Introduction
Cardiovascular diseases cause millions of
mortalities each year and remain the major causes of increased
mortality worldwide. Atherosclerosis is the leading cause of the
majority of cardiovascular diseases (1). Inflammation plays a key role in the
pathogenesis of vascular diseases, including atherosclerosis
(2). Persistent vascular
inflammation may lead to endothelial dysfunction and abnormal
expression of cell adhesion molecules, such as intercellular
adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1
(VCAM-1). In turn, the above effects can promote the adhesion of
mononuclear macrophages to vascular endothelial cells, which is
considered as a significant manifestation of early damage in
cardiovascular diseases, such as atherosclerosis (3,4).
Endothelial dysfunction, macrophage recruitment and inflammatory
cytokine circuits are critical steps in the development of
atherosclerotic diseases. Emerging evidence has suggested that
anti-inflammatory factors can prevent vascular dysfunction and
cardiovascular events (5,6). Lipopolysaccharide (LPS), a common
surface component of Gram-negative bacteria, is an effective
initiator of inflammatory responses. LPS plays a crucial role in
numerous inflammatory diseases via activating the NF-κB signaling
pathway, eventually leading to the release of excessive
inflammatory mediators from vascular endothelial cells. In
addition, LPS is considered to be a pivotal risk factor for
endothelial dysfunction (7-9).
Therefore, identifying novel drugs to inhibit LPS-induced
inflammation-related signaling pathways could improve the treatment
of atherosclerosis.
Epidemiology has shown that the Mediterranean diet
is associated with a reduced incidence of cardiovascular diseases
(10). Extra virgin olive oil
(EVOO), a nearly exclusive dietary fat in the Mediterranean diet,
is associated with general fitness benefits and a decreased
incidence of atherosclerotic diseases (11). Tyrosol
[2-(4-hydroxyphenyl)-ethanol], is the most abundant biophenol in
EVOO and has a broad range of biological effects, including
antioxidant, antiapoptotic and anti-inflammatory effects (12). In addition, tyrosol is also the
main active component of Rhodiola rosea, a plant that has
been extensively used in traditional Chinese medicine (13). A previous study showed that tyrosol
could exert salutary effects on inflammatory lung diseases via
inhibiting inflammatory responses and maintaining the alveolar
capillary barrier (14). Although,
current studies have reported the enhanced anti-inflammatory
activity of tyrosol, its anti-inflammatory effects and mechanisms
in umbilical vein endothelial cells (HUVECs) remain elusive.
Therefore, in the present study an in vitro inflammation
model was established in LPS-stimulated HUVECs to uncover the
underlying molecular mechanisms of tyrosol in atherosclerosis.
Materials and methods
Cell culture
Primary HUVECs and Tohoku Hospital Pediatrics-1
(THP-1) cells were purchased from Shanghai Zhong Qiao Xin Zhou
Biotechnology Co., Ltd. HUVECs were cultured in Endothelial Cell
Medium (cat. no. 1001; ScienCell Research Laboratories, Inc.),
according to the supplier's instructions. HUVECs between passages 4
and 6 were used for the experiments. THP-1 cells were grown in PRMI
1640 medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented
with 1% penicillin/streptomycin solution and 10% FBS (Gibco; Thermo
Fisher Scientific, Inc.).
Cell viability assay
Cell viability was assessed using a Cell Counting
Kit 8 (CCK-8) assay (Dojindo Laboratories, Inc.), according to the
manufacturer's instructions. Briefly, HUVECs were cultured in
96-well plates overnight and were then treated with different
concentrations of tyrosol (0.3, 0.5, 0.7, 1, 1.2, 1.5 and 2 mmol/l)
at 37˚C for 24 h. Subsequently, culture medium supplemented with 10
% CCK-8 reagent was added into each well and cells were cultured at
37˚C for an additional 1.5 h. The absorbance at a wavelength of 450
nm was measured using an enzyme labelling apparatus.
Cell adhesion assay
Cell adhesion assay was performed as previously
described (15). Briefly, HUVECs
were seeded into 12-well plates and cultured until reached ~90%
confluence. Prior to stimulation with LPS, cells were treated with
tyrosol (0.5 mM or 1 mM) at 37˚C for 4 h. THP-1 cells were then
labelled with the fluorescent pH indicator, BCECF-AM (5 µM; cat.
no. S1006; Beyotime Institute of Biotechnology), for 30 min and
were allowed to adhere on the endothelial cell monolayer at 37˚C
for 60 min. Following washing with PBS, residuary adherent THP-1
cells were analyzed with a fluorimeter (Tecan Group, Ltd.).
HUVECs wound healing assay
HUVECs at a density of 5x105 cells/well
were cultured in 6-well plates and grown until they reached 90%
confluence. The HUVECs monolayer was then scratched with a 200-µl
pipette tip, followed by rinsing with PBS and cell treatment with
LPS (100 ng/ml) or tyrosol (0.5 mM or 1 mM) at 37˚C for 12 h.
HUVECs were incubated in FBS-free Endothelial Cell Medium (cat. no.
1001; ScienCell Research Laboratories, Inc.) for 12 h and the cell
migration ability was assessed under an inverted microscope
(Olympus Corporation) in three randomly selected fields. Migratory
cells were analyzed using ImageJ software (version 1.5; National
Institutes of Health).
ELISA
The secretion levels of TNF-α and monocyte
chemotactic protein-1 (MCP-1) were measured using the corresponding
Human TNF-α ELISA kit (cat. no. EK0525) and Human MCP-1 ELISA kit
(cat. no. EK0441), according to the manufacturer's protocol (Wuhan
Boster Biological Technology, Ltd.).
