Introduction
Non-alcoholic fatty liver disease (NAFLD) represents
a spectrum of liver injury diseases, including simple steatosis,
non-alcoholic steatohepatitis as well as fibrosis and liver
cirrhosis (1). Notably, the
progressive form of NAFLD can lead to hepatocellular carcinoma and
liver-related mortality (2,3).
With the continuous increase in the prevalence of metabolic
syndromes, such as type 2 diabetes mellitus (T2DM), obesity and
hyperlipidemia, the morbidity of NAFLD has increased in parallel,
affecting ~25% of the population worldwide, thus significantly
contributing to the disease burden (2,4). At
present, NAFLD is considered to be one of the most common chronic
liver diseases worldwide (5).
However, to date, there have been no approved pharmacological
approaches for this condition. Therefore, the development of novel
therapeutic strategies to improve the progression of NAFLD is
important.
The pathogenesis and treatment of NAFLD have been
widely investigated; however, they are still not entirely
understood. The most accepted mechanism of NAFLD is the ‘multiple
hits’ theory. According to this theory, NAFLD can be caused by
several factors, such as insulin resistance (IR), dyslipidemia,
oxidative stress (OS), endoplasmic reticulum stress and
inflammation (6,7). Notably, dyslipidemia is a primary
feature of NAFLD (8), while OS
(free radical damage) and inflammation are considered as the most
significant mechanisms leading to hepatic cell death and tissue
injury (9,10). Therefore, regulating lipid
metabolism, OS and the inflammatory response may be a particular
approach for ameliorating the progression of NAFLD.
Ophiopogonin D (OP-D) is a significant
pharmacological ingredient of the traditional Chinese medicine
Ophiopogon japonicus. It has been reported that OP-D
exhibits several pharmacological effects, including antioxidant and
anti-inflammatory activities, while it also inhibits venous
thrombosis (11-13).
A recent study showed that OP-D can improve renal function by
inhibiting OS and inflammation in streptozotocin-induced diabetic
nephropathy rats (14). Therefore,
the current study aimed to explore the role of OP-D on NAFLD in a
mouse model of high-fat diet (HFD)-induced obesity.
Materials and methods
Drugs and reagents
OP-D was obtained from Shanghai Yuanye Biotechnology
Co., Ltd. Palmitic acid (PA) was purchased from Sigma-Aldrich
(Merck KGaA).
Animal experiments
A total of 15 male C57BL/6J mice (age, 3-4 weeks;
weight, 17-20 g) were purchased from Shanghai Model Organisms
Centre, Inc. Mice with similar body weights were allowed to adjust
to the environment for one week prior to the experiments.
Subsequently, mice (age, 4-5 weeks) were randomly divided into the
following three groups: Normal chow diet (ND); HFD; and HFD + OP-D
group. Mice were given free access to food and distilled water.
Mice fed with ND (cat. no. D12450B; 10% fat, 70% carbohydrates and
20% protein; Research Diets, Inc.) or HFD (cat. no. D12492; 60%
fat, 20% carbohydrate and 20% protein; Research Diets, Inc.).
Following feeding for 12 weeks, HFD-induced obese mice were
randomly allocated to the test groups for pharmacological studies.
Mice in the HFD + OP-D group continued to be fed with HFD and were
intragastrically administered with 5 mg/kg/day OP-D (dissolved in
0.5% of carboxymethyl cellulose) for the following 4 weeks,
according to preliminary experiments and previous studies (14,15).
Mice in the other groups were treated with the same volume of 0.5%
sodium carboxyl methyl cellulose solution (in order to protect the
gastric mucosa of mice) according to a previous study (16,17).
At the end of the experiment (16 weeks), all mice were sacrificed
by cervical dislocation. All mice were housed in specific
pathogen-free facility under a 12-h light/dark cycle, a relative
humidity of 50% and a controlled temperature of 22±1˚C. All animal
procedures were approved by the Medical Ethics Committee of Wuhan
University of Science and Technology (Wuhan, China; approval
number, 2022139).
Serum biochemical measurements
At the end of the experiment, mice were fasted
overnight and blood samples from the tail were collected to detect
blood glucose levels using a blood glucose meter (Bayer AG). The
mice were then sacrificed, and blood samples were collected by
aortic puncture with centrifugation (1,300 x g for 10 min at 4˚C)
to obtain serum, and stored at -80˚C for the blood chemistry
measurements. The levels of insulin, IL-6, IL-1β and TNFα in serum
were detected using the corresponding commercially available ELISA
kits (insulin: cat. no. KCD-E1025; IL-6: cat. no. KCD-E1002; IL-1β:
cat. no. KCD-E10010; TNFα: cat. no. KCD-E1018; Shanghai Kechuangda
Biomedical Technology Co., Ltd.), according to the manufacturer's
instructions. The serum levels of triglycerides (TG), total
cholesterol (TC), low-density lipoprotein cholesterol (LDL-C),
alanine aminotransferase (ALT) and aspartate aminotransferase (AST)
were measured using an automatic biochemical analyzer (Hitachi,
Ltd.). In addition, the serum levels of free fatty acids (FFA) were
detected by a commercially available colorimetric assay kit (cat.
No. A042-1-1, Nanjing Jiancheng Bioengineering Institute). The
homeostatic model assessment for insulin resistance (HOMA-IR) index
was calculated as follows: HOMA-IR=[fasting serum glucose (mmol/l)
x fasting serum insulin (µU/ml)]/22.5.
Liver histology
Liver tissues from mice in each group were collected
and fixed in 10% formaldehyde neutral buffer solution for 24 h at
4˚C, followed by washing with tap water, dehydration in alcohol and
embedding in paraffin. Subsequently, the 5-µm thick transversal
sections were obtained, deparaffinized, dehydrated in ethanol
(50-100%) and cleared with xylene. The sections were stained using
hematoxylin and eosin (H&E) solution for 5 min at room
temperature (Wuhan Servicebio Technology Co., Ltd.), according to
the manufacturer's protocols. For Oil-red O staining, liver tissues
were frozen in optimal cutting temperature compound at -20˚C and
10-µm thick sections were obtained, followed by staining with
Oil-red O solution (Wuhan Servicebio Technology Co., Ltd.) for 10
min at room temperature, according to the manufacturer's protocols.
Images were observed using a NIKON imaging workstation (NIKON
digital sight DS-FI2; Nikon Corporation).
Hepatic biochemical analysis
The commercially available Triglyceride Assay Kit
(cat. no. ab65336; Abcam) was used to assess the levels of liver TG
by measuring the optical density at a wavelength of 570 nm using
spectrophotometry. The index of OS, including malondialdehyde
(MDA), superoxide dismutase (SOD), catalase (CAT) and glutathione
peroxidase (GSH-Px) levels, was determined using the corresponding
commercially available kits (MDA: cat. no. A003-1-1; SOD: cat. no.
A001-1-1; CAT: cat. no. A007-1-1; GSH-Px: cat. no. A005-1-1.
Nanjing Jiancheng Bioengineering Institute).
Cell cultures and treatments
Primary hepatocytes (PMHs) were isolated from mice.
Briefly, 6-8-week-old male C57BL/6J mice (n=6; weight, 18-22 g)
were purchased from Shanghai Model Organisms Centre, Inc. Mice were
given free access to food and distilled water, and were housed in a
specific pathogen-free facility under a 12-h light/dark cycle, a
relative humidity of 50% and a controlled temperature of 22±1˚C.
