Many systematic reviews and trials compared the
effect of different H2RAs and PPIs, but there is no head-to-head
comparison of different doses of these pharmaceuticals (13-19).
The Japanese Society of Gastroenterology (JSGE) developed the
evidence-based clinical practice guidelines for DU's initial
non-eradication treatment, but there are no recommended doses of
PPIs and H2RAs (10). A
reappraisal of the available evidence to support clinical
decision-making is timely. Therefore, we did a contemporaneous
systematic review and network meta-analysis of RCTs of
pharmaceuticals in non-eradication treatment DU.
The study protocol is available on the International
Prospective Register of Systematic Reviews with a registration
number of CRD42020219564 and was prepared according to the
guidelines of the Cochrane Multiple Interventions Methods Group
(20).
We searched Cochrane Library, Embase, Medline, Web
of Science, and Clinical Trials.gov databases from their inception
until November 2022 for randomized clinical trials (RCTs)
investigating different PPIs and H2RAs in initial non-eradication
treatment of DU patients with no language restrictions. Additional
studies were searched in the reference lists of all identified
publications, including relevant meta-analyses. Regarding the
search strategy, terms included the following items: (‘Proton Pump
Inhibitors’ or ‘PPI’ or ‘PPIs’) and (‘H2 Receptor Antagonists’ or
‘H2-receptor antagonists’ or ‘H2RAs’) and (‘Initial Non-eradication
Treatment of DU patients’ or ‘Non-eradication of DU patients’).
All superiority, non-inferiority, phase II and III,
single-blinded, and double-blinded trials were included. RCTs
examining the effect of drugs (omeprazole, lansoprazole,
rabeprazole, vonoprazan, pantoprazole, ilaprazole, ranitidine,
cimetidine, famotidine) in adult patients (aged >18 years) with
DU were eligible. The first period of randomized crossover trials
was eligible for inclusion if they provided efficacy data before
crossover. The definitions of DU considered within this network
meta-analysis included endoscopically confirmed active DU. Trials
that examined the efficacy of any dose of the drugs of interest and
compared them with each other or placebo were considered
eligible.
Two investigators (X. Zhu and X. Meng) did the
literature search independently from one another. Two investigators
(B. Li and Y. Su) evaluated all abstracts identified by searching
for eligibility independently from one another. They obtained all
potentially relevant papers and evaluated them in more detail,
using pre-designed forms, to assess eligibility independently,
according to the predefined criteria. We translated papers that
were not in the English language. We resolved disagreements between
investigators by discussion.
Two reviewers (J. Zhao and J. Liu) independently
extracted data from original trial reports using a standardized
form and then double-checked the extraction. We assessed the
sources of bias using the Cochrane Collaboration's risk-of-bias
tool addressing six domains (21).
Two investigators (H. Wang and Q. Feng) independently completed the
assessments, and discrepancies were discussed with a third party
and resolved by consensus. Additionally, the Grading of
Recommendations Assessment, Development, and Evaluation (GRADE)
framework were used to assess the quality of evidence contributing
to each estimated network (22).
Two independent reviewers (X. Meng and B. Li)
assessed the risk of bias of these studies included in our
analysis. Consistancy between the two reviewers was reported. Any
disagreement was solved by a third senior investigator (X. Zhu or
J. Zhao). Studies were classified as having high risk of bias if
one or more domains were rated as high risk of bias; low if five or
more were rated as low risk of bias and none was rated as high risk
of bias, and all other cases were assumed to regard as moderate
risk.
Additionally, a funnel plot was drawn and a
deviation test in combination with Egger test was conducted. A
comparison-adjusted funnel plot was used to detect potential
publication biases in the results between small and large studies.
Global heterogeneity was assessed using the I2
statistics, which incorporated the extent of heterogeneity and
evaluated the extent of uncertainty in the estimated effect size
locally. To assess whether the results were impacted by study
characteristics (effect modifiers), the risk of bias (high,
unclear, or low) was assessed. Additionally, to assess the
robustness of the results, we used random effect models for
sensitivity analysis. Comparison-adjusted funnel plots were
obtained to investigate whether the integrated results were
different between the imprecise and precise trials (27). All analyses were conducted using R
3.6.2 via the netmeta, version 1.1-0.
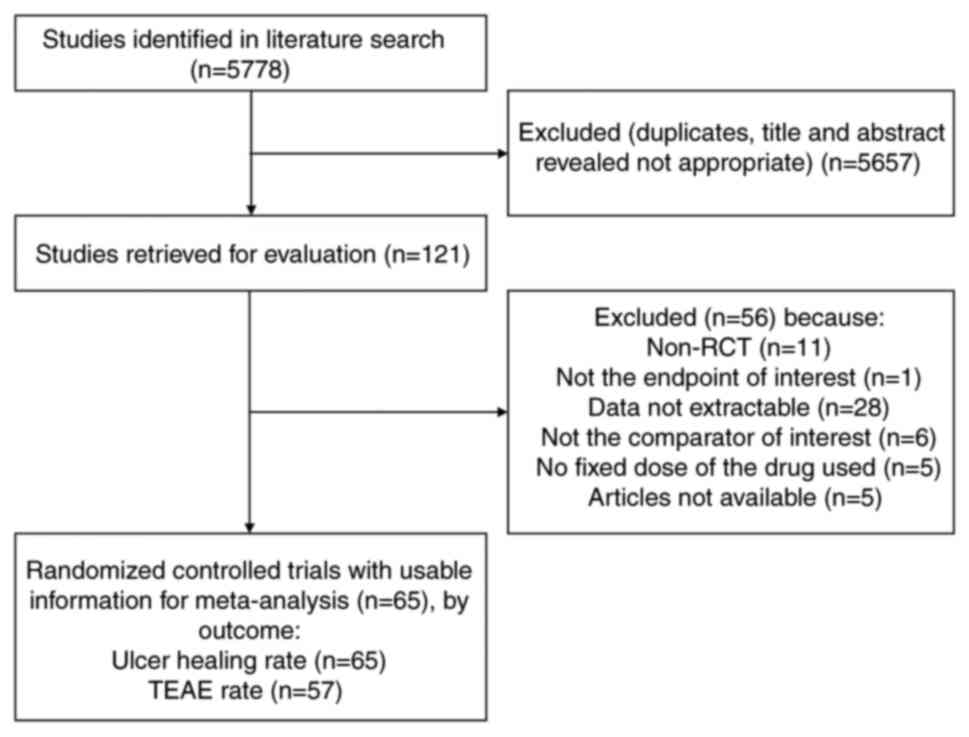
Sixty-five eligible studies, published between 1976
and 2022, corresponding to 15381 adults, were selected for pooled
analyses (18,19,28-90).
The literature search process is shown in Fig. 1. These trials evaluated 6 different
PPIs (ilaprazole, omeprazole, lansoprazole, pantoprazole,
rabeprazole, and vonoprazan) and 3 different H2RAs (cimetidine,
ranitidine and famotidine). These studies have come from many
countries (mainly China, Japan, United States, United Kingdom,
Germany) and centers. Endoscopic examination was used to define the
healing of duodenal ulcers after treatment. The patients' treatment
courses were divided into three types: 46 studies were treated for
four weeks, 14 studies were treated for six weeks, and five studies
were treated for eight weeks. The baseline characteristics of the
RCTs included are provided in Table
SI (Supplementary File). We found a total of 89 studies about
the application PPIs and H2RAs in initial non-eradication treatment
of DU patients in the past five years. Most of them are non-RCT,
data not extractable and no fixed dose of the drug used. Therefore,
only one study was included in the criteria, and the others were
not included in this study.
