Introduction
Diabetes is a chronic endocrine/metabolic disease
characterized by hyperglycemia due to insulin action and/or insulin
secretion defects. Diabetes is frequently accompanied by visceral
lesions, which may lead to serious complications and death
(1). Diabetic foot (DF), a serious
complication of diabetes mellitus (DM), causes a significant
economic, social and psychological burden due to its high
incidence, disability and mortality rates (2). The pathophysiology of foot ulcer and
soft tissue infection in diabetes is due to neuropathy, trauma and
peripheral arterial occlusive disease in numerous patients.
Diabetes neuropathy may lead to foot deformity, resulting in
increased skin pressure when walking. Once foot ulcers occur, there
is a high risk of invasive infection in the limbs. When the
condition is combined with peripheral arterial occlusive disease,
patients should be considered to have severe limb ischemia
(3). DF destroys the skin and deep
tissues of the patient's ankle joints and is frequently accompanied
by infection and/or varying degrees of lower limb arterial
occlusion. In severe cases, the muscle and bone tissue are involved
(2). In patients with DM aged
>50 years, the incidence of DF may be as high as 8.1%, and
~32/100 patients with DF who achieve healing exhibit recurrence
within 1 year (4). It is
noteworthy that the annual mortality rate of patients with DF is as
high as 11% (4). The major risk
factors for the progression of DF are male sex, prolonged history
of DM, smoking, drinking, visual impairment, comorbidities and
complications. Certain partial risk factors for DF, including
neuropathy, peripheral arterial disease, history of toe amputation,
abnormal plantar pressure and lower extremity venous insufficiency,
accelerate its progression (2,5).
Although interventions have been developed to target these risk
factors, the incidence of DF is still annually increasing (4). Therefore, it is imperative that
studies actively seek to establish new risk factors for DF. The
different causes and clinical manifestations of DF require a
multidisciplinary approach to address the ultimate goal of
treatment, prevention of amputation and maintenance of functional
foot with load-bearing capacity (6).
Previous studies have reported that the prevalence
of anemia in patients with DF was as high as 51.9-85.3% (7), and the rate of adverse outcomes was
even higher (8). However, it has
remained to be clarified whether anemia is a poor prognostic factor
for DF, and whether it affects DF outcomes and reduces the survival
rate. The aim of the present study was to analyze the clinical
characteristics and risk factors of DF in patients with type 2 DM
(T2DM) and explore the association between anemia and the risks and
outcomes of DF, as this information may provide a theoretical
clinical basis for the prevention and treatment of DF and the
improvement of its outcomes.
Materials and methods
Study population
Between January and December 2021, a total of 145
patients with T2DM were admitted to Suqian First Hospital in
(Suqian, China). Patients were divided into the DF and non-DF
groups (n=80 and 65, respectively), based on whether they had DF.
The inclusion criteria were as follows: i) Diagnosis of T2DM based
on the Guidelines for the Prevention and Treatment of T2DM in China
(9); ii) diagnosis of DF according
to the International Working Group on the DF guidelines (2); and iii) aged between 18 and 80 years.
The exclusion criteria were as follows: i) Diagnosis of type 1,
gestational or any special type of diabetes; ii) pregnancy or
lactation; iii) hematological disease; iv) recent consumption of
iron, folic acid or B12 supplements; v) DF secondary to severe
injury, such as trauma; vi) dehydration and nutritional disorders;
vii) other causes of anemia, including chronic renal failure,
chronic liver disease, malabsorption, insufficient meat intake and
strict vegetarian diet.
