Introduction
Hepatocellular carcinoma (HCC) accounted for 80% of
the 826,000 global cases of primary liver cancer and was the third
most common cause of cancer mortality in 2018(1). Chronic hepatitis and liver cirrhosis
(LC) caused by hepatitis B virus (HBV) and hepatitis C virus are
the major preneoplastic conditions of HCC (1).
HBV is a small, partially double-stranded DNA virus
that causes acute and chronic hepatitis in humans. HBV is one of
the most hazardous viral pathogens for humans, and 2 billion
individuals have been infected and >350 million are chronic
carriers of the virus (2). Despite
the improvement in the management of chronic HBV infection by
antiviral therapy and universal vaccine, HCC remains the fourth
most common cause of cancer-associated mortalities overall
worldwide (3). Patients with
untreated HBV infection are at a 5- to 100-fold higher risk for
developing HCC compared with healthy individuals (4).
Nucleotide and nucleoside analogues (NAs) function
as competitive inhibitors of the HBV reverse transcriptase, as
their incorporation into the DNA strand provokes chain termination
(5). The NAs lamivudine (LAM),
adefovir (ADF), entecavir (ETV), tenofovir disoproxil fumarate
(TDF) and tenofovir alafenamide (TAF) result in virological
remission in almost all compliant patients with chronic HBV
infection and are associated with significant improvement of liver
inflammation and fibrosis (6-8).
However, while the current antiviral agents decrease HCC risk in
patients with HBV (9-11),
they do not eliminate the risk of HCC because HBV is not eradicated
(12,13). Several groups have developed scores
for the prediction of HCC in NA-treated patients (14,15).
Liver inflammation and fibrosis caused by alcohol abuse and obesity
are established risk factors for HCC (16). However, previous prediction models
have not included these factors. A simple and non-invasive test
that reflects factors other than HBV is needed to predict
carcinogenesis. Previous studies have identified age, sex,
fibrosis-4 index (FIB-4 index), serum α-fetoprotein (AFP) level,
HBV-DNA level and HBV core promotor mutations as risk factors of
HCC occurrence in patients with HBV during NA therapy. However, the
early occurrence of HCC during NA therapy in previous studies may
have included HCC not detected by imaging before NA therapy
(17-21).
The present study aimed to identify definite predictors of HCC
occurrence by analyzing HCC occurrence ≥1 year following NA
treatment.
Materials and methods
Patients
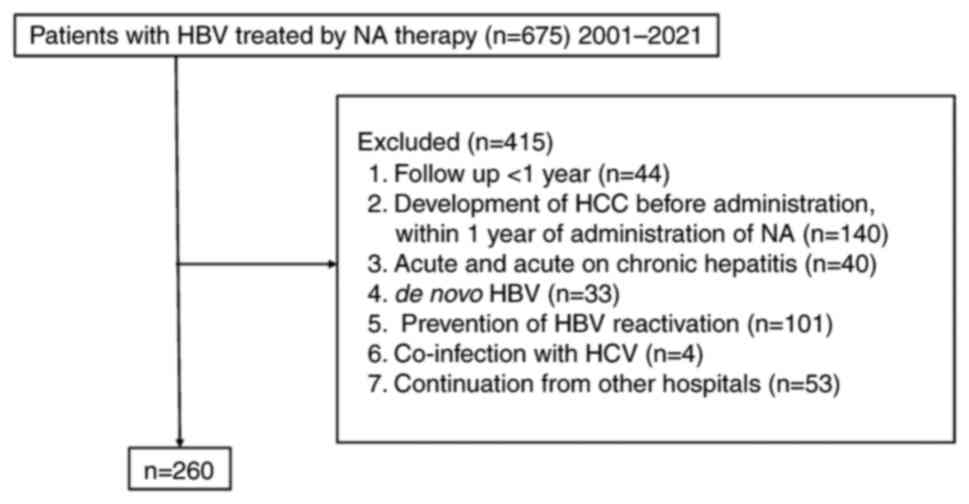
A total of 675 patients with HBV were treated with
NA at Aso Iizuka Hospital (Iizuka, Japan) between January 2001 and
January 2021. The exclusion criteria were as follows: i) follow-up
of <1 year; ii) development of HCC before administration or
within 1 year of administration of NA; iii) acute hepatitis and
acute-on-chronic hepatitis; iv) de novo HBV; v) prevention
of HBV reactivation; vi) co-infection with HCV; and vii)
continuation from other hospitals (Fig. 1). Finally, the present study
included 260 patients in this study. The present study was
conducted in accordance with the guidelines of the Declaration of
Helsinki and was approved by the Ethics Committee of Aso Iizuka
Hospital (approval no. 21141). Informed consent of individual
patients was not obtained because of the retrospective nature of
this study.
Serum levels of HBV DNA were quantified by a fully
automated system. A quantitative polymerase chain reaction (PCR)
assay (Cobas TaqMan HBV Test v2.0; Roche Diagnostics) was used
before 2017, according to the manufacturer's instructions. HBV DNA
was processed from 650 µl of sample using the cobas AmpliPrep and
cobas TaqMan 96 analyzer for automated sample extraction and
real-time PCR amplification and detection, according to the
manufacturer's instructions. The assay primers and probes target
the HBV pre-Core/Core region (preC-C) gene. Another PCR assay
(Cobas 6800/8800 system HBV; Roche Diagnostics) was used after
2017, according to the manufacturer's instructions. HBV DNA was
extracted, purified, and amplified from 500 µl of samples on the
cobas 6800/8800 analyzer system according to the manufacturer's
instructions. The viral region targeted by this assay primers and
probes is the preC-C gene.
