Introduction
Spinal cord injury (SCI) is mainly induced by
mechanical injuries, such as falls and traffic accidents, and is a
public health issue with a global prevalence of 280-1,298 per
1,000,000 individuals (1,2). The pathophysiology of SCI is
traditionally separated into two phases: Primary injury (the injury
caused by the initial traumatic event) and secondary injury
(delayed and progressive tissue injury, including the infiltration
of inflammatory cells) (3,4). Regarding the primary injury,
treatment mainly focuses on early acute care; for secondary injury,
therapeutic approaches include traditional drug therapy, surgery,
cell therapy, gene therapy and tissue engineering (5-7).
However, considering the limited efficacy and unsatisfactory
prognosis of the present treatment approaches, it would be valuable
to further investigate the pathogenesis of SCI and develop more
effective therapeutic targets.
The fibroblast growth factor (FGF) family comprises
18 secreted proteins that modulate neural development, metabolism
and function via multiple signalling pathways (8,9).
FGF23 is mainly secreted by the osteoblastic lineage and detected
in the cerebrospinal fluid, it also exhibits a regulatory effect on
neuronal properties (10-12).
For example, previous studies have revealed that FGF23 has a
fundamental role in mediating neuronal morphology, synaptic
density, presympathetic neuronal activity and inflammatory cytokine
secretion through several mechanisms, such as FGF receptor-Klotho
complexes and the NF-κB signalling pathway (11-14);
however, the role of FGF23 in SCI remains unclear. The current
study aimed to investigate the effects of FGF23 on neural
apoptosis, inflammation and locomotion recovery, as well as its
underlying mechanism in SCI.
Materials and methods
Animals
Male Sprague Dawley (SD) rats (n=60; weight, 200±20
g; age, 4-6 weeks) were purchased from Jiangsu Laboratory Animal
Centre and housed under standard conditions (12-h dark/light cycle;
temperature, 23±2˚C; ad libitum access to food and water;
relative humidity, 40-60%). All procedures were approved by the
Animal Care and Use Committee of Xiamen University (approval no.
20210401; Xiamen, China). Morbidity was used as the humane endpoint
in the present study. These endpoints were applied following the
criteria delineated in the ‘Guidelines for Endpoints in Animal
Study Proposals’ at the Zhongshan Hospital Affiliated to Xiamen
University (15). The humane
endpoints included: i) The animal exhibited symptoms including, but
not limited to, a lack of responsiveness to manual stimulation,
and/or immobility and an inability to eat or drink; ii) laboured
breathing and cyanosis; iii) diarrhoea or urinary incontinence; iv)
severe, rapid weight loss and emaciation (maximum 20% of body
weight from baseline); v) impaired mobility; vi) other situations
where a veterinarian had determined that euthanasia was
necessary.
Primary neuron culture
Primary neurons were cultured as previously
described (16). Briefly, a
pregnant SD rat was anaesthetized by intraperitoneal injection of
50 mg/kg pentobarbital sodium and rapidly sacrificed using
CO2 inhalation (50% chamber volume/min). Subsequently,
the cerebral cortex of the six embryos (embryonic day 16) was cut
into ~1-mm pieces and isolated using trypsin (Sangon Biotech Co.,
Ltd.) for 20 min at 37˚C. After being centrifuged (300 x g; 5 min;
room temperature), cells were resuspended and seeded into
poly-D-lysin-coated plates (Corning, Inc.). Cells were then
cultured in Neurobasal-A medium (Gibco; Thermo Fisher Scientific,
Inc.) supplemented with B-27™ supplement (Gibco; Thermo
Fisher Scientific, Inc.) and 100 U/ml penicillin/streptomycin
(Sangon Biotech Co., Ltd.) at 37˚C with 5% CO2. The
medium was replaced every 2 days and primary neurons were harvested
after 10 days of culture.
H2O2
stimulation
The primary neurons were plated into a 6-well plate
(5x105 cells/well) and were divided into two groups: The
H2O2 group, in which primary neurons were
treated with 100 µM H2O2 (Beijing Solarbio
Science & Technology Co., Ltd.) (17,18);
the normal group, in which the primary neurons were cultured
without H2O2 stimulation. After 24 h of
stimulation at 37˚C, cells were harvested for reverse
transcription-quantitative PCR (RT-qPCR), western blotting and
apoptosis analysis.
FGF23 regulation
The FGF23 overexpression adenovirus-associated virus
(AAV; oeFGF23), short hairpin (sh)RNA AAV (shFGF23) and negative
control (NCs) AAVs (oeNC and shNC) were purchased from Shanghai
GenePharma Co., Ltd. Briefly, primary neurons were seeded into
6-well plates (5x105 cells/well) and infected
(multiplicity of infection, 50) with overexpression NC AAV (oeNC),
oeFGF23, shRNA NC AAV (shNC) or shFGF23 at 37˚C for 24 h. The sense
sequences for shNC and shFGF23 were:
5'-GTTCTCCGAACGTGTCACGTTTCAAGAGAACGTGACACGTTCGGAGAAC-3' and
5'-GGAACAGCTATCACCTACATTCAAGAGATGTAGGTGATAGCTGTTCC-3',
respectively. Uninfected primary neurons were used as control
cells. After being cultured for 48 h at 37˚C, cells in all groups
were stimulated with 100 µM H2O2 for 24 h at
37˚C and harvested for RT-qPCR, western blotting and apoptosis
analysis.
LY294002 treatment
oeNC- or oeFGF23-infected cells were seeded into
6-well plates (5x105 cells/well) and divided into the
following groups: OeNC, cells infected with oeNC; oeFGF23, cells
infected with oeFGF23; LY294002, cells infected with oeNC and
incubated with 10 µM LY294002 (PI3K inhibitor; MedChemExpress) at
37˚C for 12 h (19); and oeFGF23 +
LY294002, cells infected with oeFGF23 and incubated with 10 µM
LY294002 at 37˚C for 12 h. Primary neurons without infection or
inhibitor treatment were used as the control group. The primary
neurons in all groups were then stimulated with 100 µM
H2O2 for 24 h at 37˚C. Finally, the cells
were harvested for RT-qPCR, western blotting and apoptosis
analysis.
RT-qPCR
RT-qPCR was performed to assess FGF23 mRNA
expression levels in primary neurons. Briefly, total RNA was
extracted from 1x106 primary neurons using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). Subsequently, RNA was reverse transcribed into cDNA using a
PrimeScript™ RT reagent kit (Takara Bio, Inc.) according
to manufacturer's protocols. qPCR amplification was performed using
a TB Green® Fast qPCR mix kit (Takara Bio, Inc.), and
relative mRNA expression levels of FGF23 were calculated using the
2-ΔΔCq method (20).
The primer sequences were as follows: FGF23 forward,
5'-TGGCCATGTAGACGGAACAC-3' and reverse,
5'-GGCCCCTATTATCACTACGGAG-3'; and GAPDH forward,
5'-CAAGTTCAACGGCACAGTCAAG-3' and reverse,
5'-ACATACTCAGCACCAGCATCAC-3'. The thermocycling conditions were as
follows: 95˚C for 30 sec (one cycle), followed by 40 cycles at 95˚C
for 5 sec and 61˚C for 20 sec.
Apoptosis assay
The apoptotic rate of primary neurons was detected
using an Annexin V-FITC/PI Cell Apoptosis Detection Kit (Beyotime
Institute of Biotechnology). Briefly, infected or treated cells
were washed twice with precooled PBS and adjusted to
2x106 cells/ml. Subsequently, 5 µl Annexin V-FITC and 5
µl PI were added to the cell suspension for 10 min at room
temperature. Flow cytometric analysis was carried out using the
FACSCanto II flow cytometer (BD Biosciences). The data were
analysed using FlowJo 7.6.1 (BD Biosciences).
