Introduction
In recent years, the incidence of infertility has
been increasing, with the World Health Organization reporting a
global prevalence of ~15% (1).
In vitro fertilization-embryo transfer (IVF-ET) is currently
the most commonly used assisted reproductive technique, but embryo
implantation rates are low (2),
with evidence suggesting that up to two-thirds of embryo
implantation failures can be attributed to poor endometrial
reception and the remaining one-third due to quality defects in
embryos (3). Good endometrial
receptivity (ER) and embryo quality are necessary for successful
implantation. ER refers to the ability of the endometrium (the
inner lining of the uterus) to support embryo implantation during
the menstrual cycle. It is influenced by various physiological
factors and mechanisms, including hormonal regulation (4), endometrial gene expression (5), endometrial morphological changes
(6), immune system modulation
(7), and endometrial
vascularization (8). Considering
the mechanisms, first, once progesterone and estrogen signaling is
disrupted, it leads to progesterone resistance and estrogen
dominance. This hormone imbalance leads to increased inflammation,
reducing the endometrium's receptivity to embryo implantation
(4). Secondly, the upregulation of
certain genes, such as Eps15 homology domain-containing 1 (EHD1)
(9) and ICAM1(10), were found to be associated with
reduced ER. Third, changes in endometrial morphology, manifesting
as disruption of the endometrial epithelial cells, have been shown
to lead to impaired ER (6).
Recently, it has been found that endometrial microbiota disturbance
can cause immune microenvironment remodeling (activation of uterine
NK cells and changes in specific subpopulations of T cells), which
negatively impacts ER (7).
Finally, adequate blood flow and angiogenesis are critical for
endometrial receptivity. Blood vessels supply oxygen, nutrients,
and signaling molecules necessary for embryo development and
implantation. Abnormalities in endometrial vascularization can
compromise implantation (8).
Clinically, endometrial receptivity is usually
evaluated from endometrial morphology, ultrasound imaging,
biochemistry, and other aspects (11). Among them, transvaginal ultrasound
assessment of endometrial receptivity is non-invasive and highly
repeatable and thus has been widely used in clinical practice
(12). However, due to the use of
ultrasound to measure the relevant indicators of endometrial
receptivity, it is affected to a certain extent by the subjective
factors of the examiner and the objective factors that differ
between different ultrasound machines and equipment (13). Therefore, there are currently no
reliable and consistent conclusions regarding transvaginal
ultrasound assessment of endometrial receptivity in predicting
clinical pregnancy outcomes in IVF-ET. In the present meta-analysis
multiple endometrial receptivity indices that can be used to
predict the outcomes of IVF-ET clinical pregnancy, such as
transvaginal ultrasound measurement of endometrial thickness,
endometrial volume, peak uterine systolic blood flow velocity to
end-diastolic blood flow velocity ratio (systolic/diastolic S/D),
pulsatility index (PI), resistance index (RI), vascularization
index (VI), flow index (FI), and vascularization flow index (VFI),
were assessed, with the aim of providing a diagnostic basis for
clinical practice.
Materials and methods
Literature inclusion and exclusion
criteria
The inclusion criteria for the meta-analysis were:
i) Study object, infertile women undergoing IVF-ET and undergoing
vaginal ultrasound; ii) intervention measures, whether the
pregnancy was successful after receiving IVF-ET; iii) outcome
indicators, endometrial thickness (cm), endometrial volume
(cm3), resistive index (RI) of the uterine artery,
pulsatility index (PI) of the uterine artery, systolic/diastolic
(S/D), vascularization index (VI), flow index (FI) and
vascularization flow index (VFI); and iv) study design,
case-control or cohort studies.
The exclusion criteria were: Repeat publications,
studies where the full text was not available, studies where data
could not be extracted, studies using animal experiments, reviews,
meta-analyses, and systematic reviews.
Search strategy
For this meta-analysis, PubMed (https://pubmed.ncbi.nlm.nih.gov), Embase
(https://www.embase.com/), and Cochrane Library
(https://www.cochranelibrary.com/)
databases were searched from establishment of the database to
January 2023. The search terms were:
(((((((((((((((((‘Ultrasonography’[Mesh]) OR (Diagnostic
Ultrasound[Title/Abstract])) OR (Diagnostic
Ultrasounds[Title/Abstract])) OR (Ultrasound
Imaging[Title/Abstract])) OR (Echotomography[Title/Abstract])) OR
(Ultrasonic Imaging[Title/Abstract])) OR (Medical
Sonography[Title/Abstract])) OR (Ultrasonographic
Imaging[Title/Abstract])) OR (Ultrasonographic
Imagings[Title/Abstract])) OR (Echography[Title/Abstract])) OR
(Ultrasonic Diagnoses[Title/Abstract])) OR (Ultrasonic
Diagnosis[Title/Abstract])) OR (Computer
Echotomo-graphy[Title/Abstract])) OR (Ultrasonic
Tomography[Title/Abstract])) OR (Ultrasound[Title/Abstract])) AND
((((((‘Embryo Transfer’[Mesh]) OR (Embryo
Transfers[Title/Abstract])) OR (Blastocyst
Transfer[Title/Abstract])) OR (Tubal Embryo
Transfer[Title/Abstract])) OR (Tubal Embryo Stage
Transfer[Title/Abstract])) OR ((((((((((‘Fertilization in
Vitro’[Mesh]) OR (In Vitro
Fertilization[Title/Abstract])) OR (In Vitro
Fertilizations[Title/Abstract])) OR (Test-Tube
Fertilization[Title/Abstract])) OR (Test Tube Fertilization
[Title/Abstract])) OR (Test Tube Fertilizations[Title/Abstract]))
OR (Fertilizations in Vitro[Title/Abstract])) OR (Test-Tube
Babies[Title/Abstract])) OR (Test Tube Babies[Title/Abstract])) OR
(Test-Tube Baby[Title/Abstract])))) AND ((pregnancy
outcome[Title/Abstract]) OR (pregnancy outcomes[Title/Abstract])))
AND (((((‘Infertility’[Mesh]) OR (Sterility[Title/Abstract])) OR
(Reproductive Sterilit[Title/Abstract])) OR
(Subfertility[Title/Abstract])) OR
(Sub-Fertility[Title/Abstract])).
Literature screening and data
extraction
Two researchers independently performed the
literature search, screening, and data extraction. When a question
or dispute arose, a consensus was reached after discussion. The
data extraction included: Author, article publication year,
country, study design, sample size, age, BMI, anti-Mullerian
Hormone (pmol/l), and the outcome indicators.
Assessment of the quality of the
literature
Two researchers independently conducted literature
quality evaluations using the Newcastle-Ottawa Scale (NOS) for
cohort studies (14). NOS includes
4 items (4 points) for ‘Research Subject Selection’, 1 item (2
points) for ‘Comparability between Groups’ and 3 items (3 points)
for ‘Result Measurement’, with a full score of 9 points, where a
score ≥7 is regarded as high-quality literature, and #x003C;7 is
divided into lower-quality literature. When the scores differed
between the two researchers, it was decided through discussion or
consultation with a third person. The present meta-analysis was
performed based on the related items of the Preferred Reporting
Items for Systematic Reviews and Meta-analysis (PRISMA) statement
(15).
Data synthesis and statistical
analysis
Data were analyzed using STATA version 15.1
(StataCorp LLC). Weighted mean differences (WMDs) were used to
assess differences in continuous variables. I2 and Q
tests were used to evaluate heterogeneity. If the heterogeneity
test was P≥0.1 and I2≤50%, there was homogeneity amongst
the studies; if they were P#x003C;0.1 and I2>50%,
there was heterogeneity, and a sensitivity analysis was performed
to identify the source. A random effects model was used for
combining effects in the present meta-analysis. Funnel plots and
Egger's tests were used to analyse publication bias.