FIB-4 index
The FIB-4 index was used to evaluate fibrosis
clinically before and after NA treatment. The FIB-4 index was
calculated using the following formula: FIB-4 index=[aspartate
aminotransferase (AST, IU/l) x age (years)/platelet count
(109/l) x alanine aminotransferase (ALT, IU/l)1/2]
(22).
HBV DNA after 6 months often shows negative readings
and the FIB-4 index after 6 months reflects other factors such as
alcohol abuse and obesity instead of HBV. Therefore, the present
study used the value of FIB-4 index at 6 months after NA
treatment.
Follow-up definitions
Patients with chronic hepatitis and cirrhosis caused
by HBV were treated using NA. Chronic hepatitis B was diagnosed as
positive HBsAg for >6 months, elevated ALT ≥31 IU/l and serum
HBV DNA ≥3.3 log IU/ml (23). LC
was diagnosed on the basis of liver histology or transient
elastography or the presence of gastro-esophageal varices. HCC
surveillance was performed by blood tests and imaging examinations
including ultrasonography, computed tomography and magnetic
resonance imaging, each 3-6 months.
Statistical analysis
Results are shown as the median (inter-quartile
range). Significant differences between groups were examined by
χ2, Fisher's Exact test or Mann-Whitney U-test.
Statistical analyses were performed using the Kaplan-Meier method,
log-rank test, receiver operating characteristic (ROC) analysis and
Cox hazard analysis using JMP statistical software (version 11.0
for Windows; SAS Institute, Inc.). All P-values were derived from
two-tailed tests, and P<0.05 was considered to indicate a
statistically significant difference. ROC and area under the curve
(AUC) values were calculated to define cut-off values for risk
factors of HCC occurrence.
Results
Characteristics of patients at the
start of NA and cumulative occurrence of HCC
Table I shows the
clinical characteristics of the 260 patients with HBV in the
present study. The median age of the overall patient group was 53.0
(range, 44.0-61.1) years, and 59.2% of patients were male. Serum
HBV-DNA concentration was 6.2 log IU/ml. The numbers of patients
that received specific NAs are as follows: LAM (n=77), ETV (n=163),
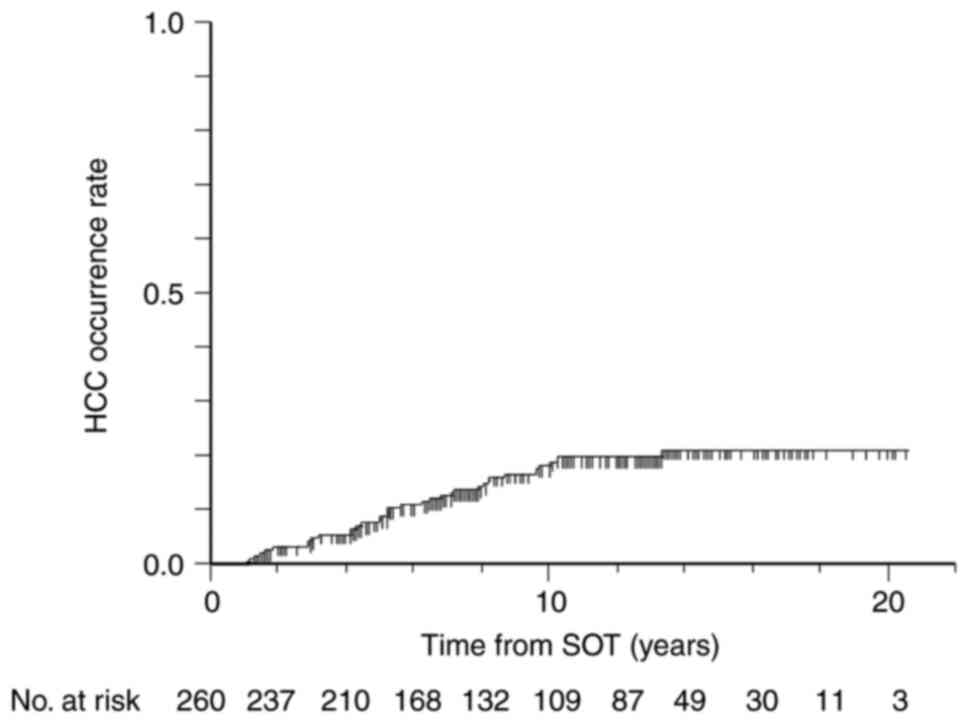
TDF (n=8), and TAF (n=12). The median follow-up duration was 8.18
(4.73-13.07) years, and 40 (15.4%) patients developed HCC during
the follow-up period (Fig. 2).
 | Table ICharacteristics of patients with or
without HCC during NA treatment. |
Table I
Characteristics of patients with or
without HCC during NA treatment.
