Introduction
Craniopharyngioma (CP) is one type of epithelial
tumor originating from embryonic remnants in the sellar region,
which most commonly occur in individuals aged 5-14 and 50-74 years
(1). Traditionally, CP can be
divided into two subtypes: Adamantinomatous CP (ACP) and papillary
CP (PCP). The latest World Health Organization classification
criteria define ACP and PCP as different types (2). ACP is driven by somatic CTNNB1
mutations, and histologically contains palisade-like basal
epithelium, loosely aggregated stellate reticulum, cell clusters
composed of cells with nucleocytoplasmic accumulation of β-catenin,
nodules of anucleated ghost cells with brightly eosinophilic
cytoplasm termed wet keratin, calcification, cholesterol clefts and
other structures. In addition, high levels of cytokines and
inflammatory markers are frequently discovered in the stroma of ACP
(3,4).
The mechanism underlying the formation of the
special pathological structures of ACP remains to be elucidated,
including wet keratin and calcification. S100 calcium-binding
protein A9 (S100A9) is a calmodulin that is closely associated with
cellular proliferation, differentiation and apoptosis, and is
abundant in inflammatory cells, such as neutrophils. Therefore,
S100A9 has also been identified as a crucial inflammatory factor
(5). S100A9 is also expressed in
keratinocytes in certain skin inflammatory diseases, and is
expressed and released by keratinocytes and activated leukocytes
during inflammation and bodily injury (6,7).
Several genes of the S100 family and proteins involved in epidermal
keratinization form the so-called epidermal differentiation complex
on human chromosome 1q21(8), and
evidence has suggested that S100A9 is closely associated with
epithelial differentiation (9). In
view of the complex inflammatory microenvironment of ACP, it was
hypothesized that epithelial differentiation associated with S100A9
may be involved in the formation of wet keratin. Therefore, the
present study investigated the expression of S100A9 in ACP and its
association with wet keratin formation, in order to obtain improved
insight into the tumor biology of ACP.
Materials and methods
Demographic and clinical data
All sample tissues were obtained from cases that
underwent surgery (including radical resection and conservative
resection) at Beijing Sanbo Brain Hospital (Beijing, China) between
September 2016 and September 2019. Cases were selected that had
complete clinical information and histological specimens, and for
whom follow-up data could be obtained. Cases were excluded that did
not have complete clinical information or enough histological
specimens, or follow-up data could not be obtained. A total of 46
eligible cases were included in the present study. The baseline
data were collected, including age, sex, radiological images,
surgical resection and postoperative recurrence (Table I). Follow-up ended in March 2022,
and tumor recurrence or death was considered a progressive event.
The patients in the present study provided written informed
consent. The present study was designed in accordance with The
Declaration of Helsinki and approved by the ethics committee of
Sanbo Brain Hospital, Capital Medical University (Beijing, China;
ethical approval no. SBNK-YJ-2020-014-01).
 | Table IBaseline data of patients. |
Table I
Baseline data of patients.
| Factor | Values |
|---|
| Sex | |
|
Male | 33 |
|
Female | 13 |
| Age, years, median
(IQR) | 11.38 (13.06) |
| Consistency | |
|
Predominantly
cystic | 29 |
|
Predominantly
solid | 17 |
| Preoperative
status | |
|
Primary | 16 |
|
Recurrent | 30 |
| Resection | |
|
Radical
resection | 42 |
|
Conservative
resection | 4 |
| Postoperative
status | |
|
Recurrence | 7 |
|
No
recurrence | 39 |
Hematoxylin and eosin (H&E)
staining
Following surgery, the tissue specimens were
immersed in 10% formalin for 24-48 h at room temperature. Then the
tissue specimens were placed into molds supplemented with liquid
paraffin, cooled and frozen to make the paraffin solid to achieve
tissue fixation, and sectioned using a paraffin microtome. The wax
blocks were cut into 4-µm sections for H&E staining. Sections
were stained with hematoxylin for 0.5-1 min, rinsed with running
water, differentiated in 1% hydrochloric acid alcohol for a few
seconds, rinsed with running water, then returned to 1% ammonia
aqueous solution for 1 min, rinsed with running water for a few
seconds, stained in eosin staining solution for a few seconds and
sealed after rinsing with running water. All the above experimental
procedures were carried out at room temperature. Light field
microscopy was used for observation.
Immunohistochemistry and
immunofluorescence
Following fixation in 10% formalin for 24-48 h at
room temperature, the samples were dehydrated in increasing ethanol
concentrations, then samples were embedded in paraffin, the wax
blocks were cut into 4-µm sections, deparaffinized in xylene and
rehydrated with graded ethanol. Antigen retrieval was performed
using Tris-EDTA buffer (pH 9.0) in a 95˚C water bath for 15 min.
Endogenous peroxidase was inactivated with 3%
H2O2 for 10 min at room temperature. and
samples were washed with phosphate-buffered saline (PBS). After
blocking using the peroxidase blocking agent for 15 min at room
temperature, the sections were incubated with the primary antibody
overnight at 4˚C and then rinsed with PBS. Horseradish
peroxidase-conjugated secondary antibody was added dropwise and
incubated at 37˚C for 30 min and then rinsed with PBS. Samples were
placed in diaminobenzidine and rinsed with PBS, and the slides were
dehydrated, dried and sealed. Light field microscope was used for
observation.
For double immunofluorescence staining, sections
(prepared as for immunohistochemistry and immunofluorescence) were
subjected to antigen retrieval using Tris-EDTA buffer (pH 9.0) in a
95˚C water bath for 20 min, and were then incubated with
immunostaining blocking solution containing 0.1% Triton X-100 for
90 min at room temperature, The diluted primary antibody was then
added to the slides and incubated overnight at 4˚C, before being
rinsed with PBS containing 0.1% Triton X-100. The diluted
fluorescence secondary antibody was added and incubated with the
slides for 90 min at room temperature, before being rinsed with PBS
containing 0.1% Triton X-100 and incubated with DAPI for 10 min.
Immunofluorescence was assessed using a Leica fluorescence
microscope (THUNDER Imager Tissue; Leica Microsystems, Inc.) and
image analysis was performed using Leica Application suite X
software (v3.1.5, Leica Microsystems, Inc.).
The primary antibodies used were S100A9 (Abcam;
1:400; ab92507), Ki67 (OriGene Technologies, Inc.; 1:200; cat. no.
ZM-0167) and β-catenin (MAX; 1:200; cat. no. MAB-0754), and the
secondary antibodies were conjugated to Alexa Fluor 488 (Abcam;
1:500; cat. no. ab150077) or 647 (Abcam; 1:500; cat. no.
ab150115).
