Introduction
The incidence of trochanteric femoral fractures has
increased owing to an aging society (1). Falling is one of the main causes of
fracture Hydroxyapatite (HA) augments are used to treat
trochanteric femoral fractures. However, the efficacy of HA
augmentation has not been fully described in trochanteric femoral
fracture surgery. In total, 85 patients were enrolled in the
present study; all had trochanteric femoral fractures between
January 2016 and October 2020, 45 with HA (HA group) and 40 without
HA (N group). The intraoperative lag screw insertion torque was
directly measured and the amount of lag screw telescoping with and
without HA augmentation after surgery was analyzed. Maximum lag
screw insertion torque (max-torque), bone mineral density in the
opposite femoral neck (n-BMD), tip apex distance (TAD) of the lag
screw, radiographic findings including fracture union, the amounts
of lag screw telescoping and occurrence of complications were
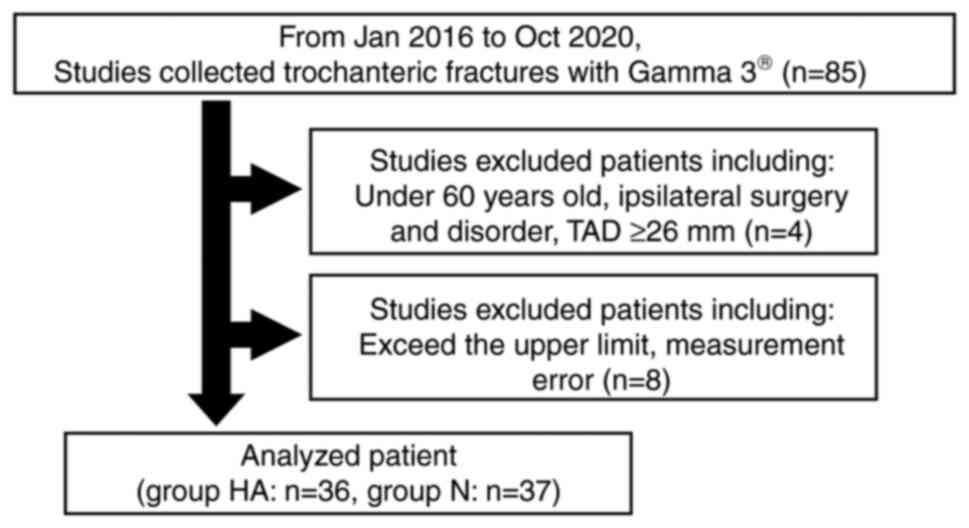
evaluated. A total of 12 patients were excluded if they were aged
under 60 years old, had ipsilateral surgery and disorders in the
hip joint, TAD of the lag screw ≥26 mm on postoperative radiographs
and had measurement errors. A total of 73 fractures could be
analyzed: HA group (n=36) and N group (n=37). Max-torque/n-BMD
ratios were higher in the HA group compared with in the N group
(7.23±2.71 vs. 5.93±1.91 g/cm2·N·m; P=0.04). The amounts
of lag screw telescoping in the HA group were smaller compared with
the N group (1.41±2.00 vs. 2.58±2.34; P=0.05). Evaluation of screw
insertion torque showed maximum screw insertion torque correlated
well with n-BMD in both groups, HA (R=0.57; P<0.01) and N group
(R=0.64; P<0.01). No correlation was found between maximum screw
insertion torque and TAD in both groups, HA (R=-0.10; P=0.62) and N
group (R=0.02; P=0.93). All fractures were radiographically united
without any complications. These results support the effectiveness
of HA augmentation, indicating higher resistance against rotational
instability and reduced lag screw telescoping in trochanteric
femoral fracture treatment.
and is related to high mortality rates (2,3).
Open reduction and internal fixation using an intramedullary nail
is the most commonly used operative method for fractures (4). Many implant designs and augmentation
methods have been reported to increase the initial fixation
strength (5,6). However, complications such as
necrosis of the femoral head and cut-out of the lag screw are
problematic due to lower bone mineral density (BMD) (7,8). The
uses of cement augmentation involve fixation of the femoral head
screw (9,10). While cement augmentation reportedly
increases the lag screw fixation force (11,12),
cement usage is a risk factor for femoral head necrosis due to the
chemical and heat reactions (9,13-16).
