Introduction
Osteoporosis is considered to be one of the most
common metabolic bone diseases. It is generally believed that the
prevalence of osteoporosis in patients increases with age. The
incidence of osteoporotic fractures will increase due to the aged
tendency of population (1). Women
are usually more susceptible to osteoporosis after menopause due to
hormonal changes and have a higher incidence than men, but men have
a higher mortality rate from fracture-related disease (2,3).
Although there is significant interindividual variation in
polygenic diseases, we can identify many osteoporosis
susceptibility genes from genetic studies. Effective promotion of
bone regeneration can significantly improve the cure rate of
osteoporotic (4). Moreover, in
orthopedic clinical treatment, bone tissue engineering technology
with mesenchymal stem cells (MSCs) as the main raw material, with
access and in vitro expansion, can provide a solution for
clinical treatment of orthopedic diseases such as osteoporosis
(5,6).
Bone Morphogenetic Proteins (BMPs) are members of
the Transforming Growth Factor Beta (TGF-β), of which BMP9 has a
higher osteogenesis potential and better clinical application
prospects (7,8). However, while promoting MSCs
osteogenic differentiation, BMP9 also promotes their adipogenic
differentiation (9). Finding
effective targets to both promote BMP9-induced osteogenic
differentiation of MSCs and suppress their adipogenic
differentiation is the key to address tissue bone engineering for
the treatment of bone diseases such as osteoporosis.
The role of p53 as an oncogene in cancer has been
studied in depth, TP53 mutation leads to the occurrence of cancer
(10). Under normal conditions,
p53 is inactivated, and would get activated in response to extra-
or intra-cellular stress or impaired function (11). Moreover, exquisite regulation of
p53 functions such as post-translational modifications is also
critical for cell fate decision (12). Numerous studies demonstrated that
p53 is a key cell proliferation regulator, while its function goes
far beyond this. The active p53 regulates the expression of related
target genes and participates in regulation of DNA damage repair,
cell cycle, apoptosis, and metabolic (13). However, p53 in development, stem
cell differentiation, and non-tumor diseases still need further
investigation. In the process of tumor treatment, radiotherapy and
chemotherapy have negative effects on bone, and bone metastasis of
cancer itself will also destroy osteogenesis. Some data suggested
that p53 act as a sequence-specific transcription factor can be an
important regulator in cell differentiation (14-16).
The role of p53 in bone formation remains controversial. Some
studies found that p53 deficiency results in osteogenesis
inhibition (17,18), whereas other studies demonstrated
that p53 is a negative regulator of osteoblast differentiation and
skeletal development (19,20). Either view affirms the involvement
of p53 in the regulation of MSCs osteogenic differentiation.
The present study systematically analyzed the effect
of p53 on MSCs differentiation by detecting MSCs osteogenic and
adipogenic differentiation markers at different stages, and
preliminarily explored the molecular mechanisms related to TGF-β1.
Our finding provides a new experimental basis for promoting the
clinical development and application of p53 and BMP9 in improving
the therapeutic of bone diseases.
Materials and methods
Reagents
p53 inhibitor Pifithrin-α (PFTα) HBr (Cat# S2929),
and TGF-β1 inhibitor LY364947 (Cat# S2805) was purchased from
Selleck Chem.
Cell culture and transfection
C2C12, C3H10T1/2, 3T3-L1 and MG-63 cells originally
obtained from ATCC and mouse embryonic fibroblasts (MEFs) extracted
from a pregnant NIH mouse were generously provided by Professor
Tongchuan He (Medical Center of the University of Chicago). All
cells were immortalized and can be sub-cultured in medium of
Dulbecco's modified Eagle medium (Saimike, Cat#SMK200.01, China)
containing 10% fetal bovine serum (Cat#SMK110.01, Saimike, China).
The recombinant adenoviruses tagged with green fluorescent protein
(GFP) were designated as AdBMP9, Adp53, AdTGF-β1, while expressing
GFP only were used as vehicle control. All recombinant adenoviruses
were generously provided by Professor Tongchuan He, which were
generated previously using the AdEasy system. MSCs were seeded in
cell culture plates, and polybrene (5 µg/ml, HY-112735, MCE) was
added to give the final concentration of the culture medium when
the cells were adherent (approximately 3 h). Then the recombinant
adenoviruses were added and cultured at 37˚C, 5% CO2.
Eight hours after transfection was recorded as a starting point.
The virus titer of AdBMP9+, AdBMP9++, and AdBMP9+++ were MOI 10,
MOI 15, MOI 20, respectively. Transfections with AdBMP9, Adp53 and
AdTGF-β1 with MOI 15 were selected in the experiment.
Western blot
Cells were lysed in RIPA buffer (MilliporeSigma,
Cat# R0278) and heated to denature the protein. An equal amount of
protein was loaded onto 4-10% polyacrylamide gel and transferred to
a 0.45-µm PVDF membrane (Thermo Fisher Scientific, Inc., Cat#
IPVH00010), then blocked with 5% (w/v) bovine serum albumin. The
membranes were incubated with the primary antibodies at 1:1,000
dilution, respectively [rabbit anti-p53 (Affinity Biosciences Ltd.,
Cat# AF0879), anti-p-p53 (p53-18) (Santa Cruz Biotechnology, Inc.,
Cat# sc-13580), mouse anti-GAPDH (ProteinTech Group Inc., Cat#
60004-1-Ig), mouse anti-RUNX2 (Santa Cruz Biotechnology, Inc., Cat#
sc-390351), mouse anti-OPN (Santa Cruz Biotechnology, Inc., Cat#
sc-21742), rabbit anti-PPARγ (Affinity Biosciences Ltd., Cat#
AF6284) in primary antibody diluent (Beyotime Institute of
Biotechnology, Cat# P0256)]. The membranes were washed in TBST and
probed with secondary antibodies, then washed in TBST for three
times and scanned using the CLiNX Scan Image system (CLiNX Science
Instruments).
Reverse transcription-quantitative PCR
(RT-qPCR)
Cells were lysed in TRIzol (Invitrogen, Cat #
15596026), and the total RNA was extracted. After measuring the
concentration with Nanodrop™ One (Thermo Fisher Scientific, Inc.),
the reverse transcription reaction was performed (Takara Bio, Inc.,
Cat# RR037A). RT-qPCR was performed with the above cDNA using
primers (Table I) and SYBR-Green
qPCR mix (Bimake, Cat# b21202).
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Gene | Species | Forward, 5'-3' | Reverse, 5'-3' |
|---|
| p53 | Mouse |
AGAGACCGCCGTACAGAAGA |
CTGTAGCATGGGCATCCTTT |
| Runx2 | Mouse |
GCCGGGAATGATGAGAACTA |
GGACCGTCCACTGTCACTTT |
| OPN | Mouse |
TGCACCCAGATCCTATAGCC |
CTCCATCGTCATCATCATCG |
| PPARγ | Mouse |
TTTTCAAGGGTGCCAGTTTC |
AATCCTTGGCCCTCTGAGAT |
| β-actin | Mouse |
TGCTGACAGGATGCAGAAGG |
CGGACTCATCGTACTCCTGC |
| TP53 | Human |
GGCCCACTTCACCGTACTAA |
GTGGTTTCAAGGCCAGATGT |
| β-actin | Human |
CCACCATGTACCCTGGCATT |
CGGACTCGTCATACTCCTGC |
Alkaline phosphatase (ALP)
staining
ALP activity was detected by a BCIP/NCT ALP assay
kit (Beyotime Institute of Biotechnology, Cat# C3206). Cells were
seeded in well plates with factors and stained on the day 5 and 7
protected from light, scanned and captured using a microscope
(Olympus Corporation).