SCI model and treatment
The SCI rat model was constructed as previously
described (21). The SD rats were
anaesthetized by intraperitoneal injection of 50 mg/kg
pentobarbital sodium and a laminectomy was performed at the T9
vertebral section to expose the spinal cord. Subsequently, the
spinal cord was contused with a force of 200 kDyne using the
Infinite Horizon Impactor (Precision Systems &
Instrumentation). Injection of AAV or PBS at the site of injury was
performed after the surgery. The rats were divided into five
groups: Sham group (n=12), which received only laminectomy without
contusion and 10 µl PBS injection; SCI group (n=12), which received
SCI surgery and 10 µl PBS injection; SCI + oeNC group (n=12), which
received SCI surgery and 10 µl oeNC injection; SCI + oeFGF23 group
(n=12), which received SCI surgery and 10 µl oeFGF23 injection; and
SCI + oeFGF23 + LY294002 group (n=12), which received SCI surgery,
10 µl oeFGF23 injection and LY294002 injection (0.3 mg/kg/day;
intravenous) (22). At 7 days
post-operation, rats were anaesthetized by intraperitoneal
injection of 50 mg/kg pentobarbital sodium and rapidly sacrificed
using CO2 asphyxiation (50% chamber volume/min) and
spinal cord lesion tissues (n=6/group) were harvested for western
blotting. In the remaining rats, Basso-Beattie-Bresnahan (BBB)
scoring (23) was performed to
assess the locomotion recovery at 1, 3, 7, 10, 14, 21 and 28 days
after surgery (n=6/group). At 28 days post-surgery, the rats were
anaesthetized by intraperitoneal injection of 50 mg/kg
pentobarbital sodium and rapidly sacrificed using CO2
asphyxiation (50% chamber volume/min), and spinal cord lesion
tissues were collected for H&E and TUNEL staining, and ELISA.
The death of the rats was confirmed following the AVMA Guidelines
for the Euthanasia of Animals: 2020 Edition, including lack of
pulse, breathing, corneal reflex and response to firm toe pinch;
inability to hear respiratory sounds and heartbeat by use of a
stethoscope; greying of the mucous membranes; and rigor mortis
(24).
H&E and TUNEL staining
The spinal cord lesion tissues were fixed with 4%
paraformaldehyde (Beyotime Institute of Biotechnology) for 24 h at
room temperature, embedded in paraffin and cut into sections (4
µm). H&E staining was accomplished using the H&E staining
kit (Wuhan Servicebio Technology Co., Ltd.) according to the
manufacturer's instructions. The images were captured using a light
microscope (Olympus Corporation). TUNEL staining was performed
using a One Step TUNEL Apoptosis Assay Kit (Beyotime Institute of
Biotechnology) according to the manufacturer's protocol. The images
were observed using an inverted fluorescence microscope (Olympus
Corporation).
ELISA
The spinal cord lesion tissues were lysed in RIPA
buffer (Beyotime Institute of Biotechnology) and the supernatant
