Introduction
Acquired immunodeficiency syndrome (AIDS) and
tuberculosis (TB) are major serious public health concerns
worldwide (1). According to
estimates from the Joint United Nations Programme on HIV and AIDS,
1.7 million people were living with HIV in 2018 and there were
about 37.9 million cases of HIV/AIDS (2). Patients with AIDS have a compromised
immune system and are susceptible to opportunistic infections, such
as invasive fungal rhinosinusitis, which without the control of
immunosuppression, the infection is aggravated and extends to the
orbit and inside the skull base, even with the timely surgical and
medical treatment (3). Of
opportunistic infections, TB is the most common and one of the
primary causes of death in patients with HIV (4,5). In
2019, 208,000 of ~1.4 million TB-related deaths were among
HIV-positive people (6). The risk
of developing TB for patients with HIV is 20-30-fold higher than
for those without HIV and the synergistic interplay of the two
pathogens accelerates the decrease in immunological function
(7). Pulmonary TB is the most
common form of active TB in patients with HIV (8). Highly active antiretroviral therapy
(HAART) is a common treatment for HIV infection that inhibits viral
replication and restores the functions of the immune system
(9,10). HAART makes HIV infection a
controllable chronic disease and plays a key role in HIV infection
prevention and treatment (11).
However, immune reconstitution leads to an excessive inflammatory
response to infectious and non-infectious agents. This phenomenon
is known as immune reconstitution inflammatory syndrome (IRIS)
(12); it occurs because of the
pathological immune inflammatory response triggered by HAART
treatment (13,14). TB-associated IRIS (TB-IRIS) is
considered to involve Mycobacterium tuberculosis antigens
and is characterized by unexplained worsening or occurrence of
symptoms or signs of TB post-ART initiation (15). For example, The increased clinical
blood C-reactive protein (CRP) level and CD4 T-cell count of
patients, and worsening of chest imaging included progression to
miliary disease, worsening consolidations, enlarged thoracic lymph
nodes, and pleural effusions (16). Previous study reported that the
overall incidence of IRIS is 18% in patients with HIV-associated
TB, with mortality of 2% attributable to TB-IRIS (17) but not all patients will develop
IRIS. Risk factors for developing TB-IRIS include low baseline CD4
T cell count before ART initiation, short interval between TB
treatment and ART initiation and high mycobacterial burden, also
caused by disseminated TB (18).
The mechanism of IRIS occurrence remains elusive and it is
therefore necessary to find new ideas and treatment approaches to
prevent and reverse IRIS in patients with HIV-associated pulmonary
TB.
It is clinically reported that IRIS occurs as a
pathological, immunological inflammatory response induced in the
host following HAART treatment (19). A previous study hypothesized that
the combined action of innate and adaptive immune systems increases
the risk of IRIS development (20). T helper cell 17 (Th17) and
regulatory T cells (Tregs) are two subsets of CD4+ T cells with
completely different immune functions (21). Th17 cells are pro-inflammatory,
while Treg cells are anti-inflammatory. The balance between these
subpopulations is crucial for preventing excessive immune
activation and autoimmune responses, which serve a role in IRIS
(22,23).
HIV infection is associated not only with increased
free radical production through multiple mechanisms that leads to
oxidative stress but also with suppression of antioxidant defense
systems (24,25). Malonaldehyde (MDA) is a product of
lipid peroxidation and a key indicator of oxidative damage in cells
(26). High levels of MDA and
reactive oxygen species (ROS) in patients with HIV/AIDS co-infected
with TB cause systemic cell damage, leading to immune system
dysregulation and further deterioration of health (9). Superoxide dismutase (SOD) is an
important antioxidant enzyme in living organisms (27).
Oxidative stress is associated with the immune
function of patients with HIV, especially immune function of T
cells, the imbalance of antioxidant defense system and lymphocyte
apoptosis in HIV patients may lead to T cells are sensitive to
apoptosis (25). Mycobacterium
tuberculosis is the primary causative agent of TB in patients
with AIDS/HIV infection. A high level of oxidative stress occurs in
patients with HIV-TB co-infection because of tissue inflammation
and poor nutrition and immunity (28). In patients with AIDS and pulmonary
TB, the association between oxidative stress and M.
tuberculosis and abnormal T cell immune function (29) are associated with development of
TB-IRIS (15). This may provide a
new direction for treating IRIS in patients with HIV-associated
pulmonary TB.
The present study aimed to investigate changes in
oxidative stress markers and Th17/Treg cell balance in the
development of IRIS in patients with HIV-associated pulmonary
TB.
Materials and methods
Study subjects
A total of 316 patients diagnosed with
HIV-associated pulmonary TB and admitted to Changsha First Hospital
Branch in Changsha, China, from August 2018 to October 2020 were
recruited. These patients were treated with HAART and followed up
regularly for 12 weeks. Those who developed IRIS during this period
were included in the IRIS group (n=60) and the remaining patients
were included in the non-IRIS group (n=256). All patients provided
written informed consent. The present study was approved by Ethics
Committee of Changsha First Hospital (approval no. ky20180103).
The participants were aged 18-84 years old, the male
to female ratio is ~1.45:1. Pulmonary TB was confirmed according to
the diagnostic criteria of pulmonary TB in the health industry
standards of China (WS 288-2008 and WS 288-2017) (30): i) nucleic acid test shows a
positive result for M. tuberculosis and ii) chest
radiological imaging shows typical clinical symptoms of active TB
infection. HIV infection was confirmed according to the diagnostic
criteria based on the Chinese Guidelines for Diagnosis and
Treatment of HIV/AIDS (2018) (31): Nucleic acid test shows a positive
result for HIV. IRIS was confirmed according to the diagnostic
criteria of the International Network for the Study of HIV-related
IRIS consensus case definition (32): i) Patient with HIV; ii) receiving
effective ART, as evidenced by a decrease in HIV-1 RNA
concentration or an increase in CD4+ T cells from baseline; iii)
clinical symptoms consistent with the inflammatory process and iv)
clinical course not consistent with the expected course of
previously or newly diagnosed opportunistic infection or drug
toxicity. The inclusion criteria were as follows: i) Patients with
pulmonary TB who received on initial anti-TB treatment and ii)
patients with HIV infection who received initial antiretroviral
treatment. The exclusion criteria were as follows: i) Patients with
other opportunistic infections, (2) pregnant or lactating patients, iii)
patients with severe psychiatric or neurological disease, iv)
patients with oncological disease and v) patients with severe
cardiovascular, cerebrovascular, hepatic, renal or other organ
impairment.
Treatment methods
HAART was administered as follows: Lamivudine (300
mg/day), tenofovir (300 mg/day) and efavirenz (600 mg/day) taken
orally. The regimen was adjusted based on drug resistance, severe
liver and kidney injury or other intolerance.
