Introduction
Hematopoietic stem cell transplantation (HSCT) is an
effective treatment for hematological tumors. With the development
of transplantation technology, an increasing number of patients
with hematological tumors are undergoing HSCT (1). Cytomegalovirus (CMV) is an
opportunistic virus, and patients after HSCT are the most
susceptible population (2). CMV
infection is one of the most common infectious complications
following HSCT, with an incidence of 5-10% (3). Although great progress has been made
in early diagnosis and treatment, CMV seropositivity is still a
risk factor for non-relapse mortality (4). The spectrum of CMV infection is quite
broad, ranging from asymptomatic viremia to CMV end-organ disease,
such as esophagitis, gastroenteritis, hepatitis, retinitis,
pneumonia and encephalitis (5).
CMV pneumonia represents a severe manifestation of CMV disease,
with a 6-month survival rate of only 30% in patients with HSCT
(6). The treatment of refractory
CMV infection and CMV multiple organ infection remains a major
therapeutic challenge. The current study presents a case of a
patient with CMV infection involving the lung, blood and bladder
after HSCT, with a good subsequent therapeutic effect. The aim of
the study was to report a diagnostic and treatment experience for
the benefit of clinicians managing similar cases, with the ultimate
goal of enhancing the early detection, diagnosis and treatment of
CMV infection after HSCT, so as to reduce organ damage and improve
the survival rate of affected patients.
Case report
A 33-year-old male patient was admitted to The First
Affiliated Hospital of Guangxi Medical University (Nanning, China)
in April 2018 due to a recurrent cough, with sputum, and shortness
of breath after activity for >6 months following HSCT. The
patient had undergone HSCT for acute myeloid leukemia in September
2017. On day 40 after HSCT, the patient developed the
aforementioned symptoms. The recorded arterial oxygen partial
pressure was 82.8 mmHg (normal range, 80-100 mmHg). Treatments
using antibacterial agents (cefoperazone sulbactam, levofloxacin
and meropenem) and antifungal agents (voriconazole and caspofungin)
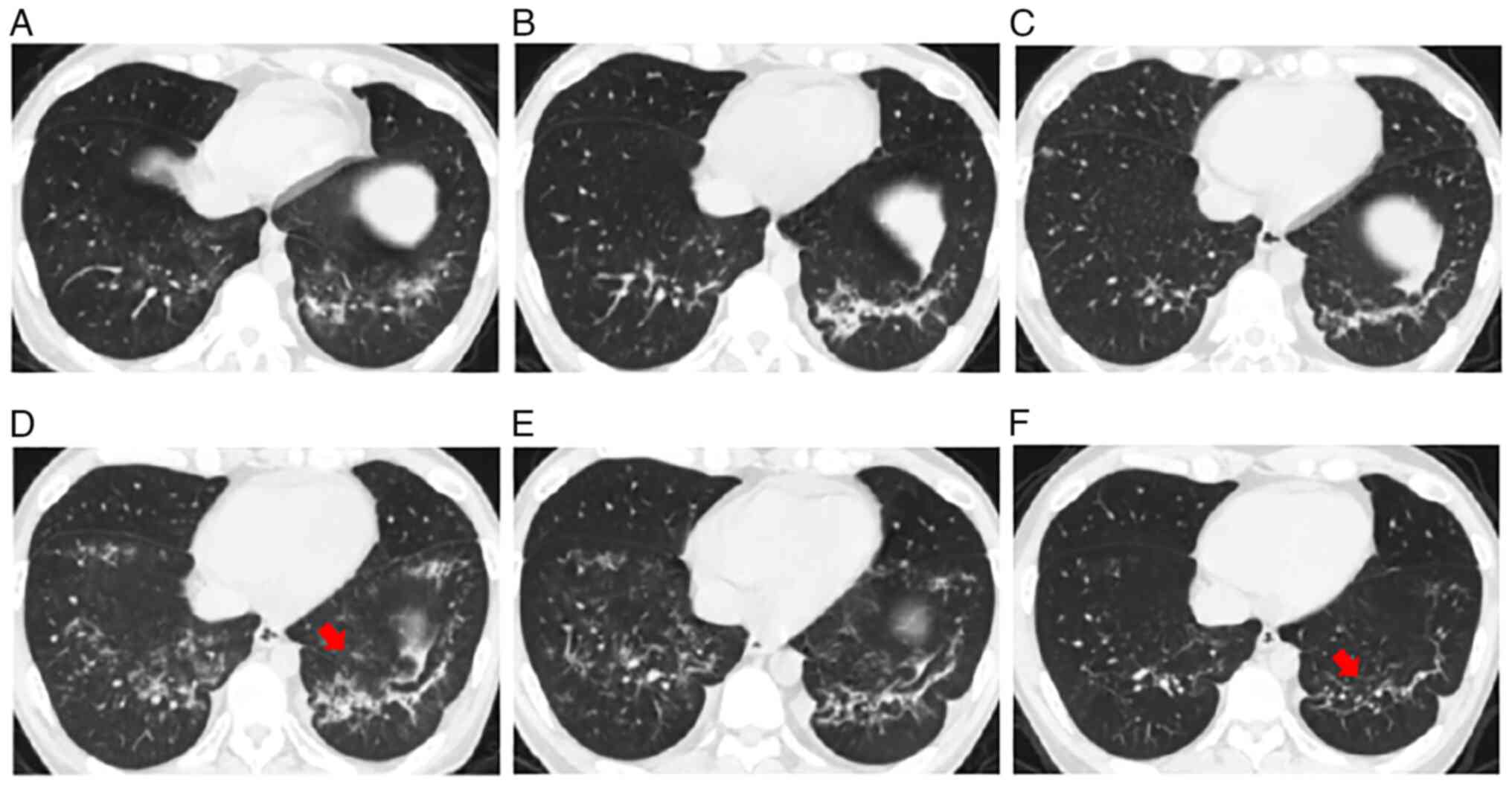
were ineffective. Chest computed tomography (CT) showed a
ground-glass shadow in the lower lung fields of both lungs
(Fig. 1A). The blood CMV DNA count
was 7.4x104 copies/ml (normal range, #x003C;500
copies/ml) according to fluorescence quantitative PCR performed by
the Clinical Laboratory of The First Affiliated Hospital of Guangxi
Medical University (Fig. 2); thus,
the diagnosis of CMV DNAemia was reached. Immediate treatment with
foscarnet (intravenous drip, 6 g every 12 h) and γ-globulin
(intravenous drip, 2.5 g every 12 h) was initiated. However, the
symptoms were not improved after 2 weeks of treatment.