Mice were anesthetized using intraperitoneal injections of sodium
pentobarbital (50 mg/kg). After the liver tissue was separated, the
mice were sacrificed by cervical dislocation. The liver tissue was
then collected, minced and filtered after portal vein perfusion.
PMHs were purified and cultured in DMEM (cat. no. G4510; Wuhan
Servicebio Technology Co., Ltd.) supplemented with 1%
penicillin/streptomycin solution and 10% FBS (cat. no. G0004; Wuhan
Servicebio Technology Co., Ltd.). To assess cell viability, PMHs
(5x103) were seeded into 96-well plates for 24 h at
37˚C. The following day, when plates reached ~80% confluency, cells
were treated with various concentrations (0-20 µmol/l) of OP-D for
an additional 24 h at 37˚C. Cell viability was assessed using a
Cell Counting Kit-8 assay (Wuhan Servicebio Technology Co., Ltd.).
Briefly, 10 µl CCK-8 solution added into each well, which were then
incubated at 37˚C with 5% CO2 for 2 h. The optical
density was measured at a wavelength of 450 nm. To establish an
in vitro model of lipid accumulation, hepatocytes were
treated with PA (400 µmol/l) for 24 h at 37˚C. Then the cells were
treated with or without 10 mol/l OP-D for another 24 h. Cultured
cells were collected, isopropyl alcohol was added into
2x106 cells according to the proportion of 200 µl
homogenizing medium, and centrifugation was performed at 4˚C and
10,000 x g for 10 min to obtain the lysed cells. The content of TG
in lysed cells was measured using the TG Colorimetric Assay kit
(cat. no. E-BC-K261-M; Elabscience Biotechnology, Inc.) following
manufacturer's protocols. The optical density was measured at a
wavelength of 510 nm.
Measurements of cytokine levels in
culture supernatants
The levels of TNF-α, IL-1β and IL-6 in PMH
supernatants were determined using ELISA, according to the
manufacturer's instructions (Shanghai Kechuangda Biomedical
Technology Co., Ltd.).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from liver tissues or cells
and isolated using TRIzol® reagent (Thermo Fisher
Scientific, Inc.) according to the protocol provided by the
manufacturer. The isolated RNA was reverse transcribed into cDNA
using the RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
qPCR was performed using SYBR Green PCR master mix (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The primer sequences
used are listed in Table SI. All
reactions were conducted in triplicate and the thermal cycling
conditions were as follows: 30 sec at 95˚C, followed by 50 cycles
of 95˚C for 5 sec and 60˚C for 30 sec. The mRNA expression levels
of target genes were calculated using the 2-ΔΔCq method
and normalized to those of GAPDH (18).
Western blot analysis
Liver tissues or cells were lysed with RIPA buffer
containing protease inhibitors (Roche Applied Science). A BCA kit
(Beyotime Institute of Biotechnology) was used to detect protein
concentrations. The extracted proteins (20 µg of each protein) were
loaded onto 10% SDS-polyacrylamide gel electrophoresis, and
transferred to polyvinylidene fluoride membranes (MilliporeSigma)
after electrophoresis. Blots were blocked with 5% bovine serum
albumin (cat. no. GC305006; Wuhan Servicebio Technology Co., Ltd.)
at 4˚C for 2 h and then incubated with primary antibodies against
GAPDH (1:2,000; cat. no. 5174), P65 (1:1,000; cat. no. 8242),
phosphorylated (p-)P65 (Ser468) (1:1,000; cat. no. 3039), IκBα
(1:1,000; cat. no. 9242), p-IκBα (1:1,000; cat. no. 2859), TNFα
(1:1,000; cat. no. 3707) (all CST Biological Reagents Co., Ltd.) at
4˚C overnight, The membranes were then incubated with
HRP-conjugated anti-IgG secondary antibodies (cat. no. A0192;
1:2,000; Beyotime Institute of Biotechnology) at room temperature
for 45 min. GAPDH was used as an internal reference. The intensity
of protein bands was assessed using the ImageJ software (version
1.8.0; National Institutes of Health).
Immunofluorescence (IF) analysis
IF staining was carried out according to standard
procedures (19). Briefly, cells
were first fixed with 95% ethanol for 15 min at -20˚C. The cells
were then incubated and blocked with blocking solution (5% BSA/PBS)
for 30 min at room temperature, followed by being incubated
overnight with a primary antibody against p65 (1:200; cat. no.
8242; Cell Signaling Technology, Inc.) at 4˚C. Subsequently,
incubation with Alexa Fluor® 647-conjugated goat
anti-rabbit IgG (1:400; cat. no. ab150079; Abcam) at 4˚C for 1 h
was performed. The coverslips were mounted on microscope slides
with fluorescence reagent containing 10 µg/ml DAPI (Thermo Fisher
Scientific, Inc.). IF images were captured using the Fluoview
FV1000 confocal microscope (Olympus Corporation).
Statistical data analysis
Data are presented as the mean ± SEM. All analyses
were performed using GraphPad Prism 8.0 (GraphPad Software;
Dotmatics) or SPSS 22.0 (IBM Corp.) software. Data among groups
were compared using one-way ANOVA followed by Dunnett's test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
OP-D attenuates body weight gain in
HFD-treated mice
OP-D is a pharmacological compound of Ophiopogon
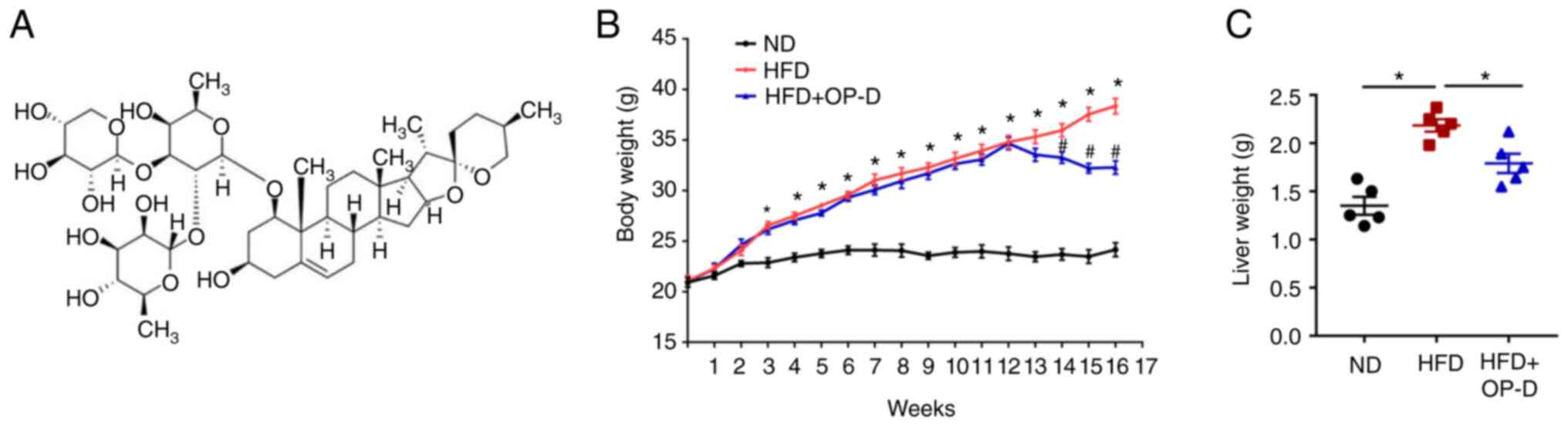
japonicus, a traditional Chinese medicine herb (14). The molecular structure of OP-D is
illustrated in Fig. 1A. The
current study aimed to explore whether OP-D administration could
inhibit body weight gain in mice fed with HFD. The results showed
that the body weight of mice in the HFD + OP-D group was notably
reduced compared with that in the HFD group at weeks 14-16
(Fig. 1B). Additionally, OP-D
administration significantly reduced liver weight in HFD-treated
mice compared with those in the HFD group (Fig. 1C).
