Introduction
Local anesthetics exert their analgesic effect by
blocking Na+ channels, thereby affecting the conduction
of nerve impulses. They are widely used in clinics as they are
safe, exhibit fewer complications than general anesthetics and have
minimal impact on physiological functions. However, side effects of
local anesthetics can occur, such as local anesthetic allergy and
toxicity, which may impact the cardiovascular and central nervous
systems (1). These side effects
are a result of a combination of adverse effects on ionotropic and
metabotropic cell signaling, as well as energy transduction
(2). The neurotoxic effects of
local anesthetics may include apoptosis, inhibition of
voltage-dependent calcium channels, calcium depletion in the
endoplasmic reticulum, mitochondrial dysfunction and DNA damage
(3,4). Nevertheless, the mechanism underlying
toxicity exerted by local anesthetics requires further study.
Tetracaine hydrochloride (TTC) is a long-lasting
local anesthetic commonly used for topical anesthesia, which is
water-soluble, has strong penetrability and a good anesthetic
effect (5). However, TTC has a
higher cytotoxicity compared with other local anesthetics due to
its high permeability (6).
Previous studies have reported that TTC exerts a cytotoxic effect
on certain cell types, such as human corneal stromal cells and
human corneal epithelial cells, inducing apoptosis and necrosis
(7,8). Koizumi et al (9) reported that large doses of
intrathecal TTC increase the concentration of glutamate in
cerebrospinal fluid, thereby causing neuronal injury in rabbits. In
addition, AMPA receptor activation was revealed to be involved in
TTC-induced neurotoxicity in the spinal cord. Song and Fan
(7) reported that TTC-induced
apoptosis may be triggered through Fas death receptors and mediated
by Bcl-2 family proteins, in a mitochondria-dependent pathway. Pang
and Fan (8) reported TTC-induced
human corneal epithelial (HCEP) cell apoptosis via a death
receptor-mediated mitochondrion-dependent pathway. Werdehausen
et al (6) reported that low
concentrations of TTC induced the apoptosis of human T-lymphoma
cells, whereas a higher concentration of TTC induced necrosis.
However, the effect of TTC on macrophages has not currently been
fully revealed. Furthermore, whether TTC cytotoxicity involves
mechanisms such as pyroptosis is also yet to be elucidated.
Macrophages are specialized, long-lived, phagocytic
cells of the innate immune system that are widely distributed in
certain tissues and organs of the body, such as the lungs, liver
and brain. Macrophages have myriad functions, including eliminating
pathogens, promoting tissue development and wound repair, and
regulating immunity, metabolism and apoptosis. Additionally, they
serve a crucial role in environmental homeostasis and the
inflammatory microenvironment (10). A number of previous studies on the
central nervous system toxicity of local anesthetics focused on
neurons, rather than the immune system (11,12).
The present study used a central nervous system immune cell line,
the murine microglial cell line BV2, which has a dual effect on
neurons. First, they can protect neurons by phagocytosing pathogens
and other harmful agents present in the brain tissue. Secondly,
under the stimulation of inflammatory factors, they can be
activated and secrete inflammatory cytokines, thereby exerting a
toxic effect on neurons (13). In
addition, the murine macrophage cell line RAW 264.7 and mouse
peritoneal macrophages (PMs) were used in the present study to
investigate the relationship between TTC and macrophages.
Pyroptosis, also known as gasdermin (GSDM)-mediated
programmed necrosis, is a form of pro-inflammatory programmed cell
death (PCD) triggered by perturbations of extracellular or
intracellular homeostasis related to innate immunity (14). It is initiated by inflammasome
activation, which serves a critical role in the defense of hosts
against danger signals (9).
Pyroptosis is induced by members of the GSDM superfamily, which
consist of GSDMA, B, C, D and E, and DFNB59; however, there is no
GSDMB gene encoded in the mouse genome. Except for DFNB59, all of
the GSDM protein family members possess an N-terminal pore-forming
domain and a C-terminal auto-inhibitory domain (15,16).
The GSDM proteins initiate proinflammatory cell death via their
pore-forming domain after its cleavage by upstream inflammatory
caspases (15,16). GSDMD and GSDME are GSDM family
proteins that have been widely studied in the context of pyroptosis
(17,18). GSDMD-dependent pyroptosis is
regulated through a canonical inflammasome pathway, which is
activated by caspase-1. Similarly, activation of murine caspase-11
and human caspase-4/-5 is involved in the noncanonical inflammasome
pathway (18). GSDME-dependent
pyroptosis is activated by apoptotic caspase-3(17). Through the release of IL-1β and
IL-18, pyroptotic cells recruit additional inflammatory cells and
induce a cascade of inflammatory responses (19). Pyroptosis is characterized by the
formation of a plasma membrane pore, swelling of the cell, rupture
of the plasma membrane and the subsequent release of intracellular
contents (15). This form of cell
death is mainly observed in professional phagocytes, such as
macrophages, monocytes and dendritic cells, but emerging evidence
suggests that pyroptosis can also be induced in cancer cells
(20). Previous studies have
reported that pyroptosis serves a significant role in
macrophage-induced inflammation (21,22).
Inflammatory responses are beneficial to humans as they help remove
pathogenic microorganisms and antagonize infection, and the release
of cytokines may contribute to tissue angiogenesis (23). However, hyperactivated pyroptosis
can result in the induction of a substantial inflammatory cascade
leading to tissue and organ damage, thereby causing inflammatory
diseases, such as lung inflammatory disease, cardiovascular disease
and kidney disease (24).
Previous reports have indicated that TTC can cause
allergic reactions and local anesthetics may have antibacterial
effects, suggesting a potential effect of TTC on macrophages
(25,26). However, the effects of TTC on
macrophages and whether pyroptosis is involved in this process have
not yet been reported. The present study aimed to find the
relationship between TTC and macrophage pyroptosis, and provide a
novel mechanism underlying the local anesthetic toxicity of TTC.
The findings may point to future novel treatments for local
anesthetic toxicity caused by TTC.
Materials and methods
Cell culture
The murine macrophage cell line RAW 264.7 and the
murine microglial cell line BV2 were purchased from Procell Life
Science & Technology Co., Ltd. (cat. nos. CL-0190 and CL-0493,
respectively). RAW 264.7 and BV2 cells were cultured in RAW
264.7-specific medium (cat. no. CM-0190; Procell Life Science &
Technology Co., Ltd.). and BV2-specific medium (cat. no. CM-0493;
Procell Life Science & Technology Co., Ltd.) with 10% FBS and
1% P/S antibiotics, respectively. Cells were incubated in a
humidified incubator at 37˚C with 5% CO2.
Cells were treated with TTC (H20084308, Chengdu
Tiantaishan Pharmaceutical Co. Ltd.) at a range of concentrations
from 100 to 400 µM for 24 h at 37˚C, with or without pretreatment
with the caspase-1 inhibitor, Belnacasan (Beln) (10 µM; cat. no.
HY-13205; MedChemExpress) and/or the caspase-11 inhibitor,
Wedelolactone (Wede) (20 µM; cat. no. HY-N0551; MedChemExpress) for
30 min at 37˚C to induce the macrophages, LPS (10 µg/ml; cat. no.
L6529; Sigma-Aldrich; Merck KGaA) was added 6 h before TTC
treatment.
Light microscopy
RAW 264.7 and BV2 cells were cultured in 100 mm
plates (cat. no. 704002; Wuxi NEST Biotechnology Co., Ltd.) in a
humidified incubator at 37˚C with 5% CO2. Once the cells
reached the logarithmic growth phase, the medium was replaced with
fresh medium containing TTC at concentrations ranging from 100-400
µM for 24 h at 37˚C. The morphology and confluence of the cells
were monitored with a light microscope.
Western blotting
The cells were homogenized in RIPA buffer (cat. no.