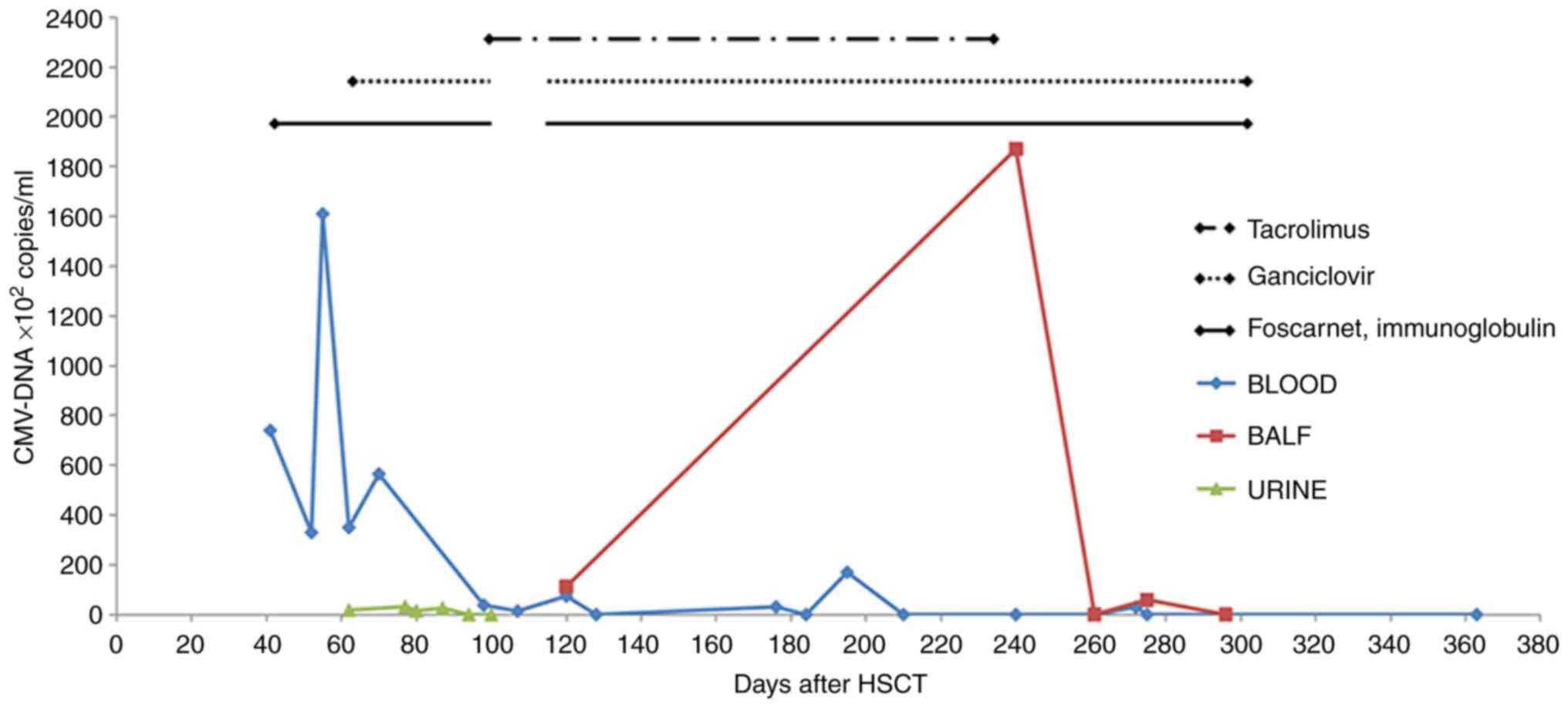 | Figure 2Number of copies of CMV DNA in blood,
BALF and urine, and the timeline for using different drugs. Blood
CMV DNA count was elevated and peaked on the early period after
HSCT, before decreasing to normal on day 103 post-HSCT. BALF CMV
DNA count was positive on day 120 post-HSCT, reached the peak on
day 240 post-HSCT, and finally returned a negative result after
anti-CMV therapy. Urine CMV DNA count was elevated on day 60
post-HSCT, fluctuated and became negative on day 100 post-HSCT. The
treatment time of tacrolimus was from 107 days to 233 days
post-HSCT, and was stopped at 233 days post-HSCT. The treatment
time of ganciclovir was from 62 days to 92 days post-HSCT, was
stopped after a negative result was returned for hematuria CMV DNA,
and the was used again from 102 days to 302 days post-HSCT. The
treatment time of foscarnet and immunoglobulin therapy was from 40
days to 92 days post-HSCT. Treatment stopped after a negative
result was returned for hematuria CMV DNA, and then was used again
from 102 days to 302 days post-HSCT. BALF, bronchoalveolar lavage
fluid; CMV, cytomegalovirus; HSCT, hematopoietic stem cell
transplantation. |
On day 55 after HSCT, the blood CMV DNA level
further increased to 1.61x105 copies/ml and the
respiratory symptoms were not relieved. On day 62 after HSCT, the
patient developed hematuria, and frequent and painful urination.