Anti-TB regimen was as follows: Isoniazid (300
mg/day), rifampicin (450 mg/day), ethambutol (750 mg/day) and
pyrazinamide (150 mg/day) taken orally. In the first and second
months, all four drugs were administered; in the third to ninth
months, isoniazid + rifampicin were administered.
ELISA determination of SOD and MDA
levels
Blood was collected from all patients before and
after HAART at week 12. Patients fasted for 8 h before blood
collection. Fasting venous blood (5 ml) was collected at 8:00 AM
and stored at 4˚C, then divided into two portions for flow
cytometry and preparing serum by centrifugation at 1,200 x g for 15
min at 4˚C, which was stored at -80˚C until use. SOD (cat. no.
ab65354) and the MDA Assay kit (cat. no. ab238537) purchased from
Abcam were used to determine the levels of serum SOD and MDA with a
fully automated biochemical immunoassay analyzer. These kits were
used according to the manufacturer's protocol.
Determination of the WBC count,
HIV-RNA and CRP levels
CRP was detected by immunoturbidimetric method
through C-reactive protein kits (E023, Nanjing Jiancheng
Bioengineering). White blood cell (WBC) were detected by automatic
blood cell counter (XN-1000V, Sysmex). The patient's 4 ml venous
blood was placed in EDTA anticoagulant tube to separate the plasma,
and the HIV-1 Qualitative Test kit (Roche, Germany) was used for
real-time fluorescence RT-qPCR analyzer (BIO-RAD, Germany) to
determine the HIV-RNA load, set positive control and negative
control, HIV-RNA copies were converted to geometric mean
representation.
Flow cytometric assay to determine
ratio of immune cells
Flow cytometric assay was used to determine the
ratio of CD4+ T, Th17 and Treg cells in whole blood samples using a
CytoFLEX Flow Cytometer (cat. no. B96622) and Kaluza C Analysis
Software 2.1.1 (both Beckman Coulter, Inc.). Briefly, 125 µl
anticoagulated blood was taken in a flow tube and 125 serum-free
medium (12753018, Gibco), 1 PMA/Ionomycin and 1 µl BFA/Monensin
Mixture (250X) (70-CS1002) (MultiSciences) were added. Another 125
µl anticoagulated blood and 125 µl serum-free medium were used as
control. The mixture was incubated at 37˚C for 4-6 h and shaken
every 1-2 h. Peripheral blood mononuclear cells (PBMCs ) were
isolated by standard Ficoll-Hypaque gradient centrifugation: Whole
blood (3 ml) in K3EDTA-diluted PBS was layered over Ficoll-paque
PLUS (3 ml; Amersham; Cytiva). After centrifugation at 1,000 x g
for 20 min at room temperature, PBMCs were harvested and washed
twice with incomplete RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc.) followed by centrifugation at 700 x g for 10 min
at 4˚C. The viable PBMCs were evaluated using 0.2% Trypan blue dye
for 3 min at room temperature. Single nucleated cells were
resuspended in medium containing 10% fetal bovine serum (16140089,
Gibco) to a concentration of 1x107 cells/ml. Next, 250
µl PBMCs in a flow tube was mixed with 1 µl PMA/Ionomycin Mixture
(250X) and 1 µl BFA/Monensin Mixture (250X). Samples containing
only PBMCs were used as controls. The mixture was incubated at 37˚C
for 4-6 h and shaken at 1-2 h intervals. A total of 100 µ cell
suspension from the sample and control tubes was mixed with 5 µl
Anti-Human CD3, FITC and 5 µl Anti-Human CD8α, PerCP-Cy5.5 (1:100;
cat. nos. ab34275, ab34275, ab95591 and ab210329; Abcam) or 5 µl of
Anti-Mouse CD3ε, FITC and 5 µl Anti-Mouse CD4, PerCP-Cy5.5 (1:100;
cat. nos. ab210317, ab210316, ab218745 and ab210373; Abcam) in a
new flow tube. The mixture was shaken and mixed well before
incubation for 15 min at room temperature in the dark. A total of
100 µl FIX & PERM Medium A (GAS001S100, Invitrogen) was added
to each tube and mixed by shaking. The mixture was incubated at
room temperature for 15 min in the dark. Next, 10X Flow Cytometry
Staining Buffer (70-S1001, MultiSciences) was diluted to 1X with
distilled water. Then, 2 ml pre-chilled 1X Flow Cytometry Staining
Buffer was added to each tube. The mixture was centrifuged at 300 x
g for 5 min at 4˚C and the supernatant was discarded. Next, 100 µl
FIX & PERM Medium B (GAS002S5, Invitrogen) and 5 µl Anti-Human
IL-17A, PE (1:100; cat. nos. ab189377, Abcam; 560438, BD
Pharmingen) or 5 µl Anti-Mouse IL-17A, PE (1:100; cat. nos.
ab189377, Abcam; 12-71777-81, eBioscience) were added to each tube.
The mixture was incubated at room temperature for 15 min in the
dark. Flow Cytometry Staining Buffer (1X, 2 ml) was added to each
tube and the mixture was centrifuged at 300 x g for 5 min at 4˚C.
The resultant supernatant was discarded. Flow Cytometry Staining
Buffer (1X, 500 µl) was added to each tube and the mixture was
resuspended and assayed on the flow cytometer.
Statistical analysis
All statistical analysis was performed with SPSS
V25.0 (IBM Corp.). Quantitative data with normal distribution are
expressed as the mean and standard deviation, each trial was
repeated three times. Paired or unpaired t test was used for
comparison of two independent samples and χ2 test was
performed for categorical data. Correlation analysis between was
performed using Pearson's correlation analysis. P<0.05 was
considered to indicate a statistically significant difference. In
addition, receiver operator characteristic (ROC) curves were used
to analyze the predictive value of oxidative stress markers and
Th17 and Treg cells for the occurrence of IRIS.