Specimen evaluation
Antibody expression was evaluated by two observers
and supervised by an experienced pathologist. Tissue samples were
divided by H&E staining into a high-inflammation group and a
low-inflammation group, according to the degree of inflammatory
cell infiltration. Tissues with extensive aggregation of
inflammatory cells were included in the high-inflammation group and
tissues with scattered distribution of inflammatory cells were
included in the low-inflammation group. To assess the degree of
inflammation, five areas containing inflammatory cells in the field
of vision that could be observed and counted were selected and the
percentage of inflammatory cells was counted. For Ki67 staining,
brown nuclei were regarded as positive, and five dense areas of
positive cells were randomly selected to count the number of
positive cells, and the percentage of positive cells was calculated
as previously described (10).
Expression of S100A9 was evaluated in wet keratin, with brown
tissue considered positive; according to the degree of staining,
the tissue was classified as strongly positive (3 points),
moderately positive (2 points) and weakly positive (1 point)
(11). A total of five fields of
vision were randomly selected, and the percentage of
S100A9-positive areas were estimated for each field; finally, the
S100A9 scores of each case were calculated, score=∑ (S100A9
percentage x degree of staining)/5, as previously described
(12,13). The percentage of areas of wet
keratin was also counted via H&E staining to analyze the
relationship between wet keratin and inflammation.
Online databases
The chromosomal location of the S100A9 gene was
determined from the Ensembl database (http://www.ensembl.org; release 109). The
protein-protein interaction was analyzed by STRING (https://cn.string-db.org/). RNA and protein data were
obtained from the pediatric brain tumor online database (The
University of Alabama at Birmingham Cancer data analysis portal;
Pediatric brain cancer in CBTTC dataset: https://ualcan.path.uab.edu/cgi-bin/CBTTC-Result.pl?genenam=S100A9);
RNA and protein data from various pediatric brain tumors, including
ACP, were used to assess the RNA and protein expression levels of
S100A9.
Data analysis
R (v4.1.1 https://mirrors.tuna.tsinghua.edu.cn/CRAN/) and
GraphPad Prism (v8.0; GraphPad Software; Dotmatics) were used for
statistical analysis. Measurement data are shown as the mean ±
standard deviation and median (IQR), normally distributed data were
assessed using the two-sample unpaired t-test and non-normally
distributed data (age comparison of S100A9 high group and low
group) were assessed using the Mann-Whitney U test. The
χ2 test was used for contingency tables. A linear
correlation analysis was performed using Spearman's test. Survival
analysis was performed using the Kaplan-Meier test and log-rank
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
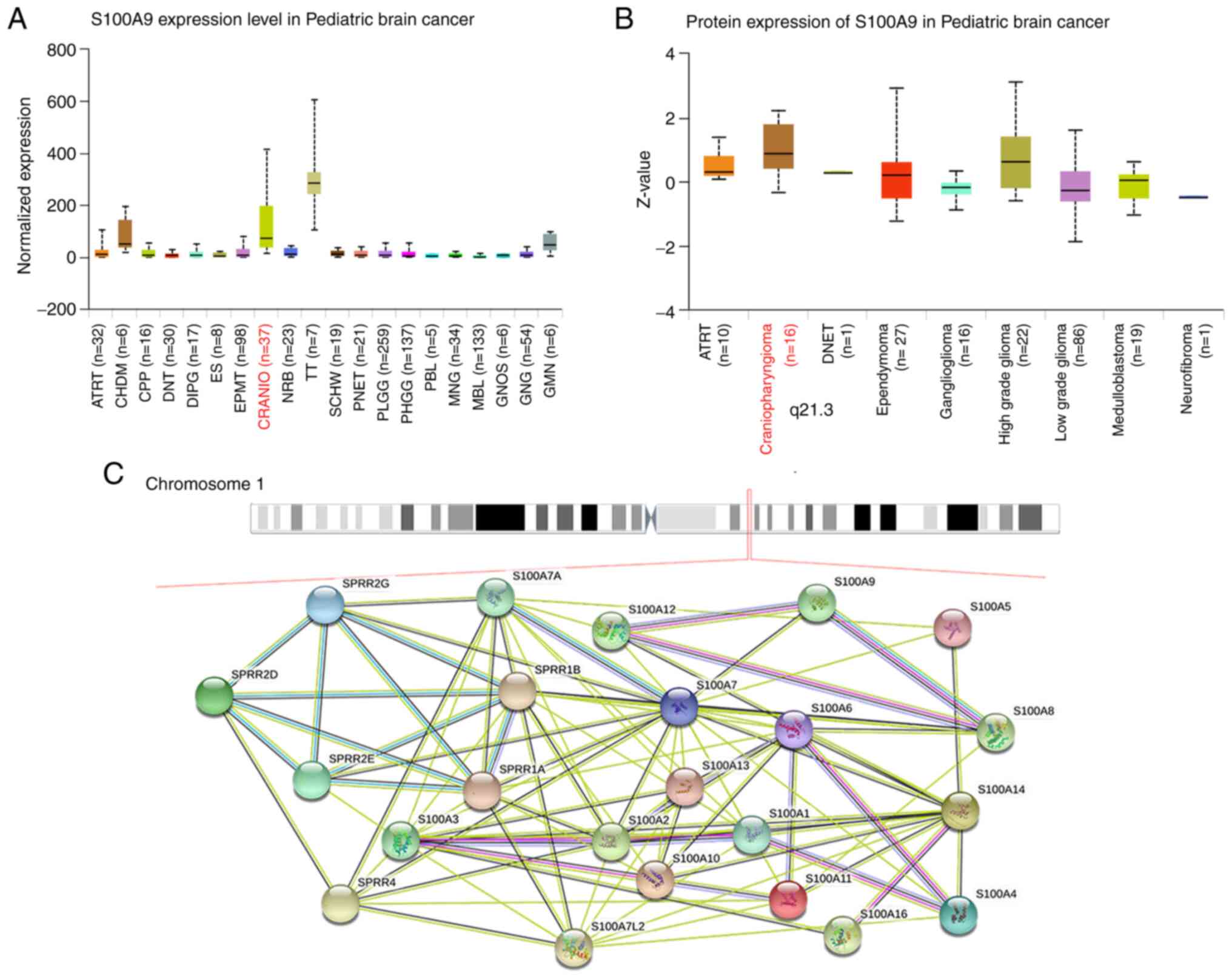
Online databases validate the
expression of S100A9 in ACP
Online databases were used to verify the RNA and
protein expression levels of S100A9 in ACP. The RNA expression of
S100A9 ranked second in ACP among all detected pediatric brain
tumors, after teratoma. Protein expression was ranked first
(Fig. 1A and B). Several genes of the S100 family and
proteins involved in epidermal cornification form a so-called
epidermal differentiation complex on human chromosome 1q21(8). The protein-protein interaction
between epidermis-related small proline-rich protein genes and
S100A family genes was found in the adjacent chromosome location,
showing a complex interaction between them (Fig. 1C). These data showed that S100A9
was indeed highly expressed in ACP and played an important role in
tumorigenesis.
