Introduction
Traumatic brain injury (TBI), caused by exposure to
explosions (blast TBI), is common among war veterans who have
served in war zones during their active duty. Some service members
face the aftermath of the explosions instantaneously. However, the
majority of veterans face the consequences further into their
veteran status. The deleterious effects of blast TBI on mental
health conditions in war veterans are becoming a matter of
significant concern (1,2). It is established that blast TBIs may
have long-term adverse effects on normal brain function, increasing
the risk of memory loss, inducing post-traumatic stress disorder
and reducing the quality of life for returning war veterans. This
is particularly the case for those who served in Operation Enduring
Freedom/Operation Iraqi Freedom/Operation New Dawn as these war
veterans were exposed to blast TBI more frequently in the combat
war zone (1,2). Although the cause of the TBI may have
occurred in the past, the possibility of blood brain barrier (BBB)
leakage persists, thus, exacerbating mental health conditions
further (for example, memory loss or reduced cognitive
function).
BBB disruption is a major pathophysiological feature
of TBI and contributes to brain edema, structural protein breakdown
and cell death. BBB leakage is also one of the major secondary
effects after a blast that has a long-term effect on the brain
(3,4). By contrast, it has also been
demonstrated that transient and size-selective modulation of the
BBB increases the movement of water from the brain parenchyma to
blood vessels, leading to a decrease in the swelling of the brain
(5). TBI can elicit a series of
secondary injuries after the initial trauma, which is mainly
mediated by microglial activation, resulting in the release of
inflammatory molecules to induce BBB leakage (6-8).
The consequent damage is critical and poses a major risk factor for
high mortality or permanent cognitive dysfunction. The BBB
maintains brain homeostasis by facilitating nutrient delivery
whilst prohibiting the entry of toxic molecules and peripheral
immune cells, thereby acting as a solute exchange barrier between
the blood and the brain. The BBB is primarily comprised of brain
microvascular endothelial cells (BMVECs) along with astrocytes,
pericytes, microglia and basal membrane. Together, these cells and
the extracellular matrix form the neurovascular unit which acts as
a dynamic tissue barrier that selectively controls BBB permeability
(9,10). All structural components in this
neurovascular unit perform specific functions in the central
nervous system (CNS) to maintain normal brain homeostasis (11-13).
Typically, the BBB serves as a gatekeeper in the CNS and
contributes a significant physiological role in permeability, ion
balance and nutrient transport. The monolayer of BMVECs is pivotal
in maintaining BBB function in the brain. Furthermore, the BBB is
regulated by various types of tight junctions (TJs), such as zonula
occludens (ZO) 1, ZO2, ZO3, occludin and claudins, which are
present on cerebral endothelial cells and form transmembrane and
cytosolic TJ-associated protein complexes (14,15).
Following TBI, the breakdown of TJ proteins in the BBB endothelium
causes leakage and increased permeability, contributing to
cytotoxicity and neuronal damage (15). Therefore, BMVECs (BMVECs) are
particularly important cell types in the microvasculature that are
implicated in TBI-induced BBB disruption. This is a primary reason
for their selection in the present study. Glial cells are another
cell type in the neurovascular unit and are also major contributors
to BBB disruption. Following activation, glial cells secrete an
array of chemokines and cytokines, triggering microvascular
remodeling and immune activation, thus resulting in inflammation
and cellular apoptosis (7,16-19).
Taken together, the adverse remodeling of human (h)BMVECs is highly
likely to be involved in the impairment of the neurovascular unit.
Since the BBB lies in an intricate network among hBMVECs and other
cerebral cell types, it is also likely to be involved in the immune
and inflammatory responses that occur following TBI. Previous
studies have attempted to alleviate the secondary injury caused by
BBB disruption in TBI or cerebral ischemia models by administering
mesenchymal stem cells; however, these studies have demonstrated
limited potential (20,21). The underlying mechanism of BBB
damage after TBI remains to be fully elucidated and there is an
urgent need to discover novel therapeutic strategies for the
treatment of TBI.
The neurotrophic factor neuregulin-1 (Nrg1)
regulates a wide variety of functions, including ensheathment and
myelination in neurons and glia (22). Nrg1 is an endogenous growth factor
that is encoded by four genes, Nrg1, Nrg2, Nrg3 and Nrg4. The
neuroprotective effect of Nrg1 has been proposed to be associated
with its anti-inflammatory action in murine brain ischemia models
(23-25).
Nrg1 signals are transduced through the ErbB family of receptor
protein tyrosine kinases, namely ErbB1 (HER1)-ErbB4 (HER4). It has
been demonstrated that the binding of Nrg1 to ErbB3 or ErbB4
results in the phosphorylation and dimerization of the ErbB
receptors (26). Furthermore, it
has been shown that the Nrg1/ErbB4 pathway can regulate visual
cortical plasticity in a Cre recombinase parvalbumin expressing
neuron knock-in mouse model (27).
Although Nrg1 has been characterized extensively in terms of
cardiac pathophysiology (28), the
role of Nrg1 signaling in the BBB remains elusive. Thymosin β4
(Tβ4) is a major G-actin sequestering molecule that has been
observed to serve various biological functions due to its
actin-binding properties (29,30).
Previous studies have demonstrated that Tβ4 treatment results in
myocardial damage repair, and that Tβ4 promotes cardiomyocyte
survival (31,32), reduces inflammation, stimulates
angiogenesis (30-32),
accelerates wound healing (33)
and attenuates oxidative stress (34). This suggests that Tβ4 can exert a
diverse range of physiological and pathological functions.
Previously, Tβ4 and its bioactive peptide, Ac-SDKP, have been found
to mediate neuronal protection and improve neurological functions
in a TBI rat model (35-38).
However, the role of Tβ4 in BBB function repair remains poorly
understood.
In the present study, the hypothesis that Tβ4 may
mitigate lipopolysaccharide (LPS)-induced neurovascular remodeling
in human BMVEC (hBMVECs) was investigated. Bacterial LPS is a
bacterial endotoxin that is a potent stimulus of inflammatory
molecule release and has been shown to affect the permeability and
transport physiology of the BBB (39). Therefore, LPS-induced alterations
in the expression of TJ proteins, production of inflammatory
molecules/cytokines, apoptosis, and Nrg1 and vascular gene
expression in hBMVECs were all examined in the present study.