Hydroxyapatite (HA) augmentation has the advantage of high
biomechanical and biochemical stability after implantation. HA
augmentation is widely used in the treatment of trochanteric
femoral fracture, distal radial fracture, proximal tibial fracture,
and spinal surgery (17,18). HA augmentation reportedly increases
peri-implant bone formation after surgery and demonstrates
increased cut-out resistance (17,19,20).
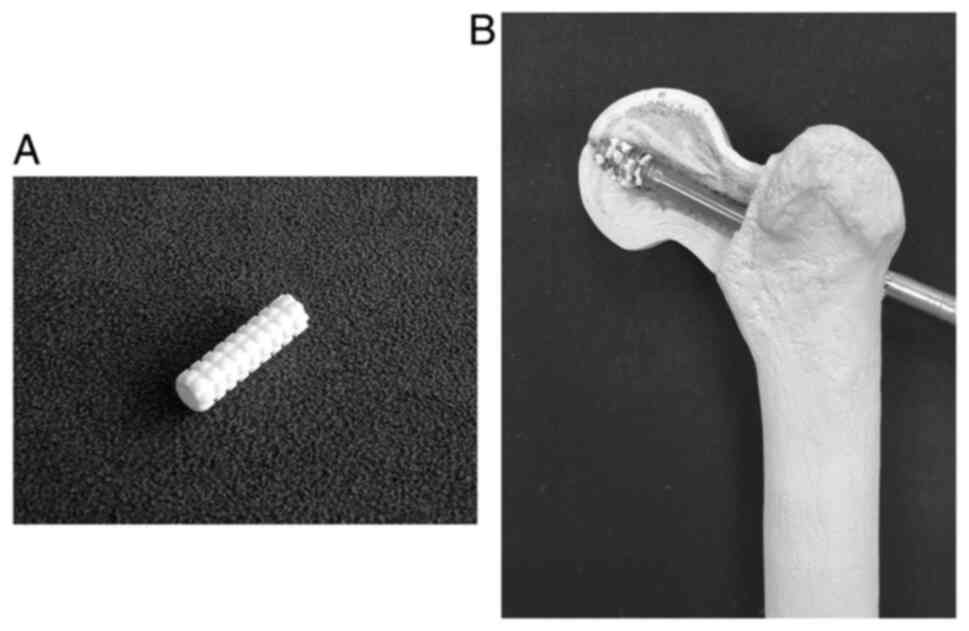
In this study, Neobrace® (Neobrace®, Aimedic
MMT Co., Ltd., Japan) was used for HA augmentation. This is a
cylinder-shaped implant with a length of 25 mm, inner diameter of
3.5 mm, and outer diameter of 7 mm (Fig. 1A). Neobrace® is composed
of pure HA particles with a porosity of 72-78% and a diameter of
150-200 µm. Neobrace® was easily collapsed by lag screw
insertion into comminuted granules and was scattered around the
screws (Fig. 1B). The granule size
was 40-70 µm, which was sufficiently strong (12-18 MPa). HA
granules have advantages such as high biocompatibility, as HA is
the main composite of bone.
Evaluation of the postoperative risk of lag screw
cut-out during surgery is difficult. It has been reported that the
insertion torque of the screw is a key predictor for evaluating the
cut-out resistance force (21-23).
Local BMD [e.g. BMD around femoral neck (n-BMD)] and force at
cut-out are highly correlated with maximum screw insertion torque
(21-23).