The CMV DNA level in the urine was 1.8x103 copies/ml
(Fig. 2), and the patient was
diagnosed with CMV cystitis. The aforementioned treatment was
ineffective; therefore, the patient was treated with ganciclovir
(intravenous drip, 0.3 g every 12 h) for 1 month to strengthen the
anti-CMV treatment. On day 98 after HSCT, the urinary symptoms had
improved, but the cough with sputum, and the shortness of breath
after physical activity remained, even though the blood and urine
analyses were now negative for CMV DNA.
On day 107 after HSCT, the patient presented with
multiple rashes on the face and extremities, leading to a diagnosis
of mild graft-versus-host disease of the skin; tacrolimus (oral, 1
mg every 12 h) and mycophenolate mofetil (oral, 0.25 g three times
a day) treatment was administered. On day 120 after HSCT, chest CT
revealed scattered ground-glass and patchy shadow lesions in the
lower lungs, which had progressed compared with the findings on
previous scans (Fig. 1B).
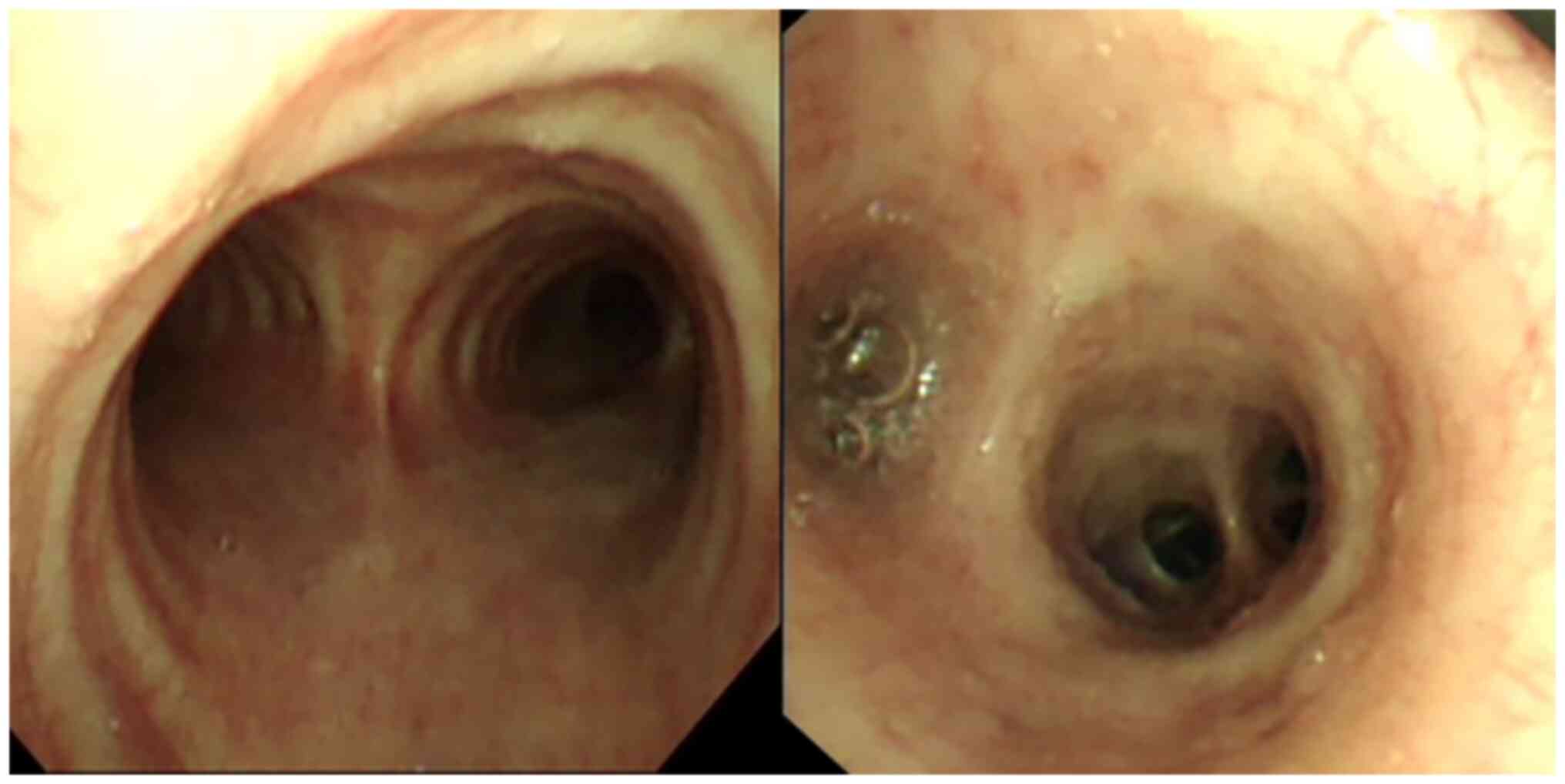
Additionally, bronchoscopy showed mucosal congestion (Fig. 3). BALF culture, (1,3)-β-D-glucan test and galactomannan test
results on BALF samples were normal, with no signs of bacterial or
fungal infection. However, the BALF exhibited CMV DNA levels of
1.12x104 copies/ml (Fig.