Data collection and measurement
The patients' body weight and height were measured
using a height and weight scale. During the weight and height
measurements, patients were requested to remove heavy clothes,
shoes, bags or any other heavy items. These data were accurate to
0.1 of their respective units. The body mass index (BMI) was
calculated by dividing the body weight in kg by the squared body
height in m2. The blood pressure was measured in the
supine position at rest using a mercury sphygmomanometer. The mean
of three measurements (at least 3 min apart) was used for the
analysis. Other basic characteristics, such as age, sex, course of
diabetes and history of smoking and drinking, were collected by
trained investigators using a standard questionnaire. Fasting
venous blood samples were collected on the first day after
admission to determine the patients' fasting plasma glucose (FPG)
levels and the related biochemical parameters, including total
cholesterol (TC), triglycerides (TG), high-density lipoprotein
cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C),
serum creatinine (Scr), albumin (Alb), hemoglobin A1c (HbA1c), Hb
and C-reactive protein (CRP) levels (VARIANT II and D-10 Systems;
Bio-Rad Laboratories, Inc.).
The estimated glomerular filtration rate (eGFR) was
calculated from the Scr levels using the Chinese version of the
simplified Modification of Diet in Renal Disease formula (10). Anemia was defined as a Hb
concentration of <130 and <120 g/l in men and women,
respectively (7,11).
Statistical analysis
Statistical analysis was performed using SPSS
version 24.0 (IBM Corp.). Continuous and categorical variables were
expressed as the mean ± SD and as n (%), respectively. Comparisons
between the groups were made using a Student's t-test and the
χ2 test for continuous and categorical data,
respectively. The median (interquartile range) was used for
non-normally distributed variables and the Wilcoxon rank-sum test
was used for comparison between the groups. Single-factor binary
logistic regression analysis was performed to assess the impact of
the clinical indicators on the risk of DF. Multivariate binary
logistic regression analysis was performed to evaluate the
association between Hb and a high DF risk. Kaplan-Meier plots were
used to examine patient survival. Log-rank tests were performed to
assess statistically significant differences between survival
curves. P≤0.05 was considered to indicate a statistically
significant difference.
Results
Study participant characteristics
A total of 145 patients with T2DM were analyzed in
the present study, including 76 women and 78 men, and divided into
two groups (DF and non-DF). The mean age was 67.92±11.75 years and
the mean diabetes duration was 12.06±7.34 years. As presented in
Table I, the duration of DM, age,
history of smoking and alcohol consumption, and Scr and CRP levels
in the DF group were significantly higher compared with those in
the non-DF group, while the eGFR, as well as the Hb, Alb and TC
levels, exhibited the opposite trend (i.e., lower in the DF group
compared to the non-DF group). No significant differences were
observed in terms of number of men, BMI, systolic blood pressure,
diastolic blood pressure, HbA1c, FPG, TG, HDL-C or LDL-C.
 | Table IComparison of the general data from
the patients with type 2 diabetes mellitus in the DF and non-DF
groups. |
Table I
Comparison of the general data from
the patients with type 2 diabetes mellitus in the DF and non-DF
groups.