P0013C; Beyotime Institute of Biotechnology), and the protein
concentration was determined using a bicinchoninic acid protein
assay kit (cat no. PC0020; Beijing Solarbio Science &
Technology). Equal quantities of cell lysate (20 µg/lane) were
separated by SDS-PAGE on a 10 or 12% gel and then transferred to a
0.45-µm polyvinylidene difluoride membrane. Membranes were blocked
with 5% skim milk at room temperature for 1 h, then incubated with
primary antibodies against GSDMA (cat no. sc-376318; 1:100; Santa
Cruz Biotechnology, Inc.), GSDMC (cat. no. 27630-1-AP; 1:1,000;
Proteintech Group, Inc.), GSDMD (cat. no. ab209845; 1:1,000;
Abcam), GSDME (cat. no. ab215191; 1:1,000; Abcam), caspase-1 (cat.
no. ab179515; 1:1,000; Abcam), caspase-11 (cat. no. ab180673;
1:1,000; Abcam), caspase-3 (cat. no. 9662; 1:1,000; Cell Signaling
Technology, Inc.), and β-actin (cat. no. 66009-1-Ig; 1:10,000;
Proteintech Group, Inc.) at 4˚C overnight. Subsequently, the
membranes were washed with 1X TBST (0.5% Tween) three times for 10
min and incubated with HRP-conjugated Affinipure goat anti-mouse
IgG (H+L) secondary antibodies (cat. no. SA00001-1; 1:10,000;
Proteintech Group, Inc.) or HRP-conjugated Affinipure goat
anti-rabbit IgG (H+L) secondary antibodies (cat. no. SA00001-2;
1:10,000; Proteintech Group, Inc.) for 1 h at room temperature.
After washing with 1X TBST three times for 10 min, the bands were
visualized using the ECL FemtoLightChemiluminescence kit (cat. no.
PE100; Chengdu Wanda Biotechnology Development Co., Ltd.). To
better show the expression levels of the full-length and cleaved
forms of the detected proteins, both short and long exposure images
are presented in the present study. For relative protein expression
quantification, the integrated optical density of the protein bands
was assessed using ImageJ software (version 1.31; National
Institutes of Health).
Cell Counting Kit-8 (CCK-8) assay
Cell viability was measured using the CCK-8 assay
kit (Dojindo Laboratories, Inc.) according to the manufacturer's
instructions. RAW 264.7 and BV2 cells were seeded in 96-well plates
(1x103 cells/well) and treated with 100-400 µM TTC, then
incubated at 37˚C with 5% CO2. After 24 h, 10 µl CCK-8
reagent was added to each well, and the cells were further
incubated for 1 h at 37˚C. Subsequently, the absorbance of samples
was measured at 450 nm using a microplate reader.
ELISA
ELISA was used to measure the levels of IL-1β
present in the cell culture medium The cell culture medium was
centrifuged at 1,000 x g for 10 min at 4˚C and the levels of IL-1β
present were measured using the mouse IL-1β ELISA Kit (cat. no.
KE10003; Proteintech Group, Inc.) according to the manufacturer's
protocol. A total of 200 µl cell culture medium was loaded into
each well of the ELISA plate. The absorbance of each sample was
measured at 450 nm using a microplate reader. Standard curves were
established using IL-1β standard samples provided in the kit and
the concentration of the cytokines in the collected medium was
measured.
Lactate dehydrogenase (LDH) release
assay
The release of LDH from the cells was measured using
the LDH cytotoxicity assay kit (cat. no. C0016; Beyotime Institute
of Biotechnology). RAW 264.7 and BV2 cells were seeded in 96-well
plates (1x103 cells/well) and treated with 100-400 µM
TTC for 24 h at 37˚C. After treatment, cells were centrifuged at
400 x g for 5 min at 4˚C, then 120 µl supernatant was collected and
analyzed using the assay kit according to the manufacturer's
instructions. Briefly, 60 µl LDH determination working solution was
added to the supernatant and incubated for 30 min at 37˚C.
Subsequently, the absorbance of samples was measured at 490 nm
using a microplate reader. All experiments were performed in
triplicate.
Extraction of murine PMs
The study was approved by the Ethical Approval for
Research Involving Animals in Southwest Medical University (Luzhou,
China; approval no. SWMU20220028) and all animal experiments were
performed according to the Guide for the Care and Use of Laboratory
Animals of the National Institutes of Health. A total of 40 male
C57BL/6 mice (weight, ~20 g; age, 6-8 weeks) were purchased from
Chongqing Tengxin Biotechnology Co., Ltd., and were kept in the
Experimental Animal Center of Southwest Medical University. The