Results
Comparison of clinical
characteristics
No statistically significant difference in sex and
age was observed between groups (Table
I). Compared with the non-IRIS group, the IRIS group did not
show statistically significant differences in HIV infection mode,
underlying disease or complications (Table I).
 | Table IGeneral characteristics of each
group. |
Table I
General characteristics of each
group.
| Characteristic | IRIS group
(n=60) | Non-IRIS group
(n=256) |
χ2/t | P-value |
|---|
| Sex,
male/female | 36/24 | 151/105 | 0.021 | 0.885 |
| Mean age,
years | 35.6±9.5 | 36.5±10.4 | 0.613 | 0.540 |
| HIV infection mode
(%) | | | 3.142 | 0.076 |
|
Blood
transfusion | 7 (11.7) | 23 (9.0) | | |
|
Intravenous
drug use | 7 (11.7) | 15 (5.9) | | |
|
Sexual
transmission | 46 (76.7) | 218 (85.2) | | |
| Underlying diseases
Y/N | | | | |
|
Diabetes | 17/43 | 86/170 | 0.612 | 0.434 |
|
Hypertension | 12/48 | 71/185 | 1.501 | 0.220 |
| Complications,
Y/N | | | | |
|
Pulmonary
infection | 5/55 | 22/234 | 0.004 | 0.948 |
|
Drug-related
liver injury | 10/50 | 64/192 | 1.882 | 0.170 |
|
Drug-induced
rash | 10/50 | 54/202 | 0.590 | 0.442 |
Comparison of inflammatory indices,
HIV-RNA, and CD4+ T cell count before and after treatment
Before HAART, no statistically significant
differences in white blood cell (WBC) count, C reactive protein
(CRP) levels, HIV-RNA and CD4+ T cell count were observed between
the IRIS and non-IRIS groups (Table
II). Following HAART treatment, WBC levels were increased,
HIV-RNA was decreased and CD4+ T cell count increased in both
groups. After HAART treatment, the CRP level was increased in the
IRIS group but decreased in the non-IRIS group and the differences
were statistically significant Compared with the non-IRIS group,
the IRIS group showed a significant increase in WBC count, CRP
level and HIV-RNA after ART but no obvious change was observed in
CD4+ T cell count.
 | Table IIInflammatory indices, HIV-RNA and