| Parameter | Non-DF group
(n=65) | DF group (n=80) | T/χ2 | P-value |
|---|
| Male sex | 30.00 (46.15) | 48.00 (60.00) | 2.766a | 0.096 |
| Age, years | 66.46±10.04 | 69.10±12.92 | -1.383b | 0.024 |
| BMI, kg/m² | 26.38±3.39 | 24.64±4.60 | 2.536b | 0.254 |
| Diabetes duration,
years | 10.00 (7.83,
12.51) | 12.00 (8.24,
13.16) | -2.383c | 0.017 |
| History of smoking,
years | 12.00 (9.24,
20.16) | 31.00 (17.64,
41.58) | 7.076a | 0.008 |
| History of drinking,
years | 8.00 (6.51,
12.31) | 21.00 (17.16,
26.25) | 4.004a | 0.045 |
| SBP, mmHg | 134.00 (21.00) | 137.50 (23.75) | -1.014c | 0.310 |
| DBP, mmHg | 76.03±9.22 | 75.66±10.01 | 0.228b | 0.426 |
| HbA1c, % | 8.00 (3.30) | 8.30 (3.35) | -0.639c | 0.523 |
| FPG, mmol/l | 8.00 (6.67) | 7.97 (5.26) | -0.634c | 0.526 |
| Scr, mg/l | 0.70 (0.31) | 0.96 (0.63) | -4.475c | <0.001 |
| eGFR, ml/(min x 1.73
m²) | 117.97±42.09 | 87.56±52.81 | 3.770b | <0.001 |
| Hb, g/l | 133.15±25.01 | 117.25±22.00 | 4.040b | <0.001 |
| Alb, g/l | 41.50 (4.65) | 35.75 (7.15) | -6.040c | <0.001 |
| TG, mmol/l | 1.35 (1.39) | 1.35 (0.91) | -1.263c | 0.207 |
| TC, mmol/l | 4.46±1.25 | 3.96±1.13 | 2.550b | 0.010 |
| HDL-C, mmol/l | 1.09 (0.47) | 1.14 (0.46) | -0.925c | 0.355 |
| LDL-C, mmol/l | 2.55±0.86 | 2.37±0.92 | 1.140b | 0.260 |
| CRP, mg/l | 4.90 (2.95) | 9.60 (14.80) | -3.872c | <0.001 |
Single-factor binary logistic
regression analysis of the clinical data and DF
As presented in Table
II, a single-factor binary logistic regression analysis was
performed with DF as the dependent variable and different clinical
indicators of patients from the two groups as independent
variables. The duration of DM, and Scr and CRP levels were
significant risk factors for DF, while the eGFR, Hb, Alb and TC
levels were protective factors against DF.
 | Table IISingle-factor binary logistic
regression analysis of the influence on the risk of DF. |
Table II
Single-factor binary logistic
regression analysis of the influence on the risk of DF.
| Variable | B | SE | Wald | Exp(B) | P-value |
|---|
| Male sex | -0.560 | 0.338 | 2.747 | 0.571 | 0.097 |
| Age, years | 0.019 | 0.014 | 1.799 | 1.020 | 0.180 |
| Diabetes duration,
years | 0.053 | 0.024 | 4.761 | 1.055 | 0.029 |
| BMI, kg/m² | -0.116 | 0.480 | 5.957 | 0.890 | 0.150 |
| SBP, mmHg | 0.012 | 0.010 | 1.317 | 1.012 | 0.251 |
| DBP, mmHg | -0.004 | 0.017 | 0.053 | 0.996 | 0.818 |
| HbA1c, % | 0.069 | 0.072 | 0.903 | 1.071 | 0.342 |
| FPG, mmol/l | -0.007 | 0.037 | 0.039 | 0.993 | 0.844 |
| Scr, mg/dl | 2.057 | 0.579 | 12.606 | 7.825 | <0.001 |
| eGFR, ml/(min x
1.73 m²) | -0.014 | 0.004 | 11.637 | 0.986 | 0.001 |
| Hb, g/l | -0.033 | 0.009 | 13.559 | 0.968 | <0.001 |
| Alb, g/l | -0.245 | 0.048 | 26.692 | 0.782 | <0.001 |
| TG, mmol/l | 0.018 | 0.032 | 0.321 | 1.018 | 0.571 |
| TC, mmol/l | -0.373 | 0.154 | 5.840 | 0.688 | 0.016 |
| HDL-C, mmol/l | -0.573 | 0.538 | 1.132 | 0.564 | 0.287 |
| LDL-C, mmol/l | -0.233 | 0.205 | 1.293 | 0.792 | 0.256 |
| CRP, mg/l | 0.078 | 0.028 | 7.921 | 1.082 | 0.005 |
Multivariate binary logistic
regression analysis following eGFR stratification
According to the single-factor binary logistic
regression analysis, the Scr level was a significant risk factor
for DF. Therefore, to exclude the influence of renal function, all
participants were stratified according to their eGFRs and divided
into two groups (H-90 and L-90). The H-90 group was defined as
having an eGFR ≥90 ml/(min x 1.73 m2) and the L-90 group
as having an eGFR <90 ml/(min x 1.73 m2).
