Introduction
Gliomas are the most common primary intracranial
tumors in adults. Among them, high-grade gliomas include anaplastic
gliomas and glioblastomas; they are characterized by rapid
progression, high malignancy, a 5-year survival rate of #x003C;10%
and a poor prognosis (1), thus
seriously threatening the life of affected patients. Currently, the
standard treatment is to perform surgical resection to the maximum
extent to preserve nerve function. After the operation,
temozolomide chemoradiotherapy and adjuvant chemotherapy (Stupp
regimen) are used. However, the median overall survival (mOS) time
for glioblastoma is only 14.6 months, the 2-year survival rate is
only 26.5%, the median progression-free survival (mPFS) time is
only 6.9 months and ~85% of patients have disease progression
within 2 years (2). At present,
there is no single standard treatment plan for recurrent high-grade
glioma. The commonly used treatment methods include reoperation,
recourse radiotherapy, temozolomide chemotherapy, bevacizumab
anti-vascular treatment, carmustine and irinotecan (3). Although new treatment models have
emerged on this basis, such as tumor electric field therapy
(4) and oncolytic virus therapy
(5), the efficacy is still not
ideal. Therefore, it is of clinical significance to continue to
search for more novel treatment models not only to improve the
therapeutic efficacy of patients with recurrent high-grade glioma
but also to improve patient prognosis.
Previous studies have indicated that the growth and
recurrence of high-grade glioma are closely related to tumor
angiogenesis (6,7). As a monoclonal antibody against
vascular endothelial cytokines, bevacizumab has been approved in
China and abroad to treat recurrent high-grade glioma, but it has
serious adverse effects, such as hypertension and bleeding
(8). As bevacizumab is expensive
and needs to be administered by intravenous infusion once every two
weeks, numerous patients have poor compliance or miss the treatment
timepoint. Various patients cannot afford the cost for economic
reasons and the treatment efficacy is not guaranteed.
Apatinib is a new oral small-molecule inhibitor of
vascular endothelial growth factor receptor-2 (VEGFR-2), which is
convenient, economical and efficient. It selectively competes for
the ATP binding site of VEGFR-2 in cells, blocks the downstream
signal transduction and inhibits angiogenesis in tumor tissues,
thereby exerting its anti-tumor effect (9). Apatinib is the first small-molecule
anti-angiogenic targeted drug worldwide proven to be safe and
effective and has achieved gratifying results in treating advanced
gastric cancer (10). The clinical
application of apatinib in other tumors and the relevant mechanisms
have also been widely explored. An increasing number of studies
indicate that apatinib has a significant anti-tumor effect in
various tumor types (11-13).
Apatinib has also been demonstrated to enhance the inhibitory
effect of temozolomide on the proliferation and invasion of glioma
cells, suggesting that apatinib and temozolomide have synergistic
anti-tumor effects in glioma (10). In recent years, accumulating data
have revealed that combining apatinib and temozolomide may
significantly improve the treatment efficacy of recurrent
high-grade glioma, but the conclusions are all based on small
samples (14-16).
The present meta-analysis aimed to analyze all of the relevant
studies in a combined manner, clarify the efficacy of apatinib plus
temozolomide in patients with recurrent high-grade glioma and
provide evidence for its utility in future clinical treatment.
Materials and methods
Data sources
The literature related to the topic of the present
study was searched without any limitations regarding the language,
and the search date was limited up to May 2023. The PubMed
(https://pubmed.ncbi.nlm.nih.gov), Web of
Science (https://webofknowledge.com), Cochrane
Library (https://www.cochranelibrary.com), Chinese National
Knowledge Infrastructure (https://www.cnki.net) and Wanfang (https://www.wanfangdata.com.cn) databases were
searched, also including a search for ahead of print articles. The
search terms were as follows: ‘Apatinib’, ‘temozolomide’, ‘TMZ’,
‘temodal’, ‘temodar’, ‘relapse’, ‘recurrent’, ‘recrudescent’,
‘glioblastoma’ and ‘glioma’. The search strategy is detailed in
Appendix S1, and abstracts
without full-text were not included in the present meta-analysis.
The contents of domestic Chinese Food and Drug
Administration-approved documents were also reviewed and the
identified literature was screened to determine whether there were
any further related publications. In addition, the safety data of
the manufacturer's (Jiangsu Hengrui Medicine Co., Ltd.) updated
instructions for apatinib were reviewed to obtain relevant
information and these were considered to be the latest data that
were analyzed. When the relevant data required to be clarified, the
authors of the studies and the manufacturer of apatinib were
contacted.
Inclusion and exclusion criteria
All included studies were required to meet the
following inclusion criteria: i) All patients in the study had
undergone surgical resection or brain biopsy and were confirmed to
be World Health Organization grade III or IV. After the operation,
they received the standard Stupp regimen chemotherapy and
radiotherapy (2), and the
subsequent maintenance chemotherapy failed. Pathology or MRI
examination confirmed that they were relapsed, and there were
clear, measurable lesions on MRI. ii) All patients in the study
were treated with apatinib combined with temozolomide [conventional
5/28 regimen (2) or dense regimen
(17)] after the recurrence was
confirmed. iii) The diagnosis, treatment plan, response criteria,
overall objective response rate (ORR) and disease control rate
(DCR) of recurrent high-grade glioma were reported in the study.
iv) The general clinical characteristics of patients, such as age
and Karnowski performance status score, were clearly described in
the study. v) The type of study was that of a randomized or
nonrandomized controlled trial.
The exclusion criteria were as follows: i) Patients
did not receive standard chemoradiotherapy after the operation; ii)
only apatinib monotherapy or temozolomide monotherapy was
administered; iii) the study's treatment results were not clearly
described; and iv) the studies involved case reports, reviews and
other meta-analyses.
Data extraction
According to the PRISMA guidelines (18), two researchers (GL and XX)
extracted relevant data from the articles' text, tables and figures
and cross-checked them. In the case of any disagreement, a third
author (SW) was consulted to discuss and solve the issue. The
specific contents extracted from the literature included the
following: i) The basic information of the included study,
including the title, first author name and publication year; ii)
baseline characteristics of the subjects, including sample size and
age; iii) specific details of treatment measures, including
treatment plan and drug dosage; and iv) outcome measures, such as
ORR, DCR, mOS and mPFS.