animals were supplied with food and water ad libitum and
maintained in a 12/12-h light/dark cycle for 2 weeks (ambient
temperature, 22-26˚C; relative humidity, 40-60%). Mice were
intraperitoneally injected with TTC (2.5 mg/kg) or an equal volume
of physiological saline once a day for 3 consecutive days. Then,
PMs were isolated as previously reported (18). First, the mice were euthanized by
cervical dislocation and soaked in 70% ethanol for 3 min, then
transferred to a clean bench. Subsequently, a small incision was
made along the ventral aspect of the mouse abdomen with a sterile
scalpel and the abdominal skin was carefully removed to expose the
peritoneum. Subsequently, using a 5-ml syringe and a needle, 5 ml
RPMI medium 1640 (Gibco; Thermo Fisher Scientific, Inc)
supplemented with 1% penicillin/streptavidin was injected into the
abdomen. The abdomen was gently massaged and the medium was
recovered into a 15-ml tube. Then, the medium was centrifuged for 5
min at 4˚C at 310 x g to pellet the cells. The supernatant was
discarded and the cells were resuspended in 1 ml complete RPMI
medium. Next, the collected cells were transferred to cell plates
and cultivated in complete RPMI medium in a humidified incubator
with 5% CO2 at 37˚C. After being incubated for 1 h, only
macrophages adhered to the culture dish. The culture supernatant
containing other cells was removed and replaced with fresh culture
medium. Then macrophages were then used for the subsequent
experiments.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism software (version 8.0; Dotmatics). Each experiment was
performed in triplicate. Data are presented as the mean ± standard
error of the mean. Statistically significant differences were
determined by one-way ANOVA followed by Tukey's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
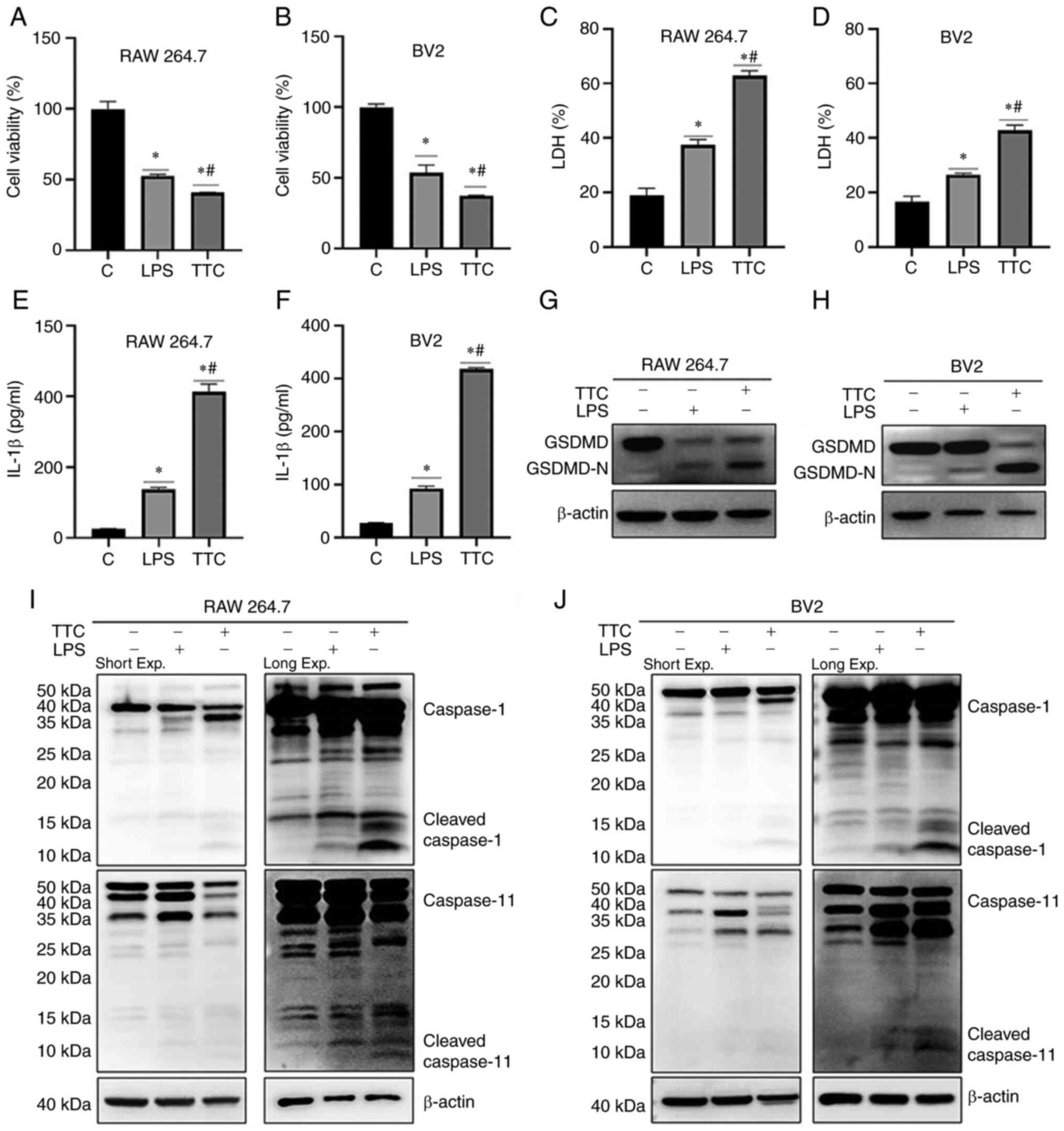
TTC induces pyroptosis in
macrophages
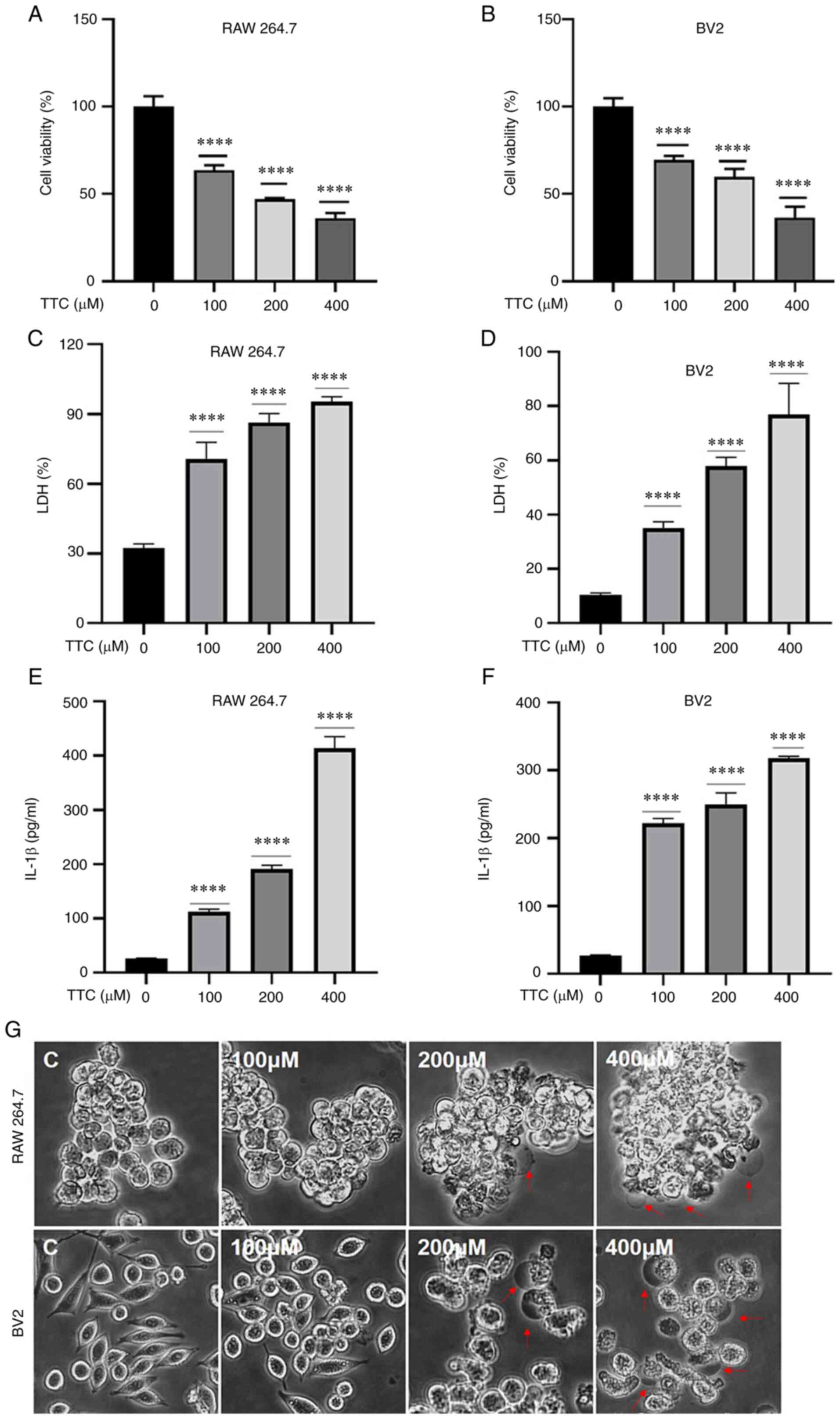
The cytotoxicity of various concentrations of TTC in
RAW 264.7 and BV2 cells was evaluated. Cell viability was measured
using a CCK-8 assay and the morphology of cells was observed using
a light microscope. The viability of both RAW 264.7 and BV2 cells
was significantly decreased by TTC in a dose-dependent manner
(Fig. 1A and B). Furthermore, both RAW 264.7 and BV2
cells treated with >200 µM TTC for 24 h underwent morphological
alterations. The dying cells showed marked swelling with large
bubbles emanating from the plasma membrane (Fig. 1G). These morphological changes were
reminiscent of pyroptosis, as reported previously (15). In addition to morphological
changes, pyroptosis is characterized by the release of certain
inflammatory factors. The levels of secreted LDH and IL-1β were
examined in the present study (Fig.
1C-F). These results demonstrated that the secretion of both
LDH and IL-1β was significantly increased after TTC treatment and
this effect was TTC-dose-dependent. As the release of LDH and IL-1β
have been perceived as hallmarks of pyroptosis (15), combined with the morphological
changes after TTC treatment, these results suggested that TTC could
induce pyroptosis in RAW 264.7 and BV2 cells.
TTC induces macrophage pyroptosis
mediated by GSDMD cleavage
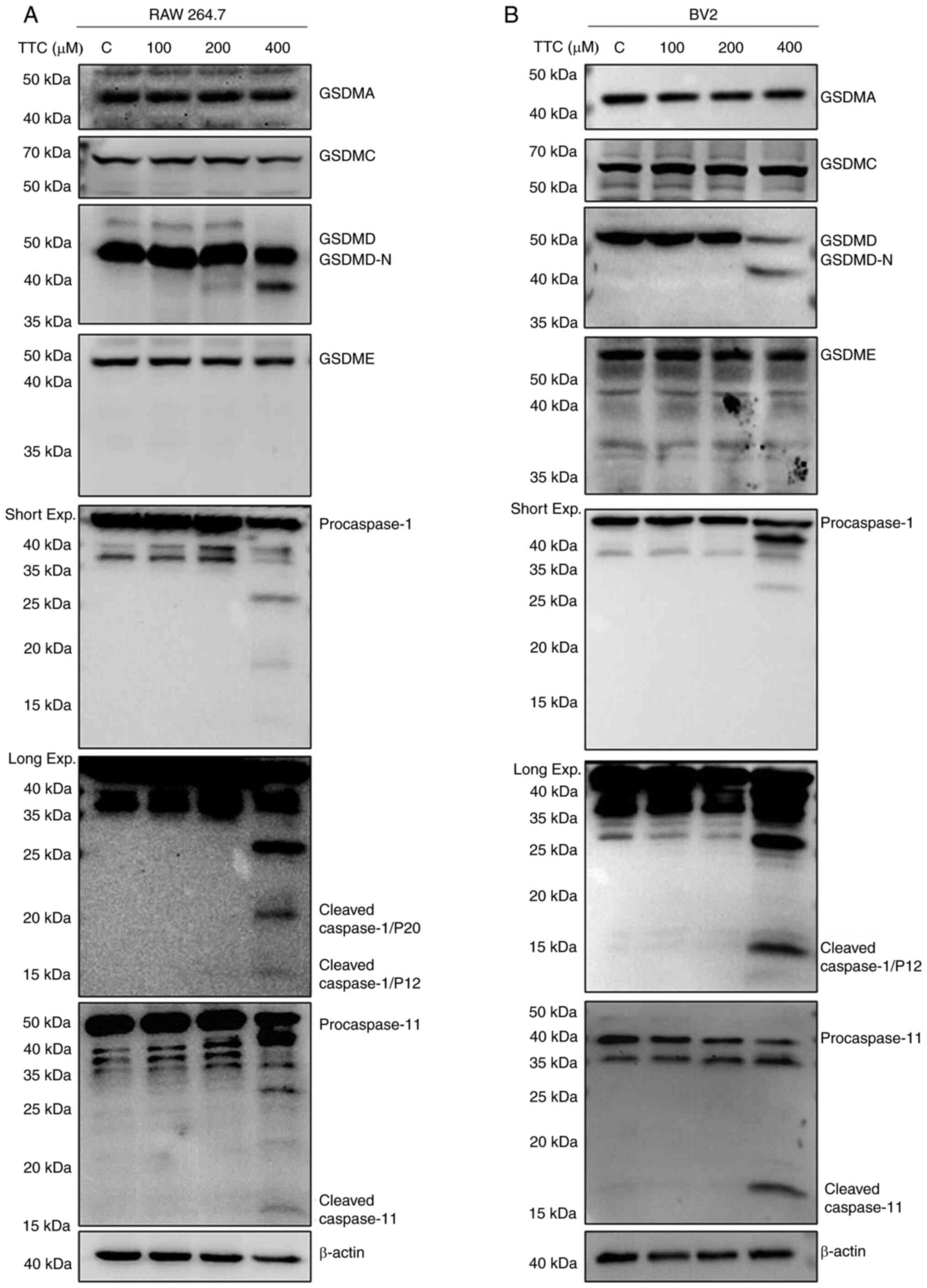
The molecular mechanism by which TTC could induce
macrophage pyroptosis was subsequently examined. It has previously
been reported that the GSDM protein family is the dominant effector
of pyroptosis (15). To determine
which GSDM protein exerts TTC-induced pyroptosis, the protein
expression levels and the cleavage status of the GSDM protein
family (GSDMA, GSDMC, GSDMD and GSDME) in RAW 264.7 and BV2 cells
were determined by western blotting (Fig. 2A and B). The full-length GSDMD protein
expression levels were decreased, whereas GSDMD N-terminal fragment
(GSDMD-N), the fragment that possesses the pore-forming activity,
was increased in macrophages treated with 400 µM TTC. However,
GSDMA, GSDMC and GSDME demonstrated no change in protein expression
levels upon TTC treatment. These findings suggested that GSDMD, the
most frequently reported effector of pyroptosis (18), could be involved in TTC-induced
pyroptosis, and TTC may influence the cleavage of GSDMD, rather
than its total expression. Therefore, TTC-induced macrophage
pyroptosis may be mediated by GSDMD cleavage.