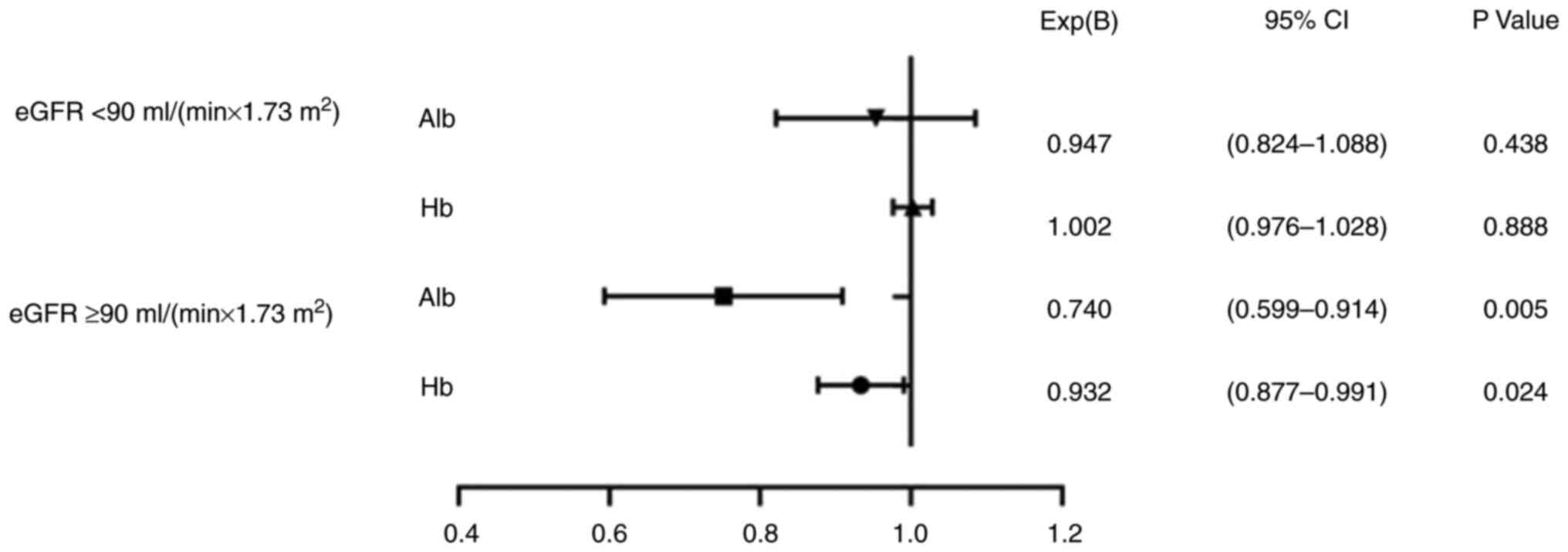
As indicated in Fig.
1, after adjusting for DM duration, and Scr, Hb, Alb, TC and
CRP levels, it was found that Hb and Alb levels were protective
factors against the risk of DF in the H-90 group (odds ratio=0.932
and 0.740, 95% CI=0.877-0.991 and 0.599-0.914, respectively,
P=0.024 for both). However, no statistical significance was
observed for the L-90 group.