Western blot analysis
For western blot analysis, total proteins were
extracted from HUVECs using RIPA Lysis and Extraction Buffer (cat.
no. 89900; Thermo Fisher Scientific, Inc.). The proteins were then
centrifuged at 14,000 x g, for 15 min at 4˚C and the protein
concentration was measured using a BCA protein assay kit (Beyotime
Institute of Biotechnology). Subsequently, 10-30 µg total
protein/sample was separated by 10% SDS-PAGE, followed by transfer
to PVDF membranes (cat. no. IPVH00010; MilliporeSigma). Following
blocking with 5% BSA for 1 h at room temperature, the membranes
were incubated with primary antibodies in a 4˚C refrigerator
overnight. The membranes were then washed thrice with TBS-1%
Tween-20 for 10 min each, followed by incubation with the
corresponding secondary antibodies at room temperature for 2 h. The
antibodies used were as follows: Mouse anti-ICAM-1 (dilution,
1:1,000; cat. no. ab20), rabbit anti-VCAM-1 (dilution, 1:1,000;
cat. no. ab181315), rabbit GAPDH (dilution, 1:1,000; cat. no.
ab181602; all from Abcam), rabbit anti-phosphorylated(p)-p65
(dilution, 1:1,000; cat. no. 3033S), rabbit anti-p65 (dilution,
1:1,000; cat. no. 8242T), rabbit anti-p-IκBα (dilution, 1:1,000;
cat. no. 2859T), mouse anti-IκBα (dilution, 1:1,000; cat. no.
4814S) (all from CST Biological Reagents Co., Ltd.) and horseradish
peroxidase-coupled secondary antibodies (dilution, 1:10,000; cat.
nos. ab6728 and ab6721; Abcam). Following detection with an ECL
reagent (Shanghai Yeasen Biotechnology, Co., Ltd.), ImageJ software
(version 1.5; National Institutes of Health) was used for
densitometric analysis. The relative expression levels of the
target proteins were normalized to those of GAPDH.
Dual luciferase reporter assay
HUVECs at a density of 104 cells/well
were seeded into 96-well plates and were then co-transfected with
100 ng NF-kB luciferase reporter plasmid (cat. no. YB003B; Shanghai
Yu Bo Biological Technology Co., Ltd.) and 10 ng pGMLR-CMV
luciferase Reporter plasmid (cat. no. 11558ES03; Shanghai Yeasen
Biotechnology, Co., Ltd.) using Lipofectamine® 3000
(cat. no. L3000015; Invitrogen; Thermo Fisher Scientific, Inc.). At
24 h post-transfection, cells were stimulated with 100 ng/ml LPS
with or without tyrosol (0.5 mM or 1 mM) at 37˚C for 24 h.
Luciferase activity was measured using the corresponding
Dual-Luciferase Assay kit (Shanghai Yeasen Biotechnology, Co.,
Ltd.). Renilla luciferase was used as internal
reference.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from HUVECs at a density of
5x105 cells/well using an RNA extraction kit
(EZBioscience). Subsequently, ~1 µg of total RNA was reverse
transcribed into cDNA using the Evo M-MLV Reverse Transcription kit
(cat. no. AG11705; Accurate Biology). The target genes ICAM-1,
VCAM-1 and the internal reference gene GAPDH were amplified by qPCR
using the 2X SYBR® Green Pro Taq HS Premix (cat. no.
AG11718; Accurate Biology) kit. The thermocycling conditions used
were as follows: For gDNA removal: 2 min at 42˚C, followed by
keeping at 4˚C; for reverse transcription PCR: 15 min at 37˚C and 5
sec at 85˚C, followed by keeping at 4˚C; and for qPCR: 95˚C for 30
sec, followed by 40 cycles at 95˚C for 5 sec and 60˚C for 30 sec,
followed by dissociation stage. All of the above steps were
performed according to the manufacturer's protocols. The primer
sequences used were as follows: VCAM-1, forward,
5'-GAGATACAACCGTCTTGG-3' and reverse, 5'-CCTTCACATAAATAAACCC-3';
ICAM-1, forward, 5'-ATGGCAACGACTCCTTCTC-3' and reverse,
5'-TGTCACCTCGGTCCCTTC-3'; and GAPDH, forward,
5'-TCTGACTTCAACAGCGACACC-3' and reverse,
5'-CTGTTGCTGTAGCCAAATTCGTT-3' (Generay Biotech Co., Ltd.). The
amplification data were quantified using the 2-ΔΔCq
method (16) and the relative
expression levels of target genes were then calculated. These
experiments were replicated in triplicate.
Flow cytometry (FCM) analysis
To promote the differentiation of THP-1 cells into
macrophages, cells were seeded into 6-well plates and were then
induced with 0.1 µg/ml phorbol myristate acetate (PMA) for at 37˚C
72 h. The suspended cells were washed away using PBS. Following
treatment of PMA-derived THP-1 cells with tyrosol at 37˚C for 4 h,
cells were then stimulated with 100 ng/ml LPS at 37˚C for 24 h,
followed by washing twice with staining buffer (BioLegend, Inc.).
Subsequently, cells were incubated with anti-human/mouse FITC-CD86
at room temperature for 20 min. After incubation, cells were
collected, washed and resuspended in cell staining buffer. The
percentage of CD86 positive cells was measured using FCM
(CytExpert; Beckman Coulter, Inc.) and the results were analyzed
using FlowJo v10.0.6 software (FlowJo LLC).