| Characteristic | All | HCC occurrence | No HCC
occurrence | P-value |
|---|
| n | 260 | 40 | 220 | |
| Age, years | 53 (44-61) | 56 (48-72) | 52 (43-60) | 0.017 |
| Male/Female, n | 154/106 | 31/9 | 123/97 | 0.014 |
| NA treatment,
n | | | | <0.01 |
|
LAM | 77 | 21 | 56 | |
|
ETV | 163 | 19 | 144 | |
|
TDF | 8 | 0 | 8 | |
|
TAF | 12 | 0 | 12 | |
| HBV-DNA, log
IU/ml | 6.2 (4.5-7.2) | 6.25
(5.33-7.05) | 6.2 (4.38-7.2) | 0.669 |
| Blood test at
SOT | | | | |
|
PLT,
x104/mm3 | 17.15
(11.9-22.48) | 11.05
(7.56-16.73) | 18.15
(13.58-23.5) | <0.01 |
|
TB,
mg/dl | 0.8 (0.6-1.2) | 1.05
(0.7-1.85) | 0.8 (0.6-1.1) | 0.077 |
|
AST,
IU/l | 49 (31-94.75) | 62 (36.75-100) | 46.5
(30.75-83.5) | 0.950 |
|
ALT,
IU/l | 50.5 (35-113) | 57
(34.5-101.25) | 50 (34.75-122) | 0.628 |
|
Alb,
g/dl | 4.0 (3.7-4.3) | 3.7 (2.98-4.1) | 4.0 (3.8-4.35) | <0.01 |
|
FIB-4
index | 2.19
(1.21-4.16) | 5.44
(2.04-7.5) | 2.03
(1.18-3.41) | <0.01 |
| Blood test at 6
months after treatment | | | | |
|
PLT,
x104/mm3 | 18.4
(3.7-52.5) | 10.7
(6.65-17.53) | 18.45
(14-23.4) | <0.01 |
|
AST,
IU/l | 27 (22-36) | 33
(25.25-43.25) | 26 (21-34) | 0.018 |
|
ALT,
IU/l | 26 (18.25-37) | 27
(21.25-35.75) | 26 (18-37) | 0.523 |
|
FIB-4
index | 1.71
(1.1-2.84) | 3.73
(1.96-5.76) | 1.54
(1.01-2.49) | <0.01 |
| Follow up duration,
years | 8.18
(4.73-13.07) | | | |
The present study detected significant differences
between patients with and without HCC occurrence, including age
(P=0.017), sex (P=0.014), type of NA (P<0.01), platelets at
start of treatment (SOT) (P<0.01) and 6 months after NA
treatment (P<0.01) and FIB-4 index at SOT (P<0.01) and 6
months after NA treatment (P<0.01). There was no significant
difference in HBV DNA between the two groups (P=0.669) (Table I).
Differences of HCC occurrence between
patients treated with the older and newer NAs
Several studies reported that the new NAs, including
ETV, TDF and TAF, reduce HCC risk to a greater extent compared with
the old NAs (LAM and ADF) (24,25).
It has been reported that LAM-resistant mutations arise in ~23% of
patients after 12 months of therapy and in up to 80% after 5 years
of treatment, and patients with LAM-resistant mutations have a
higher risk of HCC (26). In the
present study, drug resistance developed in 41 of 77 patients
(53.2%) treated with LAM and the patients with NA resistance had
higher incidence of HCC compared with the patients without NAs
resistance (P<0.01) (data not shown). In the current study, the
rate of HCC occurrence in patients treated with old NAs was higher
compared with that of patients treated with new NAs (P<0.01;
Table II). HBV DNA (P<0.01),
platelets (P<0.01), and FIB-4 index at SOT (P<0.01) were
higher in patients treated with old NAs compared with the values in
patients treated with new NAs (Table
II). Fibrosis in patients treated with old NAs was more
advanced compared with that observed in patients treated with the
newer NAs (Table II).
 | Table IICharacteristics of patients treated
with old (LAM) and new NAs (ETV, TDF and TAF). |
Table II
Characteristics of patients treated
with old (LAM) and new NAs (ETV, TDF and TAF).
|
Characteristics | Old (LAM) | New (ETV, TDF,
TAF) | P-value |
|---|
| n | 77 | 183 | |
| Age, years | 49.5 (45-56.5) | 55 (43-61.25) | 0.012 |
| Male/Female, n | 54/23 | 100/83 | <0.01 |
| HCC occurrence,
n | 22 | 20 | <0.01 |
| HBV-DNA, log
IU/ml | 6.6 (5.5-7.4) | 6.1 (4.25-7.1) | <0.01 |
| Blood test at
SOT | | | |
|
PLT,
x104/mm3 | 14.7
(8.45-17.4) | 18.95
(13.08-23.8) | <0.01 |
|
TB,
mg/dl | 1.0 (0.7-1.63) | 0.8 (0.6-1.1) | <0.01 |
|
AST,
IU/l | 67 (39.75-130) | 43 (29-74.25) | <0.01 |
|
ALT,
IU/l | 64
(42.75-157.75) | 46.5
(24.75-88.25) | 0.057 |
|
Alb,
g/dl | 3.9 (3.4-4.2) | 4.1 (3.8-4.4) | <0.01 |
|
FIB-4
index | 3.29
(1.79-6.67) | 1.89
(1.09-3.32) | <0.01 |
| FIB-4 index at 6
months after treatment | 2.04
(1.39-4.07) | 1.5
(1.02-2.52) | 0.056 |
| FIB-4 inverse
index | 1.96
(1.42-2.17) | 2.18
(1.87-2.31) | 0.028 |
Univariate and multivariate analysis
for risk factors of HCC
Using the ROC value for HCC occurrence, the cut-off
value of FIB-4 index at 6 months after NA treatment was 1.95 (AUC,
0.757; 1-specificity, 0.350; sensitivity, 0.775) (data not shown).