Meta-analysis
The results were analyzed using Stata 17.0
statistical software (StataCorp LP). The forest plot was drawn at
first using the random-effects model, combining the effects of each
study to evaluate the overall efficacy and prognosis associated
with apatinib combined with temozolomide for recurrent high-grade
glioma. For the consistency evaluation of the study, the
traditional statistical test (Cochran's Q-test) was first used for
evaluation, and the I2 test was then used for
verification. If the I2-value was ≤50%, the
fixed-effects model was used to integrate all effect sizes. If the
I2-value was >50%, the random-effects model was used
to integrate all effect sizes and indicate substantial
heterogeneity. Subsequently, further funnel plot and sensitivity
analyses were performed to identify the source of heterogeneity,
and subgroup analysis was conducted as necessary to identify
sources of heterogeneity (19).
For the evaluation of publication bias, Begg's test and Egger's
test were used (20). P#x003C;0.05
was considered to indicate a statistically significant
difference.
Results
Literature screening
Initially, 44 entries were retrieved from all
databases. After removing duplicate entries and abstracts, 15
studies were selected. After removing any studies that did not meet
the subject and inclusion criteria, 10 studies were finally
included in the comprehensive analysis (14-16,21-27).
The specific screening process is illustrated in Fig. 1. A total of 357 patients with
recurrent high-grade glioma were included in the present
meta-analysis. The name of the first author, publication year,
sample size, median age, specific interventions and outcome
indicators are listed in Table
I.
 | Table IBaseline characteristics of all
included studies. |
Table I
Baseline characteristics of all
included studies.
| First author,
year | Study type | Temozolomide
dosing | Sample size | Mean ± SD or median
age (range), years | Intervention | CR, n | PR, n | SD, n | PD, n | ORR, % | DCR, % | Median OS (95% CI),
months | Median PFS (95%
CI), months | (Refs.) |
|---|
| Wang et al,
2019 | Single-arm | Dose-dense | 20 | 50.5
(27.0-67.0) | Apatinib (500
mg/day) + temozolomide (100 mg/m2, take for 1 week, stop
for 1 week) | 1 | 8 | 9 | 2 | 9/20 (45.0) | 18/20(90) | 9 (8.2-12.2) | 6 (5.3-7.8) | (14) |
| Zhou et al,
2020 | Randomized-
controlled | Dose-dense | 12 | 48.0±7.0 | Apatinib (500
mg/day) + temozolomide (150 mg/m2, take for 1 week, stop
for 1 week) | 0 | 8 | 3 | 1 | 8/12 (66.7) | 11/12 (91.7) | NA | NA | (15) |
| | | Conventional-
dose | 14 | 50.0±8.0 | Apatinib (500
mg/day) + temozolomide (200 mg/m2, take for 5 days, stop
for 23 days) | 0 | 3 | 5 | 6 | 3/14 (21.4) | 8/14 (51.7) | NA | NA | |
| Liu et al,
2020 | Randomized-
controlled | Dose-dense | 15 | 40.3
(21.0-71.0) | Apatinib (500
mg/day) + temozolomide (150 mg/m2, take for 1 week, stop
for 1 week) | 1 | 7 | 5 | 2 | 8/15 (53.4) | 13/15 (86.7) | NA | NA | (21) |
