1. Introduction
Glaucoma is the second leading cause of blindness
worldwide (1). Among the cases of
glaucoma in China, the proportion of open-angle glaucoma (OAG)
gradually increases. IOP is the main factor leading to loss of
visual field and optic nerve atrophy (2). The treatment of glaucoma focuses on
reducing and controlling IOP through drugs, lasers, or surgery
(3).
Trabeculectomy, as a traditional invasive filtering
surgery, significantly reduces intraocular pressure (4). However, there are several
postoperative complications, including low intraocular pressure,
shallow anterior chamber, scarring of filtering bubbles,
complicated cataract, endophthalmitis, malignant glaucoma, and even
suprachoroidal hemorrhage, which directly affect the success rate
of surgery (5). As an alternative
filtering surgery, non-penetrating deep sclerectomy (NPDS) reduces
IOP by increasing aqueous outflow through a thin trabeculo-descemet
window (TDW) into a surgically created scleral lake (6,7).
NPDS does not penetrate the anterior chamber, so the incidence of
postoperative complications such as low intraocular pressure,
shallow anterior chamber, intraocular hemorrhage, and choroidal
detachment is significantly reduced, and the safety is higher
(8). However, NPDS is difficult to
perform and requires advanced surgical techniques (9).
CO2 laser-assisted sclerectomy surgery
(CLASS) is a non-invasive anti-glaucoma surgery assisted by
CO2 laser. CLASS is an optimized approach to
non-penetrating deep sclerectomy (NPDS) because it employs a
CO2 laser to ablate the scleral tissue instead of
performing a manual procedure. In this surgery, CO2
laser ablation reduces the intraocular pressure (IOP) of the deep
sclera and the outer wall of the Schlemm's canal, thereby
facilitating the drainage of aqueous humor (10,11).
Recently, CLASS has been used to gradually lower IOP in patients
with Primary Open Angle Glaucoma (POAG), pseudocapsular detachment
syndrome, and secondary glaucoma (12-14).
CLASS has a shorter learning curve, lower technical demands, and
higher safety (15) than other
filtering surgeries. Dai et al (15) evaluated the effectiveness and
safety of CLASS for treating glaucoma by systematically reviewing
and meta-analyzing related studies. In our study, we reviewed and
updated the CO2 laser-assisted sclerectomy surgery in
primary open-angle glaucoma, highlighted the history, actual
procedure, pre/post management, mechanism of IOP-lowering effect,
safety, and complications.
2. History of CLASS
As an improved glaucoma filtering surgery, NPDS can
effectively reduce the intraocular pressure of OAG (6,7).
NPDS, on the other hand, is difficult to perform and necessitates
advanced surgical techniques. Perforation of the TDM during
operation is a common complication that limits its clinical
application to a certain extent.
The efficacy and safety of various types of lasers
(16-19),
including excimer laser, holmium: YAG laser, and erbium: YAG laser,
have been assessed in NPDS. This might help overcome the technical
difficulties of NPDS, including the difficulty of the operation and
the possibility of intraoperative perforation.
In 2007, Assia et al (20) performed non-penetrating deep sclera
resection in the eyes of living rabbits and human cadavers using
the CO2 laser. They suggested that the CO2
laser was the most suitable for NPDS and could simplify the
surgical procedure of NPDS due to its precise ablation of the dry
tissue, as well as photocoagulation and effective absorption of the
micro liquid. In 2012, Ton et al (21) performed a similar experiment with
an improved CO2 laser (OT-134 system), which improved
the accuracy and safety of the surgery. Then, Geffen et al
(22)and performed a clinical
trial of CLASS. This clinical trial suggested that CLASS may offer
a simple, safe, and effective surgical method for the treatment of
OAG.
In 2016, Greifner et al (23) compared CLASS to traditional NPDS
surgery, and the results confirmed that the effect of CLASS was
equivalent to that of experienced surgeon-performed NPDS surgery.
In 2018, Jankowska-Szmul et al (12) reported that the one-year success
rate of POAG and exfoliative glaucoma following CLASS was
comparable to that of trabeculectomy but with better safety.
CLASS began in China in 2015. In 2016, Yick et
al (24) reported that the
CO2 laser parameters applicable to the Western
population were not entirely applicable to the Chinese population.
The difference in laser response made the Chinese population more
prone to iris adhesion and scarring after CLASS surgery. Their
study also reported that a larger and thicker sclera flap and a
deeper sclera pool during the operation helped improve the success
rate of CLASS in the Chinese population. In 2018, Yu et al
(25) combined CLASS with
phacoemulsification for the first time. They confirmed that this
combination could effectively reduce IOP in the early postoperative
stage of POAG with cataracts while reducing the use of IOP-lowering
drugs. By combining improved CLASS with preoperative prophylactic
iris laser, Zhang and Cheng (26)
effectively reduced the incidence of postoperative anterior
synechia. Zhang et al (14). reported in 2021 that CLASS and
trabeculectomy had similar IOP-lowering effects in the treatment of
primary open-angle glaucoma, with CLASS being safer.
3. Preoperative assessment
Before CLASS begins, a thorough medical history
inquiry and eye examination should be performed. Before surgery,
patients should have at least one thorough eye examination. The
preoperative IOP ≤30 mmHg (1 mmHg=0.133 kPa) should be maintained.
The following are specific conditions: (1) Routine medical history collection and
basic ophthalmic examination (27); Evaluate the morphology, thickness,
and disease of conjunctiva and sclera in the area to be operated.
(2) The anterior chamber angle of
the patient shall be evaluated by anterior chamber angle
microscopy, ultrasonic biomicroscopy (UBM) or anterior segment
OCT(optical coherent tomography), to avoid the operation at the
position of anterior chamber angle lesion, anterior chamber angle
stenosis or adhesion; (3) Examine
the optic nerve and evaluate the visual field.