From univariate analysis, age, male sex, old NA and FIB-4 index at
6 months were identified as risk factors of HCC. HBV DNA level
showed no association. Multivariate analysis showed that age
[hazard ratio (HR), 1.31; P=0.0453], male sex (HR, 3.14; P<0.01)
and FIB-4 index at 6 months >1.95 (HR, 4.35; P<0.01) were
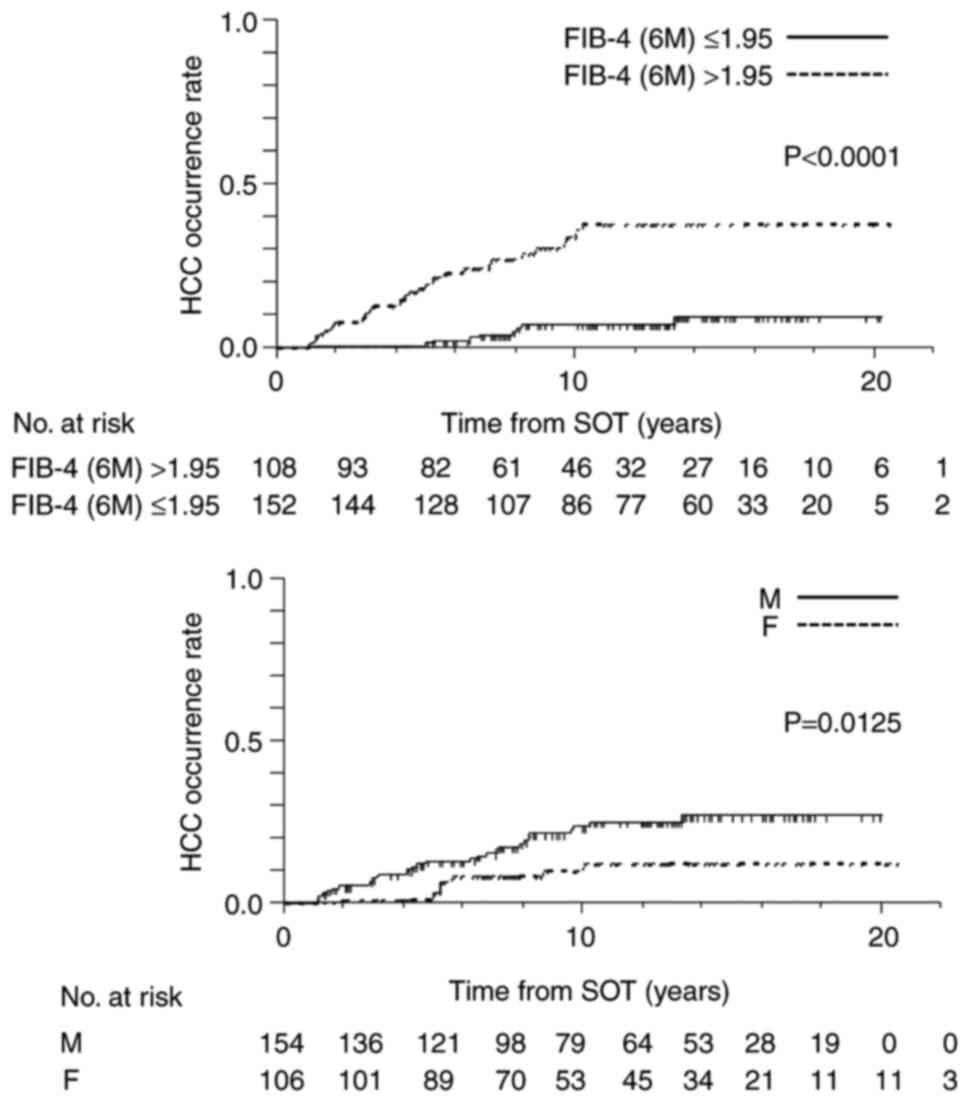
associated with the occurrence of HCC (Table III). Fig. 3 shows the cumulative incidence of
HCC based on male sex and FIB-4 index at 6 months. There were
significant differences with regard to male sex (P=0.0125) and
FIB-4 index at 6 months (P<0.0001).
 | Table IIIFactors associated with HCC
occurrence during NA treatment. |
Table III
Factors associated with HCC
occurrence during NA treatment.
| | Univariate | Multivariate |
|---|
| Factor | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age, years | 1.04 | 1.02-1.07 | <0.01 | 1.03 | 1.02-1.06 | 0.0453 |
| Male sex | 2.49 | 1.19-5.23 | 0.016 | 3.14 | 1.46-6.75 | <0.01 |
| HBV-DNA, log
IU/ml | 0.99 | 0.81-1.23 | 0.966 | | | |
| FIB-4 index
>1.95 | 5.93 | 2.82-12.47 | <0.01 | 4.35 | 1.92-9.87 | <0.01 |
| Old NA | 2.164 | 1.16-4.04 | 0.016 | 1.72 | 0.89-3.33 | 0.108 |
Multivariate analysis for risk factors
of HCC in patients with serum AFP level at 6 months after NA
treatment
Additional analysis was performed in the group in
which serum AFP level at 6 months after NA treatment was measured.