CD4+ T cell count. |
Table II
Inflammatory indices, HIV-RNA and
CD4+ T cell count.
| Group | Treatment
point | n | Mean WBC,
x109 | Mean CRP, mg/l | Mean HIV-RNA,
g/µl | Mean CD4+
cells/µl |
|---|
| IRIS | Before | 60 | 5.51± 1.26 | 64.92± 6.10 | 5.37± 1.73 | 187.05± 23.97 |
| | After | 60 |
7.51±1.31a,b | 78.99±
9.05a,b | 3.86±
1.20a,b | 277.94±
28.08a |
| Non-IRIS | Before | 256 | 5.22±0.84 | 67.75±6.85 | 5.13±1.12 | 192.5±20.24 |
| | After | 256 |
6.43±1.09a |
56.32±7.86a |
3.05±0.78a |
265.33±33.02a |
Changes in MDA and SOD levels and Th17
and Treg ratio before and after treatment
Before the initiation of HAART, no significant
differences in MDA and SOD as well as the Th17 and Treg levels were
observed between the IRIS and non-IRIS groups (Table III). Following treatment, the
IRIS group showed increased MDA and Th17 but decreased SOD and Treg
levels; all these differences were statistically significant. After
treatment, the non-IRIS group showed decreased MDA and SOD and
increased Th17and Treg levels; except for SOD, the changes of other
indicators were significant.
 | Table IIIProportion of Th17 and Treg cells and
MDA and SOD levels. |
Table III
Proportion of Th17 and Treg cells and
MDA and SOD levels.
| Group | Treatment
point | n | Mean Th17, % | Mean Treg, % | Mean MDA,
µmol/l | SOD, U/ml |
|---|
| IRIS | Before | 60 | 0.46±0.10 | 1.61±0.22 | 48.38±5.61 | 291.73±32.63 |
| | After | 60 |
0.59±0.09a,b |
1.09±0.12a,b |
52.56±8.44a,b |
139.86±27.33a,b |
| Non-IRIS | Before | 256 | 0.46±0.05 | 1.49±0.32 | 50.10±7.33 | 301.57±41.30 |
| | After | 256 |
0.54±0.08a |
1.63±0.10a |
44.05±5.75a | 299.67±41.23 |
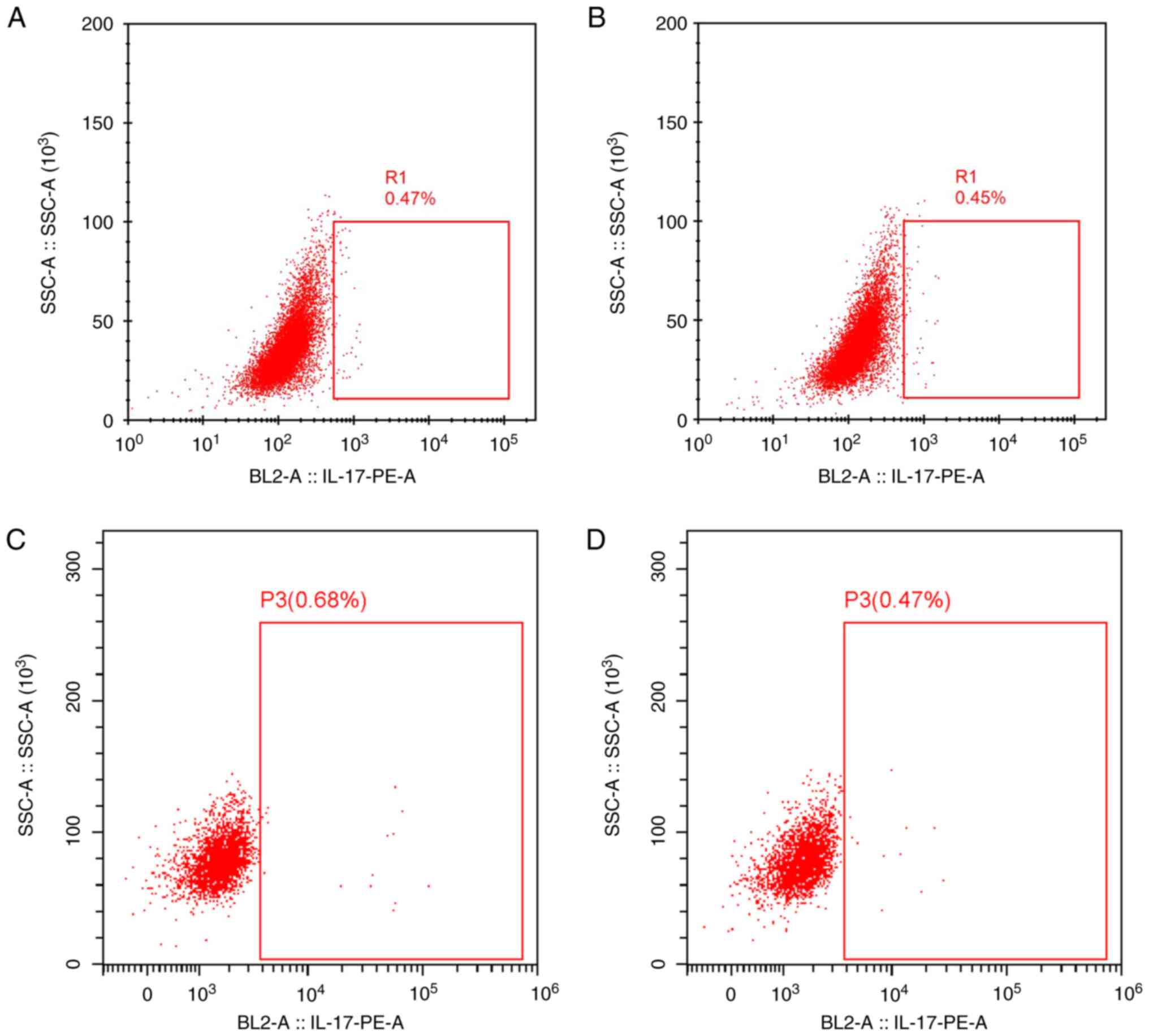
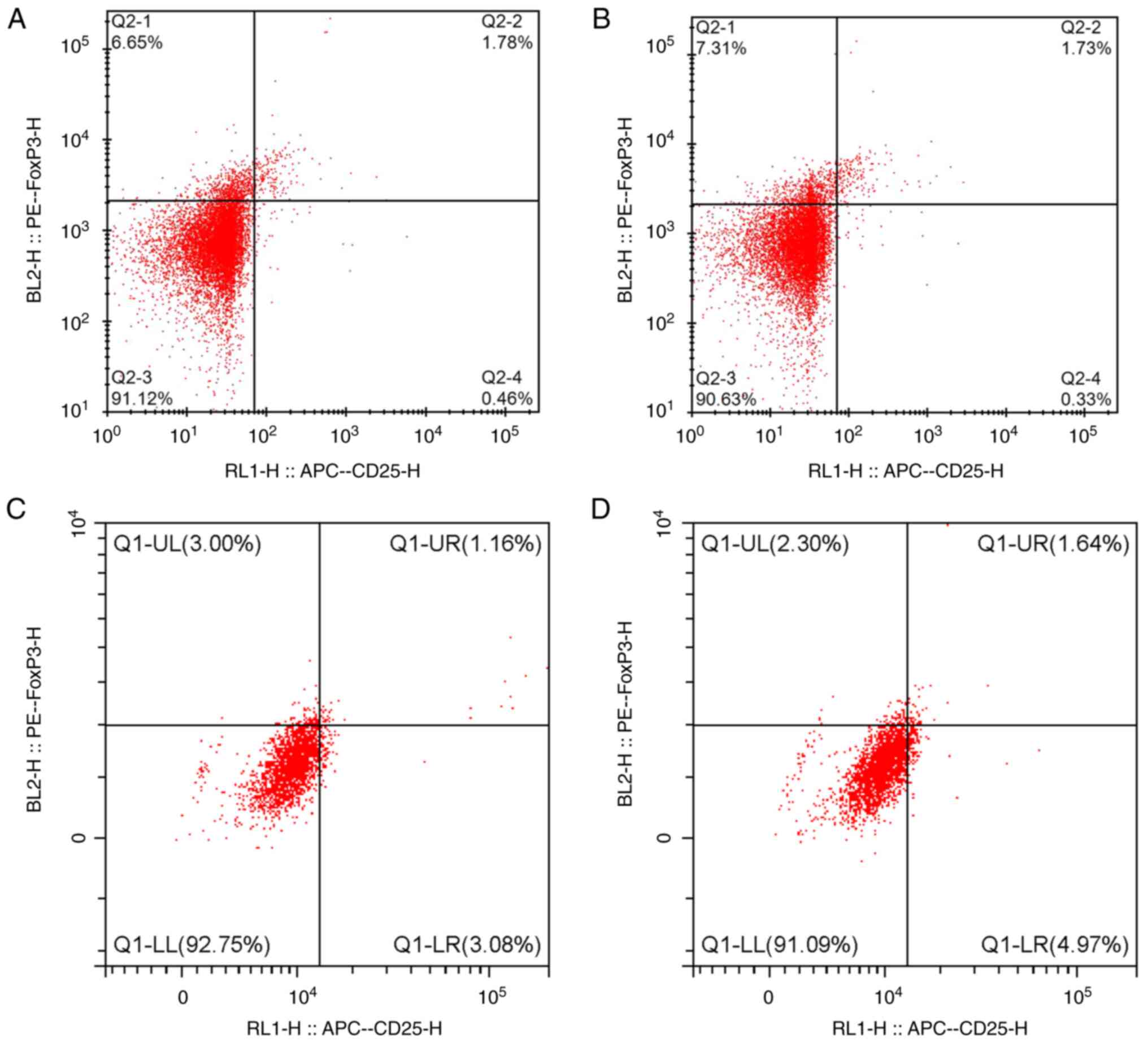
Following HAART, the Th17 and MDA levels in IRIS
group were significantly higher than that in the non-IRIS group but
the Treg and SOD levels in IRIS group were significantly lower than
that in the non-IRIS group (Table
III; Figs. 1 and 2).