OP-D improves glucose homeostasis and
IR in mice fed with HFD
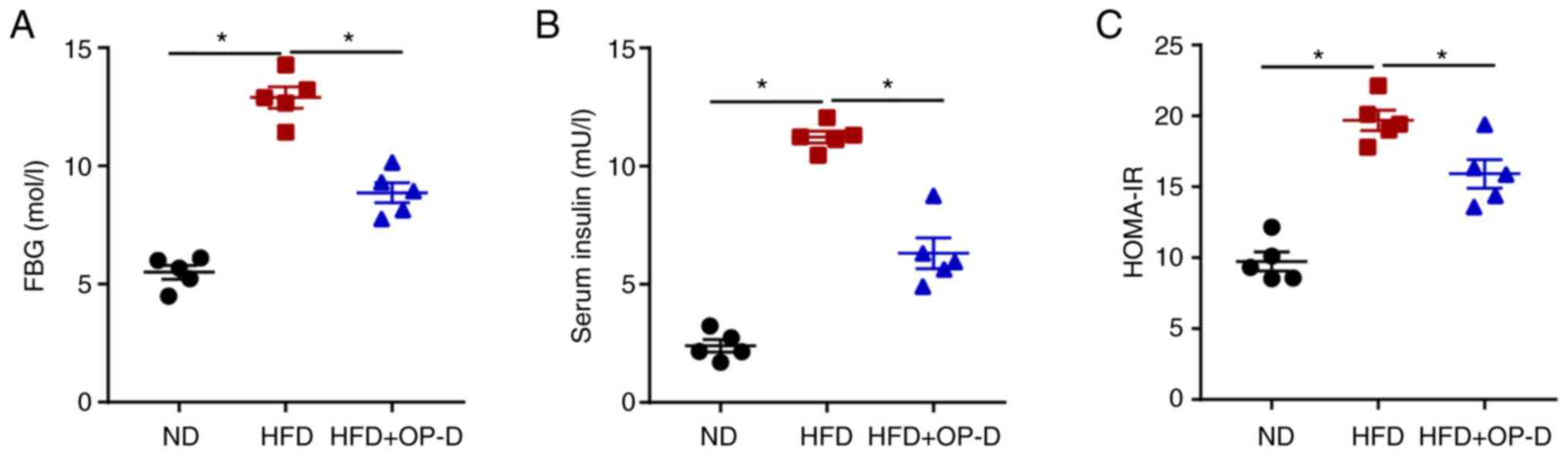
Subsequently, the present study investigated whether
OP-D could regulate glucose and insulin homeostasis in HFD-fed
mice. Consistent with previous studies (20,21),
HFD-treated mice exhibited elevated glucose and insulin levels
compared with ND control mice. The effect of the HFD on the levels
of fasting blood glucose (FBG) and insulin was significantly
reduced by OP-D treatment (Fig. 2A
and B). Furthermore, the HOMA-IR
index suggested that mice in the HFD + OP-D group displayed
significantly reduced IR compared with those in the HFD group
(Fig. 2C). These findings
indicated that OP-D could exert a significant role in improving
glucose homeostasis and IR in HFD-induced obese mice.
OP-D improves lipogenesis and hepatic
steatosis in NAFLD mice
To further explore whether OP-D could prevent
hepatic steatosis, H&E staining was first carried out. H&E
staining showed widespread vacuolation in the liver tissue of HFD
mice. However, OP-D treatment alleviated hepatic steatosis
(Fig. 3A). In addition, mice in
the HFD + OP-D group displayed a smaller area of hepatic lipid
droplets compared with mice in the HFD group (Fig. 3A). Treatment with OP-D
significantly reduced TG levels in the liver compared with the HFD
group, thus suggesting that OP-D could attenuate lipid accumulation
(Fig. 3B). Furthermore, TC, TG,
FFA and LDL-C levels were significantly reduced in the serum of HFD
mice treated with OP-D compared with mice treated with vehicle
(Fig. 3C-F). Subsequently, the
mRNA expression levels of lipid metabolism-related genes were
detected. The data showed that the expression levels of lipid
droplet formation-related genes
[3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR), fat
storage-inducing transmembrane protein (Fitm)1 and Fitm2], lipid
uptake-related genes [fatty acid-binding protein 1 (Fabp1), fatty
acid transporter 1 (Fatp1) and lipoprotein lipase (Lpl)] and
lipogenesis-related genes [fatty acid synthase (FAS), stearoyl-CoA
desaturase 1 (SCD1) and peroxisome proliferator-activated receptor
(PPAR)-γ] were significantly decreased by treatment of HFD-fed mice
with OP-D (Fig. 3G-I). By
contrast, the expression of fatty acid β-oxidation-related genes
[PPAR-α, peroxisomal acyl-coenzyme A oxidase 1 (Acox1) and
carnitine O-palmitoyl transferase 1α (Cpt1α)] were significantly
upregulated after OP-D treatment compared with the HFD group
(Fig. 3J). Furthermore, the
HFD-induced increased levels of ALT and AST in HFD mice were
significantly reduced by OP-D (Fig.
3K and L). Taken together,
these results suggested that OP-D could exert a protective role in
the development of NAFLD.