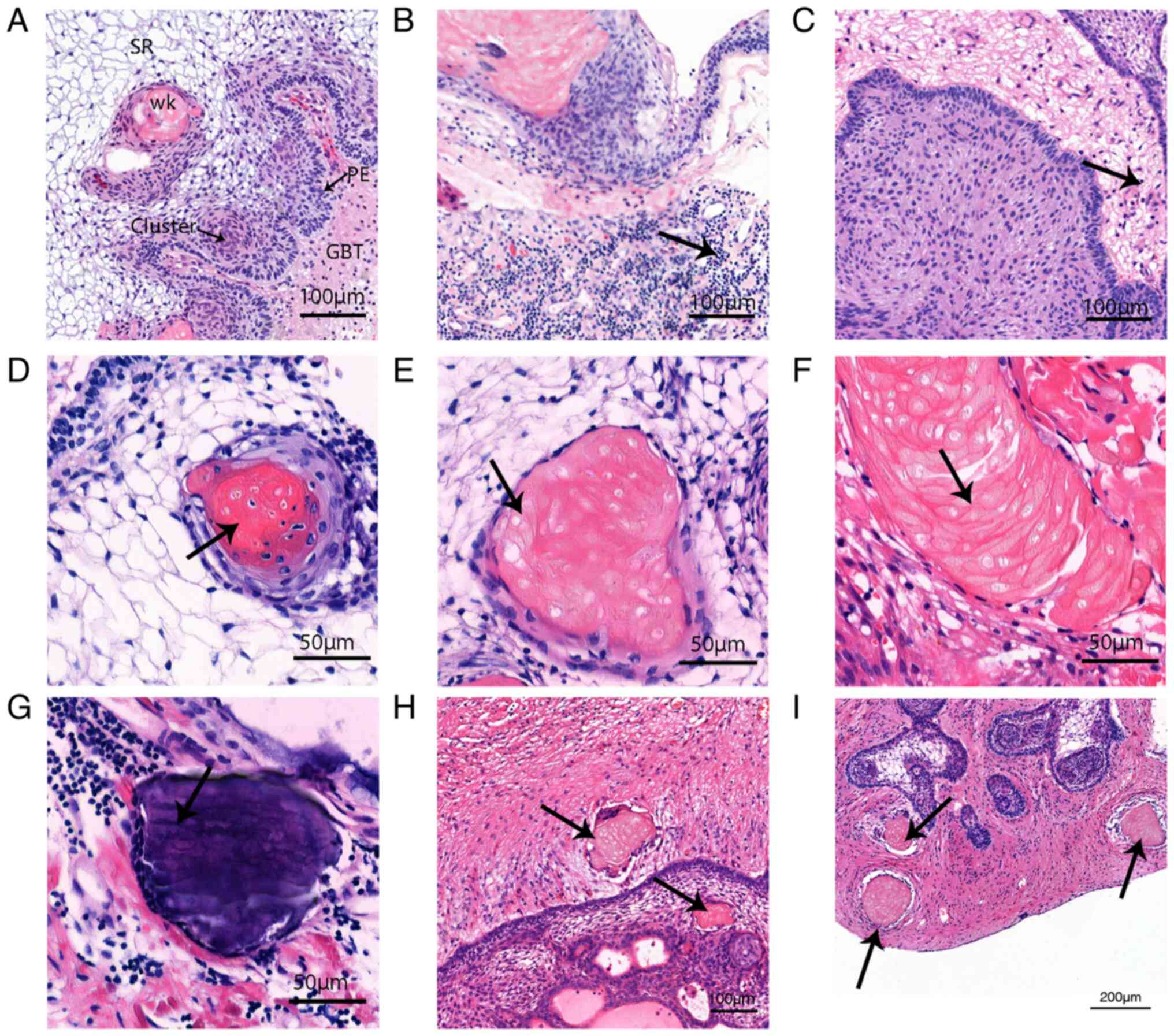
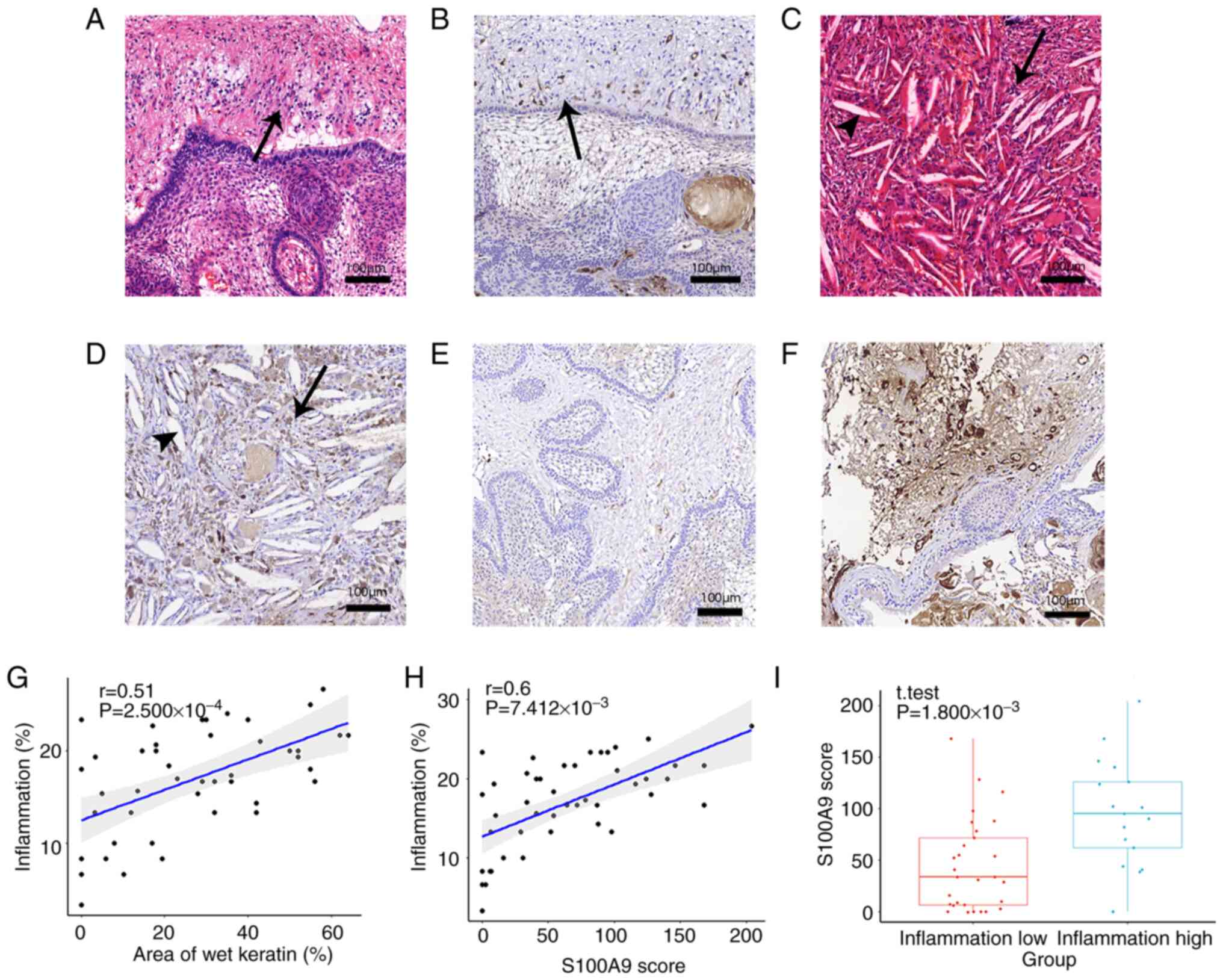
Inflammatory infiltration and wet
keratin evolution detected using H&E staining
The typical pathological structures of ACP are shown
in Fig. 2A. Extensive aggregation
of inflammatory cells was observed in the high-inflammation group
(Fig. 2B), and scattered
distribution of inflammatory cells was observed in the
low-inflammation group (Fig. 2C).