According to the Cochrane Collaboration's tool, all
of the studies were judged to be at low or unclear risk of bias for
six domains. Disagreements were resolved by discussion. The method
used to generate the randomization schedule and conceal treatment
allocation was recorded and whether blinding was implemented for
participants, personnel, and outcomes assessment, whether there was
evidence of incomplete outcomes data and whether there was evidence
of selective reporting of outcomes.
Two independent reviewers (X. Meng and B. Li)
assessed the risk of bias of these studies included in our
analysis. Consistancy between the two reviewers was reported. Any
disagreement was solved by a third senior investigator (X. Zhu or
H. Wang). Studies were classified as having high risk of bias if
one or more domains were rated as high risk of bias; low if five or
more were rated as low risk of bias and none was rated as high risk
of bias, and all other cases were assumed to regard as moderate
risk. The risk of bias assessment of the trials included in this
study is presented in Table
SII.
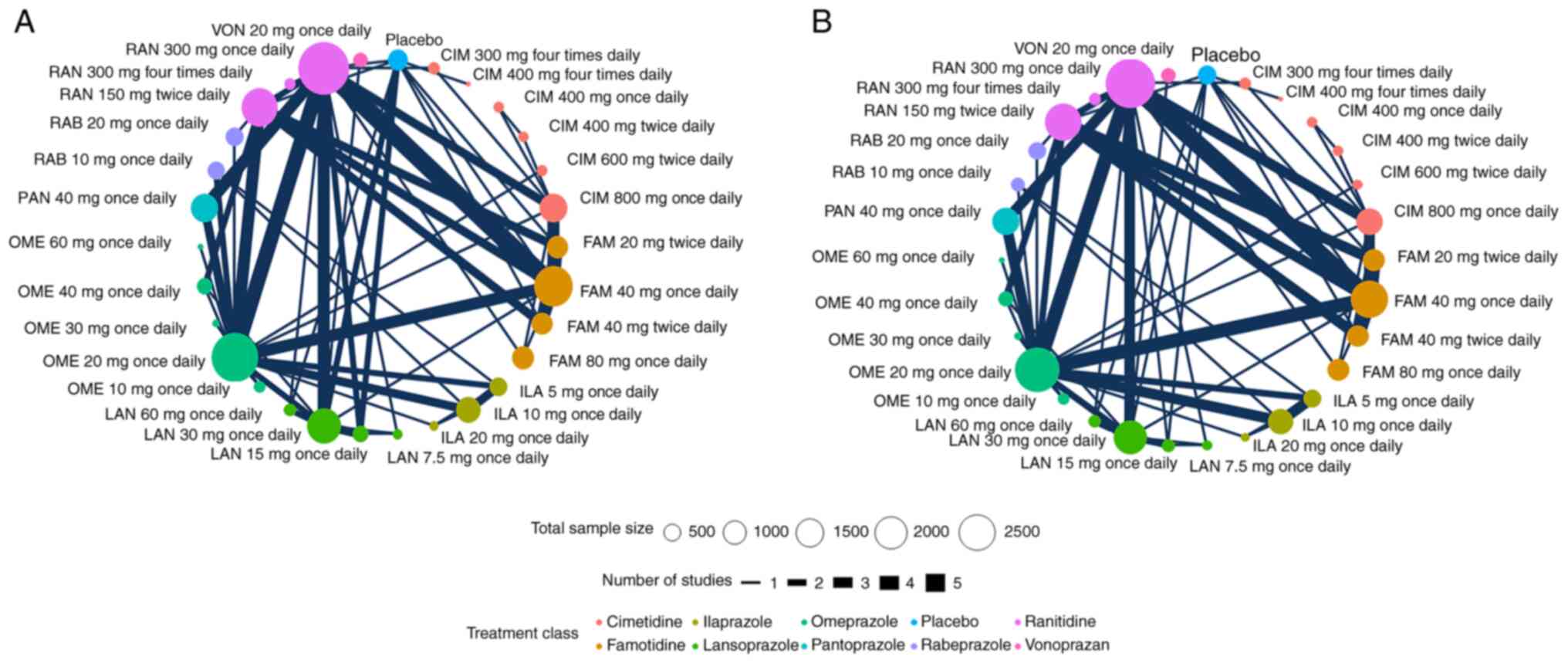
We assessed the efficacy and safety of 6 different
PPIs (ilaprazole, omeprazole, lansoprazole, pantoprazole,
rabeprazole, and vonoprazan) and 3 different H2RAs (cimetidine,
ranitidine and famotidine) for initial non-eradication treatment of
DU. There are different doses of the ilaprazole, omeprazole,
lansoprazole, pantoprazole, rabeprazole, vonoprazan, cimetidine,
ranitidine, and famotidine (3, 5, 4, 1, 2, 1, 6, 3, 4,
respectively). Fig. 2 shows the
network of eligible comparisons for ulcer healing rate.
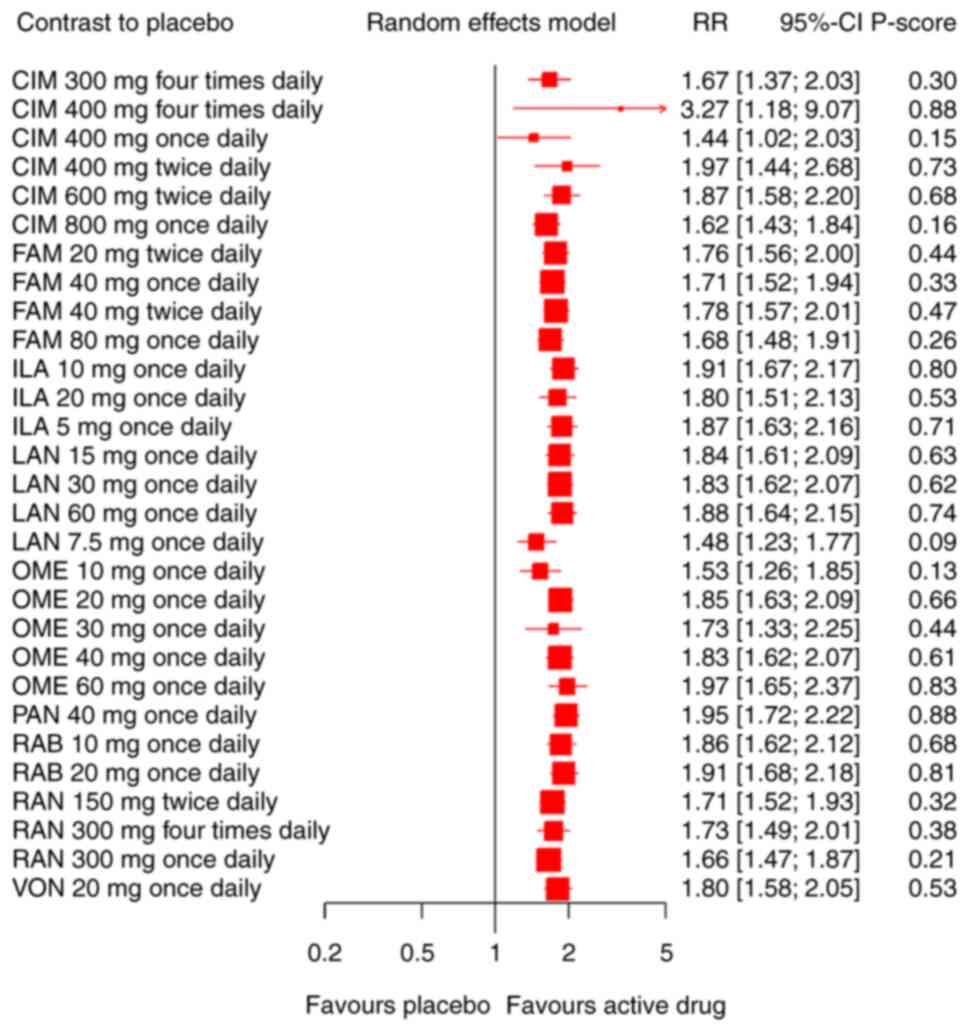
Concerning an increase in the ulcer healing rate,
our network meta-analysis included 65 RCTs involving the
administration of 6 different PPIs and three different H2RAs
patients. A placebo was used as a reference. We found significant
differences in efficacy between all the drugs and the placebo.