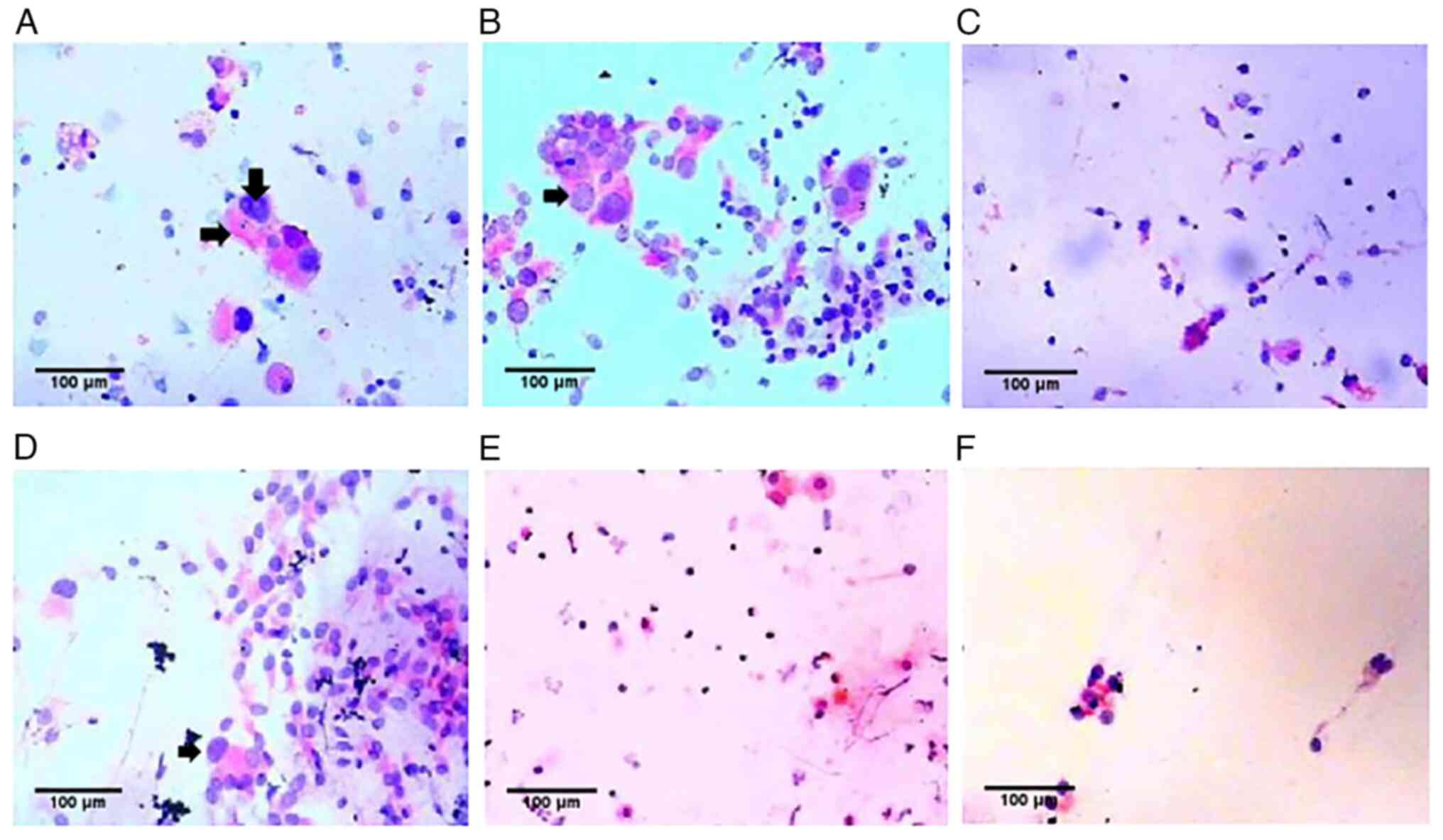
2), but was negative for CMV IgM. Under a microscope, gian T
cells could be observed in the BALF and bronchial brushes, and
their nuclei were enlarged, dark-colored and rich in cytoplasm
(H&E staining; light microscopy; Fig. 4A and B). The clinical diagnosis was therefore
CMV pneumonia and a treatment was again initiated consisting of a
combination of ganciclovir (intravenous drip, 0.3 g every 12 h) and
foscarnet (intravenous drip, 6 g every 12 h), with the addition of
γ-globulin (intravenous drip, 2.5 g every 12 h) to augment immune
function. After 1 month of treatment, although the patient
experienced some relief from the cough with sputum and from the
shortness of breath after activity, these symptoms persisted and
recurred.