The continuous evolutionary process from tumor cells to wet keratin
was observed by H&E staining (Fig.
2D-F). Some tumor cells underwent apoptosis and nucleolysis,
and all cells exhibited eosinophilic properties. The dissolved
cellular components also fused and eventually calcium deposits
appeared, suggesting that wet keratin evolved from dead tumor cells
and the calcifications in ACP were the result of calcium salt
deposition after wet keratin formation. Additionally, scattered
isolated islands of wet keratin were seen in brain tissue (Fig. 2H and I).
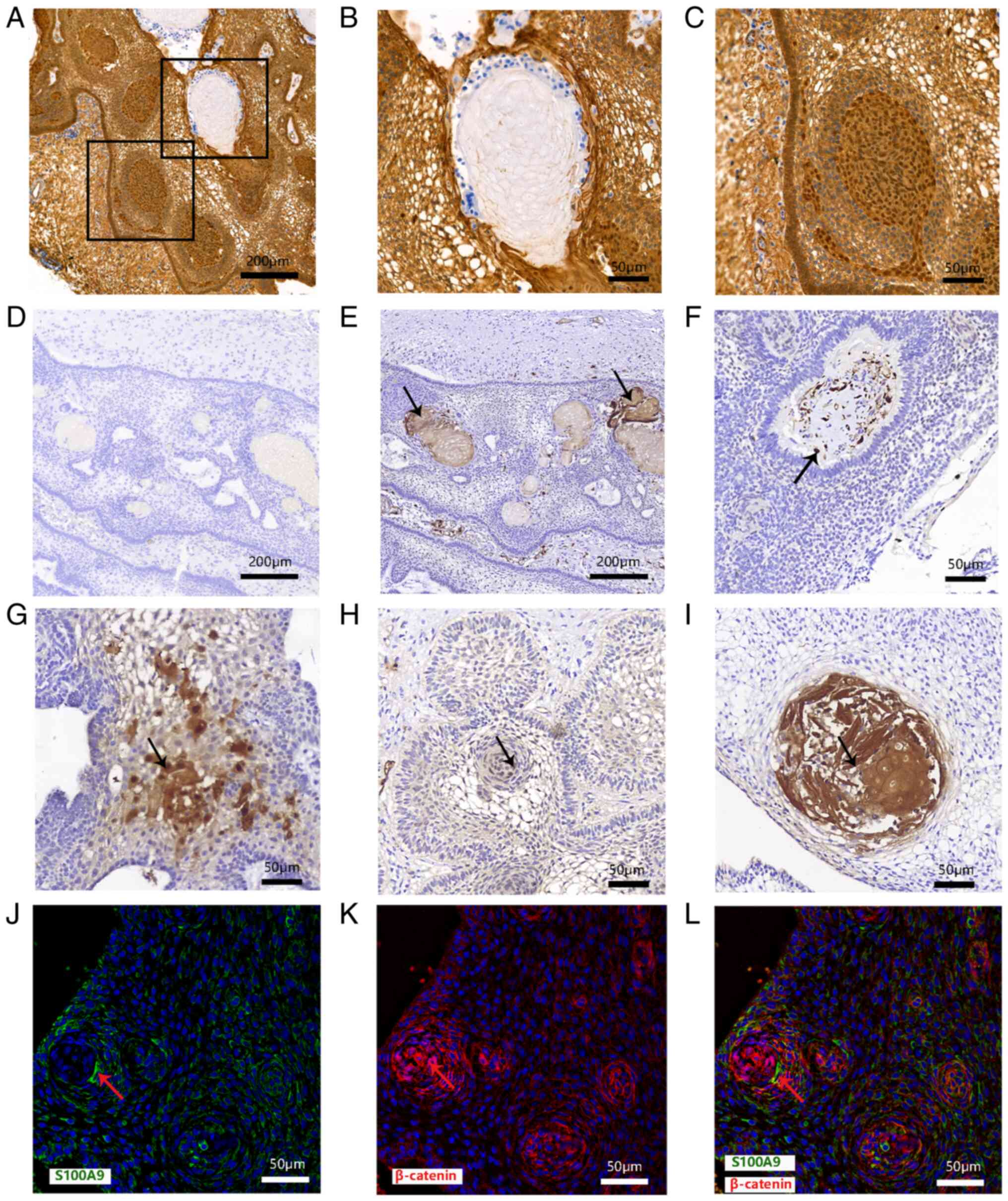
Relationship between S100A9 and
keratinization
Clusters with nucleocytoplasmic accumulation of
β-catenin is characteristic of ACP. Clusters were shown by
immunohistochemical staining but wet keratin was negative for
β-catenin (Fig. 3A-C; derived from
the same sample). Immunohistochemical results showed no obvious
dark brown staining of wet keratin on the negative control (ACP
tissue without S100A9 antibody) images (Fig. 3D). On the sections stained with the
S100A9 antibody, the results showed that S100A9 was expressed in
the wet keratin (Fig. 3E and
I) and stellate reticulum
(Fig. 3G). Some cells in clusters
also showed weak positive expression (Fig. 3H) and S100A9 was highly expressed
in the tumor stroma (Fig. 3F). In
the basal palisades epithelium, S100A9 was partly expressed
(Fig. 3H). S100A9 was also highly
expressed in wet keratin (Fig.
3I). Immunofluorescence staining showed that S100A9 was
expressed in some ACP tumor cells positive for β-catenin. These
results indicated that S100A9 was expressed in ACP intratumoral and
extratumoral cells, especially in wet keratin (Fig. 3J-L).
