Introduction
Hepatic trauma is the leading cause of death in
major abdominal trauma, and the incidence has increased
considerably in the last three decades (1). Liver rupture injuries account for
approximately 15 to 20% of abdominal injuries. According to results
from clinical data, the mortality rate of hepatic trauma is about
10% and up to 50% in severe hepatic trauma or combined with other
organ injuries (2,3). Hemorrhagic shock is the leading cause
of death in hepatic trauma, making prompt and effective hemostasis
the most critical task for the successful treatment of severe
cases.
Previously, most patients diagnosed with hepatic
trauma were treated surgically using techniques such as tamponade,
liver repair, vascular ligation, and hepatectomy (4). However, with improvements in imaging
quality and accessibility, as well as the development of
percutaneous interventions, non-operative management (NOM) has
become the primary method for treating severe hepatic trauma
(5,6). The treatment concept for hepatic
trauma has shifted, with nearly 80% of patients now preferring
non-operative treatment (7).
Transcatheter arterial embolization (TAE) has emerged as a highly
effective non-surgical treatment for severe hepatic trauma due to
its minimally invasive nature, precise visualization of the
bleeding artery, precise hemostasis, and lower degree of
operational difficulty. TAE has been widely performed, and its
effectiveness has been clinically proven (8,9).
However, while TAE can achieve effective hemostasis, some patients
may experience postoperative liver pain, fever, and other
complications related to hepatic ischemia and necrosis. These
questions are worth exploring further (10-13).
To address these questions, this study investigated the effects of
transhepatic arterial embolization on normal liver tissues in
rabbits by using an AGS and PVA, respectively. By better
understanding the impact of these techniques, we can develop
improved approaches for protecting the liver during hepatic
intervention embolization, reducing complications, and improving
the survival rate.
Materials and methods
Experimental animals and related
materials
Experimental animals for this study were New Zealand
white rabbits weighing 2-2.5 kg, obtained from Songlian
Experimental Animal Farm in Songjiang District, Shanghai. The
animal license number for this study was (SCXK (Shanghai)
2017-0008). Materials used in the study included PVA (100 µm) and
1.7F EV3 microcatheters purchased from COOK Corporation
(Bloomington, IN, USA), an absorbable gelatin sponge purchased from
Jinling Pharmaceutical Co. Ltd, and ELISA kits for TNF-α, IL-6, and
SOD purchased from Jiancheng Institute of Biological Engineering
(Nanjing, Jiangsu, China). Bcl2 and Bax antibodies were purchased
from Proteintech Corporation (Inc., Chicago, USA).
Experimental animals and study
design
This study received approval from the Animal
Experimentation Committee of Xiamen University (XMULAC20230016),
and all rabbits were treated in accordance with the National
Institutes of Health Guide for the Care and Use of Laboratory
Animals. A total of 45 New Zealand white rabbits were carefully
selected and randomly divided into three groups using the numerical
table randomization method: a control group (5 animals), a PVA
group (20 animals), and an AGS group (20 animals). The control
group underwent no experimental manipulation (1 animal per time
point), while animals in the experimental groups were evaluated at
1, 3, 7 and 21 days after embolization (5 animals per time point).
To minimize the influence of biological rhythms on the experimental
results, all experiments were conducted at 8 am. Two commonly used
interventional embolic materials were chosen for the study: AGS for
absorbable materials and PVA for non-absorbable materials.
Establishment of animal models
We established a rabbit embolism model by performing
hepatic artery embolization using AGS and PVA embolic materials.
The procedure for establishing the model was as follows: All
rabbits were fasted for 12 h before surgery and could drink water
freely. Anesthesia was administered by intramuscular injection(IM)
of ketamine (25 mg/kg) and medetomidine (0.2 mg/mg) and maintained
with 1-2% isoflurane (600 ml/min). A catheter (1.7F vascular
sheath) was delivered via the femoral artery using Seldinger's
technique, and then the catheter was selectively delivered into the
celiac trunk, the common hepatic artery, and contrast agent was
injected into the arterial DSA examination to reveal the hepatic
artery course and branches. The 1.7Fcoaxial catheter was
super-selectively cannulated into the left hepatic artery, and the
embolic material was slowly injected, with 0.1 ml of AGS in the AGS
group, 0.1 ml of PVA in the PVA group, and an equal amount of
normal saline in the control group. After completion, the same
imaging rate was used to understand the embolization (the end point
of injection was paravalvular flow arrest). At the end of the
operation, the tube was removed to stop the bleeding, the puncture
site was dressed with pressure, and the right inguinal incision was
sutured. After the interventional operation, intramuscular
penicillin and gentamicin were injected for three consecutive days
to prevent infection.
Sample collection and
preservation
Open the ear marginal vein channel of the
experimental rabbit and perform induction anesthesia by
administering propofol of 1.25 to 2 ml (5 mg/kg) through auricular
intravenous. The rabbit was then euthanized by injecting 20-30 ml
of air through the auricular intravenous to obtain blood and liver
tissue specimens. Venous blood samples were taken at different time
points and collected in tubes containing EDTA. The samples were
then centrifuged at 3000 rpm for 10 min, and the resulting serum
was stored at -80˚C. Following dissection, approximately 200 mg of
liver left outer lobe tissue was immediately collected and gently
rinsed in 5 ml of PBS for histopathological studies. The remaining
tissue was frozen in liquid nitrogen and stored at -80˚C until
tissue testing was performed.