was collected by centrifugation (10,000 x g; 15 min; 4˚C).
Subsequently, the supernatant was quantified using a BCA kit
(Beijing Solarbio Science & Technology Co., Ltd.). Rat TNF-α
(cat. no. D731168), IL-1β (cat. no. D731007) and IL-6 (cat. no.
D731010) ELISA kits (Sangon Biotech Co., Ltd.) were then used
according to the manufacturer's instructions. The absorbance at 450
nm was assessed using a microplate reader (BioTek Instruments,
Inc.).
Western blotting
The primary neurons or spinal cord lesion tissues
were lysed using RIPA buffer. After quantification using a BCA kit,
a total of 50 µg protein/lane was separated by SDS-PAGE on precast
10% gels (Willget) and transferred onto nitrocellulose membranes
(Wuhan Servicebio Technology Co., Ltd.). The membranes were then
blocked with 5% BSA (Wuhan Servicebio Technology Co., Ltd.) at 37˚C
for 1.5 h and incubated overnight at 4˚C with the following primary
antibodies: Anti-FGF23 (cat. no. DF3596; 1:500; Affinity
Biosciences), anti-cleaved (C)-caspase3 (cat. no. AF7022; 1:1,000;
Affinity Biosciences), anti-Bcl-2 (cat. no. AF6139; 1:1,000;
Affinity Biosciences), anti-phosphorylated (p)-PI3K (cat. no.
ab182651; 1:2,000; Abcam), anti-PI3K (cat. no. ab191606; 1:2,000;
Abcam), anti-p-AKT (cat. no. ab38449; 1:2,000; Abcam), anti-AKT
(cat. no. ab8805; 1:2,000; Abcam) or anti-GAPDH (cat. no. T0004;
1:4,000; Affinity Biosciences). Following incubation with
HRP-conjugated secondary antibodies for 1 h at 37˚C (cat. nos.
S0001and S0002; 1:10,000; Affinity Biosciences), the protein bands
were visualized using an Hypersensitive ECL Chemiluminescence Kit
(Wuhan Servicebio Technology Co., Ltd.) and semi-quantified using
ImageJ software (version 1.52; National Institutes of Health) using
GAPDH as the loading control.
Statistical analysis
Quantitative data are presented as the mean ±
standard deviation in triplicate and were analysed using GraphPad
Prism software (Version 7.0; Dotmatics). Unpaired Student's t-test
was used for comparisons between two groups. BBB scoring was
analysed using Kruskal-Wallis test with Dunn's post hoc test. For
other indexes, one-way ANOVA followed by Tukey's multiple
comparisons test was used for comparisons among three or more
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Cell apoptosis, FGF23 and PI3K/AKT
signalling in H2O2-stimulated primary
neurons
Primary neurons were treated with
H2O2 to establish a cellular model of SCI
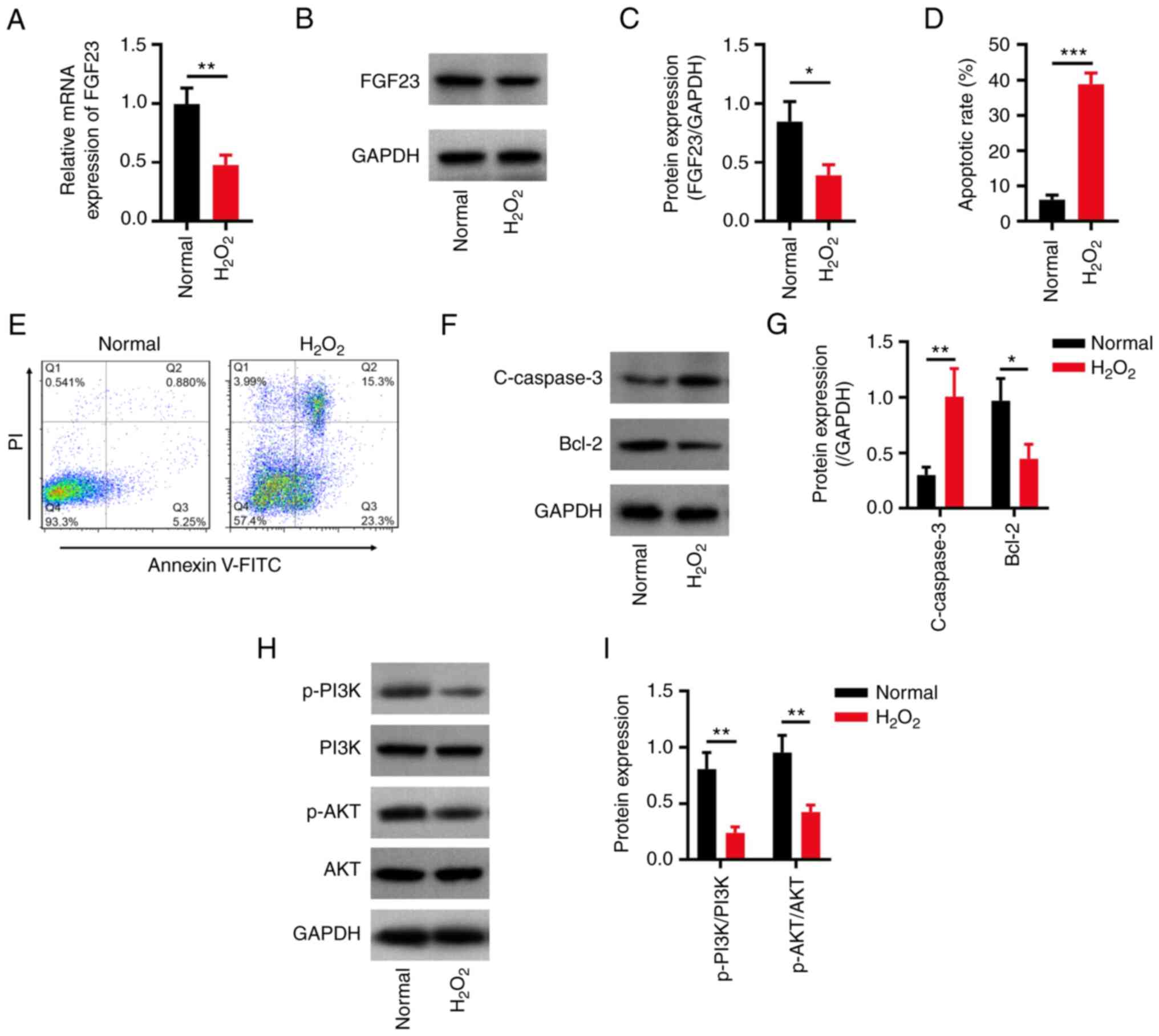
(17). The results revealed that
the apoptotic rate was higher in the H2O2
group compared with that in the normal group (P<0.001; Fig. 1A and B). Furthermore, C-caspase3 expression
levels were higher (P<0.01), whereas Bcl-2 expression levels
were lower (P<0.05) in the H2O2 group