| Ge et al,
2021 | Single-arm | Dose-dense | 31 | 53.0
(21.0-70.0) | Apatinib (500
mg/day) + temozolomide (50 mg/m2, take every day) | 3 | 5 | 17 | 6 | 8/31 (25.8) | 25/31 (80.6) | 8.2 (6.9-9.5) | 4.9 (2.8-7.0) | (16) |
| Yao et al,
2021 | Single-arm | Dose-dense | 18 | NA (18.0-65.0) | Apatinib (500
mg/day) + temozolomide (50 mg/m2, take every day) | 0 | 4 | 10 | 3 | 4/17 (23.5) | 14/17 (82.3) | 9.1 (7.6-10.6) | 4 (3.2-4.8) | (22) |
| Gao et al,
2021 | Randomized-
controlled | Conventional-
dose | 26 | NA | Apatinib (500
mg/day) + temozolomide (150 mg/m2, take for 5 days, stop
for 23 days) | 1 | 17 | 6 | 2 | 10/26 (38.5) | 22/26 (84.6) | 8.7 (8.2-9.3) | 7.6 (5.7-9.4) | (23) |
| Li et al,
2021 | Single-arm | Conventional-
dose | 51 | 53 .0
(31.0-70.0) | Apatinib (500
mg/day) + temozolomide (150 mg/m2, take for 5 days, stop
for 23 days) | 2 | 15 | 20 | 14 | 17/51 (33.3) | 37/51 (72.5) | NA | 5.9 (5.4-6.5) | (24) |
| Xu et al,
2022 | Single-arm | Dose-dense | 79 | 46.1±15.2
(15.0-70.0) | Apatinib (500
mg/day) + temozolomide (100 mg/m2, take for 1 week, stop
for 1 week) | 0 | 23 | 46 | 10 | 23/79 (29.1) | 66/79 (83.5) | 6.4 (5.3-7.5) | 4.9 (3.9-5.9) | (25) |
| Zhang et al,
2022 | Randomized-
controlled | Conventional-
dose | 37 | NA | Apatinib (500
mg/day) + temozolomide (200 mg/m2, take for 5 days, stop
for 23 days) | 0 | 5 | 24 | 8 | 5/37 (13.5) | 29/37 (78.4) | NA | NA | (26) |
| Quan et al,
2022 | Randomized-
controlled | Dose-dense | 54 | 62.5±4.2
(52.0-74.0) | Apatinib (500
mg/day) + temozolomide (100 mg/m2, take for 3 weeks,
stop for 1 week) | 0 | 34 | 17 | 3 | 34/54 (63.0) | 51/54 (94.4) | 17 | NA | (27) |
Meta-analysis results. Overall ORR in
the treatment of recurrent high-grade glioma with apatinib combined
with temozolomide
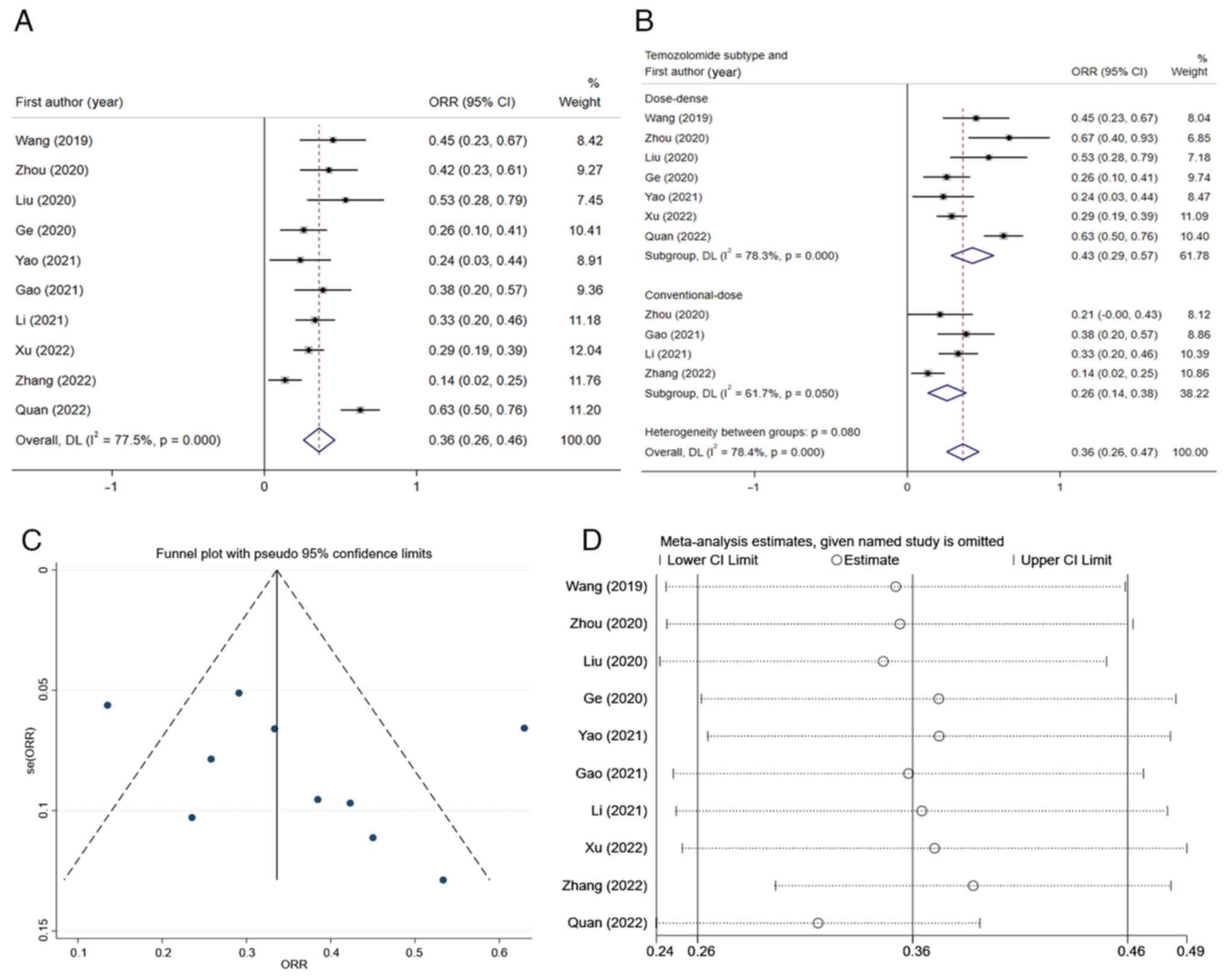
A comprehensive analysis of the results of the 10
studies included was performed. The heterogeneity test was
performed, and it was found that the I2-value was 77.5%,
thereby being >50%. Accordingly, the random-effects model was
used for the meta-analysis. The results indicated that the overall
ORR of patients with recurrent high-grade glioma who received
apatinib and temozolomide was 0.36 (95% CI, 0.26-0.46) (Fig. 2A). Furthermore, a sensitivity
analysis was performed and a funnel plot was generated to identify
the sources of heterogeneity (Fig.
2C and D). Among all studies,
there were two studies with significant deviation (26,27),
and after excluding these two studies, the I2-value was
reduced to 2.3% (Fig. 2B). The 10
studies were then stratified according to different dosage schemes
of temozolomide. The subgroup analysis indicated that the overall
ORR of the dose-dense temozolomide group was 0.43 (95% CI,
0.29-0.57), while that of the conventional-dose temozolomide was
0.26 (95% CI, 0.14-0.38) (Fig.
2B). There was a significant difference (P#x003C;0.05) between
subgroups.