Liver function and inflammatory
indexes
In the control group, blood was collected
immediately after euthanasia, while in the experimental group,
blood was collected at 1, 3, 7, and 21 days after embolization,
respectively. After centrifugation, serum was collected and
analyzed using enzyme-linked immunosorbent assay (ELISA) to measure
ten indicators of liver injury, including glutamate transaminase
(ALT), glutathione transaminase (AST), alkaline phosphatase (ALP),
bilirubin (BIL), albumin (ALB), r-glutamine transpeptidase (r-GT),
SOD, CRP, TNF-a, and IL-6. The content changes of these ten
indicators were monitored at the four time periods in the rabbit
embolism model.
Histo-pathological examination
Immediately after euthanasia, the left outer lobe of
the liver tissues from both control and experimental rabbits were
collected, fixed with formaldehyde, embedded in paraffin, and
sectioned. The sections were then stained with hematoxylin and
eosin (HE) and observed under a microscope at 100x and 400x
magnification. Images were captured for further analysis.
Western blotting
The Western Blot method was used to detect Bcl2 and
Bax proteins in hepatocytes and biliary epithelial cells in liver
tissue. To separate protein samples by electrophoresis based on
molecular protein weight, 10% or 12% SDS-PAGE gel was prepared. The
separated proteins on the gel were transferred to a polyvinylidene
fluoride (PVDF) membrane by electrotransfer. After electrotransfer,
the PVDF membrane was immersed in a blocking solution containing 5%
skim milk powder and incubated at room temperature for 1 h. The
corresponding primary antibodies, Bax and Bcl2, were added after
washing the membrane with phosphate-buffered saline with Tween 20
(PBST) and incubated overnight at 4˚C. The corresponding
horseradish peroxidase-labelled secondary antibody solution was
added after washing the membrane with PBST and incubated for 1 h at
room temperature. The PVDF membrane was then reacted with freshly
prepared enhanced chemiluminescent agent (ECL) solution for 2 min
and quickly exposed to a film in a dark room for development.
