Introduction
Periodontitis is a common clinical oral infectious
disease and one of the main causes of adult tooth loss, severely
affecting the health of patients (1). Periodontitis, which is characterized
by the damage of periodontal supporting tissue, usually results
from pathogenic microorganisms, thus contributing to the loss as
well as the extraction of teeth (2). It has been previously reported that
periodontitis is a chronic inflammation of the periodontal
supporting tissue induced by multiple factors, manifesting with
alveolar bone absorption, gingival inflammation and loss of
periodontal attachment with pathological features (3,4). The
association between microbiota and host can determine the
occurrence and the advancement of periodontal diseases. In the
aforementioned diseases, the induction of the immune response of
the host against bacteria and their products is considered as the
key factor resulting in the destruction of periodontal tissue. The
periodontal tissue is commonly impaired by the excessive release of
inflammatory factors (5-7).
Additionally, periodontal ligament contains a group of pluripotent
periodontal stem cells, periodontal ligament stem cells (hPDLSCs),
which have significant involvement in the reconstruction,
regeneration and fixation of periodontal tissue (8). Furthermore, a case from a previous
study has shown that the periodontal inflammatory microenvironment
can destroy periodontal tissue by suppressing the regeneration
ability of hPDLSCs (9). Therefore,
the suppression of inflammatory injury and improvement of the
osteogenic differentiation capability of hPDLSCs could be a
promising treatment approach for periodontitis.
Protein arginine methyltransferases (PRMTs) catalyze
the methylation of arginine residues on several proteins, including
histones and non-histone proteins (10). As a common post-translational
modification, arginine methylation has been associated with several
cellular processes, such as DNA transcription, signal transduction
and subcellular protein localization (11). PRMT5, a member of the PRMT family,
serves a crucial role in several biological processes (12). A study showed that PRMT5
downregulation could suppress the differentiation capacity of
osteoclasts and exerted a protective effect on bone in patients
underwent ovariectomy via downregulating 10-kDa (CXCL10) and
radical S-adenosyl methionine domain containing 2(13). In addition, PRMT5 silencing
promoted the osteogenic differentiation of mesenchymal stromal
cells and repressed basal interferon stimulated gene expression
(14). A previous study also found
that PRMT5 was involved and promoted inflammatory responses of
bronchial epithelial cells (15).
However, the role of PRMT5 in inflammatory response and the
osteogenic differentiation of hPDLSCs remains to be elucidated.
Therefore, the present study aimed to investigate the biological
role of PRMT5 and its potential underlying mechanism in
liposaccharide (LPS)-induced hPDLSCs. It was hypothesized that
PRMT5 inhibition can reduce inflammation and accelerate the
osteogenic differentiation of LPS-induced hPDLSCs via regulating
the STAT3/NF-κB signaling.
Materials and methods
Cell culture
hPDLSCs were obtained from ScienCell Research
Laboratories, Inc. and cultured in DMEM (Hyclone; Cytiva)
supplemented with 1% antibiotics and 10% FBS at 37˚C in the
presence of 5% CO2. hPDLSCs were treated with 0.1, 1 or
10 µM EPZ015666 (EPZ), a PRMT5 inhibitor, for three days. To
establish a periodontitis model, hPDLSCs were induced with 10 µg/ml
P. gingivalis LPS (MiliporeSigma) for 24 h. Osteoblast
differentiation was induced using conditional medium containing 1%
antibiotics, 10% FBS, α-MEM, 50 µg/ml l-ascorbic acid, 10 nM
dexamethasone and 10 mM β-glycerophosphate (MiliporeSigma).
Colivelin (1 µM), a STAT3 agonist, was used to activate the STAT3
pathway.
ELISA
The secretion levels of IL-1β, IL-6 and TNF-α in
cultured hPDLSCs were assessed using the IL-1β assay kit (cat. no.
H002), IL-6 assay kit (cat. no. H007-1-1) and TNF-α assay kit (cat.
no. H052-1) (Nanjing Jiancheng Bioengineering Institute) according
to the manufacturer's instructions. The optical density (OD) values
at a wavelength of 450 nm were measured utilizing the xMark
Microplate absorbance spectrophotometer (Bio-Rad Laboratories,
Inc.). Finally, the concentration of the above inflammatory factors
was calculated based on the corresponding standard curves.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was isolated from 1x104 hPDLSCs
using a TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions, and
its concentration was measured employing NanoDrop 2000 (Thermo
Fisher Scientific, Inc.) at 260 and 280 nm. Subsequently, RNA was
reverse transcribed into cDNA using the PrimeScript RT Master Mix
(Takara Bio, Inc.) according to the manufacturer's instructions.
qPCR was performed on the ABI PRISM 7900 Real-Time system (Applied
Biosystems; Thermo Fisher Scientific, Inc.) with the SYBR Premix
ExTaq kit (Takara Bio, Inc.) according to the manufacturer's
instructions. The thermocycling conditions were as follows: Initial
denaturation at 95˚C for 3 min; followed by 40 cycles of
denaturation at 95˚C for 30 sec, annealing at 60˚C for 30 sec and
extension at 72˚C for 30 sec. The primer sequences for PCR were:
PRMT5: 5'-CTGACACACTAGGGGCTGTG-3' (forward) and
5'-ACTAGTCTGCCCTTCTCCGT-3' (reverse); GAPDH:
5'-GGGAAACTGTGGCGTGAT-3' (forward) and 5'-GAGTGGGTGTCGCTGTTGA-3'
(reverse). The relative mRNA levels were calculated using the
2-ΔΔCq method (16).
Alkaline phosphatase (ALP) activity
assay
ALP activity was measured to assess the
differentiation ability of hPDLSCs using an ALP Assay kit (Beyotime
Institute of Biotechnology), according to the manufacturer's
instructions. The OD values were measured at a wavelength of 405 nm
using a microplate reader.