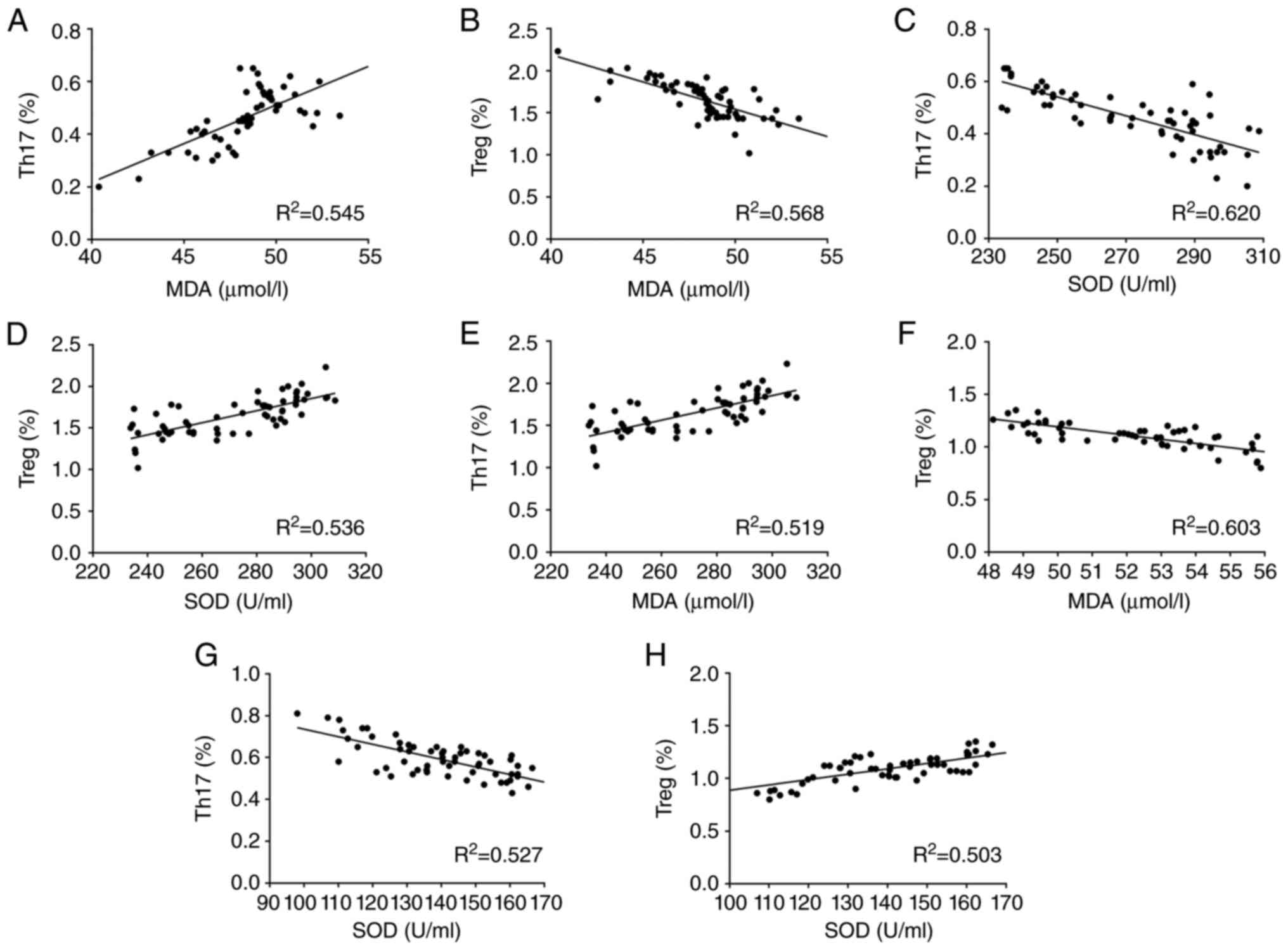
Correlation between oxidative stress
markers and Th17 and Treg levels
The correlation between oxidative stress markers and
levels of Th17 and Treg cells in the IRIS group was analyzed by
Pearson's correlation analysis (Fig.
3). Both before and after treatment, MDA had a positive
correlation with Th17, but a negative correlation with Treg. SOD
had a negative correlation with Th17, but a positive correlation
with Treg.
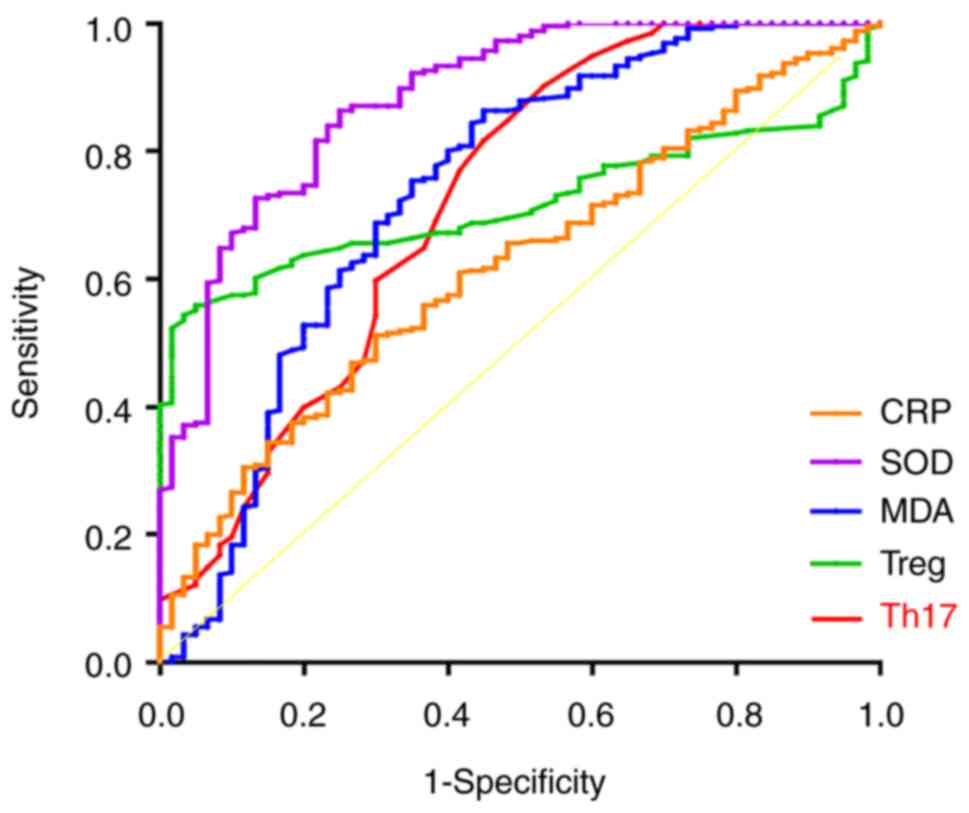
Predictive value of oxidative stress
markers and Th17 and Treg for the occurrence of IRIS
The area under the curve (AUC) values of serum MDA
and SOD levels and Th17 and Treg were 0.738, 0.883, 0.722 and
0.719, respectively. The differences were statistically significant
when receiver operating characteristic curves were plotted
(Fig. 4; Table IV). These results showed that the
above parameters have certain diagnostic value for the occurrence
of IRIS.
 | Table IVPredictive value of oxidative stress
markers and Th17 and Treg for the occurrence of immune
reconstitution inflammatory syndrome. |
Table IV
Predictive value of oxidative stress
markers and Th17 and Treg for the occurrence of immune
reconstitution inflammatory syndrome.
| Variable | Truncation
value | Sensitivity, % | Specificity, % | Area under the
curve | 95% CI | P-value |
|---|
| MDA | 48.695 | 86.3 | 55.1 | 0.738 | 0.657-0.819 | <0.001 |
| SOD | 289.795 | 86.3 | 75.2 | 0.883 | 0.835-0.932 | <0.001 |
| Th17 | 0.455 | 90.2 | 46.7 | 0.722 | 0.641-0.804 | <0.001 |
| Treg | 2.005 | 54.3 | 96.7 | 0.719 | 0.665-0.773 | <0.001 |
| CRP | 67.570 | 51.2 | 70.3 | 0.619 | 0.545-0.693 | 0.004 |
Discussion
It is estimated 251,000 people living with HIV
infection died from TB, accounting for one third of all
HIV-associated deaths and one sixth of all TB-associated deaths, in
2018(33), thus making TB
infection one of the most common causes of death in patients with
HIV and accounting for 26% of AIDS-associated deaths (34). Although ART improves the survival
of patients with HIV-associated TB and contributes to restoration
of immune function, some patients experience worsening of clinical
symptoms and exacerbation of the inflammatory response, which lead
to development of IRIS. The incidence of IRIS in patients with
HIV-associated TB has been reported to be 7-40% (35). Although the pathogenesis,
biomarkers and treatment of IRIS have been studied (12-14),
the etiology of IRIS is not clear and its treatment is limited.