Results
Literature search results
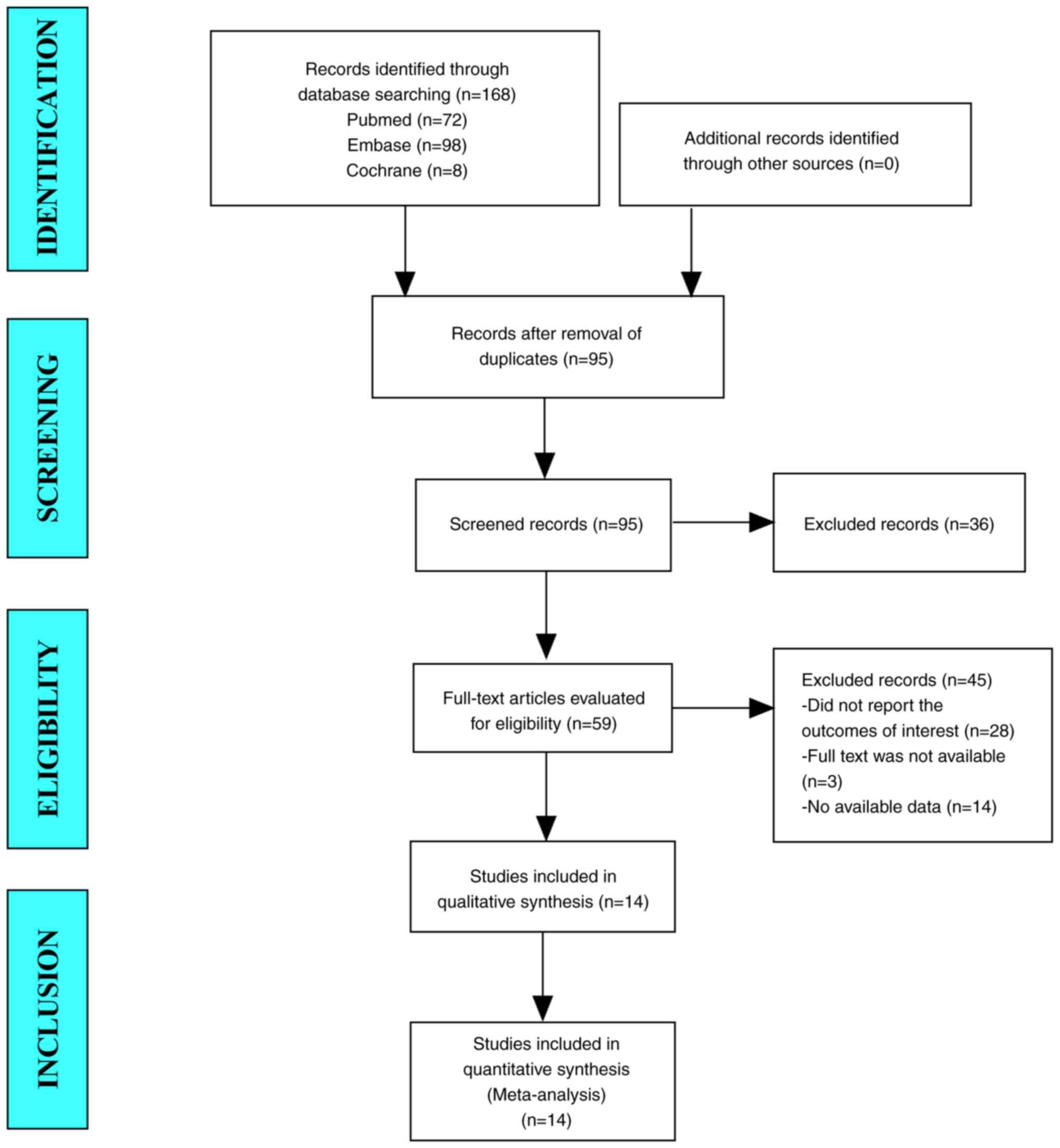
In the present study, 168 studies were retrieved
from the database. After eliminating duplicate studies, 95 studies
were included. After browsing the titles and abstracts, 59 studies
were identified. Finally, 14 articles were included in the
meta-analysis (Fig. 1).
Baseline characteristics and quality
assessment of the included studies
In total, 14 cohort studies were included in the
present meta-analysis (16-29).
The combined patient sample size was 4,842. The mean age
distribution of the pregnancy group was 30.3-34.4 years, while the
mean age distribution of the non-pregnancy group was between
31.5-35.8 years, indicating that the ages of the two groups did not
differ notably. In addition, the BMI distribution of the pregnancy
group was 21.2-23.6, while in the non-pregnancy group, it was
21.4-23.3, indicating that the BMI of the two groups were
comparable as well (Table I). The
NOS scores used for quality assessment for all 14 studies were
>7 (Table II).
 | Table IBaseline characteristics and quality
assessment of the included studies. |
Table I
Baseline characteristics and quality
assessment of the included studies.
| | Sample size | Age | BMI | Anti-Mullerian
Hormone, pmol/l | |
|---|
| First author,
year | Country | Pregnancy | Non- pregnancy | Pregnancy | Non- pregnancy | Pregnancy | Non- pregnancy | Pregnancy | Non- pregnancy | (Refs.) |
|---|
| Zhang et al,
2022 | China | 1,167 | 686 | 32.2±3.5 | 33.1±3.7 | 23.6±3.9 | 23.2±3.7 | 29.6±22.1 | 26.3±22.0 | (16) |
| Crosby et al,
2022 | Ireland | 21 | 29 | 34.4±2.1 | 34.7±2.2 | 23.4±3.0 | 23.3±2.5 | 21.8±14.9 | 16.3±12.8 | (17) |
| Tong et al,
2020 | China | 36 | 43 | 31.0±4.2 | 32.5±4.6 | 21.5±2.3 | 22.3±2.9 | 36.1±23.9 | 37.7±31.1 | (18) |
| Long et al,
2019 | China | 29 | 32 | 33.2±3.3 | 32.1±3.8 | 22.9±3.4 | 21.4±2.4 | / | / | (19) |
| Koo et al,
2018 | Korea | 20 | 15 | 33.6±3.6 | 35.8±3.1 | / | / | 24.3±18.6 | 26.4±25.7 | (20) |
| Prasad et
al, 2017 | India | 76 | 112 | 31.2±3.9 | 31.5±4.3 | / | / | / | / | (21) |
| Son et al,
2014 | Korea | 29 | 41 | 32.9±4.1 | 34.7±3.7 | 21.2±2.5 | 22.1±3.9 | / | / | (22) |
| Zhao et al,
2014 | China | 1,010 | 923 | 30.6±4.4 | 31.8±4.8 | 21.6±2.6 | 21.9±3.1 | / | / | (23) |
| Nandi et al,
2014 | UK | 7 | 9 | / | / | / | / | / | / | (24) |
| Engels et
al, 2011 | Spain | 9 | 70 | 33.0±3.6 | | / | / | / | / | (25) |
| Zácková et
al, 2009 | Czech Republic | 15 | 15 | 31.3±1.1 | 31.5±1.1 | 22.9±1.1 | 23.0±0.8 | / | / | (26) |
| Mercé et al,
2008 | Spain | 38 | 39 | 33.9±3.4 | 34.3±3.5 | / | / | / | / | (27) |
| Chien et al,
2004 | China | 91 | 226 | 32.7±3.6 | 33.6±3.9 | / | / | / | / | (28) |
| Wu et al,
2003 | China | 18 | 36 | 30.3±3.8 | 32.2±4.4 | / | / | / | / | (29) |
 | Table IINOS quality assessment of the
included studies. |
Table II
NOS quality assessment of the
included studies.