Alizarin red staining (ARS)
The cells were cultured for two weeks and were then
mineralized to form opaque calcified nodules. Subsequently, hPDLSCs
were first treated with 95% ethanol for 10 min and were then
stained with 0.1% ARS solution (MilliporeSigma) for 15 min at room
temperature. To measure the degree of mineralization, ARS released
from the cell matrix was visualized using an inverted light
microscope.
Western blot analysis
Total proteins were isolated from hPDLSCs with RIPA
buffer (Shanghai Yisheng Biotechnology Co., Ltd.) and the protein
concentration was determined using a BCA Protein Assay Kit
(Shanghai Fantai Biotechnology Co., Ltd.). Following separation by
10% SDS-PAGE (60 µg/lane), the proteins were transferred onto PVDF
membranes. The overnight incubation of membranes, which were first
blocked with 5% non-fat milk in 0.1% TBS-Tween-20 for 2 h at room
temperature, was performed at 4˚C with primary antibodies against
PRMT5 (cat. no. ab109451; 1:10,000; Abcam), inducible nitric oxide
synthase (iNOS; cat. no. ab178945; 1:1,000; Abcam),
cyclooxygenase-2 (COX-2; cat. no. ab179800; 1:1,000; Abcam), bone
morphogenetic protein 2 (BMP2; cat. no. ab284387; 1:1,000; Abcam),
osteocalcin (OCN; cat. no. ab133612; 1:1,000; Abcam), runt-related
transcription factor 2 (Runx2; cat. no. ab92336; 1:5,000; Abcam),
phosphorylated (p)-STAT3 (cat. no. ab267373; 1:1,000; Abcam), STAT3
(cat. no. ab68153; 1:1,000; Abcam), p-NF-κB (cat. no. ab239882;
1:1,000; Abcam), NF-κB (cat. no. ab220803; 1:1,000; Abcam) and
GAPDH (cat. no. ab9485; 1:2,500; Abcam). Subsequently, the
membranes were incubated with the corresponding HRP-labeled
secondary antibody (cat. no. ab6759; 1:5,000; Abcam) for 1 h at
room temperature. The protein blots were visualized using an ECL
detection system (MilliporeSigma) and analyzed with ImageJ software
(version 1.49; National Institutes of Health). The ratio of the
target protein to GAPDH light density was considered as the
relative protein expression.
Statistical analysis
All experiments were performed three times or more.
All data were analyzed with SPSS 23.0 software (IBM Corporation)
using one-way ANOVA followed by Bonferroni's multiple comparison
test. Data are expressed as the mean ± standard deviation.
P<0.05 was considered to indicate a statistically significant
difference.
Results
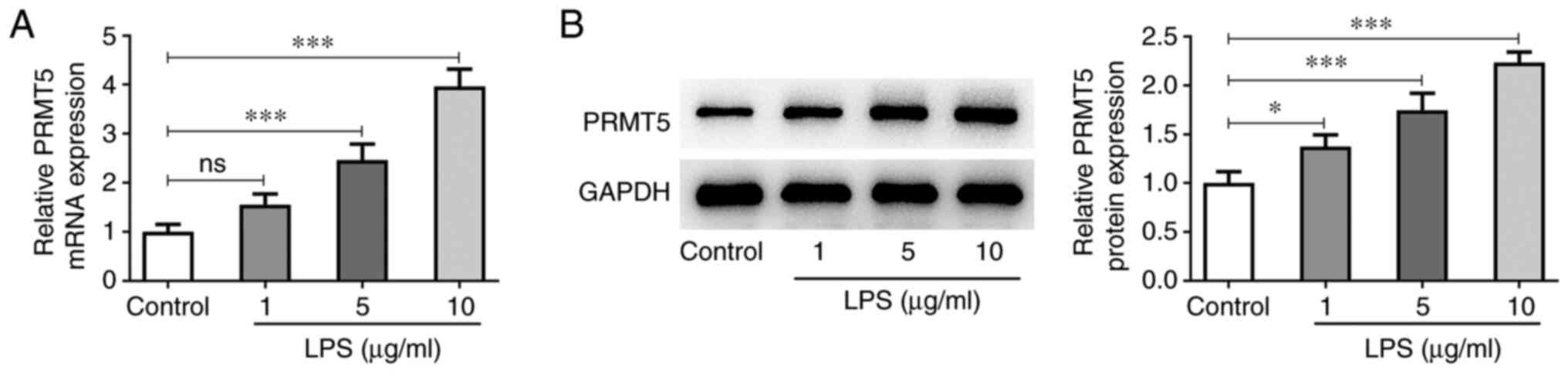
PRMT5 is upregulated in LPS-induced
hPDLSCs
To explore the biological role of PRMT5 in
LPS-induced hPDLSCs, the expression levels of PRMT5 in treated
hPDLSCs were initially assessed. As shown in Fig. 1A and B, the mRNA and protein expressions levels
of PRMT5 in LPS-induced hPDLSCs (5-10 µg/ml) were both markedly
elevated compared with the control cells. Therefore, LPS
concentration of 10 µg/ml was selected for the following
assays.
PRMT5 inhibition attenuates
inflammation in LPS-induced hPDLSCs
To investigate the function of PRMT5 in LPS-induced
hPDLSCs, cells were treated with 0.1-10 µM EPZ. The inhibitory
effect of EPZ on LPS-induced hPDLSCs is shown in Fig. 2A and B. LPS treatment of hPDLSCs significantly
increased the secretion levels of IL-1β, IL-6 and TNF-α, which were
reduced following co-treatment with 1-10 µM EPZ (Fig. 2C). In addition, western blot
analysis revealed that the protein expression levels of iNOS and
COX-2 were increased by LPS, which were also restored following
co-treatment of LPS-induced hPDLSCs with 0.1-10 µM EPZ (Fig. 2D).
