Introduction
Inflammatory bowel disease (IBD) results from the interaction between genetic and environmental factors, which may influence immune responses. Crohn's disease (CD) and ulcerative colitis (UC), the two main forms of IBD (1), consist of manifestations of chronic inflammation of the gastrointestinal tract. However, the inflammatory and symptom burden between patients and within the same individual displays considerable heterogeneity over time (2). In the past two to three decades, the incidence and prevalence of IBD have increased rapidly around the world. IBD not only seriously affects patients' quality of life, but also has the potential to induce serious complications. For example, CD can cause an intestinal fistula, and skin and biliary stones, while UC is associated with osteoporosis and possibly colon cancer if it lasts over 8-10 years (1).
Conventional treatments for IBD include 5-aminosalicylates, corticosteroids, azathioprine, or 6-mercaptopurine. Novel anti-TNF-α biologics, such as infliximab and adalimumab, have been used as the basis for moderate-to-severe IBD treatment (3). However, >30% of patients do not respond to initial anti-TNF-α therapy, and up to 45% of patients show a decreased response over time (4). In addition, anti-TNF-α agents are associated with serious adverse events, such as infusion response, neutropenia, and systemic infections. Therefore, it is important to identify novel therapies for IBD. Vedolizumab (VDZ), specifically expressed by gastrointestinal T lymphocytes, is an integrin antagonist that binds to α4β7 integrin. VDZ alleviates intestinal inflammation by selectively inhibiting interactions between integrin α4β7 and mucosal addressin cell adhesion molecule-1, thereby blocking lymphocyte migration to the intestinal tract, thus playing an effective role in moderate-to-severe IBD (5).
In recent years, VDZ has been approved for the treatment of moderate-to-severe active UC and CD patients with at least one conventional treatment failure. There are multiple randomized controlled trials (RCTs) on the use of VDZ for the treatment of IBD, whose findings on efficacy and adverse reactions differ. Therefore, this systematic review and meta-analysis was conducted to thoroughly evaluate the therapeutic value of VDZ for the treatment of IBD by selecting high-quality RCTs and providing an objective basis for its clinical application.
Materials and methods
The present systematic review and meta-analysis was registered in PROSPERO (https://www.crd.york.ac.uk/prospero) under CRD42022335987 and was performed in accordance with the updated Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines (6). The PICO format (population, intervention, comparison, outcome) was used to answer the research question: ‘Is VDZ effective and safe for the treatment of IBD?’ The population included IBD patients with UC or CD, and the intervention included VDZ or placebo. The primary outcomes were clinical remission and clinical response during induction and maintenance therapy. Secondary outcomes included clinical remission and clinical response in patients with TNF antagonist failure, corticosteroid-free remission, mucosal healing in CD patients, IBD exacerbation, and adverse event rate.
Search strategy
PubMed (https://pubmed.ncbi.nlm.nih.gov), Embase (https://www.embase.com), and the Cochrane databases (https://www.cochranelibrary.com) in English were searched until April 2022. The search terms used were as follows: ‘IBD’ or ‘UC’ or ‘Crohn's disease’ or ‘IBD’ or ‘UC’ or ‘CD’ and ‘VDZ’ or ‘α4β7 integrin’, and ‘TNF-α’ or ‘TNF antagonist failure’, and ‘RCTs’. Titles and abstracts were independently screened by two reviewers (HJT and LLZG) to exclude irrelevant articles. A secondary search was made for references for the literature review. Duplicates were excluded and full texts were then retrieved to assess eligibility.
Inclusion and exclusion criteria
The inclusion criteria were as follows: i) Adults (age, >18 years) with moderate-to-severe UC or CD (confirmed endoscopically and/or histopathologically, with a partial Mayo score of 1-12 at screening) who were either treatment-naive, previously exposed to anti-TNF agents, or had failed TNF antagonist therapy; ii) randomized, double-blinded, placebo-controlled studies to evaluate the efficacy and safety of VDZ in the treatment of IBD; iii) available studies reporting the risk estimates with their corresponding 95% confidence interval (CI) or original data allowing us to compute them; and iv) if the published studies reported data for specific subgroups, results for the whole population were considered.
The exclusion criteria were as follows: i) Not RCTs; ii) animal studies; iii) studies on children and pregnant women; iv) non-original papers; v) duplicate reports and abstracts; vi) comparison of VDZ with other biological agents; and vii) full-text or complete data were not available.
Data extraction and quality assessment
The data extracted from each study included the name of the first author, publication year, location, sample size (number of cases and total number of participants), type of IBD, categories of efficacy indicators, and types of adverse reactions. Three investigators (LLZG, LXZ, and HJT) independently extracted the data, and discrepancies were resolved by consensus.
The clinical response of CD patients was defined as CD Activity Index (CDAI)-100 response. Mucosal healing was determined using a sub-score of 0 or 1 on the Mayo endoscopic component. Adverse events included serious adverse events, such as infusion reaction and delayed hypersensitivity that led to discontinuation of the drug, headaches, nasopharyngitis, upper respiratory tract infection, arthralgia, abdominal pain, and vomiting.
The methodological quality of the included RCTs was evaluated based on the Cochrane Collaboration's risk of bias tool (7), which consists of selection bias (random sequence generation and allocation concealment), performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessment), attrition bias (incomplete outcome data), reporting bias (selective reporting) and other (bias source); and together are used to evaluate the risk of bias. The results of methodological quality were interpreted as ‘low-risk bias’, ‘high-risk bias’, and ‘unclear’ for each item according to the risk assessment criteria of bias. The study quality was assessed independently by two investigators (LXZ and CQB), and any discrepancies were addressed by a joint reevaluation of the original article.
Statistical analysis
All statistical analysis was performed using STATA version 12.0 (StataCorp LLC). The results are presented as the risk ratio (RR) and 95% CI for the comparison of VDZ and placebo treatment in IBD. A random effects model was used to calculate the pooled estimates. Statistical heterogeneity between studies was examined using the I² value, and I²>50% was considered to indicate statistically significant heterogeneity (8). Subgroup analysis based on study design, IBD category, and type of adverse reaction was conducted to explore the source of heterogeneity in this study. Sensitivity analysis was further performed to examine the reliability of the results by omitting one study at a time. Publication bias was assessed using a Begg's test and funnel plots if ≥10 studies were available, and it was considered to exist when P<0.05. The trim-and-fill method was used to reduce the potential influence of publication bias (9).
Results
Fig. 1 shows a flow diagram of the detailed selection process. A total of 1,107 potentially relevant articles were initially retrieved, 452 duplicate articles were excluded, and 513 were articles were excluded due to being reviews, conference abstracts, and animal experiments. After screening the title and abstract, 34 articles remained for full-text review. Among them, 22 were excluded (10 were meta-analyses/guideline articles, 7 were abstracts whose original articles were not available, 2 reported duplicate analysis from the same data source and three were not RCTs). Thus, a total of 12 eligible articles (10-21) were included in this meta-analysis. Of these studies, 5 were conducted in the United States (11,15,17-19), 4 in Canada (10,12,13,16), 2 in Japan (14,20), and 1 in Belgium (21). Table I shows the primary characteristics of the included studies. All 12 studies were assessed as having a low or moderate risk of bias. Table SI shows the study quality and risk of bias in each domain of the included studies.
