Introduction
Osteoarthritis (OA), a chronic degenerative joint
disease, is characterized by the growth of osteophytes, subchondral
bone sclerosis and synovial hyperplasia (1). According to current research, a
variety of risk factors, such as aging, hereditary factors,
previous joint injury and obesity are linked to the development of
OA (2). However, the mechanisms
underlying the pathogenesis of this disease remain to be fully
elucidated.
Previous studies have demonstrated the involvement
of circular RNAs (circRNAs) and microRNAs (miRNAs/miRs) in
controlling the progression of OA, including processes such as
chondrocyte death, oxidative stress, extracellular matrix (ECM)
metabolism and autophagy (3,4).
Some circRNAs are rich in miRNA binding sites, they act as ‘miRNA
sponges’ (5). For instance,
circSERPINE2 suppresses OA development by regulating the
miR-495/TGFBR2 axis (6). On the
other hand, circ33186 promotes the pathogenesis of OA by
upregulating matrix metalloproteinase (MMP)-13 expression, which is
targeted by miR-127-5p (7). This
cross-interaction between circRNAs and miRNAs in the
pathophysiology of OA suggested that they may be potential targets
for OA therapy. hsa_circ_0072568 (circ0072568) is a circRNA derived
from phosphodiesterase 4D that inhibits the tumorigenesis and
progression of colorectal cancer and oxaliplatin-resistant
colorectal cancer. RNA sequencing (RNAseq) analysis has revealed
that circ0072568 is significantly downregulated in OA tissues and
in pro-inflammatory factor-stimulated human chondrocytes (8). However, the role of circ0072568 in OA
remains unclear. The present study aimed to investigate the effects
of circ0072568 in interleukin (IL)-1β-stimulated chondrocytes. It
is hoped that the findings presented herein may provide novel
insight into OA treatment by modulating the
miR-382-5p/topoisomerase 1 (TOP1) axis.
Materials and methods
Collection of specimens
Cartilage tissues from the knee joints of patients
with OA [7 males and 3 females; age range, 54-66 years; body mass
index (BMI), 19.7-23.5 kg/m2] who had undergone total
knee arthroplasty, and healthy cartilages from patients with
femoral neck fracture (5 males and 5 females; age range, 50-52
years; BMI, 19.9-23.2) from January 2021 to January 2022 were
obtained and used for PCR analysis. The study was approved by the
Ethics Committee of Zhongda Hospital Southeast University and the
signed informed consent was obtained from all subjects (no.
ZDL22-21; date: April 23, 2022).
Primary culture of chondrocytes and
treatment
As previously described (9), cartilage tissues from the knee joints
of OA patients were collected, and then cut into small sections.
After washing five times with PBS (Beyotime Institute of
Biotechnology), the specimens were digested in 0.25% trypsin-EDTA
(Beyotime Institute of Biotechnology) solution for 30 min and
supplemented with Dulbecco's modified Eagle's medium (DMEM; Thermo
Fisher Scientific, Inc.) containing collagenase type II for 2 h at
37˚C. The released chondrocytes were seeded in 25 cm2
cell flasks. The cells were passaged at a ratio of 1:3, when they
reached 80-90% confluency. For stimulation with IL-1β, the isolated
chondrocytes were maintained in DMEM (Thermo Fisher Scientific,
Inc.) at 37˚C, 5% CO2 and incubated with IL-1β (10
ng/ml; Abcam) for 24 h.
Cell transfection
Overexpression vector for circ0072568
(oe-circ0072568) was provided by Guangzhou BersinBio Biotechnology
Co., Ltd.). miR-382-5p mimics and negative control (miR-NC) were
obtained from RiboBio (Guangzhou RiboBio Co., Ltd.) and
MyBioSource, respectively, without sequence information. miR-382-5p
inhibitor (inhib-miR-382-5p) and negative control (inhib-NC) were
obtained from Shanghai GenePharma Co., Ltd. Circ0072568 siRNA
(si-circ0072568) and its corresponding control (si-nc), as well as
TOP1 siRNA (si-TOP1) and its corresponding control (si-NC), were
designed and synthesized by Shanghai GenePharma Co., Ltd. Primary
chondrocytes, isolated from normal articular cartilage tissues,
were cultured in 96-well plates at 37˚C for 24 h and then
transfected with 200 pmol/l oe-circ0072568 [or 200 pmol/l negative
control (oe-NC)], 50 nM miR-382-5p mimics (or 50 nM miR-NC), 100 nM
inhib-miR-382-5p (or 100 nM inhib-NC), or 200 pmol/l si-TOP1 (or
200 pmol/l si-NC) using Lipofectamine 3000® (Invitrogen;
Thermo Fisher Scientific, Inc.). The aforementioned
oligonucleotides or plasmids were transfected into chondrocytes
using Lipofectamine™ 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) following the manufacturer's instructions.