Thus, we calculated max-torque/n-BMD ratio because this would be a
good surrogate parameter to indicate bone strength. Moreover, it
indicates that max-torque/n-BMD ratio should be statistically
similar to each other compared to each specimen. Our previous study
showed that the mean lag screw insertion torque (mean torque)/screw
insertion and n-BMD increased with the use of a HA tube in the
treatment of trochanteric femoral fractures (24). However, the maximum lag screw
insertion torque (max-torque)/n-BMD did not reach significance as
noise in the raw data made statistical analysis difficult. The
purpose of this study was to investigate the effectiveness of HA
augmentation in the treatment of trochanteric femoral fractures
using novel data-smoothing techniques. We measured the lag screw
insertion torque and n-BMD with and without HA augmentation during
the surgery and subsequently evaluated the effects of HA
augmentation on max-torque/n-BMD. Moreover, the amount of lag screw
telescoping with and without postoperative HA augmentation was
evaluated.
Materials and methods
Patients' selection and exclusion
This study was approved by the Research Ethics
Committee of Kainan Hospital. Written informed consent was obtained
from all participants before surgery. Between January 2016 and
October 2020, 85 patients with closed trochanteric femoral
fractures treated using Gamma3® (Stryker, MI, USA) were
enrolled in the study. The mean age of all patients was 83.8±8.4
years (median, 84; range, 54-102). A tip apex distance (TAD)-the
distance from the apex of the femoral head to the tip of the lag
screw on anteroposterior and lateral radiographs-greater than 25 mm
identified the risk for cut-out (25,26).
Therefore, patients were excluded if they were aged less than 60
years, had ipsilateral surgery and disorders in the hip joint, and
TAD of the lag screw ≥26 mm on postoperative radiographs (Fig. 2).
Measurement method and data
analysis
Surgery was conducted under lumbar or general
anesthesia. In the HA group, we used 2 Neobrace® before
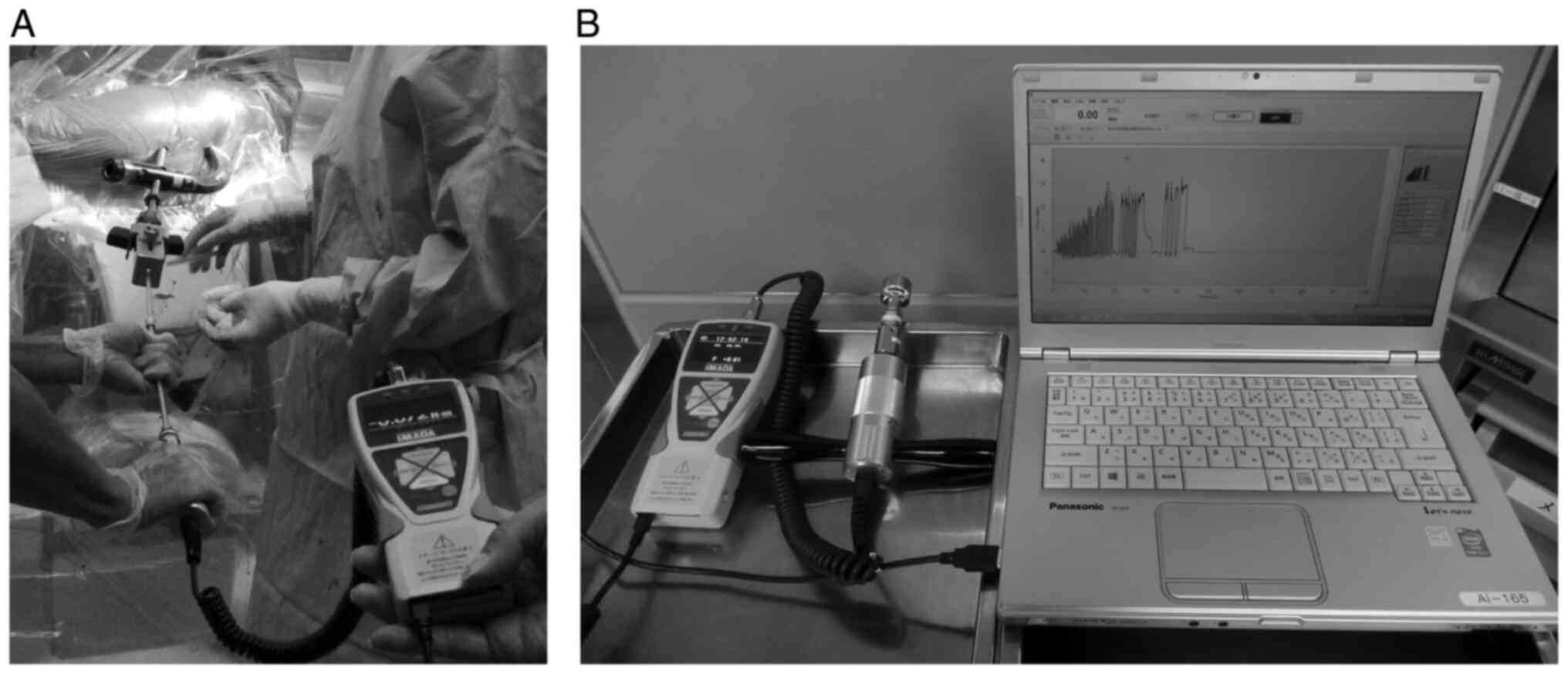
lag screw insertion via the lag screw hole (Fig. 1). The lag screw insertion torque
was obtained continuously using a digital torque measurement device
(HTGS-5N, 10N, Imada Co., Ltd., Tokyo, Japan), and the data were
recorded using the software (Force Recorder, Imada Co., Ltd.,
Japan) (Fig. 3). The sampling rate
was 2,000 Hz. The lag screw was manually inserted using a digital
torque measurement device connected to a screwdriver. Data were
excluded if the measurements exceeded the upper limit and had
measurement error (Fig. 2). The
data were described as time-series scatterplots with noise, and the