Statistical analysis
The statistical analysis was performed using SPSS
version 27.0 and GraphPad Prism version 8.0 software. The
measurement data were presented as mean ± standard deviation (s).
To compare the differences among multiple groups, we used one-way
analysis of variance (ANOVA), followed by post-hoc testing using
Dunnett's method. Within the same group, we used paired sample
t-tests to compare the differences among different time points.
P<0.05 was considered statistically significant.
Results
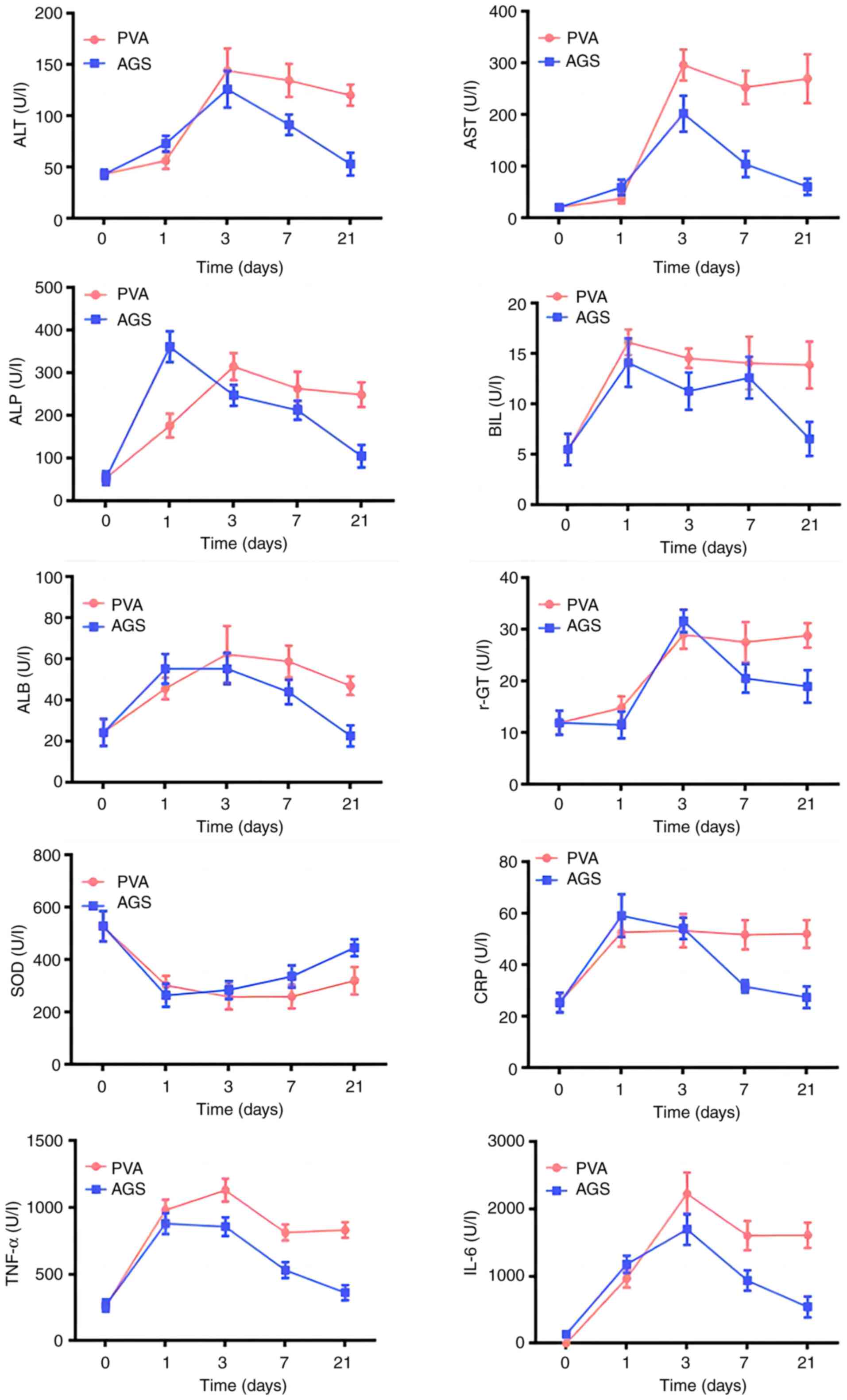
Changes in liver function at different
time periods in each group
On day 1, the PVA-treated group showed increased
levels of Alanine aminotransferase (ALT), Aspartate
aminotransferase (AST), and Gamma-glutamyltranspeptidase (γ-GT), as
well as significant increases in Alkaline phosphatase (ALP),
Albumin (ALB), Bilirubin (BIL), C-Reactive protein (CRP), Tumor
necrotic factor (TNF-α), and Interleukin-6 (IL-6) levels, and a
significant decrease in Superoxide dismutase (SOD) levels. The AGS
group also showed increased levels of ALT, AST, γ-GT, ALP, ALB,
BIL, CRP, TNF-α, and IL-6, and a significant decrease in SOD
levels. ALP and ALB levels were significantly different between the
two groups on day 1.
On day 3, the PVA-treated group showed significant
increases in ALT, AST, γ-GT, ALB, TNF-α, and IL-6 levels, while ALP
and BIL levels decreased. The AGS-treated group showed significant
increases in ALT, AST, γ-GT, ALP, ALB, and IL-6 levels, while BIL
and CRP levels had some decreases. AST and TNF-α levels were
statistically different between the two groups on day 3.
On day 7, the PVA-treated group showed decreased
levels of ALT, AST, ALP, BIL, ALB, and γ-GT, and significant
decreases in TNF-α and IL-6 levels, while the AGS-treated group
showed decreases in ALT, AST, ALP, BIL, ALB, γ-GT, and CRP, and
significant decreases in TNF-α and IL-6 levels, but a significant
decrease in SOD levels. ALT, AST, CRP, TNF-α, and IL-6 showed
statistical differences between the two groups.