Statistical analysis
Each experiment was performed in triplicate and data
analysis and output were performed using GraphPad Prism 7 (GraphPad
Software; Dotmatics) software. All data are expressed as the mean ±
standard error of the mean. The differences among multiple groups
were analyzed by one-way ANOVA analysis followed by Tukey's post
hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Tyrosol inhibits LPS-induced
monocyte-endothelial cell adhesion
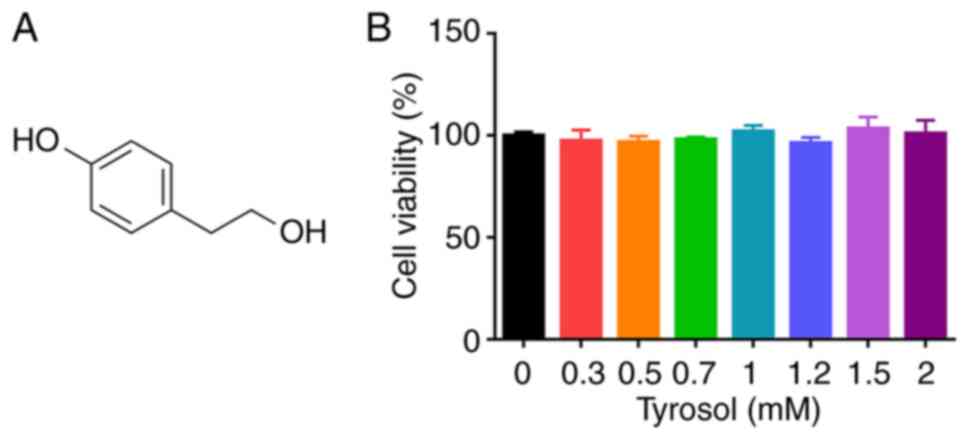
No statistically significant difference was observed
in the viability of HUVECs treated with 0-2 mM tyrosol (Fig. 1B). Eventually, the concentrations
of 0.5 and 1 mM were selected for the following experiments. It has
been reported that the induction of pro-inflammatory cytokines and
adhesion molecules during vascular inflammation can promote the
adhesion and infiltration of monocytes into the endothelium, thus
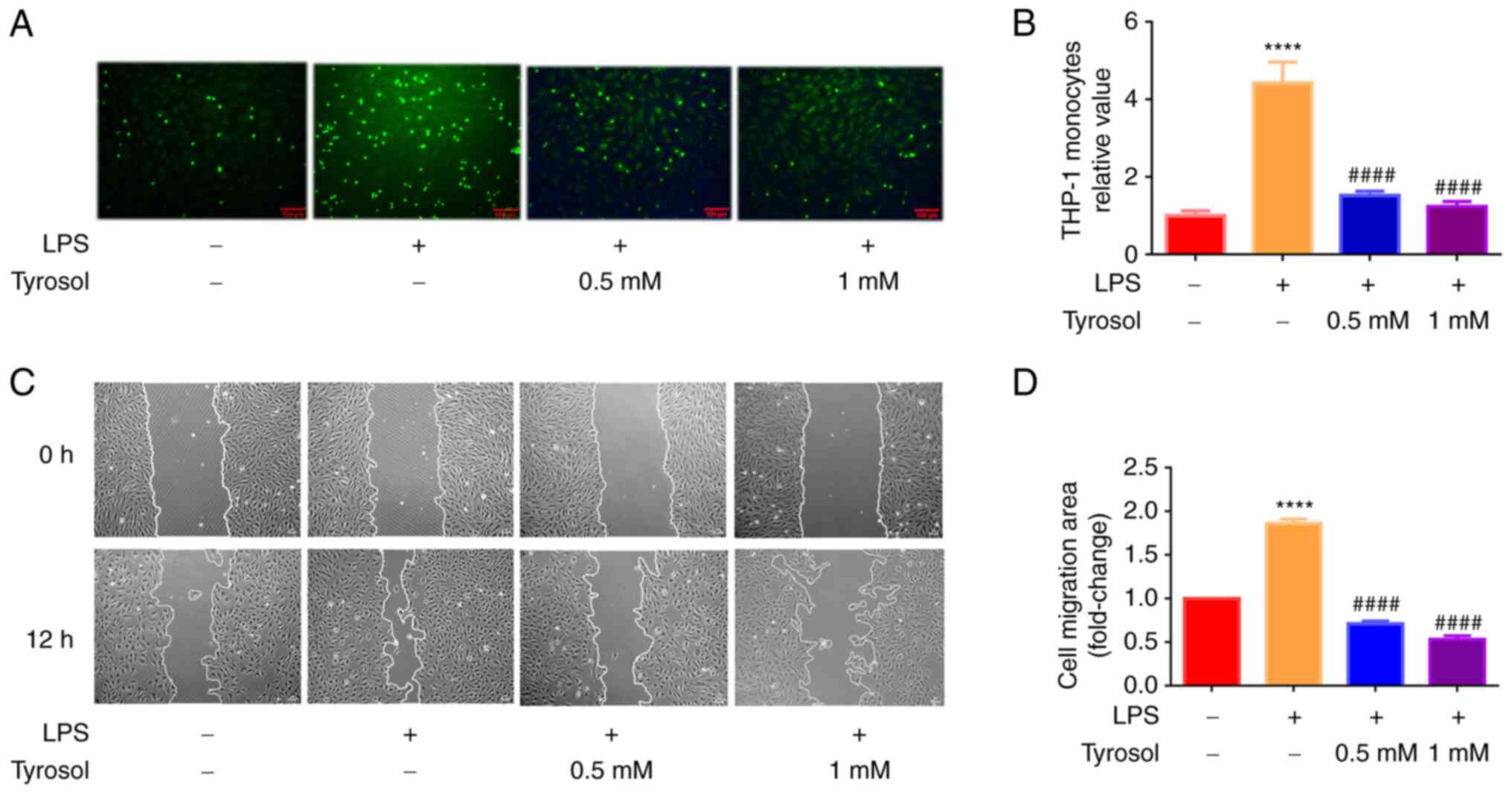
resulting in the occurrence of atherosclerosis (4). To assess the effect of tyrosol on
monocyte-endothelial cell interactions, the adhesion of monocytes
to HUVECs was evaluated by fluorescence labelling (Fig. 2A). As shown in Fig. 2B, when HUVECs were treated with 100
ng/ml LPS, the number of THP-1 cells adhering to HUVECs were
obviously increased to approximately 4.5-fold compared with the
control group. However, HUVECs pre-treated with tyrosol
significantly declined LPS-mediated enhanced cell adhesion. The
above results suggested that tyrosol could constrain the adhesion
of THP-1 cells to LPS-induced HUVECs.
Tyrosol attenuates LPS-induced HUVECs
migration
Emerging evidence has suggested that LPS, an
inflammatory mediator, induces HUVECs inflammation in vitro
(7-9).
To investigate the effect of tyrosol on cell migration, the HUVECs
migration ability was evaluated using wound healing assay.
Following cell induction with LPS for 12 h, the migration ability
of HUVECs was markedly enhanced (Fig.