Compared with the placebo, the included pharmaceuticals
significantly increased the ulcer healing rate (Fig. 3). CIM 400 mg four times daily and
PAN 40 mg once daily were ranked first (P-score=0.88) in 65 RCTs
(RR 3.27, 95% CI 1.18-9.07; RR 1.95, 95% CI 1.72-2.22
respectively). Quantifying heterogeneity/inconsistency:
tau^2=0.0006; tau=0.0254; I^2=24.4% [0.0%; 44.1%]. Results of the
pairwise comparison are indicated by the RRs and 95% CIs in
Table SIII. There were no
significant statistical differences in different doses of the CIM
(300 mg four times daily, 400 mg four times daily, 400 mg twice
daily), FAM (20 mg twice daily, 40 mg once daily, 40 mg twice
daily, 80 mg once daily), RAB (10 mg once daily, 20 mg once daily),
ILA (5 mg once daily, 10 mg once daily, 20 mg once daily), LAN (15
mg once daily, 30 mg once daily, 60 mg once daily), OME (20 mg once
daily, 40 mg once daily, 60 mg once daily).
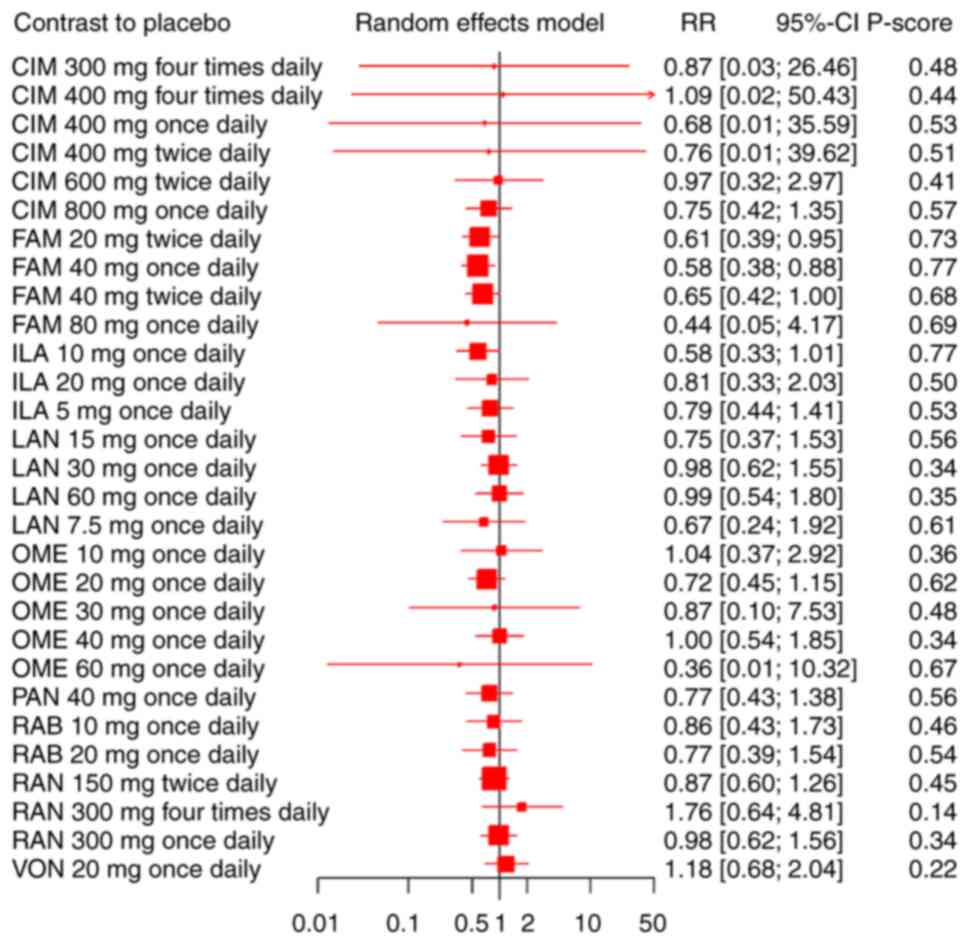
Our network meta-analysis included 57 RCTs,
reporting the administration of 6 different PPIs and three
different H2RAs among 14788 DU patients. There was no statistically
significant association between the nine drugs and the
treatment-emergent adverse event compared with the placebo
(Fig. 4). Results of the pairwise
comparisons are indicated by the RRs and 95% CIs in Table SIII. FAM 20 mg twice daily was
significantly less likely to lead to adverse events than RAN 300 mg
four times daily, VON 20 mg once daily, and placebo. FAM 40 mg once
daily was significantly less likely lead to adverse events than RAN
150 mg twice daily, RAN 300 mg four times daily, RAN 300 mg once
daily, VON 20 mg once daily and placebo. FAM 40 mg twice daily was
significantly less likely to lead to adverse events than VON 20 mg
once daily. ILA 10 mg once daily was significantly less likely to
lead to adverse events than RAN 300 mg four times daily, RAN 300 mg
once daily, and VON 20 mg once daily.
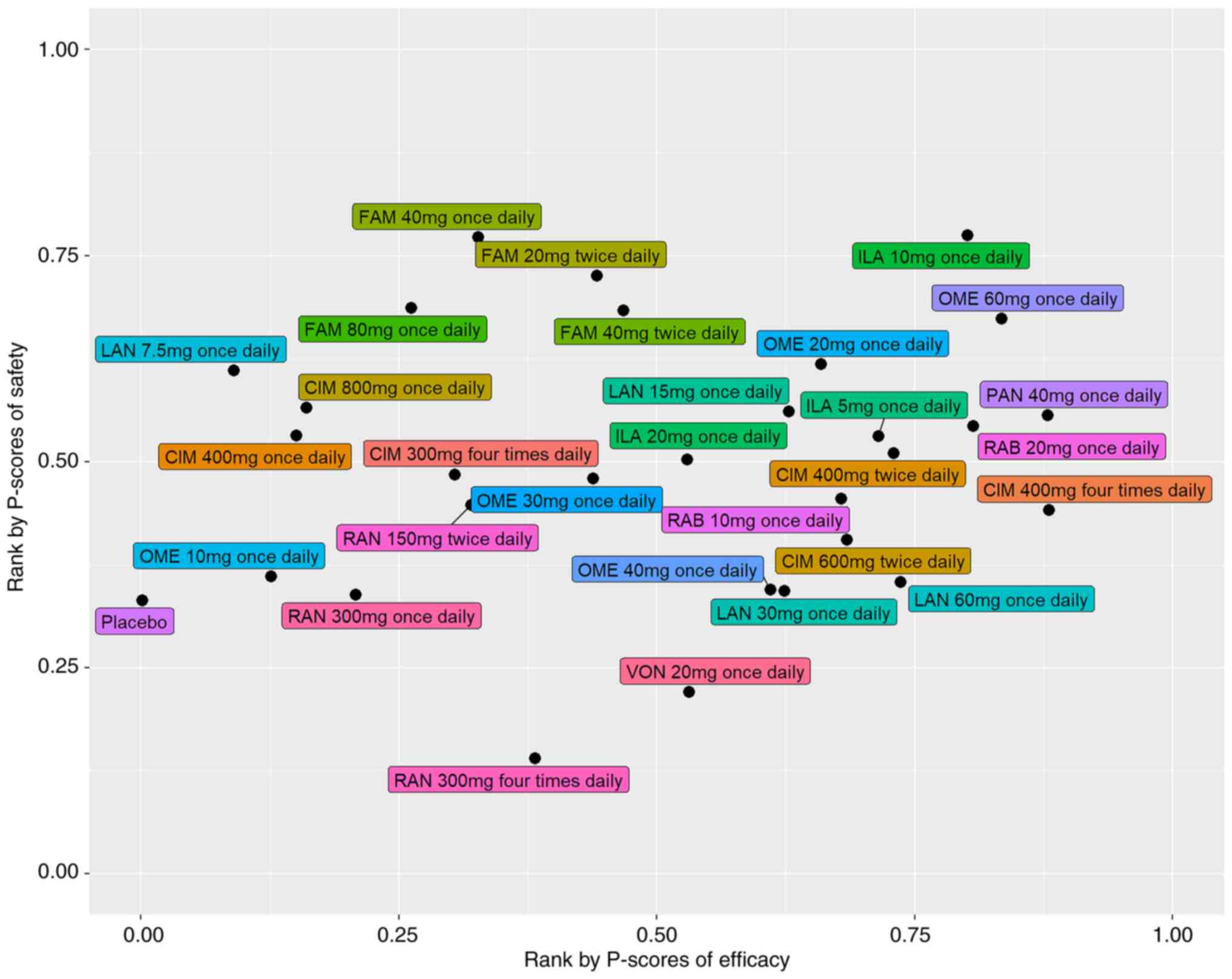
We used the calculated p-scores to rank the efficacy
and safety of the nine drugs included in our study (Table I; Fig.