4. Surgical procedure
CLASS involves the following specific steps and
points (14,26,28):
(1) Local anesthesia and fixation
of the eyeball. (2) Routine
opening of the bulbar conjunctiva and fascia bulbi (Tenon's
capsule). (3) Shrinking the pupil
before or during ablation to avoid iris herniation due to
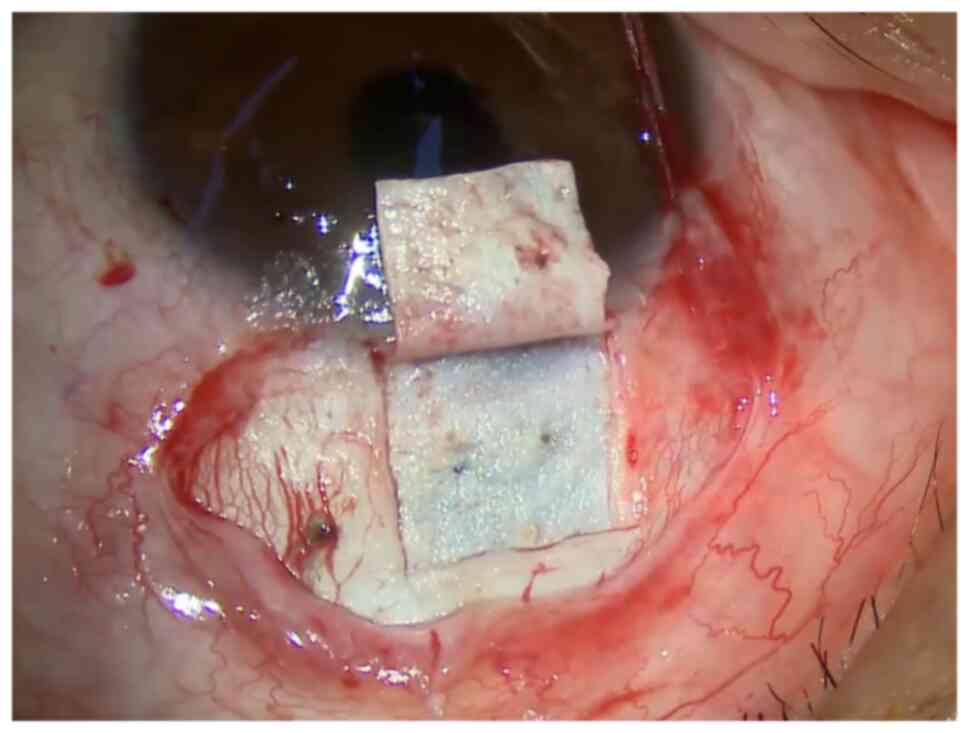
micro-perforation during ablation. (4) The standard size of the sclera flap is
5x5 mm, about 1/3-1/2 sclera thickness; the scleral flap is
separated from the front edge to 1 mm inside the transparent
cornea, exposing three key anatomical areas of the limbus,
including the transparent corneal area, the gray-blue trabecular
meshwork zone, and the white sclera area (Fig. 1). (5) Cotton pieces impregnated with
mitomycin C (MMC) at a recommended concentration of 0.2-0.4 mg/ml
are placed under the conjunctival flap and the shallow sclera flap.
If 5-fluorouracil (5-FU) is used, it is recommended to double the
duration of exposure. After the removal of the cotton pieces, MMC
or 5-FU is rinsed thoroughly. (6)
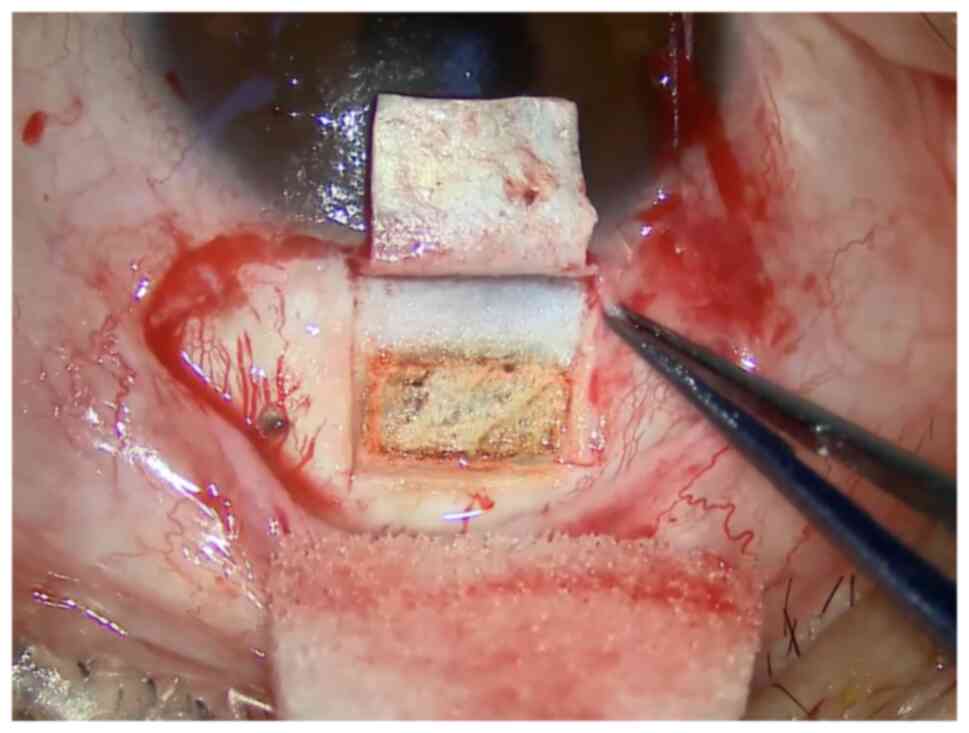
The sclera is ablated by a CO2 laser to make the scleral
cisterna (Fig. 2). It is
recommended that the area of the sclera pool should be at least 4x2
mm, with a supporting edge of at least 0.5 mm away from the edge of
the sclera flap. The initial ablation energy is recommended to be
21 W, and the rectangular laser is excited perpendicular to the
sclera. Ablation to reveal the uveal pigment. (7) MMC at a recommended concentration of
0.2-0.4 mg/ml is placed at the bottom of the deep sclera pool, and
the duration of exposure depends on the patient's condition. It is
recommended to remove the cotton piece after 30 s to 2 min and
rinse it thoroughly. If 5-FU is used, it is recommended to double
the stay time. (8) For glaucoma
patients with severe optic nerve damage and high IOP (≥30 mmHg)
before the ablation of the Schlemm's canal, it is recommended to
puncture the lateral incision and slowly reduce the IOP to nearly
the normal level to avoid the large difference in filtration
pressure after opening the outer wall of the Schlemm's canal,
resulting in the rupture of the inner wall of the Schlemm's canal
(full-thickness penetration of the ocular wall), or development of
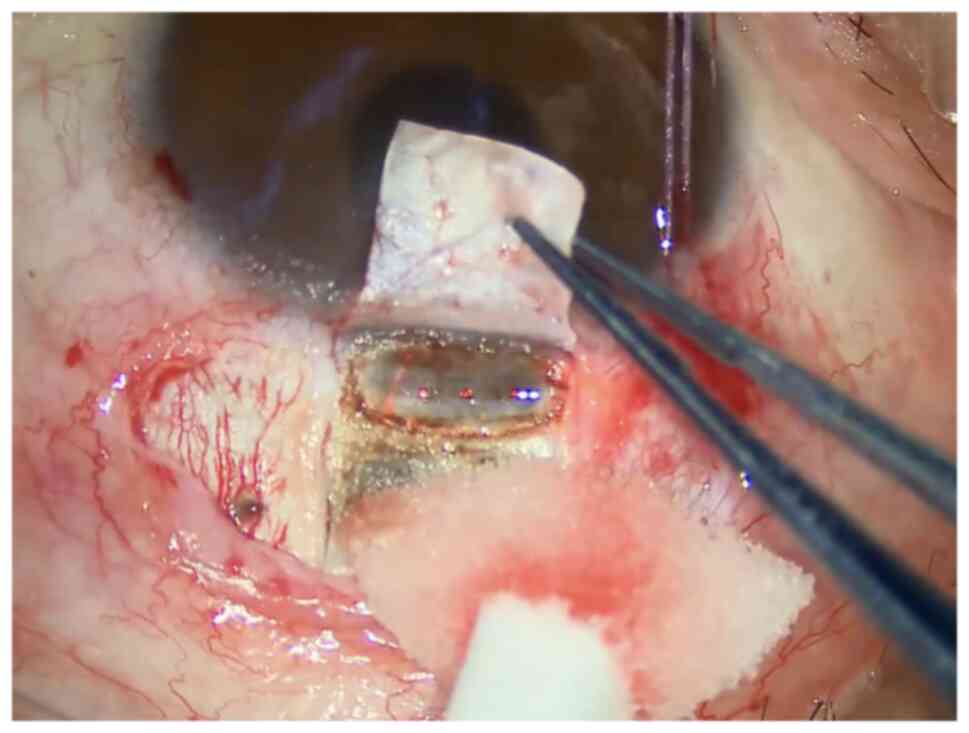
a hernia of the iris. (9) Ablation
of the outer wall of the Schlemm's canal (Fig. 3). During ablation, the bow aiming
at the light front end is aligned with the transparent and gray
junction area of the corneal limbus, and the ablation range shall
be at least 4 x1.4 mm. Continuous ablation is performed until the
outer wall of the Schlemm's canal is opened, and the aqueous humor
continues to flow steadily. For patients receiving laser treatment
before surgery, it is better if the center of the ablation area of
the Schlemm's canal faces the laser hole at the root of the iris
(LPI hole) and/or the area of peripheral laser iridoplasty (ALPI
area). If the IOP is too low during surgery, water injection in the
anterior chamber is needed to increase the IOP and observe the
outflow of aqueous humor after ablation of the Schlemm's canal.
(10) Reduction of the scleral
flap is performed using a fixed suture, adjustable suture, or no
