Introduction
Chordoid meningioma (CM) was first described by
Kepes et al (1) and is a
rare type of meningioma. It accounts for <0.5% of all meningioma
types (2). CM has an aggressive
clinical course and is classified to be a grade II meningioma
according to the World Health Organization (WHO). The recurrence
rate of the disease has been reported to be high, especially
following a subtotal resection (2-7).
CM is characterized by the presence of chordoma-like
histopathological features and lymphoplasmacytic infiltration
(2,8). With abundant chordoid elements, CMs
are composed of epithelioid or spindle cells, forming cords or
nests, in a pale and basophilic mucoid matrix (2,4,6). The
tumor cells are diffusely positive for epithelial membrane antigen
(EMA) and vimentin (9). The most
frequent symptoms of CM were headache or dizziness, followed by
visual or auditory impairment, limb weakness, and epilepsy
(10,11).
Ventricular meningiomas account for 0.5-5% of all
intracranial meningiomas (12,13).
However, intraventricular CM is rare, with only 18 cases reported
in the English-language literature as of 2022 (6,10,11,14-19).
Inflammation is uncommon in the majority of cases of ventricle CM
(17). To date, to the best of our
knowledge, only two cases of lateral ventricle CM presenting with
inflammatory syndrome have been reported (15,17),
comprising a male pediatric patient (age, 11 years) and an adult
female patient (age, 37 years). Therefore, the present case report
documents the first case of lateral ventricle CM with inflammatory
syndrome in an adult male.
Case report
A 28-year-old male was admitted to the Affiliated
Taian City Central Hospital of Qingdao University in January 2020
with a 7-day history of fever of unknown cause (body temperature,
37.5-38.3˚C) and a 3-day history of progressive headache, with
paroxysmal blurred vision in the right eye. The patient was a
resident of Taian City, Shandong Province, North China, without any
prior history of hematological or infectious diseases, or any