5). A higher p-score indicated higher efficacy or safety. Among
all the drug pharmaceuticals, CIM 400 mg four times daily and PAN
40 mg once daily had the highest efficacy, with a p-score of 0.88,
while FAM 40 mg once daily and ILA 10 mg once daily were associated
with the highest safety (p-score=0.64). RAN 300 mg four times daily
(p-score=0.14) and VON 20 mg once daily (p-score=0.22) had lower
safety than placebo (p-score=0.33).
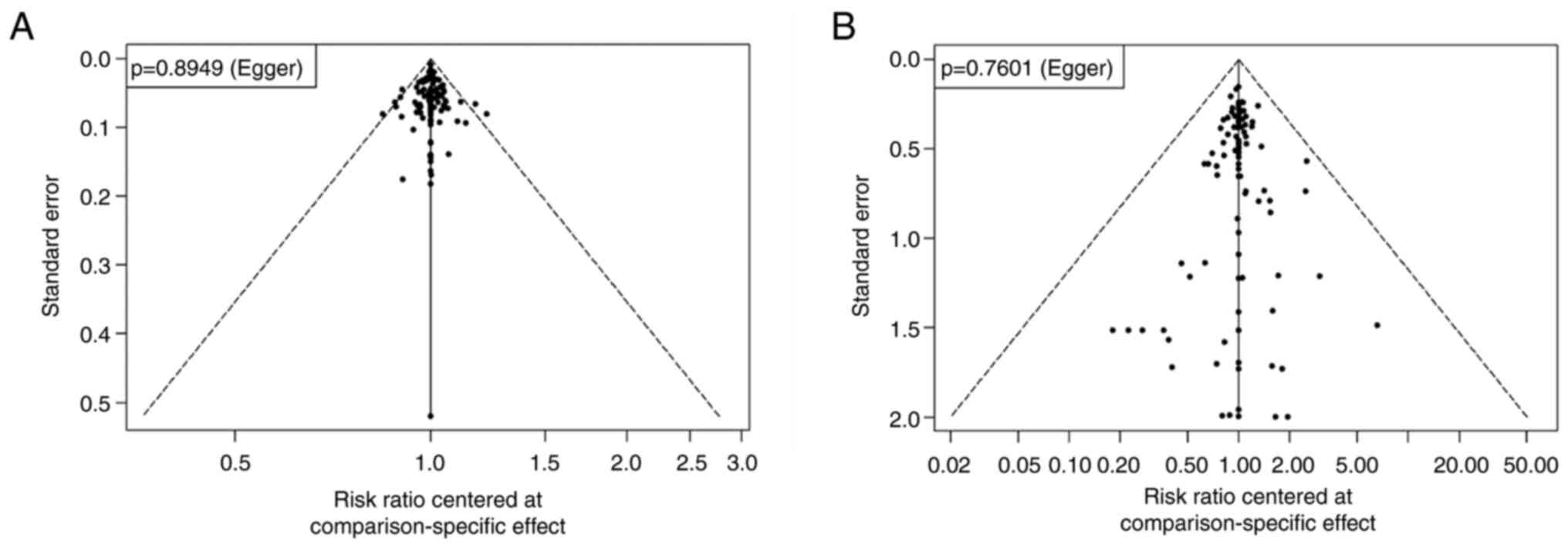
The results of the comparison-adjusted funnel plots
suggested that there may not be small-study effects for efficacy
and safety (Egger test; P>0.05) (Fig. 6).
This study is the first network meta-analysis to
specifically evaluate the efficacy and safety of different doses of
PPIs and H2RAs for the initial non-eradication treatment of
duodenal ulcer (DU) patients. The direct and indirect comparison
results showed some evidence from RCTs. Firstly, H2RAs and PPIs
were found to perform significantly better than the placebo for
increasing the ulcer healing rate. Concerning the TEAE rate, the
pharmaceuticals included in this study were comparable to the
placebo. Secondly, as refer to the ulcer healing rate, we did find
there was no significant statistical differences in different doses
of the CIM, FAM, RAB, ILA, LAN, OME. For these different
pharmaceuticals, FAM 20 mg twice daily, RAB 10 mg once daily, ILA 5
mg once daily, LAN 15 mg once daily, and OME 20 mg once daily
should be prescribed in the clinic considering the economy and
convenience of taking medicine. Thirdly, 150 mg twice daily is the
best choice for RAN, RAN 300 mg four times daily and VON 20 mg once
daily needs more studies to confirm its safety. Lastly, CIM 400 mg
four times daily and PAN 40 mg once daily had the highest P-scores
of ulcer healing rate, but there is a significant heterogeneity of
CIM 400 mg four times daily treatment. Moreover, PAN 40 mg once
daily treatment was ranked twelfth for the TEAE rate.