| | Selection | Comparability | Outcome | |
|---|
| First author,
year | Representativeness
of the sample | Sample size | Non-
respondents | Ascertainment of
exposure | Based on design and
analysis | Assessment of the
outcome | Follow- up | Adequacy of
follow-up | Overall score |
|---|
| Zhang et al,
2022 | 1 | 1 | 1 | 1 | 2 | 0 | 1 | 1 | 8 |
| Crosby et
al, 2022 | 1 | 1 | 1 | 1 | 2 | 0 | 1 | 0 | 7 |
| Tong et al,
2020 | 1 | 1 | 1 | 1 | 2 | 0 | 1 | 1 | 8 |
| Long et al,
2019 | 1 | 1 | 1 | 1 | 2 | 0 | 1 | 2 | 9 |
| Koo et al,
2018 | 1 | 1 | 1 | 1 | 2 | 0 | 1 | 0 | 7 |
| Prasad et
al, 2017 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 7 |
| Son et al,
2014 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 7 |
| Zhao et al,
2014 | 1 | 1 | 1 | 1 | 2 | 0 | 1 | 1 | 8 |
| Nandi et al,
2014 | 1 | 1 | 1 | 1 | 2 | 0 | 1 | 1 | 8 |
| Engels et
al, 2011 | 1 | 1 | 1 | 1 | 2 | 0 | 1 | 0 | 7 |
| Zácková et
al, 2009 | 1 | 1 | 1 | 1 | 2 | 0 | 1 | 0 | 7 |
| Mercé et al,
2008 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 7 |
| Chien et al,
2004 | 1 | 1 | 1 | 1 | 2 | 0 | 1 | 0 | 7 |
| Wu et al,
2003 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 7 |
Results of the meta-analysis
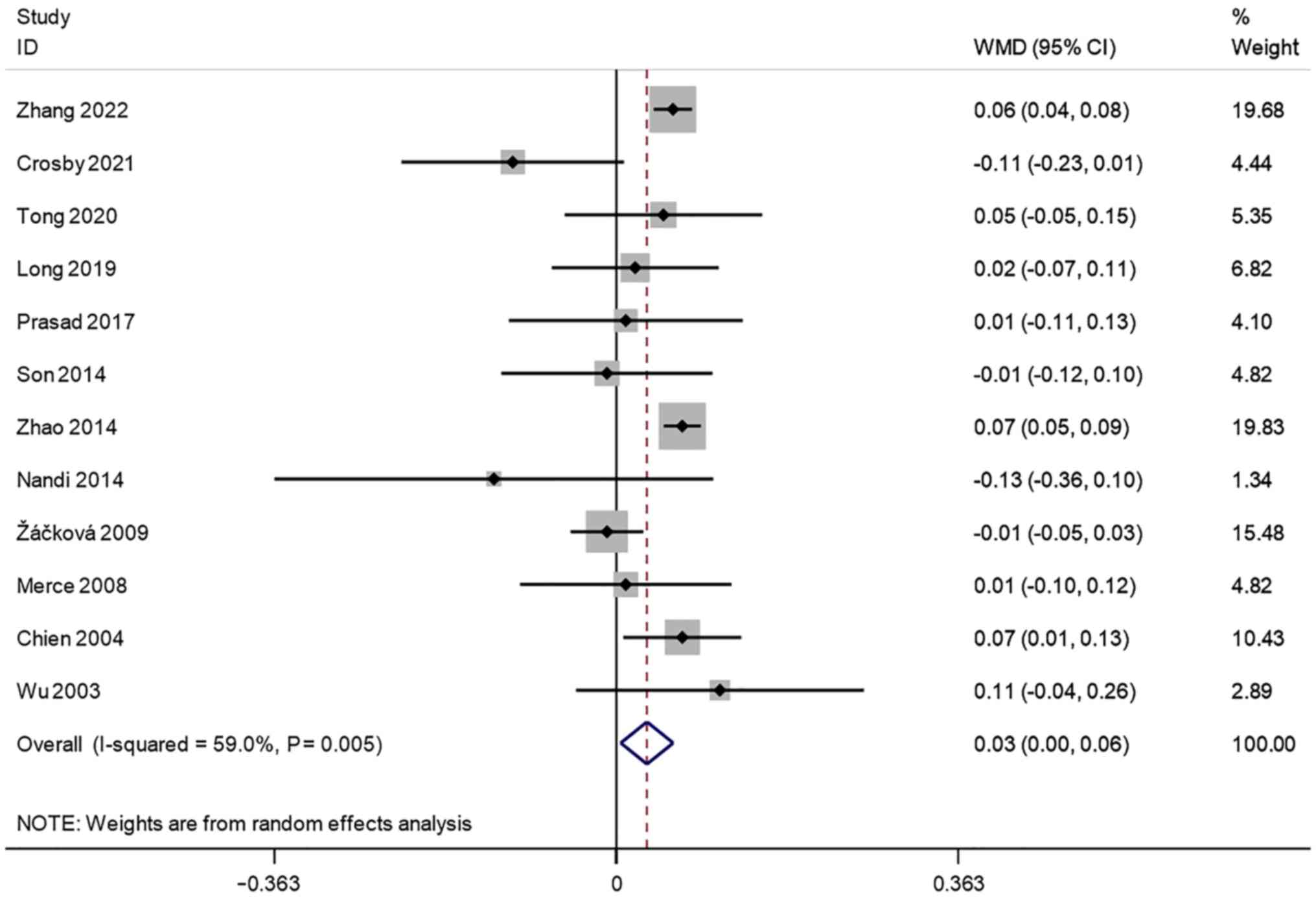
Endometrial thickness (cm). A total of 12
studies reported transvaginal ultrasound endometrial thickness in
infertile women undergoing IVF-ET. There was significant
heterogeneity (I2=59.0%, P=0.005). A meta-analysis was
performed using a random effects model. The pooled results showed
that the endometrial thickness of the pregnancy group after
receiving IVF-ET was significantly higher than that of the
non-pregnancy (WMD=0.03, 95% CI: 0.00-0.06; P=0.022) (Fig. 2).