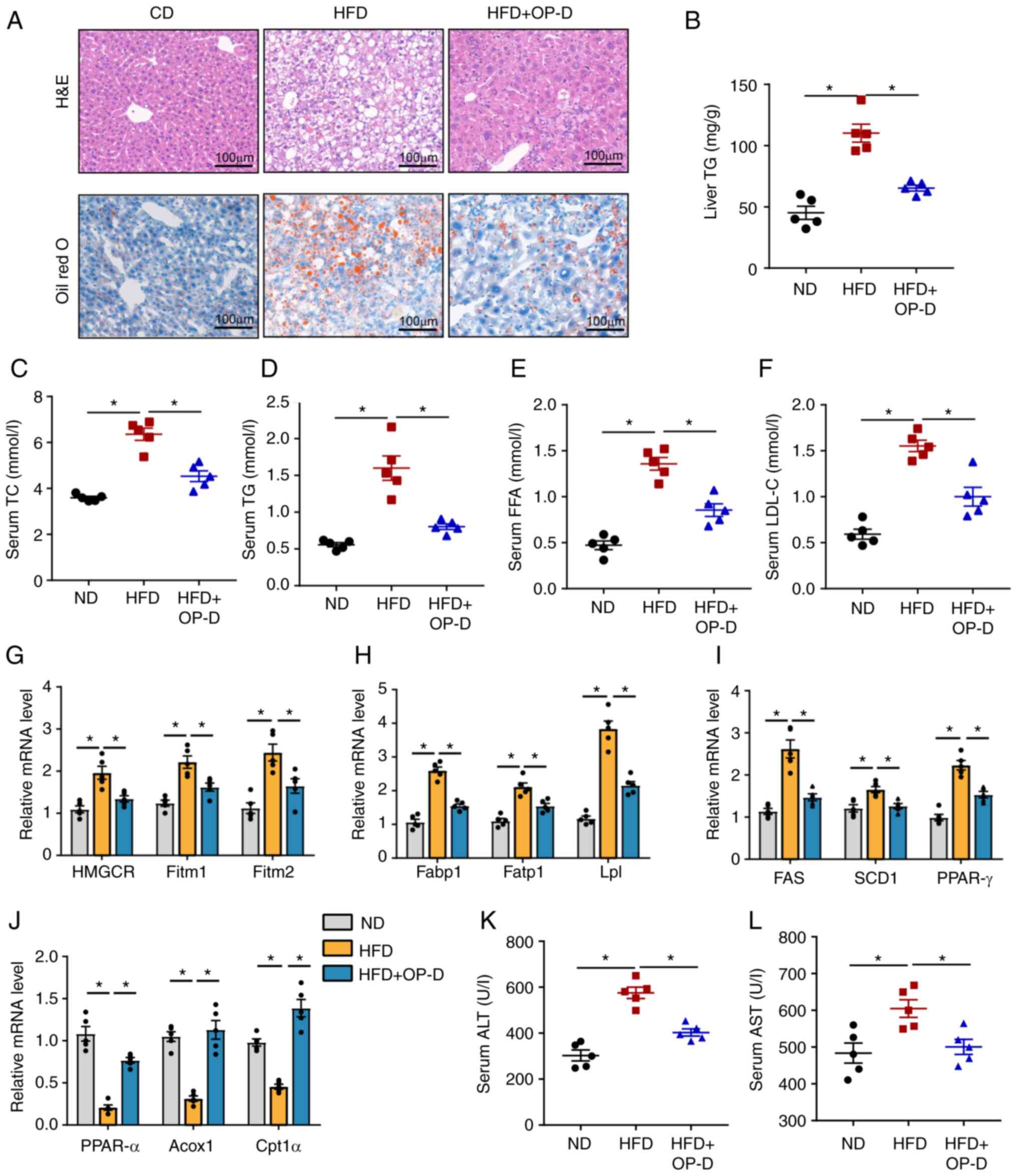 | Figure 3OP-D improves lipogenesis and hepatic
steatosis in HFD-induced NAFLD mice. (A) Representative images of
H&E (upper panel) and Oil-Red O (lower panel) stained liver
tissue. Scale bar, 100 µm. (B) Content of TG in the liver. Levels
of (C) TC, (D) TG, (E) FFA and (F) LDL-C in the serum. Relative
mRNA expression of (G) cholesterol synthesis and efflux-related
genes (HMGCR, Fitm1 and Fitm2), (H) uptake-related genes (Fabp1,
Fatp1 and Lpl), (I) fatty acid synthesis genes (FAS, SCD1 and
PPAR-γ) and (J) fatty acid oxidation genes (PPAR-α, Acox1 and
Cpt1α). Levels of (K) ALT and (L) AST in the serum (n=5).
*P<0.05. OP-D, Ophiopogonin D; HFD, high fat diet;
ND, normal chow diet; NAFLD, non-alcoholic fatty liver disease;
H&E, hematoxylin and eosin; TC, total cholesterol; TG,
triglycerides; FFA, free fatty acids; LDL-C, low-density
lipoprotein cholesterol; HMGCR, 3-hydroxy-3-methylglutaryl-coenzyme
A reductase; Fitm, fat storage-inducing transmembrane protein;
Fabp1, fatty acid-binding protein 1; Fatp1, fatty acid transport
protein 1; Lpl, lipoprotein lipase; FAS, fatty acid synthase; SCD1,
stearoyl-CoA desaturase-1; PPAR-γ, peroxisome
proliferator-activated receptor γ; PPAR-α, peroxisome
proliferators-activated receptor α; Acox1, acyl-CoA oxidase 1;
Cpt1α, carnitine palmitoyl transferase 1α; ALT, alanine
aminotransferase; AST, aspartate aminotransferase. |
OP-D attenuates OS and inflammation in
NAFLD mice
Lipid peroxidation, OS and inflammation serve a
critical role in the progression of NAFLD (22). Therefore, the levels of OS in the
liver tissue were determined in the present study. The results
revealed that the hepatic levels of MDA, an oxidative marker, were
significantly reduced following treatment of HFD-induced NAFLD mice
with OP-D (Fig. 4A). Inversely,
the levels of the antioxidative markers SOD, CAT and GSH-Px were
significantly increased in the livers of mice in the HFD + OP-D
group compared with the HFD group that did not receive OP-D
treatment (Fig. 4B-D).
Furthermore, the mRNA expression levels of nuclear respiratory
factor 1 and transcription factor A mitochondria, two mitochondrial
regulators, were significantly increased in the HFD + OP-D group
compared with the HFD group (Fig.
4E). Subsequently, the inflammatory response in mice treated
with or without OP-D was investigated. As shown in Fig. 4F-H, the serum levels of the
cytokines TNFα, IL-6 and IL-1β were significantly increased in HFD
mice compared with ND mice. However, the secretion levels of these
inflammatory mediators were restored following OP-D administration.
Additionally, OP-D administration significantly reversed the
increased mRNA expression levels of TNFα, IL-6 and Il-1β after
induction by HFD (Fig. 4I-K).
Furthermore, the significantly increased gene expression levels of
the inflammatory mediators, including matrix metalloproteinase 9
(MMP9), monocyte chemoattractant protein 1 (MCP1) and vascular cell
adhesion protein 1 (VCAM1), were also significantly reduced in the
HFD + OP-D group compared with the HFD group (Fig. 4L). These findings suggested that
OP-D could reduce OS and inflammation in HFD-induced NAFLD
mice.