locally weighted scatterplot smoothing (LOWESS) technique was
adopted to smooth the data series in this study (27). The library and application
programming interface, including NumPy, SciPy, and
statsmodels.api, were imported into Python (Python Software
Foundation, Wilmington, USA). The function
statsmodels.nonparametric.smoothers_lowess.lowess was used
to smoothen the data series (parameter: frac=0.01). This method
enabled the visualization of numerous data series of torque. As
previously described, a normal graph of the screw insertion torque
exhibits a plateau torque pattern followed by a clear peak
(27). Thus, we excluded data that
had no exact plateau and/or peak and exceeded the upper limit of
the torque gauge in this study. The function
scipy.signal.find_peaks was used to obtain the peak of the
smoothed data (parameters: height=0.3, distance=500). The highest
peak of lag screw insertion torque was defined as the
max-torque.
The n-BMD was calculated using a dual-energy X-ray
absorptiometry (DEXA) machine (DCS-900EX, Hitachi, Ltd., Tokyo,
Japan). TAD of the lag screw was measured on postoperative
radiographs at the final follow-up. Radiographical evidence of
fracture union and postoperative complications was also assessed at
the final follow-up. The amount of lag screw telescoping and
radiographic evidence of fracture union at the final follow-up were
evaluated by a single surgeon.
Statistical analysis
Both categorical and continuous variables including
patients' age, sex, operative side, follow-up duration, n-BMD,
max-torque/n-BMD, TAD of the lag screw, amount of lag screw
telescoping, radiographical evidence of fracture union, and
complications were evaluated using Fisher's exact test and Welch's
t-test. Pearson's correlation and overall agreement were calculated
to assess the reliability of each parameter, including max-torque,
n-BMD, and TAD, in the two groups. Statistical analysis was
conducted using the R statistical package, version 4.0.4 (R Core
Team, Foundation for Statistical Computing, Austria). All reported
two-sided P-values were evaluated, and P-values <0.05 were
considered statistically significant.
Results
Among the 85 patients enrolled, 12 patients were
excluded (Fig. 2). Thus, a total
of 73 patients (patients with the use of HA augments: HA group,
n=36; patients without the use of augments: N group, n=37) were
analyzed in the study. The mean age of participants was 83.4±7.9
years (range, 63-95) in the HA group and 85.7±7.7 years (range,
67-107) in the N group (P=0.22). There were 10 males and 26 females
in the HA group and 8 males and 29 females in the N group (P=0.60).