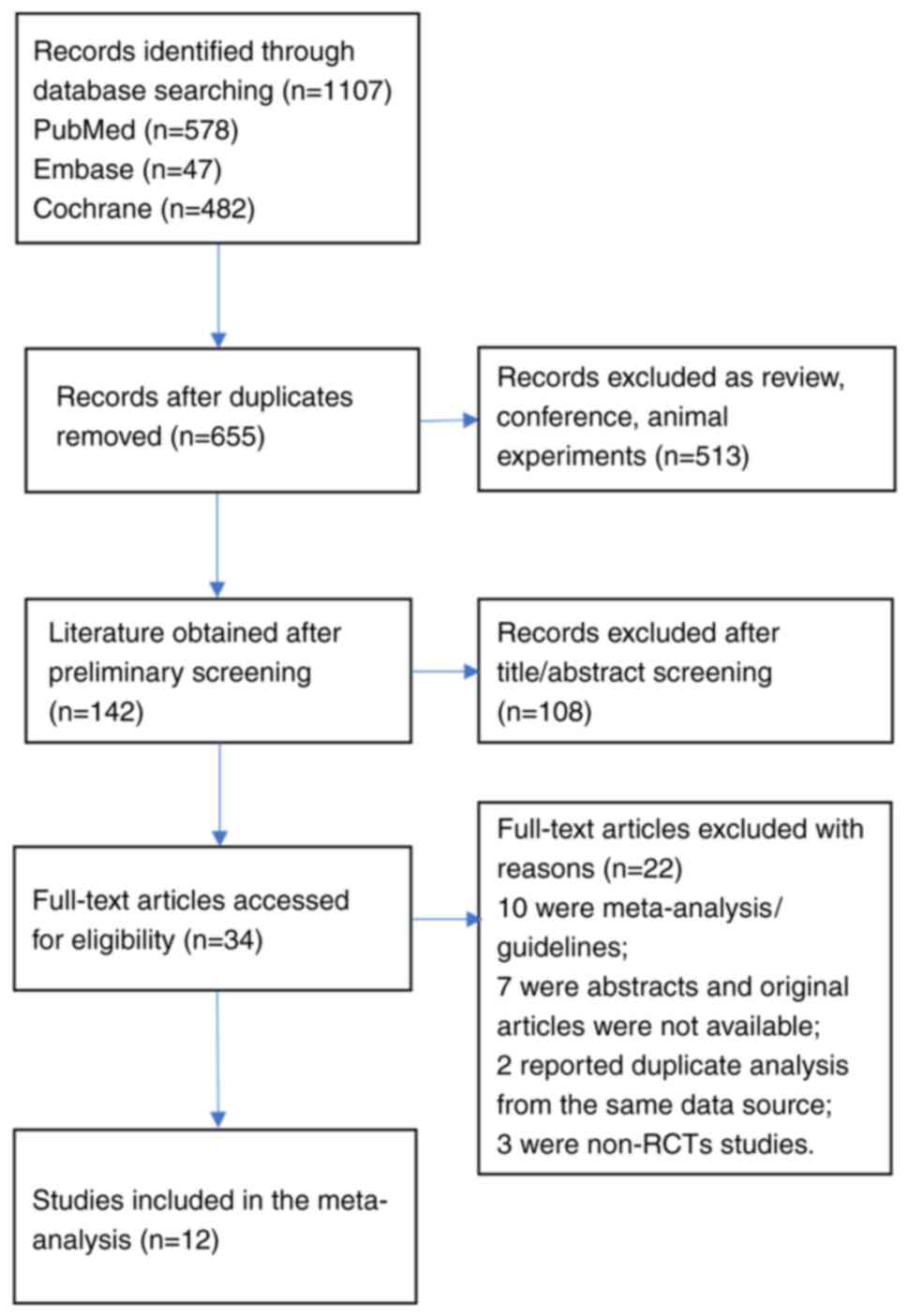 |
Figure 1
Flow diagram of the assessment of studies identified by the literature search for inclusion.
|
 |
Table I
Detailed information on the included studies.
|
Table I
Detailed information on the included studies.
| First author, year |
Country |
Diagnosis |
Intervention measures and administration methods of VDZ group |
No. of patients |
Female (%) |
Outcomes |
Follow-up |
(Refs.) |
| Feagan et al, 2005 |
Canada |
UC |
Induction Phase: |
|
|
Induction Phase: |
8 weeks |
(10) |
| |
|
|
VDZ(0.5 mg/2.0 mg/kg, iv) |
118 |
- |
Clinical response, clinical remission |
|
|
| |
|
|
Placebo |
63 |
- |
Adverse events |
|
|
| Parikh et al, 2012 |
USA |
UC |
Induction Phase: |
|
|
Clinical response; clinical remission |
253 days |
(11) |
| |
|
|
VDZ (2 mg/kg, 6 mg/kg, 10 mg/kg, iv) |
37 |
57 |
|
|
|
| |
|
|
Placebo |
9 |
67 |
|
|
|
| Feagan et al, 2013 |
Canada |
UC |
Induction Phase: |
|
|
Induction Phase: |
6 weeks |
(12) |
| |
|
|
VDZ (300 mg, iv) |
225 |
41.3 |
Clinical response, clinical remission |
|
|
| |
|
|
Placebo |
149 |
38.3 |
|
|
|
| |
|
|
Maintenance Phase: |
|
|
Maintenance Phase: |
52 weeks |
|
| |
|
|
VDZ (300 mg iv, Q4/Q8W) |
247 |
- |
Clinical response, clinical remission |
|
|
| |
|
|
Placebo |
126 |
- |
|
|
|
| Feagan et al, 2017 |
Canada |
UC |
Induction Phase: |
|
|
Induction Phase: |
6 weeks |
(13) |
| |
|
|
TNF-naïve: VDZ (300 mg, iv) |
130 |
47.0 |
Clinical response, clinical remission |
|
|
| |
|
|
Placebo |
76 |
38.2 |
TNF-failure: clinical response, |
6 weeks |
|
| |
|
|
TNF-failure: VDZ (300 mg, iv) |
82 |
39.0 |
clinical remission |
|
|
| |
|
|
Placebo |
63 |
44.4 |
|
|
|
| Motoya et al, 2019 |
Japan |
UC |
Induction Phase: |
|
|
Induction Phase: |
10 weeks |
(14) |
| |
|
|
VDZ: 300 mg iv |
164 |
39.6 |
Clinical response, clinical remission |
|
|
| |
|
|
Placebo |
82 |
32.9 |
Maintenance Phase: |
60 weeks |
|
| |
|
|
MaintenancePhase: |
|
|
Clinical response, clinical remission |
|
|
| |
|
|
VDZ300 mg iv (Q8W) |
41 |
- |
Adverse events |
|
|
| |
|
|
Placebo |
42 |
- |
|
|
|
| Sandborn et al, 2020 |
USA |
UC |
Maintenance Phase: |
|
|
Maintenance Phase: |
52 weeks |
(15) |
| |
|
|
VDZ (108 mg, SC, Q2W) |
106 |
38.7 |
Clinical response, clinical remission |
|
|
| |
|
|
VDZ (300 mg, iv, Q8W) |
54 |
42.6 |
Adverse events |
52 weeks |
|
| |
|
|
Placebo |
56 |
39.3 |
|
|
|
| Feagan et al, 2008 |
Canada |
CD |
Induction Phase: |
|
|
Induction Phase: |
57 days |
(16) |
| |
|
|
VDZ (0.5 mg/2.0 mg/kg, iv) |
127 |
56.0 |
Clinical response, clinical remission |
|
|
| |
|
|
Placebo |
58 |
48.3 |
Adverse events |
|
|
| Sandborn et al, 2013 |
USA |
CD |
Induction Phase: |
|
|
Induction Phase: |
6 weeks |
(17) |
| |
|
|
VDZ (300 mg, iv) |
220 |
52.3 |
Clinical response, clinical |
|
|
| |
|
|
Placebo |
148 |
53.4 |
remission |
|
|
| |
|
|
Maintenance Phase: |
|
|
Maintenance Phase: |
52 weeks |
|
| |
|
|
VDZ (300 mg, iv, Q4/8W) |
308 |
- |
Clinical response, clinical remission |
|
|
| |
|
|
Placebo |
153 |
- |
|
|
|
| Sands et al, 2014 |
USA |
CD |
Induction Phase: |
|
|
Induction Phase: |
10 weeks |
(18) |
| |
|
|
TNF-naïve: |
51 |
55.0 |
Clinical response, clinical remission |
|
|
| |
|
|
VDZ (300 mg, iv) |
50 |
46.0 |
TNF-failure: clinical response, |
6 weeks |
|
| |
|
|
Placebo |
|
|
clinical remission. Adverse reactions |
|
|
| |
|
|
TNF-failure: |
|
|
|
|
|
| |
|
|
VDZ (300 mg, iv) |
158 |
57.0 |
|
|
|
| |
|
|
Placebo |
157 |
61.0 |
|
|
|
| Sands et al, 2017 |
USA |
CD |
Induction Phase: |
|
|
Induction Phase: |
6 weeks |
(19) |
| |
|
|
VDZ (300 mg, iv) |
154 |
48.7 |
Clinical response, clinical remission |
|
|
| |
|
|
Placebo |
123 |
47.2 |
|
|
|
| |
|
|
Maintenance Phase: |
|
|
Maintenance Phase: |
52 weeks |
|
| |
|
|
VDZ (300 mg, iv, Q4/8W) |
137 |
- |
Clinical response, clinical remission |
|
|
| |
|
|
Placebo |
71 |
- |
TNF-failure: clinical response, |
6 weeks |
|
| |
|
|
TNF-failure: |
|
|
clinical remission |
|
|
| |
|
|
VDZ (300 mg, iv) |
263 |
56.7 |
|
|
|
| |
|
|
Placebo |
227 |
60.0 |
|
|
|
| Watanabe et al, 2020 |
Japan |
CD |
Induction Phase: |
|
|
Induction Phase: |
10 weeks |
(20) |
| |
|
|
VDZ (300 mg, iv) |
79 |
35.4 |
Clinical response, clinical remission |
|
|
| |
|
|
Placebo |
78 |
33.3 |
|
60 weeks |
|
| |
|
|
Maintenance Phase: |
|
|
Maintenance Phase: |
|
|
| |
|
|
VDZ (300 mg, iv, Q8W) |
12 |
50.0 |
Clinical response, clinical remission |
|
|
| |
|
|
Placebo |
12 |
25.0 |
Adverse events |
|
|
| Vermeire et al, 2022 |
Belgium |
CD |
Maintenance Phase: |
|
|
Maintenance Phase: |
52 weeks |
(21) |
| |
|
|
VDZ (108 mg, SC, Q2W) |
275 |
42.9 |
Clinical response, clinical remission |
|
|
| |
|
|
Placebo |
134 |
50.7 |
Adverse events. |
|
|
As shown in Table II, a total of 12 RCTs were included to discuss the efficacy and safety of VDZ compared with that of a placebo for the treatment of IBD patients. All outcomes were uniformly assessed according to the standard-defined Mayo Clinic Score on weeks 6-10 of induction therapy and weeks 52-60 of maintenance therapy.