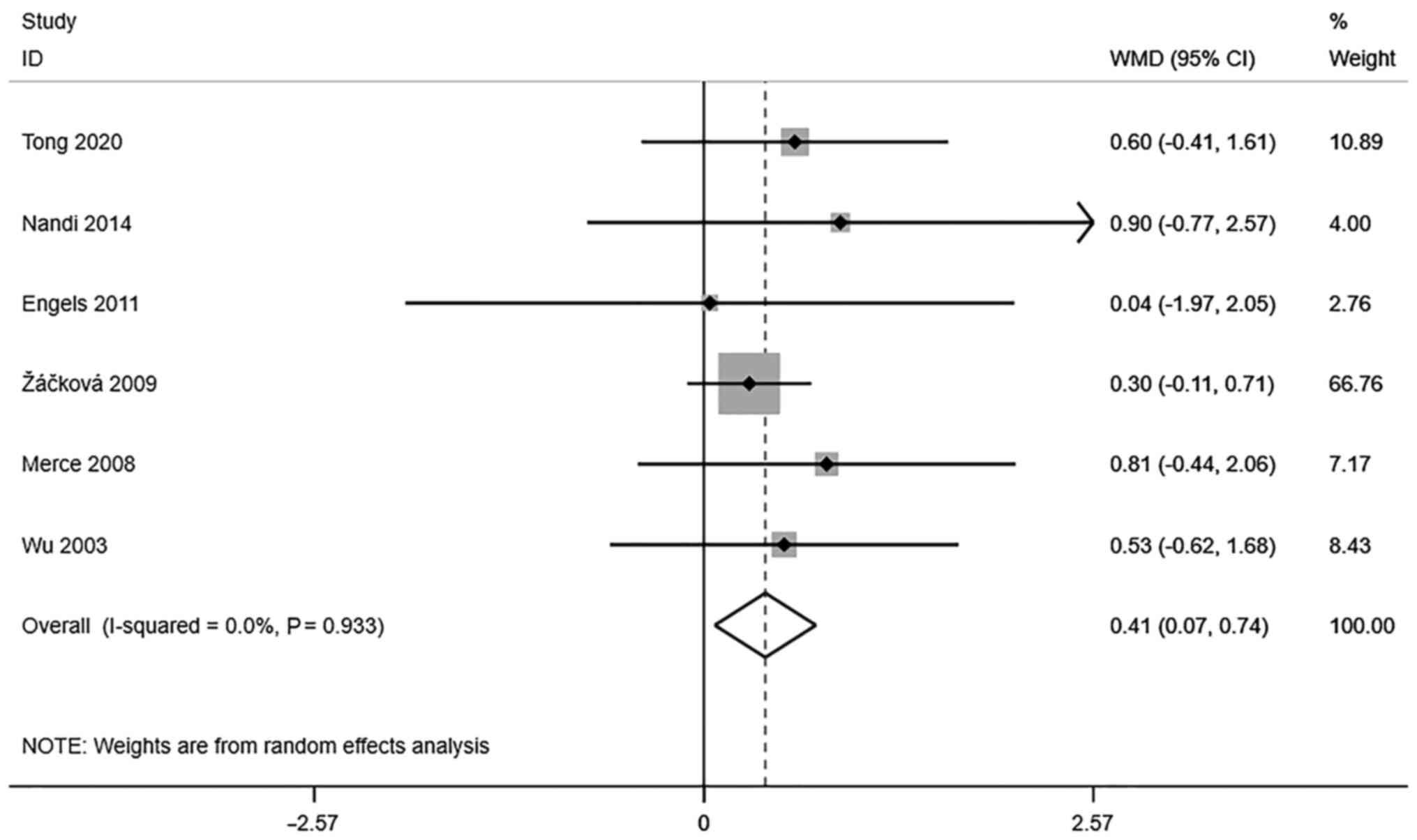
Endometrial volume (cm3). A
total of 5 studies reported transvaginal ultrasound endometrial
volume in infertile women undergoing IVF-ET. There was no
significant heterogeneity (I2=0.0%, P=0.933). The pooled
results of the random-effects model showed that the endometrial
volume of the pregnancy group after receiving IVF-ET was
significantly higher than that of the non-pregnancy group
(WMD=0.41, 95% CI: 0.07-0.74; P=0.017) (Fig. 3).
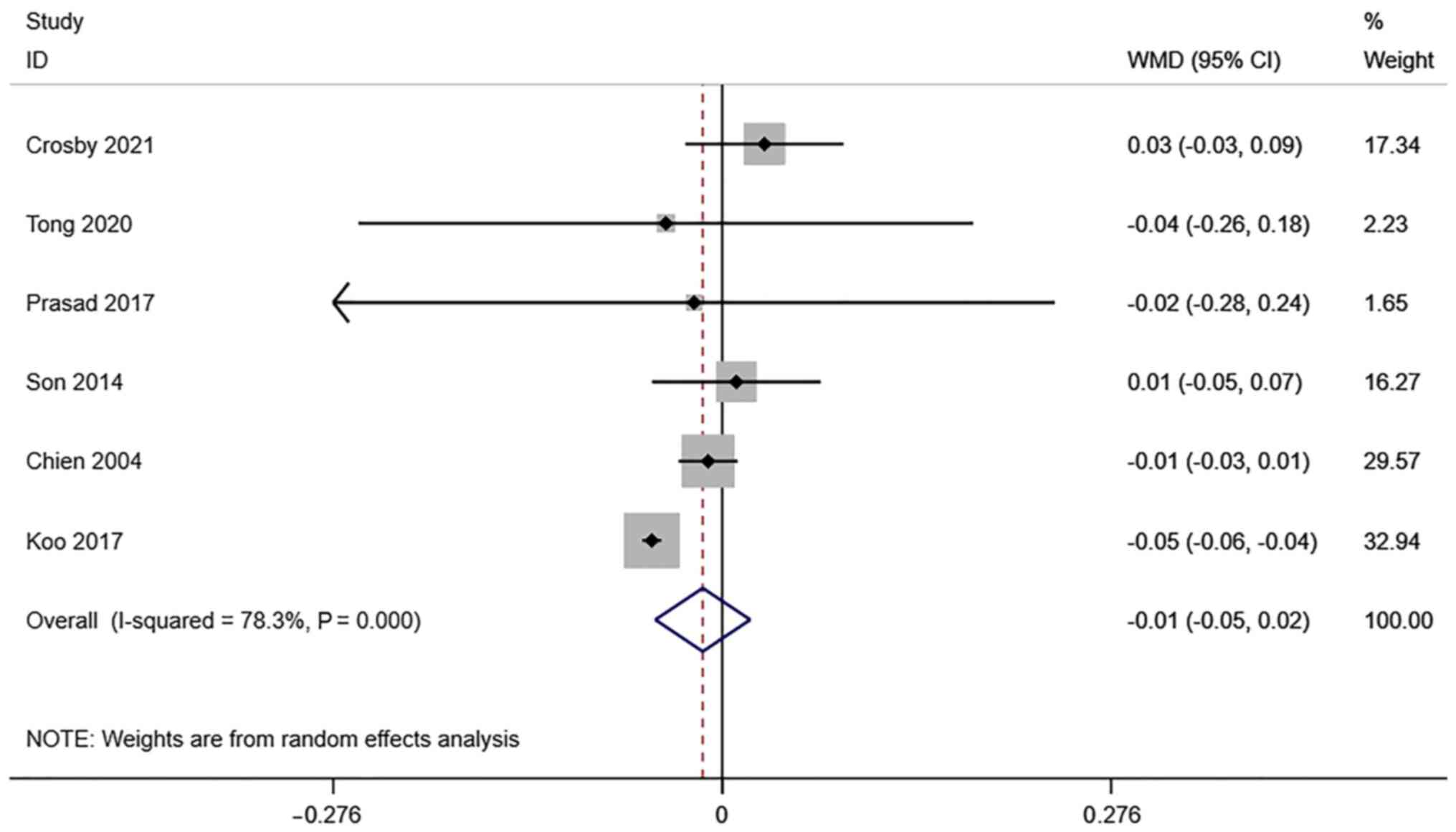
RI of the uterine artery. A total of 6
studies reported transvaginal ultrasound RI in infertile women
undergoing IVF-ET. There was significant heterogeneity
(I2=78.3%, P=0.000), and a meta-analysis was performed
using a random effects model. The pooled results showed that the
difference between RI in the pregnancy group and the non-pregnancy
group after receiving IVF-ET was not statistically significant
(WMD=-0.01, 95% CI: -0.05-0.02; P=0.422) (Fig. 4).