family history thereof. Physical examination indicated no
remarkable findings, whilst neurological examination revealed no
deficits. In addition, no visual acuity impairment or visual field
defect was recorded during the preoperative examination. Echo
scanning indicated no hepatosplenomegaly or lymphadenopathy. The
patient did not receive any intervention prior to hospitalization.
Laboratory findings revealed that the patient had inflammatory
syndrome with a C-reactive protein (CRP) levels of 58 mg/l (normal
range, 5-10 mg/l), an erythrocyte sedimentation rate (ESR) of 99
mm/h (normal range, 0-40 mm/h) and a white blood cell count of
16.78x109 cells/l (normal range, 4-10x109
cells/l). Routine blood examination indicated no anemia. In
addition, since routine urine examination suggested no
abnormalities, urinary tract infection was therefore excluded. A CT
scan of the head [performed with a Toshiba 64-slice CT-scanner
(Aquilion; Toshiba Medical Systems); imaging parameters: Voltage,
120 kV; tube current, 50 mA; scanning thickness, 1 mm] revealed a
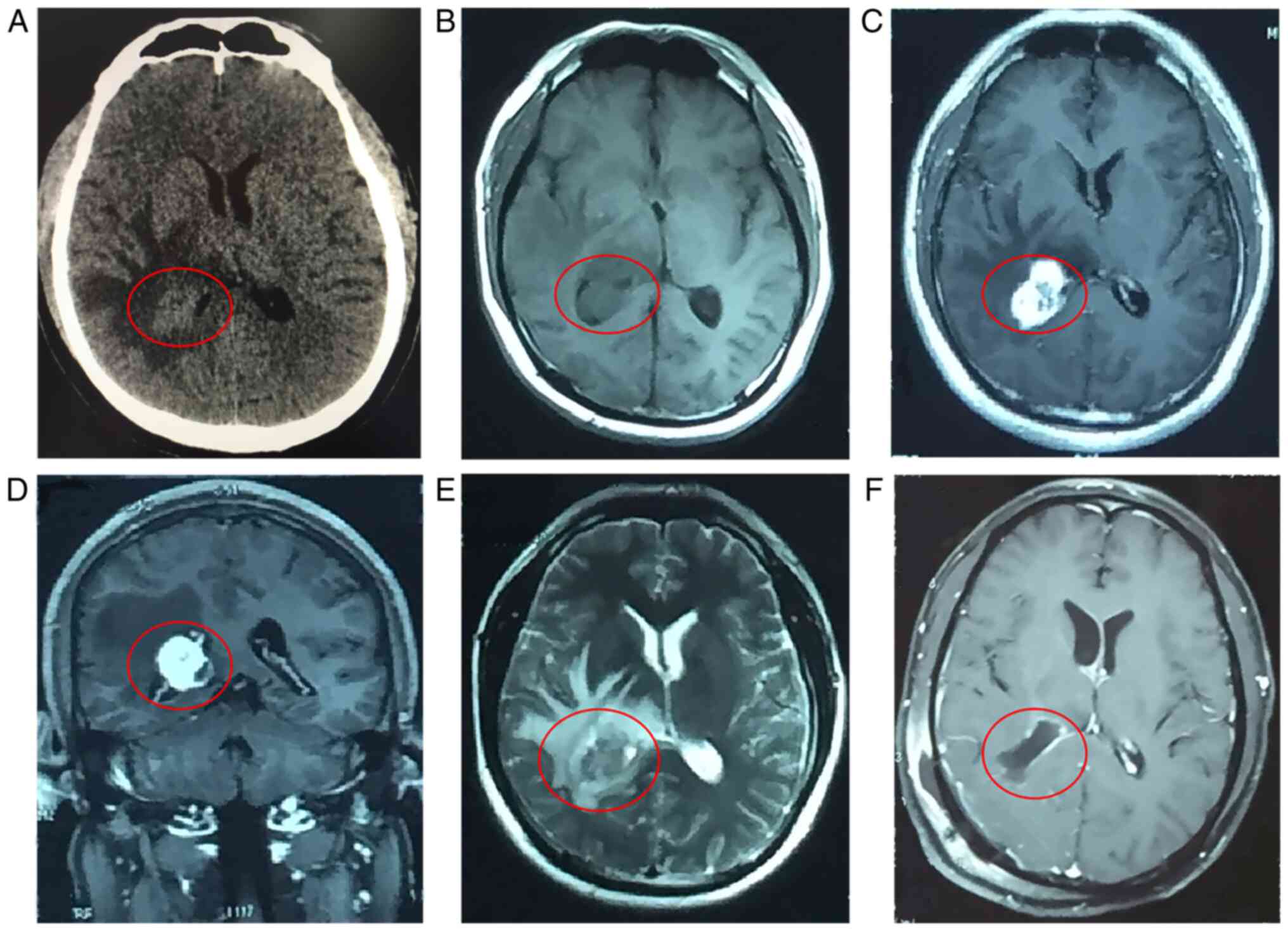
3.5-cm diameter tumor located in the right lateral ventricle
(Fig. 1A). In addition,
T1-weighted MRI images [acquired with a 3.0-T Philips Ingenia MRI
scanner (Philips Medical Systems); brain MRI with unenhanced and
contrast-enhanced T1 weighted: Repetition time (TR), 673 msec; echo
time (TE), 8.4 msec; T2 weighted: TR, 6,200 msec; TE, 123 msec],
slice thickness, 5 mm] displayed an isointense lesion that was
homogeneously enhanced following gadolinium administration
(Fig. 1B-D). On T2-weighted MRI
scans, the lesion appeared hyperintense and was surrounded by brain
edema (Fig. 1E). A preoperative
chest CT scan was also performed, revealing no abnormalities. The
patient provided written informed consent. As this was a single
case investigation, formal approval from the local ethics committee
was not required for this study.
Following hospitalization for 3 days, the lesion was
excised through a right transtrigone lateral ventricle approach.