 |
Table II
Summary of results.
|
Table II
Summary of results.
| |
Heterogeneity |
| Treatment |
Outcome |
Subgroups |
No. of studies |
Pooled RR (95% CI) |
P-value |
I2 (%) |
Ph |
| Induction therapy |
Clinical remission |
IBD |
9 |
2.09 (1.66-2.62) |
<0.001 |
0.0 |
0.810 |
| |
|
UC |
4 |
2.33 (1.61-3.38) |
<0.001 |
5.20 |
0.367 |
| |
|
CD |
5 |
1.95 (1.45-2.61) |
<0.001 |
0.0 |
0.944 |
| |
Clinical response |
IBD |
10 |
1.54 (1.34-1.78) |
<0.001 |
20.5 |
0.254 |
| |
|
UC |
5 |
1.68 (1.39-2.03) |
<0.001 |
10.9 |
0.344 |
| |
|
CD |
5 |
1.41 (1.16-1.73) |
0.001 |
16.5 |
0.309 |
| Maintenance therapy |
Clinical remission |
IBD |
8 |
1.98 (1.58-2.49) |
<0.001 |
43.9 |
0.086 |
| |
|
UC |
4 |
2.55 (1.93-3.35) |
<0.001 |
0.0 |
0.482 |
| |
|
CD |
4 |
1.59 (1.32-1.91) |
<0.001 |
0.0 |
0.566 |
| |
Clinical response |
IBD |
8 |
1.78 (1.40-2.26) |
<0.001 |
68.7 |
0.002 |
| |
|
UC |
4 |
2.23 (1.82-2.73) |
<0.001 |
0.0 |
0.807 |
| |
|
CD |
4 |
1.38 (1.10-1.74) |
0.006 |
45.3 |
0.140 |
| Failure of TNF antagonists |
Clinical remission |
All studies |
5 |
2.07 (1.48-2.89) |
<0.001 |
10.2 |
0.348 |
| |
|
Induction therapy |
3 |
1.85 (1.30-2.65) |
0.001 |
0.0 |
0.601 |
| |
|
Maintenance therapy |
2 |
3.29 (1.06-10.15) |
0.039 |
58.2 |
0.122 |
| |
Clinical response |
All studies |
5 |
1.84 (1.54-2.21) |
<0.001 |
0.0 |
0.820 |
| |
|
Induction therapy |
3 |
1.83 (1.50-2.23) |
<0.001 |
0.0 |
0.951 |
| |
|
Maintenance therapy |
2 |
1.97 (1.17-3.31) |
0.010 |
29.6 |
0.233 |
| Corticosteroid-free |
|
IBD |
8 |
1.98 (1.51-2.59) |
<0.001 |
17.4 |
0.293 |
| remission/glucocorticoid |
|
UC |
4 |
2.79 (1.84-4.21) |
<0.001 |
0.0 |
0.876 |
| free remission |
|
CD |
4 |
1.58 (1.21-2.07) |
0.001 |
0.0 |
0.461 |
| Mucosal healing |
UC |
All studies |
4 |
1.78 (1.27-2.51) |
0.001 |
68.4 |
0.023 |
| |
|
Induction therapy |
2 |
1.43 (1.05-1.95) |
0.022 |
35.1 |
0.215 |
| |
|
Maintenance therapy |
2 |
2.35 (1.66-3.34) |
<0.001 |
26.5 |
0.243 |
| Adverse event |
|
|
|
|
|
|
|
| Headache |
|
IBD |
9 |
1.09 (0.81-1.47) |
0.567 |
0.0 |
0.561 |
| |
|
UC |
4 |
0.98 (0.60-1.58) |
0.921 |
0.0 |
0.837 |
| |
|
CD |
5 |
1.08 (0.63-1.86) |
0.769 |
35.7 |
0.198 |
| |
|
Induction therapy |
5 |
1.05 (0.71-1.55) |
0.805 |
17.0 |
0.307 |
| |
|
Maintenance therapy |
4 |
1.13 (0.58-2.21) |
0.720 |
0.0 |
0.603 |
| Nasopharyngitis |
|
IBD |
7 |
1.43 (0.98-2.08) |
0.062 |
31.0 |
0.191 |
| |
|
UC |
4 |
1.28 (0.76-2.16) |
0.350 |
49.2 |
0.116 |
| |
|
CD |
3 |
1.77 (1.01-3.10) |
0.045 |
0.0 |
0.421 |
| |
|
Induction therapy |
4 |
1.49 (0.95-2.34) |
0.084 |
0.0 |
0.600 |
| |
|
Maintenance therapy |
3 |
1.38 (0.64-2.97) |
0.416 |
70.0 |
0.036 |
| Upper respiratory tract |
|
IBD |
7 |
1.30 (0.85-2.00) |
0.223 |
0.0 |
0.643 |
| infection |
|
|
|
|
|
|
|
| |
|
UC |
3 |
1.60 (0.43-5.99) |
0.488 |
38.9 |
0.195 |
| |
|
CD |
4 |
1.30 (0.81-2.09) |
0.281 |
0.0 |
0.793 |
| |
|
Induction therapy |
3 |
1.11 (0.64-1.94) |
0.706 |
0.0 |
0.468 |
| |
|
Maintenance therapy |
4 |
1.63 (0.84-3.16) |
0.149 |
0.0 |
0.569 |
| Arthralgia |
|
IBD |
5 |
1.15 (0.71-1.86) |
0.561 |
0.0 |
0.841 |
| |
|
UC |
3 |
1.46 (0.63-3.39) |
0.373 |
0.0 |
0.630 |
| |
|
CD |
2 |
1.03 (0.57-1.84) |
0.927 |
0.0 |
0.839 |
| |
|
Induction therapy |
3 |
1.16 (0.61-2.19) |
0.652 |
0.0 |
0.968 |
| |
|
Maintenance therapy |
2 |
1.31 (0.45-3.84) |
0.625 |
27.7 |
0.239 |
| Abdominal pain |
|
IBD |
4 |
0.90 (0.61-1.33) |
0.590 |
0.0 |
0.726 |
| |
|
UC |
1 |
0.73 (0.31-1.73) |
0.480 |
NA |
NA |
| |
|
CD |
3 |
0.95 (0.61-1.47) |
0.807 |
0.0 |
0.592 |
| |
|
Induction therapy |
3 |
0.88 (0.55-1.41) |
0.608 |
0.0 |
0.521 |
| |
|
Maintenance therapy |
1 |
0.93 (0.46-1.87) |
0.839 |
NA |
NA |
| Vomiting |
|
IBD |
5 |
0.87 (0.42-1.83) |
0.720 |
31.2 |
0.213 |
| |
|
UC |
3 |
0.89 (0.32-2.48) |
0.821 |
12.8 |
0.318 |
| |
|
CD |
2 |
0.86 (0.21-3.58) |
0.838 |
71.6 |
0.061 |
| |
|
Induction therapy |
3 |
1.03 (0.45-2.37) |
0.948 |
18.7 |
0.292 |
| |
|
Maintenance therapy |
2 |
0.89 (0.13-6.00) |
0.903 |
60.8 |
0.110 |
| Serious adverse events |
|
IBD |
9 |
0.90 (0.67-1.20) |
0.473 |
0.0 |
0.891 |
| |
|
UC |
4 |
1.27 (0.77-2.11) |
0.352 |
0.0 |
0.899 |
| |
|
CD |
5 |
0.76 (0.54-1.08) |
0.130 |
0.0 |
0.985 |
| |
|
Induction therapy |
5 |
0.93 (0.64-1.34) |
0.694 |
0.0 |
0.647 |
| |
|
Maintenance therapy |
4 |
0.86 (0.54-1.36) |
0.513 |
0.0 |
0.788 |
Induction therapy. Induction of clinical remission
A total of 9 studies (9,11-13,15-19) were included to compare the clinical remission of IBD during induction therapy with VDZ vs. placebo. VDZ significantly improved clinical remission during induction therapy when compared with the placebo (RR=2.09; 95% CI=1.66-2.62; P<0.001; I²=0%; P=0.81). This positive association was also seen in the subgroup analysis of both UC (RR=2.33; 95% CI=1.61-3.38; P<0.001; I²=5.2%, P=0.37) and CD (RR=1.95; 95% CI=1.45-2.61; P<0.001; I²=0%; P=0.94; Fig. 2A).