Briefly, chondrocytes were cultured for 24 h, before the constructs
were transfected into the cells using 500 µl Opti-MEM containing 20
µl Lipofectamine 2000 (1 mg/ml) in a humidified atmosphere with 5%
CO2 at 37˚C. After incubating for 6 h, culture medium
was replaced by fresh medium. Subsequently, at 48 h following
transfection, the cells were harvested for use in subsequent
experiments. The sequences of the siRNAs, mimics and inhibitors
used were as follows: miR-382-5p mimics forward,
5'-GAAGUUGUUCGUGGUGGAUUGG-3' and reverse,
5'-AAUCCACCACGAACAACUUCUU-3'; miR-NC forward,
5'-CTCGCTTCGGCAGCACA-3' and reverse, 5'-AACGCTTCACGAATTTGCGT-3';
miR-382-5p inhibitor, 5'-CGAAUCCACCACGAACAACUUC-3'; inhib-NC,
5'-CAGUACUUUUGUGUAGUACAA-3'; si-TOP1, 5'-GCAUAAAGACAAACAUAAAGA-3';
si-NC, 5'-UUCUCCGAACGUGUCACGUTT-3'. si-circ0072568,
5'-AACAGUUUUGAUGUGGACAAU-3'; si-nc, 5'-UUCUCCGAACGUGUCACGU-3'.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA extracted from tissues and cells was
incubated in the presence or absence of RNase R (3 U/µg, Epicentre)
at 37˚C for 20 min. After TRIzol® reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) and the miRNA isolation kit (Thermo
Fisher Scientific, Inc.) to extract the total RNA, reverse
transcription was performed using a miRNA cDNA kit (Invitrogen;
Thermo Fisher Scientific, Inc.), TaqMan™ MicroRNA Assays (Applied
Biosystems; Thermo Fisher Scientific, Inc.) and PrimeScript RT
Master Mix (Takara Bio, Inc.). The PCR thermal cycling parameters
were as follows: 95˚C (5 min), 45 cycles of 94˚C (30 sec) and 60˚C
(30 sec). Gene expression was quantified using the
2-ΔΔCq method (10),
using GAPDH and U6 as internal genes for circ0072568 and TOP1, as
well as miR-382-5p and miR-545-3p, respectively. The primer
sequences used are listed in Table
I.
 | Table IPrimer sequences used in the present
study. |
Table I
Primer sequences used in the present
study.
| Gene | Sequence |
|---|
| circ0072568 | Forward:
5'-GCAAGATCGAGCACCTAGCA-3' |
| | Reverse:
5'-GCTTGGAGAATTAGCCCGGA-3' |
| TOP1 | Forward:
5'-ACGAATCAAGGGTGAGAAGG-3' |
| | Reverse:
5'-CGATACTGGTTCCGGATCTT-3' |
| GAPDH | Forward:
5'-GGGAAACTGTGGCGTGAT-3' |
| | Reverse:
5'-GAGTGGGTGTCGCTGTTGA-3' |
| miR-382-5p | Forward:
5'-ACACTCCAGCTGGGAAAGTGCTTCCC-3' |
| | Reverse:
5'-CTCAACTGGTGTCGTGGA-3' |
| miR-545-3p | Forward:
5'-TCGGCAGGTCAGCAAACATTT-3' |
| | Reverse:
5'-CAGTGCGTGTCGTGGAGT-3' |
| U6 | Forward:
5'-CGCTTCACGAATTTGCGT-3' |
| | Reverse:
5'-CTCGCTTCGGCAGCACA-3' |
MTT assay
Chondrocytes (1x104 cells/well) were
cultured in the 96-well plates. 10 µl MTT stock solution (Beijing
Solarbio Science & Technology Co., Ltd.) was added to the
culture medium according to the manufacturer's instructions, and
the plate was incubated for 4 h at 37˚C. Following the addition of
dimethyl sulfoxide (DMSO; Beijing Solarbio Science & Technology
Co., Ltd.), the absorbance of the cells was read at 570 nm using a
Multiska FC microplate reader (Thermo Fisher Scientific, Inc.).
Flow cytometry
The apoptotic rate of the chondrocytes
(2x105 cells/well) at 80% confluency was analyzed using
an apoptosis detection kit [Annexin V-FITC/propidium iodide (PI);
Beyotime Institute of Biotechnology] and a BD FACS flow cytometer
(BD Biosciences).
Enzyme-linked immunosorbent assay
(ELISA)
The secretion of pro-inflammation factors was
detected using corresponding IL-6 (cat. no. PI325), TNF-α (cat. no.
PT518) and monocyte chemoattractant protein-1 (MCP-1; cat. no.
PC130) ELISA kits (Beyotime Institute of Biotechnology).
Western blot analysis
After using the bicinchoninic acid (BCA) method
(Beyotime Institute of Biotechnology) to quantify total proteins,
which were extracted from the cell using radio immunoprecipitation
assay buffer (cat. no. BP0013B; Beyotime Institute of
Biotechnology), the proteins were separated by 10% SDS-PAGE,
transferred onto PVDF membranes. Thereafter, the membranes were
blocked with 5% skimmed milk at room temperature for 2 h and then
incubated with primary antibodies at 4˚C overnight. Subsequently,
the membranes were incubated with secondary antibody IgG (1:2,000;
cat. no. ab205718; Abcam) at room temperature for 2 h. The primary
antibodies used were as follows: Anti-collagen II (1:1,000; cat.
no. ab188570; Abcam), anti-MMP-13 (1:1,000; cat. no. ab51072;
Abcam), anti-TOP1 (1:1,000; cat. no. ab131166; Abcam) and
anti-GAPDH (1:1,000; cat. no. ab8245; Abcam). Blots were developed
using an enhanced chemiluminescence system (Beyotime Institute of
Biotechnology). The density of each protein blot was compared with
that of GAPDH using ImageJ software (version 1.46r; National
Institutes of Health) and is presented as a ratio to the endogenous
control.
Nuclear-cytoplasmic fractionation
The PARIS kit (cat. no. AM1921; Invitrogen; Thermo
Fisher Scientific, Inc.) was used for nuclear-cytoplasmic
fractionation. Briefly, the nuclei were lysed using cell disruption
buffer and the supernatant of nuclear and mitochondrial DNA
extracted form chondrocytes were mixed with a 2X lysis binding
solution, followed by eluting the cytoplasm and nucleus RNA using
the corresponding kit solutions.