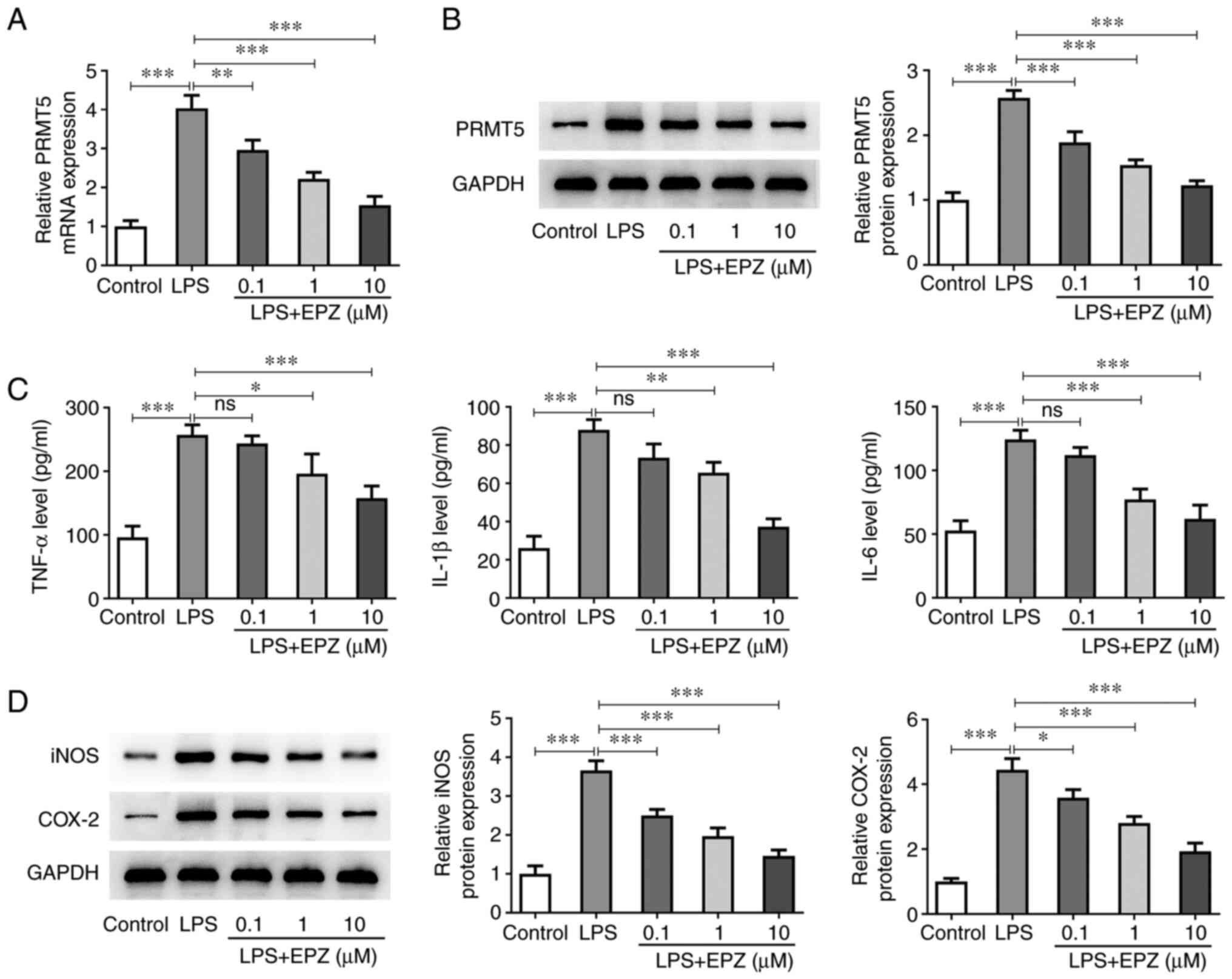 | Figure 2Downregulation of PRMT5 reduces
LPS-induced hPDLSCs inflammation. (A) mRNA expression and (B)
protein level of PRMT5 in LPS-induced hPDLSCs with 0.1-10 µM EPZ
were detected by reverse transcription-quantitative PCR and western
blotting. (C) The levels of TNF-α, IL-1β and IL-6 were evaluated by
ELISA. (D) Western blot assay was used to assess protein levels of
iNOS and COX-2. Data are expressed as mean ± standard deviation.
*P<0.05, **P<0.01,
***P<0.001. ns, not significant. PRMT5, protein
arginine methyltransferase 5; LPS, liposaccharide; hPDLSCs, human
periodontal ligament stem cells; EPZ, EPZ015666; iNOS, inducible
nitric oxide synthase; COX-2, cyclooxygenase-2. |
PRMT5 inhibition promotes the
osteogenic differentiation of LPS-induced hPDLSCs
Subsequently, the current study aimed to evaluate
the effect of PRMT5 on the differentiation ability of LPS-induced
hPDLSCs. As shown in Fig. 3A, LPS
stimulation dramatically repressed ALP activity in hPDLSCs, which
was subsequently restored by PRMT5 inhibition in a dose-dependent
manner. Furthermore, cell treatment with LPS reduced the area of
ARS in hPDLSCs. However, PRMT5 inhibition abrogated the effect of
LPS on ARS area (Fig. 3B and
C). Additionally, BMP2, OCN and
Runx2 were downregulated after cell induction with LPS. However,
the above effect was also reversed by PRMT5 inhibition (Fig. 3D).