Operative sides of the HA and N groups were 18 right and 18 left
sides and 20 right and 17 left sides, respectively (P=0.82). The
follow-up durations were 11.88±14.56 months (range, 0.23-62.00) in
the HA group and 9.87±13.14 months (range, 0.20-12.00) in the N
group (P=0.54). n-BMD was 0.45±0.11 g/cm2 (range,
0.25-0.64) in the HA group and 0.48±0.13 g/cm2 (range,
0.25-0.86) in the N group (P=0.26).
Max-torque was 3.26±1.49 N·m (range, 1.08-8.50) in
the HA group and 2.76±1.16 N·m (range, 0.92-6.03) in the N group
(P=0.11). Max-torque/n-BMD was 7.23±2.71 g/cm2·N·m
(range, 2.51-13.26) in the HA group and 5.93±1.91
g/cm2·N·m (range, 3.29-12.25) in the N group (P=0.04).
TAD was 18.89±4.38 mm (range, 9.11-25.82) in the HA group and
18.32±3.72 mm (range, 10.70-25.16) in the N group (P=0.60). The
amounts of lag screw telescoping was 1.41±2.00 mm (range, 0-8.81)
in the HA group and 2.58±2.34 mm (range, 0-7.47) in the N group
(P=0.05) (Table I).
 | Table IOverview of preoperative variables in
the HA and N groups. |
Table I
Overview of preoperative variables in
the HA and N groups.
| Preoperative
variables | HA group (n=36) | N group (n=37) | P-value |
|---|
| Age, years
(range) | 83.4±7.93
(63-95) | 85.7±7.70
(67-107) | 0.22 |
| Sex | | | 0.60 |
|
Male | 10 | 8 | |
|
Female | 26 | 29 | |
| Side | | | 0.82 |
|
Right | 18 | 20 | |
|
Left | 18 | 17 | |
| Follow-up duration,
months (range) | 11.88±14.56
(0.23-62.00) | 9.87±13.14
(0.20-12.00) | 0.54 |
| n-BMD,
g/cm2 (range) | 0.45±0.11
(0.25-0.64) | 0.48±0.13
(0.25-0.86) | 0.26 |
| Max-torque, N·m
(range) | 3.26±1.49
(1.08-8.50) | 2.76±1.16
(0.92-6.03) | 0.11 |
| Max toqrue/n-BMD,
g/cm2·N·m (range) | 7.23±2.71
(2.51-13.26) | 5.93±1.91
(3.29-12.25) | 0.04a |
| TAD, mm (range) | 18.89±4.38
(9.11-25.82) | 18.32±3.72
(10.70-25.16) | 0.60 |
| Telescoping, mm
(range) | 1.41±2.00
(0-8.81) | 2.58±2.34
(0-7.47) | 0.05 |
| Complications | N/A | N/A | |
Regarding the relationships between the max-torque
and n-BMD, the values of correlation coefficients were 0.57 in the
HA group (P<0.01) and 0.64 in the N group (P<0.01). In the
relationship between the max-torque and TAD, the values of the
correlation coefficients were -0.10 in the HA group (P=0.62) and
0.02 in the N group (P=0.93) (Table
II). All fractures were radiographically united without any
complications.
 | Table IIPearson's correlation and overall
agreement between max-torque and n-BMD and TAD. |
Table II
Pearson's correlation and overall
agreement between max-torque and n-BMD and TAD.
| Variables | Correlation | Lower limit | Upper limit | P-value |
|---|
| n-BMD | | | | |
|
HA
group | 0.57 | 0.26 | 0.77 |
<0.01a |
|
N group | 0.64 | 0.37 | 0.81 |
<0.01a |
| TAD | | | | |
|
HA
group | -0.10 | -0.45 | 0.28 | 0.62 |
|
N group | 0.02 | -0.35 | 0.38 | 0.93 |
Discussion
In this study, we examined the mechanical effects of
HA augmentation in the treatment of trochanteric femoral fractures.