compared with those in the normal group (Fig. 1C and D). Additionally, the protein (P<0.05;
Fig. 1E and F) and mRNA expression levels of FGF23
(P<0.05; Fig. 1G) were
decreased in the H2O2 group compared with
those in the normal group; the protein expression ratios of
p-PI3K/PI3K and p-AKT/AKT were both decreased in the
H2O2 group compared with those in the normal
group (both P<0.01; Fig. 1H and
I).
Effect of FGF23 on neuronal apoptosis
and PI3K/AKT signalling
The mRNA and protein expression levels of FGF23 were
higher in the oeFGF23 group compared with those in the oeNC group
(both P<0.001), whereas the mRNA and protein expression levels
of FGF23 were lower in the shFGF23 group compared with those in the
shNC group (both P<0.05) (Fig.
2A-C), which implied successful infection. The apoptotic rate
was lower in the oeFGF23 group compared with that in the oeNC group
(P<0.01), whereas it was higher in the shFGF23 group compared
with that in the shNC group (P<0.05) (Fig. 2D and E). Additionally, the protein expression
levels of C-caspase3 were lower in the oeFGF23 group compared with
those in the oeNC group and higher in the shFGF23 group compared
with those in the shNC group (both P<0.05), whereas Bcl-2 showed
the opposite trend (oeFGF23 vs. oeNC, P<0.01; shFGF23 vs. shNC,
P<0.05) (Fig. 2F and G).
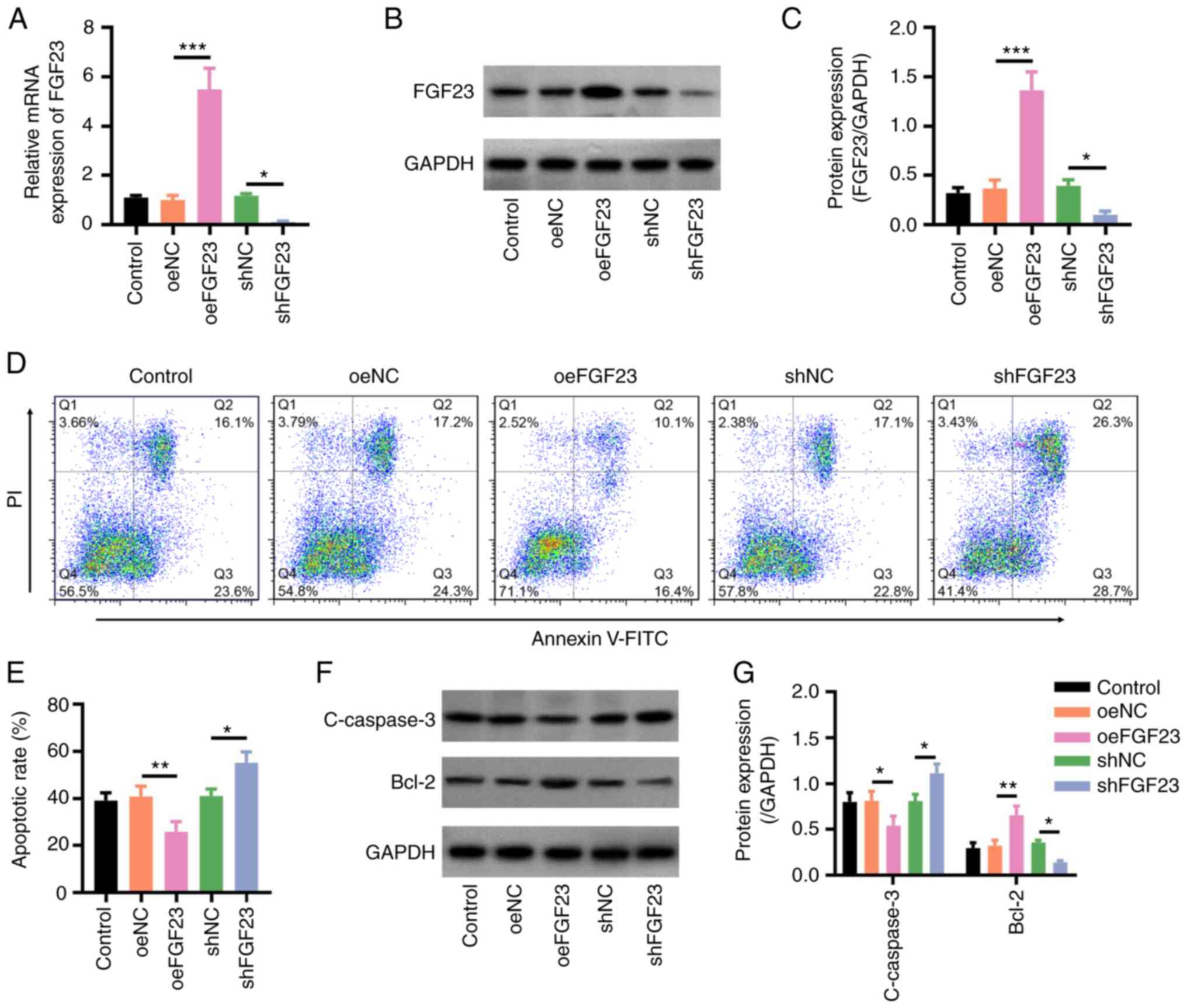 | Figure 2FGF23 overexpression inhibits
H2O2-stimulated neuronal apoptosis. (A)
Relative mRNA expression levels of FGF23 in the control, oeNC,
oeFGF23, shNC and shFGF23 groups. (B) Representative western
blotting images and (C) relative protein expression levels of
FGF23. (D) Representative flow cytometry plots of Annexin V/PI
staining, and (E) quantification of apoptotic rates. (F)
Representative western blotting images and (G) relative protein
expression levels of C-caspase3 and Bcl-2. *P<0.05,
**P<0.01 and ***P<0.001. C-, cleaved;
FGF23, fibroblast growth factor 23; NC, negative control; oe,
overexpression; sh, short hairpin RNA. |
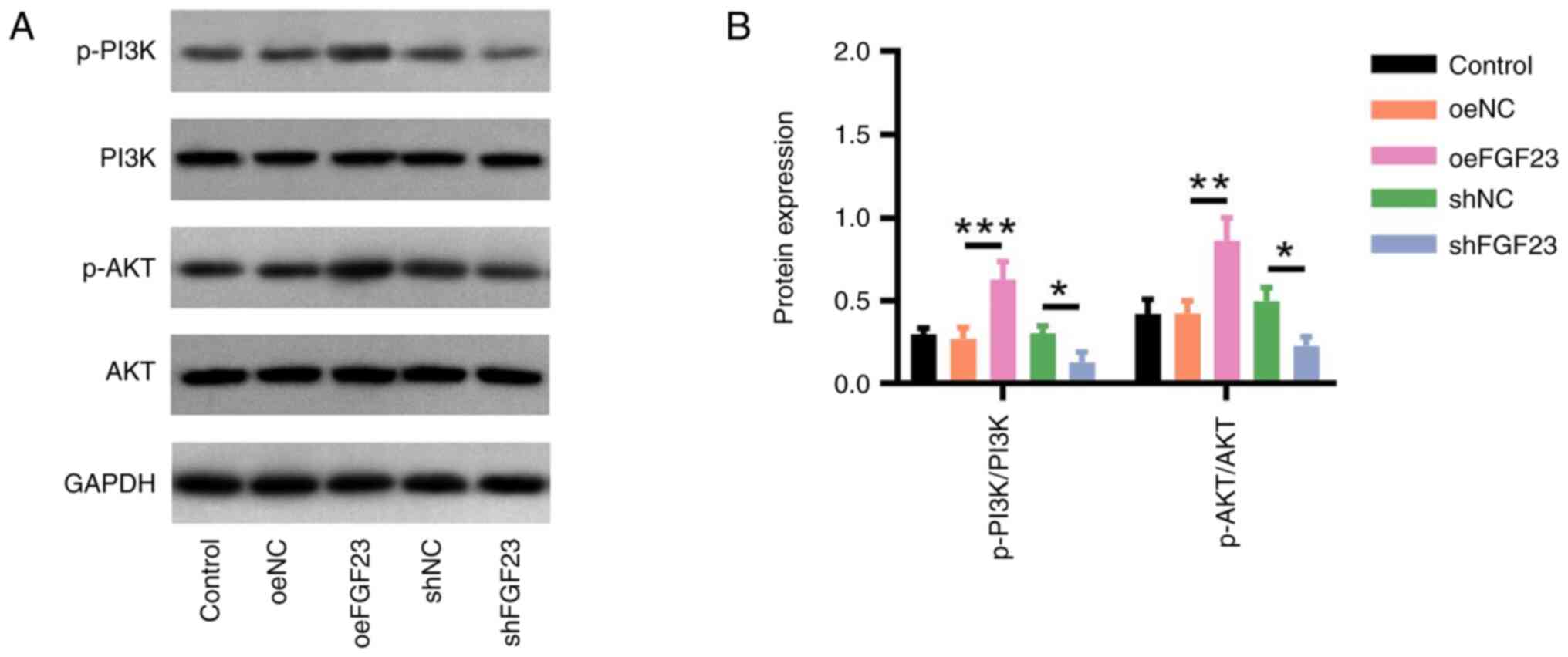
Regarding PI3K/AKT signalling, the p-PI3K/PI3K
(P<0.001) and p-AKT/AKT (P<0.01) protein ratios were higher
in the oeFGF23 group compared with those in the oeNC group, whereas
the relative protein expression levels of p-PI3K/PI3K (P<0.05)
and p-AKT/AKT (P<0.05) were lower in the shFGF23 group compared
with those in the shNC group (Fig.
3).
Effects of FGF23 and LY294002 on
PI3K/AKT signalling and neuronal apoptosis
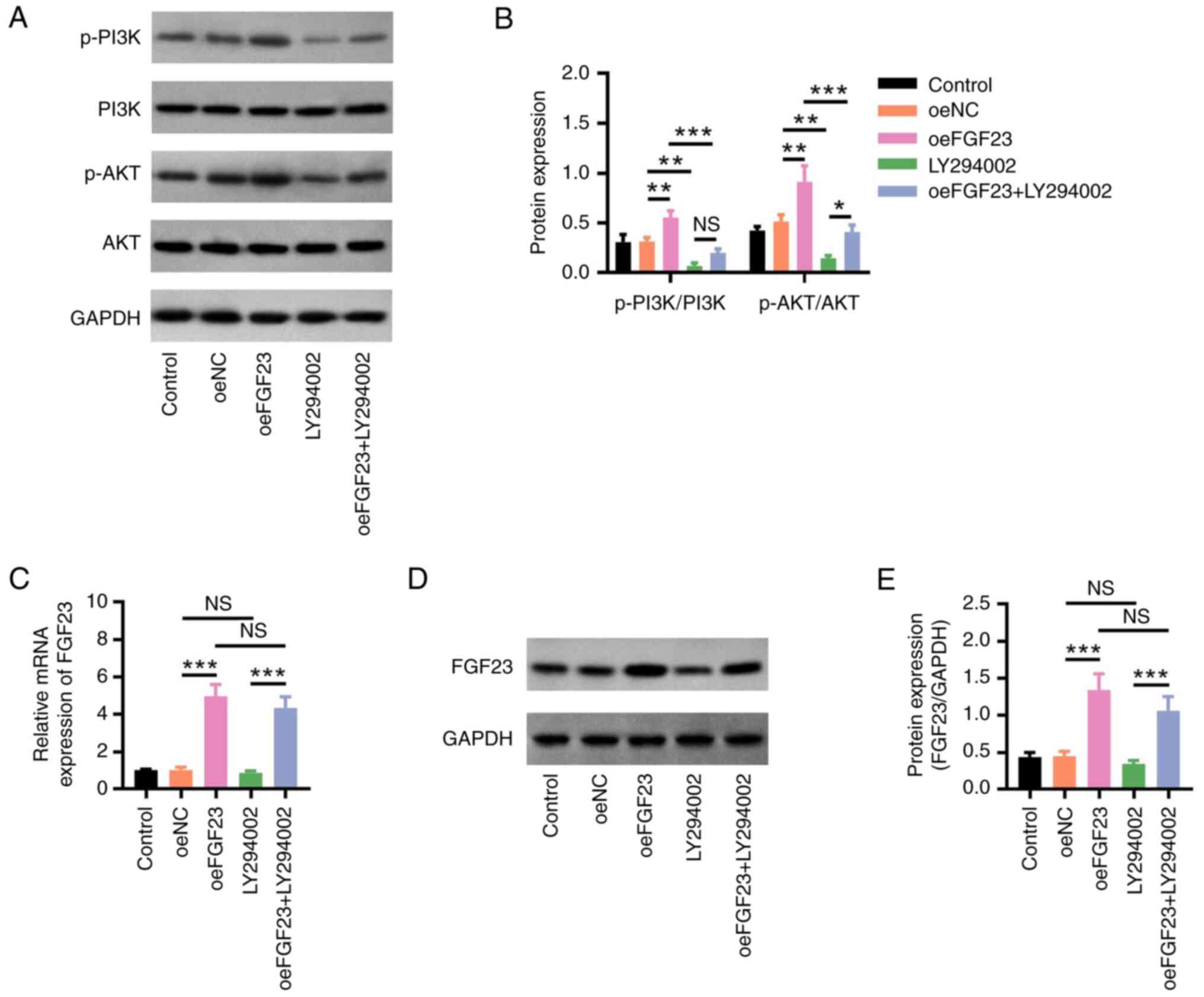
The p-PI3K/PI3K and p-AKT/AKT protein ratios were
decreased in the LY294002 group compared with those in the oeNC
group (both P<0.01); they were also lower in the oeFGF23 +
LY294002 group compared with those in the oeFGF23 group, which
implied that LY294002 decreased the effect of FGF23 on PI3K/AKT
signalling (both P<0.001) (Fig.
