Introduction
Hantavirus is responsible for causing a rare
zoonosis in the South-Eastern European countries, but apparently
with increasing incidence and geographical spread during recent
years. For 2020, 28 countries from Europe reported 1,647 cases of
hantavirus infection (0.4 cases per 100,000 population), mainly
caused by Puumala virus (98%) (1).
Several serotypes have rodents as reservoirs (2). These are categorized as follows:
Old-World Hantaviruses, which may cause hemorrhagic fever with
renal syndrome (HFRS), and New-World Hantaviruses which may cause
cardiopulmonary syndrome (CPS) (2).
HFRS often presents as a flu-like syndrome, with
fever and myalgia, which further evolves similarly to thrombotic
microangiopathy (TMA), causing anemia, severe thrombocytopenia,
coagulation disorders and acute kidney injury (AKI) (3,4).
Patients with renal impairment often have hematuria and proteinuria
up to the nephrotic range, which may be mistaken for nephritic
syndrome (4). These urine changes
are most likely caused by a defect in the filtration barrier, since
the histological appearance may include moderate glomerular
lesions, and the most frequently seen one is acute
tubulointerstitial nephritis with hemorrhage in the outer
medulla.
Both cases reported in the current study had been
diagnosed as TMA. A kidney biopsy (KB) was essential for the
diagnosis since an infection with hantavirus is often
underdiagnosed in the South-Eastern European countries due to its
low incidence. Hantaan (HNTV) serotype, which is usually found in
China and Russia, was identified as the causative agent in a
patient who had not traveled abroad, a trigger point for
reconsidering the geographical distribution of the species
involved. The current knowledge regarding the diagnosis and
management of this rare zoonosis is further reviewed.
Case report
Case 1
A 26-year-old female patient was hospitalized in a
Buzau Emergency Hospital for the abrupt onset of gastrointestinal
symptoms (fever, nausea, vomiting and diarrhea), hypotension (blood
pressure of 80/50 mmHg) and headache. After admission, the patient
was diagnosed with kidney impairment (serum creatinine, 1.4 mg/dl),
nephritic syndrome with nephrotic range proteinuria (microscopic
hematuria with dysmorphic red cells and red cells casts;
proteinuria, 8 g/day), elevated liver enzymes, severe
thrombocytopenia (11,000/mm3), anemia and leukocytosis
(Table I).
 | Table ILaboratory data in both reported
cases. |
Table I
Laboratory data in both reported
cases.
| Parameter | Case 1 | Case 2 | Reference
values |
|---|
| Hemoglobin,
g/dl | 9 | 12.8 | 11.5-17 |
| Hematocrit, fl | 30 | 35 | 43-54 |
| Leukocytes,
/mm3 | 16,000 →
a13,810 | 20,140 | 4,000-9,000 |
| Platelets,
/mm3 | 11,000 →
279,000 | 5,000 → 54,000 →
190,000 |
150,000-400,000 |
| Iron, µg/dl | 110 | 69 | 35-175 |
| Ferritin,
ng/ml | 154 | 92 | 15-150 |
| Lactate
dehydrogenase, IU/l | 527 | 812 | 208-378 |
| Haptoglobin,
g/l | 0.65 | 0.5 | 0.35-2.5 |
| Direct Coombs
test | Positive | Positive | Negative |
| C-reactive protein,
mg/l | 5 | 69.3 | 0-5 |
| Serum creatinine,
mg/dl | 1.4 → 6 → 1.35 | 9.6 → 1.68 →
0.96 | 0.5-1.3 |
| Blood Urea
Nitrogen, mg/dl | 75 → 94 → 43 | 131 → 35 → 20 | 7-20 |
| Uric acid,
mg/dl | 5.27 | 14.3 | 2.6-9.2 |
| Alanine
aminotransferase, IU/l | 150 → 82 | 64 | 0-49 |
| Aspartate
aminotransferase, IU/l | 151 → 62 | 103 | 0-34 |
| Potassium,
mmol/l | 3.3 | 4.4 | 3.5-5.5 |
| Sodium, mmol/l | 145 | 126 | 132-146 |
| Albumin, g/dl | 3.45 | 2.5 | 3.2-5.2 |
| Antinuclear
antibodies | Negative | Negative | Negative |
| Anti-double
stranded DNA | Negative | Negative | Negative |
| Anti-Ro | Negative | Negative | Negative |
| Anti-La | Negative | Negative | Negative |
| Anti-C1q | Negative | Negative | Negative |
| C3, mg/dl | 99 | 76 | 83-193 |
| C4, mg/dl | 19 | 10 | 15-57 |
|
Anti-myeloperoxidase-ANCA | Negative | Negative | Negative |
| Anti-proteinase
3-ANCA | Negative | Negative | Negative |
| Anti-glomerular
basement membrane antibodies | Negative | Negative | Negative |
| Lupus
anticoagulant | Positive | Negative | Negative |
|
Anti-β2-glycoprotein antibodies | Negative | Negative | Negative |
| Anti-cardiolipin
antibodies | Negative | Negative | Negative |
| HBV surface
antigen | Negative | Negative | Negative |
| anti-HBV surface
antibody, IU/l | >1,000 | Negative | 0-10 |
| Anti-HBV core
antibody | Negative | Negative | Negative |
| Anti-hepatitis C
antibody | Negative | Negative | Negative |
| Anti-HIV | Negative | Negative | Negative |
| IgG, mg/dl | 2,127 | 695 | 552-1,631 |
| IgA, mg/dl | 328 | 259 | 65-421 |
| IgM, mg/dl | 251 | 206 | 33-293 |
| Proteinuria,
mg/dl | 1,000 → 30 | 1,000 | 0-30 |
| Hematuria, red
blood cells/µl | 100 → 150 | 2,400 | 0-1 |
| Proteinuria,
g/day | 8 → 2 | 6.8 | <0.15 |
The stool was collected in a transport recipient by
selecting from three different places, especially from zones with
mucus or blood (if these areas of interest were present). The probe
was cultivated on selenite broth for Salmonella, and on ADCL agar
SS (agar Shigella-Salmonella). They are cultivated for 24 h. After
24 h the probe was moved from selenite broth to MacConkey medium.