suture to sew the conjunctival incision.
The operation picture of Fig. 1, Fig.
2 and Fig. 3 was from the
screenshot of the operation video of a 31-year-old male patient, on
May 21, 2021, at the Second Affiliated Hospital of Zhejiang
University School of Medicine. The surgical procedures shown in
Fig. 1, Fig. 2 and Fig. 3 correspond to standard clinical
practice, refer to the consensus of experts in the operation of
CLASS (China, 2021).
5. Postoperative management
Tobramycin and dexamethasone eye drops were used
four times per day for 1 month to reduce inflammation and prevent
infection following CLASS. Piloneurtin eye drops were used to
reduce pupil size and prevent PAS after surgery (26). Specific drug concentrations and
frequencies should be adjusted based on the patient's condition,
and medication should be taken for at least 3 months. Routine
examinations were performed 1 day, 2 weeks, 1 month, 3 months, and
6 months postoperatively to assess visual acuity, IOP, the
morphology of filtering blebs, and scleral cisterna volume. The
anterior chamber angle was examined at the 2-week postoperative
follow-up to detect early peripheral anterior iris adhesion. In
contrast, ultrasonic biological microscopy or anterior segment OCT
was used to understand the scleral cisternal morphology. If there
are any complications, the number of follow-up examinations should
be increased appropriately. If there are any abnormalities in the
operated eyes, a doctor should be consulted as soon as possible.
After surgery, the patient should avoid pressing the eyeball
(including massage) or severely coughing, as these actions may
rupture the inner wall of the TDM and the Schlemm's canal,
resulting in iris entrapment (14,29).
The following are the key points of management
within 6 months of surgery: (1)
Most patients' IOP on the first day after surgery should be <10
mmHg. If the IOP is >14 mmHg, we should look for the source and
eliminate it (26); (2) IOP can reach a peak 2-4 weeks after
surgery. When the IOP is >21 mmHg, it is recommended to check
the anterior chamber angle and scleral pool. If the anterior
chamber angle and scleral pool are normal, the patient should be
monitored for 6 weeks after surgery (24); If the IOP is still >21 mmHg 6
weeks after surgery and/or there is a significantly shrunk scleral
pool, it is recommended that an LGP treatment be performed first
(30). If the effect of LGP is not
good or the scleral cisterna are at risk of disappearing, it is
recommended to administer a 5-FU injection under the scleral flap
or conjunctiva (31); (3) When there is a risk of the scleral
cisterna disappearing, the scleral flap should first be needled,
and anti-metabolic drugs be injected (31). If the scleral cisterna is
unobstructed and the IOP is >21 mmHg, laser goniopuncture (LGP)
is recommended (30); (4) If peripheral anterior adhesion is
observed after surgery, it should be handled in time and laser
peripheral iridectomy (LPI) or Argon laser peripheral iridoplasty
(ALPI) is feasible in the early stage (32); (5)
LGP is a beneficial complementary treatment after CLASS (30,33);
(6) For patients with iris
incarceration, it is recommended to perform an internal surgery
first, and trabeculectomy is not recommended.