4A and B). FGF23 mRNA and
protein expression levels did not differ between the LY294002 and
oeNC groups nor between the oeFGF23 + LY294002 and oeFGF23 groups,
which indicated that LY294002 did not affect FGF23 expression (both
P>0.05; Fig. 4C-E).
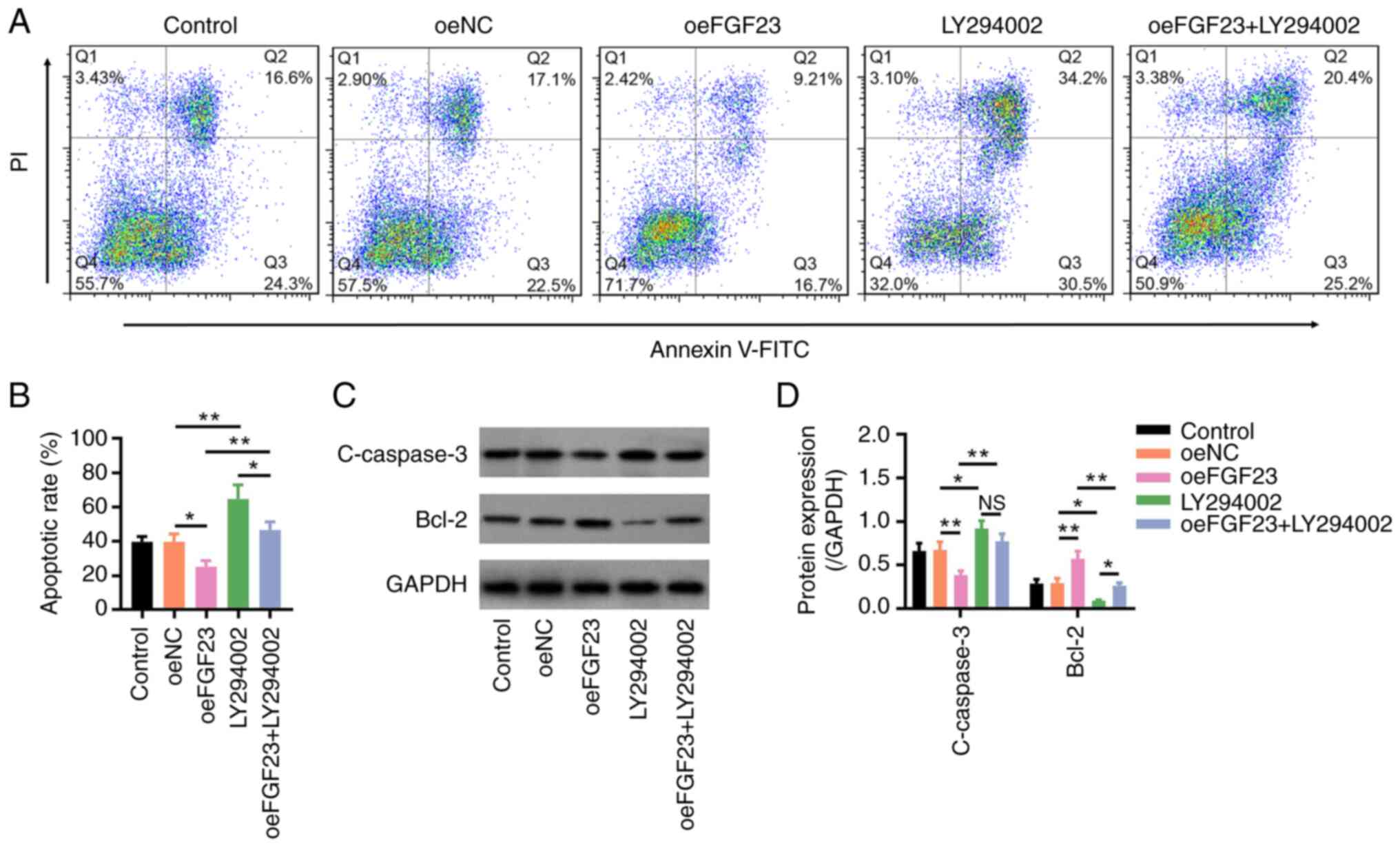
The apoptotic rate was higher in the LY294002 group
compared with that in the oeNC group and this was also increased in
the oeFGF23 + LY294002 group compared with that in the oeFGF23
group (both P<0.01; Fig. 5A and
B). In addition, the protein
expression levels of C-caspase3 were increased, whereas those of
Bcl-2 were decreased in the LY294002 group compared with those in
the oeNC group (both P<0.05) and in the oeFGF23 + LY294002 group
compared with those in the oeFGF23 group (both P<0.01) (Fig. 5C and D). These results indicated that LY294002
reduced the effect of FGF23 on neuronal apoptosis.
Effects of FGF23 and LY294002 on
PI3K/AKT signalling, locomotion recovery and inflammation in SCI
model rats
FGF23 protein expression was decreased in rats in
the SCI model group compared with those in the sham group
(P<0.05), whereas it was increased in the SCI + oeFGF23 group
compared with that in the SCI + oeNC group (P<0.001) (Fig. 6A and B). Additionally, the protein expression
levels of FGF23 did not differ significantly between the SCI +
oeFGF23 + LY294002 and SCI + oeFGF23 groups (P>0.05).
Furthermore, the p-PI3K/PI3K and p-AKT/AKT protein expression
ratios were increased in the SCI + oeFGF23 group compared with
those in the SCI + oeNC group (both P<0.05; Fig. 6C and D). The p-PI3K/PI3K (P<0.01) and
p-AKT/AKT (P<0.05) ratios were decreased in the SCI + oeFGF23 +
LY294002 group compared with those in the SCI + oeFGF23 group,
which indicated that LY294002 decreased the effect of FGF23 on
PI3K/AKT signalling in SCI model rats.