The colonies which suggested intestinal pathogens were incubated
for 24 h more on MacConkey medium and agar SS. Colonies which were
suspected to be E Coli were tested with agglutination sera
against enteropathogenic E Coli, enterotoxigenic E
Coli, enteroinvasive E Coli and enterohemorrhagic E
coli. When after 48 h one of these pathogens was present, the
antibiogram was be performed according to CLSI/EUCAST. The stool
samples were negative for Escherichia coli, Shigella
and Salmonella.
The patient became oliguric, with a further increase
in serum creatinine (≤6 mg/dl) and persistence of severe
thrombocytopenia; therefore, 3 days after hospitalization, the
patient received methylprednisolone (three intravenous injections,
500 mg each). Subsequently, the patient developed polyuria and the
platelet count, proteinuria, and serum creatinine improved
considerably. However, the patient remained with nephritic syndrome
and kidney dysfunction, developing high blood pressure (167/94
mmHg), consequently, after 3 weeks, the patient was transferred to
the Department of Nephrology of The Fundeni Clinical Institute.
When transferred, the patient had no edema, high
blood pressure (170/100 mmHg), tachycardia (heart rate 90
beats/min) and polyuria (4 l/day). The blood analysis revealed
moderate normocytic normochromic anemia with raised lactate
dehydrogenase (LDH), normal haptoglobin (0.65 g/l), a positive
direct Coombs test, normal platelet count, leukocytosis, mild
kidney impairment, normal serum albumin, mild hypokalemia and
elevated liver enzymes (Table I).
Urinalysis showed glomerular hematuria, leukocyturia and
proteinuria of 2 g/day. For urinalysis a spontaneous morning void
was collected in a sterile recipient and was examined within the
first hour after the collection. The urine sample was verified to
have the necessary quantity and then placed in a rack in the
analyzer, which was a fully automated urine chemistry analyzer
using reflectance photometry and refractometry methods. The
analyzer was loaded with strips. Urine sample material was dropped
onto each pad of a dedicated test strip within the analyzer. The
entire sequence, starting from sample aspiration, to color
comparison and final output of the results was fully automatic. The
sample barcode reader scanned the barcode of each tube and results
were transmitted to the computer system.
Regarding the antiphospholipid antibodies, the
patient was positive for the lupus anticoagulant. The screening for
autoimmune diseases (lupus, ANCA-associated vasculitis,
anti-glomerular basement membrane, cryoglobulinemia, C1q
vasculitis, Sjögren syndrome and membranous nephropathy) was
negative (including normal complement level and normal serum levels
of immunoglobulin A and M, with only a slightly elevated level for
immunoglobulin G). A disintegrin and metalloproteinase with
thrombospondin type 1 motif, member 13 (ADAMTS 13) activity was
normal; evaluation was done according to the protocol described
previously (5). The serological
screening for viral infections [hepatitis B (HBV) and C, HIV,
cytomegalovirus and Epstein-Barr virus] and leptospira was also
negative. For the serological analysis, the blood sample was
allowed to settle, then centrifuged 5 min at 1,910 x g at room
temperature, separating the serum (hemolyzed samples were
rejected). The sample was placed in a rack in the analyzer. The
rack was inserted into the input buffer on rack trays. The sample
barcode reader scans the barcode of each tube. It used the
immunochemical method with electrochemiluminescence detection
(ECLIA) and results were transmitted to the computer system.
The tumor markers for digestive, ovarian and breast
cancer were negative. An abdominal ultrasound (a 2D convex probe
with a 2.5 MHz frequency; General Electric) showed a mildly
increased volume of both kidneys with hyperechoic parenchyma and no
signs of urinary tract obstruction.
Based on the severe thrombocytopenia
(11,000/mm3) associated with anemia and stage III AKI,
TMA as part of the hemolytic uremic syndrome (HUS) was suspected.
Lactate dehydrogenase was elevated, while haptoglobin was normal
and the Coombs test was positive, which mainly excluded TMA,
although there have been reports of TMA with microangiopathic
hemolytic anemia and a positive Coombs test (i.e., Pneumococcal
infection) (6). Typical HUS was
ruled out by the negative stool cultures. Autoimmune, viral or
malignant causes of atypical HUS (aHUS) were also excluded.
Furthermore, the patient denied any intake of illicit drugs and had
had no regular treatment before being hospitalized, except for oral
contraceptives, which can be very rarely associated with TMA
(7,8), especially in renal graft female
recipients (9). Normal ADAMTS 13
activity excluded thrombotic thrombocytopenic purpura.
Since a definite etiology for AKI could not be
established, a KB was performed. Immunofluorescence was performed
on sections from unfixed, fresh frozen tissue. The biopsy cores
were frozen with OCT at -25˚C for 15 min and 4-µm-thick sections
were cut with a Leica cryostat (Leica Microsystems GmbH). The
serial sections were collected on microscopy slides and dried for
30 min. Dried sections were rehydrated for 15 min in saline
phosphate buffer (PBS) at pH 7.2 and incubated for 30 min in dark
staining tray with FITC labeled antibodies diluted 1:50: FITC IgG
antiserum (Dako; Agilent Technologies, Inc.; cat. no. F020202-2);
FITC IgA antiserum (Dako; Agilent Technologies, Inc.; cat. no.
F020402-2); FITC IgM antiserum (Dako; Agilent Technologies, Inc.;
cat. no. F020302-2); FITC C3c antiserum (Dako; Agilent
Technologies, Inc.; cat. no. F020102-2); FITC Fibrin-Fibrinogen
(Dako; Agilent Technologies, Inc.; cat. no. F011102-2); FITC C1q
Complement antiserum (Dako; Agilent Technologies, Inc.; cat. no.