GSDMD cleavage is regulated by both
caspase-1 and caspase-11 in TTC-induced macrophage pyroptosis
GSDMD-dependent pyroptosis is regulated through the
canonical inflammasome pathway by caspase-1 activation, and the
noncanonical inflammasome pathway by caspase-11 in mice and
caspase-4/-5 in humans (18). In
the present study, the involvement of GSDMD in TTC-induced
pyroptosis was demonstrated; therefore, further examination of the
expression of caspase-1 and caspase-11 by western blotting was
performed (Fig. 2A and B). These results showed that the protein
expression levels of both cleaved caspase-1 (P20 and P12) and
caspase-11 were upregulated after TTC stimulation, suggesting the
involvement of both canonical and non-canonical inflammatory
pathways. Additionally, expression of cleaved caspase-3 was also
upregulated by TTC (Fig. S1A and
B), suggesting that TTC-induced
cell death may be caused by apoptosis in addition to pyroptosis.
This is consistent with a previous study that reported apoptosis as
a major mechanism of cell death caused by TTC in HCEP cells
(8).
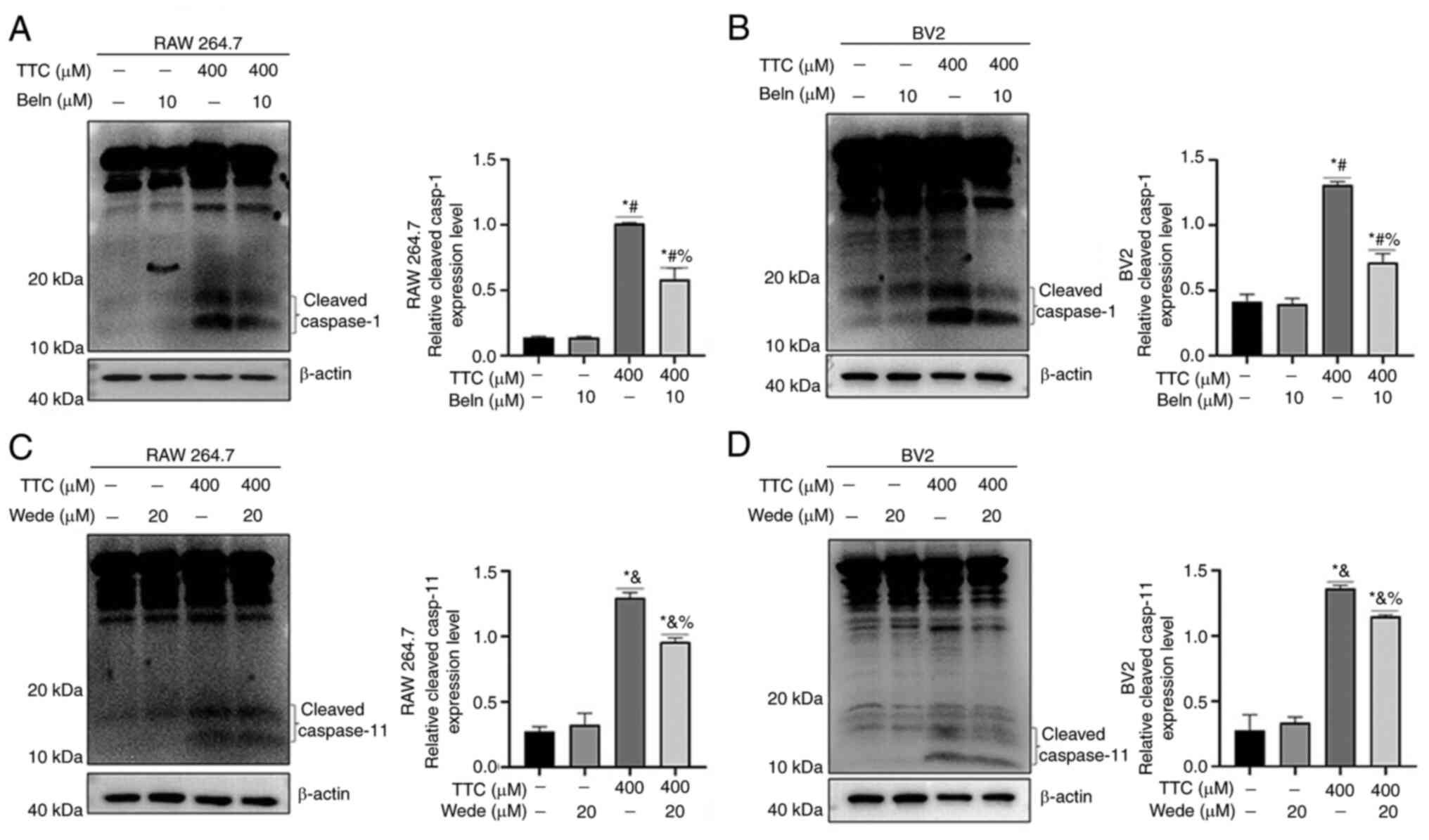
To validate these results in the present study,
cells were treated with the caspase-1 inhibitor Beln and the
caspase-11 inhibitor Wede. The inhibitory activity of Beln and Wede
on functional caspase-1 and caspase-11, respectively, was
confirmed. The results demonstrated that Beln treatment alone had
no significant effect on the protein expression levels of
caspase-1, but significantly decreased TTC-induced caspase-1
cleavage (Fig. 3A and B). Additionally, Wede caused a
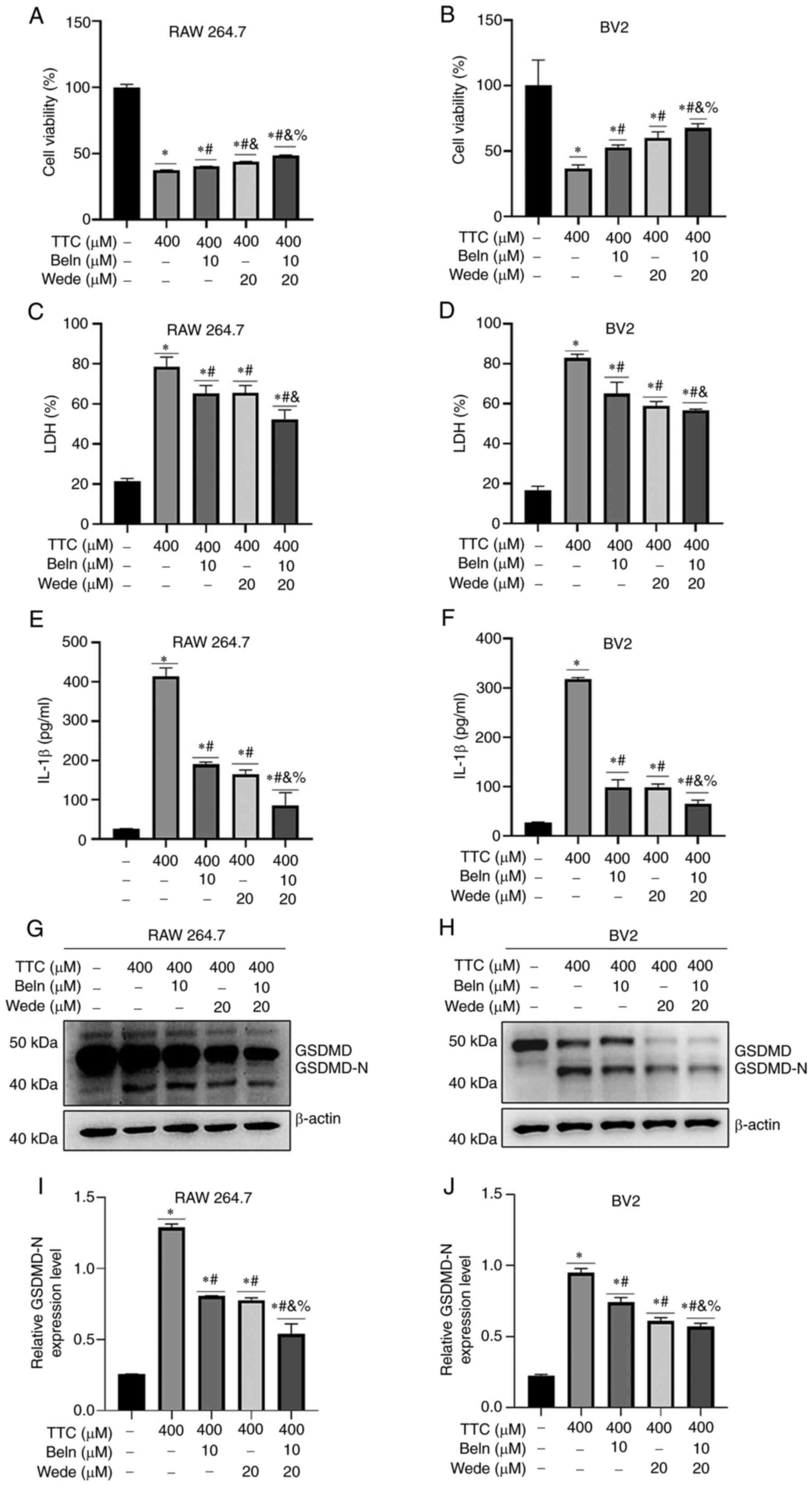
significant decrease in TTC-induced caspsase-11 cleavage (Fig. 3C and D). Inhibition of TTC-induced pyroptosis
by Beln and Wede was examined by pretreating cells with the
inhibitors for 30 min, followed by TTC exposure. These results
demonstrated that both Beln and Wede significantly abrogated the
decrease of cell viability (Fig.
4A and B), LDH release
(Fig. 4C and D), IL-1β release (Fig. 4E and F) and the upregulation of GSDMD-N induced
by TTC (Fig. 4G and H). Moreover, treatment of cells with both
Beln and Wede significantly inhibited the vast majority of GSDMD