To the best of our knowledge, the present study is
the first to report that LPS-induced hBMVEC remodeling is
associated with enhanced permeability, downregulation of TJ gene
expression, enhanced inflammatory response, NF-κB activation and
downregulation of Nrg1 expression. However, all of these
alterations were restored in LPS-stimulated hBMVECs following
pretreatment with Tβ4. These data suggest that Tβ4 and Nrg1 can
serve as targets for BBB protection against LPS stimulus, which may
offer a promising therapeutic intervention method for TBI
sequelae.
Materials and methods
Cell culture and treatment
hBMVECs (passage 3; cat no. ACBRI 376) were
purchased from Cell Systems and cultured per the manufacturers'
protocol using Complete Classic Medium with serum and CultureBoost™
(cat. no. 4Z0-500; Cell Systems; AnaBios) and Passage Reagent
Group™ (cat. no. 4Z0-800; Cell Systems; AnaBios) as per their
associated protocols. Passages 6-8 were used for all experiments.
Cells were allowed 48 h for proliferation at 37˚C in 5%
CO2 incubator to a field density of 2.5x105
cells/cm2 prior to serum starvation for 2 h with a
Complete Serum-Free Medium Kit with RocketFuel™ (cat. no.
SF-4Z0-500; Cell Systems; AnaBios). The cells were then pretreated
with Tβ4 (MilliporeSigma) at a concentration of 1 µg/ml or Nrg1
antibody (cat. no. ab191139; Abcam,) at a concentration of 2.5
µg/ml for 2 h prior to 100 ng/ml LPS (cat. no. L4391;
Sigma-Aldrich) stimulation at 37˚C for 24 h. Three separate
experimental groups were used for gene expression studies: Control,
LPS and Tβ4 + LPS. For western blotting and immunofluorescence
analyses, an extra group, Tβ4, was used. The control cells were
treated with PBS. This dose of Tβ4 was based on previously
published articles (37,38). Furthermore, similar doses were used
by other investigators in experiments with HUVECs and hiPSC-ECs
(40,41). The dose did not show any toxic
effect or damage to the cells. The dose for Nrg1 antibody (2.5
µg/ml) was published by Liu et al (42). The present study was conducted
using a research protocol approved by the Research &
Development Committee of the Central Texas Veterans Health Care
System (Waco, USA), that includes the Institutional Biosafety
Committee and the Institutional Review Board (approval no. 00711).
The Research and Development Committee is also the human research
ethics oversight committee of the institution.
RNA isolation and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA from the hBMVECs was extracted using the
RNEasy kit (Qiagen, Inc.) according to the manufacturer's
instructions. For RT, 200 ng total RNA was reverse transcribed into
cDNA using a cDNA synthesis kit (OriGene Technologies, Inc.)
following the manufacturer's instructions. qPCR was performed as
previously described (37). Change
in gene expression was evaluated using the 2-ΔΔCq method
(43). All experiments and
reactions were performed in triplicate with GAPDH used as the
internal reference. The gene specific primers used for the present
study were purchased from OriGene Technologies, Inc. The sequences
for all primers are listed in Table
I.
 | Table IPrimers used for quantitative
PCR. |
Table I
Primers used for quantitative
PCR.
| Gene name | Cat. no. | Sequence |
|---|
| ZO1 | HP206807 | FP:
GTCCAGAATCTCGGAAAAGTGCC |
| | | RP:
CTTTCAGCGCACCATACCAACC |
| ZO2 | HP208078 | FP:
ATTAGTGCGGGAGGATGCCGTT |
| | | RP:
TCTGCCACAAGCCAGGATGTCT |
| Occludin | HP206202 | FP:
ATGGCAAAGTGAATGACAAGCGG |
| | | RP:
CTGTAACGAGGCTGCCTGAAGT |
| Claudin 5 | HP206822 | FP:
ATGTGGCAGGTGACCGCCTTC |
| | | RP:
CGAGTCGTACACTTTGCACTGC |
| IL-6 | HP200567 | FP:
AGACAGCCACTCACCTCTTCAG |
| | | RP:
TTCTGCCAGTGCCTCTTTGCTG |
| TNF-α | HP200561 | FP:
CTCTTCTGCCTGCTGCACTTTG |
| | | RP:
ATGGGCTACAGGCTTGTCACTC |
| IL-1β | HP200544 | FP:
CCACAGACCTTCCAGGAGAATG |
| | | RP:
GTGCAGTTCAGTGATCGTACAGG |
| ICAM1 | HP200186 | FP:
AGCGGCTGACGTGTGCAGTAAT |
| | | RP:
TCTGAGACCTCTGGCTTCGTCA |
| VCAM1 | HP230503 | FP:
GATTCTGTGCCCACAGTAAGGC |
| | | RP:
TGGTCACAGAGCCACCTTCTTG |
| Caspase-3 | HP207674 | FP:
GGAAGCGAATCAATGGACTCTGG |
| | | RP:
GCATCGACATCTGTACCAGACC |
| Bcl-2 | HP200598 | FP:
ATCGCCCTGTGGATGACTGAGT |
| | | RP:
GCCAGGAGAAATCAAACAGAGGC |
| Nrg1 | HP228585 | FP:
GATTCCTACCGAGACTCTCCTC |
| | | RP:
TGGAAGGCATGGACACCGTCAT |
| GADPH | HP205798 | FP:
GTCTCCTCTGACTTCAACAGCG |
| | | RP:
ACCACCCTGTTGCTGTAGCCAA |
Immunofluorescence microscopy
The hBMVECs were seeded in 6-well plates coated with
attachment factor (cat. no. 4Z0 210; Cell Systems; AnaBios) and
coverslips were placed over each well. The cells were treated as
aforementioned in the Cell culture and treatment subsection. Cells
were washed with 1X PBS, fixed in 4% paraformaldehyde at room
temperature for 20 min, before being washed and permeabilized in
0.1% Triton X-100 in PBS. The cells were then blocked with 5%
Blocker BSA (Thermo Fisher Scientific, Inc.) for 60 min at room
temperature, before incubation with anti-CD31 (cat. no. 3528; Cell
Signaling Technology, Inc.; 1:800), anti-ZO3 (cat. no. 3704; clone
no. D57G7 XP; Cell Signaling Technology, Inc.; 1:1,600),
anti-occludin (cat. no. 91131; clone no. E6B4R; Cell Signaling
Technology, Inc.; 1:400) or anti-p65 (cat. no. 8242; clone no.
D14E12; Cell Signaling Technology, Inc.; 1:200) antibodies
overnight at 4˚C, in an antibody dilution buffer (1% Blocker BSA in
PBS and 0.01% Triton X-100). The cells were then washed three times
with 1X PBS and then incubated in the dark with the corresponding
HRP-linked anti-rabbit secondary antibodies (cat. no. 7074; Cell
Signaling Technology, Inc.; 1:1,000) for 1 h at room temperature.