Alizarin red S (AZR) staining
Calcium mineralization was detected using an AZR
assay kit (Saimike, Cat# SV0019). Cells treated with factors were
cultured in well plates, and osteogenic medium was replaced 2 days
later with DMEM supplemented with 5% FBS, dexamethasone (10 nM),
glycerol-2-phosphate (10 mM), and vitamin C (50 µg/ml). On day 21,
the plates were stained, scanned, captured using a microscope
(Olympus Corporation) and quantified using ImageJ software.
Oil red O (ORO) staining
Cells treated with factors were fixed and stained
using an ORO assay kit (Saimike, Cat# SR0007). On day 10, the
plates were stained, scanned, captured using a microscope (Olympus,
Japan) and quantified using ImageJ software.
Statistical analysis
Datasets presented in this study can be found in the
GEO datasets (GSE35959_RAW). All data were expressed in the form of
means ± standard deviation (SD) analyzed using GraphPad prism 8.0
software. The data in each group are obtained from three
independent experiments of biological replicates at least.
Differences between groups were evaluated by one-way analysis of
variance (ANOVA) followed by Bonferroni post hoc test. P<0.05
was considered to indicate a statistically significant difference.
Adobe Illustrator 5 software was used to generate the figures.
Results
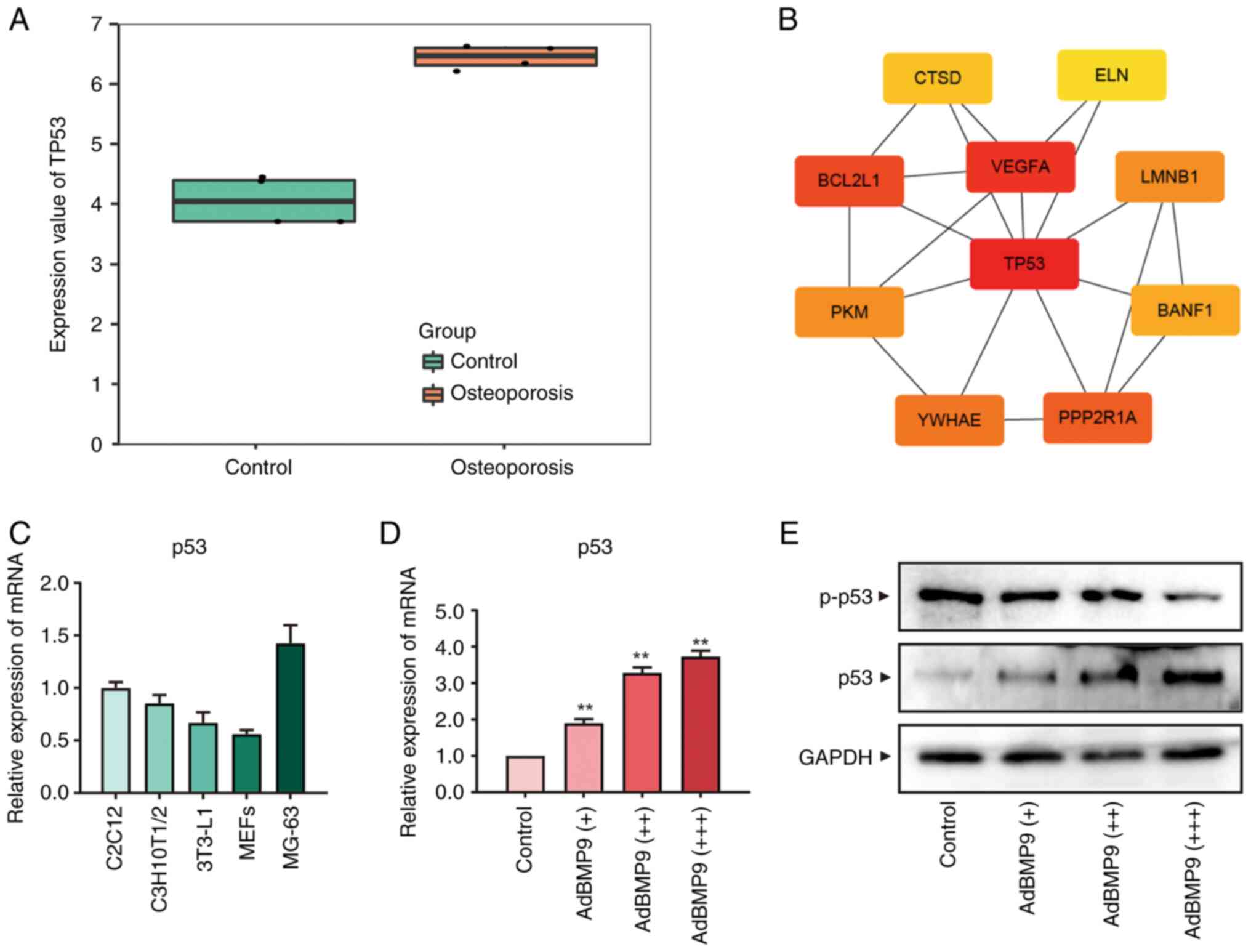
Expression of p53 induced in
progenitor cells
Transcriptome sequencing of MSCs from osteoporotic
and non-osteoporotic patient groups showed that TP53 was expressed
at higher levels in MSCs from osteoporotic patients Fig. 1A. The results of differential
expression gene analysis showed that TP53 had a high correlation
among the top 10 core central genes found in this model gene
screening (Fig. 1B).
Bioinformatics results suggested a high correlation between TP53
and osteoporosis. To examine the effect of p53 on MSCs
differentiation at the cellular level, we examined the expression
of p53 in several MSCs, including C2C12, C3H10T1/2, 3T3-L1, MEFs,
and MG-63 cells. We found that p53 were expressed in all of the
above MSCs with different degrees of differentiation (Fig. 1C). Herein, progenitor cell line
C3H10T1/2 cells were selected, which are less differentiated, have
relatively high p53 expression and are widely used for the present
study (21). Given the strong
ability of BMP9 to promote the osteo-differentiation of MSCs, this
experiment was conducted on the basis of BMP9(22). MSCs were treated with different
titers of BMP9 (Fig. S1A) to
detect the gene expression and protein level of p53 and
phosphorylation of p53. As shown in the Fig. 1D, mRNA expression and protein level
of p53 were significantly increased in MSCs at 24 and 48 h with
increasing BMP9 titers, whereas phosphorylation of p53 were
decreased (Fig. 1E). These results
suggested that p53 may work with BMP9 to regulate the osteogenic
potential of MSCs.