Patients with Asian glaucoma, including Chinese
patients, have a crowded anterior chamber structure, intractable
high IOP, and postoperative scarring (34). Domestic researchers Zhang and Cheng
(26) performed laser peripheral
iridectomy and/or laser peripheral iridoplasty during the
perioperative period and found that the incidence of PAS was
significantly reduced after laser treatment. The consensus of
Chinese CLASS experts suggests that LPI and/or ALPI laser treatment
should be performed in the center of the ablated Schlemm's canal
undergoing surgical ablation from 48 h before surgery to 1 week
after surgery for high-risk patients who are prone to local
adhesion of the anterior chamber corner (including but not limited
to patients with iris hypertrophy, relaxation, and shallow anterior
chamber). Thermal injury to the tissue surrounding the ablated area
following surgery may result in local inflammation and adhesion
(14). In addition to early
postoperative local anti-inflammatory therapy, postoperative
scarring of the filter channel should be detected and treated as
soon as possible (using LGP, followed by needle dial filter bubble
and subconjunctival injection of anti-metabolic drugs) (35,36).
6. Efficacy outcomes
CLASS
CLASS has been shown in some domestic and
international studies to be effective and safe in the treatment of
POAG (15,37) Table
I summarizes long-term CLASS outcomes from published studies.
The CS rate ranges from 45.5 to 67.9% at 12 M and from 34.1 to
73.0% at 24 M. The QS rate ranges from 69.2 to 93.1% at 12 M and
from 76.9 to 96.0% at 24 M.
 | Table IComparison of long-term outcomes of
CLASS among published studies. |
Table I
Comparison of long-term outcomes of
CLASS among published studies.
| | Complete success
rate (%) | Qualified success
rate (%) |
|---|
| No. | First author | Year | Population | N (eye) | Surgery | PAS incidence
(%) | Iris incarceration
(%) | 6 M | 12 M | 24 M | 6 M | 12 M | 24 M |
|---|
| 1 | Geffen et al
(22) | 2010 | Mixed | 37 | CLASS | 0.0 | 0.0 | 76.7 | 60.0 | | 83.3 | 86.6 | |
| 2 | Greifner et
al (23) | 2014 | Caucasian | 27 | CLASS | 0.0 | 48.0 | | | 73.0 | | | 96.0 |
| 3 | Skaat et al
(52) | 2014 | Caucasian | 15 | CLASS | 0.0 | 6.7 | | 45.5 | | | 90.9 | |
| 4 | Geffen et al
(11) | 2016 | Mixed | 97 | CLASS | 5.6 | 8.3 | | 60.2 | 57.9 | | 79.6 | 91.2 |
| 5 | Yick et al
(24) | 2016 | Chinese | 23 | CLASS | 0.0 | 0.0 | | | | 81.8 | | |
| 6 | Cutolo et al
(51) | 2017 | Caucasian | 21 | CLASS | 9.5 | 14.3 | | | | | | |
| 7 | Jankowska-Szmul
et al (12) | 2018 | Caucasian | 66 | CLASS | 0.0 | 4.5 | | 35.0 | | | 74.0 | |
| 8 | Zhang and Cheng
(26) | 2020 | Chinese | 25 | Modified CLASS | 6.9 | 0.0 | | 62.1 | 48.3 | | 89.7 | 89.7 |
| 9 | Sohajda et
al (53) | 2020 | Caucasian | 22 | CLASS | 0.0 | 0.0 | 72.7 | 64.0 | | 77.0 | 72.7 | |
| 10 | Yan et al
(40) | 2020 | Chinese | 28 | CLASS | 10.7 | 0.0 | 71.4 | 67.9 | 64.3 | 92.9 | 85.7 | 85.7 |
| 11 | Zhang et al
(14) | 2021 | Chinese | 30 | CLASS | 30.0 | 6.7 | 82.8 | 58.6 | 51.7 | 100.0 | 93.1 | 86.2 |
| 12 | Ho et al
(13) | 2021 | Asian | 13 | CLASS | 0.0 | 0.0 | 41.5 | 41.5 | 34.1 | 48.8 | 69.2 | 76.9 |
| 13 | Chen et al
(36) | 2021 | Chinese | 21 | CLASS | 73.9 | 4.3 | | 69.8 | | | 95.7 | |
Ton et al (21) were the first to conduct a
multicenter clinical study of CLASS, with a 12-month follow-up of
30 patients across three continents. The results indicated that the
average IOP of patients decreased by 42.4 and 40.7% at 6 and 12
months after surgery, respectively (P<0.001). The average number
of IOP-lowering drugs decreased from 2.5 before surgery to 0.1 and
0.6 after surgery, with a complete success rate of 76.7 and 60%,
respectively. The conditional success rates were 83.3 and 86.6%,
respectively, which indicated the short-term effectiveness and
safety of CLASS in patients with POAG and pseudo-exfoliative
glaucoma. Based on previous research, Geffen et al (11) published an open-ended study
involving seven countries and nine medical centers (22) in 2016, which included more patients
with POAG and pseudo-exfoliative glaucoma and completed a
three-year follow-up record. The results also indicated a good
IOP-lowering effect. At the same time, compared with the non-MMC
group, the complete success rates of the two groups at 24 and 36
months were 62 and 0% (P=0.03) and 52 and 0% (P=0.14),
respectively, while the qualified success rates were 91 and 80%
(P=0.97) and 86 and 75% (P=0.87), respectively. The results
indicated that the MMC group had a higher complete success rate at
both 24 and 36 months postoperatively compared to the non-MMC
group. In 2016, Yick et al (24) published the first study on the use
of CLASS in Chinese patients with late glaucoma, which preliminary
confirmed the effectiveness of CLASS surgery in the Chinese
population.