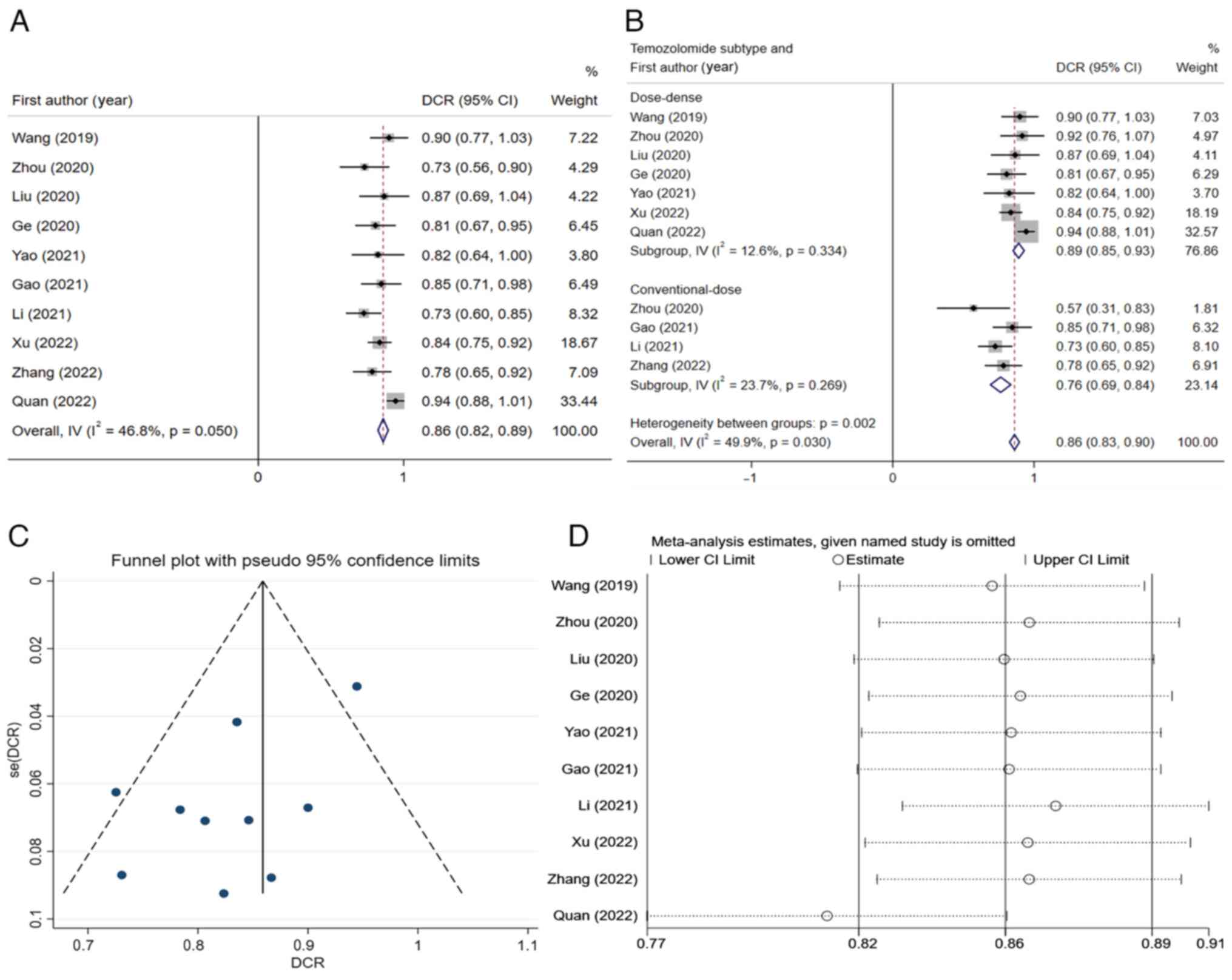
Overall DCR in the treatment of recurrent
high-grade glioma with apatinib combined with temozolomide.
Next, the combined DCR of the 10 studies was calculated and the
result indicated that the total DCR value after integration was
0.86 (95% CI, 0.82-0.89) (Fig.
3A). Since the I2-value of this result was 46.8%
≤50%), the fixed-effects model was used for integration. As could
be seen from Fig. 3A, Quan et
al (27) held significant
weight and had significant impacts on the pooled result, but the
result was not very heterogeneous, and this study could be
considered for inclusion in the analysis. The funnel plot and
sensitivity analysis results are presented in Fig. 3C and D, respectively. The studies were again
grouped according to the different dosage regimens of temozolomide,
and it was indicated that the total DCR of the dose-dense group was
0.89 (95% CI, 0.85-0.93) and the total DCR of the conventional-dose
group was 0.76 (95% CI, 0.69-0.84) (Fig. 3B). There was a significant
difference (P#x003C;0.05) between subgroups.
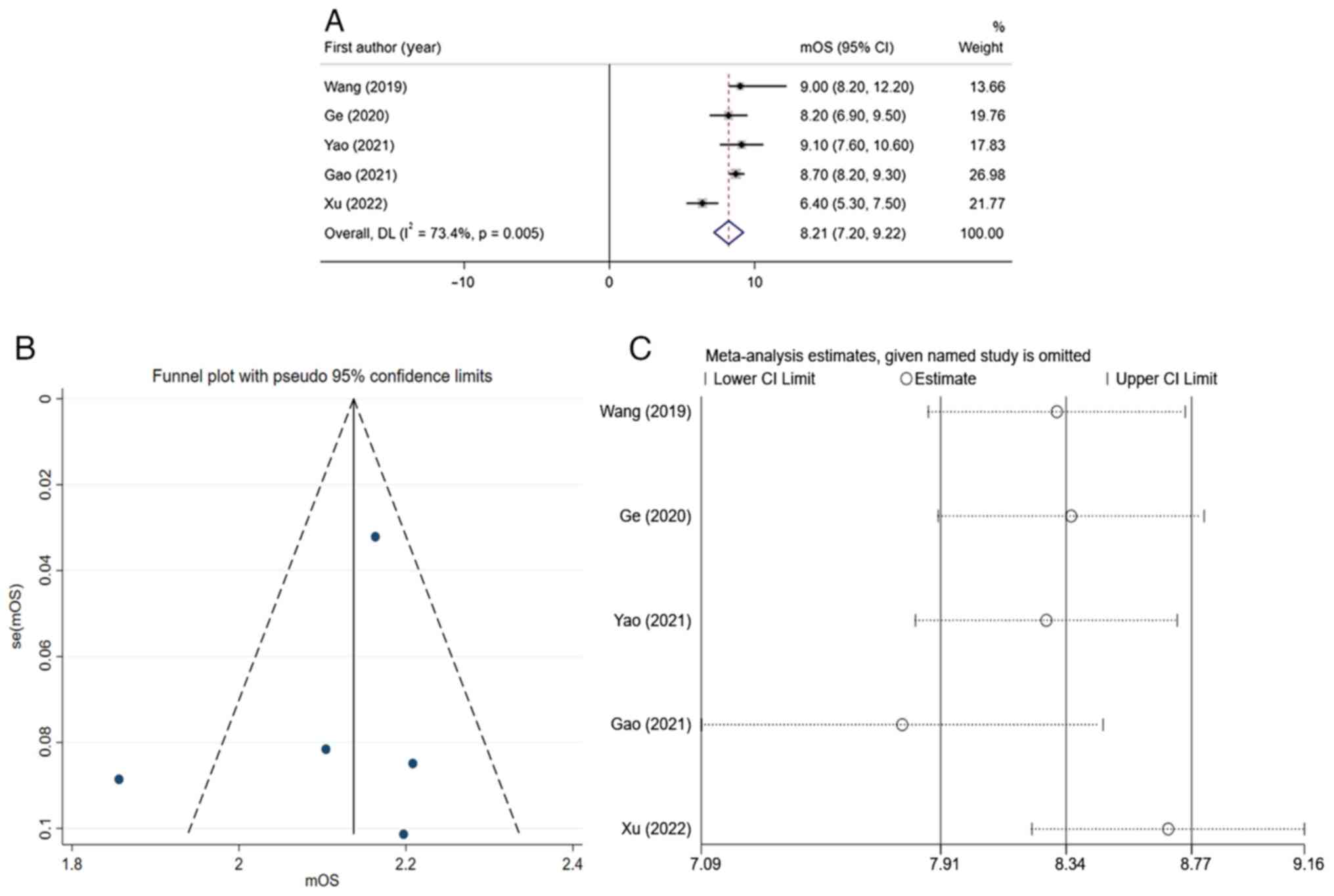
Prognostic analysis of apatinib combined with
temozolomide in the treatment of recurrent high-grade glioma.