Patients with HIV are frequently in a state of
oxidative stress, marked in part by reduced or altered levels of
antioxidants and increased levels of lipid peroxidation products
(36). TB is a chronic pathogenic
infection that leads to depletion of reducing substances in the
body, which increases the occurrence of oxidative stress (37). A previous study (38) reported that higher levels of MDA in
patients with AIDS and HIV co-infected with TB result in increased
production of ROS and that MDA induces secondary production of ROS
and subsequent uncontrolled lipid peroxidation, which may further
compromise the immune system and lead to exacerbation of disease in
these patients. Here, following treatment, the peripheral serum MDA
levels significantly increased, while the SOD levels decreased in
the IRIS group. The non-IRIS group showed a slight decrease in SOD
levels but a significant decrease in MDA levels, thus suggesting
that more severe oxidative stress was present in patients with IRIS
compared with the non-IRIS group.
Oxidative stress is associated with abnormal immune
function, especially with T cell immune function, in patients with
HIV (25). T cell imbalance is an
important factor for IRIS development in patients with AIDS. Treg
cells are hypothesized to play an important role in immune
modulation (38). Th17 cells,
which produce cytokines such as IL-17, IL-17F and IL-22, play a key
role in sustained inflammatory response and an increase in their
number can exacerbate the inflammatory response (39). Thl7 cells promote the inflammatory
response, while Treg cells suppress the inflammatory response. The
disruption of this balance may amplify the inflammatory response.
The present study revealed that the IRIS group showed a significant
increase in the CRP levels after HAART compared with the non-IRIS
group. Th17/Treg ratio is closely associated with IRIS during HIV
treatment (40). Li et al
(41) revealed that Th17
expression levels are significantly lower and Treg levels are
increased in patients with AIDS and TB compared with those in
healthy individuals. The Th17/Treg imbalance contributes to the
worsening of AIDS combined with TB. In the present study, compared
with pretreatment, the IRIS group showed a significant increase in
MDA and Th17 levels but a significant decrease in SOD and Treg
levels after treatment. The non-IRIS group showed a significant
increase in Th17 as well as Treg levels after treatment. These
findings suggested that the increase in oxidative stress in the
IRIS group was accompanied by Th17/Treg imbalance.
Previous studies have demonstrated that inhibition
of Treg-associated markers leads to significantly elevated airway
inflammation in patients with acute lung injury (42,43).
IL-17A inhibition decreases airway inflammation, while the
inhibition of IL-2-inducible T cell kinase signaling reduces airway
inflammation by modulating the Th17/Treg immune response and
oxidative stress in the lung (44). Yang (45) et al reported that in
patients with systemic lupus erythematosus (SLE), oxidative stress
aggravates inflammation and damage by decreasing Treg cells and
inducing the activation of mTORC1 to actively promote Th17 cell
differentiation and induction of local chemokine and cytokine
production. This suggests that oxidative stress could aggravate SLE
in these patients by inducing Th17/Treg imbalance. Yang et
al (46) showed decreased
Treg/Th17 ratio in peripheral blood of patients with polycystic
ovary syndrome, which is involved in the enhancement of systemic
inflammatory response, including the activation of oxidative
stress.
In the present study, correlation analysis revealed
that Th17 cells were positively correlated with MDA but negatively
correlated with SOD levels. Treg cells were negatively correlated
with MDA and positively correlated with SOD levels. These results
suggested that both oxidative stress and Th17/Treg imbalance are
related to the development of IRIS in patients with HIV-associated
pulmonary TB. In the present study, the AUC values of serum MDA and
SOD levels and Th17 and Treg proportions were 0.738, 0.883, 0.722
and 0.719, respectively; thus, these parameters are hypothesized to
have some diagnostic value for IRIS occurrence and may be
associated with oxidative stress. This may be associated with the
effect of oxidative stress on the regulation of Th17/Treg
homeostasis. The disruption of Th17/Treg homeostasis in patients
with HIV-associated pulmonary TB accelerates the imbalance of the
immune system in patients with abnormal oxidative stress (22).
Evidence suggests that the Th17/Treg balance
underlies the pathogenic mechanisms of autoimmune diseases
(47). Th17/Treg ratio is
increased in patients with rheumatoid arthritis (RA), psoriasis,
multiple sclerosis and inflammatory bowel disease (48). Monoclonal antibodies against the
human IL-6 receptor, namely tocilizumab and sarilumab, are used in
patients with RA to decrease Th17 cell levels but increase Treg
cell levels (47). The
aforementioned studies suggest that the Th17/Treg balance may be a
target for IRIS therapy. In patients with HIV, low GSH levels have
been shown to induce provirus transcription by NF-κB activation,
cell apoptosis and CD4+ T cell depletion. Therefore, replenishment
of GSH is considered to be a potential supplement to HAART
(49). The present study observed
increased MDA and decreased SOD levels in patients with IRIS. Taken
together with the findings of previous research, this suggested
that the oxidative stress markers may be therapeutic targets for
IRIS caused by HAART in patients with HIV-associated pulmonary TB.
These results will provide a basis for IRIS clinical treatment.
There were limitations to the present study. The
present study does not involve the detection indicators of upstream
and downstream signals. The sample size of the study was small.
Therefore, further validation is needed through multicenter
randomized controlled trials with a larger sample size. In
conclusion, following ART treatment, IRIS developed in patients
with HIV-associated pulmonary TB because of oxidative stress and
Th17/Treg imbalance.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Key Research
& Developmental Program of Hunan Province (grant no.