On day 21, the PVA-treated group showed a certain
decrease in the contents of ALT, AST, ALP, BIL, ALB, and γ-GT, and
a certain increase in SOD levels, while the AGS-treated group
showed a certain decrease in the contents of ALT, AST, ALP, BIL,
ALB, and γ-GT, as well as significant decreases in TNF-α and IL-6
levels, and a certain increase in SOD levels. The liver biochemical
indexes were statistically different on day 21 (Fig. 1).
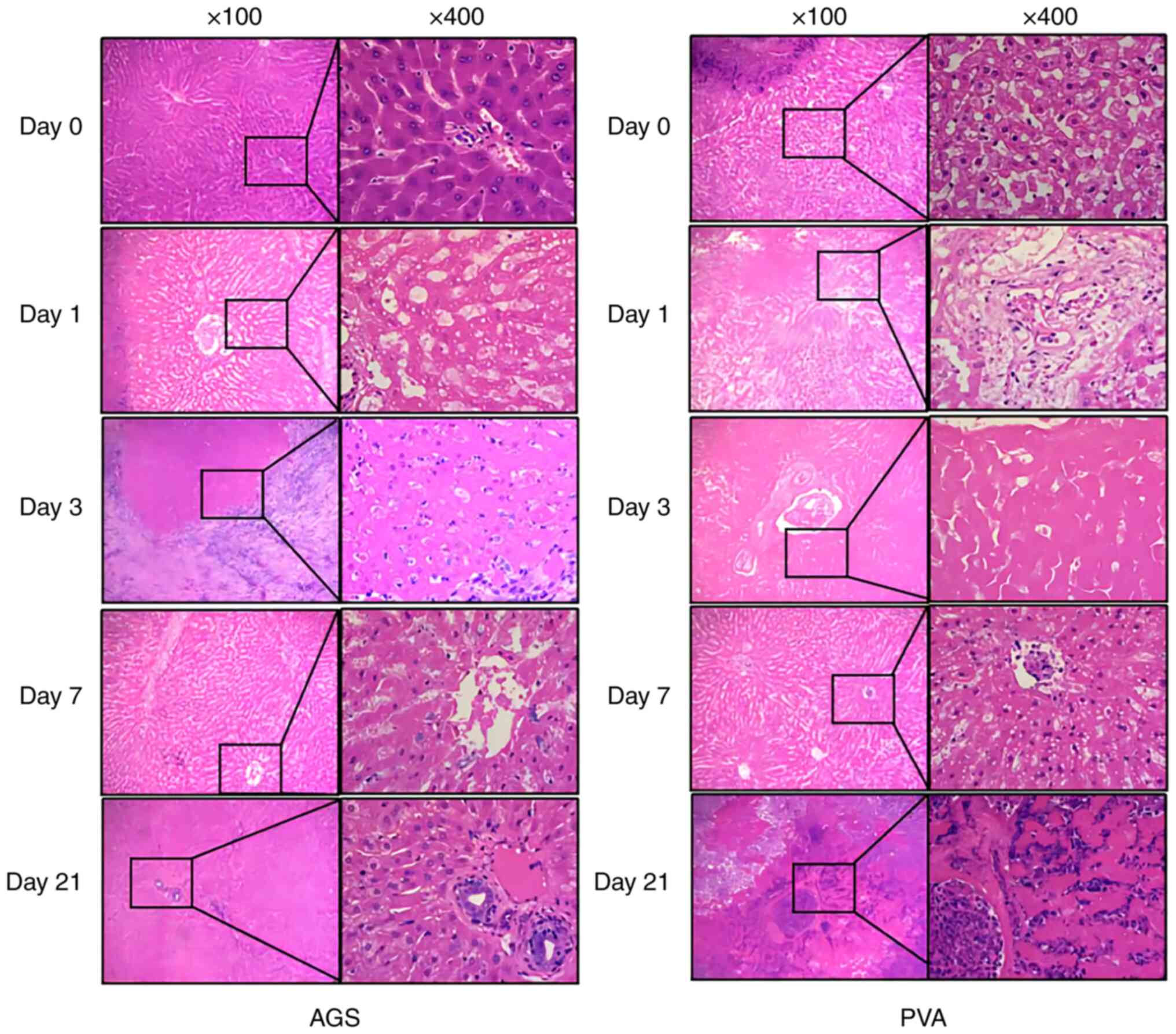
HE staining
Observation of HE staining of liver sections at
different time points in the AGS group and the PVA group revealed
the following findings. In the AGS group, on day 1, the hepatocytes
were swollen with fixed nuclei, the hepatic blood sinuses were
dilated, and the bile duct epithelium mildly degenerated. On day 3,
the hepatocytes were swollen with indistinct cell boundaries, and
focal nuclei fragmentation was observed. Massive interstitial
lymphocyte infiltration and partial loss of bile duct epithelium
were also noted. On day 7, interstitial inflammatory cell
infiltration regressed, hepatocyte degeneration was still evident,
and bile duct epithelium was degenerated and lost. On day 21, the
hepatocyte degeneration was not obvious, and the structure was
clear. The bile duct epithelium was reactive and proliferated, and
no significant interstitial inflammatory cell infiltration was
observed.
In the PVA group, on day 1, the hepatocytes were
swollen with fixed nuclei, the hepatic blood sinuses were dilated,
and the bile duct epithelium mildly degenerated. On day 3, the
hepatocytes were swollen with indistinct cell boundaries, and focal
nuclei fragmentation was observed. Massive interstitial lymphocyte
infiltration and partial loss of bile duct epithelium were also
noted. On day 7, a large area of hepatocytes and bile duct
epithelium was degenerated and necrotic, and the structure of liver
lobules disappeared, replaced by infiltration of lamellar
inflammatory cells. On day 21, the necrotic foci were further
enlarged, and the surrounding hepatic blood sinusoids were
infiltrated by lymphocytes (Fig.
2).