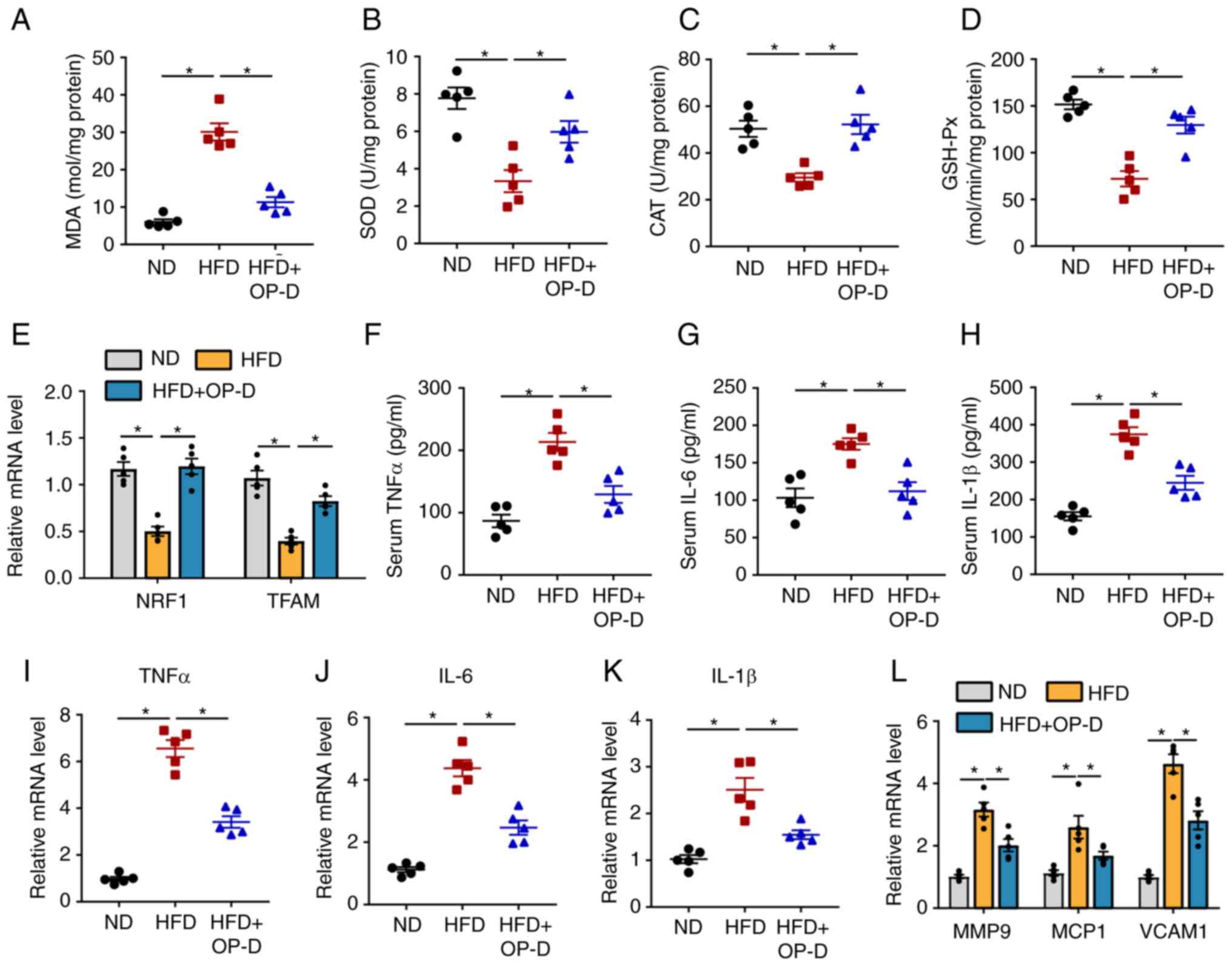 | Figure 4OP-D attenuates the levels of
oxidative stress and inflammation in NAFLD mice. (A) MDA content,
activities of (B) SOD, (C) CAT and (D) GSH-Px from the livers of
mice with different interventions. (E) Relative mRNA expression
levels of NRF1 and TFAM. Levels of (F) TNFα, (G) IL-6 and (H) IL-1β
in the serum. Relative mRNA expression levels of (I) TNF-α, (J)
IL-6 and (K) IL-1β. (L) Relative mRNA expression of MMP9, MCP1 and
VCAM1 (n=5). *P<0.05. OP-D, Ophiopogonin D; NAFLD,
non-alcoholic fatty liver disease; MDA, malondialdehyde; SOD,
superoxide dismutase; CAT, catalase; GSH-Px, glutathione
peroxidase; HFD, high fat diet; ND, normal chow diet; NRF-1,
nuclear respiratory factor 1; TFAM, mitochondrial transcription
factor A; MMP9, matrix metalloproteinase 9; MCP1, monocyte
chemoattractant protein-1; VCAM1, vascular cell adhesion protein
1. |
OP-D alleviates lipogenesis and
inflammatory responses in vitro
The current study aimed to explore whether OP-D
could exert a direct effect on PMHs. Therefore, the changes in fat
deposition were evaluated in PMHs treated with different
concentrations of PA for different time points (data not shown).
Consistently, treatment with 400 µmol/l PA for 24 h was the optimum
condition, as previously described (23). Since the CCK8 result showed that
0-20 µmol/l OP-D did not affect the cell viability of PMHs, the
concentration of 10 µmol/l OP-D for 24 h was selected as the
optimal condition (Fig. 5A).
Treatment with OP-D significantly reduced TG levels in lysed PMHs
compared with the PA-only treated group (Fig. 5B). The mRNA expression levels of
lipid synthesis-related genes, such as acetyl-CoA carboxylase 1
(ACC1), FAS and SCD1, were significantly reduced in cells treated
with OP-D compared with the PA group (Fig. 5C). Consistent with previous studies
(11,24), PA significantly increased the
levels of TNFα, IL-1β and IL-6 from PMHs, while OP-D reversed this
effect (Fig. 5D-F). Additionally,
OP-D could significantly reduce the mRNA expression levels of TNFα,
IL-6 and IL-1β compared with the PA-only treated group (Fig. 5G). Collectively, these results
indicated that OP-D could alleviate lipogenesis and inflammation
in vitro.
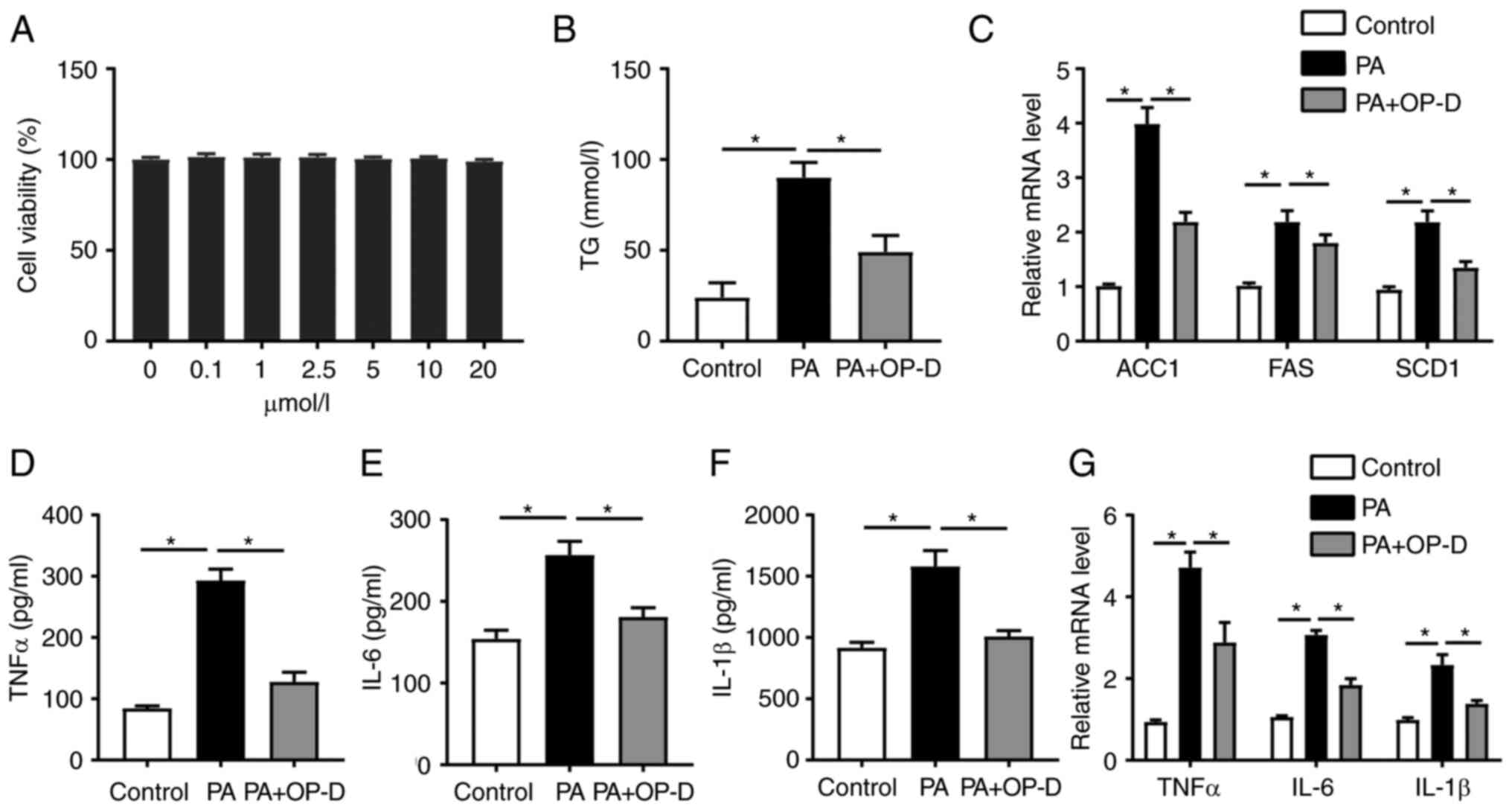 | Figure 5OP-D alleviates lipogenesis and
inflammatory responses in vitro. Primary mouse hepatocytes
were isolated and treated with 400 mmol/l PA with or without 10
µmol/l OP-D. (A) Effect of OP-D on cell viability. (B) Serum levels
of TG. (C) Relative mRNA expression levels of ACC1, FAS and SCD1.