expression, indicating a synergistic effect in reversing
TTC-induced pyroptosis compared with treatment using a single
inhibitor (Fig. 4G-J). These
results suggested that TTC-induced pyroptosis may be mediated by
both canonical and non-canonical inflammatory pathways in
macrophages.
Regulatory mechanisms underlying
TTC-induced pyroptosis differ from LPS-induced pyroptosis in
macrophages
LPS is known to induce pyroptosis in macrophages
(27). In the present study, the
mechanism of induction of macrophage pyroptosis by TTC and LPS were
compared. The results showed that TTC or LPS treatment of both RAW
264.7 and BV2 cells demonstrated a significant decrease in cell
viability (Fig. 5A and B), significant increases in the secretion
of LDH (Fig. 5C and D) and IL-1β (Fig. 5E and F), and GSDMD cleavage (Fig. 5G and H). However, when caspase-1/11 was
detected, both TTC and LPS could induce caspase-1/11 cleaveage, but
the cleavage fragments were not the same (Fig. 5I and J). Compared with LPS, TTC treatment
influenced caspase-1 and caspase-11 cleavage, rather than
upregulating the whole protein. In addition, it was demonstrated
that the cleaved fragments of caspase-1/-11 induced by TTC differed
from those induced by LPS, as the cleavage fragments of
caspase-1/-11 generated by TTC and LPS were located at different
positions on the western blot membrane, suggesting the mechanism of
TTC-induced pyroptosis might not be the same as that of LPS.
Further investigation is required to identify the mechanism of
action of the different cleavage pattern induced by TTC treatment
demonstrated in the present study and how TTC interacts with these
inflammatory caspases.
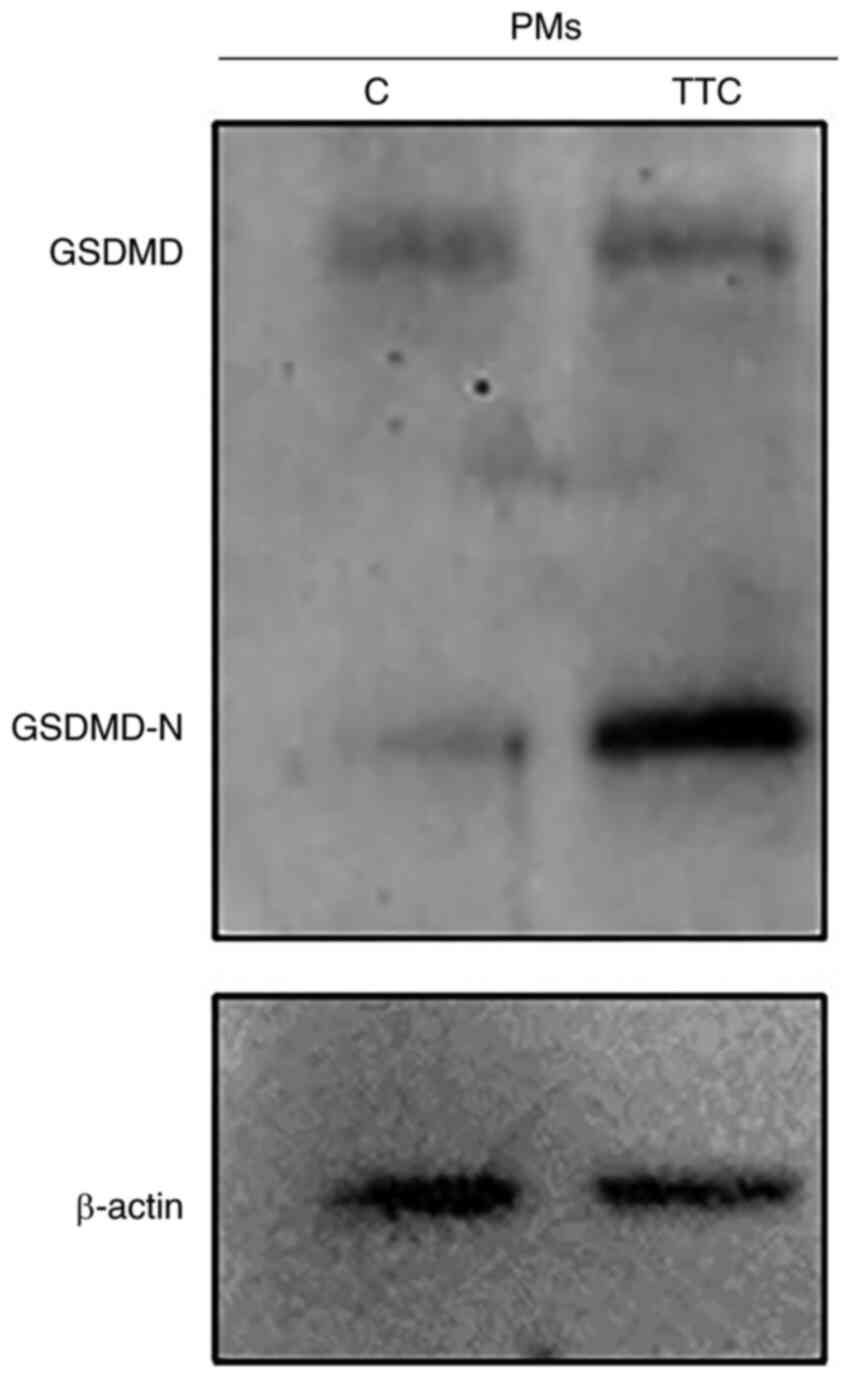
TTC induces pyroptosis in macrophages
in vivo
Next, the potential for TTC to induce pyroptosis
in vivo was investigated. TTC or an equal volume of
physiological saline was intraperitoneally injected into mice and
the PMs were extracted to analyze the protein expression levels of
GSDMD. GSDMD-N was upregulated in PMs from the TTC group compared
with control group, suggesting that TTC induced macrophage
pyroptosis in vivo (Fig.