F025402-8); FITC Kappa Light Chains (Dako; Agilent Technologies,
Inc.; cat. no. F019802-2); FITC Lambda Light Chains (Dako; Agilent
Technologies, Inc.; cat. no. F019902-2). Each slide was washed
twice in PBS 10% for 15 min, and mounted with glycerin. Each biopsy
had internal staining patterns (negative and positive internal
controls). For each biopsy a tissue section incubated with a PBS
and omitting the conjugated antibody was used as a negative
staining control. The fluorescence was assessed under Leica DM6000B
light microscope with epifluorescence module using the UV
excitation and dedicated filters for FITC (Leica Microsystems
GmbH). Representative images were recorded under a Leica DM6000B
light microscope with a Leica DFC310FX digital camera using Leica
LAS core-package software (Leica Microsystems GmbH).
Immunofluorescence (IF) was negative. Light microscopy (LM; data
not shown) revealed normal arterioles and glomeruli, dilated
proximal tubules with vesicles near the apical membrane and
erythrocytes in the lumen. However, the most striking finding was
the significant interstitial hemorrhage in the medulla, without
inflammation or interstitial fibrosis.
For electron microscopy (EM) the fragments of kidney
tissue were fixed by immersion in 4% glutaraldehyde, buffered with
0.1 mol L−1 sodium cacodylate (pH 7.3) at 4˚C overnight.
After two baths in 0.1 M cacodylate buffer, the samples were
post-fixed for 1 h in 1% OsO4 with 1.5% K4Fe(CN)6 (potassium
ferrocyanide-reduced osmium) in 0.1 M cacodylate buffer, at room
temperature. Afterwards, the samples were dehydrated through
increasing ethanol and further processed for Epon-embedding
(Agar100 resin, Agar Scientific) at 60˚C. for 24 h. Epon-embedded
kidney tissue fragments were sectioned using a Leica EM UC7
ultramicrotome (Leica Microsystems GmbH). Light microscopy was
performed on 1-µm-thick sections stained with 1% toluidine blue for
1 min at 80˚C. Representative images were recorded under a Leica
DM6000B light microscope with a Leica DFC310FX digital camera using
Leica LAS core-package software (Leica Microsystems GmbH). Selected
areas from Epon-embedded blocks were sectioned for TEM with a Leica
EM UC7 ultramicrotome and mounted on 50-mesh copper grids (Agar
Scientific). Electron microscopy imaging was performed on 60-80 nm
ultra-thin sections counterstained with uranyl acetate for 15 min
at room temperature and Reynolds lead citrate (Agar Scientific) for
5 min at room temperature. The ultrastructural analysis was
performed at 80 kV on a Morgagni 268 TEM (FEI Company), equipped
with a MegaView III CCD (Olympus Soft Imaging Solutions GmbH) and
running iTEM-SIS software (Olympus Soft Imaging Solutions GmbH). EM
showed glomeruli with mild glomerular endotheliosis, rare
tubulo-reticular inclusions in the endothelial cells and segmental
foot process effacement. In addition to the severe hemorrhage in
the medulla, EM also showed extensive endotheliosis of the
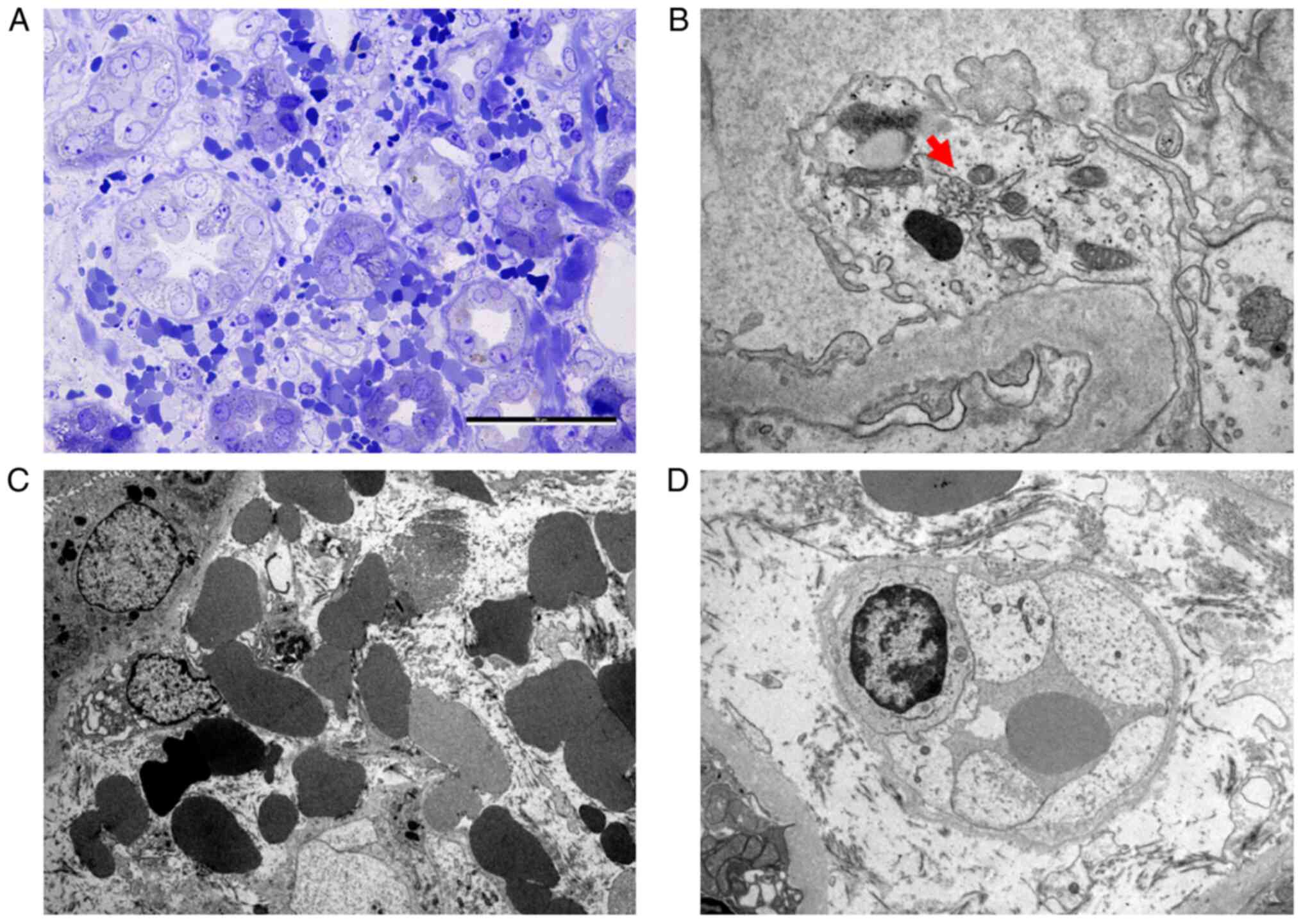
peritubular capillaries (Fig. 1).