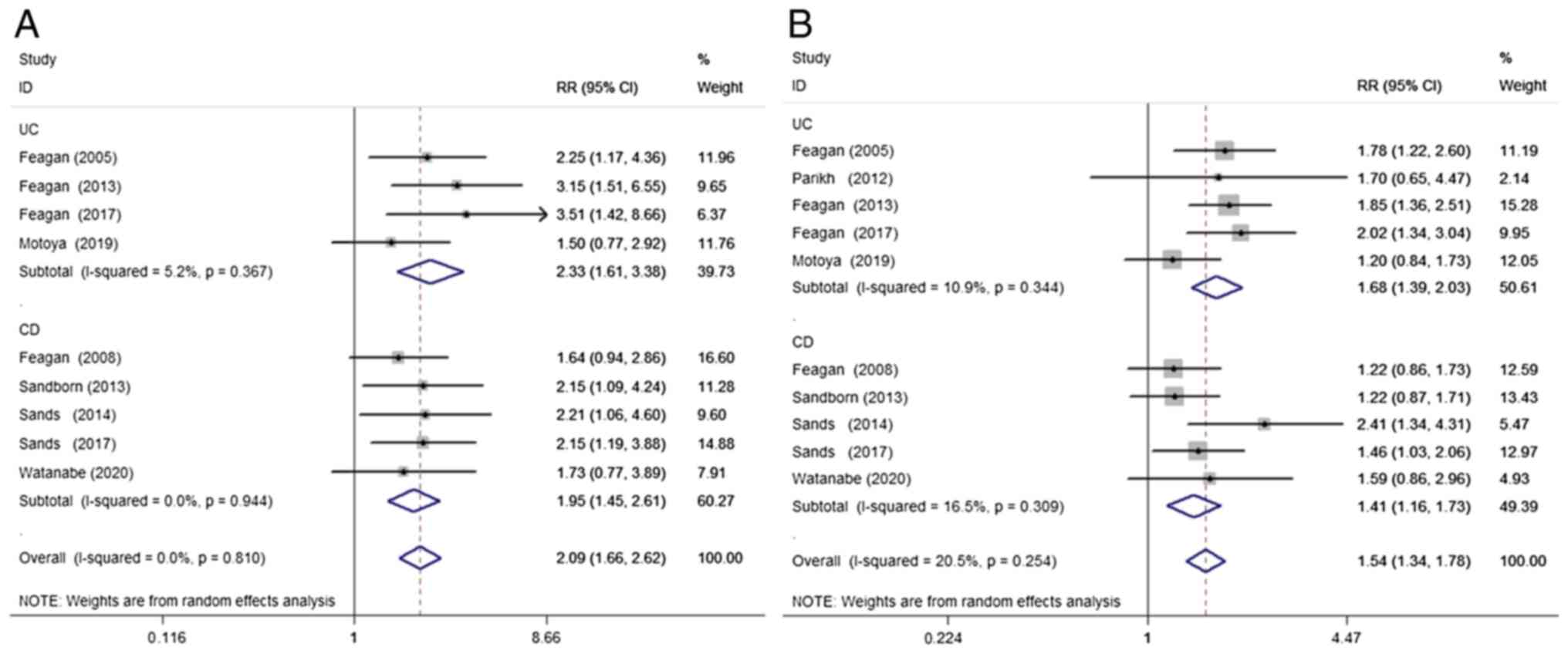 |
Figure 2
Randomized effects meta-analysis of the efficacy of vedolizumab treatment in the induction phase. (A) Clinical remission and (B) clinical response. UC, ulcerative colitis; CD, Crohn's disease; RR, risk ratio; CI, confidence interval.
|
Induction of clinical response
A total of 10 studies (9-13,15-19) were included to compare the clinical response of IBD to VDZ/placebo during induction therapy. Begg's test results showed no publication bias (P=0.363) in the 10 included studies. The funnel plots are presented in Fig. S1. When compared with the placebo, VDZ significantly improved the clinical response of IBD (RR=1.54; 95% CI=1.34-1.78; P<0.001; I²=20.5%; P=0.25), UC (RR=1.68; 95% CI=1.39-2.03; P<0.001; I²=10.9%; P=0.34) and CD (RR=1.41; 95% CI=1.16-1.73; P=0.001; I²=16.5%; P=0.31) during induction therapy (Fig. 2B).
Maintenance therapy. Maintenance of clinical remission
A total of 7 studies (11,13,14,16,18-20) were included to compare the clinical remission of IBD with VDZ/placebo during maintenance therapy. Compared with the placebo, VDZ had significant benefits for the clinical remission of IBD (RR=1.98; 95% CI=1.58-2.49; P<0.001; I²=43.9%; P=0.09) during maintenance therapy. This positive association was also seen in the subgroup analysis of both UC (RR=2.55; 95% CI=1.93-3.35; P<0.001; I²=0%; P=0.48) and CD (RR=1.59; 95% CI=1.32-1.91; P<0.001; I²=0%; P=0.57; Fig. 3A).