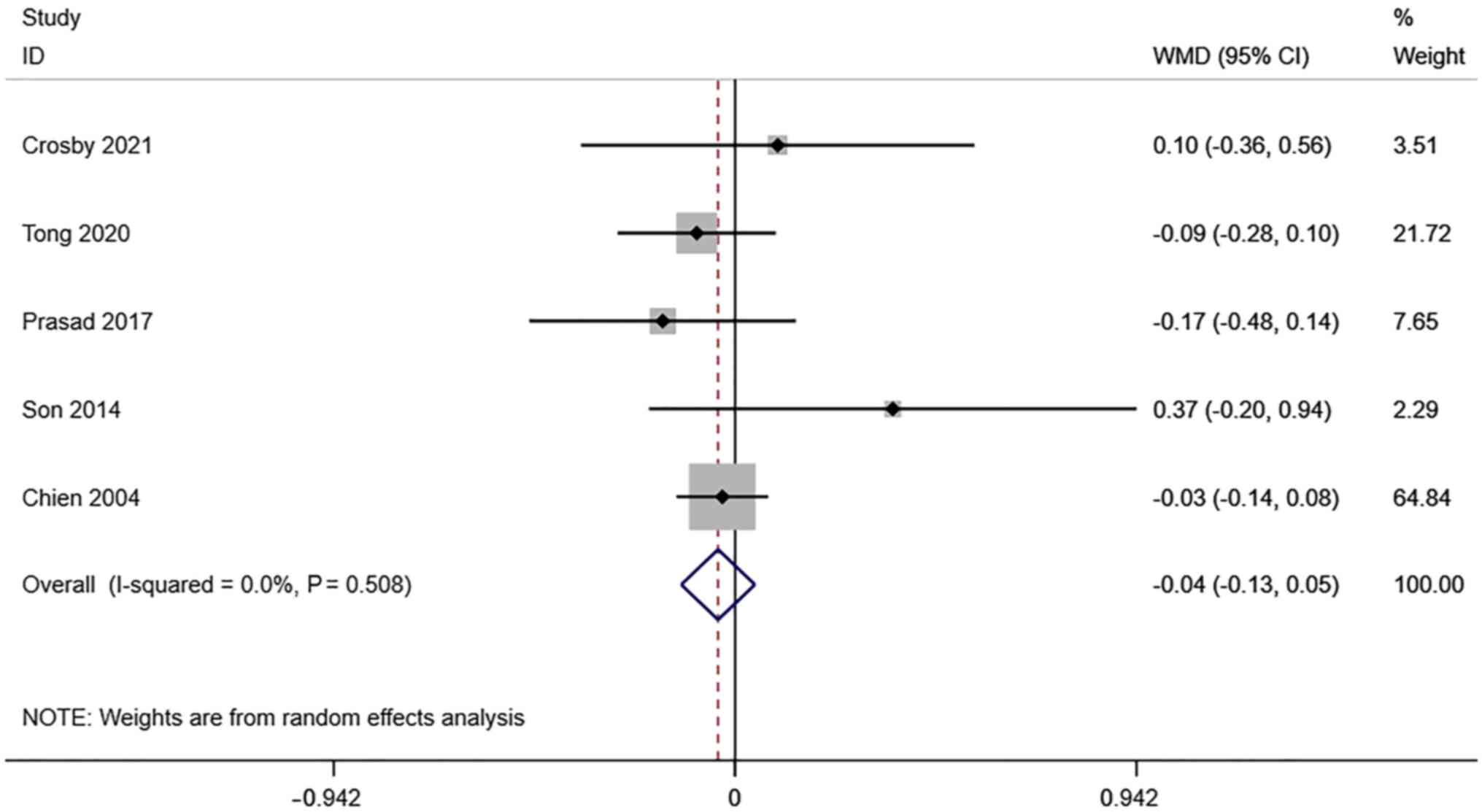
PI of the uterine artery. A total of 6
studies reported transvaginal ultrasound PI in infertile women
undergoing IVF-ET. There was no significant heterogeneity
(I2=0.0%, P=0.508). The pooled results of the
random-effects model showed that the difference between PI in the
pregnancy group and the non-pregnancy group after receiving IVF-ET
was not statistically significant (WMD=-0.04, 95% CI: -0.13-0.05;
P=0.364) (Fig. 5).
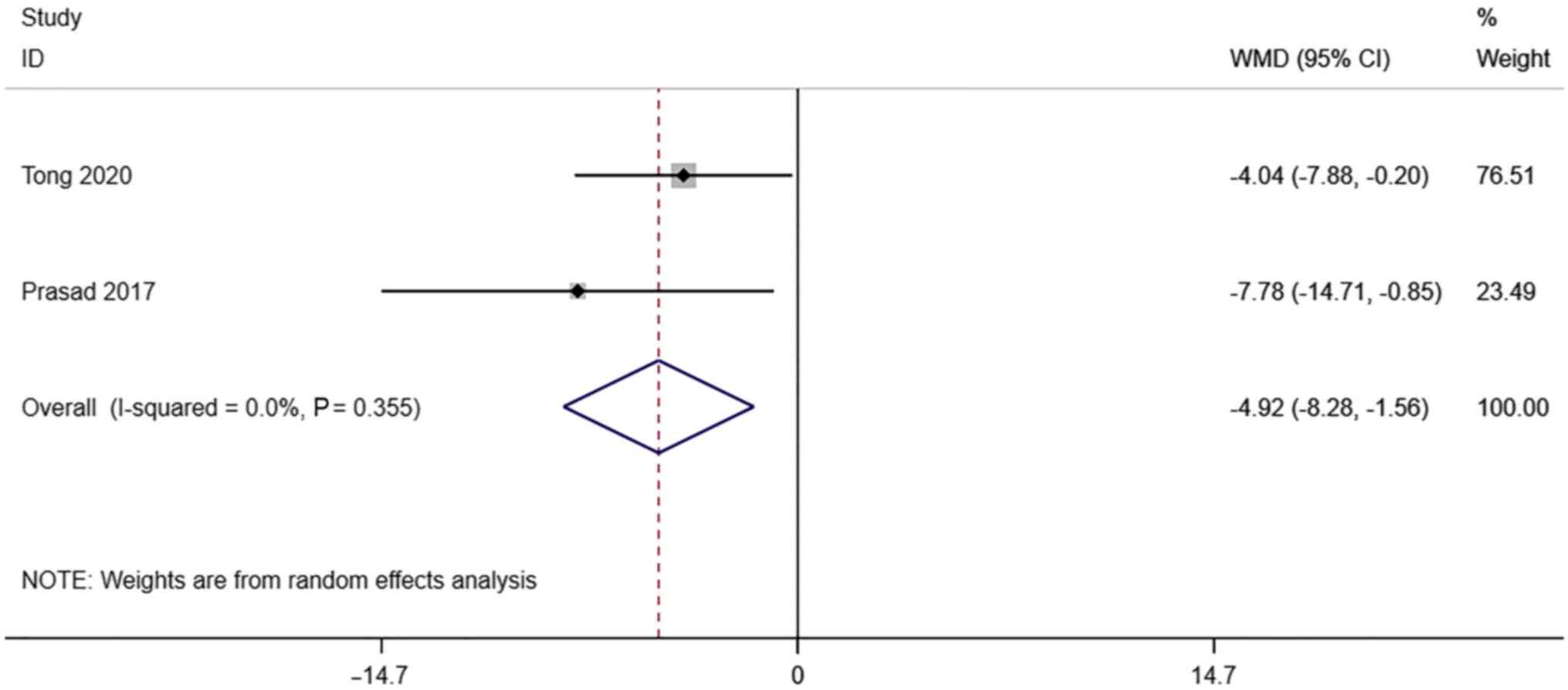
S/D. A total of 2 studies reported
transvaginal ultrasound S/D in infertile women undergoing IVF-ET.
There was no significant heterogeneity (I2=0.0%,
P=0.355). The pooled results of the random-effects model showed
that the S/D of the pregnancy group after receiving IVF-ET was
significantly lower than that of the non-pregnancy (WMD=-4.92, 95%
CI: -8.28- -1.56; P=0.004) (Fig.
6).
VI. A total of 5 studies reported
transvaginal ultrasound VI in infertile women undergoing IVF-ET.
There was no significant heterogeneity (I2=37.9%,
P=0.168). The pooled results of the random-effects model showed
that the VI of the pregnancy group after receiving IVF-ET was
significantly higher than that of the non-pregnancy (WMD=0.79, 95%
CI: 0.35-1.09; P#x003C;0.0001) (Fig.