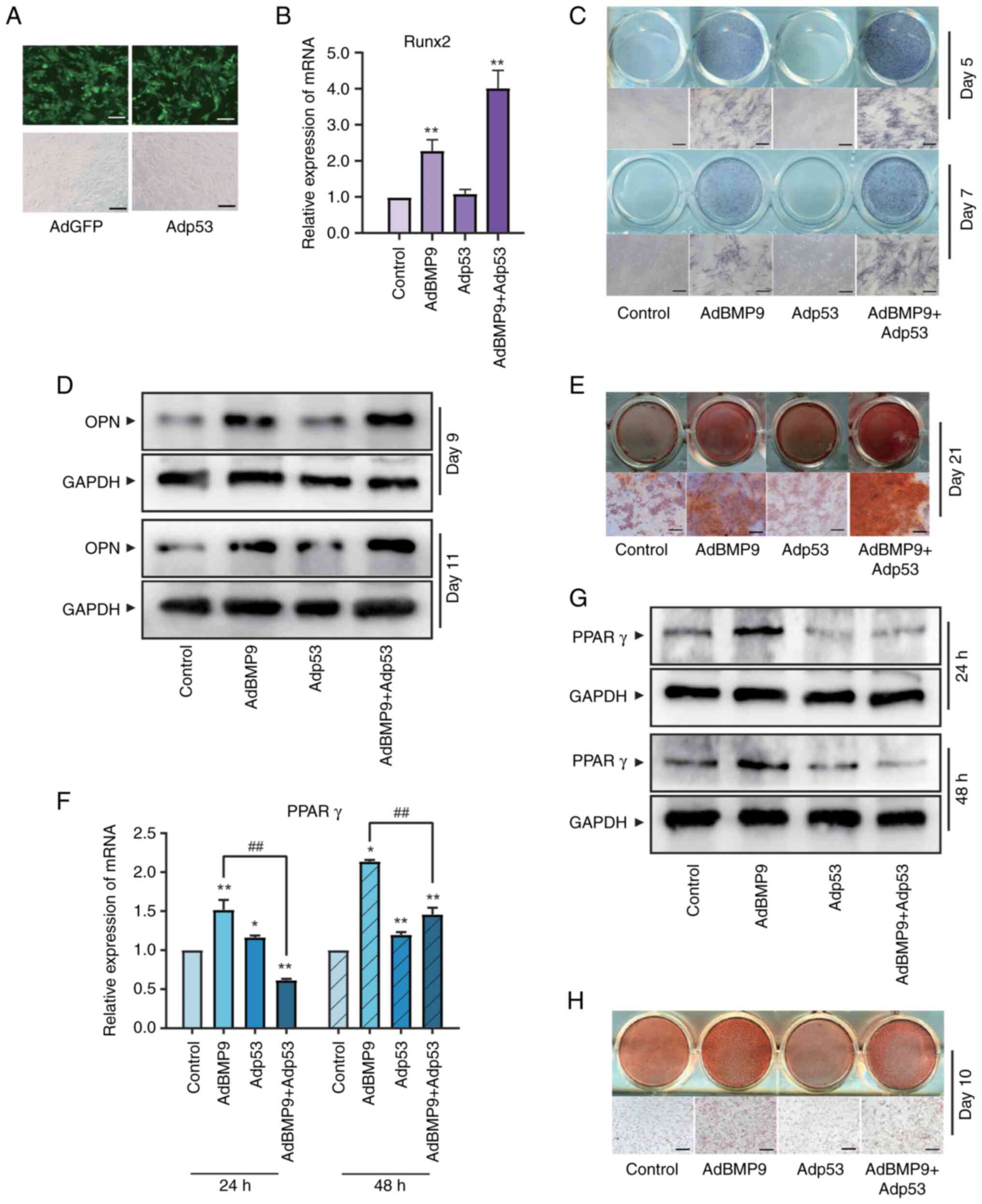
Effects of a p53-specific inhibitor on
osteogenic and adipogenic markers induced by BMP9 in MSCs
The possible relationship between BMP9-induced
osteoblast differentiation and p53 were analyzed. Recombinant
adenoviral vector Adp53 for p53 was constructed and validated
firstly (Figs. 2A and S1B). MSCs were sequentially tested for
markers of osteogenic/lipogenic differentiation at different
stages. Runt-related transfection factor 2 (Runx2), an early marker
of MSCs osteogenic differentiation (23), can be upregulated by a variety of
osteogenic factors. The induction of Runx2 by BMP9 was increased by
overexpression of p53 (Fig. 2B).
Alkaline phosphatase (ALP) is a stable and sensitive intermediate
marker of osteogenic differentiation in MSCs (24). On day 5 and 7, ALP activity in
BMP9-induced MSCs were promoted by Adp53 (Fig. 2C). Osteopontin (OPN) is an
essential bone matrix protein closely associated with bone
formation and development (25).
Western blotting on day 9 and 11 showed that Adp53 increased the
protein expression level of OPN in BMP9-induced MSCs (Fig. 2D). Alizarin Red S staining
(26) showed that p53
significantly increased calcium salt deposition (Fig. 2E). The above results suggested that
p53 contributes to the osteogenic differentiation of MSCs induced
by BMP9. Nevertheless, BMP9 promotes osteogenic differentiation in
MSCs while also inducing lipogenic differentiation.
The increased intracellular lipid accumulation that
occurs during normal physiological differentiation leads to
activation of p53, which inhibits lipogenesis by repressing the key
lipogenic transcription factor PPARγ to maintain homeostasis in
vivo (27). PPARγ were used as
an early lipogenic marker to observe the effect of p53 on
adipogenic differentiation in MSCs (28). Both RT-qPCR as well as western
blotting results demonstrated that p53 promotes the mRNA and
protein levels of the early-stage adipogenic marker PPARγ (Fig. 2F and G). Moreover, the induction of later-stage
adipogenic marker droplet formation of BMP9-induced MSCs measured
by oil red O staining (29) were
significantly inhibited by p53 (Fig.
2H). Taken together, p53 in promoting BMP9-induced MSCs
osteogenic differentiation can be accompanied by inhibition of
their adipogenesis.