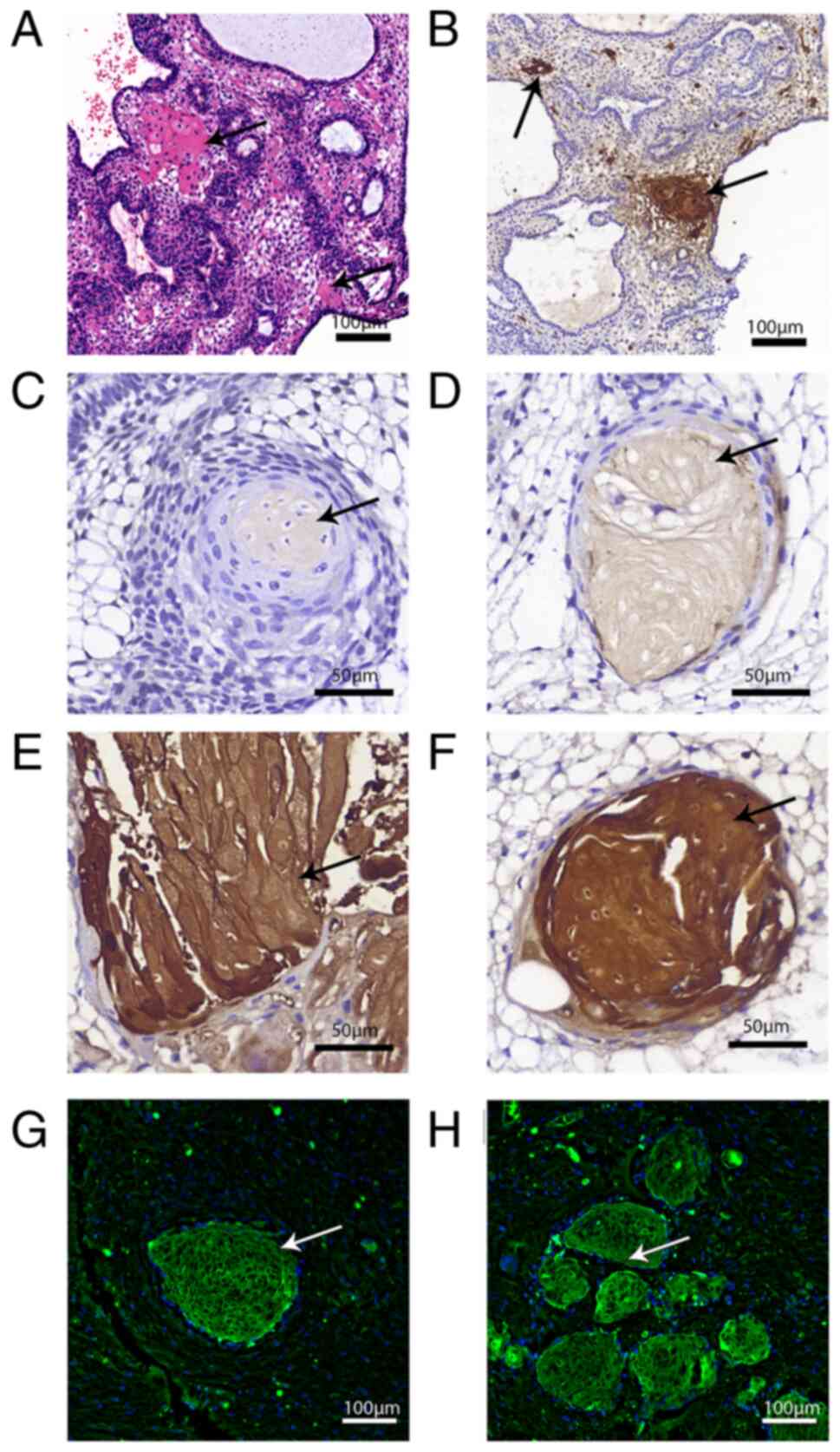
Association between S100A9 and wet
keratin
S100A9 was expressed in ACP cells, which were
eosinophilic in H&E staining (Fig.
4A), and their morphology was from a bulk of normal tumor cells
to a shape similar to wet keratin. S100A9 was also expressed in
cells are in the process of transitioning from tumor cells to wet
keratin (Fig. 4B-F). H&E
staining and immunofluorescence showed island-like structures
formed by S100A9-positive wet keratin invaded brain tissue around
the tumor (Fig. 4G and H; Fig.
2H, upper arrow and Fig. 2I,
arrows).
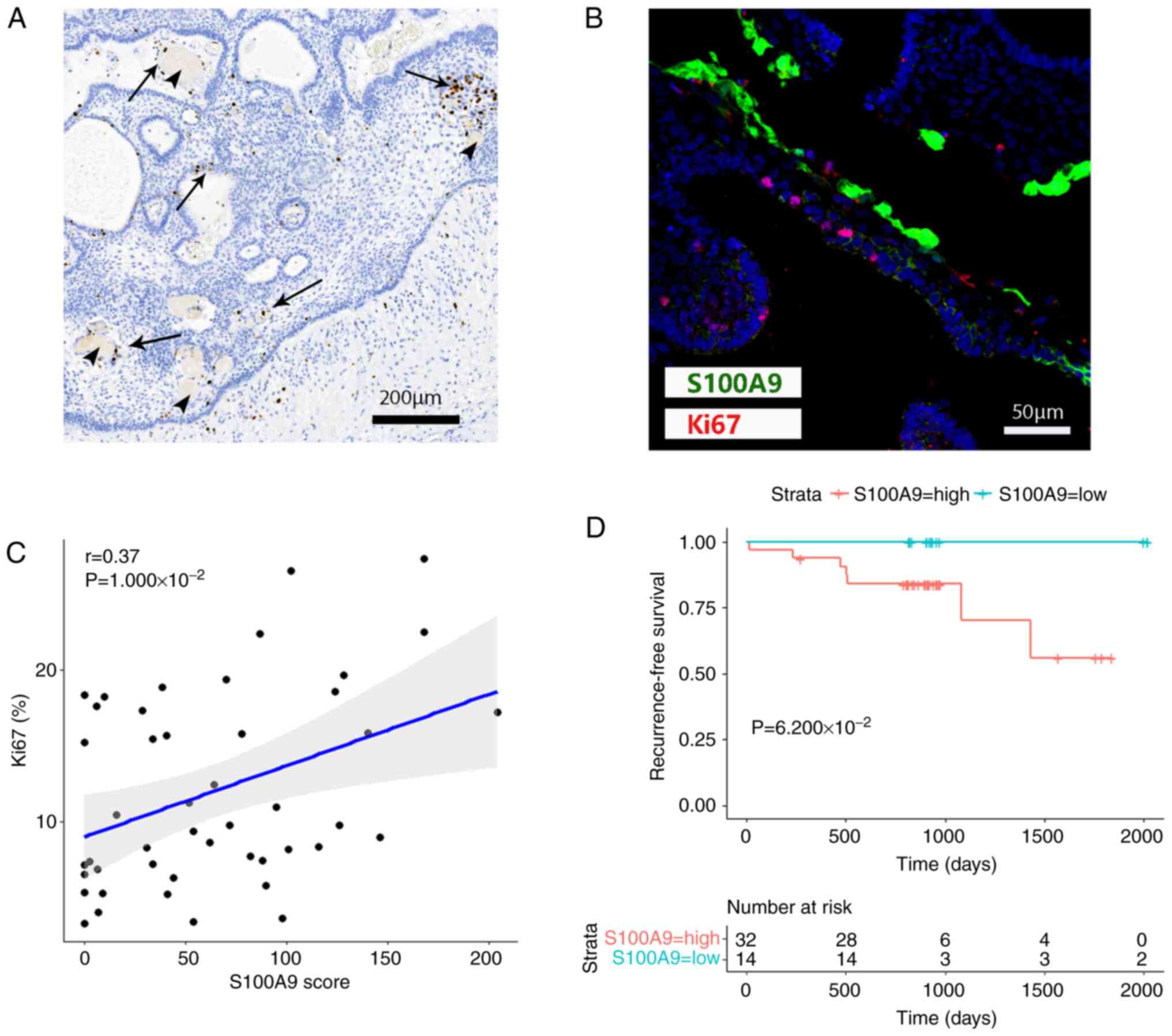
Association between S100A9 in wet
keratin and inflammation
Scattered S100A9-positive cells were seen in the
brain tissue outside the tumor and these cells became increasingly
sparse in areas most distant from the tumor, which was similar to
the degree of inflammation produced by the tumor stimulating the
brain tissue (Fig. 5A and B). S100A9 was also distributed near the
cholesterol cleft, where a large number of inflammatory cells also
accumulated (Fig. 5C and D). Areas of wet keratin and the
expression of S100A9 in wet keratin was correlated with
inflammation in ACP (Fig. 5G and
H). The scores of S100A9 in the
group with higher inflammation were higher than those in the tumor
group with lower inflammation, and the difference was statistically
significant (Fig. 5I). The
expression difference of S100A9 in the two groups is shown visually
in Fig. 5E and F.
Association between S100A9 in wet
keratin and proliferation
A number of studies have reported that S100A9
promotes cell proliferation, and inflammation is also an important
factor in causing proliferation. No Ki67-positive cells were
identified in wet keratin itself, but positive cells were found in
scattered regions around wet keratin (Fig. 6A and B). An obvious linear correlation was
found between S100A9 scores and percentage of Ki67-positive cells
(Fig. 6C).
Association between the expression
levels of S100A9 and various clinical factors
The baseline data of patients are shown in Table I. Patients were divided into two
groups according to S100A9 score with the median as the cut-off.
When the relationships between the expression levels of S100A9 and
sex, age, tumor composition, preoperative status, resection and
postoperative status were studied, no significant associations were
found between S100A9 and various clinical factors (Table II). However, an association
between S100A9 and recurrence-free survival was shown, and patients
with high S100A9 expression had a poorer prognosis (Fig. 6D). But there was no significant
difference between S100A9-High and S100A9-Low.
 | Table IIAssociation between S100A9 scores and
clinical factors. |
Table II
Association between S100A9 scores and
clinical factors.
| | S100A9 scores | |
|---|
| Clinical
factors | ≤54 | >54 | P-value |
|---|
| Sex | | |
6.079x10-1 |
|
Male | 18 | 15 | |
|
Female | 6 | 7 | |
| Age, years, median
(IQR) | 11.96 (21.63) | 11.08 (7.89) |
6.589x10-1 |
| Consistency | | |
5.949x10-1 |
|
Predominantly
cystic | 16 | 13 | |
|
Predominantly
solid | 8 | 9 | |
| Preoperative
status | | |
1.457x10-1 |
|
Primary | 6 | 10 | |
|
Recurrent | 18 | 12 | |
| Resection | | |
9.274x10-1 |
|
Radical