PPI is not only the most recommended drug in the
guide, but also the first drug in clinical practice. Although it is
accompanied by long-term side effects, such as long-term
administration of PPI will lead to gland atrophy, atrophic
gastritis and gastric polyps. But its side effects are completely
avoidable. In order to avoid such side effects or reduce the chance
of occurrence, we can also take orally rebapide tablets, teprenone
capsules and other drugs that promote the synthesis of endogenous
prostaglandins, improve gastric circulation and protect gastric
mucosa. If atrophic gastritis occurs, it can also be reversed or
prevented from further development by drugs. Gastric polyps can be
treated by endoscopic forceps, argon ion coagulation, ligation,
submucosal dissection or resection (91). Therefore, the purpose of this study
is to seek the appropriate dose and the best clinical dose to avoid
the side effects of overuse and overuse. If PPIs cannot be
prescribed, H2RAs are recommended (10). And Dr. Shi believes that with the
progress of treatment, it is very necessary to adjust the drug
dosage to obtain higher clinical efficacy (92). So this study focuses on the best
dose of PPI for DU. But there are no recommended doses of PPIs and
H2RAs. In our study, according to the P-scores of efficacy and
safety, we recommend PAN 40 mg once daily (4 weeks) as the best
choice treatment for DU patients. This is also confirmed by the
research results of Dr. Huang and Dr. Li which suggested that
pantoprazole 40 mg once daily was the best choice for the treatment
of DU patients (92,94). Similarly, a previous meta-analysis
did find PAN (40 mg/day) seems to be the most cost-effective option
in China (95). We also recommend
CIM 400 mg twice daily (4 weeks), OME 20 mg once daily (4 weeks or
6 weeks), LAN 15 mg once daily (4 weeks), ILA 5 mg once daily (4
weeks), and RAB 10 mg once daily (4 weeks or 6 weeks) can be used
as the first choice in the treatment of patients with duodenal
ulcer. If the pharmaceuticals mentioned above cannot be prescribed,
FAM 40 mg twice daily (8 weeks) is recommended. Our study provides
a reasonable dosage and optimal choice for initial non-eradication
treatment of duodenal ulcers.
This study is a network meta-analysis to explore the
efficacy and safety of different doses of pharmaceuticals
containing PPIs, H2RAs, and a placebo. Based on direct and indirect
evidence, we provide a comprehensive preliminary ranking of these
drugs regarding their effects on duodenal ulcer healing rate and
TEAE rate, which could provide a basis for future clinical
research. However, this study has some limitations, the allocation
concealment are assessed unclear in most of the studies; the
incomplete outcome data and selective reporting are assessed
unclear in some studies, there are two studies assessed as high
risk. The quality of these studies potentially threatened the
validity of our study. Notwithstanding these limitations, the
findings from this network meta-analysis represent the most
comprehensive currently available evidence base to guide the
initial non-eradication treatment of DU in adults.
At present, some studies have shown that mucosal
protection therapy combined with PPI has a good effect on DU, but
in terms of single efficacy, PPI is better than mucosal protection
therapy (96). According to
‘Evidence-based Clinical Practice Guidelines for Peptic Ulcer
Disease 2015’, PPI is recommended for line drugs (97). If PPI cannot be prescribed, H2RA is
recommended. So this study focuses on the best dose of PPI for DU.
In conclusion, these PPIs and H2RAs are effective and safe for
initial non-eradication treatment of DU. The results suggested that
pantoprazole 40 mg once daily (4 weeks) was the best choice for the
initial non-eradication treatment of DU patients, cimetidine 400 mg
twice daily (4 weeks), omeprazole 20 mg once daily (4 weeks or 6
weeks), lansoprazole 15 mg once daily (4 weeks), ilaprazole 5 mg
once daily (4 weeks), and rabeprazole 10 mg once daily (4 weeks or
6 weeks) could be used as the first choice. If the pharmaceuticals
mentioned above cannot be prescribed, famotidine 40 mg twice daily
(8 weeks) is recommended.
Not applicable.
Funding: The present study was supported by the Science and
Technology Project of Sichuan Province (grant nos. 2022YFS0409 and
2020YFS0301), The National Key Research and Development Program of
China (grant no. 2018YFC1704104) and Chengdu University of
Traditional Chinese Medicine Foundation (grant no. BSH2020014).
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
XM and XZ confirm the authenticity of all the raw
data. XM, XZ, QF and YS conceived and designed the current study,
defined the content of the research, conducted literature search,
performed statistical analysis, and prepared and edited the
manuscript. BL and HW are the guarantors of study integrity,
designed the current study, defined the content of the research and
reviewed the manuscript. JL and JZ conducted the literature search,
acquired data and performed statistical analysis. All authors read
and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Kavitt RT, Lipowska AM, Anyane-Yeboa A and
Gralnek IM: Diagnosis and treatment of peptic ulcer disease. Am J
Med. 132:447–456. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lau JY, Barkun A, Fan DM, Kuipers EJ, Yang
YS and Chan FK: Challenges in the management of acute peptic ulcer
bleeding. Lancet. 381:2033–2043. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Dadfar A and Edna TH: Epidemiology of
perforating peptic ulcer: A population-based retrospective study
over 40 years. World J Gastroenterol. 26:5302–5313. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Thorsen K, Søreide JA, Kvaløy JT,
Glomsaker T and Søreide K: Epidemiology of perforated peptic ulcer:
Age- and gender-adjusted analysis of incidence and mortality. World
J Gastroenterol. 19:347–354. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Malfertheiner P, Chan FK and McColl KE:
Peptic ulcer disease. Lancet. 374:1449–1461. 2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zheng Y, Xue M, Cai Y, Liao S, Yang H,
Wang Z, Wang X, Zhang X, Qian J and Wang L: Hospitalizations for
peptic ulcer disease in China: Current features and outcomes. J
Gastroenterol Hepatol. 35:2122–2130. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ford AC, Delaney BC, Forman D and Moayyedi
P: Eradication therapy in Helicobacter pylori positive
peptic ulcer disease: Systematic review and economic analysis. Am J
Gastroenterol. 99:1833–1855. 2004.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Treiber G and Lambert JR: The impact of
Helicobacter pylori eradication on peptic ulcer healing. Am
J Gastroenterol. 93:1080–1084. 1998.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sung JJ, Kuipers EJ and El-Serag HB:
Systematic review: The global incidence and prevalence of peptic
ulcer disease. Aliment Pharmacol Ther. 29:938–946. 2009.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kamada T, Satoh K, Itoh T, Ito M, Iwamoto
J, Okimoto T, Kanno T, Sugimoto M, Chiba T, Nomura S, et al:
Evidence-based clinical practice guidelines for peptic ulcer
disease 2020. J Gastroenterol. 56:303–322. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Savarino V, Mela GS, Zentilin P, Bisso G,
Pivari M, Vigneri S, Termini R, Fiorucci S, Usai P, Malesci A and
Celle G: Comparison of 24-h control of gastric acidity by three
different dosages of pantoprazole in patients with duodenal ulcer.
Aliment Pharmacol Ther. 12:1241–1247. 1998.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lamers CB: The changing role of
H2-receptor antagonists in acid-related diseases. Eur J
Gastroenterol Hepatol. 8 (Suppl 1):S3–S7. 1996.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ji XQ, Du JF, Chen G, Chen G and Yu B:
Efficacy of ilaprazole in the treatment of duodenal ulcers: A
meta-analysis. World J Gastroenterol. 20:5119–5123. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Poynard T, Lemaire M and Agostini H:
Meta-analysis of randomized clinical trials comparing lansoprazole
with ranitidine or famotidine in the treatment of acute duodenal
ulcer. Eur J Gastroenterol Hepatol. 7:661–665. 1995.PubMed/NCBI
|
|
15
|
Eriksson S, Långström G, Rikner L,
Carlsson R and Naesdal J: Omeprazole and H2-receptor antagonists in
the acute treatment of duodenal ulcer, gastric ulcer and reflux
oesophagitis: A meta-analysis. Eur J Gastroenterol Hepatol.
7:467–475. 1995.PubMed/NCBI
|
|
16
|
Bamberg P, Caswell CM, Frame MH, Lam SK
and Wong EC: A meta-analysis comparing the efficacy of omeprazole
with H2-receptor antagonists for acute treatment of duodenal ulcer
in Asian patients. J Gastroenterol Hepatol. 7:577–585.