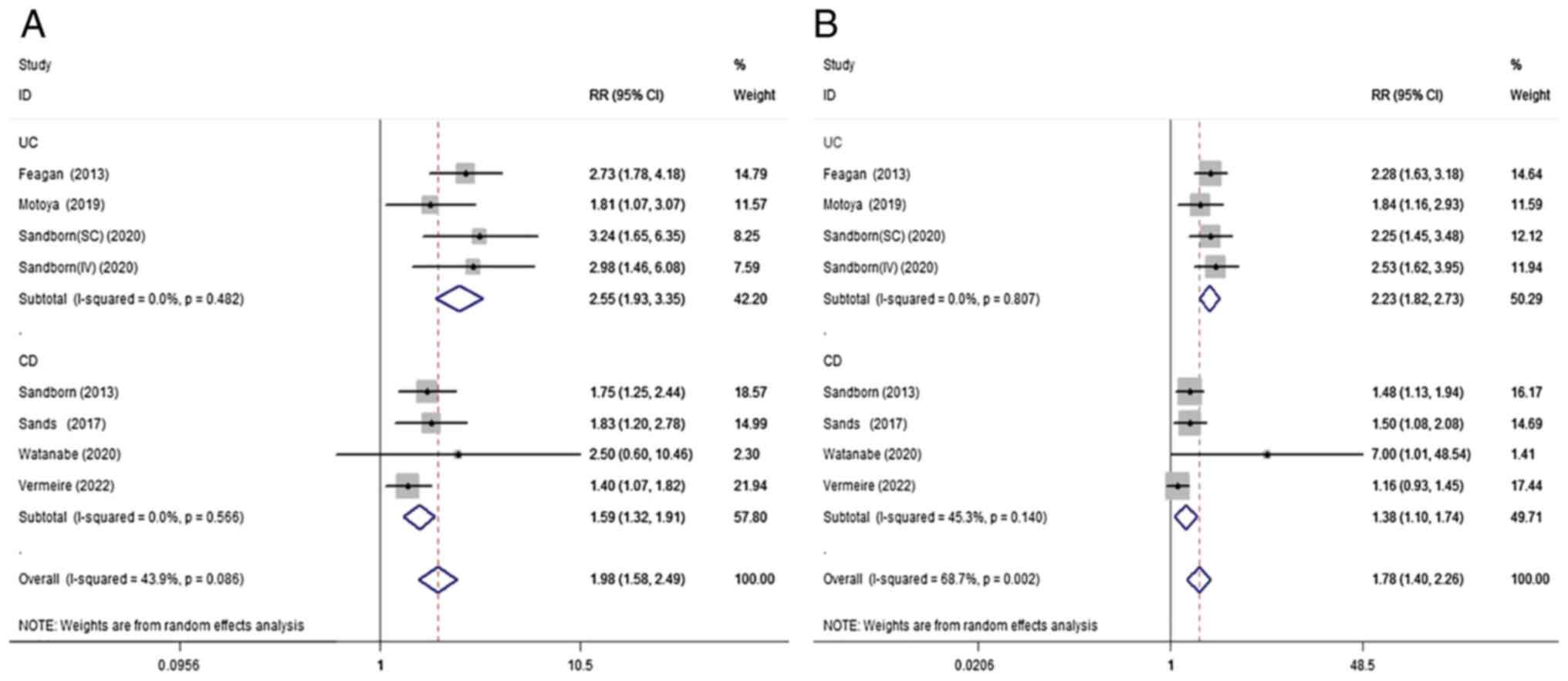 |
Figure 3
Randomized effects meta-analysis of the efficacy of vedolizumab treatment in the maintenance phase. (A) Clinical remission and (B) clinical response. UC, ulcerative colitis; CD, Crohn's disease; RR, risk ratio; CI, confidence interval.
|
Maintenance of clinical response
A total of 7 (11,13,14,16,18-20) studies were included to compare the clinical response of IBD to the VDZ/placebo during maintenance therapy. There was a positive association between VDZ induction treatment and clinical response compared with placebo during induction treatment (RR=1.78; 95% CI=1.40-2.26; P<0.001; I²=68.7%; P=0.002). Subgroup analysis showed that VDZ had similar effects in both UC (RR=2.23; 95% CI=1.82-2.73; P<0.001; I²=0%, P=0.81) and CD (RR=1.38; 95% CI=1.10-1.74; P=0.006; I²=45.3%; P=0.14; Fig. 3B).
Failure of TNF antagonists. Clinical remission of TNF antagonist failure
Three studies (12,17,18) were included to compare VDZ and placebo treatment in clinical remission of IBD patients with a history of TNF antagonist failure. VDZ significantly increased the clinical remission of IBD with TNF antagonist failure when compared with the placebo (RR=2.07; 95% CI=1.48-2.89; P<0.001; I²=10.2%; P=0.35). This positive association was also observed following subgroup analysis of both induction (RR=1.85; 95% CI=1.30-2.65; P=0.001; I²=0%; P=0.60) and maintenance therapy (RR=3.29; 95% CI=1.06-10.15; P=0.039; I²=58.2%; P=0.12; Fig. 4A).
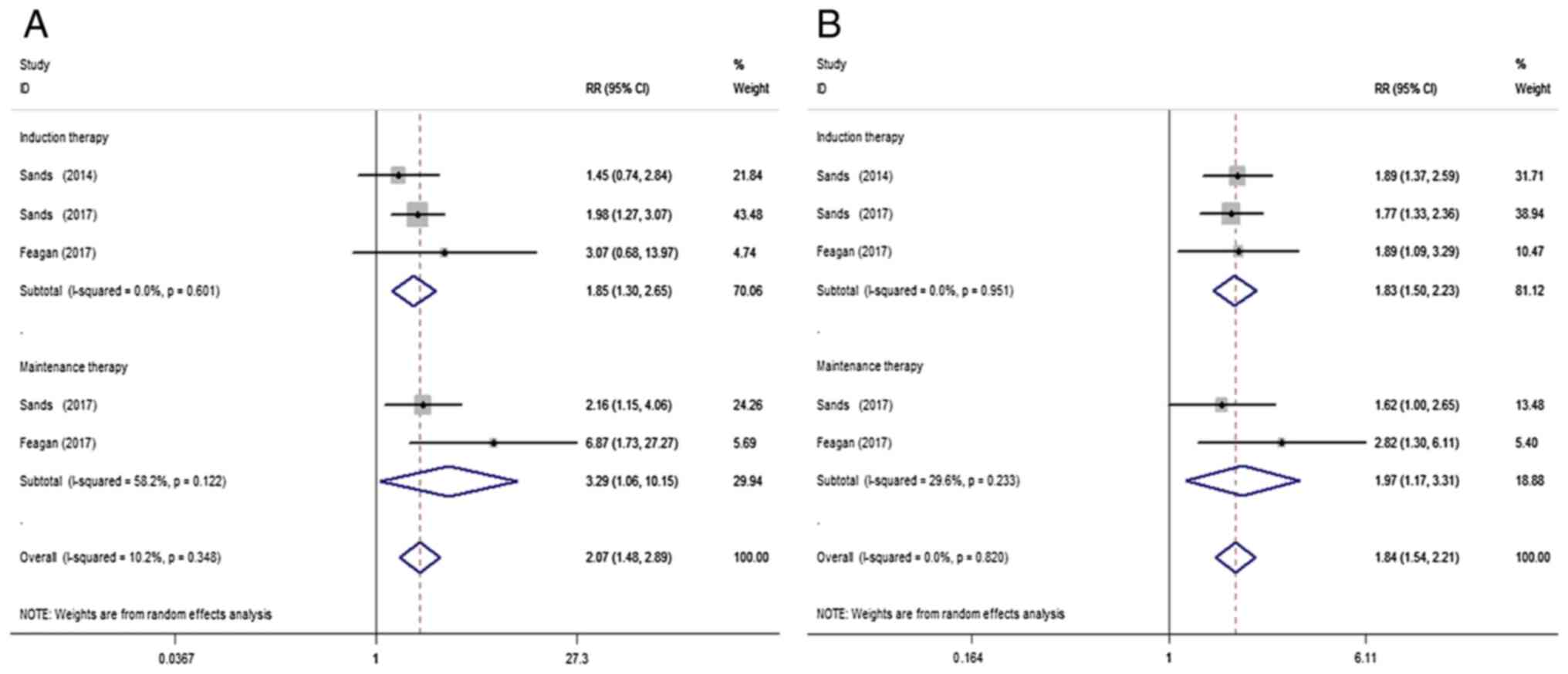 |
Figure 4
Random-effect meta-analysis of VDZ in IBD patients with a history of TNF antagonist failure. (A) Clinical remission and (B) clinical response. VDZ, vedolizumab; IBD, irritable bowel disease; RR, risk ratio; CI, confidence interval.
|
Clinical response of TNF antagonist failure
A total of 3 studies (12,17,18) were included to compare VDZ and placebo in terms of clinical response of IBD in patients with a history of TNF antagonist failure. When compared with the placebo, VDZ significantly increased the clinical response of IBD patients with TNF antagonist failure (RR=1.84; 95% CI=1.54-2.21; P<0.001; I²=0%; P=0.82) during both induction (RR=1.83; 95% CI=1.50-2.23; P<0.001; I²=0%; P=0.95) and maintenance (RR=1.97; 95% CI=1.17-3.31; P=0.010; I²=29.6%; P=0.23) therapy (Fig. 4B).