The presence of severe hemorrhagic interstitial nephritis suggested
a Hantavirus infection, which was further confirmed by the positive
serology for both IgM and IgG antibodies. The patient received
angiotensin-converting enzyme inhibitor in maximal dose (ramipril
10 mg/day) and oral steroid methylprednisolone 16 mg/day for 2
weeks, with gradual tapering during the next 2 months. The
evolution was favorable, with the normalization of kidney and liver
function, but with the persistence of the changes suggesting
interstitial nephritis (sterile leukocyturia, mild proteinuria and
hypokalemia). At discharge, the patient also received
spironolactone (25 mg/day) for hypokalemia and the prevention of
kidney fibrosis. At the 12-week follow-up, the patient presented
with normal blood and urine values.
Case 2
A 30-year-old male patient with no medical history
was hospitalized for oliguria (400 ml/day) for >48 h and altered
general status. The symptoms started 8 days before with fever
(39˚C), nausea, vomiting and diarrhea, for which the patient
self-medicated with antibiotics (levofloxacin 500 mg/day), with a
slight improvement of the fever but with the persistence of the
gastrointestinal discomfort and a progressive drop in diuresis.
The physical examination revealed a patient in
obvious distress, with slightly pale skin, no edema, bilateral
pleural rub, high blood pressure (147/92 mmHg) and oliguria with
gross hematuria.
The laboratory evaluation (Table I) showed mild normocytic
normochromic anemia with raised LDH, normal haptoglobin and
positive Coombs test, severe thrombocytopenia (5,000 elements/mmc),
positive D-dimers, inflammation (leukocytosis with neutrophilia and
raised C-reactive protein), severe kidney impairment and liver
cytolysis. Urinalysis revealed proteinuria, hematuria and
glycosuria, and 24-h proteinuria was 6.8 g with hypoalbuminemia.
The immunological tests revealed complement consumption, normal
immunoglobulins level, negative screening for autoimmune diseases
with potential kidney involvement, negative antiphospholipid
antibodies and negative serology for viruses (hepatitis B and C,
HIV, cytomegalovirus and Epstein-Barr virus) and Leptospira.
The ADAMTS 13 enzymatic activity was normal. An abdominal
ultrasound showed normal kidneys with no signs of obstruction, mild
ascites and mild bilateral pleural effusion.
Similarly to the previous case, the diagnosis was
stage III AKI. The patient also had severe thrombocytopenia, anemia
with normal haptoglobin and a positive Coombs test. A KB was
performed. The biopsy specimen showed macroscopic features of
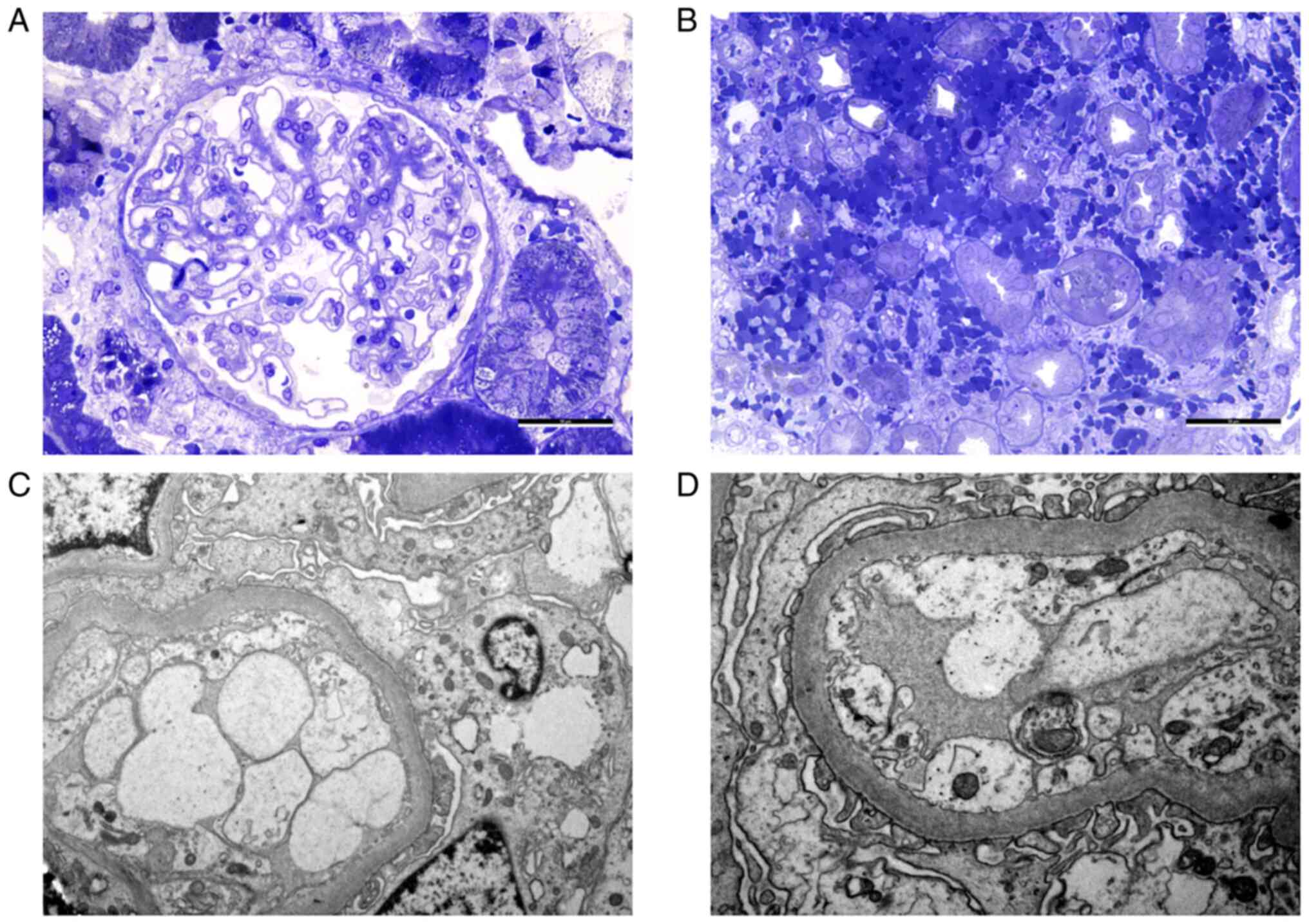
hemorrhage in the renal medulla. IF was negative. LM revealed
normal glomeruli and massive interstitial hemorrhage in the
medulla, with no associated interstitial inflammation. The EM
showed a glomerulus with segmental narrowing of the lumen due to
endothelial swelling and segmental foot process effacement
(Fig. 2). A diagnosis of
hemorrhagic interstitial nephritis with mild glomerular
abnormalities was made and Hantavirus infection was considered as a
differential diagnosis. Serology was positive for both IgM and IgG
antibodies against Dobrava virus (DOBV) strain. The patient
received fluid replacement therapy and was started on intravenous
methylprednisolone (500 mg/day for 3 days), followed by oral
methylprednisolone (32 mg/day). The patient also required renal
replacement therapy consisting of four hemodialysis sessions at 2
days apart. The evolution was good, with the recovery of the kidney
function but with polyuria during the first days, normalization of
the liver enzymes and resolution of the thrombocytopenia.
Discussion
Hantaviruses are a member of the Bunyaviridae