1992.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hou XH, Meng F, Wang J, Sha W, Chiu CT,
Chung WC, Gu L, Kudou K, Chong CF and Zhang S: 995 phase 3 Study
evaluating the efficacy and safety of Vonoprazan 20 mg versus
lansoprazole 30 mg in the treatment of endoscopically confirmed
duodenal ulcers in Asian subjects with or without Helicobacter
pylori infection. Gastroenterology. 158:S197–S198. 2020.
|
|
18
|
Hawkey CJ, Long RG, Bardhan KD, Wormsley
KG, Cochran KM, Christian J and Moules IK: Improved symptom relief
and duodenal ulcer healing with lansoprazole, a new proton pump
inhibitor, compared with ranitidine. Gut. 34:1458–1462.
1993.PubMed/NCBI View Article : Google Scholar
|
|
19
|
McFarland RJ, Bateson MC, Green JR,
O'Donoghue DP, Dronfield MW, Keeling PW, Burke GJ, Dickinson RJ,
Shreeve DR, Peers EM, et al: Omeprazole provides quicker symptom
relief and duodenal ulcer healing than ranitidine.
Gastroenterology. 98:278–283. 1990.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chaimani A, Caldwell DM, Li T, Higgins JPT
and Salanti G: Additional considerations are required when
preparing a protocol for a systematic review with multiple
interventions. J Clin Epidemiol. 83:65–74. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Cumpston M, Li T, Page MJ, Chandler J,
Welch VA, Higgins JP and Thomas J: Updated guidance for trusted
systematic reviews: A new edition of the Cochrane Handbook for
systematic reviews of interventions. Cochrane Database Syst Rev.
10(ED000142)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Salanti G, Del Giovane C, Chaimani A,
Caldwell DM and Higgins JP: Evaluating the quality of evidence from
a network meta-analysis. PLoS One. 9(e99682)2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Greco T, Edefonti V, Biondi-Zoccai G,
Decarli A, Gasparini M, Zangrillo A and Landoni G: A multilevel
approach to network meta-analysis within a frequentist framework.
Contemp Clin Trials. 42:51–59. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Devall AJ, Papadopoulou A, Podesek M, Haas
DM, Price MJ, Coomarasamy A and Gallos ID: Progestogens for
preventing miscarriage: A network meta-analysis. Cochrane Database
Syst Rev. 4(CD013792)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Burr NE, Gracie DJ, Black CJ and Ford AC:
Efficacy of biological therapies and small molecules in moderate to
severe ulcerative colitis: Systematic review and network
meta-analysis. Gut. 71:1976–1987. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Higgins JP, Jackson D, Barrett JK, Lu G,
Ades AE and White IR: Consistency and inconsistency in network
meta-analysis: Concepts and models for multi-arm studies. Res Synth
Methods. 3:98–110. 2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chaimani A, Higgins JP, Mavridis D,
Spyridonos P and Salanti G: Graphical tools for network
meta-analysis in STATA. PLoS One. 8(e76654)2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Blackwood WS, Maudgal DP, Pickard RG,
Lawrence D and Northfield TC: Cimetidine in duodenal ulcer:
Controlled trial. Lancet. 2:174–176. 1976.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Gustavsson S, Adami HO, Lööf L, Nyberg A
and Nyrén O: Rapid healing of duodenal ulcers with omeprazole:
Double-blind dose-comparative trial. Lancet. 2:124–125.
1983.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Barbara L, Corinaldesi R, Bianchi Porro G,
Lazzaroni M, Blasi A, Mangiameli A, Carratelli L, Wilkins A, Cheli
R, Bovero E, et al: Famotidine in the management of duodenal ulcer:
Experience in Italy. Digestion. 32 (Suppl 1):S24–S31.
1985.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Prichard PJ, Rubinstein D, Jones DB,
Dudley FJ, Smallwood RA, Louis WJ and Yeomans ND: Double blind
comparative study of omeprazole 10 mg and 30 mg daily for healing
duodenal ulcers. Br Med J. 290:601–603. 1985.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Simon B, Dammann HG, Jakob G, Miederer SE,
Müller P, Ottenjann R, Paul F, Scholten T, Schütz E, Seifert E, et
al: Famotidine versus ranitidine for the short-term treatment of
duodenal ulcer. Digestion. 32 (Suppl 1):S32–S37. 1985.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Arcidiacono R, Benvestito V, Bonomo GM,
Bottari M, Buscarini L, Camarri E, Celle G, Coltorti M, Di Matteo
S, Dobrilla G, et al: Comparison between ranitidine 150 mg b.d. and
ranitidine 300 mg nocte in the treatment of duodenal ulcer. Int J
Clin Pharmacol Ther Toxicol. 24:381–384. 1986.PubMed/NCBI
|
|
34
|
Bardhan KD, Bianchi Porro G, Bose K, Daly
M, Hinchliffe RF, Jonsson E, Lazzaroni M, Naesdal J, Rikner L and
Walan A: A comparison of two different doses of omeprazole versus
ranitidine in treatment of duodenal ulcers. J Clin Gastroenterol.
8:408–413. 1986.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Hirschowitz BI, Berenson MM, Berkowitz JM,
Bright-Asare P, DeLuca VA Jr, Eshelman FN, Font RG, Griffin JW Jr,
Kozarek RA, McCray RS, et al: A multicenter study of ranitidine
treatment of duodenal ulcers in the United States. J Clin
Gastroenterol. 8:359–366. 1986.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Lee FI, Reed PI, Crowe JP, McIsaac RL and
Wood JR: Acute treatment of duodenal ulcer: A multicentre study to
compare ranitidine 150 mg twice daily with ranitidine 300 mg once
at night. Gut. 27:1091–1095. 1986.PubMed/NCBI View Article : Google Scholar
|
|
37
|
McCullough AJ: A multicenter, randomized,
double-blind study comparing famotidine with ranitidine in the
treatment of active duodenal ulcer disease. Am J Med. 81:17–24.
1986.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Rohner HG and Gugler R: Treatment of
active duodenal ulcers with famotidine. A double-blind comparison
with ranitidine. Am J Med. 81:13–16. 1986.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Barbara L, Blasi A, Cheli R, Corinaldesi
R, Dobrilla G, Francavilla A, Rinetti M, Vezzaldini P, Abbiati R,
Gradnik R, et al: Omeprazole vs. ranitidine in the short-term
treatment of duodenal ulcer: An Italian multicenter study.
Hepatogastroenterology. 34:229–232. 1987.PubMed/NCBI
|
|
40
|
Dobrilla G, De Pretis G, Piazzi L, Boero
A, Camarri E, Crespi M, Fontana G, Ideo G, Manenti F, Marenco G, et
al: Comparison of once-daily bedtime administration of famotidine
and ranitidine in the short-term treatment of duodenal ulcer. A
multicenter, double-blind, controlled study. Scand J Gastroenterol
Suppl. 134:21–28. 1987.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Gitlin N, McCullough AJ, Smith JL, Mantell
G and Berman R: A multicenter, double-blind, randomized,
placebo-controlled comparison of nocturnal and twice-a-day
famotidine in the treatment of active duodenal ulcer disease.
Gastroenterology. 92:48–53. 1987.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Kimmig JM: Acute therapy of duodenal
ulcer. Comparison of famotidine with ranitidine in a single evening
dosage. Fortschritte der Medizin. 105:72–76. 1987.PubMed/NCBI
|
|
43
|
Archambault AP, Pare P, Bailey RJ, Navert
H, Williams CN, Freeman HJ, Baker SJ, Marcon NE, Hunt RH,
Sutherland L, et al: Omeprazole (20 mg daily) versus cimetidine
(1200 mg daily) in duodenal ulcer healing and pain relief.
Gastroenterology. 94:1130–1134. 1988.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Hartmann H and Fölsch UR: Famotidine
versus cimetidine in the treatment of acute duodenal ulcer.