After this incubation, the cells were again washed three times with
1X PBS. A cover glass was mounted on the slide containing a drop of
ProLong Gold Anti-fade mounting media containing DAPI (cat. no.
P36962; Thermo Fisher Scientific, Inc.) at room temperature for 15
min. Fluorescent images at x20 magnification were captured using a
Leica DMi8 inverted LED fluorescence motorized microscope. Anti-ZO3
(cat. no. 3704), anti-occludin (cat. no. 91131), anti-p65 (cat. no.
8242) and DAPI (cat. no. 4083) were purchased from Cell Signaling
Technology, Inc. Immunofluorescence-positive cells were evaluated
in 5-7 separate field images in all groups. The degradation and
restoration of ZO1, ZO3 and occludin immunofluorescence-positive
cells were counted based on immunofluorescence intensity.
Western blotting
The protein lysates were prepared from 3-5
independent cell culture preparations from each group. Cells
homogenized with cell lysis buffer (cat. no. 9803; Cell Signaling
Technology, Inc.) containing protease inhibitors were centrifuged
at 5,200 x g at 4˚C for 15 min. Protein concentration estimation
was conducted using the Bradford assay (Bio-Rad Laboratories,
Inc.). Western blotting experiments were performed as described
previously (37), with minor
modifications. A stain-free 10-15% gradient Tris-Glycine eXtended™
gel (Bio-Rad Laboratories, Inc.) was used for protein separation.
In total, 40 and 80 µg cell lysates were used for occludin and
caspase-3 analyses, respectively. After running the gel, the
protein was transferred onto a PVDF membrane using the Trans Blot
Turbo transfer system (Bio-Rad Laboratories, Inc.) according to the
manufacturer's protocol. The membrane was then blocked using
EveryBlot Blocking Buffer (cat. no. 12010020; Bio-Rad Laboratories,
Inc.) for 5 min at room temperature. Images were captured at each
step using the ChemiDoc MP imaging system (Bio-Rad Laboratories,
Inc.). Primary antibodies against occludin (cat. no. 91131) and
cleaved caspase-3 (cat. no. 9664) were purchased from Cell
Signaling Technology, Inc. Both occludin and cleaved caspase-3
antibodies were incubated with the membranes overnight with at 4˚C
at 1:1,000 dilutions. The corresponding HRP-conjugated secondary
antibody (cat. no. 7074) used for the final immunoblotting process
was purchased from Cell Signaling Technology, Inc. The dilution
used for the secondary antibody was 1:1,000 and the membrane was
incubated for 1 h at room temperature. The immunoreactive bands
were visualized using Clarity Max Western enhanced
chemiluminescence kit (cat. no. 1705062; Bio-Rad Laboratories,
Inc.), before the density of the band was semi-quantified and
analyzed using the ImageJ 4.1 software (National Institutes of
Health). The parallel blot for the GAPDH (cat. no. 2118; Cell