2C). Following cell pre-treatment with tyrosol (0.5 and 1 mM)
for 12 h, the number of migrated cells was reduced and the scratch
area without cells was markedly increased compared with cells
treated with LPS alone (Fig. 2D).
The above findings indicated that tyrosol could restrain
LPS-induced HUVECs migration.
Tyrosol mitigates the LPS-induced
expression of adhesion molecules in HUVECs
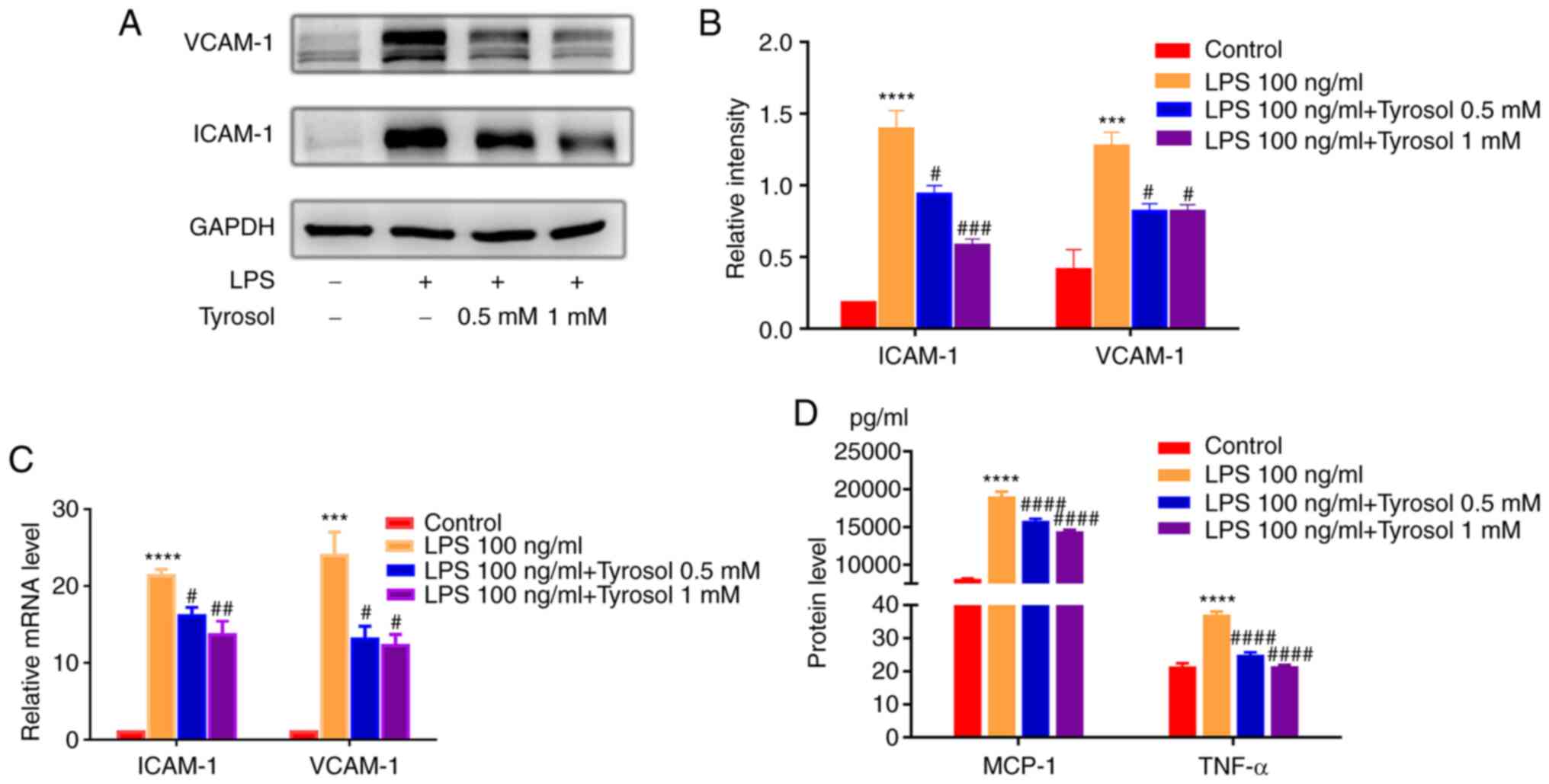
Subsequently, the expression levels of
adhesion-related molecules were detected in HUVECs to determine
whether tyrosol could exert a repressive effect on LPS-induced
endothelial cell inflammation. The protein expression levels of
ICAM-1 and VCAM-1 were also markedly decreased in tyrosol-treated
LPS-induced HUVECs (Fig. 3A and
B). Consistent with the western
blotting results, the relative mRNA expression levels of ICAM-1 and
VCAM-1 were also notably increased in LPS-treated HUVECs (Fig. 3C). However, cell pre-treatment with
tyrosol abrogated the effect of LPS on the mRNA expression levels
of the above genes.
Tyrosol ameliorates the LPS-induced
expression of pro-inflammatory cytokines
It has been reported that the excessive secretion of
cytokines and chemokines is closely associated with endothelial
dysfunction (3). In the present
study, the contents of TNF-α and MCP-1 in the culture supernatant
were significantly enhanced by ~1.7- and 2.4-fold, respectively, in
LPS-stimulated HUVECs. Notably, the LPS-induced secretion of TNF-α
and MCP-1 were markedly inhibited by tyrosol treatment (Fig. 3D).