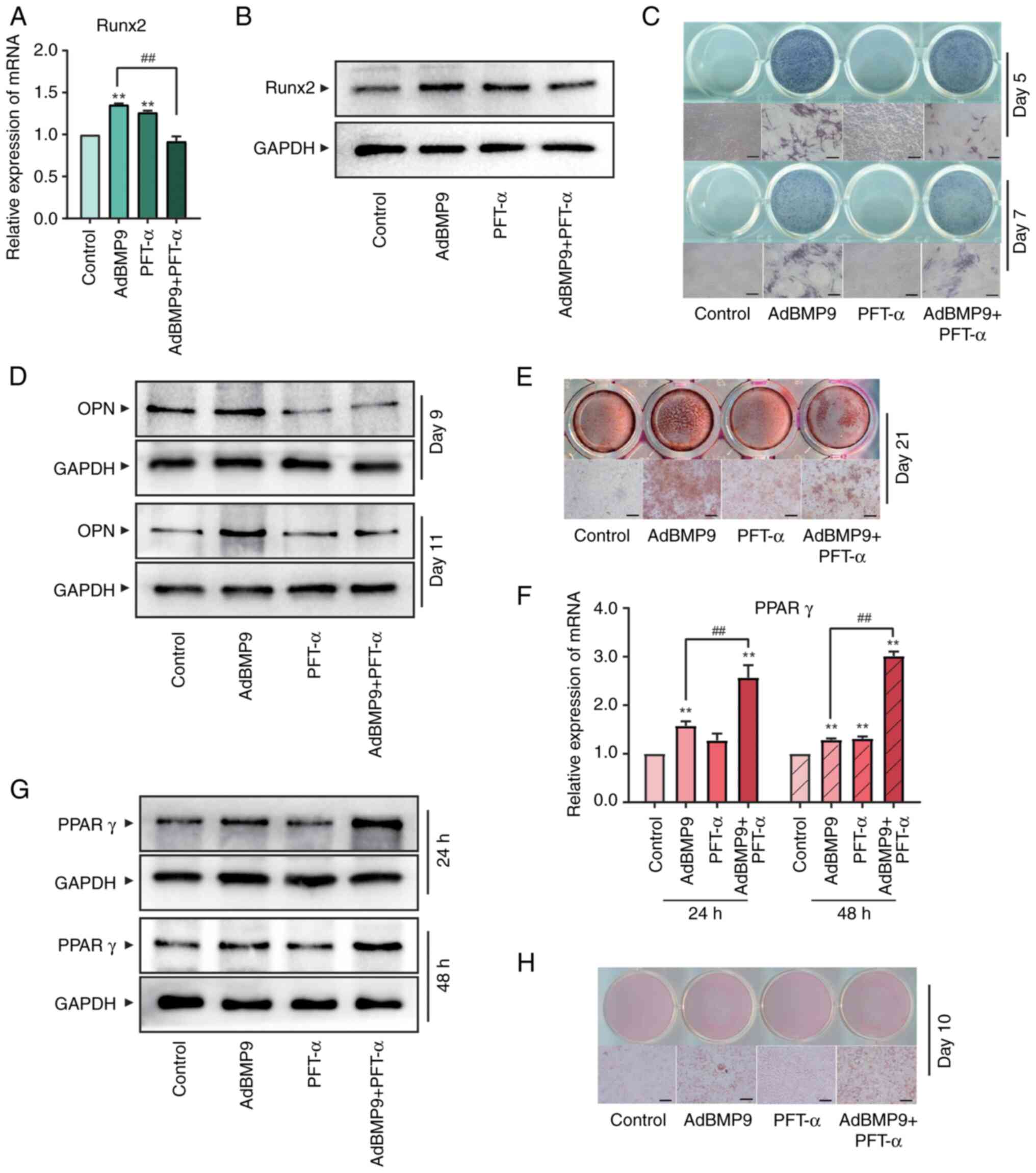
Effects of a p53-specific inhibitor on
osteogenic and adipogenic markers induced by BMP9 in MSCs
To further investigate the effect of p53 in
BMP9-induced MSCs differentiation, we used PFT-α, a selective
inhibitor of p53(30), to perform
assays from the opposite perspective. RT-qPCR and western blotting
showed that Runx2, an early marker of osteogenic differentiation,
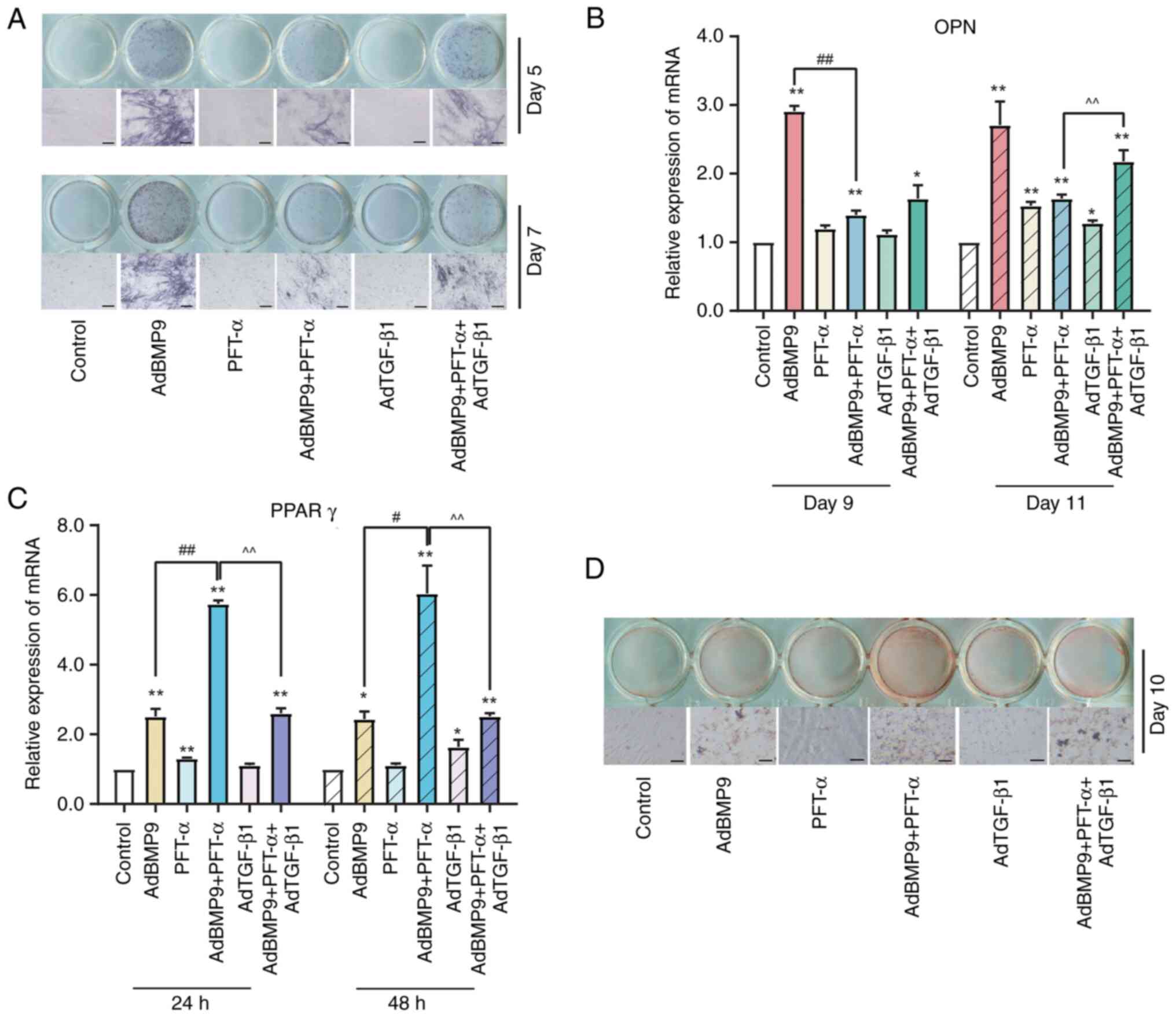
were suppressed by PFT-α (15 µM) in BMP9-induced MSCs (Fig. 3A and B). ALP staining further confirmed that
ALP activity of MSCs was reduced by PFT-α significantly (Fig. 3C). Osteopontin (OPN), an advanced
marker closely related to bone formation, also showed the
suppression role of PFT-α (Fig.
3D). In addition, MSCs were cultured in osteogenic induction
medium to evaluate the matrix mineralization of osteoblasts. As
shown in Fig. 3E, the formation of
alizarin red-positive nodules treatment with PFT-α significantly
were restrained. Taken together, these findings indicated that
PFT-α reduced the osteogenic potential of BMP9 in MSCs. In terms of
MSCs adipogenic differentiation, both early-stage marker PPARγ
expression (Fig. 3F and G) and later-stage marker oil red O
staining (Fig. 3H) showed that the
formation of BMP9-induced markers of MSCs adipogenic
differentiation were facilitated by PFT-α. Collectively, these data
suggested that silencing p53 disrupts the bone-lipid homeostasis in
MSCs and shifts them towards adipogenesis.