Levels of (D) TNFα, (E) IL-6 and (F) IL-1β in the serum. (G)
Relative mRNA expression level of TNFα, IL-6 and IL-1β (n=3).
*P<0.05. OP-D, Ophiopogonin D; PA, palmitic acid; TG,
triglycerides; ACC1, acetyl-CoA carboxylase 1, SCD1, stearoyl-CoA
desaturase-1; FAS, fatty acid synthase. |
NF-κB signaling is involved in the
regulation of OP-D in PMHs
The results suggested that OP-D could alleviate
inflammatory responses, which have a significant effect on the
progression of HFD-induced NAFLD in mice. Therefore, the present
study further explored the mechanisms underlying the effect of OP-D
on NAFLD in PMHs. It has been reported previously that the
inflammatory NF-κB signaling pathway plays a crucial role in
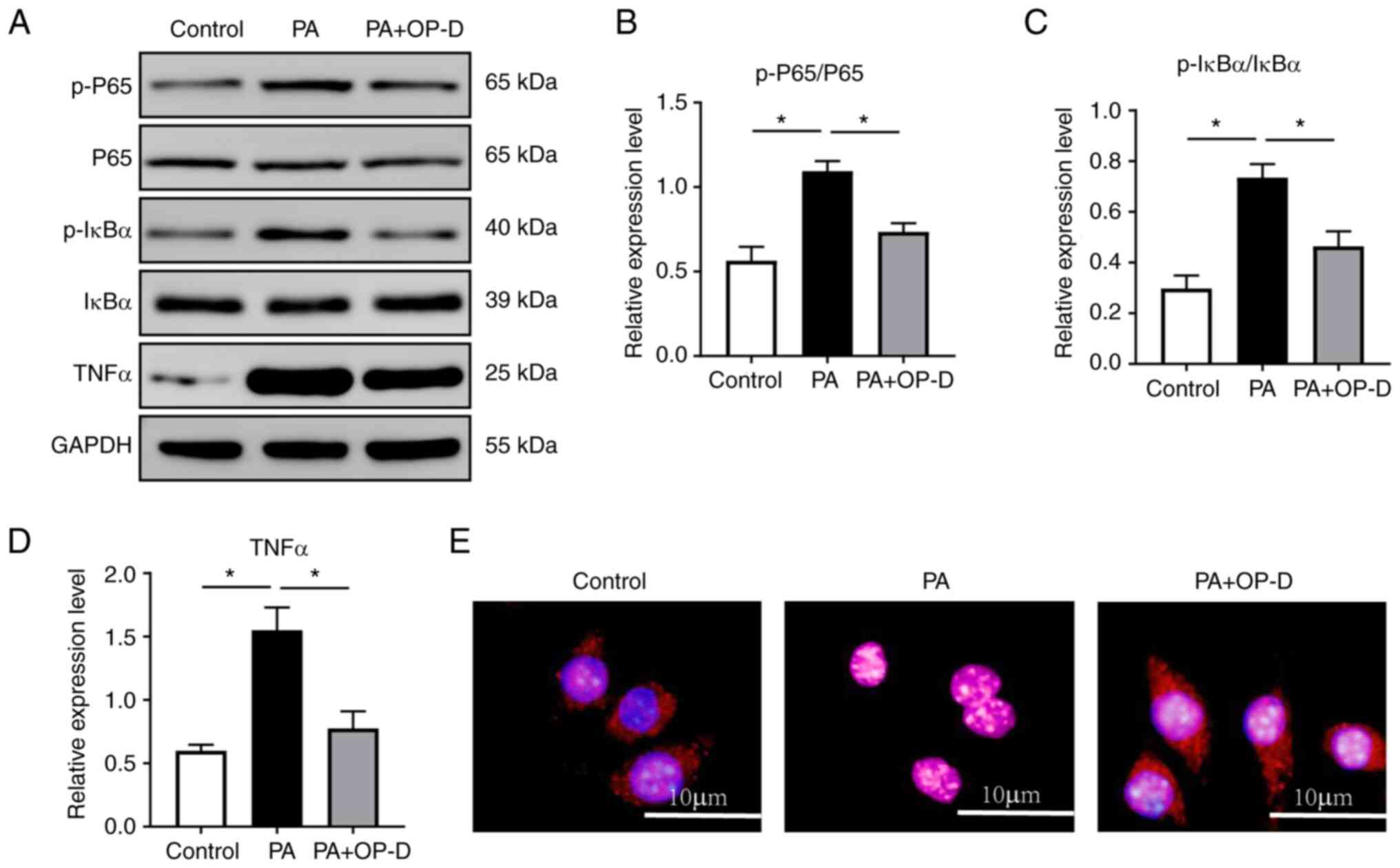
regulating inflammatory responses in NAFLD (25). In the current study, western blot
analysis demonstrated that cell treatment with OP-D significantly
reduced the PA-induced protein expression levels of p-P65, p-IκBα
and TNFα in hepatocytes (Fig.
6A-D). OP-D also inhibited the PA-induced nuclear translocation
of P65 (Fig. 6E). These results
suggested that the NF-κB signaling pathway could mediate the
beneficial effects of OP-D on NAFLD.
Discussion
NAFLD is a common pathological disease of the liver,
affecting ~25% of the global population, which increases the risk
of hepatic and extrahepatic complications (2,4).
Although several phytochemicals are currently used worldwide for
the clinical treatment of NAFLD (26,27),
the efficacy and safety of these drugs still remains unknown.
Therefore, studies on the mechanisms underlying the development of
NAFLD and potential protective therapeutic interventions are
important.
Ophiopogon japonicus is a common herb in
traditional Chinese medicine, that has been widely used for
thousands of years due to its high nutritional and medicinal value
(11-13).
However, the mechanisms underlying the effects of Ophiopogon
japonicus still require investigation. Emerging evidence has
suggested that OP-D, a steroidal glycoside of Ophiopogon
japonicus, exhibits several biological activities. Previous
studies have demonstrated that OP-D can exert an anti-inflammatory
effect on PM2.5-injured alveolar epithelial cells (28), protect human endothelial cells from
OS injury (29) and alleviate
diabetic myocardial injuries by reducing lipid accumulation and
mitochondrial injury (15).
The current study aimed to investigate the possible
therapeutic effects of OP-D on HFD-induced NAFLD. The main findings
of the present study were as follows: i) OP-D alleviated obesity in
a mouse model; ii) OP-D potentially played a beneficial role in
NAFLD via the regulation of lipogenesis, OS injury and
inflammation; and iii) the NF-κB signaling pathway was potentially
involved in the regulation of OP-D in NAFLD.