Corticosteroid-free remission
A total of 7 studies (11,13,14,16,18-20) were included to compare VDZ and placebo in terms of corticosteroid-free remission of IBD. VDZ improved corticosteroid-free remission compared with the placebo (RR=1.98; 95% CI=1.51-2.59; P<0.001; I²=17.4%; P=0.29). This positive association was also observed in the subgroup analysis of both UC (RR=2.79; 95% CI=1.84-4.21; P<0.001; I²=0%; P=0.88) and CD (RR=1.58; 95% CI=1.21-2.07; P=0.001; I²=0%; P=0.46) (Fig. S2).
Mucosal healing
A total of 2 studies (11,13) were included to compare VDZ and placebo in terms of mucosal healing in UC. VDZ significantly improved the mucosal healing of UC compared with the placebo (RR=1.78; 95% CI=1.27-2.51; P=0.001; I²=68.4%; P=0.02). This positive association was also observed in the subgroup analysis of both induction (RR=1.43; 95% CI=1.05-1.95; P=0.022; I²=35.1%; P=0.22) and maintenance (RR=2.35; 95% CI=1.66-3.34; P<0.001; I²=26.5%; P=0.24; Fig. S3) therapy.
IBD exacerbation
A total of 7 studies (9,13-15,17,19,20) were included to compare IBD exacerbation following VDZ and placebo treatment. VDZ significantly reduced the risk of IBD exacerbation compared with the placebo (RR=0.60; 95% CI=0.39-0.93; P=0.023; I²=64.6%; P=0.004). In the subgroup analysis, evidence in favor of the aforementioned association was weaker in induction therapy (RR=0.62; 95% CI=0.30-1.28; P=0.20; I²=75.7%; P=0.001) when compared with maintenance therapy (RR=0.56; 95% CI=0.37-0.87; P=0.009; I²=22.0%; P=0.28). The placebo increased the risk of exacerbation in patients with CD when compared with VDZ (RR=0.51; 95% CI=0.27-0.99; P=0.047; I²=63.9%; P=0.03), while there was no association between exacerbation and VDZ in UC patients (RR=0.69; 95% CI=0.34-1.41; P=0.31; I²=71.6%; P=0.01).
Adverse events
The safety results of VDZ compared with those of the placebo in IBD are shown in Table II.
Serious adverse events. A total of seven studies (9,13-15,17,19,20) were included to compare serious adverse events of VDZ/placebo treatment for IBD. No significant differences in serious adverse events were observed between the VDZ and placebo treatment groups (RR=0.90; 95% CI=0.67-1.20; P=0.473; I²=0%; P=0.89). There were no significant differences in the subgroup analysis.
Headache. A total of 7 studies (9,13-15,17,19,20) were included to compare the occurrence of headache caused by VDZ and placebo treatment for IBD; no significant differences were observed between the two treatments (RR=1.09; 95% CI=0.81-1.47; P=0.567; I²=0%; P=0.56).
Nasopharyngitis. A total of 6 studies (9,13-15,17,20) were included to compare the correlation between nasopharyngitis and VDZ/placebo treatment. No significant differences were observed between VDZ and placebo (RR=1.43; 95% CI=0.98-2.08; P=0.062; I²=31.0%; P=0.19). In the subgroup analysis, VDZ increased the risk of nasopharyngitis in patients with CD when compared with the placebo (RR=1.77; 95% CI=1.01-3.10; P=0.045; I²=0%; P=0.42), while there was no association between nasopharyngitis and VDZ in UC patients (RR=1.28; 95% CI=0.76-2.16; P=0.350; I²=49.2%; P=0.12). There was also no association between nasopharyngitis and VDZ/placebo treatment in either induction or maintenance therapy.
Upper respiratory tract infection. A total of 5 studies (13,14,17,19,20) were included to compare the association between VDZ/placebo and upper respiratory tract infection in IBD. No significant differences were observed between the VDZ and placebo treatment groups (RR=1.30; 95% CI=0.85-2.00; P=0.223; I²=0%; P=0.64). No significant differences were observed in the subgroup analysis either.
Arthralgia. A total of 5 studies (9,13,14,17,20) were included to compare the association between VDZ/placebo treatment and arthralgia in IBD; no significant differences were observed between the VDZ and placebo treatment groups (RR=1.15; 95% CI=0.71-1.86; P=0.561; I²=0%; P=0.84).
Abdominal pain. A total of 4 studies (9,15,17,20) were included to compare the association between VDZ/placebo treatment and abdominal pain in IBD. No significant differences were observed between the VDZ and placebo treatment groups (RR=0.90; 95% CI=0.61-1.33; P=0.590; I²=0%; P=0.73). No significant differences were observed following subgroup analysis either.
Vomiting. A total of 4 studies (9,13,17,20) were included to compare the association between VDZ/placebo and vomiting in IBD and no significant differences were identified (RR=0.87; 95% CI=0.42-1.83; P=0.720; I²=31.2%; P=0.21).
Sensitivity analysis
Sensitivity analysis was conducted to evaluate the influence of a single study on the overall risk estimate by omitting one study at a time (Table SII). In the analysis of clinical response during the maintenance phase, the heterogeneity decreased from 68.7 to 41.1% when omitting the study by Vermeire et al (21). In the analysis of IBD exacerbation, the risk ratio showed little change, which means that the result was stable. In the analysis of UC exacerbation, the heterogeneity was markedly decreased from 71.6 to 10.8% when omitting the study by Feagan et al (10). In the analysis of CD exacerbation, the heterogeneity decreased from 63.9 to 46.6% when omitting the study by Watanabe et al (20) (induction therapy). In the analysis of IBD exacerbation (induction therapy), the results were stable. In the analysis of nasopharyngitis (maintenance), the study by Sandborn et al (15) contributed to most of the heterogeneity. In the analysis of mucosal healing (UC), the heterogeneity decreased from 68.4 to 20.3% when omitting the study by Feagan et al (12) (maintenance therapy).
Discussion
In the present meta-analysis, 12 high-quality published RCTs assessing IBD patients were identified by searching several English databases to review relevant articles. The results showed that VDZ was superior to placebo for the treatment of IBD during both induction and maintenance therapy, especially for patients with TNF antagonist failure. VDZ also showed significant efficacy in IBD patients with regard to corticosteroid-free remission compared with the placebo. In terms of mucosal healing, VDZ had significant effects on UC patients during both induction and maintenance therapy. It was also found that VDZ significantly reduced the risk of IBD exacerbation when compared with the placebo groups. In subgroup analysis, evidence in favor of the association was weaker among induction therapy when compared with maintenance therapy. Placebo increased the risk of exacerbation in patients treated with CD compared with those treated with VDZ. These results fully demonstrate the effectiveness of VDZ in the induction and maintenance of IBD treatment.
Adverse reactions are very common in the treatment of biological agents. The most common adverse reactions in the VDZ treatment group were headache, nasopharyngitis, upper respiratory tract infection, and arthralgia. VDZ increased the risk of nasopharyngitis in patients with CD when compared with the placebo, but these symptoms quickly improved with symptomatic treatment. Concerning the incidence of serious adverse events and the other assessed adverse reactions, there were no significant differences between VDZ and placebo. Therefore, VDZ was shown to be a relatively safe biological agent in the treatment of IBD.
IBD is a chronic inflammatory disease of the gastrointestinal tract, which consists of two major subtypes, CD and UC (22). This disease is characterized by alternating periods of clinical relapse and remission (23). The etiology and pathogenesis of IBD remain unclear. Increasing evidence shows that persistent intestinal infections, mucosal barrier defects, mucosal immune dysregulation, and genetic and environmental factors are involved in the process of the disease (24,25). Among these, the genetic dysfunction of the mucosal immune system of susceptible hosts to intestinal microbiota plays an important role in the pathogenesis of IBD (26). The primary clinical manifestations of IBD are abdominal pain, diarrhea, stool with mucous, bloody stool, weight loss, perianal abscess, and anal fistula (27). In the 20th century, IBD was predominantly prevalent in Western countries such as North America, Europe, and Oceania. However, IBD began to emerge as a global disease at the beginning of the 21st century, accelerating in incidence in newly industrialized countries such as Asia, South America, and Africa (28).