Double-blind, randomized clinical trial comparing nocturnal
administration of 40 mg famotidine to 800 mg cimetidine. Digestion.
39:156–161. 1988.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Young MD, Lottes SR and Webb LA: An
evaluation of cimetidine and ranitidine in the pain relief and
acute healing of duodenal ulcer disease. Clin Ther. 10:543–552.
1988.PubMed/NCBI
|
|
46
|
Alcalá Santaella R, Guardia J, Pajares J,
Piqué J, Pita L, Alvárez E, Castellanos P, Guarner L, Ortiz J,
Pesquera R, et al: A multicentre, randomized, double-blind study
comparing nocte famotidine or ranitidine for the treatment of
active duodenal ulceration. Aliment Pharmacol Ther. 3:103–110.
1989.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Al-Mofleh I, Mayet I, Al-Rashed R,
Al-Faleh F, Al-Aska AK and Laajam MA: Efficacy of single-daily
doses of H2-blockers in duodenal ulcer: Comparison of cimetidine
and ranitidine in a double-blind controlled trial. Curr Ther Res
Clin Exp. 46:399–403. 1989.
|
|
48
|
Chelvam P, Goh KL, Leong YP, Leela MP, Yin
TP, Ahmad H, Jalleh R, Wong NW, Lee HB and Mahendran T: Omeprazole
compared with ranitidine once daily in the treatment of duodenal
ulcer. J Gastroenterol Hepatol. 4 (Suppl 2):S53–S61.
1989.PubMed/NCBI
|
|
49
|
Crowe JP, Wilkinson SP, Bate CM,
Willoughby CP, Peers EM and Richardson PD: Symptom relief and
duodenal ulcer healing with omeprazole or cimetidine. Aliment
Pharmacol Ther. 3:83–91. 1989.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Delvaux M, Hagège CG and Ribet A:
Comparative efficacy of famotidine and ranitidine in the treatment
of acute-phase duodenal ulcer. A French comparative therapeutic
trial. Gastroenterol Clin Biol. 13:1055–1059. 1989.PubMed/NCBI
|
|
51
|
Lauritsen K, Andersen BN, Havelund T,
Laursen LS, Hansen J, Eriksen J, Jørgensen T and Rask-Madsen J:
Effect of 10 mg and 20 mg omeprazole daily on duodenal ulcer:
Double-blind comparative trial. Aliment Pharmacol Ther. 3:59–67.
1989.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Lee FI, Booth SN, Cochran KM, Crowe J,
Dickinson RJ, Kennedy NP, Cottrell J and Mann SG: Single night-time
doses of 40 mg famotidine or 800 mg cimetidine in the treatment of
duodenal ulcer. Aliment Pharmacol Ther. 3:505–512. 1989.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Mulder CJ, Tijtgat GN, Cluysenaer OJ,
Nicolai JJ, Meyer WW, Hazenberg BP, Vogten AJ, Gerrits C and
Stuifbergen WH: Omeprazole (20 mg o.m.) versus ranitidine (150 mg
b.d.) in duodenal ulcer healing and pain relief. Aliment Pharmacol
Ther. 3:445–451. 1989.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Page MC, Lacey LA, Mills JG and Wood JR:
Can higher doses of an H2-receptor antagonist accelerate duodenal
ulcer healing? Aliment Pharmacol Ther. 3:425–433. 1989.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Rodrigo L, Viver J, Conchillo F, Barrio E,
Forné M, Zozaya JM, Alvarez A, Dieguez P, Muñoz M, Panés J, et al:
A multicenter, randomized, double-blind study comparing famotidine
with cimetidine in the treatment of active duodenal ulcer disease.
Digestion. 42:86–92. 1989.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Kang JY, Guan R, Tay HH, Yap I, Wee A,
Math MV and Labrooy SJ: Low-dose cimetidine in the acute treatment
of duodenal ulcer. Comparison of a single nocturnal dose regimen
with a twice daily regimen. J Gastroenterol Hepatol. 5:669–674.
1990.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Double blind comparative study of
omeprazole and ranitidine in patients with duodenal or gastric
ulcer: A multicentre trial. Gut. 31:653–656. 1990.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Popovic O, Dzambas D, Tasic T, Bidikov V,
Jesenski T and Janosevic S: Omeprazole 20 mg o.m. vs. famotidine 40
mg h.s. in the treatment of duodenal ulcer. Multicenter
double-blind randomized trial. Gastroenterohepatoloski Arhiv.
9:26–34. 1990.
|
|
59
|
Cochran KM: Famotidine, ranitidine and
cimetidine in the treatment of duodenal ulcer: Two large
international studies. Eur J Gastroenterol Hepatol. 3:527–531.
1991.
|
|
60
|
Londong W, Barth H, Dammann HG, Hengels
KJ, Kleinert R, Müller P, Rohde H and Simon B: Dose-related healing
of duodenal ulcer with the proton pump inhibitor lansoprazole.
Aliment Pharmacol Ther. 5:245–254. 1991.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Delle Fave G, Annibale B, Franceschi M,
Quatrini M, Cassetta MR and Torsoli A: Omeprazole versus famotidine
in the short-term treatment of duodenal ulcer disease. Aliment
Pharmacol Ther. 6:469–478. 1992.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Hotz J, Kleinert R, Grymbowski T, Hennig U
and Schwarz JA: Lansoprazole versus famotidine: Efficacy and
tolerance in the acute management of duodenal ulceration. Aliment
Pharmacol Ther. 6:87–95. 1992.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Kumar TR, Naidu MU, Shobha JC, Reddy DN,
Subhash S, Chaubal C, Prasad R and Babu S: Comparative study of
omeprazole and famotidine in the treatment of duodenal ulcer.
Indian J Gastroenterol. 11:73–75. 1992.PubMed/NCBI
|
|
64
|
Lysy J, Karmeli F, Wengrower D and
Rachmilewitz D: Effect of duodenal ulcer healing induced by
omeprazole and ranitidine on the generation of gastroduodenal
eicosanoids, platelet-activating factor, pepsinogen A, and gastrin
in duodenal ulcer patients. Scand J Gastroenterol. 27:13–19.
1992.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Wang CY, Wang TH, Lai KH, Siauw CP, Chen
PC, Yang KC, Tsai YT and Sung JL: Double-blind comparison of
omeprazole 20 mg OM and ranitidine 300 mg NOCTE in duodenal ulcer:
A Taiwan multi-centre study. J Gastroenterol Hepatol. 7:572–576.
1992.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Ahmed W, Qureshi H, Zuberi SJ and Alam SE:
Omeprazole vs ranitidine in the healing of duodenal ulcer. J Pak
Med Assoc. 43:111–112. 1993.PubMed/NCBI
|
|
67
|
Misra SC, Dasarathy S and Sharma MP:
Omeprazole versus famotidine in the healing and relapse of duodenal
ulcer. Aliment Pharmacol Ther. 7:443–449. 1993.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Judmaier G and Koelz HR: Comparison of
pantoprazole and ranitidine in the treatment of acute duodenal
ulcer. Pantoprazole-Duodenal Ulcer-Study Group. Aliment Pharmacol
Ther. 8:81–86. 1994.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Lanza F, Goff J, Scowcroft C, Jennings D
and Greski-Rose P: Double-blind comparison of lansoprazole,
ranitidine, and placebo in the treatment of acute duodenal ulcer.
Lansoprazole Study Group. Am J Gastroenterol. 89:1191–1200.
1994.PubMed/NCBI
|
|
70
|
Porro GB, Lazzaroni M, Cardelli A,
Gasperoni S, De Berardinis F, Delle Fave G, Annibale B, Galeazzi R,
Novelli G, Mazzeo F, et al: Pantoprazole vs omeprazole in the
treatment of duodenal ulcer. Argomenti di Gastroenterologia Clin.