6).
Discussion
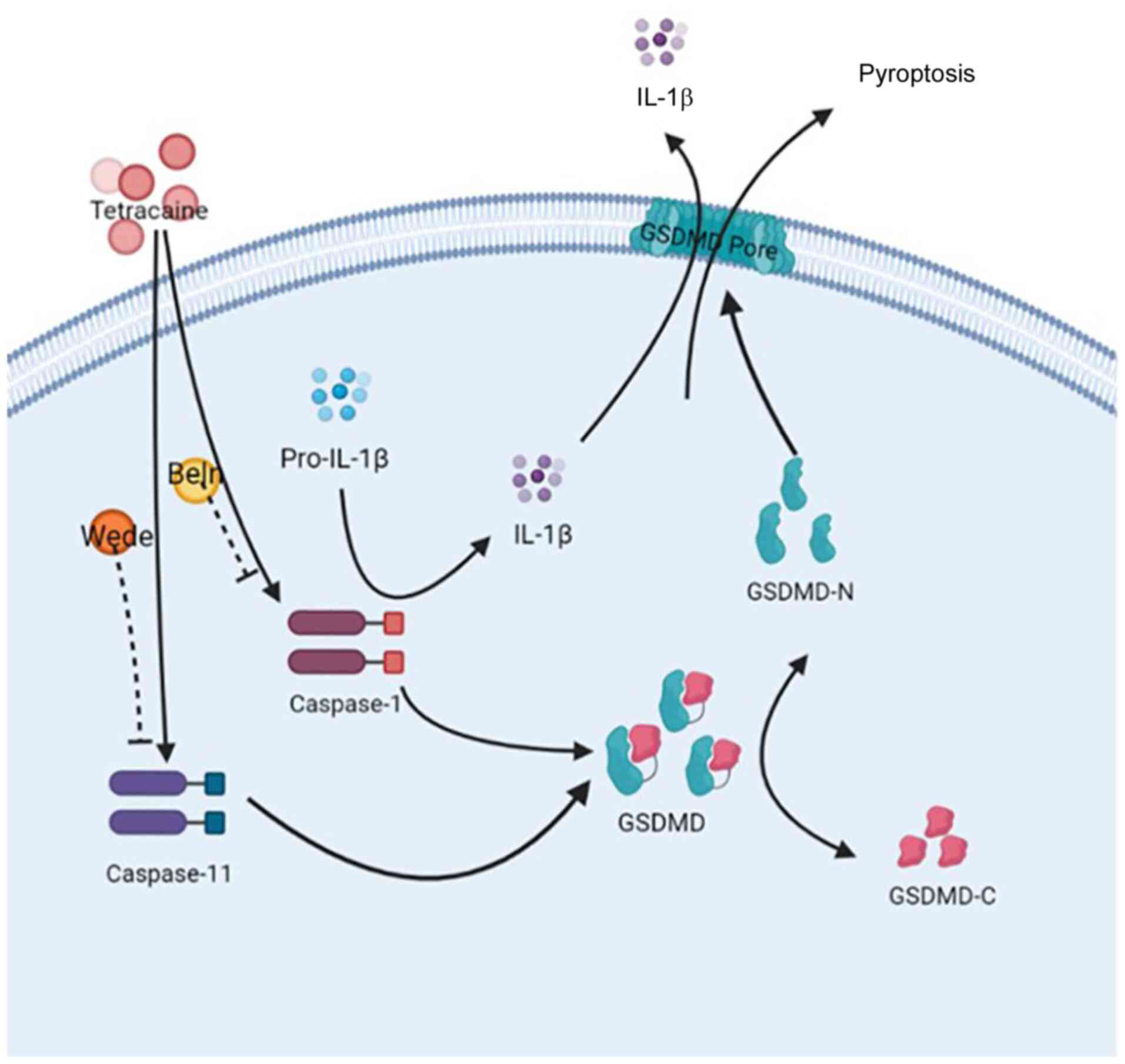
Previous studies have reported that TTC causes
cytotoxicity in various cell types mainly through the induction of
apoptosis, a type of PCD characterized by nuclear fragmentation,
plasma membrane blebbing, cell shrinkage and formation of apoptotic
bodies (7,8). The present study demonstrated that
pyroptosis was the mechanism of cell death involved in TTC
cytotoxicity (Fig. 7). Pyroptosis
is a form of PCD characterized by the formation of a plasma
membrane pore, swelling of the cell, swift plasma membrane
disruption, and release of intracellular contents and
pro-inflammatory cytokines. Although apoptosis and pyroptosis are
different forms of PCD, certain pathological factors can induce
both types of PCD as caspase-3 can cause a switch between apoptosis
and pyroptosis (28).
In the present study, an increase in the release of
IL-1β from macrophages treated with 100, 200 and 400 µM TTC was
demonstrated, while the pyroptosis-related proteins GSDMD-N,
caspase-1 and caspase-11 were only detected in macrophages treated
with 400 µM TTC. However, caspase-1 and caspase-11 inhibitors, Wede
and Beln, reduced IL-1β release, which suggests that the release of
IL-1β is associated with caspase-1 and caspase-11 expression, but
is not wholly dependent on these cytokines. A similar trend was
demonstrated by LDH release from TTC-treated cells. These results
suggested that TTC could induce multiple cell death patterns in
macrophages. In addition, necroptosis, similar to pyroptosis, is a
lytic, inflammatory type of PCD, which is mainly mediated by MLKL
proteins, and is characterized by cellular swelling, membrane
rupture and cytoplasmic and nuclear disintegration (29). Furthermore, ferroptosis is an
additional type of PCD that involves lipid peroxidation caused by
iron accumulation and is characterized by mitochondrial
condensation, reduced mitochondrial cristae and increased membrane
density (30). NETosis is a unique
form of PCD that occurs in response to various pathogens, cytokines
and other physiological stimuli, and is characterized by the
release of decondensed chromatin and granular contents into the
extracellular space, which can form web-like structures known as
neutrophil extracellular traps (31). However, it is currently unknown
whether these forms of PCD, including necroptosis, ferroptosis and
NETosis, are involved in TTC induced-cytotoxicity, and further
in-depth studies are required to elucidate these underlying
molecular mechanisms.
Local anesthetics, such as lidocaine, and general
anesthetics, such as propofol and sevoflurane, have previously been
reported to affect cell viability (32). Certain anesthetics can affect cell
pyroptosis and related molecular mechanisms (Table I) (32-41).
Combined anesthesia refers to the simultaneous or sequential use of
multiple anesthetic drugs or techniques during surgical procedures
to achieve the desired anesthetic state; it involves the
synergistic effects of different drugs to enhance the effectiveness
of anesthesia while minimizing side effects (42). Therefore, the different effects of
certain anesthetics on the process of pyroptosis may provide some
theoretical support for their use in combined anesthesia.
 | Table IEffects of different anesthetics on
the process of pyroptosis. |
Table I
Effects of different anesthetics on
the process of pyroptosis.