Luciferase reporter assay
The downstream target miRNAs of circ0072568 were
predicted using the online website StarBase v2.0 (http://starbase.sysu.edu.cn/index.php)
and circBank (http://www.circbank.cn/searchCirc.html). Direct
targets of miR-382-5p were predicted using the online databases
StarBase, miRTarBase (http://mirtarbase.mbc.nctu.edu.tw/index.html),
TargetScan (https://www.targetscan.org/vert_80/) and miRDB
(https://mirdb.org/). Primary chondrocytes
(1x104) were cultured to 70% confluency in 24-well
plates. Either wild-type or mutant circ0072568 (circ0072568-wt or
circ0072568-mut) and TOP1 (TOP1-wt or TOP1-mut) fragments were
inserted into the pGL3-firefly luciferase vectors (Shanghai
Genechem Co. Ltd.). Primary chondrocytes were cultured for 24 h
before being co-transfected with 600 ng circ0072568-wt or 600 ng
TOP1 3'UTR-wt luciferase reporter gene plasmid and 20 nmol
miR-382-5p mimics or 20 nmol miR-NC using Lipofectamine 3000
reagent for 48 h at 37˚C, and the dual-luciferase System (Promega
Corporation) was used to determine the luciferase activity. The
results were normalized to Renilla lucifierase activity.
RNA immunoprecipitation (RIP)
assay
The Ago-RIP test (MilliporeSigma) was performed
using the the Magna RIP RNA-Binding Protein Immunoprecipitation kit
and RNeasy MinElute Cleanup kit (Qiagen GmbH). The cells were lysed
in RIP buffer (Beyotime Institute of Biotechnology) containing a
protease inhibitor cocktail and RNase inhibitors, and incubated at
4˚C with anti-Ago2 (cat. no. ab186733; 1:50; Abcam) or anti-IgG
antibodies (cat. no. PP64B; 1:20; EMD Millipore) overnight. RNA was
quantified by qRT-PCR.
Statistical analysis
SPSS v22.0 software was used for statistical
analyses. An unpaired two-tailed Student's t-test and one-way ANOVA
(for ≥3 groups) followed by post hoc (Tukey's or Dunnett's) tests
was used. Data are presented as the mean ± SD of three repeats. The
correlation between circ0072568 and miR-382-5p expression in OA
tissues was assessed using Pearson's correlation analysis. A value
of P<0.05 was considered to indicate a statistically significant
difference.
Results
Effects of circ0072568 on
IL-1β-stimulated human primary chondrocytes
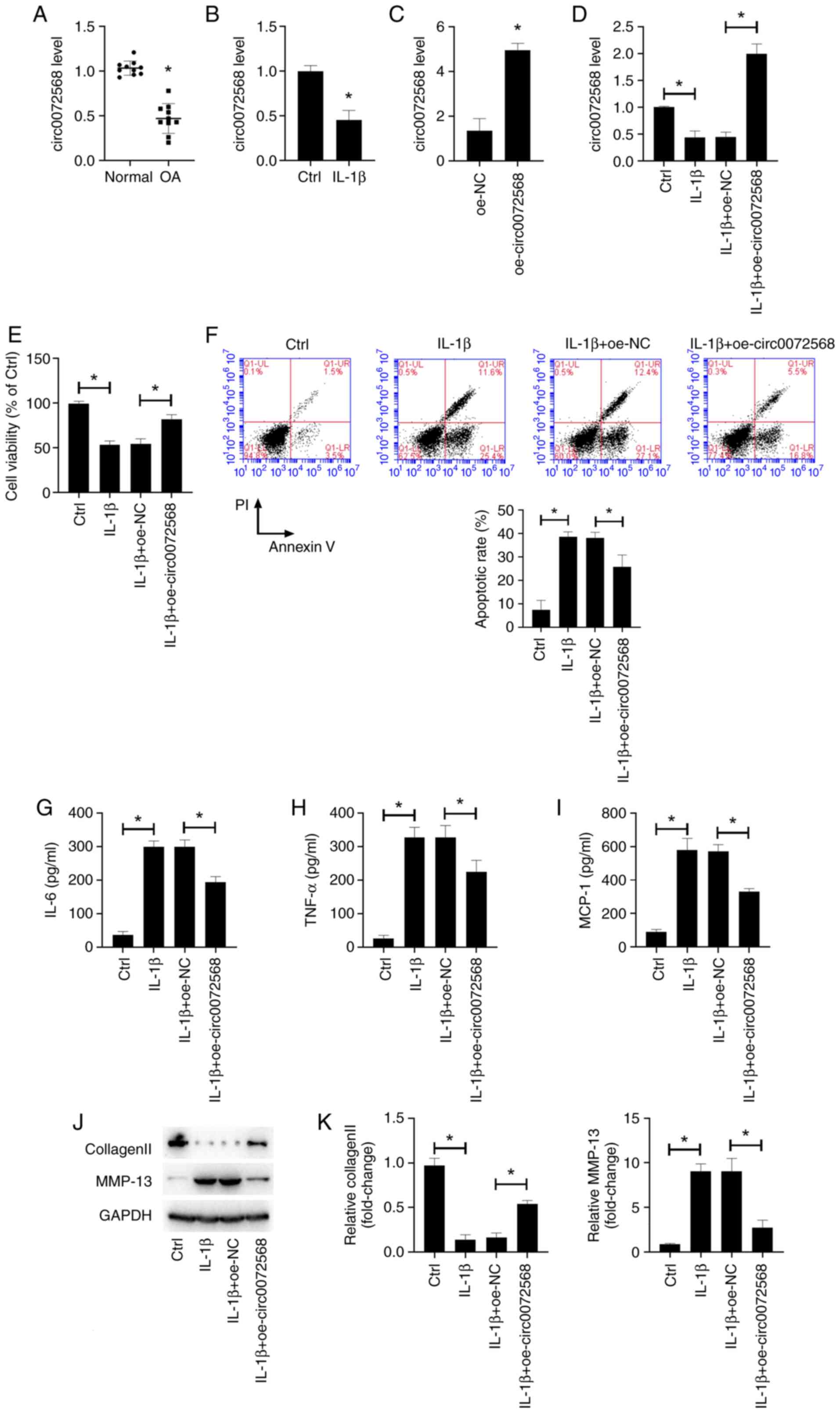
As shown in Fig.