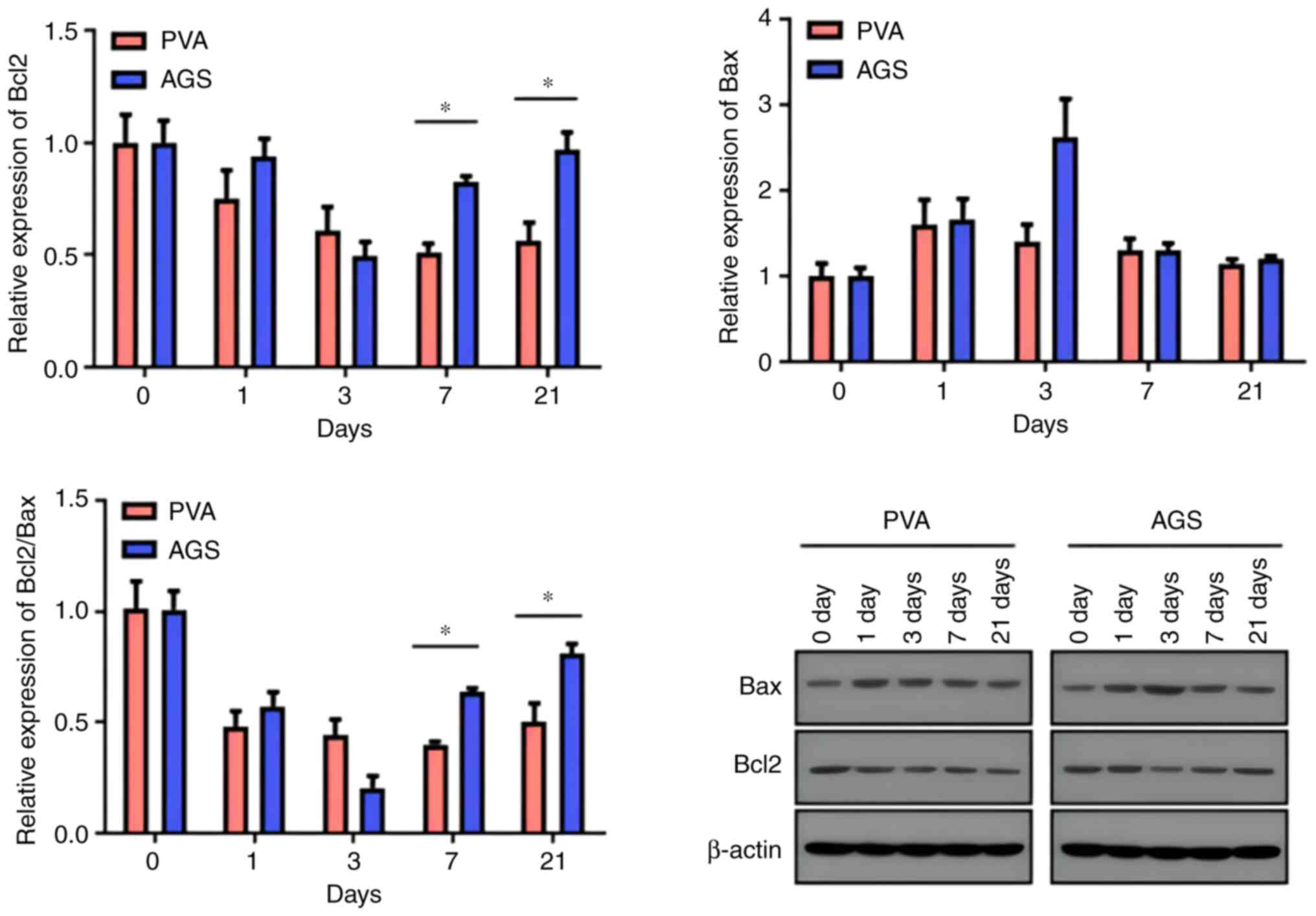
WB results
The Western Blot method was used to detect Bcl2 and
Bax proteins in hepatocytes and biliary epithelial cells in liver
tissues. Results showed that the expression of Bax protein in liver
tissues increased on day 1, day 3, day 7, and day 21 in the PVA
group, while in the AGS group, it showed an upward-downward trend
and peaked on the third day. Bax apoptotic protein expression
induced by PVA did not recover with time, while AGS-induced Bax
apoptotic protein expression showed a trend of recovery at day 7.
The expression of Bcl2 protein in liver tissues decreased on days
1, 3, 7, and 21 in the PVA group, and the expression in the AGS
group decreased significantly on day 3 and showed an increasing
trend after day 7. The expression of the anti-apoptotic protein was
inhibited in the PVA group, with no recovery trend observed with
time. Conversely, the expression of the anti-apoptotic protein was
inhibited in the AGS group, but a recovery trend was evident with
time at day 7. The Bcl2/Bax ratio can be used to measure apoptosis
and repair of liver tissues. The Bcl2/Bax ratio serves as a measure
of apoptosis and tissue repair in the liver. In the AGS group, the
Bcl2/Bax values showed an upward trend on day 7 following a decline
on days 1 and 3, while in the PVA group, the Bcl2/Bax values did
not show a significant upward trend after declining from day 1. The
Bcl2/Bax ratio in the AGS group was higher than that in the PVA
group on both day 7 and day 21, and the difference was
statistically significant (Fig. 3;
Tables I and II).
 | Table IWestern-Blot detection of expression
of proteins related to Bcl2 and Bax expression in the PVA and AGS
groups at different time points. |
Table I
Western-Blot detection of expression
of proteins related to Bcl2 and Bax expression in the PVA and AGS
groups at different time points.
| A, Bcl2 |
|---|
| | PVA | AGS |
|---|
| Time, days | Sample 1 | Sample 2 | Sample 3 | Sample 1 | Sample 2 | Sample 3 |
|---|
| 0 | 1.0945 | 1.1505 | 0.7550 | 0.8734 | 0.9375 | 1.1890 |
| 1 | 0.8153 | 0.9294 | 0.5126 | 0.7837 | 0.9605 | 1.0630 |
| 3 | 0.6867 | 0.7375 | 0.3982 | 0.4412 | 0.6223 | 0.4117 |
| 7a | 0.4880 | 0.5876 | 0.4629 | 0.8021 | 0.7951 | 0.8742 |
| 21a | 0.6708 | 0.6126 | 0.3935 | 0.8321 | 0.9635 | 1.1030 |
| B, Bax |
| | PVA | AGS |
| Time, days | Sample 1 | Sample 2 | Sample 3 | Sample 1 | Sample 2 | Sample 3 |
| 0 | 0.8727 | 1.2881 | 0.8393 | 1.0243 | 0.8225 | 1.1532 |
| 1 | 1.3121 | 2.1829 | 1.3146 | 1.5102 | 1.3702 | 2.1276 |
| 3 | 1.1881 | 1.8128 | 1.1743 | 2.2644 | 2.0603 | 3.5177 |
| 7 | 1.1628 | 1.5789 | 1.1396 | 1.2588 | 1.1940 | 1.4549 |
| 21 | 1.0259 | 1.2590 | 1.1059 | 1.1599 | 1.1589 | 1.2658 |
 | Table IIBcl2/Bax ratio in the PVA and AGS
groups at different time points. |
Table II
Bcl2/Bax ratio in the PVA and AGS
groups at different time points.