NAFLD, currently known as ‘metabolic
dysfunction-associated fatty liver disease’, is considered a
hepatic manifestation of metabolic syndrome (30). The disease is closely associated
with metabolic disorders, such as obesity, dyslipidemia, IR and
T2DM (31). Obesity and being
overweight are the most common causes of metabolic diseases and
NAFLD (32). The present study
showed that OP-D suppressed HFD-induced body weight gain in mice,
which prompts further research on the effects of OP-D on NAFLD. It
has been widely reported that IR and dyslipidemia are key events in
the progression of NAFLD (32-34).
In the current study, the results revealed that OP-D improved lipid
metabolic disorders and IR in HFD-induced obese mice. These
findings were consistent with those of a previous study that
demonstrated that OP-D can improve hyperglycemia in diabetic rats
(14).
OS plays an essential role in the occurrence and
progression of NAFLD (9,35). OS refers to the imbalance of
oxidants and antioxidants, which can lead to severe failure of cell
function and eventually to cell death (36). Previous studies have demonstrated
that the levels of the hepatic oxidation markers MDA and ROS are
increased, while those of the antioxidative stress markers CAT,
GSH-Px and SOD are decreased in NAFLD (36-38).
The results of the current study showed that OP-D reduced OS, as
evidenced by the reduced activity of MDA, and increased antioxidant
activities, as evidenced by the increased SOD, CAT and GSH-Px
activities.
The regulation of inflammatory responses has been
recognized as a pivotal pathway for maintaining homeostasis and
preventing the progression of NAFLD (9,39,40).
It has been reported that NF-κB signaling plays a critical role in
the regulation of inflammation (25). Once the NF-κB pathway is activated,
pro-inflammatory cytokines, such as TNFα, IL-1β and IL-6, are
widely expressed (41). The
present study demonstrated that OP-D alleviated inflammatory
responses in HFD-induced obese mice and verified that its
anti-inflammatory effect was associated with the NF-κB pathway
in vitro.
The current study indicated the effect of OP-D on
alleviating hepatic steatosis via antioxidant and anti-inflammatory
responses through the NF-κB signaling pathway; however, there are
still certain limitations. Firstly, the particular upstream
pathways involved in OP-D promoting P65 activation were not
elucidated, and it could not be excluded that other mechanisms,
such as mitochondrial OS, autophagy and ferroptosis could be
involved in the aforementioned process. Secondly, the effects of
OP-D on other cell types, such as Kupffer cells and sinusoidal
endothelial cells in the liver, were not investigated. Finally,
OP-D significantly reduced body weight, thus supporting the notion
of a reduction in obesity; however, it cannot be excluded that
muscle may also be reduced by OP-D administration. Therefore,
further studies on the roles of OP-D on muscle volume or muscle
strength are needed. Overall, further evidence is needed to clarify
the mechanism underlying the effect of OP-D on improving NAFLD.
It has been reported that OP-D is safe for clinical
use and the drug has little toxicity in animal subacute toxicity
experiments (42,43). However, a recent study reported
that OP-D can cause hemolysis in Kunming mice, but there is no
further data to confirm this (44). In the present study, no specific
side effects of OP-D on the HFD mice were observed. The side
effects of OP-D still need to be investigated further.
In summary, the present study demonstrated that OP-D
could alleviate NAFLD in HFD-induced obese mice by improving
lipogenesis, inflammation and oxidative stress injury. In addition,
NF-κB signaling could play an essential role in the beneficial
effects of OP-D on NAFLD. Therefore, the above findings indicated
that OP-D could be a potential therapeutic agent for NAFLD.
Supplementary Material
A list of reverse
transcription-quantitative PCR primers used in this study.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by grants from the School of
Medicine, Wuhan University of Science and Technology (grant no.
OHIC2019G03).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XH and QJ conducted the animal experiments. CS and
YC performed the in vitro experiments. XH and YC wrote the
manuscript. JZ and QW designed the study and conducted data
analysis. QW is the guarantor of this work. XH and QW confirm the
authenticity of all the raw data All authors have read and approved
the final manuscript.
Ethics approval and consent to
participate
All animal procedures were approved by the Medical
Ethics Committee of Wuhan University of Science and Technology
(Wuhan, China; approval no. 2022139).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kumar S, Duan Q, Wu R, Harris EN and Su Q:
Pathophysiological communication between hepatocytes and
non-parenchymal cells in liver injury from NAFLD to liver fibrosis.
Adv Drug Deliv Rev. 176(113869)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Liebe R, Esposito I, Bock HH, Vom Dahl S,
Stindt J, Baumann U, Luedde T and Keitel V: Diagnosis and
management of secondary causes of steatohepatitis. J Hepatol.
74:1455–1471. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Safari Z and Gérard P: The links between
the gut microbiome and non-alcoholic fatty liver disease (NAFLD).
Cell Mol Life Sci. 76:1541–1558. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chalasani N, Younossi Z, Lavine JE,
Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM and Sanyal AJ:
The diagnosis and management of nonalcoholic fatty liver disease:
Practice guidance from the American Association for the Study of
Liver Diseases. Hepatology. 67:328–357. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lan T, Hu Y, Hu F, Li H, Chen Y, Zhang J,
Yu Y, Jiang S, Weng Q, Tian S, et al: Hepatocyte glutathione
S-transferase mu 2 prevents non-alcoholic steatohepatitis by
suppressing ASK1 signaling. J Hepatol. 76:407–419. 2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Rohm TV, Meier DT, Olefsky JM and Donath
MY: Inflammation in obesity, diabetes, and related disorders.
Immunity. 55:31–55. 2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Khan RS, Bril F, Cusi K and Newsome PN:
Modulation of insulin resistance in nonalcoholic fatty liver
disease. Hepatology. 70:711–724. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Katsiki N, Mikhailidis DP and Mantzoros
CS: Non-alcoholic fatty liver disease and dyslipidemia: An update.
Metabolism. 65:1109–1123. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Farzanegi P, Dana A, Ebrahimpoor Z, Asadi
M and Azarbayjani MA: Mechanisms of beneficial effects of exercise
training on non-alcoholic fatty liver disease (NAFLD): Roles of
oxidative stress and inflammation. Eur J Sport Sci. 19:994–1003.
2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mohammed S, Nicklas EH, Thadathil N,
Selvarani R, Royce GH, Kinter M, Richardson A and Deepa SS: Role of
necroptosis in chronic hepatic inflammation and fibrosis in a mouse
model of increased oxidative stress. Free Radic Biol Med.
164:315–328. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Huang X, Wang Y, Zhang Z, Wang Y, Chen X,
Wang Y and Gao Y: Ophiopogonin D and EETs ameliorate Ang II-induced
inflammatory responses via activating PPARα in HUVECs. Biochem
Biophys Res Commun. 490:123–133. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kou J, Tian Y, Tang Y, Yan J and Yu B:
Antithrombotic activities of aqueous extract from Radix Ophiopogon
japonicus and its two constituents. Biol Pharm Bull. 29:1267–1270.
2006.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kou J, Sun Y, Lin Y, Cheng Z, Zheng W, Yu
B and Xu Q: Anti-inflammatory activities of aqueous extract from
Radix Ophiopogon japonicus and its two constituents. Biol Pharm
Bull. 28:1234–1238. 2005.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Qiao Y, Jiao H, Wang F and Niu H:
Ophiopogonin D of Ophiopogon japonicus ameliorates renal function
by suppressing oxidative stress and inflammatory response in
streptozotocin-induced diabetic nephropathy rats. Braz J Med Biol
Res. 53(e9628)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Li W, Ji L, Tian J, Tang W, Shan X, Zhao
P, Chen H, Zhang C, Xu M, Lu R and Guo W: Ophiopogonin D alleviates
diabetic myocardial injuries by regulating mitochondrial dynamics.