1A, circ0072568 expression was decreased in OA-affected
cartilage, as well as in IL-1β-stimulated primary human
chondrocytes (Fig. 1B). As
demonstrated by the results of RT-qPCR, transfection with
circ0072568 overexpression vector (oe-circ0072568) successfully
increased the circ0072568 level in chondrocytes isolated from
normal articular cartilage tissues (Fig. 1C). Additionally, the downregulation
of circ0072568 induced by exposure to IL-1β for 24 h was restored
by transfection with circ0072568 overexpression vector (Fig. 1D). The decrease in the viability of
IL-1β-stimulated primary human chondrocytes was abolished by the
overexpression of circ0072568 (Fig.
1E). The increased apoptosis in IL-1β-induced human primary
chondrocytes was also abolished by the overexpression of
circ0072568 (Fig. 1F). In
addition, circ0072568 overexpression led to the decreased secretion
of pro-inflammatory factors (Fig.
1G-I), and to decreased MMP-13 expression and enhanced collagen
II expression (Fig. 1J and
K).
ECM degradation by IL-1β-stimulated chondrocytes was
assessed by detecting the expression of proteins related to ECM
catabolism, including collagen II and MMP-13. Western blot analysis
revealed that IL-1β stimulation decreased the expression of
collagen II and increased the expression of MMP-13, indicative of
the increased ECM degradation potential (Fig. 1J and K). By contrast, circ0072568
overexpression led to an increase in collagen II expression and a
decrease in MMP-13 expression in IL-1β-stimulated chondrocytes.
Taken together, these data suggest that circ0072568 inhibits
IL-1β-induced inflammation and ECM degradation by chondrocytes.
circ0072568 functions as a sponge for
miR-382-5p
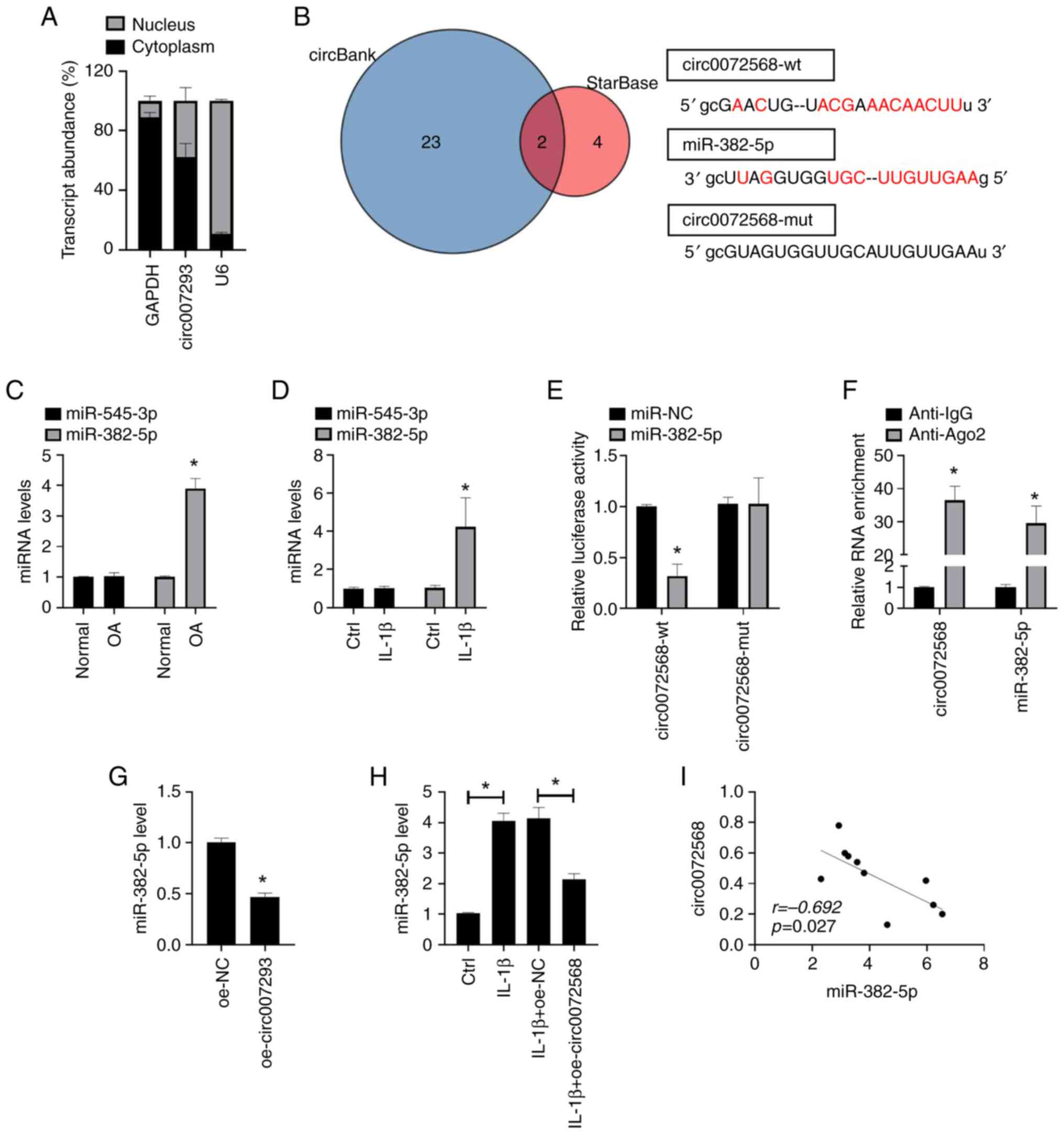
The regulatory mechanism of circRNAs as miRNA
sponges has been widely confirmed. The present study we identified
the distribution of circ0072568 in chondrocytes by subcellular
fractionation and found that circ0072568 was mainly enriched in the
cytoplasm of chondrocytes (Fig.