resection | 22 | 20 | |
|
Conservative
resection | 2 | 2 | |
| Postoperative
status | | |
7.750x10-1 |
|
Recurrence | 4 | 3 | |
|
No
recurrence | 20 | 19 | |
Discussion
In the present study, S100A9 was expressed in some
tumor cells and wet keratin. Wet keratin labeled with S100A9 was
also hypothesized to be involved in the evolution of keratin.
According to the results of the present study, it can be
hypothesized that tumor cells expressing S100A9 eventually evolve
into wet keratin in ACP.
Wet keratin is a characteristic pathological
structure of ACP, but its origin is unclear. Similar structures
have been found in calcified odontogenic cysts, an odontogenic
tumor histologically similar to ACP (14). Although no direct in vitro
experimental study of cell evolution is available, the present
study found the evolutionary process of wet keratin was traceable
from histological and pathological morphological changes in wet
keratin and is associated with ACP stem cell-like cells (15). Therefore, the present study
inferred that the evolution of wet keratin was based on the
morphological changes in wet keratin. Finally, after the formation
of wet keratin, the corresponding metabolic and necrotic substances
are deposited; for example, broken organelles, chromatin fragments,
proteins and wet keratin deposited these substances may cause
non-specific inflammation.
Previous studies have reported that the gene
encoding epidermal keratinocyte structural protein and S100
calcium-binding protein form a gene complex (‘epidermal
differentiation complex’) on human chromosome 1q21 (8,9).
This suggested that S100 calcium-binding proteins are involved in
keratinization. The present study also showed that S100A family
members have complex interactions with adjacent epidermal
differentiation genes.
It is well known that ACP is derived from residual
embryonic tissue, which is homologous to the oral epithelium and is
a typical epithelial tissue (1).
The reason for the presence of wet keratin components in epithelial
tumors remains to be elucidated. However, from the results of the
present study, it is likely that S100A9 is closely associated with
keratinization of cells in ACP to eventually form wet keratin. Wet
keratin is common in human ACP but absent in the mouse model of ACP
(16,17). Notably, the genomes of mice and
rats lack S100A2, S100A12 and two members of the S100A family genes
(18,19), This may be the potential biological
basis for the lack of wet keratin in the mouse ACP model and not
just a coincidence. In addition, S100A family members are calcium
ion regulatory proteins (18),
which, unsurprisingly, are suspected to be involved in the
formation of calcification in ACP.
Enhanced inflammation in the tumor component of ACP
is a hallmark of ACP (20-23).
Various immune cells, including lymphocytes and myeloid-derived
cells, are present in the immune microenvironment in ACP (10,24).
Neutrophils contain abundant S100A9, which serves an important role
in neutrophil N1-type polarization (25,26),
thus S100A9-positive cells in intratumoral and peritumoral brain
tissue may include ACP tumor-associated neutrophils. Accordingly,
the present study could be the first definitive report on
neutrophils in ACP, to the best of the authors' knowledge.