Our analyses identified a significantly positive relationship
between max-torque and BMD with and without HA augmentation. Our
previous study demonstrated that lag screw insertion torque was
related to screw cut-out resistance by analyzing the mean
torque/n-BMD (24). However, the
study did not show statistical significance in max-torque/n-BMD
between the groups with and without HA augmentation. We concluded
in this study that the measurement noise may cause statistical
dispersion (22). However, this
current study shows that max-torque/n-BMD was significantly higher
in the presence of HA tubes. This is the first study to use Python,
LOWESS, and the scipy.signal.find_peaks function. The
sampling rate obtained from the torque gauge was 2,000 Hz,
therefore, the total number of data series ranged from 50,000 to
100,000 plots with noise for each trial. The methods of data
smoothing and peak extraction were effectively used to grasp the
trends of the data series. LOWESS is a locally weighted
nonparametric regression analysis method. Thus, the data curves
calculated by LOWESS can be accurately fitted to the data, such as
the manually measured torque. This is the first biomechanical study
to show the effectiveness of LOWESS for analyzing nonparametric
data.
Regarding radiologic parameters, we used
intramedullary nails with neck-shaft angle of 125˚ for all cases.
We tried to calculate the neck shaft angle of the proximal femur.
However, the differences in the external rotation on radiographs
made the calculation difficult. Further examination should be
performed by using CT scans to clarify the details of radiologic
parameters. Instead of angle parameters, we measured the
telescoping amount of lag screws and found that the amounts of lag
screw telescoping were lower in the HA group than that in the N
group. Thus, it seems likely that the HA augments may increase the
fixation of lag screws in the treatment of trochanteric femoral
fractures. As for TAD, the distribution of BMD is reportedly higher
on the outer side of the femoral head than on the inner side
(28). Another study showed a TAD
>25 mm is a predictor of postoperative cut-out (26). In this study, there was no observed
association between TAD and max-torque in cases with TAD ≤25 mm.
This suggests that when the lag screw is inserted deeper than 25
mm, the lag screw insertion torque is sufficiently high enough to
prevent postoperative cut-out. Based on these results, it is likely
that HA augmentation would be the optimal approach to improve screw
pull-out strength and to reduce the risk of screw cut-out in the
treatment of trochanteric femoral fracture (22,23,29).
In this study, no complications were reported during
the follow-up period. By contrast, cement augmentation is
reportedly related to thermal osteonecrosis and bone cement
syndrome, resulting in postoperative lag screw cut-out (9,13-16).
Therefore, we believe that HA augmentation may be safer than cement
augmentation in the treatment of trochanteric femoral fractures.
Yamada et al (30) reported
that HA-coated titanium implants would induce bone regeneration
around the implant after the surgery. There may be an additional
benefit of this surgical treatment that new bone formation would be
induced. In this study, we could not find new bone formation in the
analysis of postoperative radiographs. Quantitative analysis such
as Quantitative Computed Tomography and DEXA around the screw would
be needed to evaluate this effect.
Several limitations should be considered when
interpreting the results of this study. Namely, the lack of
postoperative clinical evaluations and the small sample size, made
statistical evaluation difficult. Further studies are needed to
evaluate the exact effects of HA augmentation in the treatment of
trochanteric femoral fractures.
In conclusion, the max-torque/n-BMD ratio is
improved by HA augmentation in the treatment of trochanteric
femoral fracture surgery. Moreover, HA augmentation could increase
the bone-implant interface and decrease the risk of cut-out after
surgery.
Acknowledgements
Not applicable.
Funding
Funding: The present study was partly supported by a
Grant-in-Aid for Scientific Research (grant no. 19K18471) from the
Ministry of Education, Culture, Sports, Science and Technology of
Japan, and a Grant-in-Aid for Research (grant no. 2213035) from
Nagoya City University.
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available on the figshare repository (URL:
https://doi.org/10.6084/m9.figshare.21308985).
Authors' contributions
TU wrote the original draft, acquired, analyzed, and
interpreted the data and acquired the funding. NT conceived and
designed this study, and wrote the original draft. HI acquired,
analyzed and interpreted the data. GK wrote the original draft,
analyzed and interpreted the data, and acquired the funding. HS, YH
and IS analyzed and interpreted the data. YU, YN and HM reviewed
the original draft, analyzed and interpreted the data, and
administrated the project and resources. TU and NT confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Research Ethics
Committee of Kainan Hospital (approval no. 20230413-01). Written
informed consent was obtained from all participants before
surgery.