Tyrosol attenuates the polarization of
LPS-induced macrophages to M1 phenotype
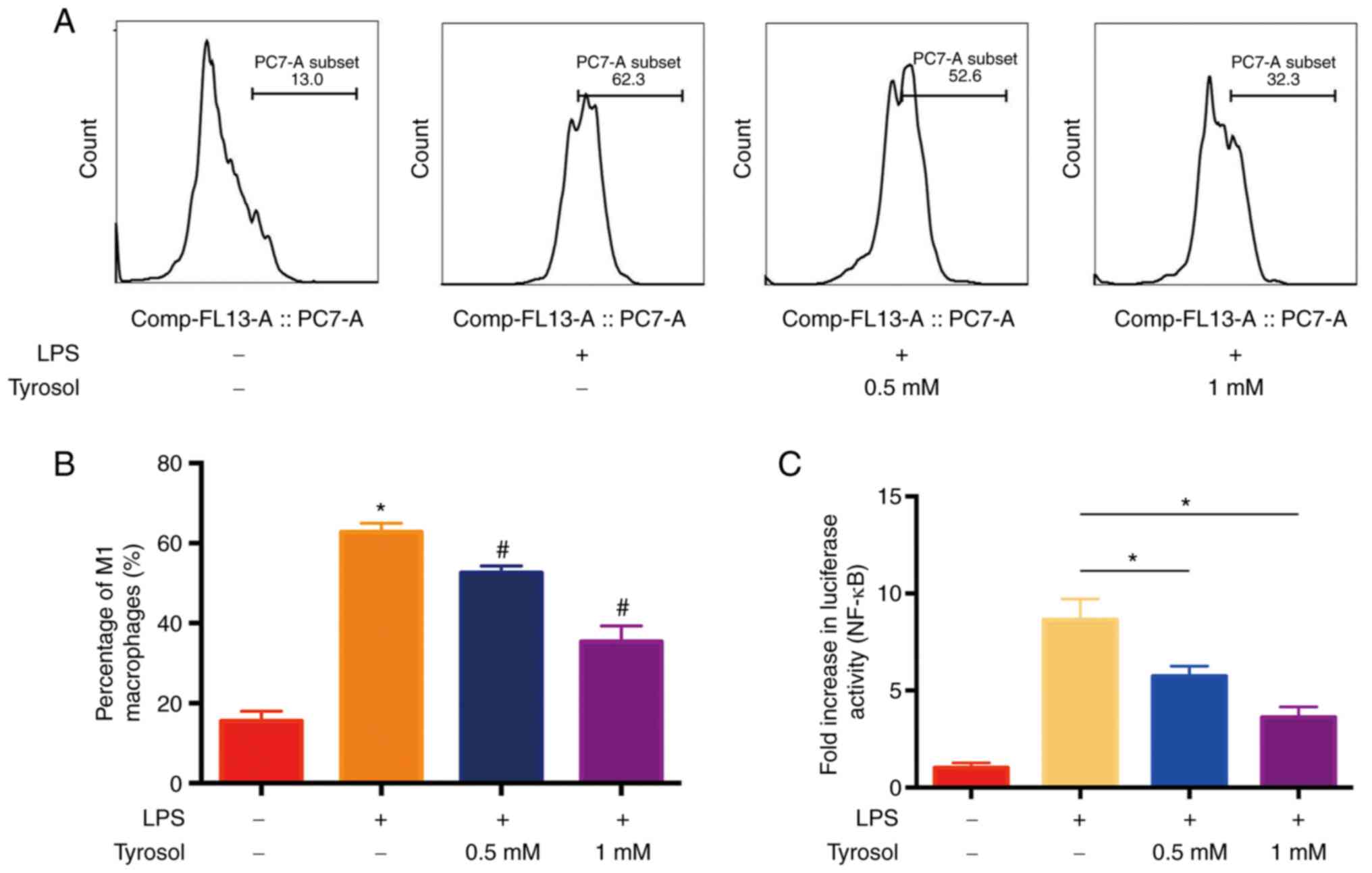
It has been reported that activated vascular
endothelial cells can accelerate circulating monocytes to enter the
subendothelial and differentiate into macrophages (3,4). In
addition, macrophages produce abundant cytokines and exacerbate
vascular inflammation. Therefore, to investigate whether tyrosol
could attenuate the polarization of macrophages to M1 phenotype,
the percentage of CD86 positive M1 cells was measured using FCM
analysis (Fig. 4A). As shown in
Fig. 4B, macrophage stimulation
with LPS alone promoted their polarization to M1 phenotype.
Additionally, the percentage of CD86-positive THP-1 macrophages was
increased by ~60%, which was dose-dependently reduced by
tyrosol.
Tyrosol suppresses the activity of
NF-κB promoter
ICAM-1 and VCAM-1 is a critical cell adhesion
molecules activated by NF-κB in HUVECs (9). The current study hypothesized that
tyrosol could promote the inactivation of NF-κB. Therefore, the
effect of LPS on the activity of NF-κB promoter was first assessed
using dual luciferase activity assay. More specifically, cell
treatment with LPS alone, notably augmented the luciferase activity
of NF-κB promoter by ~9-fold. However, the activity of NF-κB
promoter was diminished by ~6- and 4-folds following cell treatment
with 0.5 and 1 mM tyrosol, respectively (Fig. 4C).