CLASS vs. Trabeculectomy
CLASS has fewer complications, faster recovery from
visual acuity, and comparable IOP-lowering effects when compared to
traditional trabeculectomy (12,14,29).
In 2018, Jankowska-Szmul et al (12) compared CLASS to traditional
trabeculectomy. They found that, while the complete success rate of
CLASS was lower, the qualified success rate was roughly equivalent,
with fewer postoperative complications, fewer corneal endothelium
losses, and less impact on astigmatism. Therefore, CLASS may be
more suitable for early glaucoma patients or patients with less
corneal endothelium. Zhang et al (14). compared the effectiveness and
safety of CLASS and Trab in the treatment of POAG. The findings
demonstrated that CLASS was an effective and safe treatment for
POAG. The effect of lowering intraocular pressure was comparable to
Trab. There were fewer complications associated with filtering
blebs after surgery, and early postoperative visual recovery was
faster. Zhang et al (28).
compared the long-term effects of modified CO2
laser-assisted sclerectomy (MCLASS) and Trab on IOP control in POAG
patients. The intraocular pressure and types of glaucoma drugs used
in the MCLASS group were significantly lower compared to the two
groups at 24 and 36 months after surgery, and the complete and
qualified success rates of the two groups were not significantly
different. In contrast, the overall complication rate of the Trab
group was significantly higher.
CLASS combined with
phacoemulsification
In 2018, Yu et al (25) reported on the effect of CLASS
combined with cataract phacoemulsification (phaco). For the first
time, Yu et al (25)
combined CLASS with phacoemulsification. They confirmed that this
combination could effectively reduce IOP in the early postoperative
stage of the treatment of POAG with cataracts while also reducing
the use of IOP-lowering drugs. In the same year, Villavicencio
et al (37) compared the
surgical effects of CLASS surgery combined with phaco (33 cases)
and trabeculectomy combined with phaco (37 cases) for OAG. They
found that CLASS was superior to trabeculectomy in controlling IOP,
improving vision, and reducing medication and postoperative
complications in the case of combined phaco. In 2021, Ho et
al (13) demonstrated that
CLASS with or without phacoemulsification was at least as simple,
safe, and effective in the short and medium term, implying that
combined surgery should be the first choice for cataract patients.
In our previous study (38), we
reported the CLASS approach alone achieved a greater IOP reduction,
more common functional bleb formation, and a higher success rate
compared to CLASS combined with Phaco, while combination surgery
yielded a better best-corrected visual acuity (BCVA) improvement
and a lower PAS incidence than CLASS alone. Both surgical
strategies have shown favorable safety and efficacy among POAG
patients. Besides, combined surgery could be a viable option for
patients with co-existing POAG and cataracts.
Table II
summarizes 1-year outcomes of CLASS combined with or without Phaco
among published studies. These studies have confirmed that this
combination could effectively reduce IOP in the postoperative stage
of the treatment of POAG with cataracts.
 | Table IISummary of 1 year outcomes of CLASS
combined with or without Phaco amongst published studies. |
Table II
Summary of 1 year outcomes of CLASS
combined with or without Phaco amongst published studies.
| No. | First author | Year | Population | Surgery | N (eye) | Baseline IOP
(mmHg) | IOP reduction
% | Medication decrease
% | PAS (%) | LGP (%) | CS (%) | QS (%) |
|---|
| 1 | Yu et al
(25) | 2018 | Chinese | CLASS + Phaco | 17 | 23.9±8.6 | 39 | 70 | 0 | 0 | 65 | 88 |
| 2 | Villavicencio et
al (37) | 2018 | Caucasian | CLASS + Phaco | 33 | 29.5±3.7 | 45.2 | - | 0 | 33.3 | - | 97.2 |
| | | | | Trab + Phaco | 37 | 17.0±5.8 | 37.7 | - | 0 | 0 | - | 86.4 |
| 3 | Ho et al
(13) | 2021 | Asian | CLASS | 13 | 20.3 | 40.6 | - | - | - | 41.5 | 69.2 |
| | | | | CLASS + Phaco | 28 | 16.8 | 6.7 | - | - | - | - | 46.4 |
| 4 | Raja et al
(54) | 2021 | Indian | Trab + Phaco | 18 | 15.7±3.2 | - | - | - | - | 100 | 100 |
| | | | | CLASS + Phaco | 18 | 17.2±5.3 | - | - | - | - | 85.7 | 92.3 |
| 5 | Chen et al
(38) | 2022 | Chinese | CLASS | 23 | 31.0±10.0 | 54.5 | 91.3 | 0-47.8 | 30.4 | 60.9 | 87 |
| | | | | CLASS + Phaco | 23 | 19.8±6.5 | 26.2 | 85 | 0-8.7 | 34.8 | 26.1 | 32.5 |
7. Mechanism of IOP-lowering effect
The mechanism of IOP reduction after NPDS is
complicated. There may be several aqueous humor drainage pathways,
including subcon junctival bleb, trabecular meshwork, intrascleral
outflow and suprachoroidal outflow (39). It has been reported that the
subconjunctival and suprachoroidal pathway may be the main
mechanisms to achieve IOP reduction after CLASS (40).
Consensus eludes about the role of filtering bubbles
in maintaining IOP after CLASS. In a study conducted by Judyta and
Edward (41), the clinical grading
scale and OCT were used to assess the morphological manifestations
of CLASS filter bubbles following surgery. The results indicated
that the presence of the filtering blebs was also important for
maintaining intraocular pressure after CLASS. Consensus eludes
about the role of filtering bubbles in maintaining IOP after CLASS.
However, another published research reported that all patients
following deep sclerectomy with collagen implant (DSCI) and
mitomycin C (MMC)had flat filtering blebs, suggesting that there
was no obvious relationship between intraocular pressure and
filtering bleb (42).
According to some published reports, the long-term
stability of the scleral cistern is critical to the success of this
type of surgery (26,40). Regarding the imaging manifestations
of NPDS scleral cistern, some studies have found that postoperative
intraocular pressure was negatively correlated with scleral cistern
height (42,43). The collapse of the scleral cistern
has been linked to poor control of IOP (44,45).
However, Other studies have found no correlation between the size
of the scleral cistern and the decrease in IOP (41,46,47).