Prognostic analysis indicated that the total mOS of apatinib
combined with temozolomide in treating recurrent high-grade glioma
was 8.21 months (95% CI, 7.20-9.22) (Fig. 4A). Due to the significant
heterogeneity of the results (I2=73.4%, thereby being
>50%), the random-effects model was used for analysis. Funnel
plots (Fig. 4B) and sensitivity
analysis (Fig. 4C) suggested that
the bias in the study by Xu et al (25) was significant. After excluding this
study, the I2-value could be reduced to 0% (data not
shown). This indicated that the result of this study was the main
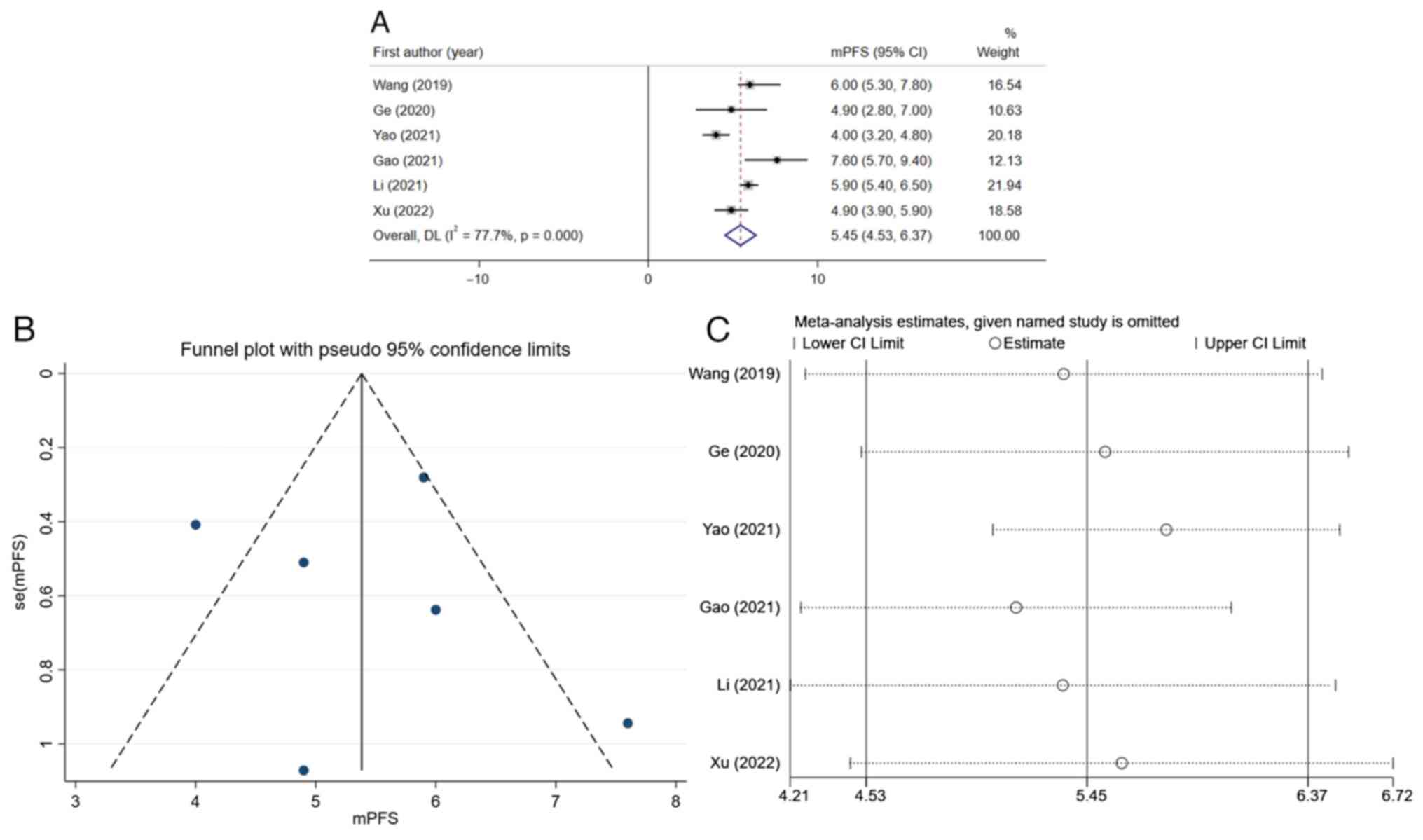
source of heterogeneity. After integrating the mPFS of various
studies, the total mPFS was determined to be 5.45 months (95% CI,
4.53-6.37) (Fig. 5A). Due to the
significant heterogeneity of the results (I2=77.7
thereby being >50%), a funnel plot was drawn (Fig. 5B) and a sensitivity analysis was
performed (Fig. 5C), indicating
significant deviations in the results of two studies (22,23).
After excluding these two studies and conducting further analysis,
it was found that the I2-value could be decreased to
19.6% (data not shown), indicating that these two studies may be
major sources of heterogeneity.