family, including over 28 serotypes which cause infections in
humans. Wild rodents are the main hosts. It is considered a rare
infectious disease but with an increasing incidence during the
recent years (10). The virus was
first identified on pulmonary tissue from the striped field mouse
Apodemus Agrarius (11).
The serotypes which are found in Europe and Asia belong to the
Old-World Hantaviruses group; Hantaan (HNTV), Puumala (PUUV), Seul,
Dobrava (DOBV), the Tula virus (TULV) and the Saaremaa virus (SAAV)
(3). The DOBV is mostly found in
the Balkan Peninsula and it is responsible for a 12% mortality rate
(4,12). These serotypes use several species
of wild rodents as hosts, such as Apodemus agrarius,
Rattus rattus, Microtus arvalis and Myodes
glareolus (13,14), but they can also spread to other
wild or domestic animals, including moose, bat, fox, cat and dog
(15-18).
The European Centre for Disease Prevention and
Control reported 1,826 cases of Hantavirus infection in 2018, most
of the cases (97%) involving PUUV (19). In the same year, the Activity
report of The National Public Health Institute from Romania
included 40 cases of suspected Hantavirus infection in patients
from the region and only one confirmed case (20). Several mild and moderate forms of
HFRS remain undiagnosed and so the incidence of the disease in
Romania may be underestimated. Regarding the current two cases, the
patients were infected by HNTV (case 1) and DOBV (case 2); they
both live in Buzau County, in a rural area, at a small distance
from each other (30 km). DOBV presence was confirmed in Central and
South-Eastern Europe, but not in Romania. It was also surprising to
identify HNTV, which is usually found in China and Russia, in case
1. It raises the question of whether the infection is not
underdiagnosed in Romania and whether the HNTV is not already
present in the Balkan peninsula. Both DOBV and HNTV have the same
reservoir, the mouse Apodemus Agrarius, which is found in
Romania.
Hantavirus is transmitted to humans through
aerosolized particles containing the virus from saliva and
droppings shed by rodents or through bite (3,4,21).
Individuals from rural areas or areas that are natural reservoirs
for rodents (farmers and hunters) are more prone to infection.
Hantaviruses cause tubular lesions by altering the
tight junctions between the tubular epithelial cells, disrupting
the podocyte cytoskeleton, and also causing an endothelial
dysfunction with increased vascular permeability. The virus
penetrates the target cells via αvβ1 and αvβ3 receptors (22). The immature dendritic cells can
serve both as viral carriers to the lymph nodes and the epithelial
cells and as antigen-presenting cells that will stimulate the T
lymphocytes (23,24). The endothelial lesions are mostly
caused by the cytokine storm caused by T lymphocytes. The
complement pathway is activated and cytokines such as IL-10 and
IL-6, IFN, TGF and TNF-α are secreted (4,25).
The role of the podocyte injury is supported by the increased serum
levels of the mediators involved in the regulation of podocyte
function, such as urokinase-type plasminogen activator receptor,
VEGF and IL-6(26).
The manifestations of HFRS can be grouped into
several stages. The disease begins with fever (3-7 days) and
flu-like symptoms (myalgia and headache), then it evolves to a
hypotensive phase (maximum 2 days) which can be accompanied by
thrombocytopenia, leukocytosis, petechial rash, gastrointestinal
hemorrhage and hematuria. These manifestations are followed by the
oliguric phase (3-7 days) with AKI development, then the polyuric
phase with the recovery of the renal function (up to a few weeks).
There are cases which develop long-term complications, such as
arterial hypertension and chronic kidney disease (27-29).