Chihara and Hayashi (47) proposed
that the presence of a scleral cistern could facilitate aqueous
humor drainage to the subconjunctival and suprachoroidal cavities
via the Schlemm's canal and trabecular meshwork opening, the gap
between the scleral flap and the scleral bed, or scleral pressure
conduction. Judyta and Edward (41) proposed that without combined
implants and MMC, the size and height of the scleral cistern would
significantly decrease over time and that there was no significant
correlation between the size of the scleral cistern and the
decrease of IOP.
Presently, the mechanism of the postoperative
drainage of aqueous humor to the suprachoroidal space through the
scleral cisterna remains controversial. Based on long-term clinical
results, Park et al (48)
and Chihara and Hayashi (47)
concluded that the mechanism of IOP reduction by CLASS was more
dependent on scleral cisterna infiltration into the suprachoroidal
space rather than scleral valve infiltration into the
subconjunctiva. Some researchers believe that if this mechanism is
correct, the area of fluid reflux will be directly proportional to
the size of the postoperative sclera cistern, influencing IOP
reduction. Other scholars believe that the hydrostatic pressure of
the suprachoroidal cavity is 0.8-4.7 mmHg lower than that of the
anterior chamber, and this pressure may form a driving force.
However, because the scleral cisterna only serves as a reservoir,
the size of the scleral cisterna has no effect on the decrease of
IOP (49).
8. Safety and complications
Theoretically, CLASS is safe and accurate under
controllable laser ablation: (1)
CO2 laser can effectively ablate the dry tissue and be
absorbed by traces of liquid. Using this principle, CLASS surgery
can efficiently ablate the deep sclera and the outer wall of the
Schlemm's canal, completely preserve the inner wall of the
Schlemm's canal and the trabecular meshwork, ensure that the
anterior chamber is not penetrated, and reduce the occurrence of
complications such as the shallow anterior chamber, anterior
chamber hemorrhage, and secondary concurrent cataract (23,50).
(2) During surgery, precise
ablation of the sclera tissue can be achieved by accurately
controlling energy and adjusting the size of the area of sclera
ablation, which greatly reduces the difficulty of the operation and
effectively reduces the operation time (11). (3)
The aqueous humor is drained into the scleral pool for absorption,
which avoids the problems of scarring and rupture infection caused
by drainage through the filtration bubble. Further, because most of
the aqueous humor is slowly and evenly absorbed from the
suprachoroidal cavity, only a small part of the aqueous humor flows
into the conjunctiva, which makes the filtered bubbles relatively
flat and diffuse. Thus, meeting the patient's postoperative
requirements for eye comfort and appearance. (4) CLASS surgery benefits from the fact
that the CO2 laser is completely absorbed by the liquid.
The aqueous humor flowing out of the Schlemm's canal can absorb
ablation energy, reducing postoperative fibrosis and tissue
adhesion by minimizing thermal damage at the bottom of the ablated
tissue. (5) Trabeculectomy can be
converted at any time if TDM penetrates during the operation.
CLASS complications include, among other things,
peripheral anterior synechia (PAS), iris incarceration, scleral
pool collapse, shallow anterior chamber, and choroidal detachment
(23). The three main causes of
elevated IOP after CLASS (36) are
PAS, iris incarceration, and scleral cisterna collapse. According
to the literature, the incidence of PAS and iris incarceration
after CLASS is relatively high, which is the primary cause of
postoperative IOP (23)
recurrence. The incidence of PAS in Chinese patients was relatively
high when compared to white patients. Jankowska-Szmul et al
(12) reported an incidence of PAS
in only 3.0% of the patients, while Cutolo et al (51) reported an incidence of 9.5%, both
were far lower than the 30.0% incidence reported by Zhang et
al (14) Table I summarizes the incidence of PAS
and iris incarceration in published studies. Inadequate aqueous
humor infiltration due to scleral pool collapse is another cause of
postoperative IOP elevation. The key to improving the long-term
efficacy of CLASS is the analysis and judgment of the causes of IOP
increase with time after CLASS, followed by taking the
corresponding intervention and treatment measures.
9. Conclusion
Over the years, the surgical treatment of glaucoma
has become more effective, safer, minimally invasive, and more
personalized. Although CLASS has several advantages, it still has
many limitations: (1) It is only
applicable to OAG, and the potential of combining it with other
surgical methods to treat refractory glaucoma needs further
exploration. (2) The occurrence of
PAS is highly likely after CLASS, which makes it necessary to
follow up closely for its timely detection and treatment. (3) Compared to trabeculectomy, CLASS is
more expensive. CLASS surgery is superior to trabeculectomy in the
incidence of early postoperative complications, the protection of
corneal endothelium, and the feasibility of reoperation. Its
long-term clinical effect still needs confirmation by long-term,
multicenter, large-sample, randomized controlled studies.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CH and XS collected and arranged the references, and
drafted and revised the manuscript. MC and KW provided guidance and
revised the paper. All authors have made a substantial contribution
to the work. All authors have read and approved the final
manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
The study involving human participants was reviewed
and approved by Ethics Committee of the Second Affiliated Hospital
of Zhejiang University (approval no. 2020-ER721). Written informed
consent was obtained from all the subjects prior to participation
and for publication of the accompanying images in this study. The
study was performed in accordance with the Helsinki Declaration of
1964 and its later amendments.
Patient consent for publication
Written informed consent was obtained from the
subject prior to participation and for publication of the
accompanying images in this study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Quigley HA: Glaucoma. Lancet.
377:1367–1377. 2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zhang N, Wang J, Li Y and Jiang B:
Prevalence of primary open angle glaucoma in the last 20 years: A
meta-analysis and systematic review. Sci Rep.
11(13762)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Schuster AK, Erb C, Hoffmann EM, Dietlein
T and Pfeiffer N: The diagnosis and treatment of glaucoma. Dtsch
Arztebl Int. 117:225–234. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hoffmann EM, Hengerer F, Klabe K, Schargus
M, Thieme H and Voykov B: Glaucoma surgery today. Ophthalmologe.