Survival curve analysis for all
patients with or without DF
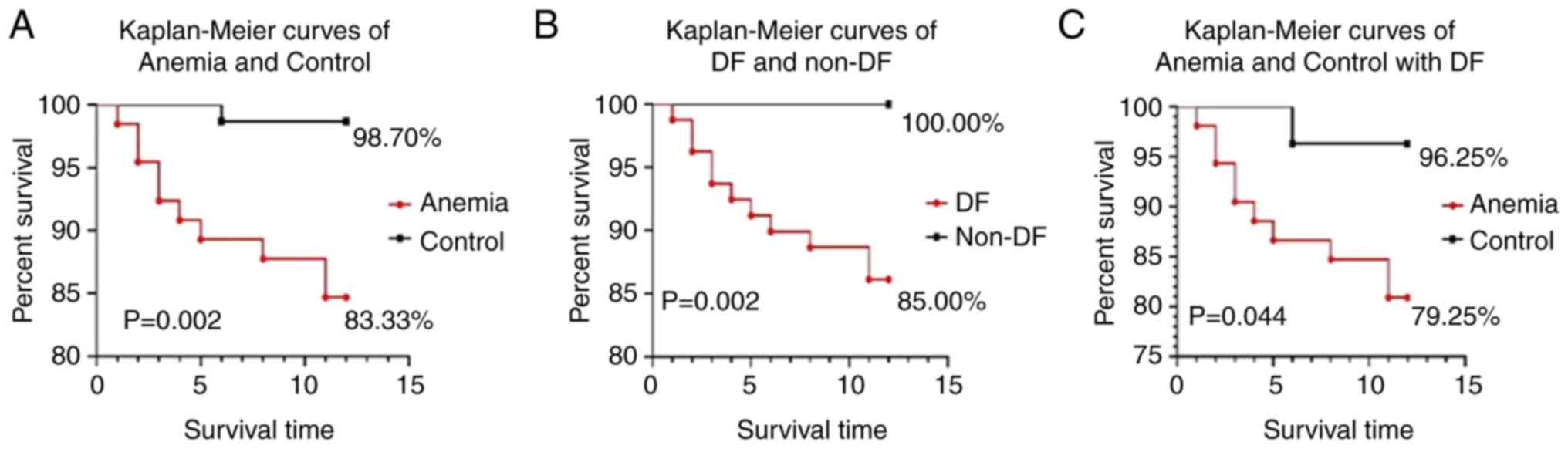
As presented in Fig.
2A, the 1-year survival rate of the patients with anemia was
significantly lower than that of the control group (83.33 vs.
93.7%; P=0.002) in all patients. Similarly, the 1-year survival
rate of the patients with DF was significantly lower than that of
the control group (85 vs. 100%, P=0.002; Fig. 2B) in all of the patients. In the DF
group, the 1-year survival rate of the patients with anemia was
significantly lower than that of the patients without anemia (79.25
vs. 96.25%, P=0.044; Fig. 2C).
Discussion
DF, one of the most serious complications of T2DM,
is associated with a variety of factors, such as loss of protective
sensation, peripheral arterial disease, foot deformities and foot
ulcers (12). It is associated
with significant costs and high disability rates, as well as poor
prognosis, all of which affect the quality of life of patients.
Recently, an increasing number of studies demonstrated a high
prevalence of anemia in patients with DF (9). The present study indicated that the
prevalence of anemia in the DF group was as high as 53%, which was
markedly higher than the 13% in the non-DF group. A previous study
indicated that low Hb levels cause a false reduction in the HbA1c
levels, reduce tissue oxygenation, weaken the antioxidant system
and accelerate nerve damage (13).
In addition, anemia and DF may share a common pathophysiological
mechanism (8). Studies suggested
that the incidence of anemia in patients with DF is high, and
anemia should be investigated as a risk factor for DF. With changes
in the microcirculation, the potential negative effects of anemia
may hinder ulcer healing, leading to higher amputation and
mortality rates (14,15). The severity of anemia has been
proven to be associated with the severity of DF (16). Therefore, determining whether Hb is
a protective factor against DF may prove useful in terms of the
prevention, delay and improvement of the occurrence and development
of DF.
In the present study, high eGFR, Hb and Alb levels
protected against DF. As the serum Hb level increased, the risk of
DF dropped to 96.8%. By contrast, the Scr, TC and CRP levels were
risk factors for the development of DF. However, there is still a
lack of studies on the survival rate in relation to the Hb level
and DF.