Signaling Technology, Inc.) control was conducted using the same
protocol for the target proteins. The dilution used for the GAPDH
antibody was 1:1,000.
Permeability assay
The permeability of the hBMVECs was assessed using
the Endothelial Transwell Permeability Assay Kit (cat. no. CB6929;
Cell Biologics, Inc.) according to manufacturer's protocols. Data
from this assay were expressed as absorption readings collected at
450 nm, which was considered the absorbance of relative
permeability. The appropriate dose of Tβ4 for the permeability
assay was determined using three separate doses, 250, 500 and 1,000
ng/ml.
Statistical analysis
Data were expressed as the mean ± standard error of
3-5 separate experiments and were analyzed using one-way analysis
of variance for multiple groups followed by Tukey's post hoc tests,
when justified using GraphPad Prism 5.0 software (Dotmatics).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effect of Tβ4 on LPS-induced TJ
disruption in hBMVECs
To examine the effects of LPS on TJ gene expression,
hBMVECs were stimulated with 100 ng/ml LPS for 24 h. This
stimulatory dose of 100 ng/ml LPS did not exert toxic effects or
damage the cells. Therefore, this dose was used for all subsequent
studies. LPS treatment significantly decreased the mRNA expression
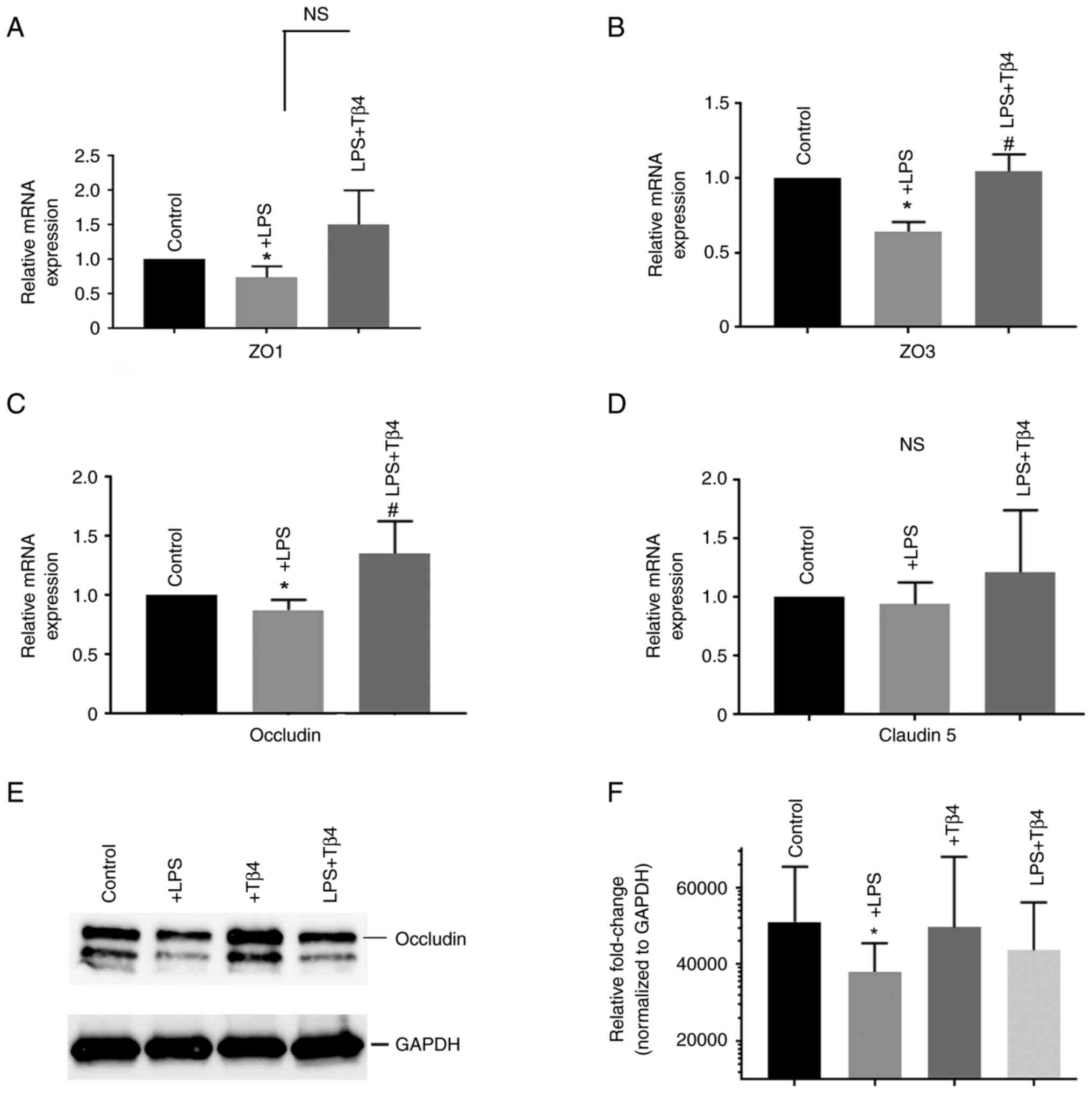
of ZO1, ZO2 and occludin (P<0.05; Fig. 1A-C) compared with that in the
untreated cells. However, pre-treatment with Tβ4 markedly prevented
this reduction in mRNA expression, with the exception of ZO1
(P<0.05). The change in mRNA expression of claudin 5 in
LPS-treated cells was not significant, despite there being a slight
reduction (Fig. 1D). Together,
these data suggest that Tβ4 pre-treatment protected against
LPS-induced TJ gene expression in hBMVECs.
Since it has been frequently applied as a
representative TJ protein marker, occludin was chosen for
examination by western blotting. The results showed that occludin
protein levels were reduced after LPS stimulation compared with
that in untreated cells (P<0.05; Fig. 1E and F). However, Tβ4 pre-treatment markedly
prevented this.
Effect of Tβ4 on the LPS-induced
inflammatory response in hBMVECs
Inflammation plays a pivotal role in BBB damage
(17,18). Therefore, the mRNA expression
levels of interleukin-6 (IL-6), tumor necrosis factor α (TNFα) and
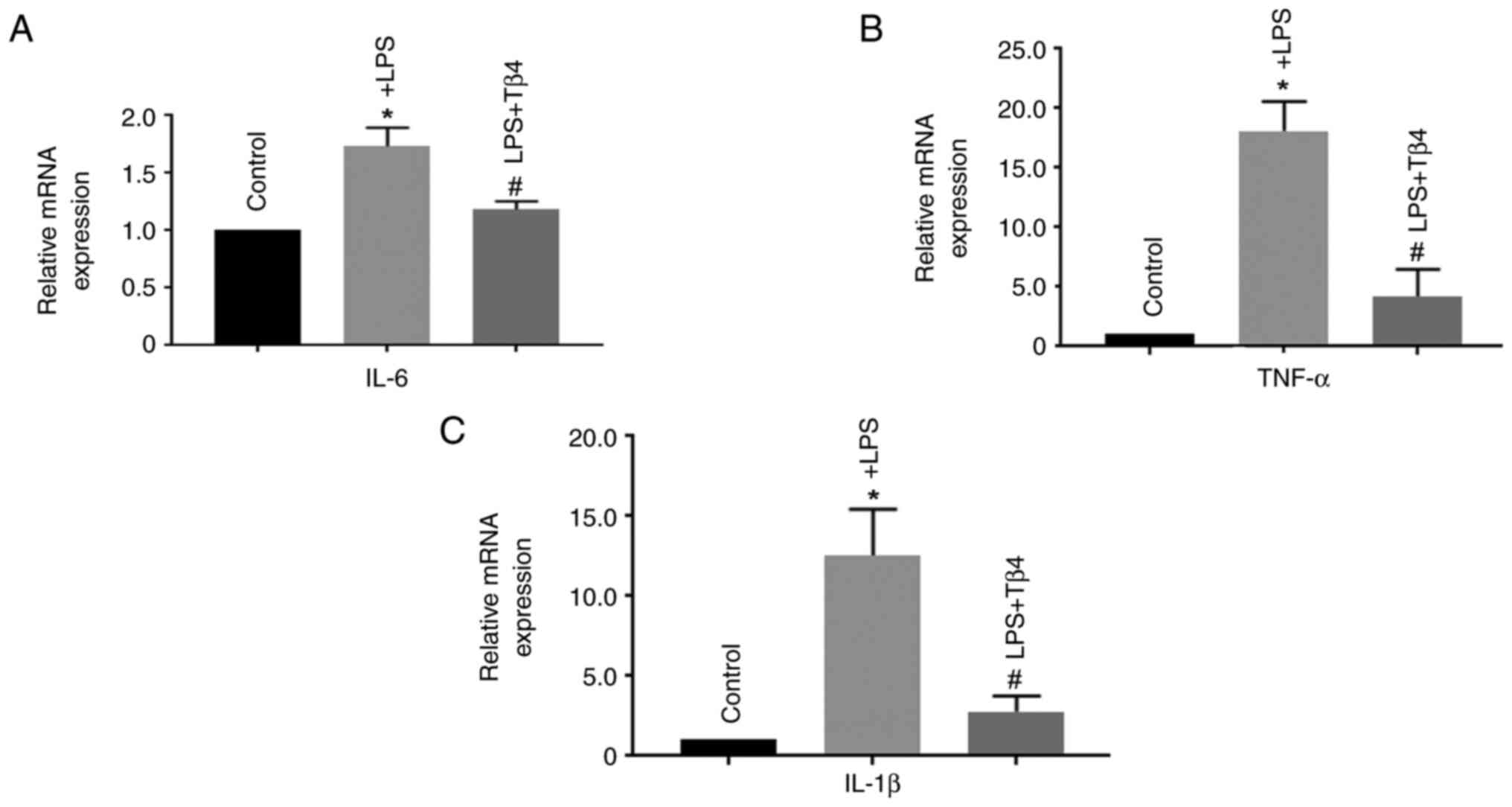
IL-1β in LPS-treated hBMVECs were measured. LPS treatment was found
to significantly increase the mRNA expression of IL-6, TNFα and
IL-1β (P<0.05) compared with that in untreated cells. By
contrast, cells pre-treated with Tβ4 were significantly more
resistant to this LPS-induced phenomenon (P<0.05; Fig. 2A-C). Together, these data suggest
that Tβ4 pre-treatment can prevent the LPS-induced inflammatory
response in hBMVECs.