2A). The analysis of public databases (StarBase v2.0 and
circBank) identified miR-545-3p and miR-382-5p as target miRNAs of
circ0072568 (Fig. 2B). However,
the expression of miR-382-5p and miR-545-3p did not differ
significantly between OA tissues and normal tissues, and the
stimulation of human chondrocytes with IL-1β did not result in
significant changes in their expression levels (both P>0.05;
Fig. 2C and D). In addition, miR-382-5p decreased the
relative luciferase activity of circ0072568-wt, but not that of
circ0072568-mut vectors (Fig. 2E).
Furthermore, the enrichment of circ0072568 in beads was found to
conjugate to the Ago2 antibody compared with the IgG controls
(Fig. 2F). oe-circ0072568 and its
negative control (oe-NC) were then transfected into chondrocytes
(Fig. S1), and the results
revealed that the overexpression of circ0072568 in chondrocytes led
to a decreased expression of miR-382-5p (Fig. 2G), abolishing the IL-1β-induced
upregulation of miR-382-5p expression (Fig. 2H). Pearson's correlation analysis
further confirmed the negative correlation between the expression
of circ0072568 and miR-382-5p in the cartilage tissues of patients
with OA (Fig. 2I).
miR-382-5p directly targets TOP1
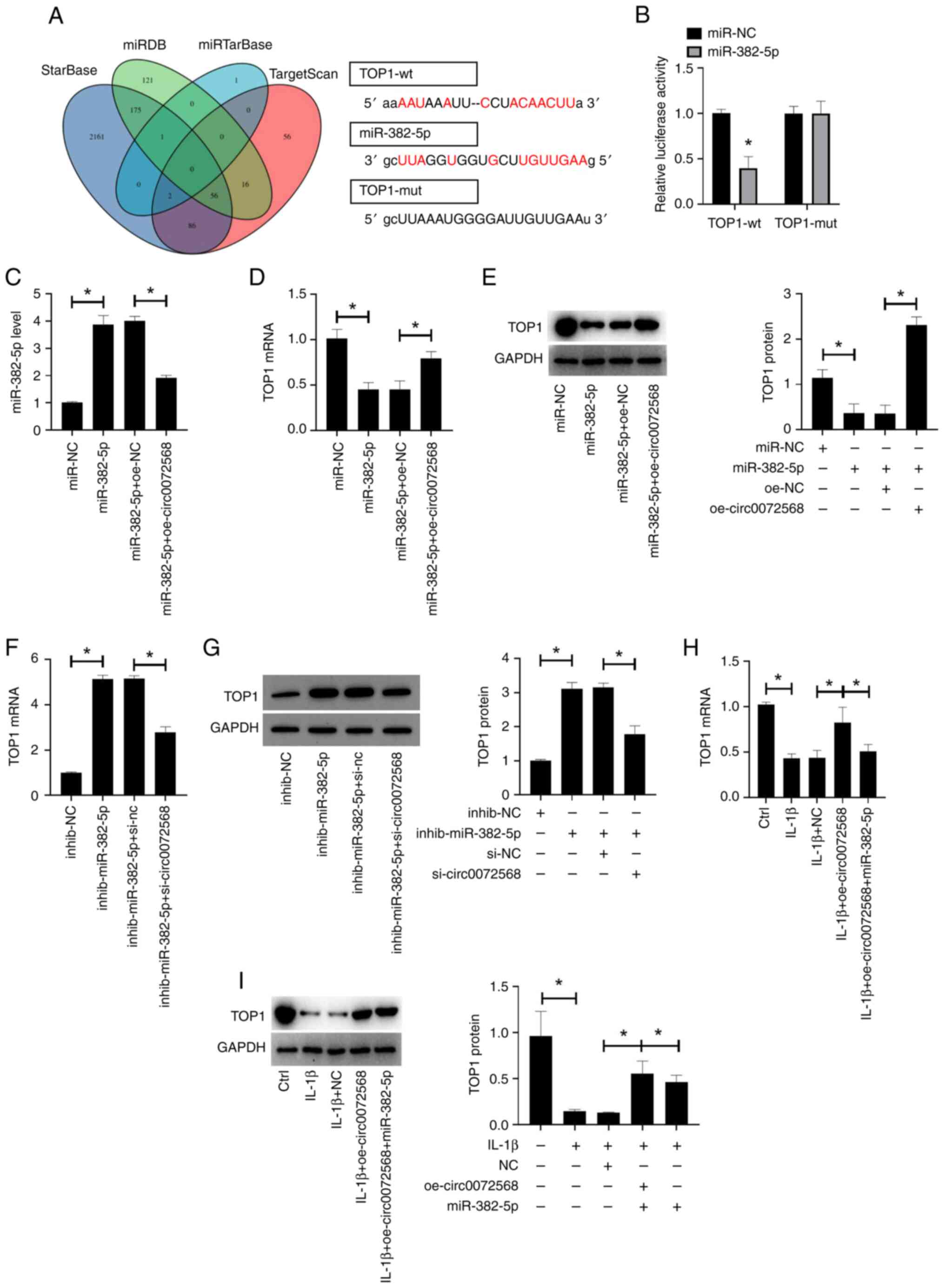
TOP1 was found to be one of the target genes of
miR-382-5p, as predicted using the StarBase, TargetScan and miRDB
databases (Fig. 3A). The relative
luciferase activity of TOP1-wt vectors was decreased in the
chondrocytes overexpressing miR-382-5p (P<0.05; Fig. 3B). The overexpression of miR-382-5p