The brain tissue adjacent to the tumor is often
infiltrated by inflammatory cells due to the mechanical stimulation
of tumor growth and the possible stimulation of biochemical
secretions (20). A large number
of inflammatory cells has also been shown to infiltrate into the
region of cholesterol crystals (20). On the whole, the severity of
inflammation was consistent with the overall distribution of S100A9
and wet keratin observed in the present study. The results of the
present study also showed that groups with a higher degree of
inflammatory infiltration had higher S100A9 expression; in
addition, both wet keratin and S100A9 were found to have a
significant linear association with inflammation. These results
demonstrated a close relationship between inflammation in ACP and
both wet keratin and S100A9. S100A9 has also been reported to act
through Toll-like receptor 4 and initiates the downstream
inflammatory pathway, which is evidently associated with immunity
and inflammation (27,28). S100A9 also serves an important role
in inflammation of multiple organs and cancer (29,30).
The results of the present study are consistent with those of
previous studies, showing that S100A9 appears to be a crucial
molecule during the inflammatory process of ACP.
The present study also showed a linear correlation
between S100A9 and tumor cell proliferation. A previous study
showed that S100A family members promote tumor proliferation, and
some researchers have used it as a potential target for therapy
(31). In the present study, the
expression of S100A9 was significantly and linearly correlated with
Ki67, reflecting that S100A9 may promote the proliferation of ACP.
Notably, S100A9 acts as an inflammation-related factor before tumor
metastasis and is associated with tumor metastasis, which suggests
that the expression pattern of S100A9 in tumor parenchyma in ACP
and the invasiveness of ACP deserve further study (32,33).
It is worth mentioning that the present study also found that wet
keratin can transcend the tumor boundary and enter the surrounding
structures to form island-like structures, which may be a mode of
invasive growth of ACP. Given the critical role of S100A9 in ACP
related to inflammation, proliferation and metastasis, therapy with
drugs such as Tasquinimod or Paquinimod may be a promising
direction.
While the present study expanded the understanding
of the mechanism underlying wet keratin and its relationship with
inflammation in ACP, evidence is still lacking. S100A9 is
associated with inflammation in ACP. A limitation of the present
study was that it did not have live ACP cells and functional assays
related to S100A9 could not be performed, thus the present study
lacked evidence of S100A9 interaction with inflammatory cells and
it was not possible to prove that S100A9 causes inflammation in
ACP. The role played by the S100 protein family in primary tumors
and metastases remains to be elucidated and needs further
exploration.
The function of S100A9 in ACP may be further
demonstrated by animal studies. At the same time, unlike human
craniopharyngioma, wet keratin has not been found in mouse models
of craniopharyngioma, which some researchers attribute to species
differences, but evidence is still insufficient (34). Whether S100A family members are
responsible for this difference may be revealed in future
studies.
The present study found that wet keratin evolved
from some tumor cells in ACP and these tumor cells expressed
S100A9. It was hypothesized that S100A9 was involved in the
evolution of wet keratin, and S100A9 was associated with
inflammation in ACP.
Acknowledgements
The authors would like to thank Mr. Zhong Ma (Sanbo
Brain Hospital, Capital Medical University, Beijing, China), for
his excellent assistance.
Funding
Funding: The present study was supported by grants from Sanbo
Brain Hospital Management Group (grant no. SBJT-KY-2020-002) and
Capital's Funds for Health Improvement and Research (grant no.
2022-2-8013).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZL conceived and designed the current study. All
authors participated in the acquisition of data. Analysis and
interpretation of data was performed by CZ, WH, DL, NL and XW. CZ
and ZL drafted the manuscript. XW and ZL critically revised the
manuscript. ZL reviewed the submitted version of manuscript and
approved the final version of the manuscript on behalf of all
authors. CZ and XW performed statistical analysis. Administrative,
technical and material support was from ZL who also supervised the
study. WH and ZL confirm the authenticity of all the raw data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The patients in the present study provided written
informed consent. The present study was designed in accordance with
The Declaration of Helsinki and approved by the ethics committee of
Sanbo Brain Hospital, Capital Medical University (approval no.
SBNK-YJ-2020-014-01).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Müller H, Merchant T, Warmuth-Metz M,
Martinez-Barbera J and Puget S: Craniopharyngioma. Nat Rev Dis
Primers. 5(75)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 World Health Organization
classification of tumors of the central nervous system: A summary.
Acta Neuropathol. 131:803–820. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Martinez-Barbera J: Molecular and cellular
pathogenesis of adamantinomatous craniopharyngioma. Neuropathol
Appl Neurobiol. 41:721–732. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Whelan R, Prince E, Gilani A and Hankinson
T: The inflammatory milieu of adamantinomatous craniopharyngioma
and its implications for treatment. J Clin Med.
9(519)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wang S, Song R, Wang Z, Jing Z, Wang S and
Ma J: S100A8/A9 in inflammation. Front Immunol.
9(1298)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Martinsson H, Yhr M and Enerbäck C:
Expression patterns of S100A7 (psoriasin) and S100A9
(calgranulin-B) in keratinocyte differentiation. Exp Dermatol.
14:161–168. 2005.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Christmann C, Zenker S, Martens L, Hübner
J, Loser K, Vogl T and Roth J: Interleukin 17 promotes expression
of alarmins S100A8 and S100A9 during the inflammatory response of
keratinocytes. Front Immunol. 11(599947)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Mischke D, Korge BP, Marenholz I, Volz A
and Ziegler A: Genes encoding structural proteins of epidermal
cornification and S100 calcium-binding proteins form a gene complex
(‘epidermal differentiation complex’) on human chromosome 1q21. J
Invest Dermatol. 106:989–992. 1996.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Argyris PP, Slama Z, Malz C, Koutlas IG,
Pakzad B, Patel K, Kademani D, Khammanivong A and Herzberg MC:
Intracellular calprotectin (S100A8/A9) controls epithelial
differentiation and caspase-mediated cleavage of EGFR in head and
neck squamous cell carcinoma. Oral Oncol. 95:1–10. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lin D, Wang Y, Zhou Z and Lin Z: Immune
microenvironment of primary and recurrent craniopharyngiomas: A
study of the differences and clinical significance. World
Neurosurg. 127:e212–e220. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Rakha E, Puls F, Saidul I and Furness P:
Torsion of the testicular appendix: Importance of associated acute
inflammation. J Clin Pathol. 59:831–834. 2006.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Vidal AP, Andrade BM, Vaisman F, Cazarin
J, Pinto LF, Breitenbach MM, Corbo R, Caroli-Bottino A, Soares F,
Vaisman M and Carvalho DP: AMP-activated protein kinase signaling
is upregulated in papillary thyroid cancer. Eur J Endocrinol.
169:521–528. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Han SA, Jang JH, Won KY, Lim SJ and Song
JY: Prognostic value of putative cancer stem cell markers (CD24,
CD44, CD133, and ALDH1) in human papillary thyroid carcinoma.