| Time, days | | PVA | | | AGS | | t-value | P-value |
|---|
| 0 | 1.2542 | 0.8932 | 0.8996 | 0.8527 | 1.1399 | 1.0311 | 0.0530 | 0.9600 |
| 1 | 0.6213 | 0.4258 | 0.3899 | 0.5190 | 0.7010 | 0.4996 | -0.9770 | 0.3840 |
| 3 | 0.5779 | 0.4068 | 0.3391 | 0.1949 | 0.3020 | 0.1170 | 2.6580 | 0.0570 |
| 7a | 0.4197 | 0.3722 | 0.4062 | 0.6372 | 0.6659 | 0.6009 | -10.0020 | 0.0100 |
| 21a | 0.6539 | 0.4866 | 0.3558 | 0.7174 | 0.8314 | 0.8714 | -3.1490 | 0.0350 |
Discussion
TAE is increasingly becoming the main therapeutic
approach for traumatic hepatic rupture haemorrhage and is more
frequent in the clinic, as a result of many clinical studies
(14,15). TAE treatment involves the delivery
of embolic material through the hepatic artery to the site of liver
injury to stop bleeding or treat the lesion, and the effectiveness
of the treatment is influenced by the skill level of the
intervening physician, the level of the embolic artery and the
property of the embolic material itself. The commonly used embolic
materials can be divided into various types, and according to their
ability to be absorbed in the body, they can be divided into
absorbable and non-absorbable embolic materials. AGS and PVA are
short- and medium-term and permanent embolic agents for large and
medium vessels in the body that are widely used in clinical
practice and have achieved certain results in treatment (16,17).
However, there are still few studies related to whether there are
differences in the pathological changes caused by local tissue
ischemia in the liver after TAE treatment with different embolic
materials, especially the structural and functional changes of the
bile ducts after ischemia.
AGS has been clinically used for a long time, and a
lot of experience has been accumulated in its use. AGS has the
advantages of weak antigenicity, good histocompatibility, and
biosafety (18,19). In addition to mechanical embolism,
the sponge-like framework of gelatin sponge can be filled with red
blood cells, which cause platelet agglutination and fibrinogen
deposition in blood vessels, forming thrombus quickly, plus it
causes vascular spasm also contribute to thrombus formation and
help blood vessel embolism. Furthermore, during embolization, it
can not only embolize the blood vessel to achieve the purpose of
hemostasis but also the AGS can promote the growth of granulation
tissue at the lesion site after adding chitosan to achieve the
effect of promoting wound recovery, which is suitable as the
material of choice for hepatic artery embolization. AGS, as a
non-permanent embolic material, usually takes a few weeks to a few
months to be completely absorbed and degrades within a certain
period of time after embolization to the designated site, resulting
in the recanalization of the target vessel at the embolized site
and thus failing to achieve the desired therapeutic effect or even
the recurrence of serious bleeding. In the study, the liver
function of the rabbits in the AGS group showed a trend of
improvement at about one week after embolization, and all the
indicators were statistically different until day 21 compared with
those in the PVA group, and a better repair of hepatocytes and the
biliary system was observed in HE. The WB results also showed that
the Bcl-2/Bax ratio rebounded on days 7 and 21, showing inhibition
of hepatocyte apoptosis and gradual repair of hepatocytes. As a
permanent embolic agent, PVA can mechanically occlude the diseased
vessels after being injected into the blood vessels, and thrombotic
materials can form and become macerated in the gaps between the
polyvinyl alcohol particles, leading to permanent vessel occlusion.
In interventional embolization for liver trauma, compared with
other embolic agents, PVA can provide a more long-lasting
hemostatic effect because it cannot be degraded in the body, which
also reduces the chance of vessel recanalization (20-22).
de Baere et al (23) found
a significant increase in liver fibrosis after embolization of the
liver vessels with PVA compared to the control group in a pig
experiment. The results of our study showed that the liver of the
rabbits in the PVA group was more severely damaged than that of the
AGS group, and there was no trend of recovery, and we could see
that the necrosis of the hepatocytes and biliary system around the
embolization was more severe under HE observation. This is also
consistent with the fact that PVA is a permanent embolic material
that cannot be recanalized after embolization. Therefore, this
conventional embolic agent embolization of the hepatic artery has a
therapeutic effect as well as serious liver damage caused by tissue
ischemia after embolization of the liver.