There is still controversy around the mechanism of
anemia in patients with DM. Alsayegh et al (17) proposed that anemia in patients with
DM is mainly associated with renal insufficiency, glomerular
hyperfiltration, chronic inflammation, microvascular injury,
autonomic neuropathy and decreased erythropoietin synthesis caused
by an abnormal renin-angiotensin-aldosterone system or other
abnormalities (18). In diabetic
patients with normal renal function, the prevalence of anemia is
high. Studies suggested that abnormal iron storage, albuminuria,
hyperglycemia and neuropathy may increase the prevalence of anemia.
After adjusting for the eGFR, no obvious association was found
between anemia and nephropathy (19,20).
The present study demonstrated that the Scr level and eGFR were
significant risk factors for DF. To further analyze the
relationship between renal insufficiency, anemia and DF, all
participants were stratified according to their eGFR and the
association between certain clinical indicators, such as Hb and Alb
levels, and the risk of DF was analyzed. In the group with eGFR ≥90
ml/(min x 1.73 m²), the odds ratios for the Alb and Hb levels were
0.740 and 0.932, respectively (P<0.05). This meant that when the
renal function was normal, both the Hb and Alb levels protected
against DF.
According to the survival curve plotted for the
present study, in the DF group, the 1-year survival rate of the
patients with anemia was significantly lower than that of the
patients without anemia (79.25 vs. 96.25%), suggesting that anemia
was a risk factor for DF-related mortality. However, the mechanism
behind the relationship between anemia and DF remains unclear. The
possible mechanisms are as follows: i) Anemia may result in reduced
skin oxygenation and capillary blood flow, increased
hypoxia-inducible factors, aggravated lower limb ischemia,
infections and hindered wound healing (21). Decreased oxygenation may also lead
to endoneurial hypoxia and a weakened antioxidant system, resulting
in increased free radical production, endothelial dysfunction and
nerve damage (13,22). ii) Anemia may cause a false
decrease in HbA1c levels, leading to insufficient hypoglycemic
strength, and accelerated progression of microvascular and
macrovascular complications (23)
that increase the prevalence of myocardial infarction, stroke, DF
and other diseases. iii) The internal environment of patients with
DF features chronic inflammation, and certain pro-inflammatory
cytokines both inhibit hematopoietic function and reduce the level
of the raw material for hematopoiesis, namely iron (24-26).
In a hyperglycemic state, the end products of glycosylation on the
red blood cell membrane continue to accumulate, leading to changes
in the red blood cell rheology and quantity (27).
The present study differed from previous research.
To the best of our knowledge, it was the first clinical study on
the association between Hb, DF risk and survival rates in Asia. It
was found that the Hb level was a protective factor for DF,
independently of renal function, which suggested that anemia is
associated with a decrease in the survival rate of patients with
DF. However, the present study had certain limitations. First, the
sample size was small and the selected respondents only resided in
China. Furthermore, the causal relationship between the Hb level
and DF, or the effect of correcting anemia on DF prognosis, could
not be elucidated. In addition, the follow-up time in the present
study was short at just 1 year. Therefore, further clinical studies
and basic experiments are needed to investigate the causal
relationship between the Hb level and DF. Finally, multiple factor
analysis was not conducted on anemia and other risk factors, such
as the risk of infection and delayed wound healing. Follow-ups
should be performed after anemia correction. Moreover, the sample
size should be expanded to determine the optimal Hb level required
to reduce the risk for DF. A multivariate analysis of anemia and
other risk factors should be supplemented. Furthermore,
interventional research will be required to explore whether the
clinical correction of anemia may reduce the incidence of DF and
improve its outcome and prognosis. This information may provide
evidence-based medicine for the early prevention of DF.
In conclusion, the present study found that, in
patients with T2DM, the Hb level was a protective factor against
DF, and a low Hb level was found to be an independent risk factor
for DF. The present study suggested that anemia is associated with
a decrease in the survival rate of patients with DF. These results
should prompt clinicians to pay close attention to the Hb levels of
patients with DM and encourage a timely correction of anemia in
that population. Such interventions may have a positive role in the
prevention of DF and improvement of its survival rate.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by Suqian Sci & Tech
Program (Suqian, China; grant no. KY202213).