2022SK2047), the Natural Science Foundation of Hunan Province
(grant no. 2018JJ2452) and the Natural Science Foundation of
Changsha city (grant no. Kp2208448).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LW and ZGZ were responsible for study concept and
design. LS and SSW performed experiments. TX and LZ performed data
collection, analysis and interpretation. ZGZ was responsible for
drafting the manuscript. LW reviewed the manuscript. All authors
have read and approved the final manuscript. LW and ZGZ confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
All experimental protocols were approved by the
Ethics Committee of Changsha First Hospital (ethical approval no.
ky20180103). The study subjects provided signed informed
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tesfaye B, Alebel A, Gebrie A, Zegeye A,
Tesema C and Kassie B: The twin epidemics: Prevalence of TB/HIV
co-infection and its associated factors in Ethiopia; A systematic
review and meta-analysis. PLoS One. 13(e0203986)2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Yuan FS, Liu L, Liu LH, Zeng YL, Zhang LL,
He F, Liu XJ, Li JM, Liu Q, Xu MJ, et al: Epidemiological and
spatiotemporal analyses of HIV/AIDS prevalence among older adults
in Sichuan, China between 2008 and 2019: A population-based study.
Int J Infect Dis. 105:769–775. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Vrinceanu D, Dumitru M, Patrascu OM,
Costache A, Papacocea T and Cergan R: Current diagnosis and
treatment of rhinosinusal aspergilloma (review). Exp Ther Med.
22(1264)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tola A, Mishore KM, Ayele Y, Mekuria AN
and Legese N: Treatment outcome of tuberculosis and associated
factors among TB-HIV Co-infected patients at public hospitals of
Harar town, eastern Ethiopia. A five-year retrospective study. BMC
Public Health. 19:1–12. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tiberi S, Carvalho AC, Sulis G, Vaghela D,
Rendon A, Mello FC, Rahman A, Matin N, Zumla A and Pontali E: . The
cursed duet today: Tuberculosis and HIV-coinfection. Presse Med.
46:e23–e39. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Liebenberg C, Luies L and Williams AA:
Metabolomics as a tool to investigate HIV/TB co-infection. Front
Mol Biosci. 8(692823)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Vecchione MB, Laufer N, Sued O, Corti M,
Salomon H and Quiroga MF: 7-oxo-DHEA enhances impaired M.
tuberculosis-specific T cell responses during HIV-TB coinfection. J
Biomed Sci. 27(20)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Schutz C, Meintjes G, Almajid F, Wilkinson
RJ and Pozniak A: Clinical management of tuberculosis and HIV-1
co-infection. Eur Respir J. 36:1460–1481. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Musisi E, Matovu DK, Bukenya A, Kaswabuli
S, Zawedde J, Andama A, Byanyima P, Sanyu I, Sessolo A, Seremba E,
et al: Effect of anti-retroviral therapy on oxidative stress in
hospitalized HIV-infected adults with and without TB. Afr Health
Sci. 18:512–522. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lu DY, Wu HY, Yarla NS, Xu B, Ding J and
Lu TR: HAART in HIV/AIDS treatments: Future trends. Infect Disord
Drug Targets. 18:15–22. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yang H, Wang Y, Guo PJ, Wu YM, Gao Y, Li
XC, Gao BC, Liu J, Li H and Yang JY: Analysis of the effect of
antiretroviral therapy and drug resistance in adult AIDS in inner
Mongolia, China: A comprehensive study. J Biol Regul Homeost
Agents. 36:783–787. 2022.
|
|
12
|
Proal AD and Marshall TG: Re-framing the
theory of autoimmunity in the era of the microbiome: Persistent
pathogens, autoantibodies, and molecular mimicry. Discov Med.
25:299–308. 2018.PubMed/NCBI
|
|
13
|
Sereti I, Sheikh V, Shaffer D, Phanuphak
N, Gabriel E, Wang J, Nason MC, Roby G, Ngeno H, Kirui F, et al:
Prospective international study of incidence and predictors of
immune reconstitution inflammatory syndrome and death in people
living with human immunodeficiency virus and severe lymphopenia.
Clin Infect Dis. 71:652–660. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Arirachakaran P, Luangworakhun S,
Charalampakis G and Dahlén G: Non-oral, aerobic, Gram-negative
bacilli in the oral cavity of Thai HIV-positive patients on
Highly-active anti-retrovirus therapy medication. J Investig Clin
Dent. 10(e12387)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Pean P, Nouhin J, Ratana M, Madec Y,
Borand L, Marcy O, Laureillard D, Fernandez M, Barré-Sinoussi F,
Weiss L, et al: High Activation of γδ T cells and the
γδ2pos T-cell subset is associated with the onset of
tuberculosis-associated immune reconstitution inflammatory
syndrome, ANRS 12153 CAPRI NK. Front Immunol.
10(2018)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Quinn CM, Poplin V, Kasibante J, Yuquimpo
K, Gakuru J, Cresswell FV and Bahr NC: Tuberculosis IRIS:
Pathogenesis, presentation, and management across the spectrum of
disease. Life (Basel). 10(262)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Namale PE, Abdullahi LH, Fine S, Kamkuemah
M, Wilkinson RJ and Meintjes G: Paradoxical TB-IRIS in HIV-infected
adults: A systematic review and meta-analysis. Future Microbiol.
10:1077–1099. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
18
|
Cevaal PM, Bekker LG and Hermans S:
TB-IRIS pathogenesis and new strategies for intervention: Insights
from related inflammatory disorders. Tuberculosis (Edinb).
118(101863)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Barber DL, Andrade BB, Sereti I and Sher
A: Immune reconstitution inflammatory syndrome: The trouble with
immunity when you had none. Nat Rev Microbiol. 10:150–156.
2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chang C, Sheikh V, Sereti I and French MA:
Immune reconstitution disorders in patients with HIV infection:
From pathogenesis to prevention and treatment. Curr HIV/AIDS Rep.
11:223–232. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhu J, Yamane H and Paul WE:
Differentiation of effector CD4 T cell populations. Ann Rev
Immunol. 28:445–489. 2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zheng Y, Zhou H, He Y, Chen Z, He B and He
M: The immune pathogenesis of immune reconstitution inflammatory
syndrome associated with highly active antiretroviral therapy in
AIDS. AIDS Res Hum Retroviruses. 30:1197–1202. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Duan Y, Bai H, Li X, Wang D and Wang Y,
Cao M, Zhang N, Chen H and Wang Y: Oncolytic adenovirus H101
synergizes with radiation in cervical cancer cells. Curr Cancer
Drug Targets. 21:619–630. 2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Stephensen CB, Marquis GS, Douglas SD,
Kruzich LA and Wilson CM: Glutathione, glutathione peroxidase, and
selenium status in HIV-positive and HIV-negative adolescents and
young adults. Am J Clin Nutr. 85:173–181. 2007.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ivanov AV, Valuev-Elliston VT, Ivanova ON,
Kochetkov SN, Starodubova ES, Bartosch B and Isaguliants MG:
Oxidative stress during HIV infection: Mechanisms and consequences.