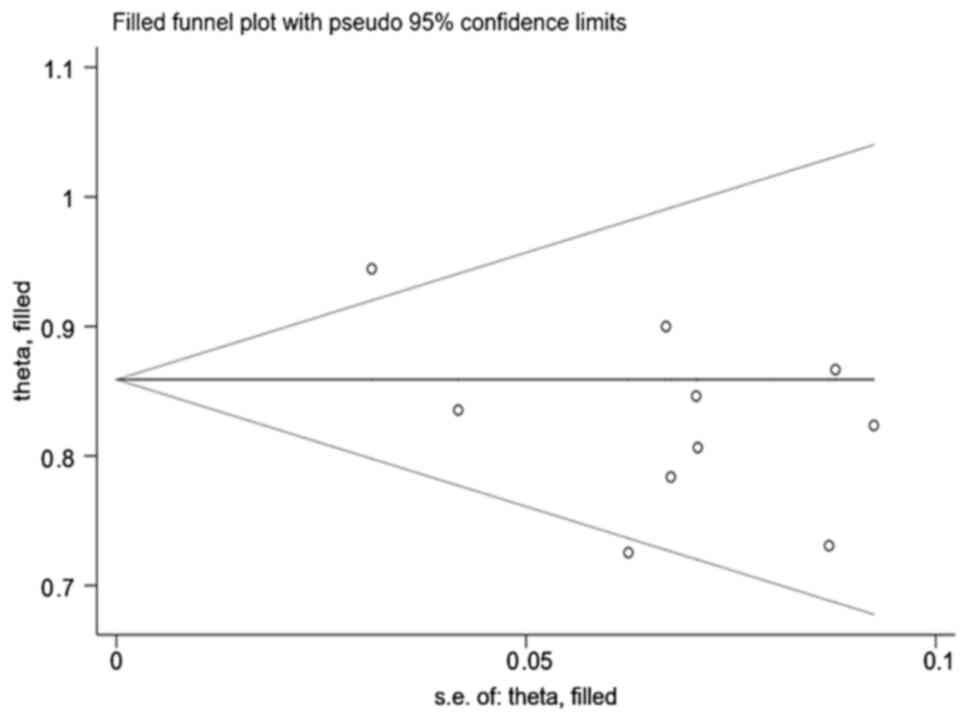
Publication bias
The analysis indicated that there was no significant
publication bias of the overall ORR in the treatment of recurrent
high-grade gliomas with apatinib and temozolomide using either the
Begg's test (P=0.152) or Egger's test (P=0.329). For each study's
DCR, publication bias was also examined using Begg's and Egger's
tests, and it was found that there was publication bias [Begg's
test (P=0.721); Egger's test (P=0.034)]. The impact of this bias on
the analysis results was further evaluated through the
trim-and-fill method, and it was found that the results were stable
(P#x003C;0.05) (Fig. 6).
Assessment of the publication bias of mOS in the prognostic
analysis of the treatment of recurrent high-grade glioma with
apatinib and temozolomide suggested that there was no significant
publication bias according to Begg's test (P=1.000) and Egger's
test (P=0.752). In addition, the results of publication bias of
mPFS indicated no significant publication bias according to Begg's
test (P=1.000) and Egger's test (P=0.885).
Discussion
Glioma is a common primary intracranial tumor, which
is difficult to treat and resect. In spite of receiving
postoperative radiotherapy and chemotherapy, most patients still
relapse, and the curative effect is poor thereafter. Currently,
there is no standard treatment strategy for recurrent high-grade
glioma (28,29). Temozolomide dose-dense chemotherapy
combined with cisplatin, carboplatin, cyclophosphamide and other
drugs has been tried clinically for the treatment of recurrent
high-grade glioma, but the effect is not ideal (30).
Improvement of the clinical efficacy of recurrent
high-grade glioma and the quality of life of patients remains an
unsolved problem. Malignant glioma is a tumor rich in
neovascularization, and high expression of VEGF and its receptor
VEGFR2 may promote the proliferation, infiltration and metastasis
of glioma cells (31). Both
apatinib and bevacizumab are antiangiogenic agents, but the former
has more advantages. Apatinib is an oral small-molecule tyrosine
kinase inhibitor that targets the intracellular domain of VEGFR2
(32,33). Unlike bevacizumab, apatinib may
promote tumor cell apoptosis through the intracellular autocrine
VEGF signaling pathway. In addition, apatinib may also inhibit
c-Kit, c-Src, platelet-derived growth factor receptor-β and MET,
directly exerting anti-tumor effects (34,35).
Bevacizumab has been widely used for clinical treatment, but its
efficacy is still unclear. Apatinib has attracted the attention of
researchers, and cell experiments have confirmed that apatinib is
able to inhibit the proliferation, invasion and metastasis of
glioma cells, promote tumor cell apoptosis and synergistically
increase the anti-tumor effects of temozolomide (10).
After screening, 357 patients with high-grade,
relapsed glioma were included in the present meta-analysis. These
patients had relapsed after receiving the standard Stupp regimen.
In certain studies, the regimen of apatinib combined with
dose-dense temozolomide was used when relapse occurred (14-16,21,22,25,27).
Other studies continued the conventional 5/28 temozolomide regimen
based on apatinib (15,23,24,26).
It was found that the overall ORR and DCR of high-grade glioma
treated with apatinib and temozolomide reached 36 and 86%,
respectively. After subgroup analysis, it was found that the
overall ORR (43%) and DCR (89%) of the apatinib with dose-dense
temozolomide group (n=229) were higher than those of the apatinib
with conventional-dose temozolomide group (n=128; ORR=26%;
DCR=76%). The results indicate that the therapeutic effect of the
combination of apatinib and dose-dense temozolomide may be better
than that of the conventional regimen. Further prognostic analysis
indicated that the overall mOS of patients with recurrent
high-grade glioma treated with apatinib combined with temozolomide
chemotherapy was 8.21 months and the mPFS was 5.45 months. At
present, there is no relevant meta-analysis or large-sample
clinical study that analyzed the efficacy of bevacizumab combined
with temozolomide or bevazicumab alone in the treatment of
high-grade glioma. Duerinck et al (36) found that the ORR, DCR, mOS and mPFS
of 313 patients with recurrent glioblastoma treated with single
bevacizumab were 35.5%, 63.5%, 6 and 3 months, respectively.
Another study found that the ORR of patients with recurrent
high-grade gliomas was only 20%, the mOS was 4.3 months and the
mPFS was 3.5 months after treatment with bevacizumab combined with
dose-dense temozolomide (37).