On day 233 after HSCT, the patient presented with a
fever and dry cough. Bone marrow examination revealed active
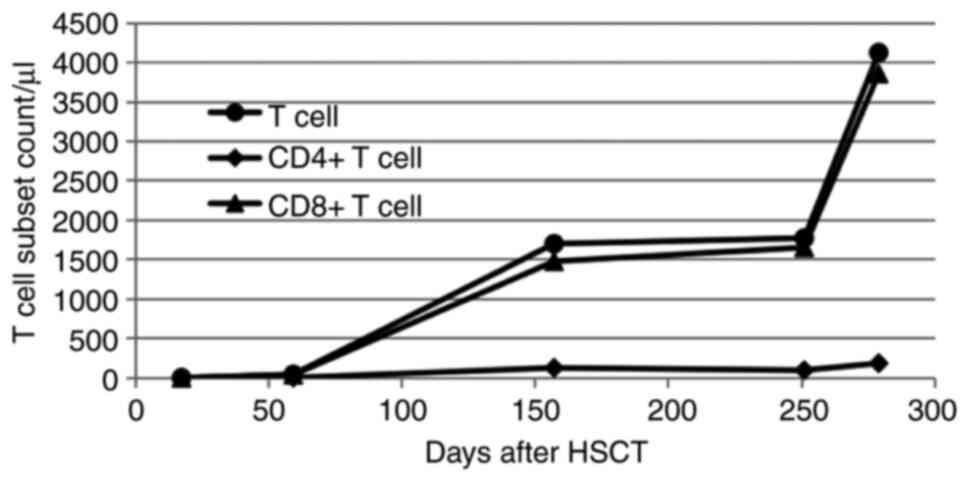
hyperplasia and no evidence of a relapse of the leukemia. The total
T-cell count was 1,774/µl, with CD4+ T cells at 98/µl
and CD8+ T cells at 1,654/µl according to flow cytometry
performed by the Clinical Laboratory of The First Affiliated
Hospital of Guangxi Medical University (Fig. 5). The BALF CMV DNA level was
1.87x105 copies/ml (Fig.
2), and the gian T cells had disappeared from the BALF
(Fig. 4C) but were still apparent
in the bronchial brushes (Fig.
4D). Chest CT showed that the ground-glass and patchy shadows
of both lungs were visible (Fig.
1C). Given the patient's compromised immune system and
subsequent inadequate infection control, the tacrolimus treatment
was discontinued and ganciclovir (intravenous drip, 0.3 g every 12
h) and foscarnet (intravenous drip, 6 g every 12 h), plus
γ-globulin (intravenous drip, 2.5 g every 12 h) therapy was
administered. After 1 month of treatment, the patient exhibited
improvement in the fever and cough symptoms, and subsequently the
number of T lymphocytes increased significantly (Fig. 5).
On day 272 after HSCT, chest CT showed that the
lesion had more lesions (Fig. 1D)
and the BALF CMV DNA level was 5.96x103 copies/ml. On
day 300 after HSCT, after 1 month of intensive antiviral therapy
with ganciclovir, the BALF was negative for CMV DNA, the gian T
cells had disappeared from the BALF (Fig. 4E) and the bronchial brushes
(Fig. 4F), and chest CT revealed
that the lung lesions in both lower lungs had been absorbed
(Fig. 1E). The patient was
discharged from the hospital. At 7 months post-discharge, the
patient had no cough with sputum or shortness of breath, the blood
CMV DNA results were still negative, and there was further lung
lesion uptake on chest CT (Fig.
1F). The patient will be followed up by re-examination every 3
months, and the prognosis is good with no recurrence.
Discussion
Early diagnosis of the present case was challenging
due to the patient's atypical symptoms and multiple organ CMV
infection. The patient in the present study first experienced
shortness of breath, a cough and hypoxemia. Blood CMV DNA testing
was positive, and the patient's lung changes on a chest CT scan
were mild, leading to the diagnosis of CMV DNAemia. As the disease
progressed, hematuria, and frequent and painful urination were
brought on by the CMV infection that had progressed to the bladder.