Patient consent for publication
Written informed consent for the publication of any
data and/or accompanying images was obtained from all patients
preoperatively.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kannus P, Parkkari J, Sievänen H, Heinonen
A, Vuori I and Järvinen M: Epidemiology of hip fractures. Bone. 18
(1 Suppl):S57–S63. 1996.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hayes WC, Myers ER, Robinovitch SN, Van
Den Kroonenberg A, Courtney AC and McMahon TA: Etiology and
prevention of age-related hip fractures. Bone. 18 (1
Suppl):77S–86S. 1996.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Holt G, Smith R, Duncan K, Finlayson DF
and Gregori A: Early mortality after surgical fixation of hip
fractures in the elderly: An analysis of data from the scottish hip
fracture audit. J Bone Joint Surg Br. 90:1357–1363. 2008.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bartoníček J and Rammelt S: The history of
internal fixation of proximal femur fractures Ernst Pohl-the genius
behind. Int Orthop. 38:2421–2426. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Usami T, Takada N, Nishida K, Sakai H,
Iwata H, Sekiya I, Ueki Y, Murakami H and Kuroyanagi G: Banding
with lesser trochanter fragment using nonabsorbable tape in
trochanteric femoral fractures. SICOT J. 7(33)2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Socci AR, Casemyr NE, Leslie MP and
Baumgaertner MR: Implant options for the treatment of
intertrochanteric fractures of the hip: Rationale, evidence, and
recommendations. Bone Joint J. 99-B:128–133. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yu X, Wang H, Duan X, Liu M and Xiang Z:
Intramedullary versus extramedullary internal fixation for unstable
intertrochanteric fracture, a meta-analysis. Acta Orthop Traumatol
Turc. 52:299–307. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Barrios C, Broström LA, Stark A and
Walheim G: Healing complications after internal fixation of
trochanteric hip fractures: The prognostic value of osteoporosis. J
Orthop Trauma. 7:438–442. 1993.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Cheng CL, Chow SP, Pun WK and Leong JC:
Long-term results and complications of cement augmentation in the
treatment of unstable trochanteric fractures. Injury. 20:134–138.
1989.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kammerlander C, Gebhard F, Meier C, Lenich
A, Linhart W, Clasbrummel B, Neubauer-Gartzke T, Garcia-Alonso M,
Pavelka T and Blauth M: Standardised cement augmentation of the
PFNA using a perforated blade: A new technique and preliminary
clinical results. A prospective multicentre trial. Injury.
42:1484–1490. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Fensky F, Nüchtern JV, Kolb JP, Huber S,
Rupprecht M, Jauch SY, Sellenschloh K, Püschel K, Morlock MM,
Rueger JM and Lehmann W: Cement augmentation of the proximal
femoral nail antirotation for the treatment of osteoporotic
pertrochanteric fractures-a biomechanical cadaver study. Injury.
44:802–807. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Erhart S, Schmoelz W, Blauth M and Lenich
A: Biomechanical effect of bone cement augmentation on rotational
stability and pull-out strength of the proximal femur nail
antirotation™. Injury. 42:1322–1327. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lu JX, Huang ZW, Tropiano P, Clouet
D'Orval B, Remusat M, Dejou J, Proust JP and Poitout D: Human
biological reactions at the interface between bone tissue and
polymethylmethacrylate cement. J Mater Sci Mater Med. 13:803–809.
2002.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Stańczyk M and van Rietbergen B: Thermal
analysis of bone cement polymerisation at the cement-bone
interface. J Biomech. 37:1803–1810. 2004.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Boner V, Kuhn P, Mendel T and Gisep A:
Temperature evaluation during PMMA screw augmentation in
osteoporotic bone-an in vitro study about the risk of thermal
necrosis in human femoral heads. J Biomed Mater Res B Appl
Biomater. 90:842–848. 2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Fliri L, Lenz M, Boger A and Windolf M: Ex
vivo evaluation of the polymerization temperatures during cement
augmentation of proximal femoral nail antirotation blades. J Trauma
Acute Care Surg. 72:1098–1101. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Shin SJ and Lee JH and Lee JH: Influence
of hydroxyapatite stick on pedicle screw fixation in degenerative
lumbar spine: Biomechanical and radiologic study. Clin Spine Surg.