Role of Tβ4 in the LPS-induced
activation of adhesion molecules in hBMVECs
Adhesion molecules, such as intercellular adhesion
molecule 1 (ICAM1) and vascular cell adhesion molecule 1 (VCAM1),
are critical for BBB integrity and are activated during BBB injury
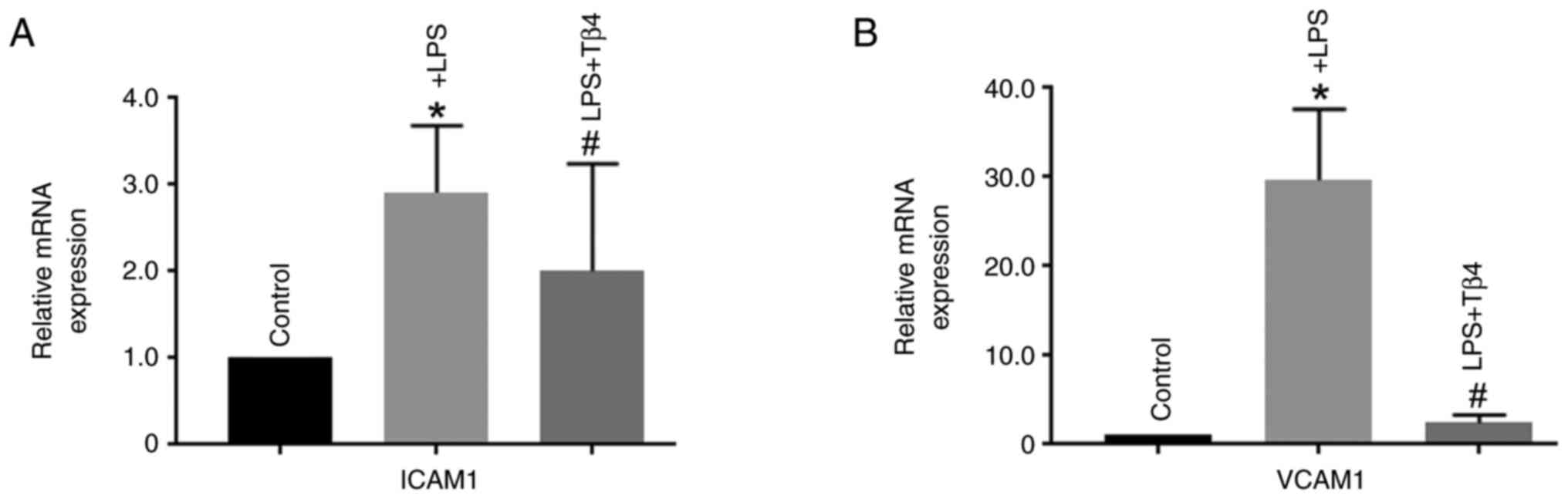
(16,19). The present study demonstrated that
both ICAM1 and VCAM1 mRNA expression levels were significantly
increased after LPS treatment (P<0.05) compared with that in
untreated cells. However, Tβ4 pre-treatment significantly prevented
this effect (P<0.05; Fig. 3A
and B). These data suggest that
Tβ4 may confer protection against LPS-induced vascular damage by
reducing ICAM1 and VCAM1 gene expression in hBMVECs.
Anti-apoptotic effects of Tβ4 on
LPS-stimulated hBMVECs
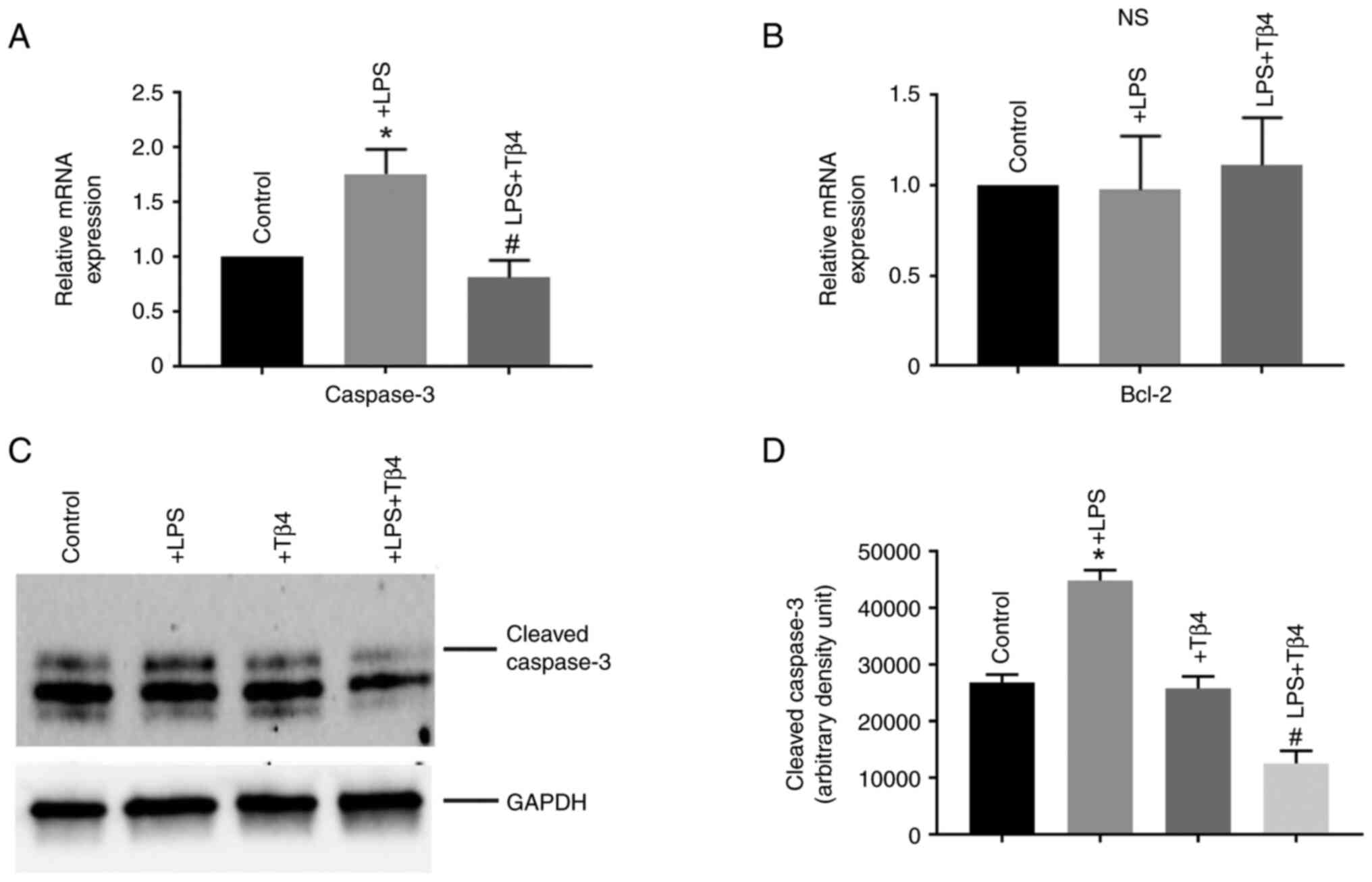
The Caspase-3 mRNA expression level in hBMVECs
following LPS treatment was next measured. LPS stimulation
significantly increased caspase-3 mRNA expression compared with
that in untreated cells. By contrast, Tβ4 pre-treatment
significantly prevented this LPS-induced increase in caspase-3 mRNA
expression (P<0.05; Fig. 4A).