Table IV shows the clinical
characteristics of these patients. There were significant
differences in sex (P=0.029), content of NA (P<0.01), platelets
at SOT (P<0.01) and 6 months after NA treatment (P<0.01) and
FIB-4 index at SOT (P<0.01) and 6 months after NA treatment
(P<0.01) between patients with and without HCC occurrence. Serum
AFP level at SOT (P=0.011) and 6 months after NA treatment
(P<0.01) were higher in patients with HCC occurrence compared
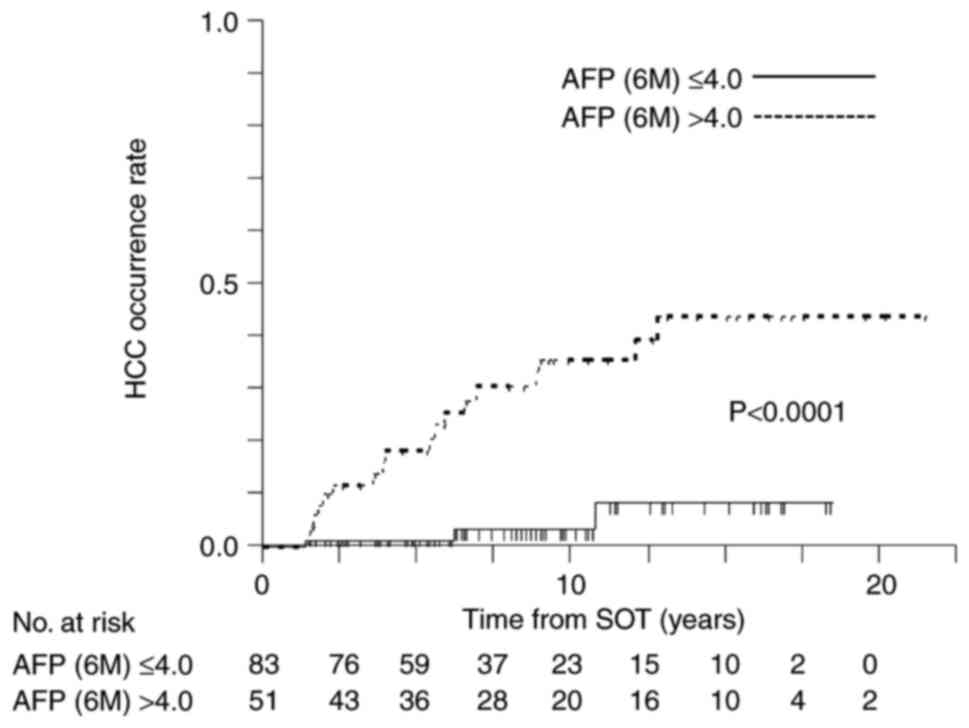
with those in patients with no HCC. The cut-off value of serum AFP
level at 6 months after treatment was 4 ng/ml (AUC, 0.842;
1-specificity, 0.3; sensitivity, 0.905) (data not shown). FIB-4
index at 6 months >1.95 (HR, 8.27; P=0.014) and serum AFP level
at 6 months >4 ng/ml (HR, 4.26; P=0.033) were identified as risk
factors in multivariate analysis (Table V). Fig. 4 shows Kaplan-Meier analysis of the
cumulative HCC occurrence stratified on the basis of cut-off values
of serum AFP level at 6 months after treatment (P<0.0001).
 | Table IVCharacteristics of patients with or
without HCC during NA treatment with measured serum AFP levels. |
Table IV
Characteristics of patients with or
without HCC during NA treatment with measured serum AFP levels.
| Characteristic | HCC occurrence | No HCC
occurrence | P-value |
|---|
| n | 21 | 110 | |
| Age, years | 56 (53-61) | 55 (42.8-62.3) | 0.217 |
| Male/Female, n | 17/4 | 60/50 | 0.029 |
| NA Treatment,
n | | | <0.01 |
|
LAM | 9 | 5 | |
|
ETV | 12 | 86 | |
|
TDF | 0 | 7 | |
|
TAF | 0 | 12 | |
| HBV-DNA, log
IU/ml | 6.0 (4.63-7) | 6.1 (4.1-7.1) | 0.984 |
| Blood test at
SOT | | | |
|
PLT,
x104/mm3 | 9.4 (6.7-12.6) | 18.85
(12.78-23.58) | <0.01 |
|
AST,
IU/l | 62 (40-102) | 42.5 (28-72.5) | 0.914 |
|
ALT,
IU/l | 61 (33-98) | 44
(31.75-82.25) | 0.433 |
|
FIB-4
index | 6.18
(3.52-7.76) | 1.99
(1.08-3.8) | <0.01 |
|
AFP,
ng/ml | 31.2
(7.33-72.3) | 3.5 (2.6-6.4) | 0.011 |
| Blood test at 6
months after treatment | | | |
|
PLT,
x104/mm3 | 9.4 (6.4-13.0) | 19.1
(14.08-23.48) | <0.01 |
|
AST,
IU/l | 33 (27-40.5) | 26 (20-33.25) | <0.01 |
|
ALT,
IU/l | 28 (21.5-40) | 25.5 (18-34.5) | 0.202 |
|
FIB-4
index | 4.09
(2.22-5.61) | 1.46
(0.9-2.51) | <0.01 |
|
AFP,
ng/ml | 7.6
(4.8-16.05) | 3.35
(2.4-4.325) | <0.01 |
 | Table VFactors associated with HCC
occurrence during NA treatment with the addition of serum AFP
level. |
Table V
Factors associated with HCC
occurrence during NA treatment with the addition of serum AFP
level.
| | Multivariate |
|---|
| Parameter | HR | 95% CI | P-value |
|---|
| Age, years | 1.01 | 0.95-1.06 | 0.786 |
| Male sex | 2.34 | 0.72-7.59 | 0.157 |
| FIB-4 index at 6
months >1.95 | 8.27 | 1.51-45.09 | 0.014 |
| Old NA | 1.71 | 0.61-4.75 | 0.310 |
| AFP at 6 months
>4 ng/ml | 4.26 | 1.13-16.13 | 0.033 |
Discussion
Current antiviral therapies cannot achieve complete
eradication of HBV; therefore, these treatments decrease HCC risk
but do not eliminate the risk of HCC in patients with chronic HBV
infection (12,13,27).