Thrombocytopenia is the result of increased
peripheral consumption and is regarded as a marker of severity. The
virus facilitates the adhesion of the platelets to the surface of
infected endothelial cells through the β3-integrin receptor,
resulting in the alteration of platelet activation and
thrombocytopenia. Furthermore, endothelial lesions can promote
coagulation activation and fibrinolysis, resulting in increased
prothrombin and D-dimer levels (30,31).
The second patient developed more severe thrombocytopenia and
positive D-dimer values. The patient also had a more severe
clinical evolution with AKI requiring dialysis, in agreement with
the literature data that state that platelet count is a predictor
for disease severity and progression (32,33).
PUUV infection causes complement alternative pathway activation,
resulting in reduced component 3 of the complement system (C3) and
increased C5b-9 serum levels in the acute phase of the disease in
patients who have chest X-ray abnormalities (34-36).
The second case, infected with DOBV, developed bilateral pleural
effusions and low complement levels (C3 and component 4 of the
complement system).
Hantavirus infection can result in other acute
extra-renal complications, such as meningoencephalitis, pituitary
gland hemorrhage, pericarditis, myocarditis, pulmonary edema, and
disseminated intravascular coagulation.
The most frequent chronic complications are
hypothyroidism, arterial hypertension, membranoproliferative
glomerulonephritis and chronic tubulointerstitial nephritis
(37-42).
The first patient developed hypertension during the polyuric phase
of AKI, which showed that the pathogenesis was not the hypervolemia
of the oliguric phase. One of the proposed mechanisms for the
development of hypertension is the injury of the small vessels of
the kidneys, accompanied by significant interstitial and tubular
inflammation. Any factors that cause vasoconstriction in the renal
medulla and adjacent cortex can induce hypertension. This theory is
supported by histology (congestion and hemorrhage around the
vessels in the outer medulla and the cortico-medullary junction)
and by the endothelial injury caused by the virus (38). At 4 weeks after discharge, the
first patient developed mild proteinuria and leukocyturia with
sterile urine culture, indicating persistent tubulointerstitial
nephritis.
Kidney biopsies usually show lesions of acute
tubulointerstitial nephritis. The pathognomonic lesion is the
interstitial hemorrhage in the outer medulla (42,43).
IF is usually negative. EM reveals diffuse foot processes
effacement, cells with enlarged Golgi apparatus, denudation of the
tubular epithelial cells and endothelial swelling (43). The KB specimens obtained during the
acute phase of the disease from the present patients raised the
suspicion of Hantavirus infection immediately after tissue removal,
as they revealed macroscopic features of hemorrhage in the medulla.
The first patient, with higher proteinuria, had resorption droplets
at the apical pole of the proximal tubular epithelial cells. EM
showed extensive foot process effacement and detachment of the
podocytes from the glomerular basement membrane, which explained
the nephrotic range proteinuria seen in both cases and emphasized
the role of the alterations of the cytoskeleton of the podocytes in
the pathogenesis of the disease.
The infection with Hantavirus has to be suspected in
all cases with fever together with AKI and thrombocytopenia, and
confirmed by serology testing. The most frequently used laboratory
methods for the detection of antibodies are indirect ELISA for IgM
and IgG and the immunochromatographic test.
The treatment consists mostly of conservative
measures and symptomatic therapy. A series of studies supported the
use of Ribavirin, but only when administered in the early phases
(44-46).
Corticosteroids can be used, as they target the secretion of
cytokines and complement activation (47). In the present study, both patients
received steroids. Their evolution was eventually favorable. DOBV
and HNTV serotypes usually have more severe manifestations. Only
the second case evolved as a severe form. The different evolution
could have been determined by the earlier administration of
corticosteroids in the first case (5 days after onset), compared to
the second one (9 days after the onset and after starting
dialysis).
Hantavirus endemic nephropathy belongs to a group of
rare zoonoses in Romania and South-Eastern Europe. Dobrava and
Puumala are the most frequent serotypes that cause the disease in
this geographical area. The first case had positive serology for
the Hantaan strain, which draws attention to a possible
underdiagnosis of the disease in these regions and also to the need
for more epidemiological studies, since they may pose major risks
to public health. The disease manifests with fever and evolves with
AKI, anemia, severe thrombocytopenia and coagulation disorders. The
symptoms are similar to TMA secondary to the hemolytic uremic
syndrome, this being one of the major differential diagnoses. Due
to the rarity of infection in the South-Eastern European countries,
we face multiple challenges, one being the absence of any initial
suspicion. Furthermore, it is necessary to include this infection
in the algorithm of differential diagnosis of disorders
characterized by thrombocytopenia, anemia, liver cytolysis and
acute kidney injury.
Acknowledgements
The authors wish to thank to Professor Mihaela
Gherghiceanu (Victor Babes National Institute of Pathology,
Bucharest, Romania), Dr Ilinca Radu (Carol Davila University of
Medicine and Pharmacy, Bucharest, Romania) and Mrs Emilia Gheoda
(Principal Biochemist, Synevo Romania Srl, Romania), for all the
support in writing this manuscript.
Funding
Funding: Publication of this study was supported by the
University of Medicine and Pharmacy Carol Davila, through the
institutional program Publish not Perish.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
GL, IV and ML conceived and designed the present
study. RS was responsible for methodology. CA, IA and MB were
responsible for data curation. IA, GI and GL were responsible for
analysis and interpretation of data. AA, MB and RS were responsible
for writing, review and editing. GL was responsible for
supervision. GI, AA and ML were responsible for project
administration. GL and AA confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Fundeni Hospital (Bucharest, Romania; approval no.
21713).
Patient consent for publication
Written informed consent was obtained from each
patient for the publication of the present study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
European Centre for Disease Prevention and
Control. https://www.ecdc.europa.eu/en/publications-data/hantavirus-infection-annual-epidemiological-report-2020.
Accessed June 9, 2023.
|
|
2
|
Zeier M, Handermann M, Bahr U, Rensch B,
Müller S, Kehm R, Muranyi W and Darai G: New ecological aspects of
hantavirus infection: A change of a paradigm and a challenge of
prevention-a review. Virus Genes. 30:157–180. 2005.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Avšič-Županc T, Saksida A and Korva M:
Hantavirus infections. Clin Microbiol Infect. 21S:e6–e16.