| First author,
year | Anesthetic | Tissue/cell
type | Disease | Effect | Mechanism of
action | (Refs.) |
|---|
| Ding et al,
2021 | Lidocaine | Spinal cord | Spinal cord
injury | Promotion | Upregulates
NLRP3/ASC/caspase-1 | (32) |
| Ding et al,
2021 |
Dexmedetomidine | Spinal cord | Spinal cord
injury | Suppression | Inhibits priming
and inflammasome activation, and reduces pyroptosis via protein
kinase C-δ phosphorylation | (32) |
| Shi et al,
2022 | Remimazolam | Brain | Cerebral I/R
injury | Suppression | Inhibits NLRP3
inflammasome-dependent pyroptosis | (33) |
| Ye et al,
2018 | Ketamine | Primary mouse
hippocampal neuron | Hippocampal
neurotoxicity | Promotion | Promotes
NLRP3/caspase-1 complex recruitment to mitochondria | (34) |
| Li et al,
2022 | Esketamine | Primary mouse
astrocyte | POCD | Suppression | Inhibits
pyroptosis-associated proteins via inhibition of the STING/TBK1
signaling pathway | (35) |
| Sun et al,
2019 | Propofol | Bone marrow-derived
macrophages, J774 | Propofol infusion
syndrome | Promotion | Activates
NLRP3/ASC/caspase-1 pathway via mitochondrial reactive oxygen
species | (36) |
| Liu et al,
2020 | | NR8383 | Acute lung
injury | Suppression | Inhibits the
expression of pyroptotic proteins and enhances the expression of
sirtuin 1 | (37) |
| Dai et al,
2021 | Sevoflurane | Primary rat
hippocampal neuron | Neuroinflammation,
neurocognitive impairment | Promotion | Activates
NF-κB-mediated pyroptosis | (38) |
| Deng et al,
2022 | | AC16 | I/R injury | Suppression | Activates the
AMPK/ULK1 pathway to trigger autophagic flux and suppress
NLRP3-mediated pyroptotic cell death | (39) |
| Chen et al,
2022 | | H19-7 | Ischemic brain
injury | Suppression | Regulates the
Mafb/DUSP14 axis to mitigate oxygen-glucose deprivation-induced
pyroptosis | (40) |
| Zheng et al,
2022 | | RAW 264.7 | Acute lung
injury | Suppression | Phosphorylates and
activates the GSK-3β to suppress pyroptotic cell death | (41) |
Activation of inflammatory caspases, such as murine
or human caspase-1, or murine caspase-11 and its human homologs
caspase-4/-5, preceding the induction of pyroptosis is a tightly
regulated process (43,44). A previous study reported that
caspase-1 is activated when the central scaffold of canonical
inflammasomes, made of NLRP3, NLRP1, AIM2, NAIP-NLR4 and pyrin,
detects its cognate ligands (45).
Aberrant or excessive activation of caspase-1 can cause, or be
associated with, certain autoinflammatory, autoimmune or metabolic
diseases (44). In mice, the
caspase-11 non-canonical inflammasome has been reported to sense
infections caused by certain bacteria, such as Escherichia
coli, Salmonella Typhimurium, Legionella
pneumophila and Burkholderia thailandensis, by
responding to the presence of cytoplasmic LPS (46). Additionally, caspase-11 activation
serves an important role in the mediation of endotoxic shock and
sepsis (47). In the present
study, TTC-induced macrophage pyroptosis was demonstrated to be
mediated through GSDMD cleavage by both caspase-1 and caspase-11.
Furthermore, as the cleavage fragments of caspase-1/-11 generated
after TTC and LPS treatment showed different patterns, the cleaved
caspase-1 and caspase-11 caused by TTC and LPS may be different The
mechanism of action of how TTC interacts with inflammatory caspases
and whether it participates in other cellular pathways has yet to
be explored. In addition, the alternative mechanism of TTC-induced
macrophage pyroptosis demonstrated in the present study may
influence the future development of therapeutics that target
pyroptosis. As GSDMD cleavage is definitive, the development of
therapies targeting GSDMD may a better alternative than targeting
inflammatory caspases.
Previous studies on the central nervous system
toxicity of local anesthetics have focused on the effects of
anesthetics on neurons as opposed to the immune system (11,12).
The present study used the BV2 central nervous system immune cell
line, which exerts a dual effect on neurons. As the present study
demonstrated that TTC could cause BV2 pyroptosis, it could be
suggested that TTC can indirectly exert toxic effects on neurons by
inducing BV2 pyroptosis to release inflammatory factors, which can
further trigger central nervous system toxicity Traditionally,
benzodiazepines are used to antagonize the symptoms of local
anesthetic poisoning by enhancing the inhibitory effects of
γ-aminobutyric acid-ergic neurons in the limbic system (48). However, these drugs cause certain
side effects, which in severe cases can affect the cognitive
function of the patients (48).
Presently used drugs such as disulfiram (49), dimethyl fumarate (50) and Prussian blue nanozyme (51) inhibit the pyroptotic pathway to
alleviate diseases, such as familial Mediterranean fever,
experimental autoimmune encephalitis and neurodegeneration. Hence,
novel drugs that target the pyroptosis pathway could potentially
provide a new future direction for the prevention and treatment of
local anesthetic toxicity.
In conclusion, the present study demonstrated that
the local anesthetic TTC induced macrophage pyroptosis through
GSDMD cleavage and was mediated by both canonical and non-canonical
inflammatory caspases. These results could potentially provide a
promising strategy for the future prevention and treatment of local
anesthetic toxicity.
Supplementary Material
TTC induces apoptosis in macrophages.
RAW 264.7 and BV2 cells were treated with TTC under indicated
concentrations for 24 h. (A and B) Caspase-3 and β-actin as an
internal control were analyzed by western blotting in (A) RAW 264.7
and (B) BV2 cells.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by funding from the Sichuan
Science and Technology Program (grant nos. 2022NSFSC1594 and
2022YFS0632), the Joint Foundation of Luzhou Government and
Southwest Medical University (grant no. 2021LZXNYD-D08) and the
Scientific Research Foundation of Southwest Medical University
(grant no. 2021ZKZD011).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RZ and WG performed the experiments and drafted the
manuscript. PY, ZQ, DZ, JJ and LL prepared materials, and collected
and analyzed data. XJ and JF contributed to the design of the work,
revised the manuscript and approved the final version of the
manuscript to be published. All authors read and approved the final
manuscript. RZ and JF confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
The study was approved by the Ethical Approval for
Research Involving Animals in Southwest Medical University (Luzhou,
China; approval no. SWMU20220028) and all animal experiments were
performed according to the Guide for the Care and Use of Laboratory
Animals of the National Institutes of Health.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wadlund DL: Local anesthetic systemic
toxicity. AORN J. 106:367–377. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Macfarlane AJR, Gitman M, Bornstein KJ,
El-Boghdadly K and Weinberg G: Updates in our understanding of
local anaesthetic systemic toxicity: A narrative review.
Anaesthesia. 76 (Suppl 1):S27–S39. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhao W, Liu Z, Yu X, Lai L, Li H, Liu Z,
Li L, Jiang S, Xia Z and Xu SY: iTRAQ proteomics analysis reveals
that PI3K is highly associated with bupivacaine-induced
neurotoxicity pathways. Proteomics. 16:564–575. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yu XJ, Zhao W, Li YJ, Li FX, Liu ZJ, Xu
HL, Lai LY, Xu R and Xu SY: Neurotoxicity comparison of two types
of local anaesthetics: Amide-bupivacaine versus ester-procaine. Sci
Rep. 7(45316)2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Grant SA and Hoffman RS: Use of
tetracaine, epinephrine, and cocaine as a topical anesthetic in the
emergency department. Ann Emerg Med. 21:987–997. 1992.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Werdehausen R, Braun S, Fazeli S, Hermanns
H, Hollmann MW, Bauer I and Stevens MF: Lipophilicity but not
stereospecificity is a major determinant of local
anaesthetic-induced cytotoxicity in human T-lymphoma cells. Eur J
Anaesthesiol. 29:35–41. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Song Z and Fan TJ: Tetracaine induces
apoptosis through a mitochondrion-dependent pathway in human
corneal stromal cells in vitro. Cutan Ocul Toxicol. 37:350–358.
2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Pang X and Fan TJ: Cytotoxic effect and
possible mechanisms of Tetracaine on human corneal epithelial cells
in vitro. Int J Ophthalmol. 9:497–504. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Koizumi Y, Matsumoto M, Yamashita A,
Tsuruta S, Ohtake T and Sakabe T: The effects of an AMPA receptor
antagonist on the neurotoxicity of tetracaine intrathecally
administered in rabbits. Anesth Analg. 102:930–936. 2006.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Barreda DR, Neely HR and Flajnik MF:
Evolution of myeloid cells. Microbiol Spectr. 4:2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Mahajan A and Derian A: Local anesthetic
toxicity. In: StatPearls StatPearls Publishing Copyright ©. 2023,
StatPearls Publishing LLC., Treasure Island (FL) ineligible
companies. Disclosure: Armen Derian declares no relevant financial
relationships with ineligible companies, 2023.
|
|
12
|
Groban L: Central nervous system and
cardiac effects from long-acting amide local anesthetic toxicity in
the intact animal model. Reg Anesth Pain Med. 28:3–11.
2003.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Henn A, Lund S, Hedtjärn M, Schrattenholz
A, Pörzgen P and Leist M: The suitability of BV2 cells as
alternative model system for primary microglia cultures or for
animal experiments examining brain inflammation. ALTEX. 26:83–94.