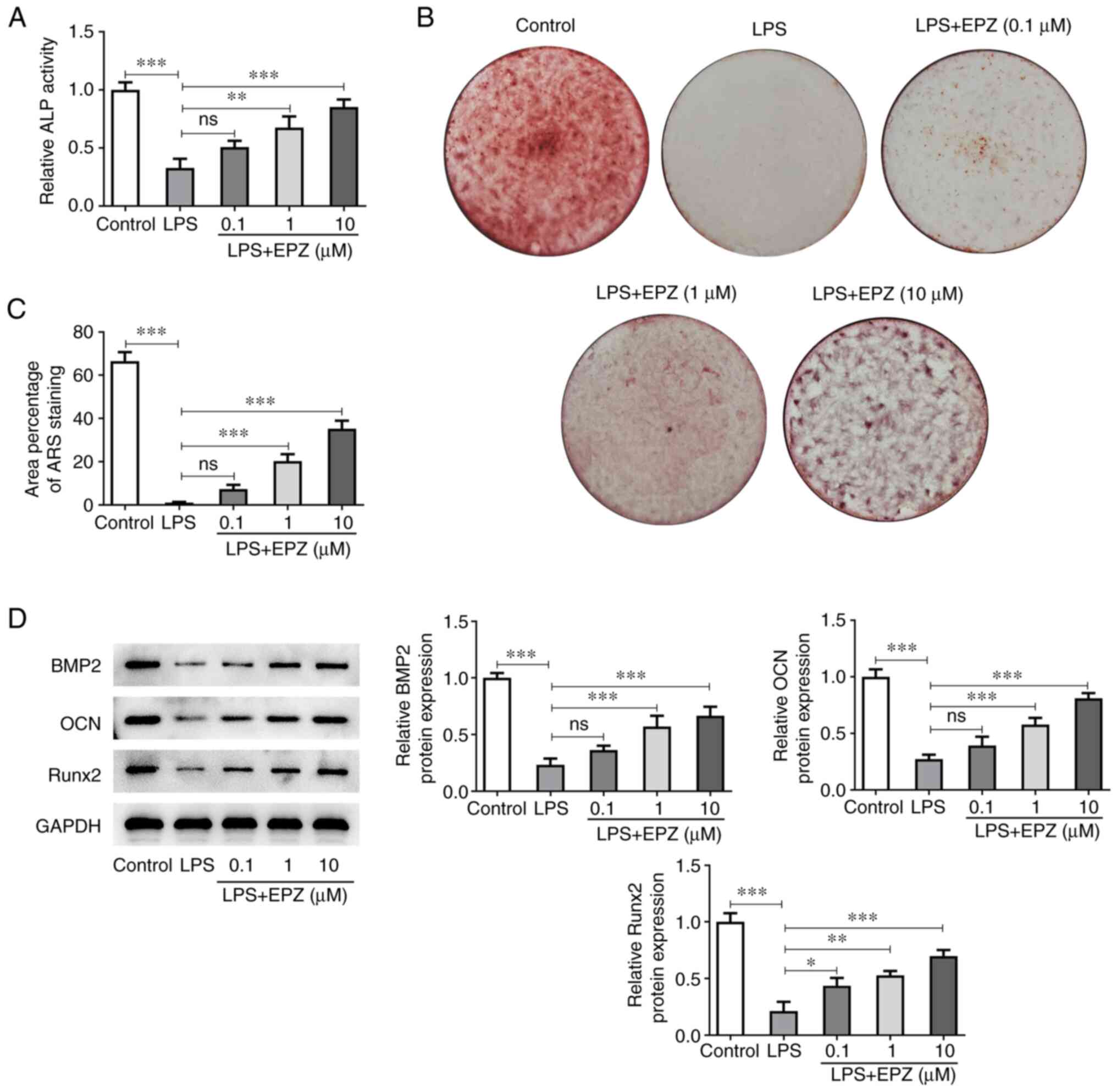 | Figure 3Inhibition of PRMT5 promotes
LPS-induced osteogenic differentiation of hPDLSCs. (A) ALP activity
in LPS-induced hPDLSCs with 0.1-10 µM EPZ. (B and C) The area of
Alizarin red staining in LPS-induced hPDLSCs with 0.1-10 µM EPZ.
(D) Western blot assay was used to assess protein levels of BMP2,
OCN and Runx2. Data are expressed as mean ± standard deviation.
*P<0.05, **P<0.01,
***P<0.001, ns, not significant. PRMT5, protein
arginine methyltransferase 5; LPS, liposaccharide; hPDLSCs, human
periodontal ligament stem cells; ALP, alkaline phosphatase; ARS,
Alizarin red staining; EPZ, EPZ015666; BMP2, bone morphogenetic
protein 2; OCN, osteocalcin; Runx2, runt-related transcription
factor 2. |
Inhibition of PRMT5 suppresses the
activation of the STAT3/NF-κB pathway in LPS-induced hPDLSCs
Subsequently, the present study aimed to uncover the
potential mechanism underlying the regulatory effect of PRMT5 on
LPS-induced hPDLSCs. As shown in Fig.
4, hPDLSCs treatment with LPS markedly enhanced the protein
expression levels of p-STAT3 and p-NF-κB. However, PRMT5 inhibition
abrogated the effect of LPS on the expression of p-STAT3 and
p-NF-κB in LPS-induced hPDLSCs. The total expression levels of
STAT3 and NF-κB remained unchanged.