2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lupuşoru G, Lupuşoru M, Ailincăi I, Bernea
L, Berechet A, Spătaru R and Ismail G: Hanta hemorrhagic fever with
renal syndrome: A pathology in whose diagnosis kidney biopsy plays
a major role (review). Exp Ther Med. 22(984)2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Muia J, Gao W, Haberichter SL, Dolatshahi
L, Zhu J, Westfield LA, Covill SC, Friedman KD and Sadler JE: An
optimized fluorogenic ADAMTS13 assay with increased sensitivity for
the investigation of patients with thrombotic thrombocytopenic
purpura. J Thromb Haemost. 11:1511–1518. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Brocklebank V, Wood KM and Kavanagh D:
Thrombotic microangiopathy and the kidney. Clin J Am Soc Nephrol.
13:300–317. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hauglustaine D, Van Damme B, Vanrenterghem
Y and Michielsen P: Recurrent hemolytic uremic syndrome during oral
contraception. Clin Nephrol. 15:148–153. 1981.PubMed/NCBI
|
|
8
|
Hauglustaine D, Vanrenterghem Y,
Michielsen OP and van Damme B: Oestrogen containing oral
contraceptives, decreased prostacyclin production, and haemolytic
uraemic syndrome. Lancet. 1:328–329. 1981.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Shirai H, Yashima J, Tojimbara T and Honda
K: Thrombotic microangiopathy caused by oral contraceptives in a
kidney transplant recipient. Nephrology. 21:41–43. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
van Regenmortel MHV, Fauquet CM, Bishop
DHL, Carsten EB, Estes MK, Lemon SM, Maniloff J, Mayo MA, McGeoch
DJ, Pringle CR and Wickner RB: Virus Taxonomy: Seventh Report of
the International Committee on Taxonomy of Viruses, 1st edition.
Academic Press, San Diego, CA, pp35-38, 2000.
|
|
11
|
Lee HW, Lee PW and Johnson KM: Isolation
of the etiologic agent of Korean hemorrhagic fever. J Infect Dis.
190:298–308. 1978.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Avsic-Zupanc T, Petrovec M, Furlan P, Kaps
R, Elgh F and Lundkvist A: Hemorrhagic fever with renal syndrome in
the Dolenjska region of Slovenia-a 10-year survey. Clin Infect Dis.
28:860–865. 1999.PubMed/NCBI View
Article : Google Scholar
|
|
13
|
Steer A: Pathogenesis of renal changes in
epidemic hemorrhagic fever. In: The Kidney IAP Monograph. Mostofi
FK and Smith DE (eds). Williams & Wilkins, Baltimore, MD,
pp476-486, 1996.
|
|
14
|
Jokinen EJ and Lähdevirta JCY:
Nephropathiaepidemica: Immunohistochemical study of pathogenesis.
Clin Nephrol. 9:1–5. 1978.PubMed/NCBI
|
|
15
|
Zaki SR, Greer PW, Coffield LM, Goldsmith
CS, Nolte KB, Foucar K, Feddersen RM, Zumwalt RE, Miller GL, Khan
AS, et al: Hantavirus pulmonary syndrome. Pathogenesis of an
emerging infectious disease. Am J Pathol. 146:552–579.
1995.PubMed/NCBI
|
|
16
|
Kim S, Kang ET, Kim YG, Han JS, Lee JS,
Kim JI, Hall WC, Dalrymple JM and Peters CJ: Localization of
Hantaan viral envelope glycoproteins by monoclonal antibodies in
renal tissues from patients with Korean hemorrhagic fever H. Am J
Clin Pathol. 100:398–403. 1993.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Groen J, Bruijn JA, Gerding MN, Jordans
JG, Moll van Charante AW and Osterhaus AD: Hantavirus antigen
detection in kidney biopsies from patients with nephropathia
epidemica. Clin Nephrol. 46:379–383. 1996.PubMed/NCBI
|
|
18
|
Poljak M and Avsic Zupanc T:
Immunohistochemical detection of Hantaan virus antigen in renal
tissue from patient with hemorrhagic fever with renal syndrome.
Nephron. 67(252)1994.PubMed/NCBI View Article : Google Scholar
|
|
19
|
European Centre for Disease Prevention and
Control: Hantavirus infection-Annual epidemiological report for
2018. https://www.ecdc.europa.eu/en/publications-data/hantavirus-infection-annual-epidemiological-report-20182020.
Accessed January 25, 2022).
|
|
20
|
National Institute for Public Health: INSP
Report for 2018. https://www.insp.gov.ro/index.php/informatii-publice/send/7-informatii-publice/722-raport-insp-2018.
Accessed 25 January 25, 2022).
|
|
21
|
Douron E, Moriniere B, Matheron S, Girard
PM, Gonzalez JP, Hirsch F and McCormick JB: HFRS after a wild
rodent bite in the Haute-Savoie-and risk of exposure to
Hantaan-like virus in a Paris laboratory. Lancet. 1:676–677.
1984.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Gavrilovskaya IN, Brown EJ, Ginsberg MH
and Mackow ER: Cellular entry of hantaviruses which cause
hemorrhagic fever with renal syndrome is mediated by beta3
integrins. J Virol. 73:3951–3959. 1999.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Peebles RS Jr and Graham BS: Viruses,
dendritic cells and the lung. Respir Res. 2:245–249.
2001.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
Schönrich G, Rang A, Lütteke N, Raftery
MJ, Charbonnel N and Ulrich RG: Hantavirus-induced immunity in
rodent reservoirs and humans. Immunol Rev. 225:163–189.
2008.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Saksida A, Wraber B and Avšič-Županc T:
Serum levels of inflammatory and regulatory cytokines in patients
with hemorrhagic fever with renal syndrome. BMC Infect Dis.