Availability of data and materials
Not applicable.
Authors' contributions
JL, MG and SM were responsible for the
conceptualization of the study. ZZ, YL,CC and JW were responsible
for data organization and statistical analysis. JL and ZZ wrote the
original draft. ZZ, JW, SM and MG wrote, reviewed and edited the
manuscript. MG and SM checked and confirm the authenticity of all
the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was in accordance with the
Declaration of Helsinki and was approved by the ethics committee of
Suqian First Hospital (Suqian, China; clinical trial no. 20220003).
The participants all provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang Z, Zhang L and Xu H: Effect of
Astragalus polysaccharide in treatment of diabetes mellitus: A
narrative review. J Tradit Chin Med. 39:133–138. 2019.PubMed/NCBI
|
|
2
|
Lipsky BA, Senneville E, Abbas ZG,
Aragón-Sánchez J, Diggle M, Embil JM, Kono S, Lavery LA, Malone M,
van Asten SA, et al: Guidelines on the diagnosis and treatment of
foot infection in persons with diabetes (IWGDF 2019 update).
Diabetes Metab Res Rev. 36 (Suppl 1)(e3280)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bandyk DF: The diabetic foot:
Pathophysiology, evaluation, and treatment. Semin Vasc Surg.
31:43–48. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Jiang Y, Wang X, Xia L, Fu X, Xu Z, Ran X,
Yan L, Li Q, Mo Z, Yan Z, et al: A cohort study of diabetic
patients and diabetic foot ulceration patients in China. Wound
Repair Regen. 23:222–230. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bus SA, van Netten JJ, Lavery LA,
Monteiro-Soares M, Rasmussen A, Jubiz Y and Price PE: International
Working Group on the Diabetic Foot. IWGDF guidance on the
prevention of foot ulcers in at-risk patients with diabetes.
Diabetes Metab Res Rev. 32 (Suppl 1):S16–S24. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Brocco E, Ninkovic S, Marin M, Whisstock
C, Bruseghin M, Boschetti G, Viti R, Forlini W and Volpe A:
Diabetic foot management: Multidisciplinary approach for advanced
lesion rescue. J Cardiovasc Surg (Torino). 59:670–684.
2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Shareef AM, Ahmedani MY and Waris N:
Strong association of anemia in people with diabetic foot ulcers
(DFUs): Study from a specialist foot care center. Pak J Med Sci.
35:1216–1220. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Costa RHR, Cardoso NA, Procopio RJ,
Navarro TP, Dardik A and de Loiola Cisneros L: Diabetic foot ulcer
carries high amputation and mortality rates, particularly in the
presence of advanced age, peripheral artery disease and anemia.
Diabetes Metab Syndr. 11 (Suppl 2):S583–S587. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Jia W, Weng J, Zhu D, Ji L, Lu J, Zhou Z,
Zou D, Guo L, Ji Q, Chen L, et al: Standards of medical care for
type 2 diabetes in China 2019. Diabetes Metab Res Rev.
35(e3158)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Schwandt A, Denkinger M, Fasching P,
Pfeifer M, Wagner C, Weiland J, Zeyfang A and Holl RW: Comparison
of MDRD, CKD-EPI, and Cockcroft-Gault equation in relation to
measured glomerular filtration rate among a large cohort with
diabetes. J Diabetes Complications. 31:1376–1383. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Bekele A, Teji Roba K, Egata G and
Gebremichael B: Anemia and associated factors among type-2 diabetes
mellitus patients attending public hospitals in Harari Region,
Eastern Ethiopia. PLoS One. 14(e0225725)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bus SA, Lavery LA, Monteiro-Soares M,
Rasmussen A, Raspovic A, Sacco ICN and van Netten JJ: International
Working Group on the Diabetic Foot. Guidelines on the prevention of
foot ulcers in persons with diabetes (IWGDF 2019 update). Diabetes
Metab Res Rev. 36 (Suppl 1)(e3269)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wu F, Jing Y, Tang X, Li D, Gong L, Zhao
H, He L, Li Q and Li R: Anemia: An independent risk factor of
diabetic peripheral neuropathy in type 2 diabetic patients. Acta
Diabetol. 54:925–931. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yammine K, Hayek F and Assi C: Is there an
association between anemia and diabetic foot ulcers? A systematic
review and meta-analysis. Wound Repair Regen. 29:432–442.