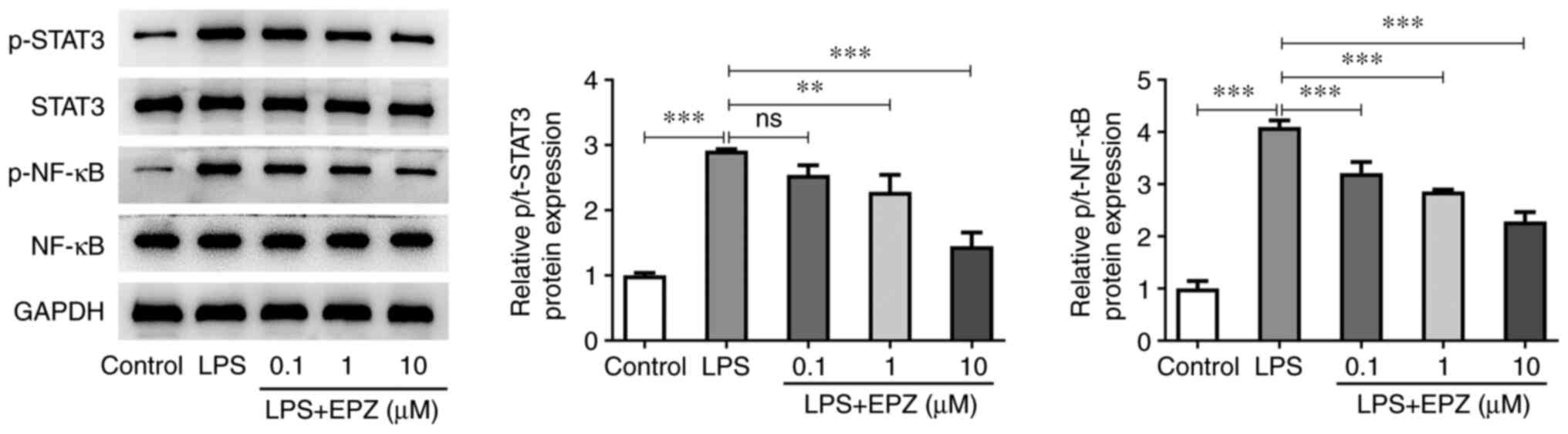 | Figure 4Inhibition of PRMT5 suppresses the
activation of STAT3/NF-κB pathway in LPS-induced hPDLSCs. Western
blot assay was used to measure the protein levels of STAT3, NF-κB,
p-STAT3 and p-NF-κB in LPS-induced hPDLSCs with 0.1-10 µM EPZ. Data
are expressed as mean ± standard deviation. **P<0.01,
***P<0.001, ns, not significant. PRMT5, protein
arginine methyltransferase 5; LPS, liposaccharide; hPDLSCs, human
periodontal ligament stem cells; p-, phosphorylated; EPZ,
EPZ015666. |
PRMT5 inhibition restrains
inflammation and promotes the osteogenic differentiation of
LPS-induced hPDLSCs via blocking the activation of the STAT3/NF-κB
pathway
To evaluate the role of the STAT3/NF-κB signaling
pathway in PRMT5-mediated LPS injury, LPS-induced hPDLSCs were
co-treated with PRMT5 inhibitor and the STAT3 agonist, colivelin.
Western blot analysis showed that cell co-treatment with EPZ and
colivelin upregulated p-STAT3 and p-NF-κB in LPS-induced hPDLSCs
compared with EPZ-treated cells (Fig.
5A). Additionally, EPZ plus with colivelin elevated the
contents of IL-1β, IL-6, TNF-α, iNOS and COX-2 in LPS-induced
hPDLSCs compared with EPZ-treated cells (Fig. 5B and C). Furthermore, colivelin diminished the
relative ALP activity and ARS area compared with EPZ-treated
hPDLSCs (Fig. 5D-F). Finally,
co-treatment with EPZ and colivelin markedly reduced the expression
levels of BMP2, OCN and Runx2 in LPS-induced hPDLSCs compared with
EPZ-treated hPDLSCs (Fig. 5G).
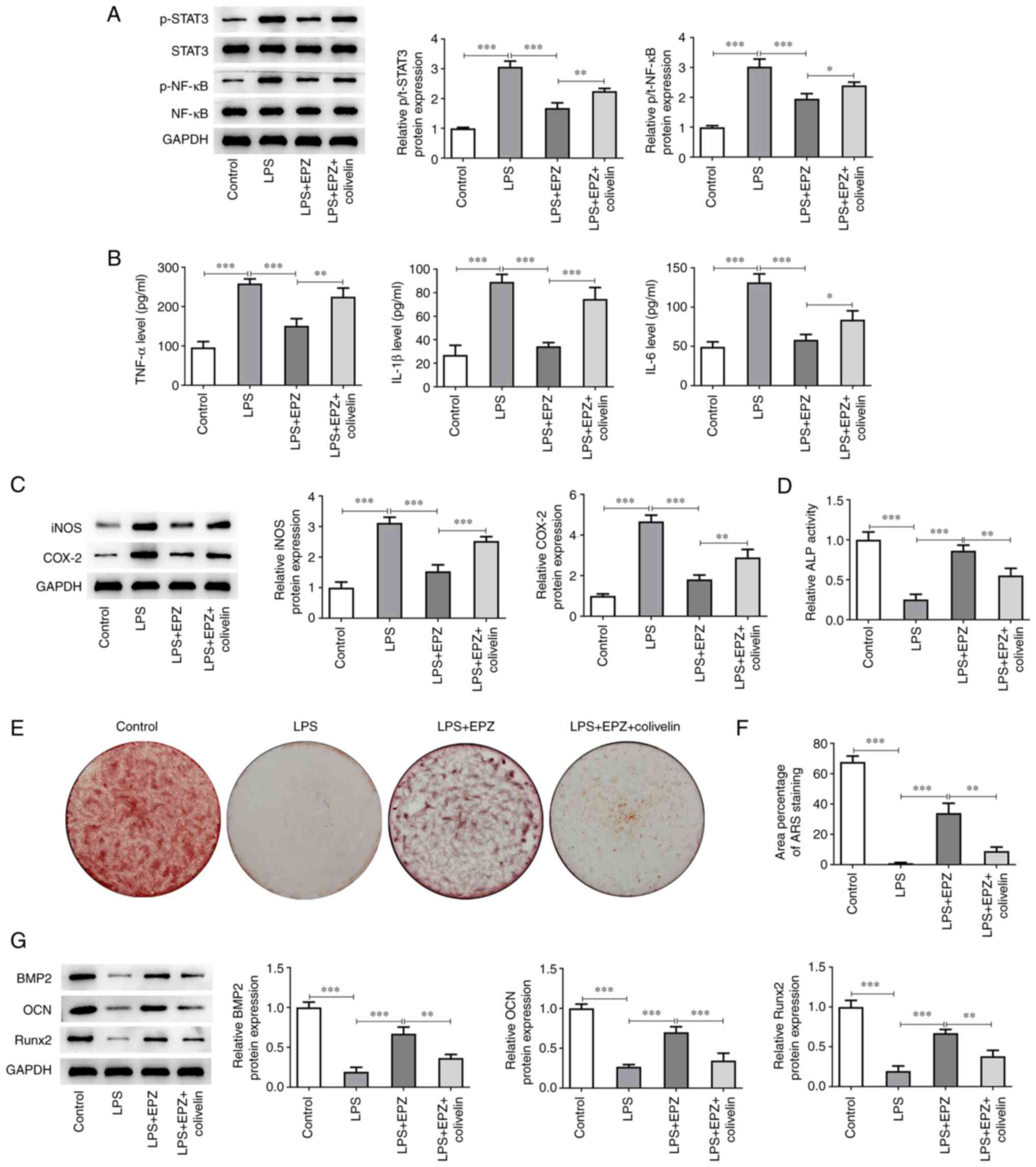 | Figure 5Downregulation of PRMT5 restrains
LPS-induced hPDLSCs inflammation and promotes osteogenic
differentiation by blocking the activation of STAT3/NF-κB pathway.