Additionally, blood and urine tests revealed the presence of CMV
DNA, which is a sign of CMV cystitis. After receiving therapy, the
patient continued to have frequent coughing fits, expectoration and
post-exercise breathlessness. CT showed ground-glass and patchy
shadows in the lungs, and the BALF CMV DNA test was positive. The
condition was clinically determined to be CMV pneumonia in
accordance with the diagnostic standards for CMV pneumonia
following organ donation (7).
Finally, the patient was diagnosed with CMV pneumonia complicated
by CMV DNAemia and CMV cystitis.
During diagnosis and treatment, a notable phenomenon
was found in the present report: The CMV DNA in the blood was not
consistent with that in the BALF. After the patient was treated
with anti-CMV drugs, the blood CMV DNA result became negative.
However, the patient's BALF CMV DNA result was positive. It has
previously been reported that the CMV DNA level in the blood of
patients with CMV pneumonia is inconsistent with that in the BALF,
and that the positive CMV DNA detection rate in the BALF is greater
than that in the blood (8). In the
present case, the patient's blood was negative for CMV DNA after
anti-CMV therapy. However, at that time, the patient still had
respiratory symptoms (cough, expectoration and shortness of
breath), and chest CT showed a small number of ground-glass shadow
in the lungs twice. Since the symptoms and CT findings were
atypical, it was easy to diagnose as a pulmonary bacterial
infection in the clinic. However, there was no significant
improvement after aggressive anti-inflammatory treatment.
Meanwhile, the patient was suffering from CMV cystitis. It was
considered that the CMV had involved the organs and that the lung
anti-inflammation treatment was ineffective, indicating that this
patient may have CMV pneumonia. A bronchoscopy, CMV DNA testing of
the BALF and cytological analyses of the bronchus brushes were
promptly conducted, and CMV pneumonia was finally diagnosed.
Therefore, even if the blood CMV DNA positivity of patients with
HSCT becomes negative after treatment, doctors should be alert to
the possibility of CMV pneumonia when patients have respiratory
symptoms such as a cough, expectoration, shortness of breath and/or
ground-glass shadows on chest CT. In particular, fiberoptic
bronchoscopy, BALF CMV DNA detection and brush cytological
examination should be completed as soon as possible to eliminate
CMV infection. In addition, the present study found that gian T
cell degeneration in the BALF and bronchial brushes could be used
as an index for diagnosis and treatment. It has been reported that
compared with normal cells, bronchial brush cells that are infected
with CMV morphologically undergo gian T cell degeneration, and in
particular, the size of the nucleus increases by 2.61-4.25 times
(9). No CMV inclusion bodies were
found in the present study, but the cells were enlarged, the
heteromorphic cells had large nuclei and the nuclear-cytoplasmic
ratio was increased. Bone marrow examination showed no recurrence
of leukemia. It was considered that the change in cells was caused
by CMV infection. After treatment, reexamination of the BALF and
brush cytology revealed that the gian T cells were gradually
disappearing, whereas chest CT findings and clinical manifestations
were improved.
After the patient was diagnosed, the effect of
treatment was still not good. The following two reasons should be
considered: First, the patient's CMV infection status included CMV
viremia, CMV pneumonia and CMV cystitis. The patient's condition
was complicated, and it was necessary to treat not only the CMV
infection but also the associated complications. The patient
developed CMV anemia 40 days after HSCT. Anti-CMV therapy failed to
treat the CMV in time, which led to CMV pneumonia and CMV cystitis.
The patient had recurrent pneumonia, which was considered to be a
bacterial or fungal infection according to clinical experience;
however, the anti-bacterial and fungal treatment was not effective,
thus CMV infection was considered and relevant examinations were
performed to finally confirm the diagnosis of CMV pneumonia.
Second, after HSCT, the T-cell immune function of the patient had
not fully recovered, and the antiviral treatment effect was of slow
onset. HSCT CMV pneumonia is more likely to occur within 100 days
after HSCT. This is the immune reconstruction period following
HSCT; it takes between 3 months and 1 year for the CD8+
T-cell count to return normal, whereas it takes longer for the
CD4+ T-cell count to return to normal, and even several
years before a low CD4+/CD8+ ratio is
maintained (10). In the present
case, the patient had recurrent episodes of CMV pneumonia and a
long treatment time during antiviral therapy, which was considered
to be related to the decrease of T lymphocyte subsets and low
immunity. It has been reported that the median number of
CD8+ T cells in refractory CMV-infected patients after
HSCT is 371 cells/µl at 1 month after HSCT, and that the median
number of CD4+ T cells is still #x003C;100 cells/µl at 4
months post-HSCT (11). Anti-CMV
therapy relies mainly on T-cell immunity, and tacrolimus inhibits
the proliferation of CD4+ T cells and CD8+ T
cells (12). In the present case,
the patient was administered tacrolimus as anti-rejection treatment
after HSCT. Meanwhile, the early immunity did not fully recover,
and the number of CD4+ T cells and CD8+ T
cells was low, resulting in CMV pneumonia and CMV cystitis. After
stopping the tacrolimus, the patient's T cell count, especially the
CD8+ T cell count, increased markedly, and immunity
gradually recovered. The effect of the antiviral treatment on the
CMV pneumonia was significantly improved.