in human chondrocytes transfected with miR-382-5p mimic was
abolished following transfection of the cells with circ0072568
overexpression vector (all P<0.05; Fig. 3C). While the transfection of
chondrocytes with miR-382-5p mimic decreased the expression of TOP1
in chondrocytes, this effect was reversed by co-transfection with
circ0072568 overexpression vector (all P<0.05; Fig. 3D and E). In addition, transfection of the
chondrocytes with miR-382-5p inhibitor increased TOP1 expression in
chondrocytes, whereas this effect was reversed by co-transfection
with si-circ0007256 (all P<0.05; Fig. 3F and G). IL-1β stimulation markedly decreased
the expression of TOP1 in chondrocytes (all P<0.05), and this
effect was reversed by the overexpression of circ0072568 (all
P<0.05). Additionally, circ0072568 regulated TOP1 expression via
miR-382-5p in chondrocytes (Fig.
3H and I).
Protective effects of circ0072568 on
IL-1β-stimulated chondrocytes are mediated via the regulation of
the miR-382-5p/TOP1 axis
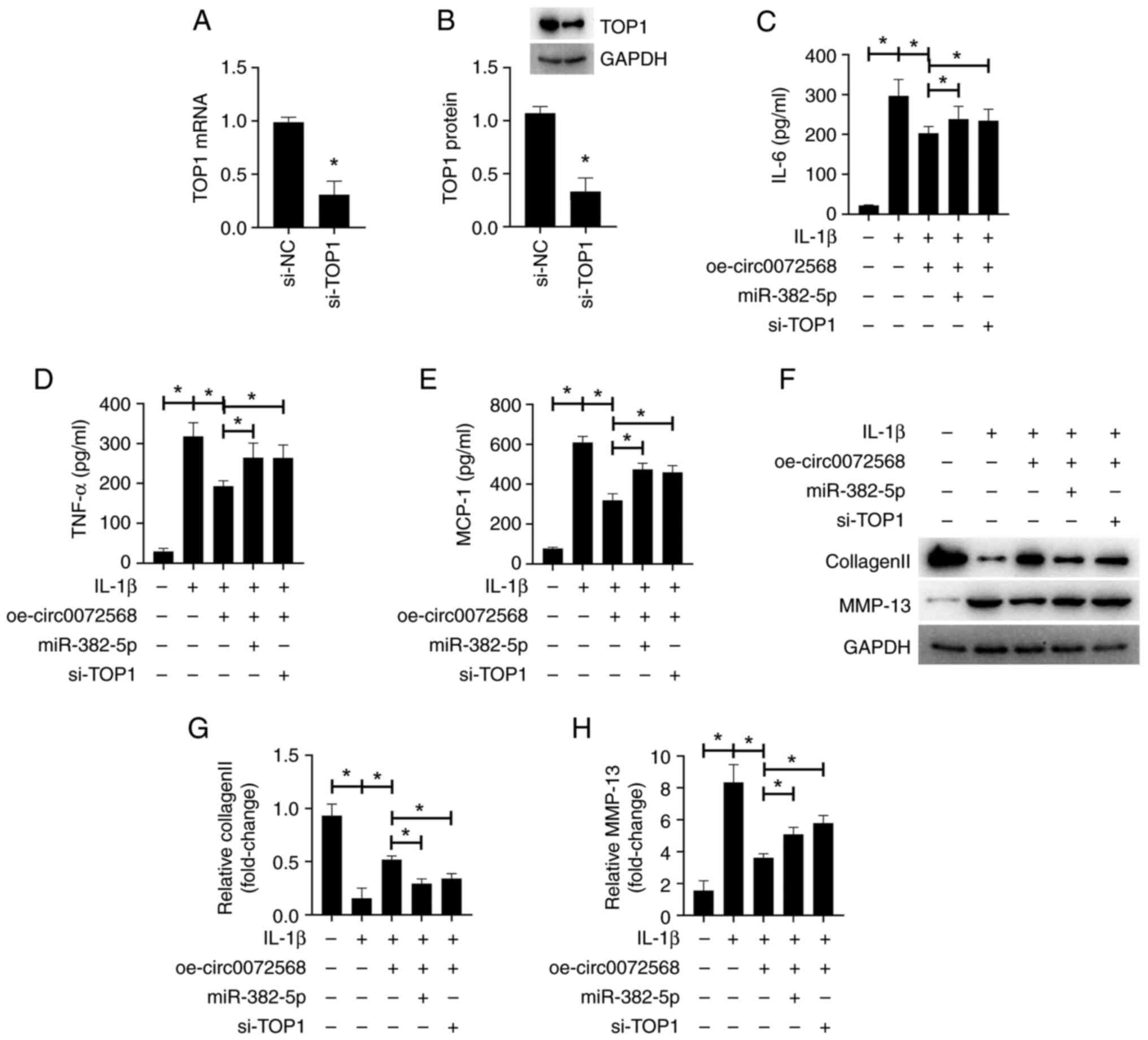
miR-382-5p overexpression or TOP1 knockdown
abolished the anti-inflammatory effects of circ0072568
overexpression on IL-1β-stimulated chondrocytes. miR-382-5p
overexpression or TOP1 knockdown abolished the suppressive effects
of circ0072568 overexpression on the levels of pro-inflammatory
factors (Fig. 4A-E), and reversed
the inhibition of MMP-13 expression and the increase in collagen II
expression, which was induced by circ0072568 overexpression
(Fig. 4F-H).