Interventional embolization has been used in a large
number of clinical studies and practical work, and the advantages
of TAE over conventional open abdomen have been studied, but the
choice of embolization material is crucial in performing TAE
treatment because of the different characteristics of various
embolic materials and their application conditions (18). The liver is subject to the dual
blood supply of the hepatic artery and portal vein, so it is rich
in blood transport (24), and the
liver has a better compensatory function after performing TAE, but
for biliary epithelial cells, the blood supply to the porta hepatis
or Hepatic portal vein and intrahepatic bile ducts depends only on
the source of tiny branches of the hepatic artery, and the
interventional treatment repeatedly embolizes these tiny arterial
branches, thus may cause ischemic necrosis and fibrous tissue
proliferation in the corresponding bile ducts, to the extent that
some of the bile ducts are narrowed, while distal bile ducts are
dilated due to biliary stasis, gallstone formation, and increased
pressure in the bile ducts, thus causing more severe damage to the
biliary system compared to the hepatocytes (25). A meta-analysis showed that the
incidence of biliary fistula after performing TAE was about 5.7%,
so we should be alert to the complications of the biliary system
during TAE (26). In this study,
it was found that the liver function of the AGS group compared to
the PVA group in the 21-day control not only had better recovery of
liver function, where the observation of HE and WB results also
indicated that the function of their intrahepatic biliary
epithelial cells in the AGS group also had some repair, and their
biliary system complications The incidence of complications related
to the biliary system was also relatively low. The use of permanent
embolic agent PVA during TAE would have a better hemostatic effect
due to the inability to revascularization, but at the same time,
the damage to the liver and biliary tract would be more serious. In
contrast, for AGS, the recanalized vessels can have a certain
repair effect on the pre-ischemic liver and biliary tract, so the
chance of related complications is lower, but it also undoubtedly
increases the chance of bleeding after recanalization.
In conclusion, the current study has some
limitations due to the small size of the New Zealand White rabbits,
making it difficult to establish a standard model of liver trauma,
and an embolization model based on liver trauma could not be
established, therefore, we ultimately chose to establish an
embolism model for the study. Future studies may need to consider
using larger experimental animals to establish a liver trauma model
and different embolic materials for validation to make the
experimental results more representative of the real clinical
situation.
In summary, this study investigated the effects of
AGS and PVA on normal liver tissue by embolizing New Zealand
rabbits and analyzed the differences in the effects of these two
materials on normal liver tissue after embolization. The absorbable
embolic material, gelatin sponge, caused less ischemic necrosis to
the liver tissue after embolization than the non-absorbable embolic
material, PVA, and the liver had some self-repair after
revascularization. In contrast, PVA caused more damage to the liver
tissue after embolization, and no obvious repair process was
observed. Therefore, the choice of embolization material in
clinical practice should be based on the patient's specific
situation, and a more reasonable option should be chosen by
balancing the therapeutic effect and possible complications after
embolization.
Acknowledgements
Not applicable.
Funding
Funding: This research was supported by the Fujian Provincial
Natural Science Foundation (grant no. 2020J01133).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SZ, JL and SW contributed to the conception and
design, data collection, data analysis/statistics, data
interpretation and funding collection. SW and JL contributed to the
acquisition of data and preparation of the manuscript. XX, XP, TH
and JH contributed to the analysis and interpretation of data. All
authors have read and approved the final manuscript. SZ and JL
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was approved by the Animal
Experimentation Committee of Xiamen University (approval no.
XMULAC20230016; Zhangzhou, China), and all rabbits were handled in
accordance with the Guide for the Care and Use of Laboratory
Animals published by the US National Institutes of Health (NIH
Publication No. 85-23, revised 1996).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tarchouli M, Elabsi M, Njoumi N,
Essarghini M, Echarrab M and Chkoff MR: Liver trauma: What current
management? Hepatobiliary Pancreat Dis Int. 17:39–44.
2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Chakraverty S, Flood K, Kessel D,
McPherson S, Nicholson T, Ray CE Jr, Robertson I and van Delden OM:
CIRSE guidelines: Quality improvement guidelines for endovascular
treatment of traumatic hemorrhage. Cardiovasc Intervent Radiol.
35:472–482. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Tignanelli CJ, Joseph B, Jakubus JL,
Iskander GA, Napolitano LM and Hemmila MR: Variability in
management of blunt liver trauma and contribution of level of
American College of Surgeons Committee on Trauma verification
status on mortality. J Trauma Acute Care Surg. 84:273–279.
2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bertens KA, Vogt KN, Hernandez-alejandro R
and Gray DK: Non-operative management of blunt hepatic trauma: Does
angioembolization have a major impact? Eur J Trauma Emerg Surg.
41:81–86. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Cimbanassi S, Chiara O, Leppaniemi A,
Henry S, Scalea TM, Shanmuganathan K, Biffl W, Catena F, Ansaloni
L, Tugnoli G, et al: Nonoperative management of abdominal
solid-organ injuries following blunt trauma in adults: Results from
an International Consensus Conference. J Trauma Acute Care Surg.