118:239–247. 2021.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
5
|
Jampel HD, Solus JF, Tracey PA, Gilbert
DL, Loyd TL, Jefferys JL and Quigley HA: Outcomes and bleb-related
complications of trabeculectomy. Ophthalmology. 119:712–722.
2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hondur A, Onol M and Hasanreisoglu B:
Non-penetrating glaucoma surgery: Meta-analysis of recent results.
J Glaucoma. 17:139–146. 2008.
|
|
7
|
Cheng JW, Cheng SW, Cai JP, Li Y and Wei
RL: Systematic overview of the efficacy of non penetrating glaucoma
surgery in the treatment of open angle glaucoma. Med Sci Monit.
17:RA155–RA163. 2011.
|
|
8
|
Slagle G, Groth SL, Montelongo M and
Sponsel WE: Non-penetrating deep sclerectomy for progressive
glaucoma: Long-term (5-year) follow-up of intraocular pressure
control and visual field survival. J Curr Glaucoma Pract. 14:3–9.
2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Elhofi A and Helaly HA: Non-penetrating
deep sclerectomy versus trabeculectomy in primary congenital
glaucoma. Clin Ophthalmol. 14:1277–1285. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Geffen N, Assia EI and Melamed S:
Laser-Assisted techniques for penetrating and non-penetrating
glaucoma surgery. Dev Ophthalmol. 59:100–112. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Geffen N, Mimouni M, Sherwood M and Assia
EI: Mid-term clinical results of CO2 laser-assisted
Sclerectomy Surgery (CLASS) for open-angle glaucoma treatment. J
Glaucoma. 25:946–951. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Jankowska-Szmul J, Dobrowolski D and
Wylegala E: CO2 laser-assisted sclerectomy surgery
compared with trabeculectomy in primary open-angle glaucoma and
exfoliative glaucoma. A 1-year follow-up. Acta Ophthalmol.
96:e582–e591. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ho DCW, Perera SA, Hla MH and Ho CL:
Evaluating CO2 laser-assisted sclerectomy surgery with
mitomycin C combined with or without phacoemulsification in adult
Asian glaucoma subjects. Int Ophthalmol. 41:1445–1454.
2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhang H, Tang Y, Yan X, Ma L, Geng Y, Li F
and Tang G: CO2 laser-assisted deep sclerectomy surgery
compared with trabeculectomy in primary open-angle glaucoma:
Two-year results. J Ophthalmol. 2021(6639583)2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Dai L, Li AL, Yu L and Ye J: Reply:
Efficacy and safety of CO2 laser-assisted sclerectomy
surgery for glaucoma: A systematic review and meta-analysis. Arq
Bras Oftalmol. 84:418–420. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Argento C, Sanseau AC, Badoza D and
Casiraghi J: Deep sclerectomy with a collagen implant using the
excimer laser. J Cataract Refract Surg. 27:504–506. 2001.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Klink T, Lieb W and Grehn F: Erbium-YAG
laser-assisted preparation of deep sclerectomy. Graefes Arch Clin
Exp Ophthalmol. 238:792–796. 2000.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Schuman JS, Chang W, Wang N, de Kater AW
and Allingham RR: Excimer laser effects on outflow facility and
outflow pathway morphology. Invest Ophthalmol Vis Sci.
40:1676–1680. 1999.PubMed/NCBI
|
|
19
|
Traverso CE, Murialdo U, Di Lorenzo G,
Venzano D, De Palma G, Gandolfo E, Calabria GA and Zingirian M:
Photoablative filtration surgery with the excimer laser for primary
open-angle glaucoma: A pilot study. Int Ophthalmol. 16:363–365.
1992.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Assia EI, Rotenstreich Y, Barequet IS,
Apple DJ, Rosner M and Belkin M: Experimental studies on
non-penetrating filtration surgery using the CO2 laser.
Graefes Arch Clin Exp Ophthalmol. 245:847–854. 2007.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ton Y, Geffen N, Kidron D, Degani J and
Assia EI: CO2 laser-assisted sclerectomy surgery part I:
Concept and experimental models. J Glaucoma. 21:135–140.
2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Geffen N, Ton Y, Degani J and Assia EI:
CO2 laser-assisted sclerectomy surgery, Part II:
Multicenter clinical preliminary study. J Glaucoma. 21:193–198.
2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Greifner G, Roy S and Mermoud A: Results
of CO2 laser-assisted deep sclerectomy as compared with
conventional deep sclerectomy. J Glaucoma. 25:e630–e638.
2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yick DWF, Lee JWY, Tsang S, Yeung BYM and
Yuen CYF: Preliminary results of CO2 laser-assisted
sclerectomy surgery (CLASS) in the treatment of advanced glaucoma
in a Chinese population. Medicine (Baltimore).
95(e5294)2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yu X, Chen C, Sun M, Dong D, Zhang S, Liu
P, Yuan R and Ye J: CO2 laser-assisted deep sclerectomy
combined with phacoemulsification in patients with primary
open-angle glaucoma and cataract. J Glaucoma. 27:906–909.
2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhang Y and Cheng G: Modified
CO2 laser-assisted sclerectomy surgery in Chinese
patients with primary open-angle glaucoma and pseudoexfoliative
glaucoma: A Two-year follow-up study. J Glaucoma. 29:367–373.
2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Prum BE Jr, Rosenberg LF, Gedde SJ,
Mansberger SL, Stein JD, Moroi SE, Herndon LW Jr, Lim MC and
Williams RD: Primary open-angle glaucoma Preferred Practice
Pattern(®) Guidelines. Ophthalmology. 123:P41–P111. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhang Y, Mao J, Zhou Q, Li L, Zhang S,
Bian A and Cheng G: Comparison of long-term effects after modified
CO2 laser-assisted deep sclerectomy and conventional
trabeculectomy in chinese primary open-angle glaucoma. Ophthalmol
Ther. 11:321–331. 2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Bettin P and Di Matteo F: Postoperative
management of penetrating and non-penetrating external filtering
procedures. Dev Ophthalmol. 59:53–66. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Shaarawy T, Mansouri K, Schnyder C,
Ravinet E, Achache F and Mermoud A: Long-term results of deep
sclerectomy with collagen implant. J Cataract Refract Surg.