Discussion
Currently, alleviating pain and improving function
is the main treatment strategy for OA. However, this treatment is
purely symptomatic, and does not affect the progression of OA
(11). Since there is no effective
therapy that can completely cure OA, the elucidation of the
underlying mechanisms of OA has obvious clinical significance. As a
type of endogenous non-coding RNAs, circRNAs have been increasingly
recognized for their role in the development of tumors (12), as well as in the pathogenesis of
degenerative diseases, including intervertebral disc degeneration
and OA (13). In the present
study, circ0072568 was inhibited in the cartilage tissue of
individuals with OA and was linked to the onset and development of
OA. Circ0072568 was also shown to prevent OA by inhibiting
miR-328-5p and TOP1.
The PDE4E gene is the source of circ0072568, and has
been linked to tumor invasion, and cell proliferation and death
(14). In the present study,
circ0072568 expression was decreased in IL-1β-stimulated
chondrocytes. Moreover, it was found that the overexpression of
circ0072568 affected ECM metabolism and the inflammatory response
in chondrocytes. These data suggest that circ0072568 may be a novel
and effective molecular target in OA.
As a basic building block of the ceRNA network,
circRNAs have been found to function as ‘miRNA sponges’ (15). miR-382-5p functions an oncogene or
cancer suppressor gene (16) is
also involved in the regulation of inflammation (17,18).
In the present study, miR-382-5p expression was significantly
incresaed in OA tissues and IL-1β-stimulated human chondrocytes,
suggesting that it has a pro-inflammatory effect in OA.
It has been demonstrated that TOP1 is downregulated
in the synovium and blood samples of patients with OA (19). This is consistent with the findings
of the present study. miRNAs can directly target the mRNAs of the
target gene via complementary base pairing (20). Previous studies have confirmed that
TOP1 can be regulated by miRNAs, including miR-24(21), miR-21-5p (22) and miR-9(23). The present study found that TOP1
may be one of the potential targets of miR-382-5p. There is
increasing evidence to indicate that the regulatory network of the
circRNAs/miRNAs/mRNAs, such as circ0092516/miR-337-3P/PTEN
(9) and
circHIPK3/miR-124/SOX8(24), plays
a vital role in regulating the progression of OA. As demonstrated
herein, the protective effects of circ0072568 against the
inflammatory response and ECM degradation in IL-1β-stimulated
chondrocytes were achieved, at least in part, by the regulation of
hte miR-382-5p/TOP1 axis. Future studies are required however, to
further explore whether IL-1β regulates the chondrocyte phenotype
by mediating TOP1.
However, both miR-382-5p and TOP1 can only partially
explain the role of circ0072568 in OA. Further regulatory networks
for circ0072568 need to be studied in vitro and in
vivo, using animal models. Previous research has indicated that
inhibiting ECM degradation and the secretion of inflammatory
factors may delay the progression of OA to a certain extent
(25,26). In this study, we mainly used
chondrocytes as the modeling cell line. Further studies are
required to explore the role of circ0072568 in OA from the
perspective of macrophage infiltration and its role in OA
progression.
In conclusion, the present study demonstrated a
novel regulatory network, namely the circ0072568/miR-382-5p/TOP1
axis, which promoted the development of OA by accelerating ECM
degradation and inflammation. These results may provide aid the
development of novel potential treatment strategies for OA.
Supplementary Material
Results of cell transfection. (A)
Expression of miR-382-5p in chondrocytes transfected with
miR-382-5p mimic and appropriate negative control (miR-NC). (B)
Expression of miR-382-5p in chondrocytes transfected with
miR-382-5p inhibitor (inhib-miR-382-5p) and appropriate negative
control (inhib-NC). (C) Expression of circ0072568 in chondrocytes
transfected with oe-circ0072586 and appropriate negative control
(oe-NC). (D) Expression of circ0072568 in chondrocytes transfected
with circ0072586 siRNA (si-circ0072568) and appropriate negative
control (si-nc).
Acknowledgements
Not applicable.
Funding
Funding: Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Author's contributions
QC, CL and JH conducted the experiments. QC and RG
were involved in the writing, data analysis and revision of the
manuscript. CL and JH were involved in data analysis. All authors
have read and approved the final manuscript. QC and CL confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Zhongda Hospital Southeast University of Science and
Technology (approval no. ZDL22-21).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sacitharan PK: Ageing and Osteoarthritis.
Subcell Biochem. 91:123–159. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Geyer M and Schönfeld C: Novel insights
into the pathogenesis of osteoarthritis. Curr Rheumatol Rev.