The tumor was removed by Simpson grade I resection (Fig. 1F). Antiemetic (ondansetron, 4 mg
i.v.) was given as needed. 5% glucose dissolved in normal saline
was used as nutritional support. The patient was given appropriate
dehydration (mannitol, 150 ml twice a day for 5 days) due to
postoperative cerebral edema. During the postoperative period,
despite the absence of antibiotics, the patient exhibited gradual
improvement of the symptoms and remained afebrile, accompanied by a
decline in the ESR and CRP levels in addition to the white blood
cell count. On day 3 after surgery, the headache and blurred vision
disappeared. The laboratory values returned to normal 11 days after
surgery (Table I) and the patient
was discharged the following day.
 | Table IChanges in laboratory findings prior
to and after the operation. |
Table I
Changes in laboratory findings prior
to and after the operation.
| Laboratory
findings | Erythrocyte sediment
rate, mm/h (normal range, 0-20) | C-reactive protein,
mg/l (normal range, 5-10) | White blood cell
count, x109/l (normal range, 4-10) |
|---|
| Day 1 after
admission | 58 | 99 | 16.78 |
| Day 1 post-OP | 31 | 78 | 17.29 |
| Day 3 post-OP | 17 | 46 | 8.13 |
| Day 11 post-OP | 2 | 5 | 5.02 |
Histopathological examination of the excised tumor
was performed as follows: Tissue specimens were fixed in a 10%
buffered formaldehyde solution and embedded in paraffin wax.
Sections of all specimens (3 µm thickness) were cut and stained
with haematoxylin and eosin (H&E) according to a standard
protocol. After H&E staining, a camera was used to acquire
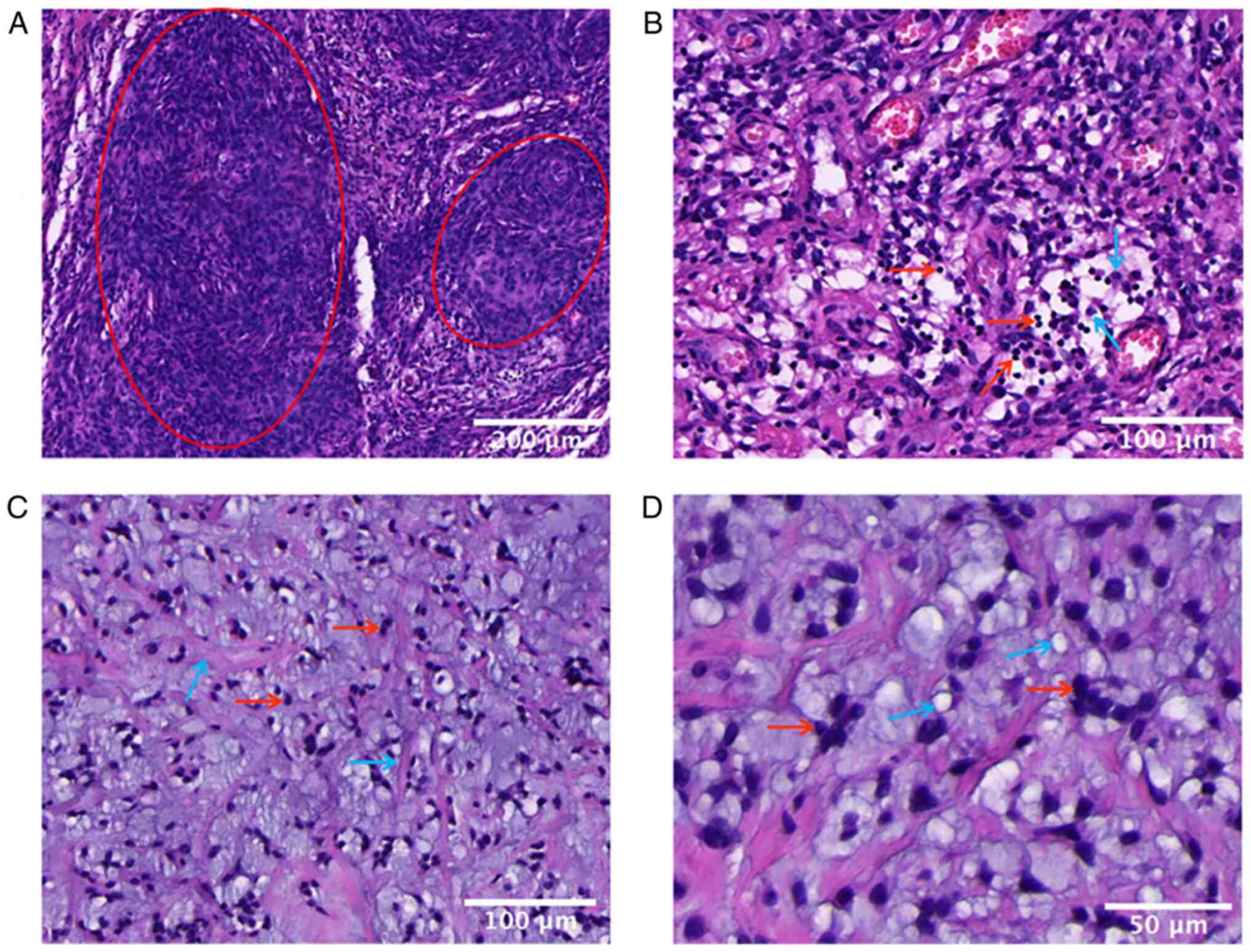