The expression of the anti-apoptotic gene, B-cell lymphoma 2
(Bcl-2), was not significantly altered following LPS-stimulation
(Fig. 4B). These data suggested
that Tβ4 pre-treatment protected against LPS-induced apoptosis of
hBMVECs by inhibiting caspase-3.
The change in expression of cleaved caspase-3 was
further determined by western blotting. The results showed that
cleaved caspase-3 was significantly higher after LPS stimulation
(P<0.05; Fig. 4C and D). However, Tβ4 pre-treatment
significantly reduced levels of cleaved caspase-3 protein
expression compared with LPS treated cells.
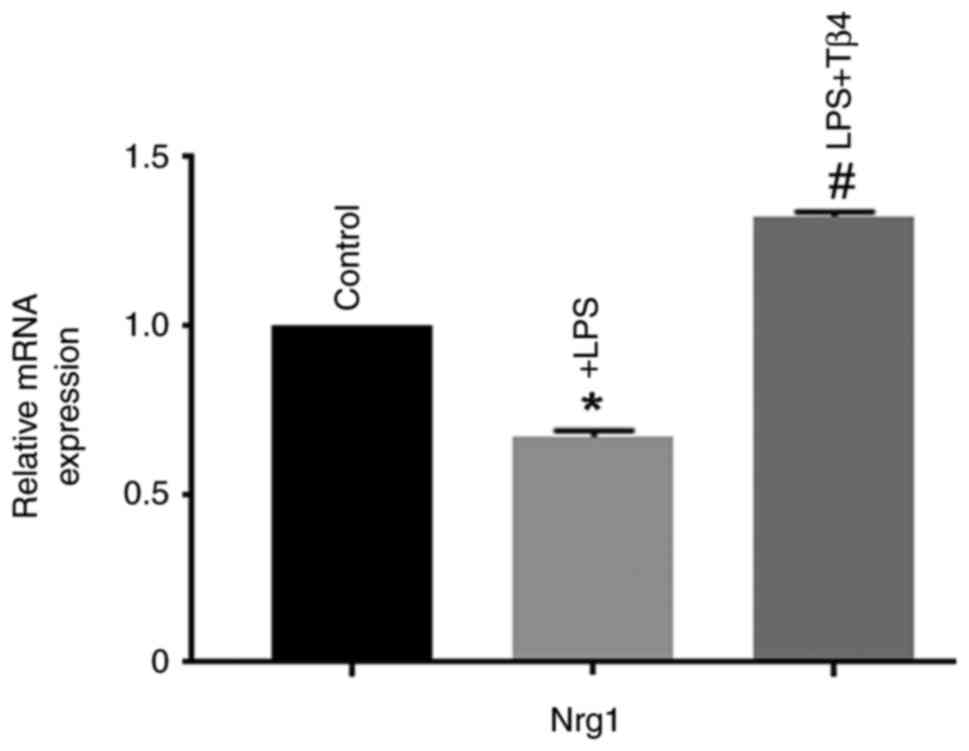
Effect of Tβ4 on LPS-stimulated Nrg1
expression in hBMVECs
Nrg1 is an endogenous growth factor belonging to the
family of epidermal growth factors (22) and has previously demonstrated
neuroprotective effects after ischemic stroke (23-25).
The mRNA expression level of Nrg1 in LPS-treated hBMVECs was
measured, which showed a significant reduction in Nrg1 mRNA
expression (P<0.05; Fig. 5)
compared with that in untreated cells. Tβ4 pre-treatment
significantly preserved Nrg1 expression (P<0.05; Fig. 5), which was higher compared with
that in LPS-treated cells, suggesting that it is a key target
molecule. These data suggest that Tβ4 pre-treatment has the
potential to prevent LPS-induced injury in hBMVECs by protecting
Nrg1 expression.