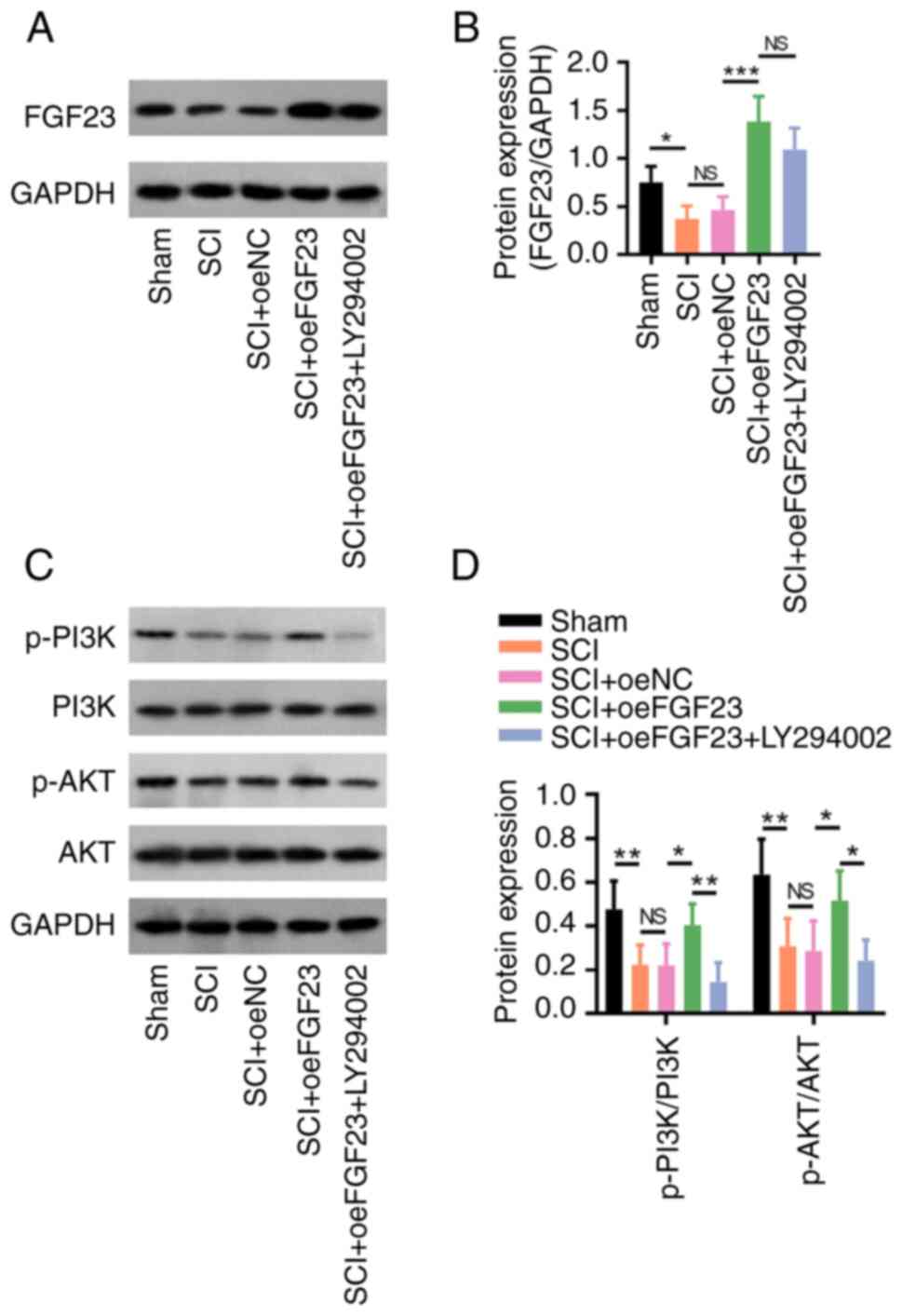 | Figure 6FGF23 activates PI3K/AKT signalling
in SCI model rats. (A) Representative western blotting images and
(B) relative protein expression levels of FGF23 in the sham, SCI,
SCI + oeNC, SCI + oeFGF23 and SCI + oeFGF23 + LY294002 groups. (C)
Representative western blotting images and (D) protein expression
ratios of p-PI3K/PI3K and p-AKT/AKT. *P<0.05,
**P<0.01 and ***P<0.001. FGF23,
fibroblast growth factor 23; NC, negative control; NS, not
significant; oe, overexpression; p-, phosphorylated; SCI, spinal
cord injury. |
Moreover, laceration and inflammatory cell
infiltration were reduced in the SCI + oeFGF23 group compared with
those in the SCI + oeNC group, whereas they increased in the SCI +
oeFGF23 + LY294002 group compared with those in the SCI + oeFGF23
group (Fig. 7A). The BBB score (on
day 28) was increased in the SCI + oeFGF23 group compared with that
in the SCI + oeNC group (P<0.05) but decreased in the SCI +
oeFGF23 + LY294002 group compared with that in the SCI + oeFGF23
group (P<0.01) (Fig. 7B).
Furthermore, TNF-α (P<0.01; Fig.
7C) and IL-1β (P<0.05; Fig.
7D) levels were lower, whereas IL-6 levels were not altered
(P>0.05; Fig. 7E) in the SCI +
oeFGF23 group compared with those in the SCI + oeNC group. The
TNF-α (P<0.001), IL-1β (P<0.01) and IL-6 (P<0.05) levels
were higher in the SCI + oeFGF23 + LY294002 group compared with
those in the SCI + oeFGF23 group. Overall, FGF23 overexpression
suppressed tissue injury and inflammation, and improved locomotion
recovery in rats with SCI, which was reversed by LY294002.
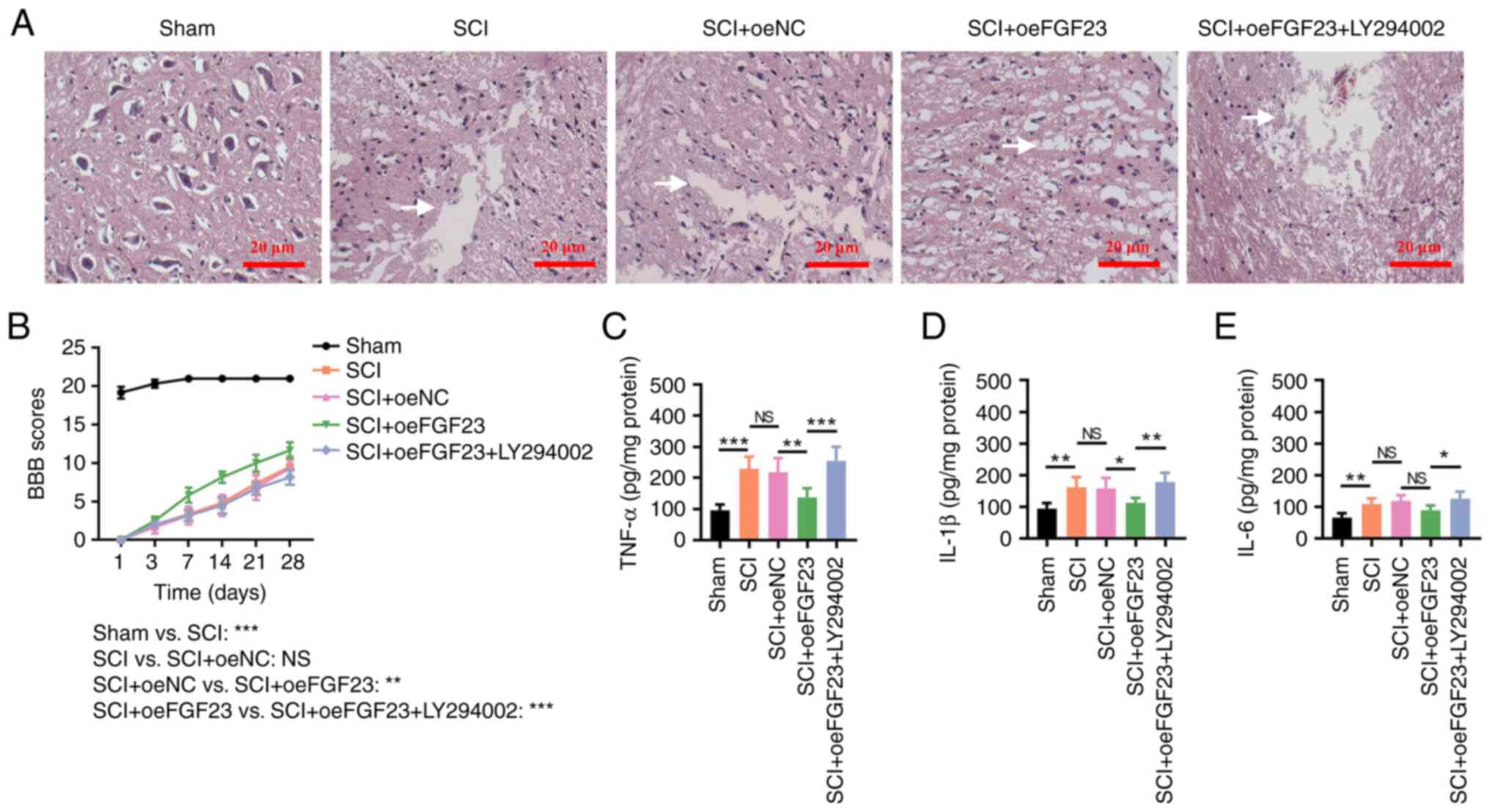 | Figure 7FGF23 improves tissue injury,
locomotor function and inflammation in SCI model rats. (A)
Histomorphological changes in the sham, SCI, SCI + oeNC, SCI +
oeFGF23 and SCI + oeFGF23 + LY294002 groups; the white arrow
indicates laceration. (B) BBB scores at 1, 3, 7, 14, 21 and 28 days
after surgery;the comparison between groups was carried out on day
28. (C) TNF-α, (D) IL-1β and (E) IL-6 levels. The intercellular
space could indicate the presence of a laceration; the larger the
intercellular space the more aggravated the laceration. For
instance, in Fig. 7A, there is no intercellular space in Sham
group, and this means that there is no laceration. However, the
intercelluar space is obvious in SCI group, which means that there
is laceration. *P<0.05, **P<0.01 and
***P<0.001. BBB, Basso-Beattie-Bresnahan; FGF23,
fibroblast growth factor 23; NC, negative control; NS, not
significant; oe, overexpression; SCI, spinal cord injury. |
The number of TUNEL-positive cells was decreased in
the SCI + oeFGF23 group compared with that in the SCI + oeNC group
(P<0.05); however, the number of TUNEL-positive cells was
increased in the SCI + oeFGF23 + LY294002 group compared with that
in the SCI + oeFGF23 group (P<0.001; Fig. S1). This finding indicated that
FGF23 suppressed apoptosis in rats with SCI, which was reversed by
LY294002.
Discussion
The FGF protein family exhibits a protective effect
against neural injury. For example, one study reported that FGF2
enhanced the axonal regeneration of human dental pulp cells
(25). Another study revealed that
FGF1 could prevent motor neuron apoptosis (26). FGF23 may revitalize neural
viability through the activation of
Na+/K+-ATPase (12), which also regulates neural
morphology and synaptic growth via the FGF-receptor-mediated AKT
pathway (11). However, to the
best of our knowledge, the effects of FGF23 on SCI has not yet been
reported. The present study revealed that FGF23 reduced the
apoptosis of H2O2-treated neuronsFGF23
exhibits a protective role in primary neurons, and it may inhibit
the apoptosis of neurons through activating the PI3K/AKT signalling
(11,12,27,28).
It should be noted that in the present study, inflammatory
cytokines were only detected in vivo but not in
vitro; the reason for this issue was that the primary neurons
used in the in vitro study were collected from the cerebral
cortex of the foetal rat, which did not secrete inflammatory
cytokines (29). Hence, the levels
of inflammatory cytokines were not measured in the present in
vitro study.
PI3K/AKT signalling activation has been confirmed to
have a protective role in SCI. For example, one study reported that
the PI3K/AKT signalling pathway can suppress neurotoxic microglia