11(142)2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hägele S, Müller A, Nusshag C, Reiser J,
Zeier M and Krautkrämer E: Motility of human renal cells is
disturbed by infection with pathogenic hantaviruses. BMC Infect
Dis. 18(645)2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
McCAUGHEY C and Hart CA: Hantaviruses. J
Med Microbiol. 49:587–599. 2000.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Linderholm M and Elgh F: Clinical
characteristics of hantavirus infections on the Eurasian continent.
Curr Top Microbiol Immunol. 256:135–151. 2001.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Krüger DH, Ulrich R and Lundkvist AA:
Hantavirus infections and their prevention. Microbes Infect.
3:1129–1144. 2001.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Gavrilovskaya IN, Gorbunova EE and Mackow
ER: Pathogenic hantaviruses direct the adherence of quiescent
platelets to infected endothelial cells. J Virol. 84:4832–4839.
2010.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Laine O, Mäkelä S, Mustonen J, Huhtala H,
Szanto T, Vaheri A, Lassila R and Joutsi-Korhonen L: Enhanced
thrombin formation and fibrinolysis during acute Puumala hantavirus
infection. Thromb Res. 126:154–158. 2010.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wang M, Wang J, Wang T, Li J, Hui L and Ha
X: Thrombocytopenia as a predictor of severe acute kidney injury in
patients with Hantaan virus infections. PLoS One.
8(e53236)2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Rasche FM, Uhel B, Krüger DH, Karges W,
Czock D, Hampl W, Keller F, Meisel H and von Müller L:
Thrombocytopenia and acute renal failure in Puumala hantavirus
infections. Emerg Infect Dis. 10:420–425. 2004.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Paakkala A, Mustonen J, Viander M, Huhtala
H and Pasternack A: Complement activation in nephropathia epidemica
caused by Puumala hantavirus. Clin Nephrol. 53:424–431.
2000.PubMed/NCBI
|
|
35
|
Sane J, Laine O, Mäkelä S, Paakkala A,
Jarva H, Mustonen J, Vapalahti O, Meri S and Vaheri A: Complement
activation in Puumala hantavirus infection correlates with disease
severity. Ann Med. 44:468–475. 2012.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Vaheri A, Henttonen H, Voutilainen L,
Mustonen J, Sironen T and Vapalahti O: Hantavirus infections in
Europe and their impact on public health. Rev Med Virol. 23:35–49.
2012.PubMed/NCBI View
Article : Google Scholar
|
|
37
|
Stojanovic M, Pekic S, Cvijovic G, Miljic
D, Doknic M, Nikolic-Djurovic M, Micic D, Hrvacevic R, Nesic V and
Popovic V: High risk of hypopituitarism in patients who recovered
from hemorrhagic fever with renal syndrome. J Clin Endocrinol
Metab. 93:2722–2728. 2008.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Miettinen MH, Mäkelä SM, Ala-Houhala IO,
Huhtala HS, Kööbi T, Vaheri AI, Pasternack AI, Pörsti IH and
Mustonen JT: Ten-year prognosis of Puumala hantavirus-induced acute
interstitial nephritis. Kidney Int. 69:2043–2048. 2006.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Mäkelä S, Jaatinen P, Miettinen M, Salmi
J, Ala-Houhala I, Huhtala H, Hurme M, Pörsti I, Vaheri A and
Mustonen J: Hormonal deficiencies during and after Puumala
hantavirus infection. Eur J Clin Microbiol Infect Dis. 29:705–713.
2010.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Miettinen M, Mäkelä S, Haapala M,
Helanterä A, Helin H, Vänttinen T and Mustonen J:
Glomerulonephritis emerging shortly after Puumala hantavirus
infection: A report on 7 patients. Clin Nephrol. 75:550–556.
2011.PubMed/NCBI View
Article : Google Scholar
|
|
41
|
Tulumovic D, Imamovic G, Mesic E, Hukic M,
Tulumovic A, Imamovic A and Zerem E: Comparison of the effects of
Puumala and Dobrava viruses on early and long-term renal outcomes
in patients with haemorrhagic fever with renal syndrome. Nephrology
(Carlton). 15:340–343. 2010.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Mustonen J, Mäkelä S, Helin H, Helanterä
A, Miettinen M, Partanen J and Pasternack A: Mesangiocapillary
glomerulonephritis caused by Puumala hantavirus infection. Nephron.
89:402–407. 2001.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Satoskar A and Tibor Nadasdy TFS: Acute
postinfectious glomerulonephritis and glomerulonephritis caused by
persistent bacterial infection. In: Heptinstall's Pathology of the
Kidney. Jannette JC, Olson JL and Silva FG (eds). 7th edition.
Lippincott Williams & Wilkins, Philadelphia, PA, pp678-798,
2014.
|
|
44
|
Huggins JW: Prospects for treatment of
viral hemorrhagic fevers with ribavirin, a broad-spectrum antiviral
drug. Rev Infect Dis. 11 (Suppl 4):S750–S761. 1989.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Huggins JW, Hsiang CM, Cosgriff TM, Guang
MY, Smith JI, Wu ZO, LeDuc JW, Zheng ZM, Meegan JM, Wang QN, et al:
Prospective, double-blind, concurrent, placebo-controlled clinical
trial of intravenous ribavirin therapy of hemorrhagic fever with
renal syndrome. J Infect Dis. 164:1119–1127. 1991.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Rusnak JM, Byrne WR, Chung KN, Gibbs PH,
Kim TT, Boudreau EF, Cosgriff T, Pittman P, Kim KY, Erlichman MS,
et al: Experience with intravenous ribavirin in the treatment of
hemorrhagic fever with renal syndrome in Korea. Antiviral Res.
81:68–76. 2009.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Vial PA, Valdivieso F, Ferres M, Riquelme
R, Rioseco ML, Calvo M, Castillo C, Díaz R, Scholz L, Cuiza A, et
al: High-dose intravenous methylprednisolone for hantavirus
cardiopulmonary syndrome in Chile: A double-blind, randomized
controlled clinical trial. Clin Infect Dis. 57:943–951.
2013.PubMed/NCBI View Article : Google Scholar
|