Anti-TNF-α is the first-line treatment for the management of moderate-to-severe IBD at present (29). TNF-α is a type of cytokine that can trigger and amplify the intestinal inflammatory process and prevent inflammatory response by binding to proteins or cells, playing an important role in the inflammatory cascade and decreasing inflammation by inducing apoptosis (30). Relieving clinical symptoms of IBD patients is helpful for better controlling the disease, reducing disease complications, and improving the quality of life of patients with IBD (31). However, a large proportion of patients do not respond to TNF therapy; termed primary non-responders (32). In the present meta-analysis, 30-50% of patients initially responded to treatment but subsequently lost their response which coincided with the onset of symptoms. For these patients, a higher drug dose, use of alternative drugs, or surgical intervention was needed (33). In the past few decades, a variety of biological agents have been developed for the treatment of IBD. Due to the different treatment periods and adverse reactions associated with these drugs, the optimal treatment for IBD remains contested. Circulating leukocyte migration to the gastrointestinal tract is hypothesized to play a role in the pathogenesis of IBD. Integrins are expressed on immune cells and may interact with cell adhesion molecules (CAM) to block leukocyte transportation to the gastrointestinal tract. Therefore, this specific therapy provides an alternative treatment for the systemic immunosuppression of IBD (34). α4β1 Integrin, which plays a role in memory and effector T lymphocytes homing to the brain and inflamed intestinal tissue (35), has also been shown to be potentially effective in the management of moderate-to-severe Crohn's disease. However, subsequent clinical studies found a significantly increased risk of progressive multifocal leukoencephalopathy (PML) following the use of α4β1 integrin in patients previously exposed to the John Cunningham virus (34,36). VDZ is a humanized IgG1 monoclonal antibody that targets the α4β7 heterodimer. This antibody selectively inhibits the adhesion of α4β7-expressing cells to mucosal addressin cell adhesion molecule-1 and fibronectin, thereby preventing leukocyte adhesion to the intestinal endothelium without affecting α4β1. VDZ does not inhibit vascular CAM-1 (VCAM-1) and therefore does not lead to PML (37). VDZ is also recommended by 2021 IBD clinical guidelines for patients with UC that have not been treated with biological therapy and for UC or CD patients with failure of conventional or anti-TNF-α therapy (38).
The present study had some limitations. First, although all included studies were assessed as having a low or moderate risk of bias, there was a slight heterogeneity among individual outcome indicators, which may have biased the results to a certain extent. Secondly, due to the limitation of existing studies, further subgroup analysis was not performed. For example, all included RCTs were published abroad. Although the research data from Asia (Japan) were updated and included, there was no domestic data. Furthermore, there were no RCTs evaluating the mucosal healing of UC patients following VDZ treatment. With regard to different types of VDZ administration, there were only 2 RCTs evaluating the efficacy and safety of the subcutaneous injection of VDZ in IBD patients. Dose-response analysis of VDZ treatment was also limited since a few studies reported the comparison among different dosages. In addition, research on the efficacy and safety of VDZ in the treatment of pediatric IBD remains incomplete. All included studies were conducted in adults, even though the incidence of pediatric IBD in industrialized or Western countries is increasing annually (39,40). Thirdly, the limitation of possible publication bias should be taken into consideration; it is easier to report studies with positive results, even though no publication bias was observed by the Begg's test.
The present meta-analysis is an updated and expanded version of a previous meta-analysis (38). The first meta-analysis conducted by Wang et al (41) showed that VDZ was more effective than the placebo as induction and maintenance therapies for IBD. However, VDZ was found to be associated with a higher rate of serious adverse events (21.7 vs. 14.3%) in patients with CD. Furthermore, that study also included only 6 RCTs to evaluate the efficacy and safety of VDZ in IBD, whereas the present study included 12 RCTs with 4,865 patients, which may yield more convincing results. In addition, the previous study did not further evaluate corticosteroid-free remission, mucosal healing, and VDZ efficacy in IBD patients with TNF antagonist failure. Schreiber et al (42) reached similar conclusions to those of the present study, but none of the included studies met the randomized controlled double-blind criteria and instead included data from peer-reviewed full-text manuscripts and abstracts. Mosli et al (43) only included 4 RCTs, thus subgroup analysis was not performed to further evaluate the efficacy of VDZ in UC patients, and the adverse reactions of VDZ were not specifically evaluated.
This meta-analysis study also had several strengths. First, the heterogeneity between studies was low in most of the analyses. Secondly, the largest RCT studies so far were included, containing new data from Asian countries and other countries, which may provide a more comprehensive analysis of the efficacy of VDZ in the treatment of IBD patients. Thirdly, sensitivity analysis was performed to investigate the stability of the results. Finally, most of the present meta-analyses did not analyze the common adverse reactions of IBD patients treated with VDZ in specific subgroups. The data on common adverse effects were summarized, and subgroup analysis showed that placebo increased the risk of exacerbation in patients with CD when compared with VDZ.
Although the present study clarified the effective role of VDZ in the treatment of IBD patients, several questions still need to be addressed in future studies. Large domestic RCTs with long-term follow-ups are required to further compare different types of administration and different dosages of VDZ in both adult and pediatric patients.
In conclusion, this meta-analysis comprehensively evaluated the efficacy and safety of VDZ in IBD patients. It was found that VDZ is a safe and effective biological agent for IBD, particularly for patients with TNF antagonist failure.
Supplementary Material
Funnel plot of publication bias of clinical response to VDZ induction therapy in IBD. VDZ, vedolizumab; IBD, irritable bowel disease; RR, risk ratio.
Random-effects meta-analysis of studies that VDZ treatment of IBD corticosteroid-free remission. VDZ, vedolizumab; IBD, irritable bowel disease; RR, risk ratio; CI, confidence interval.
Random-effects meta-analysis of studies that VDZ treatment of IBD mucosal healing. VDZ, vedolizumab; IBD, irritable bowel disease; RR, risk ratio; CI, confidence interval.
Bias risk of included RCTs assessed by Cochrane Collaboration’s tool.