(A) Western blot assay was used to measure the protein levels of
STAT3, NF-κB, p-STAT3 and p-NF-κB in LPS-induced hPDLSCs with EPZ
or colivelin. (B) The levels of IL-1β, IL-6, TNF-α were evaluated
by ELISA. (C) Western blot assay was used to assess protein levels
of iNOS and COX-2. (D) ALP activity in LPS-induced hPDLSCs with EPZ
or colivelin. (E and F) The area of Alizarin red staining in
LPS-induced hPDLSCs with EPZ or colivelin. (G) Western blot assay
was used to assess protein levels of BMP2, OCN and Runx2. Data are
expressed as mean ± standard deviation. *P<0.05,
**P<0.01, ***P<0.001. PRMT5, protein
arginine methyltransferase 5; LPS, liposaccharide; hPDLSCs, human
periodontal ligament stem cells; p-, phosphorylated; EPZ,
EPZ015666; iNOS, inducible nitric oxide synthase; COX-2,
cyclooxygenase-2 ALP, alkaline phosphatase; ARS, Alizarin red
staining. |
Discussion
Periodontitis may result in the damage of the
alveolar bone. Therefore, bone regeneration is considered as one
the most significant parts of the diagnosis and treatment of
periodontal diseases (17). In
addition, PDLSCs serve a significant role in bone regeneration due
to their high proliferation, self-renewal and multi-directional
differentiation abilities (18).
hPDLSCs are derived from mesenchymal stem cells of the periodontal
tissue and can differentiate into several tissues under in
vitro culture conditions, which in turn can be used as the cell
source of periodontal tissue regeneration (19). Nevertheless, changes in the
microenvironment can affect the differentiation ability of hPDLSCs
(20). A previous study
demonstrated that the proliferation ability of hPDLSCs under an
inflammatory environment was significantly increased, while their
differentiation capability was greatly reduced (21). Therefore, it is of critical
importance to attenuate the effects of inflammation on hPDLSCs in
periodontitis. In the present study, PRMT5 inhibition decreased
LPS-induced hPDLSCs inflammation and osteogenic differentiation via
inactivating the STAT3/NF-κB signaling pathway, thus suggesting
that PRMT5 could act as a potential target for attenuating
LPS-induced periodontitis.
LPS is commonly used to induce periodontitis injury
and it has been reported to induce cell inflammation, apoptosis,
autophagy as well as endoplasmic reticulum stress in oral diseases
(22). In the current study an
in vitro periodontitis model was established following
hPDLSCs stimulation with LPS. The results showed that cell
treatment with LPS significantly aggravated inflammation and
inhibited osteogenic differentiation in hPDLSCs. PRMT5 is an
arginine methyltransferase that serves a significant role in
osteogenic differentiation and inflammatory responses (23). A study showed that PRMT5 inhibition
relieved cartilage degradation via inactivating MAPK and NF-κB
signaling (24). Another study
also revealed that PRMT5 inhibition prevented inflammation and
migration of fibroblast-like synoviocytes in rheumatoid arthritis
(RA). It was therefore considered as a promising treatment approach
for RA (25). Qiao et al
(26) suggested that RA was
closely associated with periodontitis and that patients suffering
from periodontitis could be more vulnerable to RA. Another study
also showed that PRMT5 inhibition could alleviate the development
of periodontitis via inhibiting the activation and metastasis of
dendritic cells (27). Therefore,
it was hypothesized that PRMT5 could be also involved in
LPS-induced inflammatory response and osteogenic differentiation of
hPDLSCs. The results of the present study demonstrated that PRMT5
was upregulated in LPS-induced hPDLSCs. Additionally, PRMT5
inhibition could attenuate LPS-induced cell inflammation and
rehabilitate the osteogenic differentiation of hPDLSCs, thus
supporting the protective effect of PRMT5 inhibition on LPS-induced
hPDLSCs injury.
A previous study revealed that PRMT5 could act as a
critical regulator of STAT3 activation. Therefore, PRMT5 depletion
or inhibition could significantly inhibit the activation of
STAT3(28). Chen et al
(29) also showed that suppression
of deubiquitinase ubiquitin-specific peptidase 5 restrained the
inflammatory response in chronic periodontitis via inhibiting STAT3
signaling. In addition, Jiang et al (30) revealed that angiopoietin-like
protein 2 (ANGPTL2) downregulation activated STAT3 and NF-κB
signaling, and inhibited Akt signaling under inflammatory
environment. However, treatment with a STAT3 inhibitor suppressed
the inflammatory response of ANGPTL2-induced periodontal ligament
cells. It was therefore hypothesized that STAT3/NF-κB signaling
could be involved in PRMT5-mediated hPDLSCs. It is documented that
LPS can activate the STAT pathways according to previous studies
(31,32). In addition, some studies have
reported LPS can specifically phosphorylate STAT3 (33-35).
In the present study, LPS activated the phosphorylation of STAT3
and NF-κB in hPDLSCs. The activation of the STAT3/NF-κB pathway
could reverse the effects of PRMT5 inhibition on LPS-induced
hPDLSCs inflammation and osteogenic differentiation, which was
consistent with previous studies. The present study found that
PRMT5 inhibition can reduced the production of inflammatory
cytokines, meanwhile the addition of STAT3 agonist colivelin
enhanced the decreased inflammatory cytokine, which indicated that
PRMT5 inhibition indirectly inhibited the activation of some
inflammatory cytokine by inhibiting the pathway of STAT3/NF-κB. In
addition, the present study did not explore the substrate PRMT5
enzyme catalyzed and the mechanism of PRMT5 and how to catalyze the
substrate in LPS-induced hPDLSCs will continue to be studied.