30:E819–E826. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hofmann A, Gorbulev S, Guehring T, Schulz
AP, Schupfner R, Raschke M, Huber-Wagner S and Rommens PM: CERTiFy
Study Group. Autologous iliac bone graft compared with biphasic
hydroxyapatite and calcium sulfate cement for the treatment of bone
defects in tibial plateau fract: A prospective, randomized,
open-label, multicenter study. J Bone Joint Surg Am. 102:179–193.
2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Tami AE, Leitner MM, Baucke MG, Mueller
TL, van Lenthe GH, Müller R and Ito K: Hydroxyapatite particles
maintain peri-implant bone mantle during osseointegration in
osteoporotic bone. Bone. 45:1117–1124. 2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ohe M, Moridaira H, Inami S, Takeuchi D,
Nohara Y and Taneichi H: Pedicle screws with a thin hydroxyapatite
coating for improving fixation at the bone-implant interface in the
osteoporotic spine: Experimental study in a porcine model. J
Neurosurg Spine. 28:679–687. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Reynolds KJ, Cleek TM, Mohtar AA and Hearn
TC: Predicting cancellous bone failure during screw insertion. J
Biomech. 46:1207–1210. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Suhm N, Hengg C, Schwyn R, Windolf M,
Quarz V and Hänni M: Mechanical torque measurement predicts load to
implant cut-out: A biomechanical study investigating DHS anchorage
in femoral heads. Arch Orthop Trauma Surg. 127:469–474.
2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ab-Lazid R, Perilli E, Ryan MK, Costi JJ
and Reynolds KJ: Does cancellous screw insertion torque depend on
bone mineral density and/or microarchitecture? J Biomech.
47:347–353. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Iwata H, Takada N, Kuroyanagi G, Ikuta K,
Usami T, Sekiya I and Murakami H: Effect of hydroxyapatite tubes on
the lag screw intraoperative insertion torque for the treatment of
intertrochanteric femoral fractures. Injury. 52:3377–3381.
2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Baumgaertner MR, Curtin SL, Lindskog DM
and Keggi JM: The value of the tip-apex distance in predicting
failure of fixation of peritrochanteric fractures of the hip. J
Bone Joint Surg Am. 77:1058–1064. 1995.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kane P, Vopat B, Heard W, Thakur N, Paller
D, Koruprolu S and Born C: Is tip apex distance as important as we
think? A biomechanical study examining optimal lag screw placement.
Clin Orthop Relat Res. 472:2492–2498. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Pintus E, Sorbolini S, Albera A, Gaspa G,
Dimauro C, Steri R, Marras G and Macciotta NP: Use of locally
weighted scatterplot smoothing (LOWESS) regression to study
selection signatures in piedmontese and Italian brown cattle
breeds. Anim Genet. 45:1–11. 2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Tamaddon M, Chen SM, Vanaclocha L, Hart A,
El-Husseiny M, Henckel J and Liu C: Decrease in local volumetric
bone mineral density in osteoarthritic joints is associated with
the increase in cartilage damage: A peripheral quantitative CT
study. Fron Mater. 4(37)2017.
|
|
29
|
Hasegawa K, Yamamura S and Dohmae Y:
Enhancing screw stability in osteosynthesis with hydroxyapatite
granules. Arch Orthop Trauma Surg. 117:175–176. 1998.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yamada M, Ueno T, Tsukimura N, Ikeda T,
Nakagawa K, Hori N, Suzuki T and Ogawa T: Bone integration
capability of nanopolymorphic crystalline hydroxyapatite coated on
titanium implants. Int J Nanomedicine. 7:859–873. 2012.PubMed/NCBI View Article : Google Scholar
|