and astrocytes to improve SCI recovery (30), and another study revealed that the
PI3K/AKT pathway can attenuate neural pyroptosis during SCI
(31). In addition, it has been
reported that FGF23 may be a stimulator of PI3K/AKT signalling
(28,32,33).
A previous study revealed that FGF23 could interact with PI3K/AKT
signalling in diabetic mice (32).
Additionally, another study reported that FGF23 induced the
activation of PI3K/AKT signalling in transgenic α-Klotho mice
(28). Additionally, FGF23 can
modulate PI3K/AKT signalling in osteoblasts (33). In terms of neuron regulation, a
previous study showed that FGF23 increased the activity of
hippocampal cells via stimulation of PI3K/AKT signalling (11). Based on the aforementioned body of
evidence, PI3K/AKT regulation experiments were further performed in
the current study, which demonstrated that FGF23 exhibited a
protective effect on apoptosis via upregulation of PI3K/AKT
signalling in H2O2-treated neurons, which was
partly in agreement with previous findings (11,28,33).
However, the underlying mechanism of the regulatory role of FGF23
in PI3K/AKT signalling still requires further investigation.
In addition to the aforementioned findings, results
from the present study further demonstrated that FGF23 decreased
neuroinflammation via activation of PI3K/AKT signalling in SCI
model rats. The possible explanations are as follows: i) FGF23
maintains the property of neurons in SCI model rats, further
promoting the formation of glia limitans, which might help to
restrict the recruitment of inflammatory factors; thus, FGF23
decreases inflammation in SCI model rats (34,35);
and ii) PI3K/AKT inhibits gasdermin D-mediated microglia pyroptosis
directly to suppress inflammation (31). Additionally, the present study
investigated the effect of FGF23 on locomotion recovery in a rat
model of SCI and revealed that FGF23 improved the locomotion
recovery in a PI3K/AKT-dependent manner, which might improve neural
inflammation and apoptosis. Furthermore, the effect of LY294002 on
SCI and its compensation effect on FGF23 was assessed in
vitro; however, the effect of LY294002 monotherapy on SCI rats
was not assessed in vivo. The reason for this was that the
effect of LY294002 in SCI model rats had already been assessed in
the previous studies (19,22), hence, the current study did not
re-assess this documented issue.
In conclusion, FGF23 alleviated neuronal apoptosis
and inflammation, and it promoted locomotion recovery via
activation of PI3K/AKT signalling in SCI, indicating its potential
as a treatment option for SCI; however, further studies are
warranted for validation.
Supplementary Material
FGF23 suppresses apoptosis, which is
reversed by LY294002 in SCI model rats. (A) Representative images
and (B) quantification of the TUNEL-positive cells among the sham,
SCI, SCI + oeNC, SCI + oeFGF23 and SCI + oeFGF23 + LY294002 groups.
*P<0.05 and ***P<0.001. FGF23,
fibroblast growth factor 23; NC, negative control; NS, not
significant; oe, overexpression; p., phosphorylated; SCI, spinal
cord injury.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZL and WF substantially contributed to the
conception and design of the study. YC and BY were responsible for
the acquisition of the data. SL and LH contributed to the data
analysis. FX contributed to the interpretation of data. ZL, WF and
YC confirm the authenticity of all the raw data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
All procedures followed in the present study were
approved by the Animal Care and Use Committee of Xiamen University
(approval no. 20210401).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hachem LD and Fehlings MG: Pathophysiology
of spinal cord injury. Neurosurg Clin N Am. 32:305–313.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lee BJ and Jeong JH: Review: Steroid use
in patients with acute spinal cord injury and guideline update.
Korean J Neurotrauma. 18:22–30. 2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Anjum A, Yazid MD, Fauzi Daud M, Idris J,
Ng AMH, Selvi Naicker A, Ismail OHR, Athi Kumar RK and Lokanathan
Y: Spinal cord injury: Pathophysiology, multimolecular
interactions, and underlying recovery mechanisms. Int J Mol Sci.
21(7533)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Eli I, Lerner DP and Ghogawala Z: Acute
traumatic spinal cord injury. Neurol Clin. 39:471–488.