color images of the H&E staining. The histological analysis
revealed meningothelial cells arranged in characteristic cords
(Fig. 2A), with a large number of
lymphocytes and plasma cells around the tumor (Fig. 2B). In particular, the tumor cells
were embedded in a prominent myxoid background (Fig. 2C and D). Immunohistochemical analysis was then
performed as follows: Sections of freshly excised tissue were fixed
in 4% neutral buffered formalin, embedded in paraffin and sectioned
at 5-µm intervals. To retrieve antigens, sections were then placed
in sodium citrate buffer and heated in a pressure cooker for 3 min.
Endogenous peroxidase was blocked with 3% hydrogen peroxide for 10
min at room temperature. Sections were incubated with antibody
diluted in PBS at 4˚C overnight and then with the HRP-conjugated
secondary antibody at 37˚C for 30 min. Slides were visualized by
using 3,3'-diaminobezidine, counterstained with light hematoxylin,
dehydrated and mounted with coverslips. Immunohistochemical
staining was performed with the Envision technique using monoclonal
antibodies (Maixin Biological Technology) against EMA (1:50
dilution; cat. no. Kit-0011), glial fibrillary acidic protein
(GFAP; 1:100 dilution; cat. no. MAB-0769), S100 (1:300 dilution;
cat. no. Kit-0007), transcription termination factor 1 (TTF-1;
1:100 dilution; cat. no. MAB-0677), CD43 (1:200 dilution; cat. no.
MAB-0892) and Ki-67 (1:300 dilution; cat. no. MAB-0672) as primary
antibodies. The Elivision Super kit (Maixin Biological Technology)
was used for secondary antibody incubation and the DAB plus kit
(Maixin Biological Technology) was used to develop immunostaining.