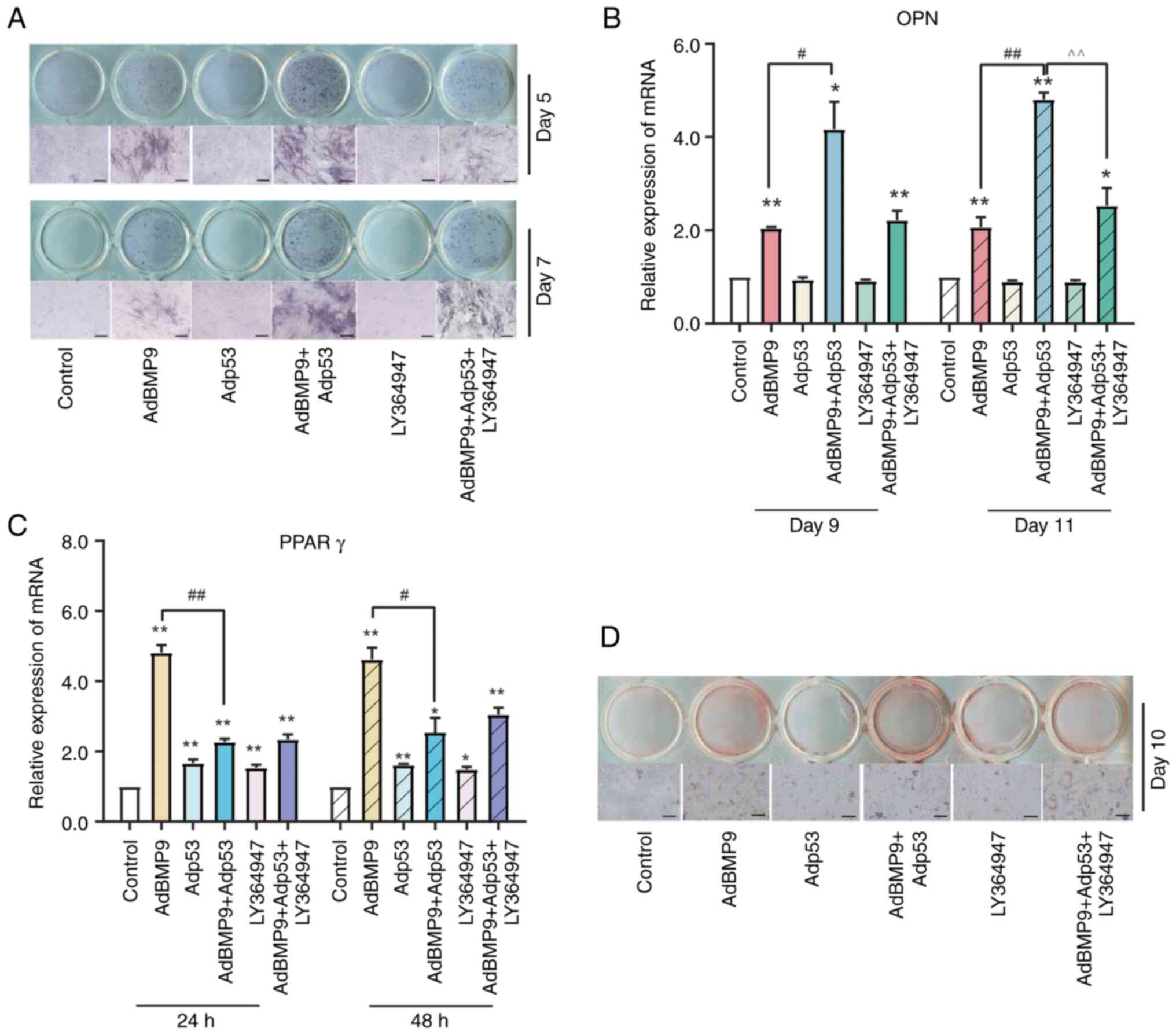
Effects of LY364947 and/or p53 on
BMP9-induced differentiation in MSCs
TGF-β1 has bidirectional regulation of osteogenesis
and adipogenesis and a shared downstream target Smad signaling with
BMP9(31). Our previous study
showed that TGF-β1 selective inhibitor LY364947 could suppress the
BMP9 induced MSCs osteo-differentiation (32). ALP staining showed that ALP
activity induced by BMP9 in MSCs could be enhanced by Adp53,
however, the TGF-β1 selective inhibitor LY364947(33) practically eliminated this effect
(Fig. 4A). PCR results showed that
the effect of BMP9 on increasing mRNA level of OPN were induced by
Adp53, whereas reduced by LY364947 (Fig. 4B). Although inhibition of TGF-β1
may reverse the promotion of BMP9 osteogenic potential by p53, it
is not clear whether the specific manner of the effect is related
to adipose differentiation. PCR analysis of PPARγ expression showed
that LY364947 attenuated the inhibitory effect of p53 on
BMP9-induced adipogenic differentiation (Fig. 4C). Moreover, Oil red O staining
showed that p53 inhibited the formation of lipid droplets in
BMP9-induced differentiation of MSCs, which could be partially
reversed by LY264947 (Fig. 4D).
The above results demonstrated that the regulatory actions of p53
on the osteogenic potential of BMP9 in MSCs were associated with
the level of TGF-β1.
Effects of TGF-β1 and/or PFT-α on
BMP9-induced differentiation in MSCs
On the contrary, ALP activity induced by BMP9 in
MSCs can be reduced by p53 inhibitor PFT-α, and exogenous TGF-β1
can partially reverse this effect (Figs. 5A and S1C). In addition, BMP9-induced OPN mRNA
levels could be reduced by PFT-α, to the extent that this effect
was in part reversed by TGF-β1 overexpression (Fig. 5B). In terms of lipogenic
differentiation, PFT-α promotes BMP9-induced mRNA expression of
PPARγ, whereas attenuated by exogenous TGF-β1 (Fig. 5C). The oil red O staining showed
the same trend in lipid droplet formation (Fig. 5D). The above results evidenced that
TGF-β1 may mediate the regulation of BMP9-induced MSCs adipogenic
differentiation by p53.