30:1225–1231. 2004.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Heuer DK, Parrish RK II, Gressel MG,
Hodapp E, Palmberg PF and Anderson DR: 5-fluorouracil and glaucoma
filtering surgery: II. A pilot study. Ophthalmology. 91:384–394.
1984.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Romano JH, Hitchings RA and Pooinasawmy D:
Role of Nd:YAG peripheral iridectomy in the management of ocular
hypertension with a narrow angle. Ophthalmic Surg. 19:814–816.
1988.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Mermou A, Karlen ME, Schnyder CC,
Sickenberg M, Chiou AG, Hédiguer SE and Sanchez E: Nd:Yag
goniopuncture after deep sclerectomy with collagen implant.
Ophthalmic Surg Lasers. 30:120–125. 1999.PubMed/NCBI
|
|
34
|
Lee RY, Chon BH and Lin SC, He M and Lin
SC: Association of ocular conditions with narrow angles in
different ethnicities. Am J Ophthalmol. 160:506–515 e1.
2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Xin Z, Chen J, Wang D, Wu X and Han Y:
CO2 laser-assisted sclerectomy surgery in the treatment
of open-angle glaucoma in Chinese patients. Eur J Ophthalmol: Jan
12, 2022 (Epub ahead of print).
|
|
36
|
Chen M, Gu Y, Yang Y, Zhang Q, Liu X and
Wang K: Management of intraocular pressure elevation after
CO2 laser-assisted sclerectomy surgery in patients with
primary open-angle glaucoma. Front Med (Lausanne).
8(806734)2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Villavicencio JCI, Baltodano FPQ, Ramirez
Jimenez IM, Mendez ALG and Ponte-Davila MC: Comparative clinical
results of phacoemulsification combined with CO2
Laser-Assisted Sclerectomy vs. Phacoemulsification combined with
trabeculectomy in patients with open-angle glaucoma. J Clin Exp
Ophthalmol. 9(5)2018.
|
|
38
|
Chen M, Yu N, Huang C, Zhang Q, Liu X and
Wang K: CO2 Laser-Assisted sclerectomy surgery alone or
combined with phacoemulsification in primary open-angle glaucoma:
Comparison of 1-year outcomes. Ophthalmol Ther. 11:1719–1733.
2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Johnson DH and Johnson M: Glaucoma surgery
and aqueous outflow: How does nonpenetrating glaucoma surgery work?
Arch Ophthalmol. 120:67–70. 2002.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Yan X, Zhang H, Li F, Ma L, Geng Y and
Tang G: Surgical site characteristics after CLASS followed by
ultrasound biomicroscopy and clinical grading scale: A 2-year
follow-up. Eye (Lond). 35:2283–2293. 2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Jankowska-Szmul J and Wylegala E: The
CLASS surgical site characteristics in a clinical grading scale and
anterior segment optical coherence tomography: A one-year
follow-up. J Healthc Eng. 2018(5909827)2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Mavrakanas N, Mendrinos E and Shaarawy T:
Postoperative IOP is related to intrascleral bleb height in eyes
with clinically flat blebs following deep sclerectomy with collagen
implant and mitomycin. Br J Ophthalmol. 94:410–413. 2010.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Fernández-Buenaga R, Rebolleda G,
Casas-Llera P, Muñoz-Negrete FJ and Pérez-López M: A comparison of
intrascleral bleb height by anterior segment OCT using three
different implants in deep sclerectomy. Eye (Lond). 26:552–556.
2012.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Roters S, Lüke C, Jonescu-Cuypers CP,
Engels BF, Jacobi PC, Konen W and Krieglstein GK: Ultrasound
biomicroscopy and its value in predicting the long term outcome of
viscocanalostomy. Br J Ophthalmol. 86:997–1001. 2002.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Chihara E, Okazaki K, Takahashi H, Shoji
T, Adachi H and Hayashi K: Modified deep sclerectomy (D-lectomy
MMC) for primary open-angle glaucoma: Preliminary results. J
Glaucoma. 18:132–139. 2009.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Khairy HA, Atta HR, Green FD, van der Hoek
J and Azuara-Blanco A: Ultrasound biomicroscopy in deep
sclerectomy. Eye (Lond). 19:555–560. 2005.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Chihara E and Hayashi K: Relation between
the volume of the lake and intraocular pressure reduction after
nonfiltering glaucoma surgery: A spectral-domain anterior segment
optical coherence tomography study. J Glaucoma. 20:497–501.
2011.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Park M, Tanito M, Nishikawa M and Chihara
E: Ultrasound biomicroscopy of intrascleral lake after
viscocanalostomy and cataract surgery. J Glaucoma. 13:472–478.
2004.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Emi K, Pederson JE and Toris CB:
Hydrostatic pressure of the suprachoroidal space. Investig
Ophthalmol Vis Sci. 30:233–238. 1989.PubMed/NCBI
|
|
50
|
Gedde SJ, Schiffman JC, Feuer WJ, Herndon
LW, Brandt JD and Budenz DL: Tube Versus Trabeculectomy Study
Group. Three-Year Follow-up of the Tube Versus Trabeculectomy
Study. Am J Ophthalmol. 148:670–684. 2009.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Cutolo CA, Bagnis A, Scotto R, Bonzano C
and Traverso CE: Prospective evaluation of CO2
laser-assisted sclerectomy surgery (CLASS) with Mitomycin C.
Graefes Arch Clin Exp Ophthalmol. 256:181–186. 2018.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Skaat A, Goldenfeld M, Cotlear D and
Melamed S: CO2 laser-assisted deep sclerectomy in
glaucoma patients. J Glaucoma. 23:179–184. 2014.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Sohajda Z, Széll N, Revák Á, Papp J and
Tóth-Molnár E: Retinal nerve fibre layer thickness change after
CO2 laser-assisted deep sclerectomy surgery. Clin
Ophthalmol. 14:1749–1757. 2020.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Raja SV, Ponnat AK, Balagiri K and
Pallamparthy S: Retrospective analysis of the comparison between
carbon dioxide laser-assisted deep sclerectomy combined with
phacoemulsification and conventional trabeculectomy with
phacoemulsification. Indian J Ophthalmol. 69:2741–2745.
2021.PubMed/NCBI View Article : Google Scholar
|