Thus, HCC represents the main challenge in the management of
patients with HBV, as HCC may occur even under effective long-term
antiviral therapy and is the only factor that currently affects
liver-related mortality in diagnosed and treated patients with HBV
(12).
HCC risk is the highest among untreated chronic
patients with HBV with LC (1,28,29).
The FIB-4 index has been used as a non-invasive and surrogate
marker for advanced hepatic fibrosis (22) and shows high predictive values for
the development of HCC risk in patients with chronic HBV infection
(30,31). Previous studies have reported FIB-4
index as a risk factor for HCC occurrence in patients with HBV
during NA therapy but the early occurrence of HCC during NA therapy
in previous studies may have included HCC not detected by imaging
before NA therapy (17,18). The present study analyzed HCC
occurrence ≥1 year following NA treatment and identified FIB-4
index at 6 months after NA treatment >1.95 as a predictor of HCC
occurrence during long-term NA therapy. The follow up duration in
the current study was longer compared with previous studies and
accurate cut off value of FIB-4 index was obtained.
In the present study, baseline HBV DNA level was not
associated with HCC risk, which was consistent with some previous
studies (32,33). However, these findings were in
contrast to studies assessing HCC risk in untreated patients
(34,35). Several studies have demonstrated
that the new NAs, including ETV, TDF and TAF, reduce HCC risk to a
greater extent compared with older NAs (LAM and ADF) (24,25).
In the present study, HCC occurrence in patients treated by old NAs
was more frequent compared with that in patients treated by new
NAs, and old NAs were one of the contributing factors for HCC
occurrence as determined by univariate analysis. This result might
be because of the advanced fibrosis in patients treated with old
NAs compared with that in patients treated with new NAs. Overall,
the present study showed the importance of the new NAs to reduce
HCC risk.
The current results identified four factors, sex,
age, FIB-4 index at 6 months after NA treatment and serum AFP level
at 6 months after NA treatment, that were associated with HCC risk
during NA therapy. Older age is a well-known risk factor for HCC
(36). Elevated AFP serum level is
one of the common risk factors for HCC occurrence in patients with
HBV and hepatitis C virus (16,37-39).
In the group where serum AFP levels were measured, the present
study showed that FIB-4 index >1.95 and serum AFP level >4
ng/ml at 6 months after NA treatment may be useful markers to
select patients at higher risk of carcinogenesis. Previous
prediction models such as GAG-HCC, CU-HCC and REACH-B (18-21)
have identified these factors. The limitations of these studies
were that the patients were enrolled regardless of receiving
antiviral therapy and the difficulty of diagnosing LC. Only
patients receiving antiviral therapy were enrolled in the current
study.
The PAGE-B score was recently developed and
validated in mostly Caucasian European patients under ETV or TDF
therapy. In this score, which includes age, sex and platelet
counts, probably reflecting the severity of liver disease, the
addition of cirrhosis does not substantially improve the
discrimination (14). While PAGE-B
is useful, FIB-4 index >1.95 and serum AFP level >4 ng/ml at
6 months after NA treatment are simpler predictors.
The present study has several limitations. The study
group was from a single center, which limited the numbers of HCC
events during NA. Additionally, the present study did not consider
the involvement of HBV core promoter mutations, BMI, alcohol
consumption and diabetes due to insufficient information.
In conclusion, FIB-4 index >1.95 and serum AFP
level >4 ng/ml at 6 months were predictors for the development
of HCC in patients with HBV during NA treatment. Further study of
hepatocarcinogenesis during NA is required with a longer follow-up
period and larger numbers of participants. Regular careful
examinations are required if the FIB-4 index and serum AFP at 6
months after NA treatment are high.
Acknowledgements
The authors would like to thank Mrs. Yukie Ishibashi
(Department of Hepatology, Iizuka Hospital, Iizuka, Japan) for
assistance with manuscript preparation.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AK, MM, MY, KT, AM and KM designed the study. AK,
MM, KT and YK assisted with data analyses. AK wrote the initial
draft of the manuscript. MY and KM contributed to analysis and
interpretation of data. MY and KM assisted in the preparation and
critical review of the manuscript. AK and MY confirm the
authenticity of all the raw data. All authors agreed to be
accountable for all aspects of the work. All authors read and
approved the final version of the manuscript
Ethics approval and consent to
participate
The research was conducted in accordance with the
principles of the Declaration of Helsinki. The study protocol
conforms to the ethical guidelines of the 1975 Declaration of
Helsinki. This study was approved by the ethics committee of Aso
Iizuka Hospital (approval no. 21141; Iizuka, Japan). Informed
consent of individual patients was not obtained because of the
retrospective nature of this study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rumgay H, Ferlay J, de Martel C, Georges
D, Ibrahim AS, Zheng R, Wei W, Lemmens VEPP and Soerjomataram I:
Global, regional and national burden of primary liver cancer by
subtype. Eur J Cancer. 161:108–118. 2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Trépo C, Chan HL and Lok A: Hepatitis B
virus infection. Lancet. 384:2053–2063. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Global Burden of Disease Cancer
Collaboration. Fitzmaurice C, Allen C, Barber RM, Barregard L,
Bhutta ZA, Brenner H, Dicker DJ, Chimed-Orchir O, Dandona R, et al:
Global, regional, and national cancer incidence, mortality, years
of life lost, years lived with disability, and disability-adjusted
life-years for 32 cancer groups, 1990 to 2015: A systematic
analysis for the global burden of disease study. JAMA Oncol.
