google Scholar
|