Appropriate positive and negative controls for each antibody were
run in parallel. The Ki-67- stained sections were evaluated by an
experienced neuropathologist (HY). All counts were performed at a
magnification of 9,400 (field size, 0.16 mm2) and five
viable fields from the area of maximum labelling were chosen for
counting. Distinct nuclear Ki-67 staining of the tumor cells was
recorded as positive. The Ki-67 labelling index (Ki-67 LI) was
calculated as the percentage of Ki-67-positive tumor cells in the
evaluated area. Vascular components and haematogenous cells were
excluded, and evaluated areas also excluded necrotic, degenerate
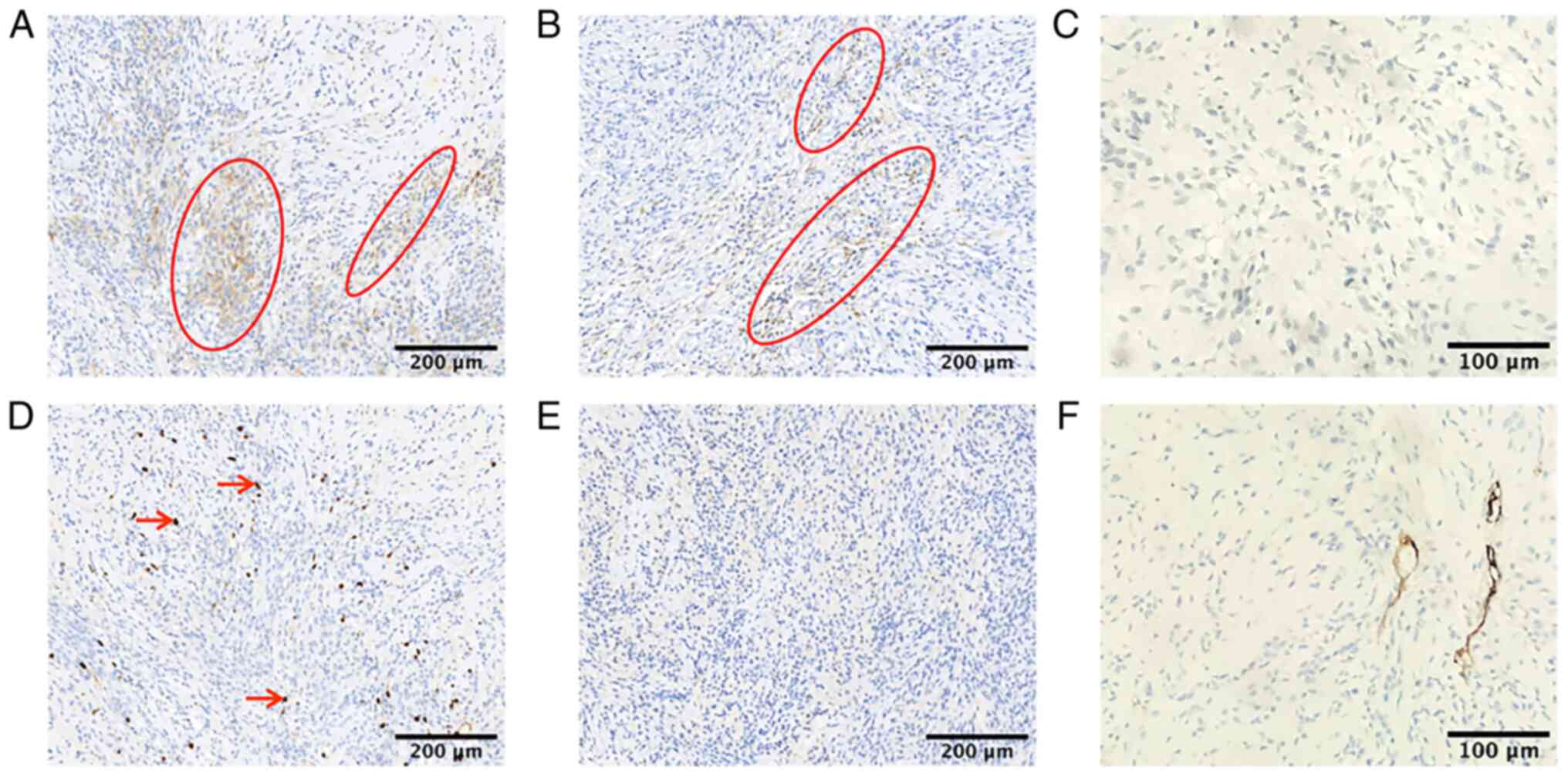
and poorly preserved areas. As presented in Fig. 3, immunohistochemistry revealed
focal cells with positive staining for EMA (Fig. 3A) and S100 protein (Fig. 3B). In addition, the Ki-67
proliferation index was <10% (Fig.
3D), whilst the tumor cells were negative for GFAP, TTF-1 and
CD34 (Fig. 3C, E and F).