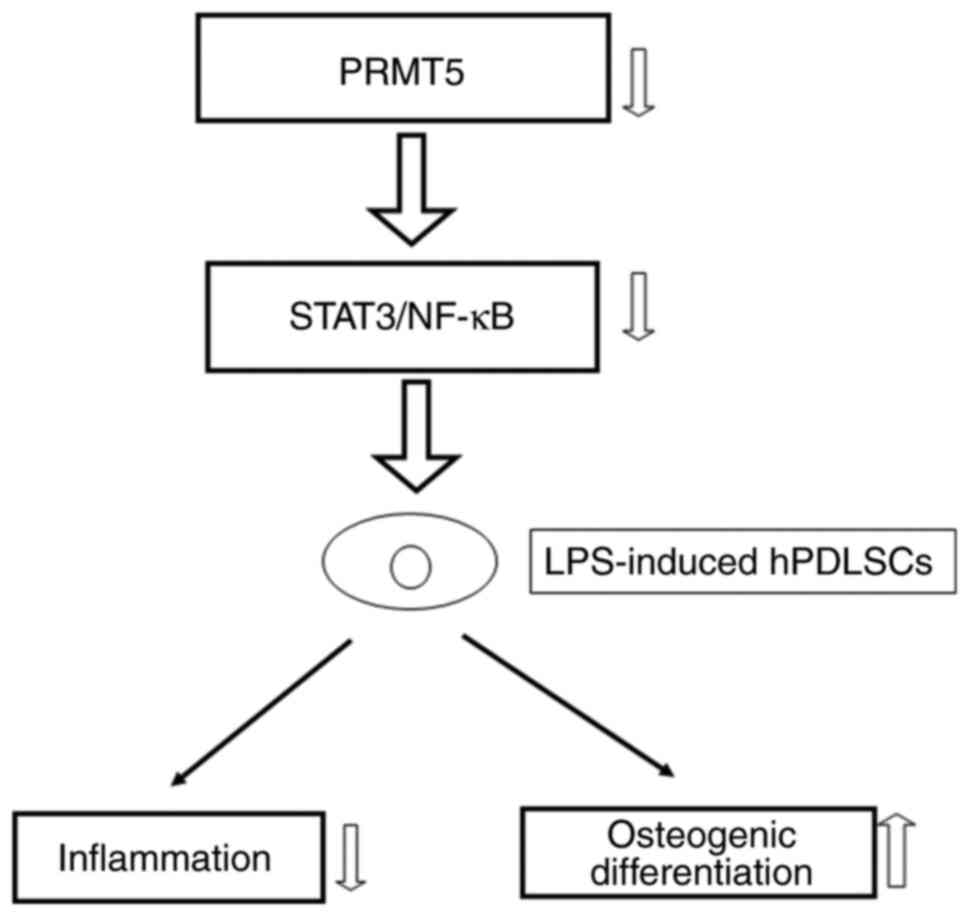
In summary, the current study highlighted the
essential protective effect of PRMT5 inhibition on LPS-induced cell
inflammation and osteogenic differentiation. In addition, the
results supported the important role of the PRMT5-regulated
STAT3/NF-κB pathway in LPS-induced hPDLSCs (Fig. 6), thus suggesting that PRMT5 could
be a promising therapy approach for improving periodontitis.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
KZ and XT designed the study, drafted and revised
the manuscript. CL and JS analyzed the data and searched the
literature. KZ and XT confirm the authenticity of all the raw data.
KS, XT, CL and JS performed the experiments. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Slots J: Periodontitis: Facts, fallacies
and the future. Periodontol 2000. 75:7–23. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kwon T, Lamster IB and Levin L: Current
concepts in the management of periodontitis. Int Dent J.
71:462–476. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Fischer RG, Lira Junior R, Retamal-Valdes
B, Figueiredo LC, Malheiros Z, Stewart B and Feres M: Periodontal
disease and its impact on general health in Latin America. Section
V: Treatment of periodontitis. Braz Oral Res.
34(e026)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bartold PM: Lifestyle and periodontitis:
The emergence of personalized periodontics. Periodontol 2000.
78:7–11. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Cecoro G, Annunziata M, Iuorio MT, Nastri
L and Guida L: Periodontitis, low-grade inflammation and systemic
health: A scoping review. Medicina (Kaunas). 56(272)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Van Dyke TE and Sima C: Understanding
resolution of inflammation in periodontal diseases: Is chronic
inflammatory periodontitis a failure to resolve? Periodontol 2000.
82:205–213. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Verrusio C, Iorio-Siciliano V, Blasi A,
Leuci S, Adamo D and Nicolò M: The effect of orthodontic treatment
on periodontal tissue inflammation: A systematic review.
Quintessence Int. 49:69–77. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tran Hle B, Doan VN, Le HT and Ngo LT:
Various methods for isolation of multipotent human periodontal
ligament cells for regenerative medicine. In Vitro Cell Dev Biol
Anim. 50:597–602. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Liu W, Konermann A, Guo T, Jäger A, Zhang
L and Jin Y: Canonical Wnt signaling differently modulates
osteogenic differentiation of mesenchymal stem cells derived from
bone marrow and from periodontal ligament under inflammatory
conditions. Biochim Biophys Acta. 1840:1125–1134. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hwang JW, Cho Y, Bae GU, Kim SN and Kim
YK: Protein arginine methyltransferases: Promising targets for
cancer therapy. Exp Mol Med. 53:788–808. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Couto E Silva A, Wu CY, Citadin CT,
Clemons GA, Possoit HE, Grames MS, Lien CF, Minagar A, Lee RH,
Frankel A, et al: Protein arginine methyltransferases in
cardiovascular and neuronal function. Mol Neurobiol. 57:1716–1732.