Oxid Med Cell Longev. 2016(8910396)2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Awodele O, Olayemi SO, Nwite JA and
Adeyemo TA: Investigation of the levels of oxidative stress
parameters in HIV and HIV-TB co-infected patients. J Infect Dev
Ctries. 6:79–85. 2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Rosa AC, Corsi D, Cavi N, Bruni N and
Dosio F: Superoxide dismutase administration: A review of proposed
human uses. Molecules. 26(1844)2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Makinde O, Rotimi K, Ikumawoyi V, Adeyemo
T and Olayemi S: Effect of vitamin A and vitamin C supplementation
on oxidative stress in HIV and HIV-TB co-infection at Lagos
University Teaching Hospital (LUTH) Nigeria. Afr Health Sci.
17:308–314. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Safe IP, Amaral EP, Araújo-Pereira M,
Lacerda MV, Printes VS, Souza AB, Beraldi-Magalhães F, Monteiro WM,
Sampaio VS, Barreto-Duarte B, et al: Adjunct N-acetylcysteine
treatment in hospitalized patients with HIV-associated tuberculosis
dampens the oxidative stress in peripheral blood: Results from the
RIPENACTB study trial. Front Immunol. 11(602589)2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Chen L, Wang X, Jia X, Lan Y, Yi H, Wang X
and Xu P: Investigation of 3-year inpatient TB cases in Zunyi,
China: Increased TB burden but improved bacteriological diagnosis.
Front Public Health. 10(941183)2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
AIDS and Hepatitis C Professional Group,
Society of Infectious Diseases, Chinese Medical Association and
Chinese Center for Disease Control and Prevention. Chinese
guidelines for diagnosis and treatment of HIV/AIDS (2018). Zhonghua
Nei Ke Za Zhi. 57:867–884. 2018.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
32
|
Meintjes G, Lawn SD, Scano F, Maartens G,
French MA, Worodria W, Elliott JH, Murdoch D, Wilkinson RJ, Seyler
C, et al: Tuberculosis-associated immune reconstitution
inflammatory syndrome: Case definitions for use in resource-limited
settings. Lancet Infect Dis. 8:516–523. 2008.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Melgar M, Nichols C, Cavanaugh JS, Kirking
HL, Surie D, Date A, Ahmedov S, Maloney S and Fukunaga R: CDC
Country Offices' Tuberculosis/HIV Advisors et al.
Tuberculosis preventive treatment scale-up among antiretroviral
therapy patients-16 countries supported by the U.S. president's
emergency plan for AIDS relief, 2017-2019. MMWR Morb Mortal Wkly
Rep. 69:329–334. 2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ji YJ, Liang PP, Shen JY, Sun JJ, Yang JY,
Chen J, Qi TK, Wang ZY, Song W, Tang Y, et al: Risk factors
affecting the mortality of HIV-infected patients with pulmonary
tuberculosis in the cART era: a retrospective cohort study in
China. Infect Dis Poverty. 7(25)2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wu Y, Chen J and Lu H: Advances in the
study of the development of immunodeficiency inflammatory syndrome
in people infected with mycobacteria tuberculosis combined with the
human immunodeficiency virus. Chin J Inf Chemo. 18:230–235.
2018.(In Chinese).
|
|
36
|
Surur AS, Teni FS, Wale W, Ayalew Y and
Tesfaye B: Health related quality of life of HIV/AIDS patients on
highly active anti-retroviral therapy at a university referral
hospital in. Ethiopia. 17(737)2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Bauer DC, Black DM, Bouxsein ML, Lui LY,
Cauley JA, de Papp AE, Grauer A, Khosla S, McCulloch CE and Eastell
R: Foundation for the National Institutes of Health (FNIH) Bone
Quality Project. Treatment-related changes in bone turnover and
fracture risk reduction in clinical trials of anti-resorptive
drugs: A meta-regression. J Bone Miner Res. 33:634–642.
2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Kleinman AJ, Sivanandham R, Pandrea I,
Chougnet CA and Apetrei C: Regulatory T cells as potential targets
for HIV cure research. Front Immunol. 9(734)2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
De Biasi S, Bianchini E, Nasi M, Digaetano
M, Gibellini L, Carnevale G, Borghi V, Guaraldi G, Pinti M, Mussini
C and Cossarizza A: Th1 and Th17 proinflammatory profile
characterizes invariant natural killer T cells in virologically
suppressed HIV+ patients with low CD4+/CD8+ ratio. AIDS.
13:2599–2610. 2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Qu YZ, Li M, Zhao YL, Zhao ZW, Wei XY, Liu
JP, Gao L and Gao GD: Astragaloside IV attenuates cerebral
ischemia-reperfusion-induced increase in permeability of the
blood-brain barrier in rats. Eur J Pharmacol. 606:137–141.
2009.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Li Y and Sun W: . Effects of Th17/Treg
cell imbalance on HIV replication in patients with AIDS complicated
with tuberculosis. Experimental and Therapeutic Medicine.
15:2879–83. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Sun L and Liu Y: Clinical factors of blood
transfusion-related acute lung injury and changes in levels of
Treg-related cytokines. Emerg Med Int. 2022(7344375)2022.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Zhou M, Zhang Y, Tang R, Liu H, Du M, Gao
Z, Ji Z and Fang H: HMGB1/TLR4 signaling affects regulatory T cells
in acute lung injury. J Inflamm Res. 14:1551–1561. 2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Nadeem A, Al-Harbi NO, Ahmad SF, Al-Harbi
MM, Alhamed AS, Alfardan AS, Assiri MA, Ibrahim KE and Albassam H:
Blockade of interleukin-2-inducible T-cell kinase signaling
attenuates acute lung injury in mice through adjustment of
pulmonary Th17/Treg immune responses and reduction of oxidative
stress. Int Immunopharmacol. 83(106369)2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Yang J, Yang X, Zou H and Li M: Oxidative
stress and Treg and Th17 dysfunction in systemic lupus
erythematosus. Oxid Med Cell Longev. 2016:1–9. 2016.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Yang Y, Xia J, Yang Z, Wu G and Yang J:
The abnormal level of HSP70 is related to Treg/Th17 balance in PCOS
patients. J Ovarian Res. 14(155)2021.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Lee GR: The Balance of Th17 versus Treg
Cells in Autoimmunity. Int J Mol Sci. 19(730)2018.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Littman DR and Rudensky AY: Th17 and
regulatory T cells in mediating and restraining inflammation. Cell.
140:845–858. 2010.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Tyagi P, Pal VK, Agrawal R, Singh S,
Srinivasan S and Singh A: Mycobacterium tuberculosis
reactivates HIV-1 via exosome-mediated resetting of cellular redox
potential and bioenergetics. mBio. 11(e03293)2020.PubMed/NCBI View Article : Google Scholar
|