This pathological examination led to the diagnosis of grade II CM
according to the WHO.
A follow-up was conducted 24 months after the
operation. At 24 months postoperatively, the patient had recovered
well and no longer suffered from fever, headache or blurred
right-eye vision. The patient did not experience any complications
or additional symptoms associated with the disease and the tumor
exhibited no signs of recurrence. Finally, the laboratory values
were in their normal ranges at 24 months after discharge.
Therefore, the patient received no further treatment, such as
adjuvant radiotherapy.
Since the symptoms of discomfort disappeared after
tumor resection. The last follow-up date was January 10, 2022, the
patient was able to have a normal life and he was satisfied with
the operation.
Discussion
CM is an atypical meningioma classified as a grade
II meningioma according to the WHO (20). It has been previously reported that
following the subtotal resection of CM, there is a high tendency
for recurrence (21). CM has a
higher incidence rate in middle-aged patients (3,18,22),
and is a type of meningioma that predominantly exhibits areas with
chordoma-like features (17). A
chordoma-like appearance and a meningothelial cell-like pattern in
certain areas of the tumor form the basis for the diagnosis of CM
(21). Histopathologically, CMs
are composed of fusiform-shaped epithelioid cells with eosinophilic
cytoplasm arranged in ‘chains and cords’ patterns, in a basophilic
extracellular matrix (23). The
pathological characteristics of CMs are relatively comparable with
several other tumor types, such as chordoid glioma, chordoma,
papillary ependymoma and epithelioid hemangioendothelioma. Chordoid
glioma exhibits strong positivity for GFAP expression (22). Chordomas originate from embryonic
notochordal remnants and are commonly located in the midline along
the spinal axis, and typically exhibit positive immunohistochemical
staining for cytokeratins, S100 and EMA (24), whilst the expression of nuclear
brachyury is considered a hallmark for the diagnosis. By contrast,
papillary ependymomas exhibit finger-shaped structures, which are
lined with a single layer of cuboidal cells with smooth surfaces
and positivity for GFAP expression (25). Intracranial epithelioid
hemangioendothelioma is characterized by the presence of
epithelioid tumor cells with prominent intracytoplasmic vacuolation
and typical disposition in the cords or nests in a myxoid stroma.
These endothelial cells tend to express the CD34 marker strongly
(26). Therefore, cytological
features accompanied by histological examinations are beneficial
for the diagnosis of CM.
The first cases of CM in children and adolescents
were reported by Kepes et al (1), which indicated that CM in the seven
pediatric cases was commonly associated with systemic symptoms,
such as iron-refractory hypochromic/microcytic anemia,
hepatosplenomegaly, bone marrow plasmacytosis and
dysgammaglobulinemia (Castleman syndrome), but not with fever.
However, the cause of this systemic inflammation in patients with
CM remains elusive. Denaro et al (27) suggested that the prominent
lymphoplasmacellular infiltration in CM may be the cause. In
previous case report, IL-6 was detected in samples derived from
patients with CM, suggesting that the manifestation of inflammation
may be associated with the excessive production of pyrogenic
cytokines (15,28). The present case report of CM in an
adult male patient provided histopathological evidence of chronic
lymphocytic infiltrates. Removal of the mass, which was able to
eliminate the source of pyrogenic cytokines, was able to explain
why the fever immediately subsided in this patient. Fever may be
one of the manifestations of tumors with inflammatory cell
infiltrations (2,8). Considering that in the present case,
the expression of pyrogens was not measured, their association with
systemic inflammation could not be verified. It is possible that
the pyrogens are secreted by the tumor itself, which is supported
by the alleviation of these manifestations after tumor resection.
Therefore, the febrile state may be largely caused by the tumor
itself.
The paroxysmal blurred vision disappeared on day 3
after the operation. In the present case, the cause of blurred
vision was difficult to explain. However, it may be due to the fact
that CM is able to compress a part of the optic radiation.