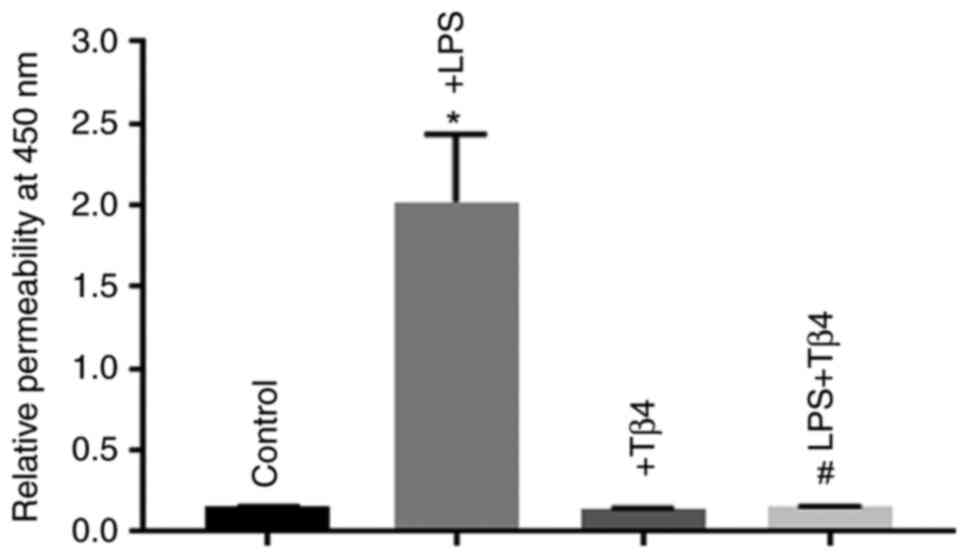
Effects of Tβ4 on LPS-stimulated
hBMVEC monolayer permeability
Permeability is one of the critical functional
parameters of the BBB in maintaining CNS homeostasis (7). Disruption of the BBB due to injury
can result in increased BBB permeability, which promotes several
neurological diseases (7,11). This suggests that LPS treatment may
either disrupt the function of or loosen the TJ proteins, increase
inflammation, and promote apoptosis. However, in the present study,
Tβ4 pre-treatment was shown to preserve TJ protein expression,
prevent inflammation and apoptosis. Therefore, the effects of Tβ4
on LPS-induced damage of the BBB components was next examined by
measuring BBB permeability. The dose-response of Tβ4 in LPS-treated
hBMVECs was determined using three doses, 250, 500 and 1,000 ng/ml.
The data indicated that 1,000 ng/ml elicits the greatest protection
on permeability (Fig. S1). The
hBMVECs were then treated with LPS for 24 h in the presence or
absence of 1,000 ng/ml Tβ4. LPS treatment was found to
significantly increase the permeability of the hBMVECs (P<0.05;
Fig. 6). By contrast, this
LPS-induced increase in hBMVEC permeability was significantly
prevented if the cells were pre-treated with Tβ4, suggesting that
Tβ4 may offer protection against LPS-induced dysfunction in
endothelial cell permeability (P<0.05; Fig. 6).
Effects of Tβ4 on ZO1, ZO3, occludin
and p65 expression in hBMVECs stimulated with LPS
To determine the effect of Tβ4 on LPS-induced
degradation of ZO1, ZO3 and occludin proteins in hBMVECs,
immunofluorescence analyses were performed. The data demonstrated
that LPS treatment significantly reduced ZO1, ZO3 and occludin
protein expression, which was indicated by the low levels of green
fluorescence compared with that in untreated cells (Fig. 7A-C). Prior treatment with Tβ4
preserved ZO1, ZO3 and occludin expression, as indicated by the
green fluorescence (Fig. 7A-C).
The fluorescence-positive cells were evaluated in 5-7 separate
field images in all groups for the determination of statistical
significance. The semi-quantification of green
fluorescence-positive cells for ZO1, ZO3 and occludin is shown in
Fig. 7D (P<0.05).
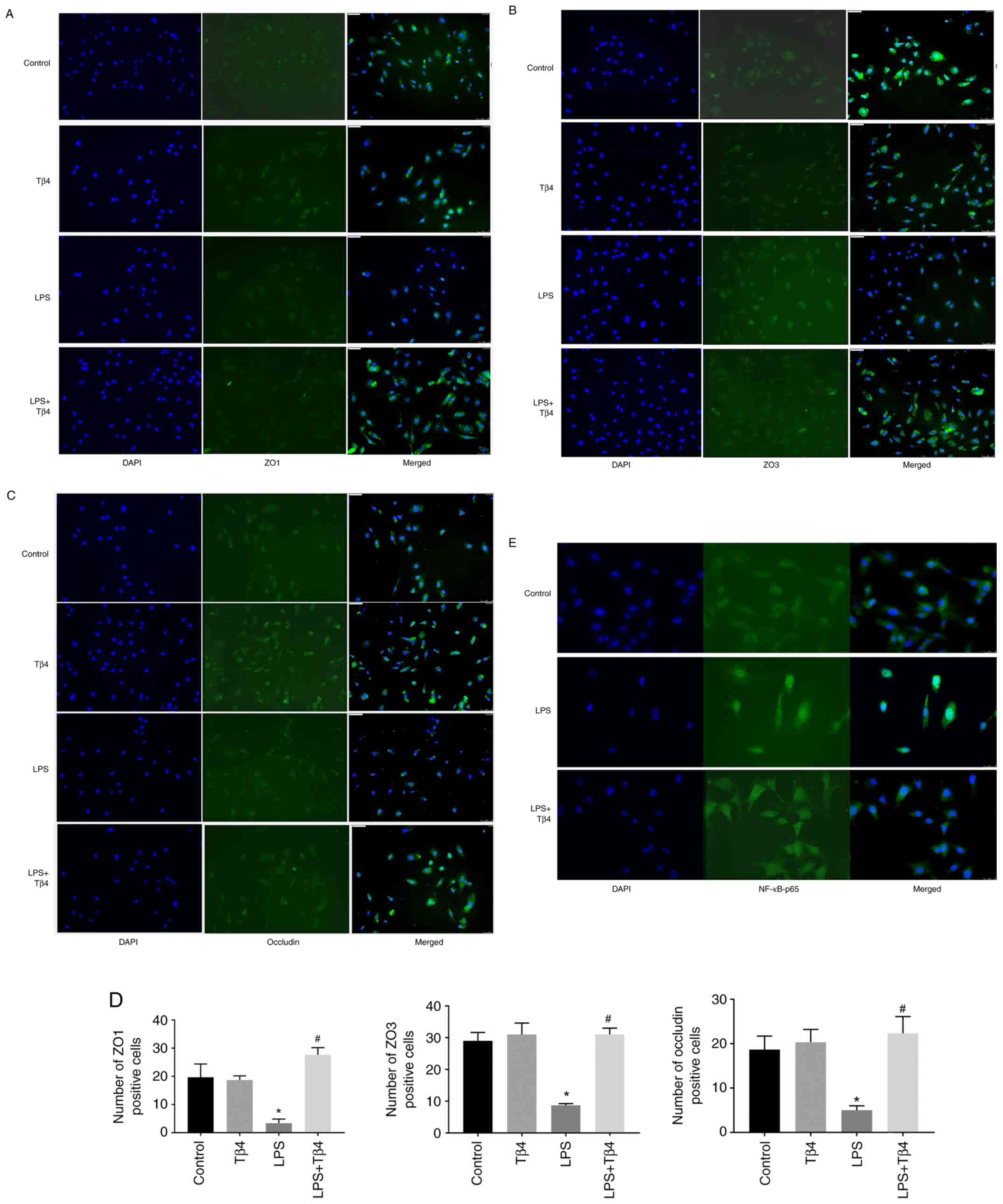 | Figure 7Effect of Tβ4 on ZO1, ZO3, occludin
and p65 expression in hBMVECs stimulated with LPS. Cultured hBMVECs
were pre-treated with Tβ4 for 2 h and then stimulated with LPS for
24 h. Dual staining immunofluorescence analysis of (A) ZO1, (B) ZO3
and (C) Occludin (green staining; DAPI, blue staining). (D)
Semi-quantification of number of green fluorescence-positive cells.