2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Stopa N, Krebs JE and Shechter D: The
PRMT5 arginine methyltransferase: Many roles in development, cancer
and beyond. Cell Mol Life Sci. 72:2041–2059. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Dong Y, Song C, Wang Y, Lei Z, Xu F, Guan
H, Chen A and Li F: Inhibition of PRMT5 suppresses osteoclast
differentiation and partially protects against ovariectomy-induced
bone loss through downregulation of CXCL10 and RSAD2. Cell Signal.
34:55–65. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kota SK, Roening C, Patel N, Kota SB and
Baron R: PRMT5 inhibition promotes osteogenic differentiation of
mesenchymal stromal cells and represses basal interferon stimulated
gene expression. Bone. 117:37–46. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kim JH, Yoo BC, Yang WS, Kim E, Hong S and
Cho JY: The role of protein arginine methyltransferases in
inflammatory responses. Mediators Inflamm.
2016(4028353)2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Tomokiyo A, Wada N and Maeda H:
Periodontal ligament stem cells: Regenerative potency in
periodontium. Stem Cells Dev. 28:974–985. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Isola G, Polizzi A, Alibrandi A,
Indelicato F and Ferlito S: Analysis of Endothelin-1 concentrations
in individuals with periodontitis. Sci Rep. 10(1652)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ren S, Yao Y, Zhang H, Fan R, Yu Y, Yang
J, Zhang R, Liu C, Sun W and Miao L: Aligned fibers fabricated by
near-field electrospinning influence the orientation and
differentiation of hPDLSCs for periodontal regeneration. J Biomed
Nanotechnol. 13:1725–1734. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Shang F, Liu S, Ming L, Tian R, Jin F,
Ding Y, Zhang Y, Zhang H, Deng Z and Jin Y: Human umbilical cord
MSCs as new cell sources for promoting periodontal regeneration in
inflammatory periodontal defect. Theranostics. 7:4370–4382.
2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yu B, Hu J, Li Q and Wang F: CircMAP3K11
contributes to proliferation, apoptosis and migration of human
periodontal ligament stem cells in inflammatory microenvironment by
regulating TLR4 via miR-511 sponging. Front Pharmacol.
12(633353)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Pussinen PJ, Kopra E, Pietiäinen M, Lehto
M, Zaric S, Paju S and Salminen A: Periodontitis and
cardiometabolic disorders: The role of lipopolysaccharide and
endotoxemia. Periodontol 2000. 89:19–40. 2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Motolani A, Martin M, Sun M and Lu T: The
structure and functions of PRMT5 in human diseases. Life (Basel).
11(1074)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Dong Y, Wang P, Yang Y, Huang J, Dai Z,
Zheng W, Li Z, Yao Z, Zhang H and Zheng J: PRMT5 inhibition
attenuates cartilage degradation by reducing MAPK and NF-κB
signaling. Arthritis Res Ther. 22(201)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Chen D, Zeng S, Huang M, Xu H, Liang L and
Yang X: Role of protein arginine methyltransferase 5 in
inflammation and migration of fibroblast-like synoviocytes in
rheumatoid arthritis. J Cell Mol Med. 21:781–790. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Qiao Y, Wang Z, Li Y, Han Y, Zhou Y and
Cao X: Rheumatoid arthritis risk in periodontitis patients: A
systematic review and meta-analysis. Joint Bone Spine. 87:556–564.
2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Mi W, Qiao S, Zhang X, Wu D, Zhou L and
Lai H: PRMT5 inhibition modulates murine dendritic cells activation
by inhibiting the metabolism switch: A new therapeutic target in
periodontitis. Ann Transl Med. 9(755)2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Cai C, Gu S, Yu Y, Zhu Y, Zhang H, Yuan B,
Shen L, Yang B and Feng XH: PRMT5 enables robust STAT3 activation
via arginine symmetric dimethylation of SMAD7. Adv Sci (Weinh).
8(2003047)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Chen Q, Su J and Chen X: Role of
ubiquitin-specific protease 5 in the inflammatory response of
chronic periodontitis. Oral Dis. 29:1234–1241. 2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Jiang C, Yao S, Guo Y, Ma L, Wang X, Chen
Y, Zhang H and Cao Z: Angiopoietin-like protein 2 deficiency
promotes periodontal inflammation and alveolar bone loss. J
Periodontol. 93:1525–1539. 2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Severgnini M, Takahashi S, Rozo LM, Homer
RJ, Kuhn C, Jhung JW, Perides G, Steer M, Hassoun PM, Fanburg BL,
et al: Activation of the STAT pathway in acute lung injury. Am J
Physiol Lung Cell Mol Physiol. 286:L1282–L1292. 2004.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Qin H, Wilson CA, Lee SJ, Zhao X and
Benveniste EN: LPS induces CD40 gene expression through the
activation of NF-kappaB and STAT-1alpha in macrophages and
microglia. Blood. 106:3114–3122. 2005.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lee SB, Lee WS, Shin JS, Jang DS and Lee
KT: Xanthotoxin suppresses LPS-induced expression of iNOS, COX-2,
TNF-α, and IL-6 via AP-1, NF-κB, and JAK-STAT inactivation in RAW
264.7 macrophages. Int Immunopharmacol. 49:21–29. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Kuriakose S, Muleme H, Onyilagha C, Okeke
E and Uzonna JE: Diminazene aceturate (Berenil) modulates LPS
induced pro-inflammatory cytokine production by inhibiting
phosphorylation of MAPKs and STAT proteins. Innate Immun.
20:760–773. 2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lee HH, Jang E, Kang SY, Shin JS, Han HS,
Kim TW, Lee DH, Lee JH, Jang DS and Lee KT: Anti-inflammatory
potential of Patrineolignan B isolated from Patrinia scabra in
LPS-stimulated macrophages via inhibition of NF-κB, AP-1, and
JAK/STAT pathways. Int Immunopharmacol. 86(106726)2020.PubMed/NCBI View Article : Google Scholar
|