Gross total resection should be the goal for the
surgical management of primary CM, since the extent of resection is
considered to be the most significant prognostic factor for the
treatment of CM (7). In addition,
the extent of the resection remains to be the most effective
predictor of long-term tumor control rate (22). Since the risk of recurrence in
patients with CM is high, careful follow-up should be recommended
for each patient. When Simpson grade ≥II resection cannot be
achieved, adjuvant radiotherapy is recommended after surgery
(11,29,30).
In the present case, the immediate resolution of fever post-surgery
may be associated with the removal of the tumor, which may be the
main source of pyrogens.
Of note, the present study has certain limitations.
The expression of IL-6 or that of other types of pyrogens was not
determined. Furthermore, although not necessary for the diagnosis,
molecular analysis of the tumor, such as detection of 2p deletion
and DNA methylation profiling in meningioma cells, could've been
useful for verifying the presence of known molecular features of
this tumor or for identifying possible novel molecular alterations.
However, the appropriate equipment for detecting this was not
available in the hospital of the present case.
Intraventricular CM presenting with inflammatory
syndrome is exceptionally rare. To the best of our knowledge, the
present case report was the second to report a case of lateral
ventricle CM presenting with inflammation in an adult, but it was
the first case in a male adult patient. The tumor was removed by
Simpson grade I resection, leading to the immediate resolution of
fever and inflammatory syndrome.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SZ, GW and XC performed the operation. YH acquired
data and drafted the manuscript. YZ interpreted clinical findings
and revised the manuscript. HY completed the pathological
diagnosis. SZ and YH checked and confirm the authenticity of all
the raw data. All authors have read and approved the final version
of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient consented to the use of their clinical
information/data and images for the purpose of research and to
their publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kepes JJ, Chen WY, Connors MH and Vogel
FS: ‘Chordoid’ meningeal tumors in young individuals with
peritumoral lymphoplasmacellular infiltrates causing systemic
manifestations of the Castleman syndrome. A report of seven cases.
Cancer. 62:391–406. 1988.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Couce ME, Aker FV and Scheithauer BW:
Chordoid meningioma: A clinicopathologic study of 42 cases. Am J
Surg Pathol. 24:899–905. 2000.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Tena-Suck ML, Collado-Ortìz MA,
Salinas-Lara C, García-López R, Gelista N and Rembao-Bojorquez D:
Chordoid meningioma: A report of ten cases. J Neurooncol. 99:41–48.
2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lin JW, Ho JT, Lin YJ and Wu YT: Chordoid
meningioma: A clinicopathologic study of 11 cases at a single
institution. J Neurooncol. 100:465–473. 2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lui PC, Chau TK, Wong SS, Lau PP, Tse GM,
Thomas TM and Ng HK: Cytology of chordoid meningioma: A series of
five cases with emphasis on differential diagnoses. J Clin Pathol.
60:1024–1028. 2007.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Epari S, Sharma MC, Sarkar C, Garg A,
Gupta A and Mehta VS: Chordoid meningioma, an uncommon variant of
meningioma: A clinicopathologic study of 12 cases. J Neurooncol.
78:263–269. 2006.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Choy W, Ampie L, Lamano JB, Kesavabhotla
K, Mao Q, Parsa AT and Bloch O: Predictors of recurrence in the
management of chordoid meningioma. J Neurooncol. 126:107–116.
2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Di Ieva A, Laiq S, Nejad R, Schmitz EM,
Fathalla H, Karamchandani J, Munoz DG and Cusimano MD: Chordoid
meningiomas: Incidence and clinicopathological features of a case
series over 18 years. Neuropathology. 35:137–147. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mawrin C and Perry A: Pathological
classification and molecular genetics of meningiomas. J Neurooncol.
99:379–391. 2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Jie D, Liu Z, He W, Wang S, Teng H and Xu
J: Clinical features, radiological findings, and prognostic factors
for primary intracranial chordoid meningioma. Front Neurol.
13(1002088)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yang Y, Li D, Cao XY, Hao SY, Wang L, Wu Z
and Zhang JT: Clinical features, treatment, and prognostic factors
of chordoid meningioma: Radiological and pathological features in
60 cases of chordoid meningioma. World Neurosurg. 93:198–207.