2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Karsy M and Hawryluk G: Modern medical
management of spinal cord injury. Curr Neurol Neurosci Rep.
19(65)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Huang H, Young W, Skaper S, Chen L,
Moviglia G, Saberi H, Al-Zoubi Z, Sharma HS, Muresanu D, Sharma A,
et al: Clinical neurorestorative therapeutic guidelines for spinal
cord injury (IANR/CANR version 2019). J Orthop Translat. 20:14–24.
2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Patsakos EM, Bayley MT, Kua A, Cheng C,
Eng J, Ho C, Noonan VK, Querée M and Craven BC: Can-SCIP Guideline
Expert Panel. Development of the Canadian spinal cord injury best
practice (Can-SCIP) guideline: Methods and overview. J Spinal Cord
Med. 44 (Suppl 1):S52–S68. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Alam R, Mrad Y, Hammoud H, Saker Z, Fares
Y, Estephan E, Bahmad HF, Harati H and Nabha S: New insights into
the role of fibroblast growth factors in Alzheimer's disease. Mol
Biol Rep. 49:1413–1427. 2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Klimaschewski L and Claus P: Fibroblast
growth factor signalling in the diseased nervous system. Mol
Neurobiol. 58:3884–3902. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Vervloet MG: Shedding light on the complex
regulation of FGF23. Metabolites. 12(401)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hensel N, Schön A, Konen T, Lübben V,
Förthmann B, Baron O, Grothe C, Leifheit-Nestler M, Claus P and
Haffner D: Fibroblast growth factor 23 signaling in hippocampal
cells: Impact on neuronal morphology and synaptic density. J
Neurochem. 137:756–769. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Oshima N, Onimaru H, Yamagata A, Ito S,
Imakiire T and Kumagai H: Rostral ventrolateral medulla neuron
activity is suppressed by Klotho and stimulated by FGF23 in newborn
Wistar rats. Auton Neurosci. 224(102640)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Musgrove J and Wolf M: Regulation and
effects of FGF23 in chronic kidney disease. Annu Rev Physiol.
82:365–390. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhang X, Guo K, Xia F, Zhao X, Huang Z and
Niu J: FGF23C-tail improves diabetic nephropathy by
attenuating renal fibrosis and inflammation. BMC Biotechnol.
18(33)2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Guidelines for Endpoints in Animal Study
Proposals. Available at: https://oacu.oir.nih.gov/system/files/media/file/2022-04/b13_endpoints_guidelines.pdf.
|
|
16
|
Yang H, Wang H, Shu Y and Li X: miR-103
promotes neurite outgrowth and suppresses cells apoptosis by
targeting prostaglandin-endoperoxide synthase 2 in cellular models
of Alzheimer's disease. Front Cell Neurosci. 12(91)2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wang N, Yang Y, Pang M, Du C, Chen Y, Li
S, Tian Z, Feng F, Wang Y, Chen Z, et al: MicroRNA-135a-5p promotes
the functional recovery of spinal cord injury by targeting SP1 and
ROCK. Mol Ther Nucleic Acids. 22:1063–1077. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Luo Z, Wu F, Xue E, Huang L, Yan P, Pan X
and Zhou Y: Hypoxia preconditioning promotes bone marrow
mesenchymal stem cells survival by inducing HIF-1α in injured
neuronal cells derived exosomes culture system. Cell Death Dis.
10(134)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhao R, Wu X, Bi XY, Yang H and Zhang Q:
Baicalin attenuates blood-spinal cord barrier disruption and
apoptosis through PI3K/Akt signaling pathway after spinal cord
injury. Neural Regen Res. 17:1080–1087. 2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Li X, Zhang C, Haggerty AE, Yan J, Lan M,
Seu M, Yang M, Marlow MM, Maldonado-Lasunción I, Cho B, et al: The
effect of a nanofiber-hydrogel composite on neural tissue repair
and regeneration in the contused spinal cord. Biomaterials.
245(119978)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chen J, Wang Z, Zheng Z, Chen Y, Khor S,
Shi K, He Z, Wang Q, Zhao Y, Zhang H, et al: Neuron and
microglia/macrophage-derived FGF10 activate neuronal FGFR2/PI3K/Akt
signaling and inhibit microglia/macrophages TLR4/NF-κB-dependent
neuroinflammation to improve functional recovery after spinal cord
injury. Cell Death Dis. 8(e3090)2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wang H, Zheng Z, Han W, Yuan Y, Li Y, Zhou
K, Wang Q, Xie L, Xu K, Zhang H, et al: Metformin promotes axon
regeneration after spinal cord injury through inhibiting oxidative
stress and stabilizing microtubule. Oxid Med Cell Longev.
2020(9741369)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
AVMA Guidelines for the Euthanasia of
Animals: 2020 Edition. Available at: https://olaw.nih.gov/avma-guidelines-2020.htm.
|
|
25
|
Nagashima K, Miwa T, Soumiya H, Ushiro D,
Takeda-Kawaguchi T, Tamaoki N, Ishiguro S, Sato Y, Miyamoto K, Ohno
T, et al: Priming with FGF2 stimulates human dental pulp cells to
promote axonal regeneration and locomotor function recovery after
spinal cord injury. Sci Rep. 7(13500)2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Vargas MR, Pehar M, Cassina P,
Martínez-Palma L, Thompson JA, Beckman JS and Barbeito L:
Fibroblast growth factor-1 induces heme oxygenase-1 via nuclear
factor erythroid 2-related factor 2 (Nrf2) in spinal cord
astrocytes: Consequences for motor neuron survival. J Biol Chem.
280:25571–25579. 2005.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kma L and Baruah TJ: The interplay of ROS
and the PI3K/Akt pathway in autophagy regulation. Biotechnol Appl
Biochem. 69:248–264. 2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Xiao Z, King G, Mancarella S, Munkhsaikhan
U, Cao L, Cai C and Quarles LD: FGF23 expression is stimulated in
transgenic alpha-Klotho longevity mouse model. JCI Insight.
4(e132820)2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Mills S: Histology for Pathologists (5th
edition). Wolters Kluwer health, chapter 9, pp224-232, 2019.
|
|
30
|
Jiang D, Gong F, Ge X, Lv C, Huang C, Feng
S, Zhou Z, Rong Y, Wang J, Ji C, et al: Neuron-derived
exosomes-transmitted miR-124-3p protect traumatically injured
spinal cord by suppressing the activation of neurotoxic microglia
and astrocytes. J Nanobiotechnology. 18(105)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Xu S, Wang J, Zhong J, Shao M, Jiang J,
Song J, Zhu W, Zhang F, Xu H, Xu G, et al: CD73 alleviates
GSDMD-mediated microglia pyroptosis in spinal cord injury through
PI3K/AKT/Foxo1 signaling. Clin Transl Med. 11(e269)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Luo W, Jiang Y, Yi Z, Wu Y, Gong P and
Xiong Y: 1a,25-Dihydroxyvitamin D3 promotes osteogenesis
by down-regulating FGF23 in diabetic mice. J Cell Mol Med.
25:4148–4156. 2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Xiao Z, Huang J, Cao L, Liang Y, Han X and
Quarles LD: Osteocyte-specific deletion of Fgfr1 suppresses FGF23.
PLoS One. 9(e104154)2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
O'Shea TM, Burda JE and Sofroniew MV: Cell
biology of spinal cord injury and repair. J Clin Invest.
127:3259–3270. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Hellenbrand DJ, Quinn CM, Piper ZJ,
Morehouse CN, Fixel JA and Hanna AS: Inflammation after spinal cord
injury: A review of the critical timeline of signaling cues and
cellular infiltration. J Neuroinflammation. 18(284)2021.PubMed/NCBI View Article : Google Scholar
|