Pathol Res Pract. 213:956–963. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kusama K, Katayama Y, Oba K, Ishige T,
Kebusa Y, Okazawa J, Fukushima T and Yoshino A: Expression of hard
alpha-keratins in pilomatrixoma, craniopharyngioma, and calcifying
odontogenic cyst. Am J Clin pathol. 123:376–381. 2005.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wang CH, Qi ST, Fan J, Pan J, Peng JX, Nie
J, Bao Y, Liu YW, Zhang X and Liu Y: Identification of tumor
stem-like cells in admanatimomatous craniopharyngioma and
determination of these cells' pathological significance. J
Neurosurg. 1–11. 2019.PubMed/NCBI View Article : Google Scholar : (Epub ahead of
print).
|
|
16
|
Andoniadou CL, Gaston-Massuet C, Reddy R,
Schneider RP, Blasco MA, Le Tissier P, Jacques TS, Pevny LH,
Dattani MT and Martinez-Barbera JP: Identification of novel
pathways involved in the pathogenesis of human adamantinomatous
craniopharyngioma. Acta Neuropathol. 124:259–271. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Andoniadou CL, Matsushima D, Mousavy
Gharavy SN, Signore M, Mackintosh AI, Schaeffer M, Gaston-Massuet
C, Mollard P, Jacques TS, Le Tissier P, et al: Sox2(+)
stem/progenitor cells in the adult mouse pituitary support organ
homeostasis and have tumor-inducing potential. Cell Stem Cell.
13:433–445. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Cmoch A, Groves P, Palczewska M and Pikuła
S: S100A proteins in propagation of a calcium signal in norm and
pathology. Postepy Biochem. 58:429–436. 2012.PubMed/NCBI
|
|
19
|
Fuellen G, Foell D, Nacken W, Sorg C and
Kerkhoff C: Absence of S100A12 in mouse: Implications for
RAGE-S100A12 interaction. Trends Immunol. 24:622–624.
2003.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Apps JR, Carreno G, Gonzalez-Meljem JM,
Haston S, Guiho R, Cooper JE, Manshaei S, Jani N, Hölsken A,
Pettorini B, et al: Tumour compartment transcriptomics demonstrates
the activation of inflammatory and odontogenic programmes in human
adamantinomatous craniopharyngioma and identifies the MAPK/ERK
pathway as a novel therapeutic target. Acta Neuropathol.
135:757–777. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Pettorini BL, Inzitari R, Massimi L,
Tamburrini G, Caldarelli M, Fanali C, Cabras T, Messana I,
Castagnola M and Rocco C: The role of inflammation in the genesis
of the cystic component of craniopharyngiomas. Childs Nerv Syst.
26:1779–1784. 2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Desiderio C, Martelli C, Rossetti DV, Di
Rocco C, D'Angelo L, Caldarelli M, Tamburrini G, Iavarone F,
Castagnola M, Messana I, et al: Identification of thymosins β4 and
β 10 in paediatric craniopharyngioma cystic fluid. Childs Nerv
Syst. 29:951–960. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Gong J, Zhang H, Xing S, Li C, Ma Z, Jia G
and Hu W: High expression levels of CXCL12 and CXCR4 predict
recurrence of adamanti-nomatous craniopharyngiomas in children.
Cancer Biomark. 14:241–251. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Coy S, Rashid R, Lin JR, Du Z, Donson AM,
Hankinson TC, Foreman NK, Manley PE, Kieran MW, Reardon DA, et al:
Multiplexed immunofluorescence reveals potential PD-1/PD-L1 pathway
vulnerabilities in craniopharyngioma. Neuro Oncol. 20:1101–1112.
2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sprenkeler EGG, Zandstra J, van Kleef ND,
Goetschalckx I, Verstegen B, Aarts CEM, Janssen H, Tool ATJ, van
Mierlo G, van Bruggen R, et al: S100A8/A9 is a marker for the
release of neutrophil extracellular traps and induces neutrophil
activation. Cells. 11(236)2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mihaila AC, Ciortan L, Macarie RD, Vadana
M, Cecoltan S, Preda MB, Hudita A, Gan AM, Jakobsson G, Tucureanu
MM, et al: Transcriptional profiling and functional analysis of
N1/N2 neutrophils reveal an immunomodulatory effect of
S100A9-blockade on the pro-inflammatory N1 subpopulation. Front
Immunol. 12(708770)2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Vogl T, Tenbrock K, Ludwig S, Leukert N,
Ehrhardt C, van Zoelen MA, Nacken W, Foell D, van der Poll T, Sorg
C and Roth J: Mrp8 and Mrp14 are endogenous activators of Toll-like
receptor 4, promoting lethal, endotoxin-induced shock. Nat Med.
13:1042–1049. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
28
|
Shichita T, Ito M, Morita R, Komai K,
Noguchi Y, Ooboshi H, Koshida R, Takahashi S, Kodama T and
Yoshimura A: MAFB prevents excess inflammation after ischemic
stroke by accelerating clearance of damage signals through MSR1.
Nat Med. 23:723–732. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
29
|
Sreejit G, Abdel-Latif A, Athmanathan B,
Annabathula R, Dhyani A, Noothi SK, Quaife-Ryan GA, Al-Sharea A,
Pernes G, Dragoljevic D, et al: Neutrophil-derived S100A8/A9
amplify granulopoiesis after myocardial infarction. Circulation.
141:1080–1094. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Mohr T, Zwick A, Hans MC, Bley IA, Braun
FL, Khalmurzaev O, Matveev VB, Loertzer P, Pryalukhin A, Hartmann
A, et al: The prominent role of the S100A8/S100A9-CD147 axis in the
progression of penile cancer. Front Oncol.
12(891511)2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Li HB, Wang JL, Jin XD, Zhao L, Ye HL,
Kuang YB, Ma Y, Jiang XY and Yu ZY: Comprehensive analysis of the
transcriptional expressions and prognostic value of S100A family in
pancreatic ductal adenocarcinoma. BMC Cancer.
21(1039)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Lukanidin E and Sleeman JP: Building the
niche: The role of the S100 proteins in metastatic growth. Semin
Cancer Biol. 22:216–225. 2012.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Liu Y, Kosaka A, Ikeura M, Kohanbash G,
Fellows-Mayle W, Snyder LA and Okada H: Premetastatic soil and
prevention of breast cancer brain metastasis. Neuro Oncol.
15:891–903. 2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Gaston-Massuet C, Andoniadou CL, Signore
M, Jayakody SA, Charolidi N, Kyeyune R, Vernay B, Jacques TS,
Taketo MM, Le Tissier P, et al: Increased Wingless (Wnt) signaling
in pituitary progenitor/stem cells gives rise to pituitary tumors
in mice and humans. Proc Nati Acad Sci USA. 108:11482–11487.
2011.PubMed/NCBI View Article : Google Scholar
|