7).
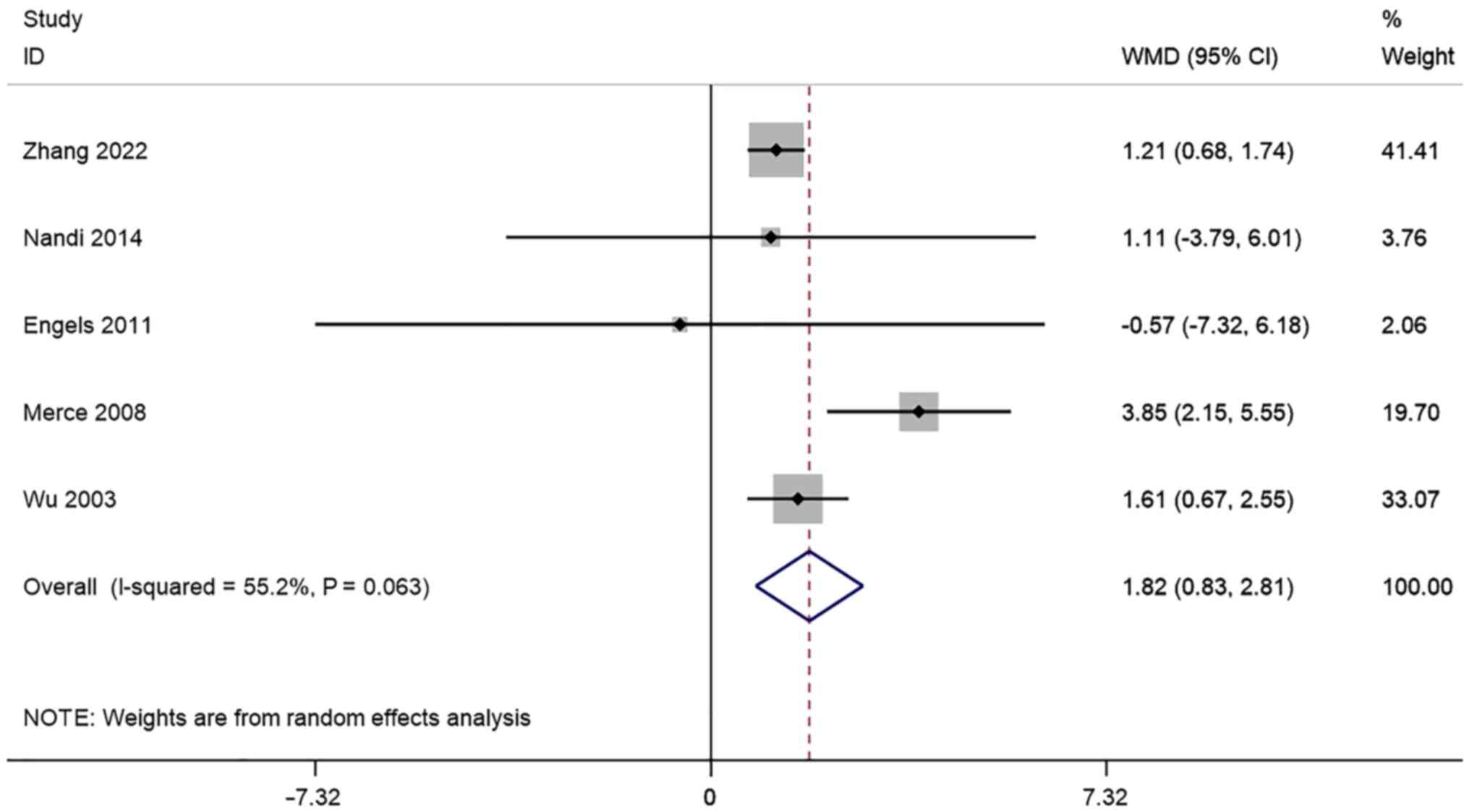
FI. A total of 5 studies reported
transvaginal ultrasound FI in infertile women undergoing IVF-ET.
There was significant heterogeneity (I2=55.2%, P=0.063)
and a meta-analysis was performed using a random effects model. The
pooled results showed that the FI of the Pregnancy group after
receiving IVF-ET was significantly higher than that of the
non-pregnancy (WMD=1.82, 95% CI: 0.83-2.81; P=0.000) (Fig. 8).
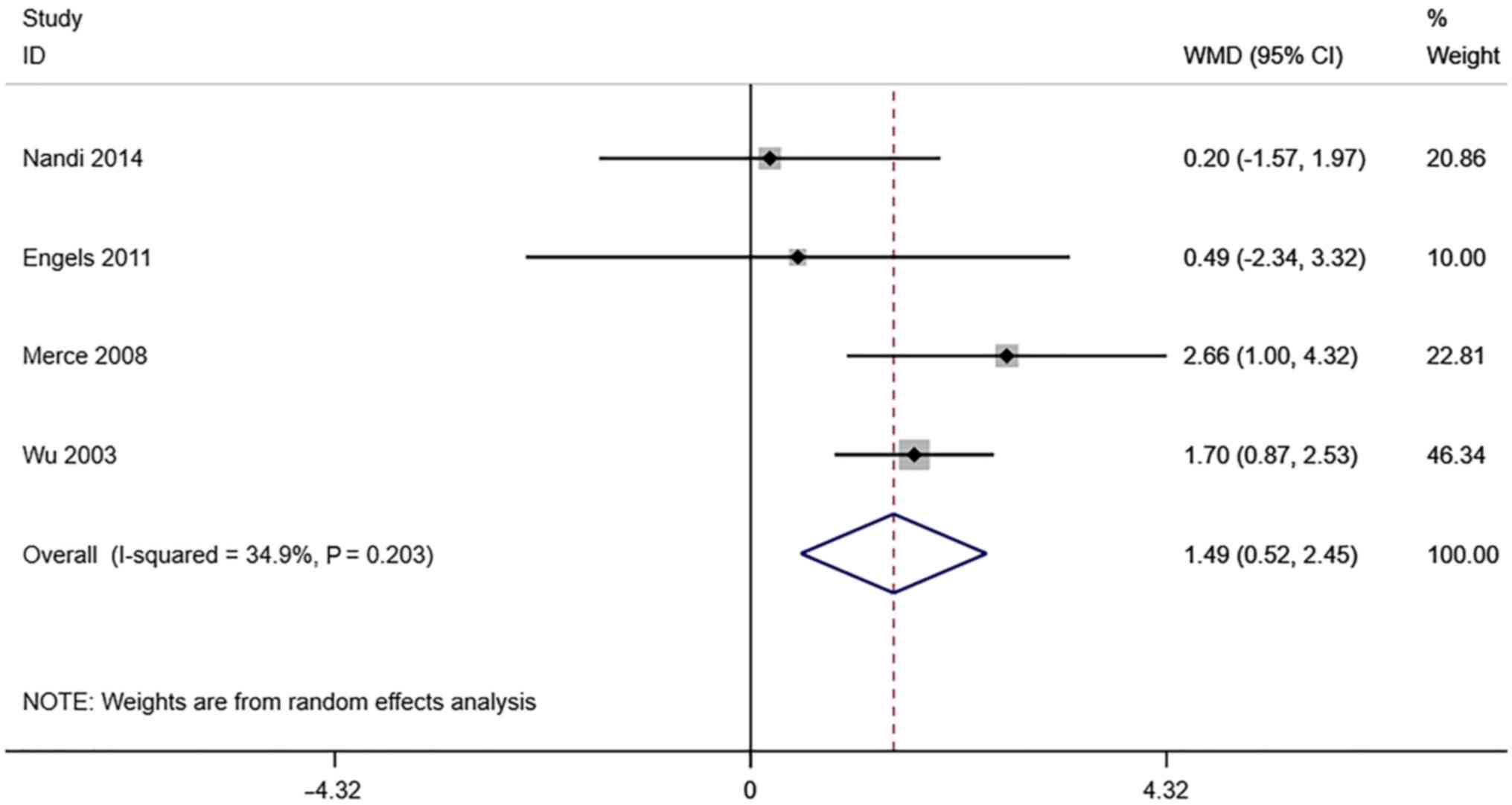
VFI. A total of 4 studies reported
transvaginal ultrasound VFI in infertile women undergoing IVF-ET.