14:98–107. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Mumtaz PT, Taban Q, Dar MA, Mir S, Haq ZU,
Zargar SM, Shah RA and Ahmad SM: Deep insights in circular RNAs:
From biogenesis to therapeutics. Biol Proced Online.
22(10)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ehrlich GD: Circular RNAs as diagnostic
biomarkers for osteoarthritis. Genet Test Mol Biomarkers.
23:701–702. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Patop IL, Wüst S and Kadener S: Past,
present, and future of circRNAs. EMBO J. 38(e100836)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhang Q, Qiao X and Xia W: CircSERPINE2
weakens IL-1β-caused apoptosis and extracellular matrix degradation
of chondrocytes by regulating miR-495/TGFBR2 axis. Biosci Rep.
40(BSR20201601)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhou ZB, Huang GX, Fu Q, Han B, Lu JJ,
Chen AM and Zhu L: circRNA.33186 contributes to the pathogenesis of
osteoarthritis by sponging miR-127-5p. Mol Ther. 27:531–541.
2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Shen S, Wu Y, Chen J, Xie Z, Huang K, Wang
G, Yang Y, Ni W, Chen Z, Shi P, et al: CircSERPINE2 protects
against osteoarthritis by targeting miR-1271 and ETS-related gene.
Ann Rheum Dis. 78:826–836. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Huang Z, Ma W, Xiao J, Dai X and Ling W:
CircRNA_0092516 regulates chondrocyte proliferation and apoptosis
in osteoarthritis through the miR-337-3p/PTEN axis. J Biochem.
169:467–475. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sharma L: Osteoarthritis of the Knee. N
Engl J Med. 384:51–59. 2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhang HD, Jiang LH, Sun DW, Hou JC and Ji
ZL: CircRNA: A novel type of biomarker for cancer. Breast Cancer.
25:1–7. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Marques-Rocha JL, Samblas M, Milagro FI,
Bressan J, Martínez JA and Marti A: Noncoding RNAs, cytokines, and
inflammation-related diseases. FASEB J. 29:3595–3611.
2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Liu F, Ma J, Wang K, Li Z, Jiang Q, Chen
H, Li W and Xia J: High expression of PDE4D correlates with poor
prognosis and clinical progression in pancreaticductal
adenocarcinoma. J Cancer. 10:6252–6260. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zang J, Lu D and Xu A: The interaction of
circRNAs and RNA binding proteins: An important part of circRNA
maintenance and function. J Neurosci Res. 98:87–97. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sun LP, Xu K, Cui J, Yuan DY, Zou B, Li J,
Liu JL, Li KY, Meng Z and Zhang B: Cancer-associated
fibroblast-derived exosomal miR-382-5p promotes the migration and
invasion of oral squamous cell carcinoma. Oncol Rep. 42:1319–1328.
2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Xiang W, Jiang L, Zhou Y, Li Z, Zhao Q, Wu
T, Cao Y and Zhou J: The lncRNA Ftx/miR-382-5p/Nrg1 axis improves
the inflammation response of microglia and spinal cord injury
repair. Neurochem Int. 143(104929)2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhang X, Zhang Y, Meng Q, Sun H, Wu S, Xu
J, Yun J, Yang X, Li B, Zhu H, et al: MicroRNA-382-5p is involved
in pulmonary inflammation induced by fine particulate matter
exposure. Environ Pollut. 262(114278)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Rialdi A, Campisi L, Zhao N, Lagda AC,
Pietzsch C, Ho JSY, Martinez-Gil L, Fenouil R, Chen X, Edwards M,
et al: Topoisomerase 1 inhibition suppresses inflammatory genes and
protects from death by inflammation. Science.
352(aad7993)2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Krol J, Loedige I and Filipowicz W: The
widespread regulation of microRNA biogenesis, function and decay.
Nature reviews Genetics. 11:597–610. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
21
|
Bu H, Baraldo G, Lepperdinger G and
Jansen-Dürr P: mir-24 activity propagates stress-induced senescence
by down regulating DNA topoisomerase 1. Exp Gerontol. 75:48–52.
2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chen JC, Hsieh YY, Lo HL, Li A, Chou CJ
and Yang PM: In vitro and in silico mechanistic insights into
miR-21-5p-Mediated topoisomerase drug resistance in human
colorectal cancer cells. Biomolecules. 9(467)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kania EE, Carvajal-Moreno J, Hernandez VA,
English A, Papa JL, Shkolnikov N, Ozer HG, Yilmaz AS, Yalowich JC
and Elton TS: hsa-miR-9-3p and hsa-miR-9-5p as post-transcriptional
modulators of DNA topoisomerase IIα in human leukemia K562 cells
with acquired resistance to etoposide. Mol Pharmacol. 97:159–170.
2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wu Q, Yuan ZH, Ma XB and Tang XH: Low
expression of CircRNA HIPK3 promotes osteoarthritis chondrocyte
apoptosis by serving as a sponge of miR-124 to regulate SOX8. Eur
Rev Med Pharmacol Sci. 24:7937–7945. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Guilak F, Nims RJ, Dicks A, Wu CL and
Meulenbelt I: Osteoarthritis as a disease of the cartilage
pericellular matrix. Matrix Biol. 71-72:40-502018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Singh P, Marcu KB, Goldring MB and Otero
M: Phenotypic instability of chondrocytes in osteoarthritis: On a
path to hypertrophy. Ann N Y Acad Sci. 1442:17–34. 2019.PubMed/NCBI View Article : Google Scholar
|