It is difficult to treat CMV pneumonia complicated
with CMV hematuria and CMV cystitis after HSCT. According to the
therapeutic effect on the current patient, a suitable treatment
plan was proffered. The guidelines for the management of CMV
infection after SCT from the 2017 European Leukemia Infection
Conference indicated that the first-line treatment for CMV
infection after HSCT is intravenous injections of ganciclovir or
foscarnet (3). Immunoglobulin can
be added in the treatment of CMV pneumonia, although it is not
recommended to add immunoglobulin in the treatment of any other CMV
diseases except pneumonia. The indicated second-line therapy is
cidofovir or ganciclovir and foscarnet (3). When the current patient was diagnosed
with CMV anemia and CMV cystitis, foscarnet sodium was chosen as
the first-line drug according to the guidelines. Furthermore,
immunoglobulin was added. Intravenous propionic immunoglobulin
contains a high dose of anti-CMV IgG antibodies that specifically
bind and kill CMV, and enhance immune persistence with a median
half-life of 51.2 days (13). The
role of immunoglobulin in preventing CMV infection is
controversial. A study previously showed that immunoglobulin not
only had no preventive function in CMV diseases, but that it also
led to interstitial pneumonia (14). In addition, it was also reported
that immunoglobulin had a good clinical therapeutic effect in the
treatment of CMV diseases (15).
In the present study, after 1 month of treatment with ganciclovir
and foscarnet combined with immunoglobulin, the patient's blood and
urine were negative for CMV DNA. The notable feature of this
patient's treatment was that when a diagnosis of CMV pneumonia was
made, ganciclovir and foscarnet combined with immunoglobulin were
chosen for treatment, but the treatment effect was poor. This may
be due to two main reasons: First, the patient developed
graft-versus-host disease after HSCT and had been treated with
tacrolimus; tacrolimus can inhibit T-cell proliferation and
reconstruction (16). Second, the
T-cell immune function of the patient after HSCT had not been fully
recovered. The patient was diagnosed with CMV pneumonia 120 days
after HSCT. At this time, the patient's immunity was being rebuilt,
the number of T cells was small and the immune function was
compromised. This led to the poor anti-CMV efficacy of ganciclovir
and foscarnet sodium combined with immunoglobulin. It was observed
that the number of T cells in the patient increased markedly 250
days after transplantation once tacrolimus was stopped. At this
time, the immune function recovered, and the anti-CMV effect of the
ganciclovir and foscarnet sodium combined with immunoglobulin was
notable. The patient's CMV DNA test was negative, and chest CT
showed that the lung lesions had been absorbed; therefore, the
patient was considered cured and discharged.
When the patient was diagnosed with CMV infection,
treatment with foscarnet and immunoglobulin was implemented, but
the symptoms were still apparent. After adding ganciclovir, the
antiviral effect was marked. We suggest that ganciclovir combined
with foscarnet should be used as soon as possible to strengthen the
anti-CMV treatment and immunoglobulin to improve immunity when the
anti-CMV effect is not good. Therefore, we recommend that
ganciclovir and foscarnet combined with immunoglobulin should be
used as the first-line treatment for HSCT CMV multi-organ
infection.
In summary, it took a long time to diagnose and
treat the current patient, although he was eventually cured. The
CMV DNA in the blood was inconsistent with that in the BALF, which
delayed the early diagnosis of CMV pneumonia. Attention should be
focused on the early diagnosis of CMV infection. When CMV pneumonia
cannot be completely ruled out in patients with HSCT, it is crucial
to conduct a pathogenic examination, CMV DNA detection and
cytological examination of the BALF. After treatment in the present
case, the gian T cells in the alveolar lavage fluid gradually
disappeared and the brush cytology returned to normal, suggesting
that gian T cell degeneration can serve as a diagnostic and
therapeutic index. In this case, the course of the disease was
long, and the anti-CMV treatment effect was not good. It was
considered that the immune function of the T cells after
transplantation had not been fully recovered, and that the
immunosuppression was caused by the use of tacrolimus. Therefore,
we propose a plan for treatment standardization. In the treatment
of patients with CMV multiple organ infection after HSCT, attention
should be focused on the side effects of tacrolimus, and
ganciclovir and foscarnet combined with immunoglobulin should be
used as soon as possible.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by grants from The Natural
Science Foundation of China (no. 81360012), The Guangxi Natural
Science Foundation of China (no. 2016GXNSFAA380269) and The Guangxi
Education Science Planning Project of China (no. YCSW2019116).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QiL and QuL made important contributions in
designing this study, acquiring and analyzing the data, and writing
the manuscript. RM, QX and YZ participated in the interpretation of
the data. CW, YW and KH participated in collecting the data and
analyzing patient data. ML made substantial contributions in
conceptualizing and designing the study, in acquiring and analyzing
the data, and in critically revising the manuscript. All authors
contributed to the writing of the final manuscript. QiL, QuL, RM,
QX, YZ, CW, YW, KH and ML confirm the authenticity of all the raw
data. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee
associated with the Faculty of Medicine at the First Affiliated
Hospital of Guangxi Medical University (approval no. 2023-E379-01;
Nanning, China).