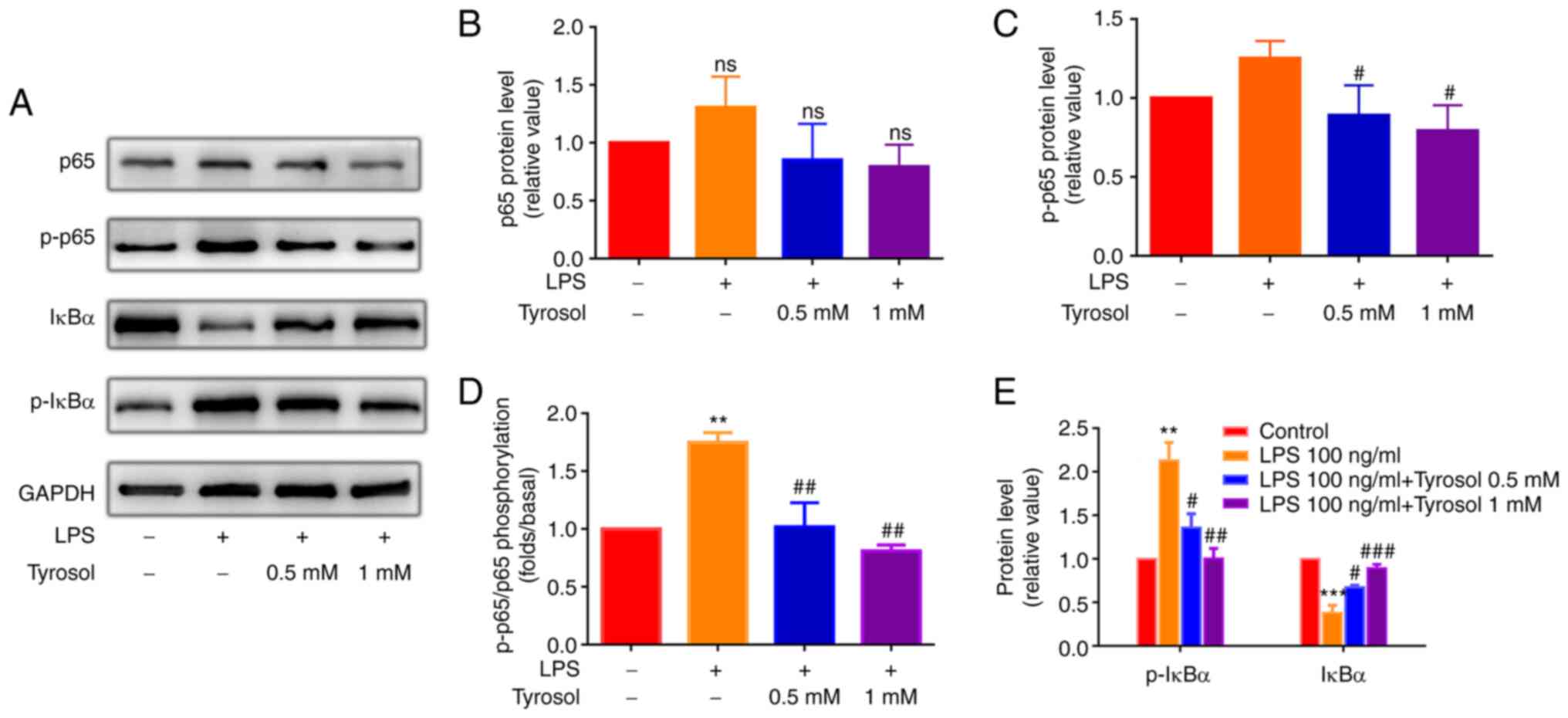
Tyrosol restrains the activation of
NF-κB induced by LPS
NF-κB is considered as the principal signaling
pathway involved in the regulation of inflammatory responses and
the expression of inflammatory genes in endothelial cells (9). Therefore, to explore the effects of
tyrosol on the NF-κB signaling pathway in LPS-treated HUVECs, the
expression levels of p-p65, p65, p-IκBα and IκBα were determined
using western blot analysis (Fig.
5A). No significant differences were observed in the total
levels of p65 (Fig. 5B), whereas,
the protein expression and phosphorylation levels of p-p65
(Fig. 5C and D) and p-IκBα (Fig. 5E) were notably enhanced in HUVECs
induced by LPS, which were restored in cells pre-treated 0.5 mM or
1 mM tyrosol. The aforementioned findings demonstrated that cell
treatment with tyrosol could inhibit the LPS-induced activation of
NF-κB signaling.
Discussion
It has been reported that vascular endothelial cells
serve a vital role in acute and chronic inflammatory responses
caused by injury or infection (17). Persistent vascular inflammation can
cause endothelial dysfunction, which in turn may result in the
development of vascular diseases, such as atherosclerosis (18). The inflammatory response in local
blood vessels commonly leads to atherosclerosis, which is a major
underlying cause of various cardiovascular diseases. In the present
study, HUVECs treatment with 0-2 mM tyrosol, isolated from EVOO or
Rhodiola rosea, had minor effect on cell viability. Mounting
evidence has suggested that monocyte adhesion, vascular endothelial
cell migration and macrophage polarization exert a crucial role in
the development of atherosclerosis (19). The results of the current study
demonstrated that tyrosol markedly reduced the adhesion of
monocytic THP-1 cells onto LPS-induced HUVECs, the migration
ability of endothelial cells and the conversion of macrophages into
M1 phenotype.
Interactions between cell adhesion molecules serve a
critical role in activating, promoting and maintaining macrophage
recruitment in the inflammatory vascular lesion zone (4). Therefore, drugs inhibiting the
expression of adhesion-related molecules and pro-inflammatory
cytokines could be a potent approach for treating inflammatory
vascular diseases. It has been also reported that the adhesion rate
of monocytes onto endothelial cells can be affected by
downregulation of VCAM-1 and ICAM-1, two major adhesion molecules
(15). Previous studies (2-4)
revealed that the upregulation of adhesion molecules could be
associated with the formation of atherosclerosis, while inhibition
of their expression could greatly attenuate aortic plaque
formation. Pro-inflammatory cytokines are a momentous pathogenic
factor involved in the progression of several diseases, including
atherosclerosis. In addition, a previous study showed that MCP-1
could serve a significant role in atherosclerosis via recruiting
immune cells to invade the arterial wall (20). The results of the present study
showed that tyrosol improved THP-1 cell adhesion onto HUVECs via
inhibiting the expression of adhesion-related molecules and
pro-inflammatory cytokines in LPS-induced HUVECs.
Inflammatory responses are present in all stages of
atherosclerosis (21). A study
demonstrated that pro-inflammatory signaling networks in vascular
endothelial cells could regulate the expression of adhesion
molecules and leukocyte cytokines by activating NF-κB (22). Notably, arterial endothelium at
sites prone to plaque formation, such as arterial branches and
non-laminar flow areas, promoted the structural activation of NF-κB
(23). Therefore, inhibition of
NF-κB signaling could prevent the development of atherosclerosis.