3:524–548. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
El-Serag HB: Epidemiology of viral
hepatitis and hepatocellular carcinoma. Gastroenterology.
142:1264–1273.e1. 2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tong S and Revill P: Overview of hepatitis
B viral replication and genetic variability. J Hepatol. 64 (1
Suppl):S4–S16. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
European Association for the Study of the
Liver. Electronic address: simpleeasloffice@easloffice.eu;
European Association for the Study of the Liver. EASL 2017 clinical
practice guidelines on the management of hepatitis B virus
infection. J Hepatol. 67:370–398. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Terrault NA, Lok ASF, McMahon BJ, Chang
KM, Hwang JP, Jonas MM, Brown RS Jr, Bzowej NH and Wong JB: Update
on prevention, diagnosis, and treatment of chronic hepatitis B:
AASLD 2018 hepatitis B guidance. Hepatology. 67:1560–1599.
2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Marcellin P, Gane E, Buti M, Afdhal N,
Sievert W, Jacobson IM, Washington MK, Germanidis G, Flaherty JF,
Aguilar Schall R, et al: Regression of cirrhosis during treatment
with tenofovir disoproxil fumarate for chronic hepatitis B: A
5-year open-label follow-up study. Lancet. 381:468–475.
2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hosaka T, Suzuki F, Kobayashi M, Seko Y,
Kawamura Y, Sezaki H, Akuta N, Suzuki Y, Saitoh S, Arase Y, et al:
Long-term entecavir treatment reduces hepatocellular carcinoma
incidence in patients with hepatitis B virus infection. Hepatology.
58:98–107. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Nguyen MH, Yang HI, Le A, Henry L, Nguyen
N, Lee MH, Zhang J, Wong C, Wong C and Trinh H: Reduced Incidence
of Hepatocellular Carcinoma in Cirrhotic and Noncirrhotic Patients
With Chronic Hepatitis B Treated With Tenofovir-A Propensity
Score-Matched Study. J Infect Dis. 219:10–18. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kumada T, Toyoda H, Tada T, Kiriyama S,
Tanikawa M, Hisanaga Y, Kanamori A, Niinomi T, Yasuda S, Andou Y,
et al: Effect of nucleos(t)ide analogue therapy on
hepatocarcinogenesis in chronic hepatitis B patients: A propensity
score analysis. J Hepatol. 58:427–433. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Papatheodoridis GV, Chan HL, Hansen BE,
Janssen HL and Lampertico P: Risk of hepatocellular carcinoma in
chronic hepatitis B: Assessment and modification with current
antiviral therapy. J Hepatol. 62:956–967. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lampertico P, Maini M and Papatheodoridis
G: Optimal management of hepatitis B virus infection-EASL special
conference. J Hepatol. 63:1238–1253. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Papatheodoridis G, Dalekos G, Sypsa V,
Yurdaydin C, Buti M, Goulis J, Calleja JL, Chi H, Manolakopoulos S,
Mangia G, et al: PAGE-B predicts the risk of developing
hepatocellular carcinoma in Caucasians with chronic hepatitis B on
5-year antiviral therapy. J Hepatol. 64:800–806. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wong VWS and Janssen HLA: Can we use HCC
risk scores to individualize surveillance in chronic hepatitis B
infection? J Hepatol. 63:722–732. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Marrero JA, Fontana RJ, Fu S, Conjeevaram
HS, Su GL and Lok AS: Alcohol, tobacco and obesity are synergistic
risk factors for hepatocellular carcinoma. J Hepatol. 42:218–224.
2005.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Tada T, Kumada T, Toyoda H, Tsuji K,
Hiraoka A and Tanaka J: Impact of FIB-4 index on hepatocellular
carcinoma incidence during nucleos(t)ide analogue therapy in
patients with chronic hepatitis B: An analysis using time-dependent
receiver operating characteristic. J Gastroenterol Hepatol.
32:451–458. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Tseng TC, Choi J, Nguyen MH, Peng CY,
Siakavellas S, Papatheodoridis G, Wang CC, Lim YS, Lai HC, Trinh
HN, et al: One-year fibrosis-4 index helps identify minimal HCC
risk in non-cirrhotic chronic hepatitis B patients with antiviral
treatment. Hepatol Int. 15:105–113. 2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yang HI, Yuen MF, Chan HL, Han KH, Chen
PJ, Kim DY, Ahn SH, Chen CJ, Wong VW and Seto WK: REACH-B Working
Group. Risk estimation for hepatocellular carcinoma in chronic
hepatitis B (REACH-B): Development and validation of a predictive
score. Lancet Oncol. 12:568–574. 2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wong VW, Chan SL, Mo F, Chan TC, Loong HH,
Wong GL, Lui YY, Chan AT, Sung JJ, Yeo W, et al: Clinical scoring
system to predict hepatocellular carcinoma in chronic hepatitis B
carriers. J Clin Oncol. 28:1660–1665. 2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yuen MF, Tanaka Y, Fong DY, Fung J, Wong
DK, Yuen JC, But DY, Chan AO, Wong BC, Mizokami M and Lai CL:
Independent risk factors and predictive score for the development
of hepatocellular carcinoma in chronic hepatitis B. J Hepatol.