Effects of p53 and BMP9 on regulating
the expression of TGF-β1
The role of TGF-β1 on p53 in MSCs osteogenic
differentiation were confirmed. However, the exact relationship
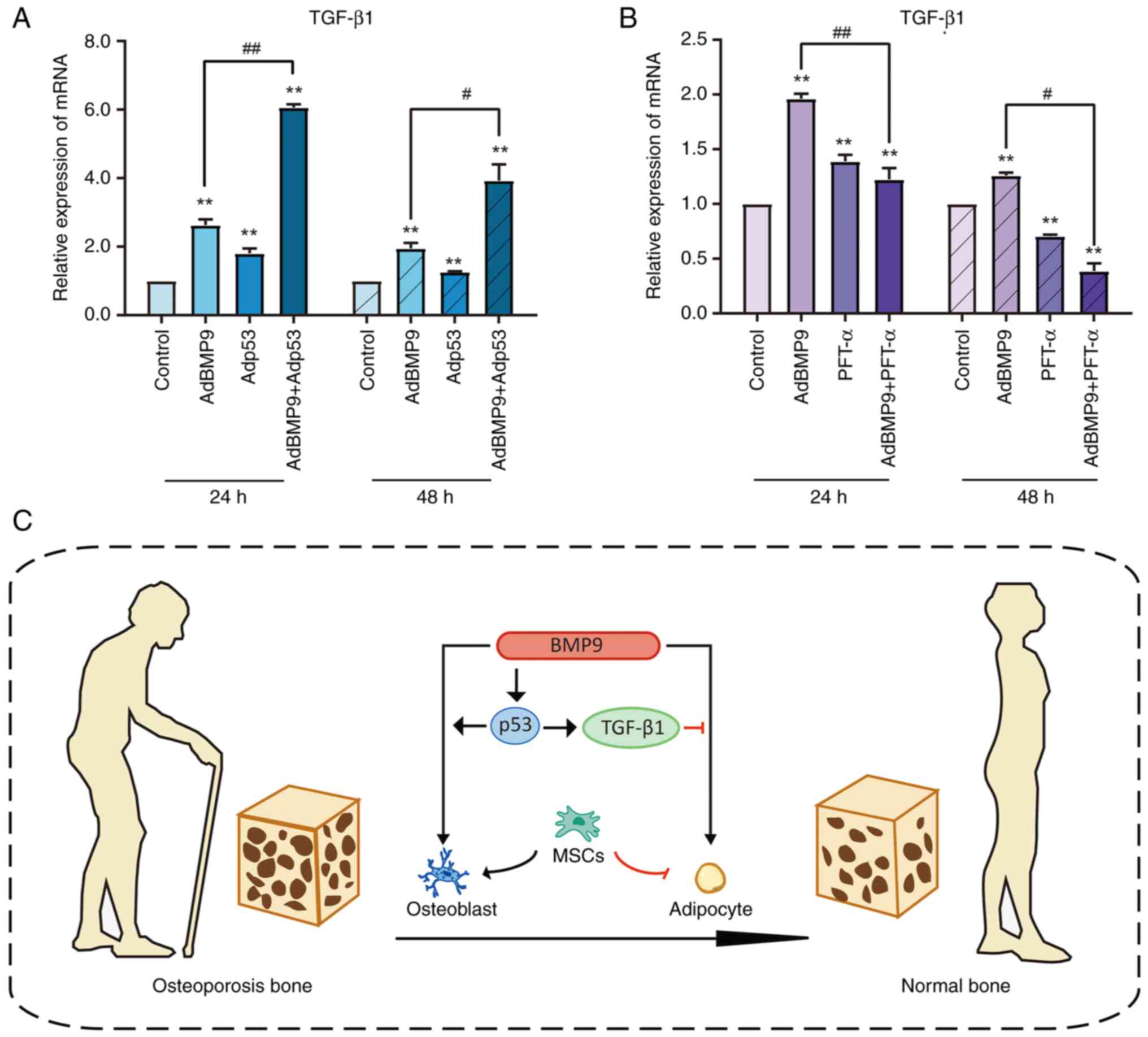
between them needs to be further clarified. PCR showed that BMP9
promotes TGF-β1 mRNA expression, exogenous p53 promotes the
upregulation of TGF-β1 by BMP9 (Fig.
6A), while inhibition of p53 suppresses the effect of BMP9 on
TGF-β1 mRNA levels (Fig. 6B).
Results above further suggested that TGF-β1 is involved in
regulating MSCs differentiation engaged by p53.
Discussion
Bones are a living dynamic tissue constantly
regenerating themselves to stay healthy. In certain pathological
conditions, such as osteoporosis, insufficient bone mass could
seriously affect the quality of survival of patients and even
induce other diseases (34).
Promotion of MSCs osteo-differentiation is a potential therapeutic
breakthrough for the clinical treatment of osteoporosis. Exploring
the molecular mechanisms that enhance the ability of MSCs to
osteodifferentiate could provide therapeutic options for clinical
bone diseases (35). In the
present study, we systematically investigated the role of p53 in
MSCs differentiation induced by BMP9 and the underlying molecular
mechanisms, expecting to provide new ideas for the treatment of
bone diseases.
BMP9 is the strongest osteogenic inducer of the BMPs
family, which can bind to type II receptors and phosphorylate Smad
proteins to regulate the expression of downstream transcription
factors, thereby inducing osteogenic differentiation of MSCs, has
been termed as a classical signaling (36). Moreover, other signaling factors
such as COX-2, Wnt/β-catenin, MAPK, etc. interact with BMP9 to
regulate its expression (37,38).
The bioinformatics analysis of the current study revealed that p53
has a high expression in the osteoporosis model, therefore we
speculate that p53 can possibly be a new regulator in the
osteogenic differentiation process.
As an important oncogene, p53 contributes to the
regulation of cell proliferation and DNA damage repair.
Nevertheless, the regulatory role of p53 in the body goes far
beyond these. p53 is also present in some newly discovered
biological processes, such as ferroptosis (39,40).
In terms of osteogenesis, several researchers have identified a
negative regulatory effect of p53 on osteoblast differentiation,
whereas some others suggested that mutations in p53 can increase
osteoblast differentiation (41,42).
The present study found that p53 was expressed in all MSCs tested
and overexpression of p53 enhanced the potential of BMP9 induced
osteogenic differentiation, while suppression of p53 decreased the
expression level of early, intermediate, and late osteogenic
markers, whereas p53 alone had no significant effect on MSCs
differentiation in this study. It has been shown in other studies
that p53 knockout mice display more bone differentiation and bone
erosion (19). PFT-α alone in the
present study also resulted in upregulation of the Runx2
expression. However, PFT-α with BMP9 resulted in a trend of
inhibition. These may be caused by different microenvironmental
conditions. Taken together, our results support the notion that p53
may regulate BMP9 induced MSCs osteogenic differentiation.
The phenomenon of the bone-adipose imbalance can be
seen in many diseases (43). In
cases of heavy corticosteroid use, for example, bone formation
decreases, while adipose tissue in the bone marrow increases
(44). In the present study p53
was evidenced to modulate bone lipid homeostasis to influence MSCs
differentiation. After infection with adenovirus overexpressing
p53, microscopic observation revealed increased lipid droplet
formation and increased expression of PPARγ in C3H10T1/2 cells
(27). These effects were
attenuated after treatment with p53 inhibitor, which revealed that
p53 may facilitate BMP9-induced osteogenic differentiation by
inhibiting lipogenic differentiation. Taken together, our study
analyzed the effect of p53 on MSCs differentiation from the
perspective of bone-lipid balance for the first time, which will be
provide a new perspective in bone marrow microenvironment on bone
formation.
The Smad dependent TGF-β and BMP signaling share
common principles in bone remodeling and therapies (45,46).
The combination of p53 and BMP9 could better enhance the expression
level of TGF-β1 than BMP9 alone. The TGF-β1 mRNA level was
suppressed even when BMP9 was overexpressed and inhibition of p53.