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of this report and any accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gratwohl A, Baldomero H, Aljurf M,
Pasquini MC, Bouzas LF, Yoshimi A, Szer J, Lipton J, Schwendener A,
Gratwohl M, et al: Worldwide network of blood and marrow
transplantation. Hematopoietic stem cell transplantation: A global
perspective. JAMA. 303:1617–1624. 2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Moss P and Rickinson A: Cellular
immunotherapy for viral infection after HSC transplantation. Nat
Rev Immunol. 5:9–20. 2005.PubMed/NCBI View
Article : Google Scholar
|
|
3
|
Ljungman P, de la Camara R, Robin C,
Crocchiolo R, Einsele H, Hill JA, Hubacek P, Navarro D, Cordonnier
C and Ward KN: 2017 European Conference on Infections in Leukaemia
group. Guidelines for the management of cytomegalovirus infection
in patients with haematological malignancies and after stem cell
transplantation from the 2017 European conference on infections in
leukaemia (ECIL 7). Lancet Infect Dis. 19:e260–e272.
2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Teira P, Battiwalla M, Ramanathan M,
Barrett AJ, Ahn KW, Chen M, Green JS, Saad A, Antin JH, Savani BN,
et al: Early cytomegalovirus reactivation remains associated with
increased transplant-related mortality in the current era: A CIBMTR
analysis. Blood. 127:2427–2438. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhou X, Jin N and Chen B: Human
cytomegalovirus infection: A considerable issue following
allogeneic hematopoietic stem cell transplantation. Oncol Lett.
21(318)2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Erard V, Guthrie KA, Seo S, Smith J, Huang
M, Chien J, Flowers ME, Corey L and Boeckh M: Reduced mortality of
cytomegalovirus pneumonia after hematopoietic cell transplantation
due to antiviral therapy and changes in transplantation practices.
Clin Infect Dis. 61:31–39. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ljungman P, Boeckh M, Hirsch HH, Josephson
F, Lundgren J, Nichols G, Pikis A, Razonable RR, Miller V and
Griffiths PD: Disease Definitions Working Group of the
Cytomegalovirus Drug Development Forum. Definitions of
cytomegalovirus infection and disease in transplant patients for
use in clinical trials. Clin Infect Dis. 64:87–91. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Rozy A, Duk K, Szumna B, Skronska P,
Gawryluk D and Chorostowska-Wynimko J: Effectiveness of PCR and
immunofluorescence techniques for detecting human cytomegalovirus
in blood and bronchoalveolar lavage fluid. Adv Exp Med Biol.
921:21–26. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Seok JY, An J, Ha SY, Chung DH, Lee S and
Kim H: Morphologic analysis of cytomegalovirus infected cells in
bronchial washing cytology: Comparison of liquid-based preparation
and conventional smear. J Pathol Transl Med. 50:147–154.
2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Elfeky R, Lazareva A, Qasim W and Veys P:
Immune reconstitution following hematopoietic stem cell
transplantation using different stem cell sources. Expert Rev Clin
Immunol. 15:735–751. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Alexandersson A, Koskenvuo M, Tiderman A,
Lääperi M, Huttunen P, Saarinen-Pihkala U, Anttila VJ,
Lautenschlager I and Taskinen M: Viral infections and immune
reconstitution interaction after pediatric allogenic hematopoietic
stem cell transplantation. Infect Dis (Lond). 51:772–778.
2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Li Y, Guptill JT, Russo MA, Massey JM,
Juel VC, Hobson-Webb LD, Howard JF, Chopra M, Liu W and Yi JS:
Tacrolimus inhibits Th1 and Th17 responses in MuSK-antibody
positive myasthenia gravis patients. Exp Neurol. 312:43–50.
2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Melamed IR, Borte M, Trawnicek L,
Kobayashi AL, Kobayashi RH, Knutsen A, Gupta S, Smits W,
Pituch-Noworolska A and Strach M: Pharmacokinetics of a novel human
intravenous immunoglobulin 10% in patients with primary
immunodeficiency diseases: Analysis of a phase III, multicentre,
prospective, open-label study. Eur J Pharm Sci. 118:80–86.
2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Alexander BT, Hladnik LM, Augustin KM,
Casabar E, McKinnon PS, Reichley RM, Ritchie DJ, Westervelt P and
Dubberke ER: Use of cytomegalovirus intravenous immune globulin for
the adjunctive treatment of cytomegalovirus in hematopoietic stem
cell transplant recipients. Pharmacotherapy. 30:554–561.
2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Malagola M, Greco R, Santarone S, Natale
A, Iori AP, Quatrocchi L, Barbieri W, Bruzzese A, Leotta S, Carotti
A, et al: CMV management with specific immunoglobulins: A
multicentric retrospective analysis on 92 allotransplanted
patients. Mediterr J Hematol Infect Dis.
11(e2019048)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Törlén J, Gaballa A, Remberger M, Mörk LM,
Sundberg B, Mattsson J and Uhlin M: Effect of graft-versus-host
disease prophylaxis regimens on T and B cell reconstitution after
allogeneic hematopoietic stem cell transplantation. Biol Blood
Marrow Transplant. 25:1260–1268. 2019.PubMed/NCBI View Article : Google Scholar
|