There was no significant heterogeneity (I2=34.9%,
P=0.203). The pooled results of the random-effects model showed
that the VFI of the pregnancy group after receiving IVF-ET was
significantly higher than that of the non-pregnancy (WMD=1.49, 95%
CI: 0.52-2.45; P=0.003) (Fig.
9).
Sensitivity analysis
Sensitivity analysis was performed by eliminating
each included study one by one and performing a summary analysis of
the remaining studies. The results found that none of the studies
had an excessive impact on the results of the meta-analysis, which
suggests that the results of this meta-analysis are stable and
reliable.
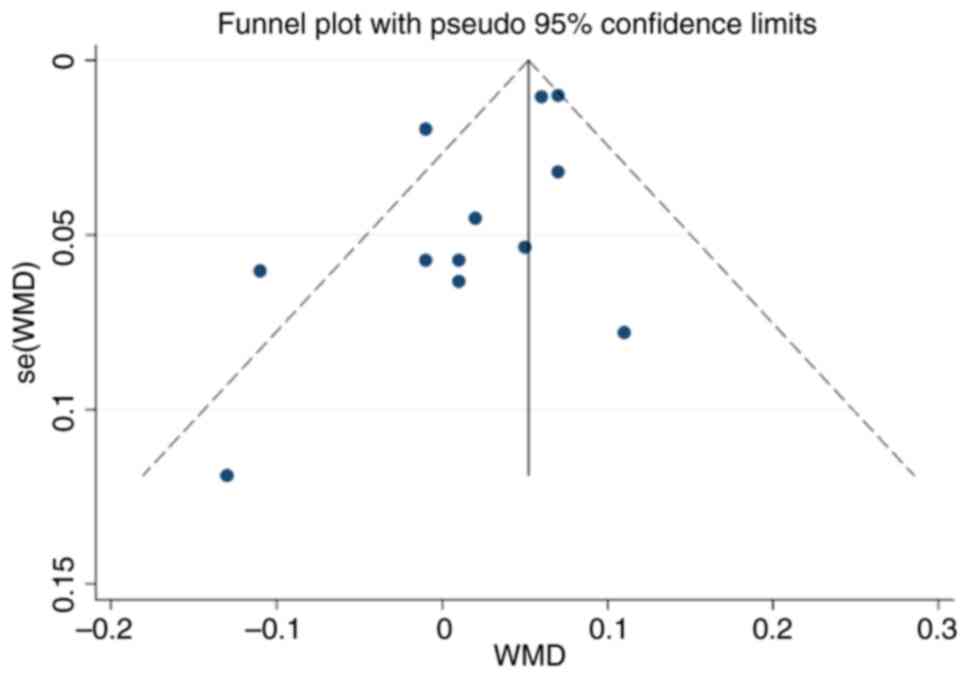
Publication bias
The funnel plot of this study is shown in Fig. 10. The funnel plot was largely
symmetrical, and Egger's test demonstrated P=0.055, which indicated
that there was no obvious publication bias in this study.
Discussion
In recent years, IVF-EF has attracted increasing
attention as an important means of treating infertility, and
endometrial receptivity is one of the important factors affecting
embryo implantation. As a common means of assessing endometrial
receptivity, ultrasound has been widely used to evaluate
endometrial receptivity to predict IVF-ET clinical pregnancy
outcomes given its advantages of being non-invasive, providing
real-time information, the ease of reproducibility, convenience,
and the fact that it is relatively inexpensive. The validity and
accuracy of different endometrial receptivity measures in
predicting clinical pregnancy outcomes are contested due to
inconsistent results in existing clinical studies. The present
meta-analysis included 14 articles for a total of 4,842 infertile
women, to pool the measures of endometrial receptivity on
transvaginal ultrasound, which may be used to predict pregnancy
outcomes following IVF-ET.
The pooled results showed that the endometrial
thickness and endometrial volume of the pregnancy group after
receiving IVF-ET were all significantly higher than that of
non-pregnancy. These results suggest that changes in endometrial
thickness and endometrial volume can be observed by ultrasound to
predict pregnancy outcomes of IVF-ET. In addition, measurements of
the endometrial volume provide a reliable method for assessing the
size of the endometrial cavity; however, its effective use requires
extensive clinical experience and may require multiple attempts
before the test is successfully completed, which may challenge the
accuracy of predicting pregnancy outcomes (30).
Compared with a single uterine spiral artery, the
uterine artery reflects the blood flow perfusion of the entire
uterus, and the uterine artery S/D is not a commonly used
measurement index to assess endometrial receptivity during IVF-ET
and typically requires the assessment of uterine artery PI, RI, and
other indicators for a comprehensive judgment, and measuring
uterine artery PI and RI on IVF-ET days is more useful in
determining whether the endometrium is in a state suitable for
embryo adhesion and completion of implantation (31). The pooled results showed that the
S/D of the pregnancy group after receiving IVF-ET were
significantly lower than that of the non-pregnancy, while the
difference between RI and PI in the pregnancy group and the
non-pregnancy group after receiving IVF-ET was not statistically
significant. In the analysis of S/D, although the pooled results
were consistent with the results of the two included individual
studies, the objectivity of the included studies deserves further
exploration as there were only two included studies. In addition,
pooled results also showed that the VI, FI, and VFI of the
pregnancy group after receiving IVF-ET was significantly higher
than that of the non-pregnancy. This indicates that as a pregnancy
progresses, the number of blood vessels in the endometrium
increases, blood flow increases and blood perfusion increases.
Observation of vascular and blood flow changes can predict
pregnancy outcomes in infertile women undergoing IVF-ET.
The present meta-analysis has some limitations. The
measurement of endometrial receptivity-related indicators by
transvaginal ultrasound will be affected by objective factors such
as the patient being examined, the equipment used, and the
treatment measures. Additionally, the lack of studies for certain
outcomes may result in less reliable results.
In conclusion, vaginal ultrasound may be used to
predict the pregnancy outcomes of infertile women undergoing IVF-ET
by measuring the thickness and volume of the endometrium, combined
with the S/D, VI, FI, and VFI of the uterine artery.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JW wrote the manuscript and analyzed the data. JS,
XW and QW collected data (literature search and data extraction)
and participated in data analysis. QW provided general supervision
of the research group. JW and QW confirm the authenticity of all
the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ahmadi A, Ramazanzadeh R, Derakhshan S,
Khodabandehloo M, Farhadifar F, Roshani D, Mousavi A, Hedayati MA
and Taheri M: Prevalence of Listeria monocytogenes infection in
women with spontaneous abortion, normal delivery, fertile and
infertile. BMC Pregnancy Childbirth. 22(974)2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zhu F, Zhao B, Wu J, Yin S, Ma T, Li Z,
Zhu X, Wang T, Yang B and Che D: Effect of transcutaneous
electrical acupoint stimulation on pregnancy outcomes in women with
in vitro fertilization-embryo transfer: A systematic review and
meta-analysis. Front Cell Dev Biol. 10(1068894)2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wang J, Xia F, Zhou Y, Wei X, Zhuang Y and
Huang Y: Association between endometrial/subendometrial vasculature
and embryo transfer outcome: A meta-analysis and subgroup analysis.