The results of the present study revealed that tyrosol could
suppress endothelial inflammation by inhibiting the NF-κB signaling
pathway. LPS activates NF-κB signaling via Toll-like receptors
(24). Therefore, once the NF-κB
signaling pathway is activated, IκBα dissociates from NF-κB and the
two molecules are then phosphorylated (24). Upon p65 dissociation, IκBα is
phosphorylated by IKK, followed by immediate ubiquitination and
degradation (25,26), thus resulting in the activation of
the distinct NF-κB signaling pathway. In the present study, western
blot analysis showed that cell treatment with tyrosol significantly
inhibited LPS-induced phosphorylation and subsequent degradation of
IκBα and phosphorylation of p65 in HUVECs, suggesting that tyrosol
could exert a protective effect against inflammation in endothelial
cells via inhibiting NF-κB signaling. The present study also aimed
to uncover the possible mechanisms underlying the effect of tyrosol
on regulating p65 expression. Therefore, the activity of p65
promoter was detected in tyrosol-treated HUVECs via subcloning
reporter gene elements into Renilla plasmid reporter
constructs, which were then transfected into HUVECs. The dual
luciferase assay showed that the activity of NF-κB promoter was
progressively reduced. However, further investigation is required
to verify whether there are binding sites for tyrosol on NF-κB
promoter.
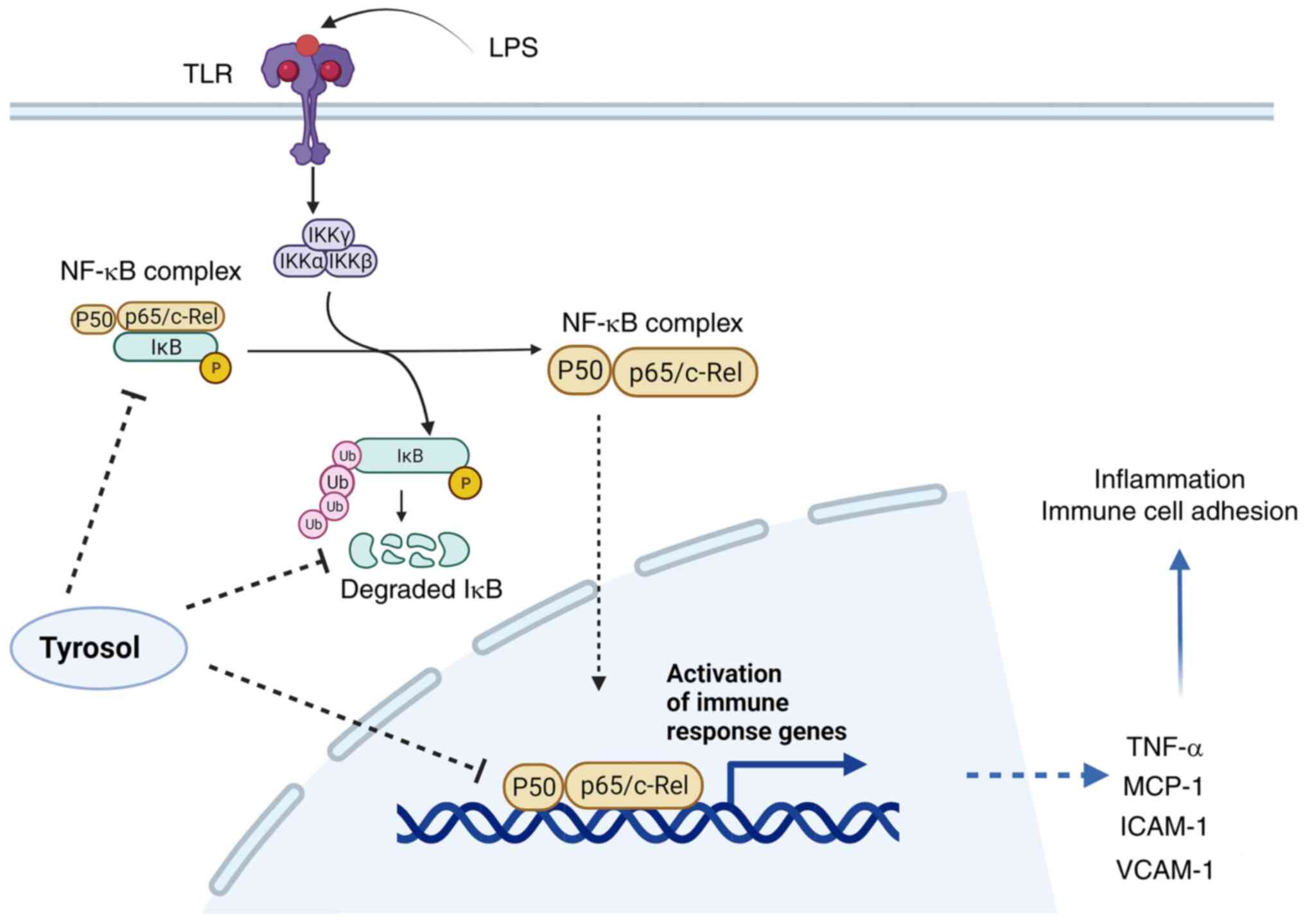
Collectively, the results of the current study
demonstrated that tyrosol could alleviate endothelial inflammatory
responses in vitro, which could be partially caused by
inhibition of NF-κB signaling activation. In addition, cell
treatment with tyrosol notably reduced the percentage of
LPS-induced M1 macrophages. The aforementioned findings could
provide novel insights into the role of tyrosol as a potential
therapeutic approach for treating several diseases associated with
endothelial inflammation (Fig. 6).
However, the present study had some limitations. The beneficial
effects of tyrosol were only investigated in a LPS-stimulated
endothelial cell culture model. The pathophysiology of
atherosclerosis is complex and therefore further investigations are
needed to clarify its underlying molecular mechanisms.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Clinical
Characteristics of Health System in Putuo District (grant no.
2020tszk01); 2021Shanghai University of Traditional Chinese
Medicine Graduate Student Innovation Cultivation Program (grant no.
Y2021055); Shanghai Health Commission Scientific Research Project
(grant no. 20204Y0154; 202240309); and Science and Technology
Innovation Projects in Shanghai Putuo District Health System (grant
no. ptkwws202003).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WZ, HW and TL contributed to the design of the
present study. WZ, HW, DS and TP performed the experiments and
analyzed the data. JL and WS drafted the manuscript and made
substantial contributions to conception and design. WZ and TL
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kobiyama K and Ley K: Atherosclerosis.