Bias risk of included RCTs assessed by Cochrane Collaboration’s tool.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Authors' contributions
HT, LG, and CB designed the study. ST performed the literature search and screened the articles for relevancy. LG, LZ and HT abstracted the data. LZ and CB assessed the methodological quality of the studies. LG and HT performed the statistical analysis and drafted the manuscript. ST was responsible for revising the manuscript. HT, CB and LG confirm the authenticity of the data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
1
|
Seyedian SS, Nokhostin F and Malamir MD: A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J Med Life. 12:113–122. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lamb CA, Kennedy NA, Raine T, Hendy PA, Smith PJ, Limdi JK, Hayee B, Lomer MCE, Parkes GC, Selinger C, et al: British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 68 (Suppl 3):s1–s106. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Jeong DY, Kim S, Son MJ, Son CY, Kim JY, Kronbichler A, Lee KH and Shin JI: Induction and maintenance treatment of inflammatory bowel disease: A comprehensive review. Autoimmun Rev. 18:439–454. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Singh S, Fumery M, Sandborn WJ and Murad MH: Systematic review with network meta-analysis: First-and second-line pharmacotherapy for moderate-severe ulcerative colitis. Aliment Pharmacol Ther. 47:162–175. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wyant T, Fedyk E and Abhyankar B: An overview of the mechanism of action of the monoclonal antibody vedolizumab. J Crohns Colitis. 10:1437–1444. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al: The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 372(n71)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, et al: The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 343(d5928)2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chen B and Benedetti A: Quantifying heterogeneity in individual participant data meta-analysis with binary outcomes. Syst Rev. 6(243)2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Duval S and Tweedie R: Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis,'. Biometrics. 56:455–463. 2000.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Feagan BG, Greenberg GR, Wild G, Fedorak RN, Paré P, McDonald JW, Dubé R, Cohen A, Steinhart AH, Landau S, et al: Treatment of ulcerative colitis with a humanized antibody to the alpha4beta7 integrin. N Engl J Med. 352:2499–2507. 2005.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Parikh A, Leach T, Wyant T, Scholz C, Sankoh S, Mould DR, Ponich T, Fox I and Feagan BG: Vedolizumab for the treatment of active ulcerative colitis: A randomized controlled phase 2 dose-ranging study. Inflamm Bowel Dis. 18:1470–1479. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Feagan BG, Rutgeerts P, Sands BE, Hanauer S, Colombel JF, Sandborn WJ, Van Assche G, Axler J, Kim HJ, Danese S, et al: Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 369:699–710. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Feagan BG, Rubin DT, Danese S, Vermeire S, Abhyankar B, Sankoh S, James A and Smyth M: Efficacy of Vedolizumab induction and maintenance therapy in patients with ulcerative colitis, regardless of prior exposure to tumor necrosis factor antagonists. Clin Gastroenterol Hepatol. 15:229–239.e5. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Motoya S, Watanabe K, Ogata H, Kanai T, Matsui T, Suzuki Y, Shikamura M, Sugiura K, Oda K, Hori T, et al: Vedolizumab in Japanese patients with ulcerative colitis: A Phase 3, randomized, double-blind, placebo-controlled study. PLoS One. 14(e0212989)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sandborn WJ, Baert F, Danese S, Krznarić Ž, Kobayashi T, Yao X, Chen J, Rosario M, Bhatia S, Kisfalvi K, et al: Efficacy and safety of vedolizumab subcutaneous formulation in a randomized trial of patients with ulcerative colitis. Gastroenterology. 158:562–572.e12. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Feagan BG, Greenberg GR, Wild G, Fedorak RN, Paré P, McDonald JW, Cohen A, Bitton A, Baker J, Dubé R, et al: Treatment of active Crohn's disease with MLN0002, a humanized antibody to the alpha4beta7 integrin. Clin Gastroenterol Hepatol. 6:1370–1377. 2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sandborn WJ, Feagan BG, Rutgeerts P, Hanauer S, Colombel JF, Sands BE, Lukas M, Fedorak RN, Lee S, Bressler B, et al: Vedolizumab as induction and maintenance therapy for Crohn's disease. N Engl J Med. 369:711–721. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sands BE, Feagan BG, Rutgeerts P, Colombel JF, Sandborn WJ, Sy R, D'Haens G, Ben-Horin S, Xu J, Rosario M, et al: Effects of vedolizumab induction therapy for patients with Crohn's disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology. 147:618–627.e3. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sands BE, Sandborn WJ, Van Assche G, Lukas M, Xu J, James A, Abhyankar B and Lasch K: Vedolizumab as induction and maintenance therapy for Crohn's disease in patients naïve to or who have failed tumor necrosis factor antagonist therapy. Inflamm Bowel Dis. 23:97–106. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Watanabe K, Motoya S, Ogata H, Kanai T, Matsui T, Suzuki Y, Shikamura M, Sugiura K, Oda K, Hori T, et al: Effects of vedolizumab in Japanese patients with Crohn's disease: A prospective, multicenter, randomized, placebo-controlled Phase 3 trial with exploratory analyses. J Gastroenterol. 55:291–306. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Vermeire S, D'Haens G, Baert F, Danese S, Kobayashi T, Loftus EV, Bhatia S, Agboton C, Rosario M, Chen C, et al: Efficacy and safety of subcutaneous vedolizumab in patients with moderately to severely active Crohn's disease: Results from the VISIBLE 2 Randomised trial. J Crohns Colitis. 16:27–38. 2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Szigethy E, McLafferty L and Goyal A: Inflammatory bowel disease. Child Adolesc Psychiatr Clin N Am. 19:301–318. ix. 2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wallace KL, Zheng LB, Kanazawa Y and Shih DQ: Immunopathology of inflammatory bowel disease. World J Gastroenterol. 20:6–21. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Manichanh C, Borruel N, Casellas F and Guarner F: The gut microbiota in IBD. Nat Rev Gastroenterol Hepatol. 9:599–608. 2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Latella G and Papi C: Crucial steps in the natural history of inflammatory bowel disease. World J Gastroenterol. 18:3790–3799. 2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Xu XR, Liu CQ, Feng BS and Liu ZJ: Dysregulation of mucosal immune response in pathogenesis of inflammatory bowel disease. World J Gastroenterol. 20:3255–3264. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Rosen MJ, Dhawan A and Saeed SA: Inflammatory bowel disease in children and adolescents. JAMA Pediatr. 169:1053–1060. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, Panaccione R, Ghosh S, Wu JCY, Chan FKL, et al: Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet. 390:2769–2778. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG and Long MD: ACG clinical guideline: Ulcerative colitis in adults. Am J Gastroenterol. 114:384–413. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hering NA and Schulzke JD: Therapeutic options to modulate barrier defects in inflammatory bowel disease. Dig Dis. 27:450–454. 2009.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Strik AS, Bots SJ, D'Haens G and Löwenberg M: Optimization of anti-TNF therapy in patients with inflammatory bowel disease. Expert Rev Clin Pharmacol. 9:429–439. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ben-Horin S and Chowers Y: Tailoring anti-TNF therapy in IBD: Drug levels and disease activity. Nat Rev Gastroenterol Hepatol. 11:243–255. 2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ben-Horin S and Chowers Y: Review article: Loss of response to anti-TNF treatments in Crohn's disease. Aliment Pharmacol Ther. 33:987–995. 2011.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Gubatan J, Keyashian K, Rubin SJS, Wang J, Buckman CA and Sinha S: Anti-Integrins for the treatment of inflammatory bowel disease: Current evidence and perspectives. Clin Exp Gastroenterol. 14:333–342. 2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Hemler ME, Huang C, Takada Y, Schwarz L, Strominger JL and Clabby ML: Characterization of the cell surface heterodimer VLA-4 and related peptides. J Biol Chem. 262:11478–11485. 1987.PubMed/NCBI
|
|
36
|
Lanzarotto F, Carpani M, Chaudhary R and Ghosh S: Novel treatment options for inflammatory bowel disease: Targeting alpha 4 integrin. Drugs. 66:1179–1189. 2006.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Soler D, Chapman T, Yang LL, Wyant T, Egan R and Fedyk ER: The binding specificity and selective antagonism of vedolizumab, an anti-alpha4beta7 integrin therapeutic antibody in development for inflammatory bowel diseases. J Pharmacol Exp Ther. 330:864–875. 2009.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Amiot A, Bouguen G, Bonnaud G, Bouhnik Y, Hagege H and Peyrin-Biroulet L: French National Consensus Clinical guidelines for the management of IBD study group. Clinical guidelines for the management of inflammatory bowel disease: Update of a French national consensus. Dig Liver Dis. 53:35–43. 2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW and Kaplan GG: Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 142:46–54 e42. 2012.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Ye Y, Manne S, Treem WR and Bennett D: Prevalence of inflammatory bowel disease in pediatric and adult populations: Recent estimates from large national databases in the United States,2007-2016. Inflamm Bowel Dis. 26:619–625. 2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Wang MC, Zhang LY, Han W, Shao Y, Chen M, Ni R, Wang GN, Wei FX, Zhang YW, Xu XD and Zhang YC: PRISMA-efficacy and safety of vedolizumab for inflammatory bowel diseases: A systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 93(e326)2014.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Schreiber S, Dignass A, Peyrin-Biroulet L, Hather G, Demuth D, Mosli M, Curtis R, Khalid JM and Loftus EV Jr: Systematic review with meta-analysis: Real-world effectiveness and safety of vedolizumab in patients with inflammatory bowel disease. J Gastroenterol. 53:1048–1064. 2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Mosli MH, MacDonald JK, Bickston SJ, Behm BW, Tsoulis DJ, Cheng J, Khanna R and Feagan BG: Vedolizumab for induction and maintenance of remission in ulcerative colitis: A Cochrane systematic review and meta-analysis. Inflamm Bowel Dis. 21:1151–1159. 2015.PubMed/NCBI View Article : Google Scholar
|


