J Ultrasound Med. 37:149–163. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
de Ziegler D, Fanchin R, de Moustier B and
Bulletti C: The hormonal control of endometrial receptivity:
Estrogen (E2) and progesterone. J Reprod Immunol. 39:149–166.
1998.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Liu S, Hong L, Lian R, Xiao S, Li Y, Diao
L and Zeng Y: Transcriptomic analysis reveals endometrial dynamics
in normoweight and overweight/obese polycystic ovary syndrome
women. Front Genet. 13(874487)2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhang D, Xu G, Zhang R, Zhu Y, Gao H, Zhou
C, Sheng J and Huang H: Decreased expression of aquaporin 2 is
associated with impaired endometrial receptivity in controlled
ovarian stimulation. Reprod Fertil Dev. 28:499–506. 2016.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Wang W, Feng D and Ling B: Biologia
Futura: Endometrial microbiome affects endometrial receptivity from
the perspective of the endometrial immune microenvironment. Biol
Futur. 73:291–300. 2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Elsokkary M, Eldin AB, Abdelhafez M, Rateb
A, Samy M, Eldorf A, Islam BA, Raafat TA, Gomaa IA, Taema M, et al:
The reproducibility of the novel utilization of five-dimensional
ultrasound and power Doppler in the prediction of endometrial
receptivity in intracytoplasmic sperm-injected women: A pilot
prospective clinical study. Arch Gynecol Obstet. 299:551–558.
2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhou Q, Yan G, Ding L, Liu J, Yu X, Kong
S, Zhang M, Wang Z, Liu Y, Jiang Y, et al: EHD1 impairs
decidualization by regulating the Wnt4/β-catenin signaling pathway
in recurrent implantation failure. EBioMedicine. 50:343–354.
2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Basatvat S, Russell JM, Saare M, Thurston
LM, Salumets A and Fazeli A: Potential innate immunity-related
markers of endometrial receptivity and recurrent implantation
failure (RIF). Reprod Biol. 21(100569)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Haas J and Casper RF: Observations on
clinical assessment of endometrial receptivity. Fertil Steril.
118:828–831. 2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Cheng F, Xv BM, Liu YL, Sun R, Wang L and
Yi JL: Endometrial microstimulation effects on endometrial
receptivity assessed by transvaginal color Doppler sonography. BMC
Womens Health. 22(508)2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhang CH, Chen C, Wang JR, Wang Y, Wen SX,
Cao YP and Qian WP: An endometrial receptivity scoring system
basing on the endometrial thickness, volume, echo, peristalsis, and
blood flow evaluated by ultrasonography. Front Endocrinol
(Lausanne). 13(907874)2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Cook DA and Reed DA: Appraising the
quality of medical education research methods: The medical
education research study quality instrument and the
Newcastle-Ottawa Scale-education. Acad Med. 90:1067–1076.
2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Bernardo WM: PRISMA statement and
PROSPERO. Int Braz J Urol. 43:383–384. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhang Q, Wang X, Zhang Y, Lu H and Yu Y:
Nomogram prediction for the prediction of clinical pregnancy in
freeze-thawed embryo transfer. BMC Pregnancy Childbirth.
22(629)2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Crosby DA, Glover LE, Downey P, Mooney EE,
McAuliffe FM, O'Farrelly C, Brennan DJ and Wingfield M: Mid-luteal
uterine artery Doppler indices in the prediction of pregnancy
outcome in nulliparous women undergoing assisted reproduction. Hum
Fertil (Camb). 25:670–676. 2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Tong R, Zhou Y, He Q, Zhuang Y, Zhou W and
Xia F: Analysis of the guidance value of 3D ultrasound in
evaluating endometrial receptivity for frozen-thawed embryo
transfer in patients with repeated implantation failure. Ann Transl
Med. 8(944)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Long Y, Liang R and Zhang J, Fang F, Cheng
C, Lu Q and Zhang J: Identification and characterization of uterine
micro-peristalsis in women undergoing in vitro fertilization and
embryo transfer via dynamic ultrasound features. Arch Gynecol
Obstet. 300:1729–1739. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Koo HS, Park CW, Cha SH and Yang KM:
Serial evaluation of endometrial blood flow for prediction of
pregnancy outcomes in patients who underwent controlled ovarian
hyperstimulation and in vitro fertilization and embryo transfer. J
Ultrasound Med. 37:851–857. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Prasad S, Goyal R, Kumar Y, Nayar P,
Hajela S, Kumaran A, Vairagi R and Chauhan S: The relationship
between uterine artery two-dimensional color Doppler measurement
and pregnancy outcome: A prospective observational study. J Reprod
Infertil. 18:251–256. 2017.PubMed/NCBI
|
|
22
|
Son JB, Jeong JE, Joo JK, Na YJ, Kim CW
and Lee KS: Measurement of endometrial and uterine vascularity by
transvaginal ultrasonography in predicting pregnancy outcome during
frozen-thawed embryo transfer cycles. J Obstet Gynaecol Res.
40:1661–1667. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhao J, Zhang Q, Wang Y and Li Y:
Endometrial pattern, thickness and growth in predicting pregnancy
outcome following 3319 IVF cycle. Reprod Biomed Online. 29:291–298.
2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Nandi A, Martins WP, Jayaprakasan K,
Clewes JS, Campbell BK and Raine-Fenning NJ: Assessment of
endometrial and subendometrial blood flow in women undergoing
frozen embryo transfer cycles. Reprod Biomed Online. 28:343–351.
2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Engels V, Sanfrutos L, Pérez-Medina T,
Álvarez P, Zapardiel I, Bueno B, Godoy-Tundidor S and Bajo-Arenas
JM: Evaluation of endometrial and subendometrial vascularization
and endometrial volume by 3-D power Doppler ultrasound and its
relationship with age and pregnancy in intrauterine insemination
cycles. Gynecol Obstet Invest. 72:117–122. 2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zácková T, Järvelä IY, Tapanainen JS and
Feyereisl J: Assessment of endometrial and ovarian characteristics
using three dimensional power Doppler ultrasound to predict
response in frozen embryo transfer cycles. Reprod Biol Endocrinol.
7(151)2009.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Mercé LT, Barco MJ, Bau S and Troyano J:
Are endometrial parameters by three-dimensional ultrasound and
power Doppler angiography related to in vitro fertilization/embryo
transfer outcome? Fertil Steril. 89:111–117. 2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chien LW, Lee WS, Au HK and Tzeng CR:
Assessment of changes in utero-ovarian arterial impedance during
the peri-implantation period by Doppler sonography in women
undergoing assisted reproduction. Ultrasound Obstet Gynecol.
23:496–500. 2004.PubMed/NCBI View
Article : Google Scholar
|
|
29
|
Wu HM, Chiang CH, Huang HY, Chao AS, Wang
HS and Soong YK: Detection of the subendometrial vascularization
flow index by three-dimensional ultrasound may be useful for
predicting the pregnancy rate for patients undergoing in vitro
fertilization-embryo transfer. Fertil Steril. 79:507–511.
2003.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Schild RL, Knobloch C, Dorn C, Fimmers R,
van der Ven H and Hansmann M: Endometrial receptivity in an in
vitro fertilization program as assessed by spiral artery blood
flow, endometrial thickness, endometrial volume, and uterine artery
blood flow. Fertil Steril. 75:361–366. 2001.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Li YW, Liang XW, Fang JH and Chen ZY:
Application of ultrasound markers measured at different time points
of COH cycle in the prediction of ovarian response for
individualised ovulation induction. J Obstet Gynaecol.
42:1467–1473. 2022.PubMed/NCBI View Article : Google Scholar
|