From the data of RTOG 0625 clinical trial (38), the total ORR of bevacizumab
combined with dose-dense temozolomide in the treatment of recurrent
glioblastoma was only 19%, mOS was 4.7 months and mPFS was 9.4
months. Although the results of the present meta-analysis were
based on the conclusion of small-sample trials, they indirectly
indicate that the combination of apatinib and temozolomide may be
better than bevacizumab and temozolomide.
At present, there is no large-sample study that
evaluated the efficacy of apatinib combined with temozolomide in
the treatment of recurrent high-grade glioma. Based on extensive
database retrieval, the present meta-analysis found that apatinib
combined with temozolomide has a certain efficacy through data
analysis of 10 studies, providing medical evidence for subsequent
clinical application. The present study had certain limitations.
Firstly, most of the studies were single group studies and only a
small number were randomized controlled clinical trials. The sample
size included in the current meta-analysis was not large and the
stability and reliability of the results cannot be guaranteed.
Secondly, during the integration of the ORR, subgroup analysis and
prognostic analysis, significant heterogeneity was found among
studies, which may affect accuracy and credibility. Thirdly, when
analyzing the publication bias of each effect size, bias of the DCR
was found. Although the significant impact of bias on the results
was excluded through the trim-and-fill method, it may still affect
the validity of the results. Lastly, the subjects included in the
present meta-analysis were mainly Chinese individuals and the
efficacy in populations from other countries or regions remains
undetermined.
In conclusion, the present analysis indicated that
the combination of apatinib and temozolomide chemotherapy has
certain efficacy and potential to improve prognosis, and it may
offer a new modality for the treatment of recurrent high-grade
glioma in the future. Although the synergistic effect of apatinib
and temozolomide has been demonstrated in cell experiments, its
clinical application remains insufficient. The present
meta-analysis study provided a comprehensive analysis based on
existing research data, thereby obtaining objective results.
However, there are not several relevant clinical large-sample
trials or controlled trials with other drug schemes to verify its
efficacy. If more prospective or randomized controlled studies were
available in the future, more accurate and stable results may be
obtained by further analysis.
Supplementary Material
Search strategy.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
GL designed the study. GL, JC and XX screened the
literature and extracted the data. GL and XX confirm the
authenticity of all the raw data. GL and FZ conducted the
meta-analysis. SW acquired, analyzed, verified and reviewed the
results. All authors have read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wu H, Deng Z, Wang H, Li X, Sun T, Tao Z,
Yao L, Jin Y, Wang X, Yang L, et al: MGMT autoantibodies as a
potential prediction of recurrence and treatment response biomarker
for glioma patients. Cancer Med. 8:4359–4369. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJB, Belanger K, Brandes A, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hervey-Jumper SL and Berger MS:
Reoperation for recurrent high-grade glioma: A current perspective
of the literature. Neurosurgery. 75:491–499. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Stupp R, Wong ET, Kanner AA, Steinberg D,
Engelhard H, Heidecke V, Kirson ED, Taillibert S, Liebermann F,
Dbalý V, et al: NovoTTF-100A versus physician's choice chemotherapy
in recurrent glioblastoma: A randomized phase Ⅲ trial of a novel
treatment modality. Eur J Cancer. 48:2192–2202. 2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lang FF, Conrad C, Gomez-Manzano C, Yung
WKA, Sawaya R, Weinberg JS, Prabhu SS, Rao G, Fuller GN, Aldape KD,
et al: Phase I study of DNX-2401(Delta-24-RGD) oncolytic
adenovirus: Replication and immunotherapeutic effects in recurrent
malignant glioma. J Clin Oncol. 36:1419–1427. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Tuettenberg J, Friedel C and Vajkoczy P:
Angiogenesis in malignant glioma-a target for antitumor therapy?
Crit Rev Oncol Hematol. 59:181–193. 2006.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Rainer E, Wang H, Traub-Weidinger T,
Widhalm G, Fueger B, Chang J, Zhu Z, Marosi C, Haug A, Hacker M and
Li S: The prognostic value of [123I]-vascular
endothelial growth factor ([123I]-VEGF) in glioma. Eur J
Nucl Med Mol Imaging. 45:2396–2403. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kreisl TN, Kim L, Moore K, Duic P, Royce
C, Stroud I, Garren N, Mackey M, Butman JA, Camphausen K, et al:
Phase Ⅱ trial of single-agent bevacizumab followed by bevacizumab
plus irinotecan at tumor progression in recurrent glioblastoma. J
Clin Oncol. 27:740–745. 2009.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Liang Q, Kong L, Du Y, Zhu X and Tian J:
Antitumorigenic and antiangiogenic efficacy of apatinib in liver
cancer evaluated by multimodality molecular imaging. Exp Mol Med.
51:1–11. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wang C, Jiang M, Hou H, Lin Q, Yan Z and
Zhang X: Apatinib suppresses cell growth and metastasis and
promotes antitumor activity of temozolomide in glioma. Oncol Lett.
16:5607–5614. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Li J, Qin S, Xu J, Xiong J, Wu C, Bai Y,
Liu W, Tong J, Liu Y, Xu R, et al: Randomized, double-blind,
Placebo-controlled phase III trial of Apatinib in patients with
chemotherapy-refractory advanced or metastatic adenocarcinoma of
the stomach or Gastroesophageal junction. J Clin Oncol.
34:1448–1454. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhang L, Shi M, Huang C, Liu X, Xiong JP,
Liu GC, Liu W, Zhang Y, LI K, Yu H and Jiang H: A phase II,
multicenter, placebo-controlled trial of apatinib in patients with
advanced nonsquamous non-small cell lung cancer (NSCLC) after two
previous treatment regimens. J Clin Oncol. 30
(15_suppl)(7548)2012.
|
|
13
|
Hu X, Cao J, Hu W, Wu C, Pan Y, Cai L,
Tong Z, Wang S, Li J, Wang Z, et al: Multicenter phase II study of
apatinib in non-triple-negative metastatic breast cancer. BMC
Cancer. 14(820)2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wang Y, Meng X, Zhou S, Zhu Y, Xu J and
Tao R: Apatinib plus temozolomide for recurrent glioblastoma: An
uncontrolled, open-label study. Onco Targets Ther. 12:10579–10585.