J Ethnopharmacol. 271(113853)2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Qin Y, Dong H, Sun J, Zhang Y, Li J, Zhang
T, Chen G, Wang S, Song S, Wang W, et al: Evaluation of MTBH, a
novel hesperetin derivative, on the activity of hepatic cytochrome
P450 isoform in vitro and in vivo using a cocktail method by
HPLC-MS/MS. Xenobiotica. 51:1389–1399. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ma L, Wei S, Yang B, Ma W, Wu X, Ji H, Sui
H and Chen J: Chrysosplenetin inhibits artemisinin efflux in
P-gp-over-expressing Caco-2 cells and reverses P-gp/MDR1 mRNA
up-regulated expression induced by artemisinin in mouse small
intestine. Pharm Biol. 55:374–380. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Donaldson JG: Immunofluorescence staining.
Curr Protoc Cell Biol. Chapter 4(Unit 4.3)2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zandani G, Anavi-Cohen S, Yudelevich T,
Nyska A, Dudai N, Madar Z and Gorelick J: Chiliadenus iphionoides
Reduces body weight and improves parameters related to hepatic
lipid and glucose metabolism in a high-fat-diet-induced mice model
of NAFLD. Nutrients. 14(4552)2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wu YK, Ren ZN, Zhu SL, Wu YZ, Wang G,
Zhang H, Chen W, He Z, Ye XL and Zhai QX: Sulforaphane ameliorates
non-alcoholic fatty liver disease in mice by promoting FGF21/FGFR1
signaling pathway. Acta Pharmacol Sin. 43:1473–1483.
2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Arroyave-Ospina JC, Wu Z, Geng Y and
Moshage H: Role of oxidative stress in the pathogenesis of
non-alcoholic fatty liver disease: Implications for prevention and
therapy. Antioxidants (Basel). 10(174)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhang X, Shang X, Jin S, Ma Z, Wang H, Ao
N, Yang J and Du J: Vitamin D ameliorates high-fat-diet-induced
hepatic injury via inhibiting pyroptosis and alters gut microbiota
in rats. Arch Biochem Biophys. 705(108894)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wang Y, Huang X, Ma Z, Wang Y, Chen X and
Gao Y: Ophiopogonin D alleviates cardiac hypertrophy in rat by
upregulating CYP2J3 in vitro and suppressing inflammation in vivo.
Biochem Biophys Res Commun. 503:1011–1019. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
De Gregorio E, Colell A, Morales A and
Marí M: Relevance of SIRT1-NF-κB Axis as therapeutic target to
ameliorate inflammation in liver disease. Int J Mol Sci.
21(3858)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Bansod S, Saifi MA and Godugu C: Molecular
updates on berberine in liver diseases: Bench to bedside. Phytother
Res. 35:5459–5476. 2021.PubMed/NCBI View
Article : Google Scholar
|
|
27
|
Bagherniya M, Nobili V, Blesso CN and
Sahebkar A: Medicinal plants and bioactive natural compounds in the
treatment of non-alcoholic fatty liver disease: A clinical review.
Pharmacol Res. 130:213–240. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wang Y, Li D, Song L and Ding H:
Ophiopogonin D attenuates PM2.5-induced inflammation via
suppressing the AMPK/NF-κB pathway in mouse pulmonary epithelial
cells. Exp Ther Med. 20(139)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Qian J, Jiang F, Wang B, Yu Y, Zhang X,
Yin Z and Liu C: Ophiopogonin D prevents H2O2-induced injury in
primary human umbilical vein endothelial cells. J Ethnopharmacol.
128:438–445. 2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Eslam M, Sanyal AJ and George J:
International Consensus Panel. MAFLD: A consensus-driven proposed
nomenclature for metabolic associated fatty liver disease.
Gastroenterology. 158:1999–2014.e1. 2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Godoy-Matos AF, Silva Júnior WS and
Valerio CM: NAFLD as a continuum: From obesity to metabolic
syndrome and diabetes. Diabetol Metab Syndr. 12(60)2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wasilewska N and Lebensztejn DM:
Non-alcoholic fatty liver disease and lipotoxicity. Clin Exp
Hepatol. 7:1–6. 2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Fujii H and Kawada N: Japan Study Group of
Nafld Jsg-Nafld. The role of insulin resistance and diabetes in
nonalcoholic fatty liver disease. Int J Mol Sci.
21(3863)2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Rhee EJ: Nonalcoholic fatty liver disease
and diabetes: An epidemiological perspective. Endocrinol Metab
(Seoul). 34:226–233. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ma Y, Lee G, Heo SY and Roh YS: Oxidative
stress is a key modulator in the development of nonalcoholic fatty
liver disease. Antioxidants (Basel). 11(91)2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Li Q, Tan JX, He Y, Bai F, Li SW, Hou YW,
Ji LS, Gao YT, Zhang X, Zhou ZH, et al: Atractylenolide III
ameliorates non-alcoholic fatty liver disease by activating Hepatic
Adiponectin Receptor 1-Mediated AMPK Pathway. Int J Biol Sci.
18:1594–1611. 2022.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Irie M, Sohda T, Anan A, Fukunaga A,
Takata K, Tanaka T, Yokoyama K, Morihara D, Takeyama Y, Shakado S
and Sakisaka S: Reduced glutathione suppresses oxidative stress in
nonalcoholic fatty liver disease. Euroasian J Hepatogastroenterol.
6:13–18. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Polimeni L, Del Ben M, Baratta F, Perri L,
Albanese F, Pastori D, Violi F and Angelico F: Oxidative stress:
New insights on the association of non-alcoholic fatty liver
disease and atherosclerosis. World J Hepatol. 7:1325–1336.
2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Yang Y, Lu Y, Han F, Chang Y, Li X, Han Z,
Xue M, Cheng Y, Sun B and Chen L: Saxagliptin regulates M1/M2
macrophage polarization via CaMKKβ/AMPK pathway to attenuate NAFLD.
Biochem Biophys Res Commun. 503:1618–1624. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Luo P, Qin C, Zhu L, Fang C, Zhang Y,
Zhang H, Pei F, Tian S, Zhu XY, Gong J, et al: Ubiquitin-Specific
Peptidase 10 (USP10) inhibits hepatic steatosis, insulin
resistance, and inflammation through Sirt6. Hepatology.
68:1786–1803. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Castoldi A, Naffah De Souza C, Câmara NO
and Moraes-Vieira PM: The macrophage switch in obesity development.
Front Immunol. 6(637)2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Tang XL, Lin Y, Wang YG and Gao Y: Effects
of ophiopogonin D on fatty acid metabolic enzymes in
cardiomyocytes. Zhongguo Zhong Yao Za Zhi. 46:3672–3677.
2021.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
43
|
Wu FM, Yang HY, Yang RS, Li M, Bao XH and
Zhou J: Study on Quality Evaluation of Ophiopogonis Radix in
Sichuan. Zhong Yao Cai. 39:1803–1808. 2016.PubMed/NCBI(In Chinese).
|
|
44
|
Xu HH, Jiang ZH, Sun YT, Qiu LZ, Xu LL,
Tang XL, Ma ZC and Gao Y: Differences in the hemolytic behavior of
two isomers in ophiopogon japonicus in vitro and in vivo and their
risk warnings. Oxid Med Cell Longev. 2020(8870656)2020.PubMed/NCBI View Article : Google Scholar
|