84:517–531. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
David Richardson J, Franklin GA, Lukan JK,
Carrillo EH, Spain DA, Miller FB, Wilson MA, Polk HC Jr and Flint
LM: Evolution in the management of hepatic trauma: A 25-year
perspective. Ann Surg. 232:324–330. 2000.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Carrillo EH, Platz A, Miller FB,
Richardson JD and Polk HC Jr: Non-operative management of blunt
hepatic trauma. Br J Surg. 85:461–468. 1998.PubMed/NCBI
|
|
8
|
Morrison JJ, Galgon RE, Jansen JO, Cannon
JW, Rasmussen TE and Eliason JL: A systematic review of the use of
resuscitative endovascular balloon occlusion of the aorta in the
management of hemorrhagic shock. J Trauma Acute Care Surg.
80:324–334. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Van den Esschert JW, van Lienden KP, Alles
LK, van Wijk AC, Heger M, Roelofs JJ and van Gulik TM: Liver
regeneration after portal vein embolization using absorbable and
permanent embolization materials in a rabbit model. Ann Surg.
255:311–318. 2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kozar RA, Moore JB, Niles SE, Holcomb JB,
Moore EE, Cothren CC, Hartwell E and Moore FA: Complications of
nonoperative management of high-grade blunt hepatic injuries. J
Trauma. 59:1066–1071. 2005.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Green CS, Bulger EM and Kwan SW: Outcomes
and complications of angioembolization for hepatic trauma: A
systematic review of the literature. J Trauma Acute Care Surg.
80:529–537. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Padia SA, Ingraham CR, Moriarty JM,
Wilkins LR, Bream PR Jr, Tam AL, Patel S, McIntyre L, Wolinsky PR
and Hanks SE: Society of Interventional radiology position
statement on endovascular intervention for Trauma. J Vasc Interv
Radiol. 31:363–369.e2. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Dabbs DN, Stein DM and Scalea TM: Major
hepatic necrosis: A common complication after angioembolization for
treatment of high-grade liver injuries. J Trauma. 66:621–627;
discussion 627-629. 2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Melloul E, Denys A and Demartines N:
Management of severe blunt hepatic injury in the era of computed
tomography and transarterial embolization: A systematic review and
critical appraisal of the literature. J Trauma Acute Care Surg.
79:468–474. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tamura S, Maruhashi T, Kashimi F, Kurihara
Y, Masuda T, Hanajima T, Kataoka Y and Asari Y: Transcatheter
arterial embolization for severe blunt liver injury in
hemodynamically unstable patients: A 15-year retrospective study.
Scand J Trauma Resusc Emerg Med. 29(66)2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Oh JS, Lee HG, Chun HJ, Choi BG and Choi
YJ: Evaluation of arterial impairment after experimental gelatin
sponge embolization in a rabbit renal model. Korean J Radiol.
16:133–138. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chun JY, Mailli L, Abbasi MA, Belli AM,
Gonsalves M, Munneke G, Ratnam L, Loftus IM and Morgan R:
Embolization of the internal Iliac artery before EVAR: Is it
effective? Is It Safe? Which technique should be used? Cardiovasc
Intervent Radiol. 37:329–336. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Luz JHM, Gomes FV, Coimbra E, Costa NV and
Bilhim T: Preoperative portal vein embolization in hepatic Surgery:
A review about the embolic materials and their effects on liver
regeneration and outcome. Radiol Res Pract.
2020(9295852)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Dabus G, Pizzolato R, Lin E, Kreusch A and
Linfante I: Endovascular treatment for traumatic scalp
arteriovenous fistulas: Results with Onyx embolization. J
Neurointerv Surg. 6:405–408. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kim SK, Chun HJ, Choi BG, Lee HG, Bae SH
and Choi JY: Transcatheter venous embolization of a massive hepatic
arteriovenous shunt complicating hepatocellular carcinoma using an
Amplatzer Vascular Plug. Jpn J Radiol. 29:156–160. 2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wolak ML, Murphy EC and Powell SZ:
Tumefactive cyst with a vascular blush as a late complication after
combined embolization and stereotactic radiosurgery treatments for
a cerebral arteriovenous malformation. Acta Neurochir (Wien).
149:705–712; discussion 712. 2007.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Derdeyn CP, Moran CJ, Cross DT, Dietrich
HH and Dacey RG Jr: Polyvinyl alcohol particle size and suspension
characteristics. AJNR Am J Neuroradiol. 16:1335–1343.
1995.PubMed/NCBI
|
|
23
|
de Baere T, Denys A and Paradis V:
Comparison of four embolic materials for portal vein embolization:
Experimental study in pigs. Eur Radiol. 19:1435–1442.
2009.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ellis H: Anatomy of the liver. Surgery
(Oxford). 29:589–592. 2011.
|
|
25
|
Broelsch CE, Emond JC, Thistlethwaite JR,
Whitington PF, Zucker AR, Baker AL, Aran PF, Rouch DA and Lichtor
JL: Liver transplantation, including the concept of reduced-size
liver transplants in children. Ann Surg. 208:410–420.
1988.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Virdis F and Reccia I, Di Saverio S, Kumar
J, Bini R, Pilasi C and Reccia I: Clinical outcomes of primary
angioembolisation in severe hepatic trauma: A systematic review of
the literature. Chin J Traumatol. 25:257–263. 2022.PubMed/NCBI View Article : Google Scholar
|