Circ Res. 123:1118–1120. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wolf D and Ley K: Immunity and
inflammation in atherosclerosis. Circ Res. 124:315–327.
2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Oates JC: Endothelial dysfunction in
injury and inflammation. Am J Med Sci. 349(2)2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tabas I and Bornfeldt KE: Macrophage
phenotype and function in different stages of atherosclerosis. Circ
Res. 118:653–667. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Moreira DM, da Silva RL, Vieira JL, Fattah
T, Lueneberg ME and Gottschall CA: Role of vascular inflammation in
coronary artery disease: Potential of anti-inflammatory drugs in
the prevention of atherothrombosis. Inflammation and
anti-inflammatory drugs in coronary artery disease. Am J Cardiovasc
Drugs. 15:1–11. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
van Bussel BC, Henry RM, Ferreira I, van
Greevenbroek MM, van der Kallen CJ, Twisk JW, Feskens EJ,
Schalkwijk CG and Stehouwer CD: A healthy diet is associated with
less endothelial dysfunction and less low-grade inflammation over a
7-year period in adults at risk of cardiovascular disease. J Nutr.
145:532–540. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wu C, Zhao W, Zhang X and Chen X:
Neocryptotanshinone inhibits lipopolysaccharide-induced
inflammation in RAW264.7 macrophages by suppression of NF-κB and
iNOS signaling pathways. Acta Pharm Sin B. 5:323–329.
2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yang L, Guo H, Li Y, Meng X, Yan L, Zhang
D, Wu S, Zhou H, Peng L, Xie Q and Jin X: Oleoylethanolamide exerts
anti-inflammatory effects on LPS-induced THP-1 cells by enhancing
PPARα signaling and inhibiting the NF-κB and ERK1/2/AP-1/STAT3
pathways. Sci Rep. 6(34611)2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
De Martin R, Hoeth M, Hofer-Warbinek R and
Schmid JA: The transcription factor NF-kappa B and the regulation
of vascular cell function. Arterioscler Thromb Vasc Biol.
20:E83–E88. 2000.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kris-Etherton P, Eckel RH, Howard BV, St
Jeor S and Bazzarre TL: Nutrition Committee Population Science
Committee and Clinical Science Committee of the American Heart
Association. AHA science advisory: Lyon diet heart study. Benefits
of a mediterranean-style, national cholesterol education
program/American heart association step I dietary pattern on
cardiovascular disease. Circulation. 103:1823–1825. 2001.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Nocella C, Cammisotto V, Fianchini L,
D'Amico A, Novo M, Castellani V, Stefanini L, Violi F and Carnevale
R: Extra virgin olive oil and cardiovascular diseases: Benefits for
human health. Endocr Metab Immune Disord Drug Targets. 18:4–13.
2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Mateos R, Sarria B and Bravo L:
Nutritional and other health properties of olive pomace oil. Crit
Rev Food Sci Nutr. 60:3506–3521. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Cui J, Guo T, Chao J, Wang M and Wang J:
Potential of the endophytic fungus phialocephala fortinii Rac56
found in rhodiola plants to produce salidroside and p-tyrosol.
Molecules. 21(502)2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kim YY, Lee S, Kim MJ, Kang BC, Dhakal H,
Choi YA, Park PH, Choi H, Shin TY, Choi HG, et al: Tyrosol
attenuates lipopolysaccharide-induced acute lung injury by
inhibiting the inflammatory response and maintaining the alveolar
capillary barrier. Food Chem Toxicol. 109:526–533. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhao S, Liang M, Wang Y, Hu J, Zhong Y, Li
J, Huang K and Li Y: Chrysin suppresses vascular endothelial
inflammation via inhibiting the NF-κB signaling pathway. J
Cardiovasc Pharmacol Ther. 24:278–287. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Leuenberger PM: Ultrastructure of the
ageing retinal vascular system, with special reference to
quantitative and qualitative changes of capillary basement
membranes. Gerontologia. 19:1–15. 1973.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Gimbrone MA Jr and García-Cardeña G:
Endothelial cell dysfunction and the pathobiology of
atherosclerosis. Circ Res. 118:620–636. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Gimbrone MA Jr, Obin MS, Brock AF, Luis
EA, Hass PE, Hébert CA, Yip YK, Leung DW, Lowe DG, Kohr WJ, et al:
Endothelial interleukin-8: A novel inhibitor of
leukocyte-endothelial interactions. Science. 246:1601–1603.
1989.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Olszak IT, Poznansky MC, Evans RH, Olson
D, Kos C, Pollak MR, Brown EM and Scadden DT: Extracellular calcium
elicits a chemokinetic response from monocytes in vitro and in
vivo. J Clin Invest. 105:1299–1305. 2000.PubMed/NCBI View
Article : Google Scholar
|
|
21
|
Fernandez DM and Giannarelli C: Immune
cell profiling in atherosclerosis: Role in research and precision
medicine. Nat Rev Cardiol. 19:43–58. 2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Baker RG, Hayden MS and Ghosh S: NF-κB,
inflammation and metabolic disease. Cell Metab. 13:11–22.
2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Brown JD, Lin CY, Duan Q, Griffin G,
Federation A, Paranal RM, Bair S, Newton G, Lichtman A, Kung A, et
al: NF-κB directs dynamic super enhancer formation in inflammation
and atherogenesis. Mol Cell. 56:219–231. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wang W, Deng M, Liu X, Ai W, Tang Q and Hu
J: TLR4 activation induces nontolerant inflammatory response in
endothelial cells. Inflammation. 34:509–518. 2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Karunakaran D, Nguyen MA, Geoffrion M,
Vreeken D, Lister Z, Cheng HS, Otte N, Essebier P, Wyatt H, Kandiah
JW, et al: RIPK1 Expression associates with inflammation in early
atherosclerosis in humans and can be therapeutically silenced to
reduce NF-κB activation and atherogenesis in mice. Circulation.
143:163–177. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Gilmore TD and Wolenski FS: NF-κB: Where
did it come from and why? Immunol Rev. 246:14–35. 2012.PubMed/NCBI View Article : Google Scholar
|