50:80–88. 2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Vallet-Pichard A, Mallet V, Nalpas B,
Verkarre V, Nalpas A, Dhalluin-Venier V, Fontaine H and Pol S:
FIB-4: An inexpensive and accurate marker of fibrosis in HCV
infection. Comparison with liver biopsy and fibrotest. Hepatology.
46:32–36. 2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Drafting Committee for Hepatitis
Management Guidelines, the Japan Society of Hepatology. Japan
society of hepatology guidelines for the management of hepatitis B
virus infection: 2019 Update. Hepatol Res. 50:892–923.
2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kobashi H, Miyake Y, Ikeda F, Yasunaka T,
Nishino K, Moriya A, Kubota J, Nakamura S, Takaki A, Nouso K, et
al: Long-term outcome and hepatocellular carcinoma development in
chronic hepatitis B or cirrhosis patients after nucleoside analog
treatment with entecavir or lamivudine. Hepatol Res. 41:405–416.
2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Köklü S, Tuna Y, Gülşen MT, Demir M,
Köksal AŞ, Koçkar MC, Aygün C, Coban S, Ozdil K, Ataseven H, et al:
Long-term efficacy and safety of lamivudine, entecavir, and
tenofovir for treatment of hepatitis B virus-related cirrhosis.
Clin Gastroenterol Hepatol. 11:88–94. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Tacke F and Kroy DC: Treatment for
hepatitis B in patients with drug resistance. Ann Transl Med.
4(334)2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Papatheodoridis GV, Lampertico P,
Manolakopoulos S and Lok A: Incidence of hepatocellular carcinoma
in chronic hepatitis B patients receiving nucleos(t)ide therapy: A
systematic review. J Hepatol. 53:348–356. 2010.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Schafer DF and Sorrell MF: Hepatocellular
carcinoma. Lancet. 353:1253–1257. 1999.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Okuda K: Hepatocellular carcinoma. J
Hepatol. 32 (1 Suppl):S225–S237. 2000.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kim JH, Kim JW, Seo JW, Choe WH and Kwon
SY: Noninvasive tests for fibrosis predict 5-year mortality and
hepatocellular carcinoma in patients with chronic hepatitis B. J
Clin Gastroenterol. 50:882–888. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Suh B, Park S, Shin DW, Yun JM, Yang HK,
Yu SJ, Shin CI, Kim JS, Ahn E, Lee H, et al: High liver fibrosis
index FIB-4 is highly predictive of hepatocellular carcinoma in
chronic hepatitis B carriers. Hepatology. 61:1261–1268.
2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Cho JY, Paik YH, Sohn W, Cho HC, Gwak GY,
Choi MS, Lee JH, Koh KC, Paik SW and Yoo BC: Patients with chronic
hepatitis B treated with oral antiviral therapy retain a higher
risk for HCC compared with patients with inactive stage disease.
Gut. 63:1943–1950. 2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zoutendijk R, Reijnders JG, Zoulim F,
Brown A, Mutimer DJ, Deterding K, Hofmann WP, Petersen J, Fasano M,
Buti M, et al: Virological response to entecavir is associated with
a better clinical outcome in chronic hepatitis B patients with
cirrhosis. Gut. 62:760–765. 2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu
SN, Huang GT and Iloeje UH: REVEAL-HBV Study Group. Risk of
hepatocellular carcinoma across a biological gradient of serum
hepatitis B virus DNA level. JAMA. 295:65–73. 2006.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Chen G, Lin W, Shen F, Iloeje UH, London
WT and Evans AA: Past HBV viral load as predictor of mortality and
morbidity from HCC and chronic liver disease in a prospective
study. Am J Gastroenterol. 101:1797–1803. 2006.PubMed/NCBI View Article : Google Scholar
|
|
36
|
European Association for the Study of the
Liver. Electronic address: simpleeasloffice@easloffice.eu;
European Association for the Study of the Liver. EASL clinical
practice guidelines: Management of hepatocellular carcinoma. J
Hepatol. 69:182–236. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Oze T, Hiramatsu N, Yakushijin T, Miyazaki
M, Yamada A, Oshita M, Hagiwara H, Mita E, Ito T, Fukui H, et al:
Post-treatment levels of α-fetoprotein predict incidence of
hepatocellular carcinoma after interferon therapy. Clin
Gastroenterol Hepatol. 12:1186–1195. 2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Tada T, Kumada T, Toyoda H, Kiriyama S,
Tanikawa M, Hisanaga Y, Kanamori A, Kitabatake S, Yama T and Tanaka
J: Post-treatment levels of α-fetoprotein predict long-term
hepatocellular carcinoma development after sustained virological
response in patients with hepatitis C. Hepatol Res. 47:1021–1031.
2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Kuwano A, Yada M, Nagasawa S, Tanaka K,
Morita Y, Masumoto A and Motomura K: Serum α-fetoprotein level at
treatment completion is a useful predictor of hepatocellular
carcinoma occurrence more than one year after hepatitis C virus
eradication by direct-acting antiviral treatment. J Viral Hepat.
29:35–42. 2022.PubMed/NCBI View Article : Google Scholar
|