7:271–275. 1994.
|
|
71
|
Avner DL, Dorsch ER, Jennings DE and
Greski-Rose PA: A comparison of three doses of lansoprazole (15, 30
and 60 mg) and placebo in the treatment of duodenal ulcer. The
Lansoprazole Study Group. Aliment Pharmacol Ther. 9:521–528.
1995.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Beker JA, Bianchi Porro G, Bigard MA,
Delle Fave G, Devis G, Gouerou H and Maier C: Double-blind
comparison of pantoprazole and omeprazole for the treatment of
acute duodenal ulcer. Eur J Gastroenterol Hepatol. 7:407–410.
1995.PubMed/NCBI
|
|
73
|
Chang FY, Chiang CY, Tam TN, Ng WW and Lee
SD: Comparison of lansoprazole and omeprazole in the short-term
management of duodenal ulcers in Taiwan. J Gastroenterol Hepatol.
10:595–601. 1995.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Ekström P, Carling L, Unge P, Anker-Hansen
O, Sjöstedt S and Sellström H: Lansoprazole versus omeprazole in
active duodenal ulcer. A double-blind, randomized, comparative
study. Scand J Gastroenterol. 30:210–215. 1995.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Rehner M, Rohner HG and Schepp W:
Comparison of pantoprazole versus omeprazole in the treatment of
acute duodenal ulceration-a multicentre study. Aliment Pharmacol
Ther. 9:411–416. 1995.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Schepp W and Classen M: Pantoprazole and
ranitidine in the treatment of acute duodenal ulcer. A multicentre
study. Scand J Gastroenterol. 30:511–514. 1995.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Miszputen SJ, De PVM, Kondo M, Ferrari A,
Zaterka S, Eisig JN, Chinzon D, De M, Maguilnik I, Vieira FEF, et
al: Comparative multicentric study between omeprazole and
ranitidine in duodenal ulcer. GED. 16:127–134. 1997.
|
|
78
|
Papp J, Döbrönte Z, Juhász L and Lonovics
J: Evaluation of the effectiveness of pantoprazole and ranitidine
in the treatment of duodenal ulcer. Result of an international
multicenter study. Orv Hetil. 138:2863–2866. 1997.PubMed/NCBI(In Hungarian).
|
|
79
|
Bardhan KD, Crowe J, Thompson RP, Trewby
PN, Keeling PN, Weir D and Crouch SL: Lansoprazole is superior to
ranitidine as maintenance treatment for the prevention of duodenal
ulcer relapse. Aliment Pharmacol Ther. 13:827–832. 1999.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Dekkers CP, Beker JA, Thjodleifsson B,
Gabryelewicz A, Bell NE and Humphries TJ: Comparison of rabeprazole
20 mg versus omeprazole 20 mg in the treatment of active duodenal
ulcer: A European multicentre study. Aliment Pharmacol Ther.
13:179–186. 1999.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Dobrilla G, Piazzi L and Fiocca R:
Lansoprazole versus omeprazole for duodenal ulcer healing and
prevention of relapse: A randomized, multicenter, double-masked
trial. Clin Ther. 21:1321–1332. 1999.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Breiter JR, Riff D and Humphries TJ:
Rabeprazole is superior to ranitidine in the management of active
duodenal ulcer disease: Results of a Double-Blind, randomized North
American study. Am J Gastroenterol. 95:936–942. 2000.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Meneghelli UG, Zaterka S, de Paula Castro
L, Malafaia O and Lyra LG: Pantoprazole versus ranitidine in the
treatment of duodenal ulcer: A multicenter study in Brazil. Am J
Gastroenterol. 95:62–66. 2000.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Shim CS, Cho JY, Chung IS, Yang YS, Kim SW
and Choi MG: Rabeprazole 10 mg versus omeprazole 20 mg in the
treatment of duodenal ulcer: The Korean multicenter, comparative
trial. Korean J Gastrointestinal Endoscopy. 24:76–83. 2002.
|
|
85
|
Ji S, Kim HS, Kim JW, Jee MK, Park KW, Uh
Y, Lee DK, Song JS, Baik SK and Kwon SO: Comparison of the efficacy
of rabeprazole 10 mg and omeprazole 20 mg for the healing rapidity
of peptic ulcer diseases. J Gastroenterol Hepatol. 21:1381–1387.
2006.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Ho KY, Kuan A, Zaño F, Goh KL, Mahachai V,
Kim DY and Yoon HM: Randomized, parallel, double-blind comparison
of the ulcer-healing effects of ilaprazole and omeprazole in the
treatment of gastric and duodenal ulcers. J Gastroenterol.
44:697–707. 2009.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Wang L, Zhou L, Lin S, Hu H and Xia J: A
New PPI, Ilaprazole compared with omeprazole in the treatment of
duodenal ulcer a randomized double-blind multicenter trial. J Clin
Gastroenterol. 45:322–329. 2011.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Wang L, Zhou L, Hu H, Lin S and Xia J:
Ilaprazole for the treatment of duodenal ulcer: A randomized,
double-blind and controlled phase III trial. Curr Med Res Opin.
28:101–109. 2012.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Miwa H, Uedo N, Watari J, Mori Y, Sakurai
Y, Takanami Y, Nishimura A, Tatsumi T and Sakaki N: Randomised
clinical trial: Efficacy and safety of vonoprazan vs. lansoprazole
in patients with gastric or duodenal ulcers-results from two phase
3, non-inferiority randomised controlled trials. Aliment Pharmacol
Ther. 45:240–252. 2017.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Fan L, Xianghong Q, Ling W, Ying H, Jielai
X and Haitang H: Ilaprazole compared with rabeprazole in the
treatment of duodenal ulcer: A randomized, double-blind,
active-controlled, multicenter study. J Clin Gastroenterol.
53:641–647. 2019.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Wu HN: Comparison of different
combinations of triple therapy in the treatment of Helicobacter
pylori positive duodenal ulcer. Yunnan Med. 38:242–244.
2017.
|
|
92
|
Huang HY, Liu GF, Qi CX, Zhao YH and Wu J:
Comparison of the efficacy of proton pump inhibitor high-dose
combination and bismuth combination in the eradication of
Helicobacter pylori. Clin Focus. 37:230–233. 2022.
|
|
93
|
Shi RL: Evaluation of rational use of
proton pump inhibitors in 300 inpatients. Chin Modern Doctor.
60:92–95. 2022.
|
|
94
|
Li CY, Zhang J, Wang XH and Wang YN:
Investigation and analysis of proton pump inhibitors in 3005
hospitalized patients. Evaluation and Analysis of Drug Use in
Chinese Hospitals. 17:406–409. 2017.
|
|
95
|
Zhang J, Ge L, Hill M, Liang Y, Xie J, Cui
D, Li X, Zheng W and He R: Standard-dose proton pump inhibitors in
the initial Non-eradication treatment of duodenal ulcer: Systematic
review, network Meta-analysis, and Cost-effectiveness analysis.
Front Pharmacol. 9(1512)2018.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Huang YZ: Clinical effect of gastric
mucosal protector combined with proton pump inhibitor in the
treatment of peptic ulcer with hemorrhage. Chin Med Guide.
20:29–32. 2022.
|
|
97
|
Satoh K, Yoshino J, Akamatsu T, Itoh T,
Kato M, Kamada T, Takagi A, Chiba T, Nomura S, Mizokami Y, et al:
Evidence-based clinical practice guidelines for peptic ulcer
disease. J Gastroenterol. 51:177–94. 2016.PubMed/NCBI View Article : Google Scholar
|