Inhibition of TGF-β1 with LY364947 impaired the osteogenic
differentiation of BMP9-induced C3H10T1/2 cells by p53, and
overexpression of TGF-β1 partially restored the inhibitory effect
of PFT-α on the osteogenic marker ALP, which was largely related to
the concentration of TGF-β1. The same results were obtained for the
intermediate and late-stage osteogenic marker OPN, suggesting that
p53 influences all stages of osteogenic differentiation. To further
corroborate this mechanism, we examined the levels of PPARγ under
the same treatment factors as well as oil red O staining, and the
variation of these indicators strongly suggested their relevance.
Our hypothesized molecular mechanism is shown in Fig. 6C. The expression of TGF-β1 is
promoted by p53, and TGF-β1 may promote BMP9-induced
osteo-differentiation and thus lipogenic differentiation by
inhibiting p53. Thus, our data suggested that p53 may mediate the
promotion of BMP9-induced osteogenic activity through upregulation
of TGF-β1. TGF-β1 is a multifunctional protein that controls
proliferation, differentiation, and other functions in many cell
types. Clarifying the relationship between p53 and TGF-β1 is
beneficial to further study the effect of bone marrow
microenvironment on bone formation, and provide solutions for
clinical treatment of bone diseases such as osteoporosis.
Subsequent experiments are expected to perform more verification by
conditional knockout of p53 mice, collection of more clinical
specimens for sequencing and bioinformatics analysis to improve our
study and beneficial to clinical.
In conclusion, the present study demonstrated that
BMP9 can further induce osteogenic differentiation of MSCs by
inhibiting lipogenic differentiation in a p53-dependent manner, and
one of the critical mechanisms might be TGF-β1 signaling. Our
findings provide another target for the treatment of osteoporosis.
Furthermore, this work presents a compelling case for the further
investigation of p53 as a crucial regulator for the osteogenic
differentiation of MSCs.
Supplementary Material
Proof of transfection success. (A)
RT-qPCR of BMP9 expression in C3H10T1/2 cells transfected with
AdGFP vs. AdBMP9 in titers of MOI 10, MOI 15, and MOI 20 at 48 h.
(B) Western blot of p53 level in C3H10T1/2 cells transfected with
AdGFP vs. Adp53 in titers of MOI 15 at 48 h. (C) Western blot of
p53 level in C3H10T1/2 cells transfected with AdGFP vs. AdTGF-β1 in
titers of MOI 15 at 48 h. *P<0.05 vs. AdGFP. MOI,
multiplicity of infection. RT-qPCR, reverse
transcription-quantitative PCR.
Acknowledgements
The authors would like to thank Professor Tongchuan
He (Molecular Oncology Laboratory, The University of Chicago
Medical Center, Chicago, Illinois, USA) for his generous provision
of the MSCs [MEFS (47,48), C2C12, C3H10T1/2, 3T3-L1 and MG-63]
and recombinant adenoviruses.
Funding
Funding: This work was supported by the National Key Research
and Development Program of China (grant no. 2016YFC1000803) and the
National Natural Science Foundation of China (NSFC, grant no.
81572226).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available in the GEO repository, https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE35959.
All other datasets used and/or analyzed during the current study
are available from the corresponding author on reasonable
request.
Authors' contributions
XY, PL and BH confirm the authenticity of all the
raw data. XY conceived the study, conducted the experiments, and
wrote the manuscript. PL performed western blotting, RT-qPCR, and
staining assays. YD performed bioinformatics assays. YY preformed
the molecular cloning experiments. HL performed western blotting
and RT-qPCR assays. BH conceived and supervised the study. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pinheiro MB, Oliveira J, Bauman A,
Fairhall N, Kwok W and Sherrington C: Evidence on physical activity
and osteoporosis prevention for people aged 65+ years: A systematic
review to inform the WHO guidelines on physical activity and
sedentary behavior. Int J Behav Nutr Phys Act.
17(150)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Yang TL, Shen H, Liu A, Dong SS, Zhang L,
Deng FY, Zhao Q and Deng HW: A road map for understanding molecular
and genetic determinants of osteoporosis. Nat Rev Endocrinol.
16:91–103. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sözen T, Özışık L and Başaran NÇ: An
overview and management of osteoporosis. Eur J Rheumatol. 4:46–56.
2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Cheng C, Wentworth K and Shoback DM: New
frontiers in osteoporosis therapy. Ann Rev Med. 71:277–288.
2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Shao J, Zhang W and Yang T: Using
mesenchymal stem cells as a therapy for bone regeneration and
repairing. Biol Res. 48:1–7. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lu LX, Zhang XF, Wang YY, Ortiz L, Mao X,
Jiang ZL, Xiao ZD and Huang NP: Effects of
hydroxyapatite-containing composite nanofibers on osteogenesis of
mesenchymal stem cells in vitro and bone regeneration in vivo. ACS
Appl Mater Interfaces. 5:319–330. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wu M, Chen G and Li YP: TGF-β and BMP
signaling in osteoblast, skeletal development, and bone formation,
homeostasis and disease. Bone Res. 4:1–21. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Mostafa S, Pakvasa M, Coalson E, Zhu A,
Alverdy A, Castillo H, Fan J, Li A, Feng Y, Wu D, et al: The
wonders of BMP9: From mesenchymal stem cell differentiation,
angiogenesis, neurogenesis, tumorigenesis, and metabolism to
regenerative medicine. Genes Dis. 6:201–223. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wang Y, Ma C, Sun T and Ren L: Potential
roles of bone morphogenetic protein-9 in glucose and lipid
homeostasis. J Physiol Biochem. 76:503–512. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Liu Y and Gu W: The complexity of
p53-mediated metabolic regulation in tumor suppression. Semin
Cancer Biol. 85:4–32. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lavin MF and Gueven N: The complexity of
p53 stabilization and activation. Cell Death Differ. 13:941–950.
2006.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Liu Y, Tavana O and Gu W: p53
modifications: Exquisite decorations of the powerful guardian. J
Mol Cell Biol. 11:564–577. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Vousden KH: Outcomes of p53
activation-spoilt for choice. J Cell Sci. 119:5015–5020.
2006.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Huang Q, Liu M, Du X, Zhang R, Xue Y,
Zhang Y, Zhu W, Li D, Zhao A and Liu Y: Role of p53 in preadipocyte
differentiation. Cell Biol Int. 38:1384–1393. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Jain AK, Allton K, Iacovino M, Mahen E,
Milczarek RJ, Zwaka TP, Kyba M and Barton MC: p53 regulates cell
cycle and microRNAs to promote differentiation of human embryonic
stem cells. PLoS Biol. 10(e1001268)2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Molchadsky A, Shats I, Goldfinger N,
Pevsner-Fischer M, Olson M, Rinon A, Tzahor E, Lozano G, Zipori D,
Sarig R and Rotter V: p53 plays a role in mesenchymal
differentiation programs, in a cell fate dependent manner. PLoS
One. 3(e3707)2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Mao X, Li X, Hu W, Hao S, Yuan Y, Guan L
and Guo B: Downregulated brain and muscle aryl hydrocarbon receptor
nuclear translocator-like protein-1 inhibits osteogenesis of BMSCs
through p53 in type 2 diabetes mellitus. Biol Open.