2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhou PC, Pan HH, Liang TS and Yang DK:
Short-term efficacy of apatinib combined with dose-dense
temozolomide in treatment of recurrent malignant glioma after
postoperative chemoradiotherapy. J Med Forum. 41:73–79. 2020.(In
Chinese).
|
|
16
|
Ge J, Li C, Xue F, Qi S, Gao Z, Yu C and
Zhang J: Apatinib plus Temozolomide: An effective salvage treatment
for recurrent glioblastoma. Front Oncol. 10(601175)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wei W, Chen X, Ma XM, Wang D and Guo Z:
The efficacy and safety of various dose-dense regimens of
temozolomide for recurrent high-grade glioma: A systematic review
with meta-analysis. J Neurooncol. 125:339–349. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372(n71)2021.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Higgins JPT, Thompson SG, Deeks JJ and
Altman DG: Measuring inconsistency in meta-analyses. BMJ.
327:557–560. 2003.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Egger M, Smith GD, Schneider M and Minder
C: Bias in meta-analysis detected by a simple, graphical test. BMJ.
315:629–634. 1997.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Liu ZJ, Sun HW, Song ZY, Guo S, Gao M,
Zhou G and Liu X: Apatinib combined with dose-dense temozolomide in
the treatment of recurrent malignant glioma. Chin J Pract Nerv Dis.
23:958–962. 2020.(In Chinese).
|
|
22
|
Yao H, Liu JG, Zhang C, Shao Y, Li X, Feng
M, Wang X, Gan W, Zhou Y and Huang Y: Clinical study of apatinib
plus temozolomide for the treatment of recurrent high-grade
gliomas. J Clin Neurosci. 90:82–88. 2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Gao M, Li MY, Ma QQ, Shi H and Yang Y:
Brucea javanica oil emulsion combined with apatinib and
temozolomide in the treatment of recurrent high-grade glioma. J
Basic Clin Oncol. 34:36–40. 2021.(In Chinese).
|
|
24
|
Li TY, Hai L, Wu H, Hong X, Lu X and Sun
X: Clinical research of apatinib in the treatment of durg-resistant
recurrent glioblastoma. Chin J Clin Oncol. 48:619–623. 2021.(In
Chinese).
|
|
25
|
Xu KY, Jiao Y, Wang JK, Liu X, Sun H, Luo
W and Zhao H: Effect of apatinib combined with temozolomide in the
treatment of recurrent high-grade glioma. Henan J Surgery.
28:30–32. 2022.(In Chinese).
|
|
26
|
Zhang M, Gao LY, Liu X, Dong F, Su Q,
Zhang Y, Li F, Wang H and Han P: Low-dose Apatinib improves the
prognosis of patients with recurrent high-grade gliomas. Evid Based
Complement Alternat Med. 2022(3181133)2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Quan LL, Song HB and Song LL: Short term
and long-term efficacy of bevacizumab monoclonal antibody and
apatinib combined with temozolomide in treatment of recurrent
high-grade glioma. Chin J Mod Med. 32:11–15. 2022.(In Chinese).
|
|
28
|
Ostrom QT, Cioffi G, Gittleman H, Patil N,
Waite K, Kruchko C and Barnholtz-Sloan JS: CBTRUS statistical
report: Primary brain and other central nervous system tumors
diagnosed in the United States in 2012-2016. Neuro Oncol. 21 (Suppl
5):v1–v100. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wick W, Weller M, Van den bent M, Sanson
M, Weiler M, von Deimling A, Plass C, Hegi M, Platten M,
Reifenberger G, et al: MGMT testing-the challenges for
biomarker-based glioma treatment. Nat Rev Neurol. 10:372–385.
2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Nabors LB, Portnow J, Ahluwalia M,
Baehring J, Brem H, Brem S, Butowski N, Campian JL, Clark SW,
Fabiano AJ, et al: Central nervous system cancers, version 3.2020,
NCCN clinical practice guidelines in oncology. J Natl Compr Canc
Netw. 18:1537–1570. 2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Jain HV, Nör JE and Jackson TL: Modeling
the VEGF-Bcl-2-CXCL8 pathway in intratumoral agiogenesis. Bull Math
Biol. 70:89–117. 2008.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Peng S, Zhang Y, Peng H, Ke Z, Xu L, Su T,
Tsung A, Tohme S, Huang H, Zhang Q, et al: Intracellular autocrine
VEGF signaling promotes EBDC cell proliferation, which can be
inhibited by Apatinib. Cancer Lett. 373:193–202. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Peng H, Zhang Q, Li J, Zhang N, Hua Y, Xu
L, Deng Y, Lai J, Peng Z, Peng B, et al: Apatinib inhibits VEGF
signaling and promotes apoptosis in intrahepatic
cholangiocarcinoma. Oncotarget. 7:17220–17229. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Tong XZ, Wang F, Liang S, Zhang X, He JH,
Chen XG, Liang YG, Mi YJ, To KKW and Fu LW: Apatinib (YN968D1)
enhances the efficacy of conventional chemotherapeutical drugs in
side population cells and ABCB1-overexpressing leukemia cells.
Biochem Pharmacol. 83:586–597. 2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Roviello G, Ravelli A, Fiaschi AI,
Cappelletti MR, Gobbi A, Senti C, Zanotti L, Polom K, Reynolds AR,
Fox SB and Generali D: Apatinib for the treatment of gastric
cancer. Expert Rev Gastroenterol Hepatol. 10:887–892.
2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Duerinck J, Clement PM, Bouttens F, Andre
C, Neyns B, Staelens Y, Fraeyenhove FV, Baurain JF, Luce S, D'hondt
L, et al: Patient outcome in the Belgian medical need program on
bevacizumab for recurrent glioblastoma. J Neurol. 262:742–751.
2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Verhoeff JJC, Lavini C, van Linde ME,
Stalpers LJA, Majoie CBLM, Reijneveld JC, van Furth WR and Richel
DJ: Bevacizumab and dose-intense temozolomide in recurrent
high-grade glioma. Ann Oncol. 21:1723–1727. 2010.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Gilbert MR, Pugh SL, Aldape K, Sorensen
AG, Mikkelsen T, Penas-Prado M, Bokstein F, Kwok Y, Lee RJ and
Mehta M: NRG oncology RTOG 0625: A randomized phase II trial of
bevacizumab with either irinotecan or dose-dense temozolomide in
recurrent glioblastoma. J Neurooncol. 131:193–199. 2017.PubMed/NCBI View Article : Google Scholar
|