2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Gao W, Li Y, Liu X, Wang S, Mei P, Chen Z,
Liu K, Li S, Xu XW, Gan J, et al: TRIM21 regulates pyroptotic cell
death by promoting Gasdermin D oligomerization. Cell Death Differ.
29:439–450. 2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Orning P, Lien E and Fitzgerald KA:
Gasdermins and their role in immunity and inflammation. J Exp Med.
216:2453–2465. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Broz P, Pelegrín P and Shao F: The
gasdermins, a protein family executing cell death and inflammation.
Nat Rev Immunol. 20:143–157. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ding J, Wang K, Liu W, She Y, Sun Q, Shi
J, Sun H, Wang DC and Shao F: Pore-forming activity and structural
autoinhibition of the gasdermin family. Nature. 535:111–116.
2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Fang Y, Tian S, Pan Y, Li W, Wang Q, Tang
Y, Yu T, Wu X, Shi Y, Ma P and Shu Y: Pyroptosis: A new frontier in
cancer. Biomed Pharmacother. 121(109595)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Evavold CL, Ruan J, Tan Y, Xia S, Wu H and
Kagan JC: The pore-forming protein gasdermin D regulates
interleukin-1 secretion from living macrophages. Immunity.
48:35–44.e6. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Jorgensen I, Rayamajhi M and Miao EA:
Programmed cell death as a defence against infection. Nat Rev
Immunol. 17:151–164. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhang L, Xing R, Huang Z, Zhang N, Zhang
L, Li X and Wang P: Inhibition of synovial macrophage pyroptosis
alleviates synovitis and fibrosis in knee osteoarthritis. Mediators
Inflamm. 2019(2165918)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Fisch D, Bando H, Clough B, Hornung V,
Yamamoto M, Shenoy AR and Frickel EM: Human GBP1 is a
microbe-specific gatekeeper of macrophage apoptosis and pyroptosis.
EMBO J. 38(e100926)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Frangogiannis NG: Regulation of the
inflammatory response in cardiac repair. Circ Res. 110:159–173.
2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhang Y, Chen X, Gueydan C and Han J:
Plasma membrane changes during programmed cell deaths. Cell Res.
28:9–21. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Scully AC and Boynton JR: Antibiotics and
local anesthetics: Drug considerations for the child patient. J
Mich Dent Assoc. 99:36–41, 71. 2017.PubMed/NCBI
|
|
26
|
Kose AA, Karabaggli Y, Kiremitci A, Kocman
E and Cetin C: Do local anesthetics have antibacterial effect on
Staphylococcus aureus under in vivo conditions? An experimental
study. Dermatol Surg. 36:848–852. 2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Liu Z, Wang M, Wang X, Bu Q, Wang Q, Su W,
Li L, Zhou H and Lu L: XBP1 deficiency promotes hepatocyte
pyroptosis by impairing mitophagy to activate mtDNA-cGAS-STING
signaling in macrophages during acute liver injury. Redox Biol.
52(102305)2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wang Y, Gao W, Shi X, Ding J, Liu W, He H,
Wang K and Shao F: Chemotherapy drugs induce pyroptosis through
caspase-3 cleavage of a gasdermin. Nature. 547:99–103.
2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Bertheloot D, Latz E and Franklin BS:
Necroptosis, pyroptosis and apoptosis: An intricate game of cell
death. Cell Mol Immunol. 18:1106–1121. 2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Jiang X, Stockwell BR and Conrad M:
Ferroptosis: Mechanisms, biology and role in disease. Nat Rev Mol
Cell Biol. 22:266–282. 2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Moujalled D, Strasser A and Liddell JR:
Molecular mechanisms of cell death in neurological diseases. Cell
Death Differ. 28:2029–2044. 2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ding XD, Cao YY, Li L and Zhao GY:
Dexmedetomidine reduces the lidocaine-induced neurotoxicity by
inhibiting inflammasome activation and reducing pyroptosis in rats.
Biol Pharm Bull. 44:902–909. 2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Shi M, Chen J, Liu T, Dai W, Zhou Z, Chen
L and Xie Y: Protective effects of remimazolam on cerebral
ischemia/reperfusion injury in rats by inhibiting of NLRP3
inflammasome-dependent pyroptosis. Drug Des Devel Ther. 16:413–423.
2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ye Z, Li Q, Guo Q, Xiong Y, Guo D, Yang H
and Shu Y: Ketamine induces hippocampal apoptosis through a
mechanism associated with the caspase-1 dependent pyroptosis.
Neuropharmacology. 128:63–75. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Li Y, Wu ZY, Zheng WC, Wang JX, Yue-Xin
Song RX and Gao JG: Esketamine alleviates postoperative cognitive
decline via stimulator of interferon genes/TANK-binding kinase 1
signaling pathway in aged rats. Brain Res Bull. 187:169–180.
2022.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Sun L, Ma W, Gao W, Xing Y, Chen L, Xia Z,
Zhang Z and Dai Z: Propofol directly induces caspase-1-dependent
macrophage pyroptosis through the NLRP3-ASC inflammasome. Cell
Death Dis. 10(542)2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Liu Z, Meng Y, Miao Y, Yu L and Yu Q:
Propofol reduces renal ischemia/reperfusion-induced acute lung
injury by stimulating sirtuin 1 and inhibiting pyroptosis. Aging
(Albany NY). 13:865–876. 2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Dai J, Li X, Wang C, Gu S, Dai L, Zhang J,
Fan Y and Wu J: Repeated neonatal sevoflurane induced
neurocognitive impairment through NF-κB-mediated pyroptosis. J
Neuroinflammation. 18(180)2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Deng L, Jiang L, Wei N, Zhang J and Wu X:
Anesthetic sevoflurane simultaneously regulates autophagic flux and
pyroptotic cell death-associated cellular inflammation in the
hypoxic/re-oxygenated cardiomyocytes: Identification of sevoflurane
as putative drug for the treatment of myocardial
ischemia-reperfusion injury. Eur J Pharmacol.
936(175363)2022.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Chen C, Zuo J and Zhang H: Sevoflurane
post-treatment mitigates oxygen-glucose deprivationinduced
pyroptosis of hippocampal neurons by regulating the Mafb/DUSP14
axis. Curr Neurovasc Res. 19:245–254. 2022.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Zheng F, Wu X, Zhang J and Fu Z:
Sevoflurane suppresses NLRP3 inflammasome-mediated pyroptotic cell
death to attenuate lipopolysaccharide-induced acute lung injury
through inducing GSK-3β phosphorylation and activation. Int
Immunopharmacol. 109(108800)2022.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Litz RJ, Bleyl JU, Frank M and Albrecht
DM: Combined anesthesia procedures. Anaesthesist. 48:359–372.
1999.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
43
|
Kayagaki N, Stowe IB, Lee BL, O'Rourke K,
Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT,
et al: Caspase-11 cleaves gasdermin D for non-canonical
inflammasome signalling. Nature. 526:666–671. 2015.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Shi J, Zhao Y, Wang K, Shi X, Wang Y,
Huang H, Zhuang Y, Cai T, Wang F and Shao F: Cleavage of GSDMD by
inflammatory caspases determines pyroptotic cell death. Nature.
526:660–665. 2015.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Lamkanfi M and Dixit VM: Mechanisms and
functions of inflammasomes. Cell. 157:1013–1022. 2014.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li
P, Hu L and Shao F: Inflammatory caspases are innate immune
receptors for intracellular LPS. Nature. 514:187–192.
2014.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Hagar JA, Powell DA, Aachoui Y, Ernst RK
and Miao EA: Cytoplasmic LPS activates caspase-11: Implications in
TLR4-independent endotoxic shock. Science. 341:1250–1253.
2013.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Dickerson DM and Apfelbaum JL: Local
anesthetic systemic toxicity. Aesthet Surg J. 34:1111–1119.
2014.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Hu JJ, Liu X, Xia S, Zhang Z, Zhang Y,
Zhao J, Ruan J, Luo X, Lou X, Bai Y, et al: FDA-approved disulfiram
inhibits pyroptosis by blocking gasdermin D pore formation. Nat
Immunol. 21:736–745. 2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Humphries F, Shmuel-Galia L,
Ketelut-Carneiro N, Li S, Wang B, Nemmara VV, Wilson R, Jiang Z,
Khalighinejad F, Muneeruddin K, et al: Succination inactivates
gasdermin D and blocks pyroptosis. Science. 369:1633–1637.
2020.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Ma X, Hao J, Wu J, Li Y, Cai X and Zheng
Y: Prussian blue nanozyme as a pyroptosis inhibitor alleviates
neurodegeneration. Adv Mater. 34(e2106723)2022.PubMed/NCBI View Article : Google Scholar
|