9(bio051482)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hüttinger-Kirchhof N, Cam H, Griesmann H,
Hofmann L, Beitzinger M and Stiewe T: The p53 family inhibitor
ΔNp73 interferes with multiple developmental programs. Cell Death
Differ. 13:174–177. 2006.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Velletri T, Huang Y, Wang Y, Li Q, Hu M,
Xie N, Yang Q, Chen X, Chen Q, Shou P, et al: Loss of p53 in
mesenchymal stem cells promotes alteration of bone remodeling
through negative regulation of osteoprotegerin. Cell Death Differ.
28:156–169. 2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wang X, Kua HY, Hu Y, Guo K, Zeng Q, Wu Q,
Ng HH, Karsenty G, de Crombrugghe B, Yeh J and Li B: p53 functions
as a negative regulator of osteoblastogenesis, osteoblast-dependent
osteoclastogenesis, and bone remodeling. J Cell Biol. 172:115–125.
2006.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Shea CM, Edgar CM, Einhorn TA and
Gerstenfeld LC: BMP treatment of C3H10T1/2 mesenchymal stem cells
induces both chondrogenesis and osteogenesis. J Cell Biochem.
90:1112–1127. 2003.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wu N, Zhao Y, Yin Y, Zhang Y and Luo J:
Identification and analysis of type II TGF-β receptors in
BMP-9-induced osteogenic differentiation of C3H10T1/2 mesenchymal
stem cells. Acta Biochim Biophys Sin (Shanghai). 42:699–708.
2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Bruderer M, Richards RG, Alini M and
Stoddart MJ: Role and regulation of RUNX2 in osteogenesis. Eur Cell
Mater. 28:269–286. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sharma U, Pal D and Prasad R: Alkaline
phosphatase: An overview. Indian J Clin Biochem. 29:269–278.
2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Icer MA and Gezmen-Karadag M: The multiple
functions and mechanisms of osteopontin. Clin Biochem. 59:17–24.
2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Springsteen G and Wang B: Alizarin Red S
as a general optical reporter for studying the binding of boronic
acids with carbohydrates. Chem Commun (Camb). 17:1608–1609.
2001.PubMed/NCBI View
Article : Google Scholar
|
|
27
|
Molchadsky A, Ezra O, Amendola PG, Krantz
D, Kogan-Sakin I, Buganim Y, Rivlin N, Goldfinger N, Folgiero Y,
Falcioni R, et al: p53 is required for brown adipogenic
differentiation and has a protective role against diet-induced
obesity. Cell Death Differ. 20:774–783. 2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Tontonoz P and Spiegelman BM: Fat and
beyond: The diverse biology of PPARgamma. Annu Rev Biochem.
77:289–312. 2008.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Mehlem A, Hagberg CE, Muhl L, Eriksson U
and Falkevall A: Imaging of neutral lipids by oil red O for
analyzing the metabolic status in health and disease. Nat Protoc.
8:1149–1154. 2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Misra UK and Pizzo SV: PFT-alpha inhibits
antibody-induced activation of p53 and pro-apoptotic signaling in
1-LN prostate cancer cells. Biochem Biophys Res Commun.
391:272–276. 2010.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Li XL, Liu YB, Ma EG, Shen WX, Li H and
Zhang YN: Synergistic effect of BMP9 and TGF-β in the proliferation
and differentiation of osteoblasts. Genet Mol Res. 14:7605–7615.
2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Deng Y, Li L, Zhu JH, Li PP, Deng YX, Luo
HH, Yang YY, He BC and Su Y: COX-2 promotes the osteogenic
potential of BMP9 through TGF-β1/p38 signaling in mesenchymal stem
cells. Aging (Albany NY). 13:11336–11351. 2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Karkampouna S, Goumans MJ, Dijke PT,
Dooley S and Julio MK: Inhibition of TGFβ type I receptor activity
facilitates liver regeneration upon acute CCl4 intoxication in
mice. Arch Toxicol. 90:347–357. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Hasegawa T and Ishii M: Visualizing bone
tissue in homeostatic and pathological conditions. Proc Jpn Acad
Ser B Phys Biol Sci. 96:43–49. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Manzini BM, Machado LMR, Noritomi PY and
DA Silva JVL: Advances in bone tissue engineering: A fundamental
review. J Biosci. 46(17)2021.PubMed/NCBI
|
|
36
|
Lamplot JD, Qin J, Nan G, Wang J, Liu X,
Yin L, Tomal J, Li R, Shui W, Zhang H, et al: BMP9 signaling in
stem cell differentiation and osteogenesis. Am J Stem Cells.
2:1–21. 2013.PubMed/NCBI
|
|
37
|
Wang H, Hu Y, He F, Li L, Li PP, Deng Y,
Li FS, Wu K and He BC: All-trans retinoic acid and COX-2 cross-talk
to regulate BMP9-induced osteogenic differentiation via
Wnt/β-catenin in mesenchymal stem cells. Biomed Pharmacother.
118(109279)2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zheng W, Gu X, Sun X, Wu Q and Dan H: FAK
mediates BMP9-induced osteogenic differentiation via Wnt and MAPK
signaling pathway in synovial mesenchymal stem cells. Artif Cells
Nanomed Biotechnol. 47:2641–2649. 2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Liu Y and Gu W: p53 in ferroptosis
regulation: The new weapon for the old guardian. Cell Death Differ.
29:895–910. 2022.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Hafner A, Bulyk ML, Jambhekar A and Lahav
G: The multiple mechanisms that regulate p53 activity and cell
fate. Nat Rev Mol Cell Biol. 20:199–210. 2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Li B, Zhang YW, Liu X, Ma L and Yang JX:
Molecular mechanisms of intermuscular bone development in fish: A
review. Zool Res. 42:362–376. 2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Boregowda SV, Krishnappa V, Strivelli J,
Haga CL, Booker CN and Phinney DG: Basal p53 expression is
indispensable for mesenchymal stem cell integrity. Cell Death
Differ. 25:679–692. 2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Yue R, Zhou B, Shimada IS, Zhao Z and
Morrison SJ: Leptin receptor promotes adipogenesis and reduces
osteogenesis by regulating mesenchymal stromal cells in adult bone
marrow. Cell Stem Cell. 18:782–796. 2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Rosen ED and MacDougald OA: Adipocyte
differentiation from the inside out. Nat Rev Mol Cell Biol.
7:885–896. 2006.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Trivedi T, Pagnotti GM, Guise TA and
Mohammad KS: The role of TGF-β in bone metastases. Biomolecules.
11(1643)2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Zou ML, Chen ZH, Teng YY, Liu SY, Jia Y,
Zhang KW, Sun ZL, Wu JJ, Yuan ZD, Feng Y, et al: The smad dependent
TGF-β and BMP signaling pathway in bone remodeling and therapies.
Front Mol Biosci. 8(593310)2021.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Tang N, Song WX, Luo J, Luo X, Chen J,
Sharff KA, Bi Y, He BC, Huang JY, Zhu GH, et al: BMP9-induced
osteogenic differentiation of mesenchymal progenitors requires
functional canonical Wnt/β-catenin signalling. J Cell Mol Med.
13:2448–2464. 2009.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Huang E, Bi Y, Jiang W, Luo X, Yang K, Gao
JL, Gao Y, Luo Q, Shi Q, Kim SH, et al: Conditionally immortalized
mouse embryonic fibroblasts retain proliferative activity without
compromising multipotent differentiation potential. PLoS One.
7(e32428)2012.PubMed/NCBI View Article : Google Scholar
|