2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Cushing H and Eisenhardt L: Meningiomas,
their classification, regional behaviour, life history, and
surgical end results. Springfield, IL, Charles C Thomas, 1938.
|
|
13
|
Rohringer M, Sutherland GR, Louw DF and
Sima AA: Incidence and clinicopathological features of meningioma.
J Neurosurg. 71:665–672. 1989.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Song KS, Park SH, Cho BK, Wang KC, Phi JH
and Kim SK: Third ventricular chordoid meningioma in a child. J
Neurosurg Pediatr. 2:269–272. 2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Arima T, Natsume A, Hatano H, Nakahara N,
Fujita M, Ishii D, Wakabayashi T, Doyu M, Nagasaka T and Yoshida J:
Intraventricular chordoid meningioma presenting with Castleman
disease due to overproduction of interleukin-6. Case report. J
Neurosurg. 102:733–737. 2005.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wilson JL, Ellis TL and Mott RT: Chordoid
meningioma of the third ventricle: A case report and review of the
literature. Clin Neuropathol. 30:70–74. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Nambiar A, Pillai A, Parmar C and Panikar
D: Intraventricular chordoid meningioma in a child: Fever of
unknown origin, clinical course, and response to treatment. J
Neurosurg Pediatr. 10:478–481. 2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sadashiva N, Poyuran R, Mahadevan A, Bhat
DI, Somanna S and Devi BI: Chordoid meningioma: A
clinico-pathological study of an uncommon variant of meningioma. J
Neurooncol. 137:575–582. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wind JJ, Jones RV and Roberti F: Fourth
ventricular chordoid meningioma. J Clin Neurosci. 17:1301–1303.
2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 world health organization
classification of tumors of the central nervous system: A summary.
Acta Neuropathol. 131:803–820. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wang XQ, Mei GH, Zhao L, Li ST, Gong Y,
Zhong J, Chen H and Jiang CC: Clinical features and treatment of
intracranial chordoid meningioma: A report of 30 cases.
Histopathology. 62:1002–1017. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hasegawa S, Yoshioka S, Urabe S and
Kuratsu J: Rapidly enlarging chordoid meningioma with abundant
mucin production. Neuropathology. 26:438–441. 2006.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Shih AR, Cote GM, Chebib I, Choy E,
DeLaney T, Deshpande V, Hornicek FJ, Miao R, Schwab JH, Nielsen GP
and Chen YL: Clinicopathologic characteristics of poorly
differentiated chordoma. Mod Pathol. 31:1237–1245. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Dohrmann GJ and Collias JC: Choroid plexus
carcinoma. J Neurosurg. 43:225–232. 1975.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ellis TS, Schwartz A, Starr JK and Riedel
CJ: Epithelioid hemangioendothelioma of the lumbar vertebral
column: case report and review of literature. Neurosurgery.
38:402–407. 1996.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Denaro L, Di Rocco F, Gessi M, Lauriola L,
Lauretti L, Pallini R, Fernandez E and Maira G: Pyrogenic cytokine
interleukin-6 expression by a chordoid meningioma in an adult with
a systemic inflammatory syndrome. Case report and review of the
literature. J Neurosurg. 103:555–558. 2005.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Cassereau J, Lavigne C, Michalak-Provost
S, Ghali A, Dubas F and Fournier HD: An intraventricular clear cell
meningioma revealed by an inflammatory syndrome in a male adult: A
case report. Clin Neurol Neurosurg. 110:743–746. 2008.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Prokopienko M, Wierzba-Bobrowicz T,
Grajkowska W, Stępień T and Sobstyl M: Chordoid meningioma. Case
report and review of the literature. Niger J Clin Pract. 25:1–4.
2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Tahta A, Genc B, Cakir A and Sekerci Z:
Chordoid meningioma: Report of 5 cases and review of the
literature. Br J Neurosurg. 37:41–44. 2023.PubMed/NCBI View Article : Google Scholar
|