2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Den JL, Gay LM and Barshes NR: Severe
anemia, anorexia, and uremia associated with diabetic foot
infections: A case series. Foot (Edinb). 53(101926)2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yammine K, Akiki S, Assi C and Hayek F:
Anemia prevalence among patients with diabetic foot ulcers
necessitating surgery on admission: A preliminary, retrospective
comparative study. Wounds. 34:216–219. 2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Alsayegh F, Waheedi M, Bayoud T, Al Hubail
A, Al-Refaei F and Sharma P: Anemia in diabetes: Experience of a
single treatment center in Kuwait. Prim Care Diabetes. 11:383–388.
2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Pappa M, Dounousi E, Duni A and Katopodis
K: Less known pathophysiological mechanisms of anemia in patients
with diabetic nephropathy. Int Urol Nephrol. 47:1365–1372.
2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Gu L, Lou Q, Wu H, Ouyang X and Bian R:
Lack of association between anemia and renal disease progression in
Chinese patients with type 2 diabetes. J Diabetes Investig.
7:42–47. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Mikhail A, Brown C, Williams JA, Mathrani
V, Shrivastava R, Evans J, Isaac H and Bhandari S: Renal
association clinical practice guideline on Anaemia of chronic
kidney disease. BMC Nephrol. 18(345)2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sahay M, Kalra S, Badani R, Bantwal G,
Bhoraskar A, Das AK, Dhorepatil B, Ghosh S, Jeloka T, Khandelwal D,
et al: Diabetes and Anemia: International Diabetes Federation
(IDF)-Southeast Asian Region (SEAR) position statement. Diabetes
Metab Syndr. 11 (Suppl 2):S685–S695. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
He BB, Xu M, Wei L, Gu YJ, Han JF, Liu YX,
Bao YQ and Jia WP: Relationship between Anemia and chronic
complications in Chinese patients with type 2 diabetes mellitus.
Arch Iran Med. 18:277–283. 2015.PubMed/NCBI
|
|
23
|
Adamsson Eryd S, Svensson AM, Franzen S,
Eliasson B, Nilsson PM and Gudbjornsdottir S: Risk of future
microvascular and macrovascular disease in people with type 1
diabetes of very long duration: A national study with 10-year
follow-up. Diabet Med. 34:411–418. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hoepers AT, Menezes MM and Frode TS:
Systematic review of anaemia and inflammatory markers in chronic
obstructive pulmonary disease. Clin Exp Pharmacol Physiol.
42:231–239. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Chuan F, Zhang M, Yao Y, Tian W, He X and
Zhou B: Anemia in patients with diabetic foot ulcer: Prevalence,
clinical characteristics, and outcome. Int J Low Extrem Wounds.
15:220–226. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chaparro CM and Suchdev PS: Anemia
epidemiology, pathophysiology, and etiology in low- and
middle-income countries. Ann N Y Acad Sci. 1450:15–31.
2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Blaslov K, Kruljac I, Mirosevic G, Gacina
P, Kolonic SO and Vrkljan M: The prognostic value of red blood cell
characteristics on diabetic retinopathy development and progression
in type 2 diabetes mellitus. Clin Hemorheol Microcirc. 71:475–481.
2019.PubMed/NCBI View Article : Google Scholar
|