(E) Immunofluorescence analysis of p65 (green staining; DAPI, blue
staining). Objective magnification, x20. ZO, zonula occludens; Tβ4,
thymosin β4; hBMVECs, human brain microvascular endothelial cells;
LPS, lipopolysaccharide. *P<0.05 vs. untreated cells
(control). #P<0.05 vs. LPS treated cells. |
To assess whether Tβ4 provided protection against
inflammation, the expression level of p65, a central regulator for
inflammation, was measured. A clear translocation of p65 protein
into the nucleus was observed after LPS treatment, whereas Tβ4
pre-treatment significantly prevented this, as indicated by the
green fluorescence outside of the nucleus (P<0.05; Fig. 7E). Therefore, the data indicated
that the LPS-induced NF-κB activation is prevented by Tβ4. The
hBMVEC-specific marker, CD31, was used as a positive control
confirming the correct cell-type was being used in the present
study and is shown in Fig.
S2.
Together, these data indicate that Tβ4 pre-treatment
protects against LPS-induced BBB damage by preserving ZO1, ZO3 and
occludin expression, whilst suppressing inflammation in
hBMVECs.
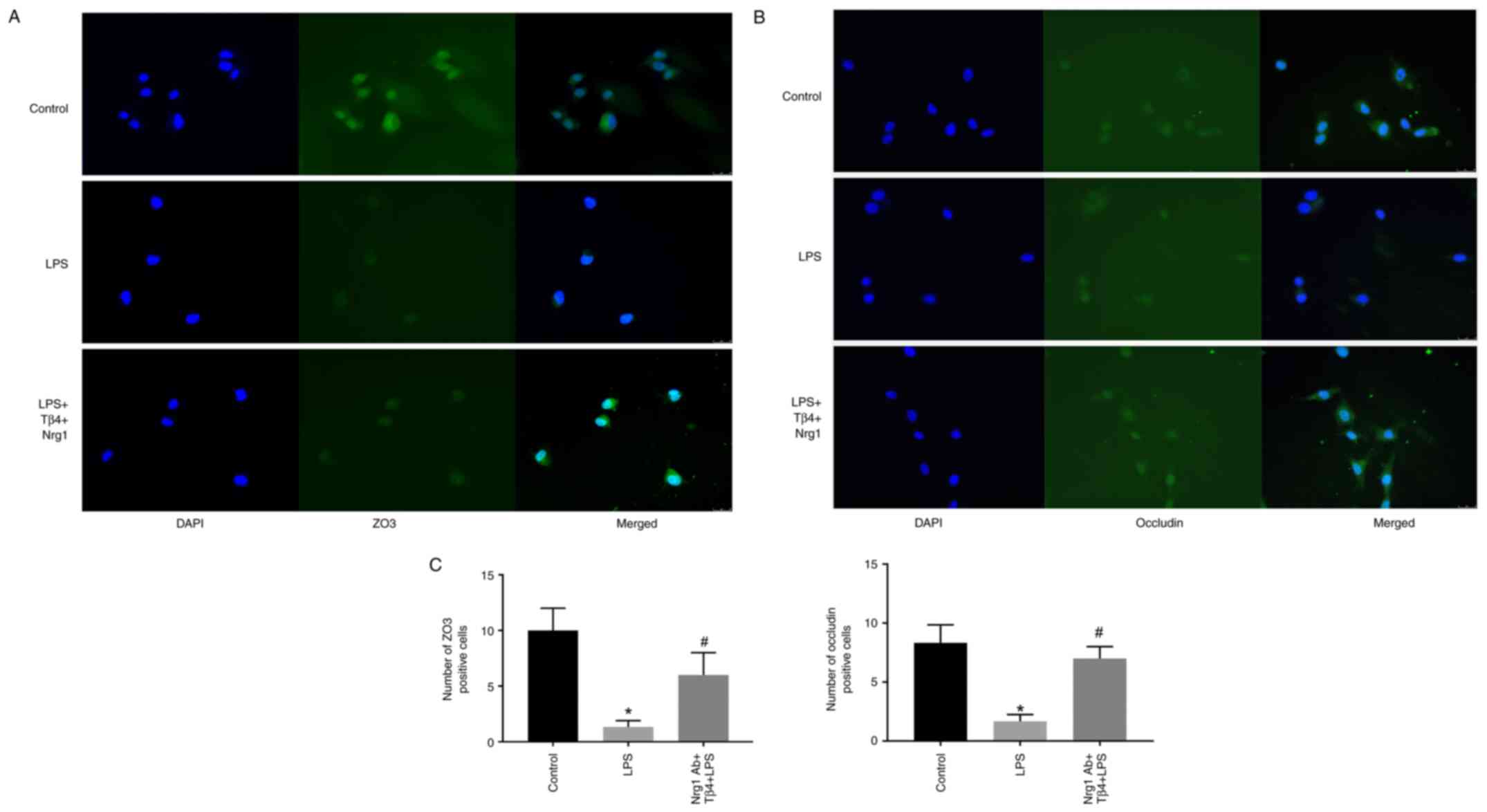
Effects of the Ngr1 antibody on
LPS-stimulated ZO3 and occludin in hBMVECs
To ascertain the role of Nrg1 in hBMVEC remodeling,
cells were pre-treated with the Nrg1 antibody before being treated
with LPS and Tβ4. The treatment appears to block the interaction of
Tβ4 and Nrg1. The fluorescence-positive cells were evaluated in 5-7
separate field images in all groups for statistical significance
determination. Pre-treatment with the Nrg1 antibody significantly
prevented the LPS-induced reduction of both ZO3 and occludin
protein level compared with that in LPS treatment group (P<0.05;
Fig. 8A and B). The semi-quantification of green
fluorescence-positive cells for ZO3 and occludin is shown in
Fig. 8C (P<0.05). These data
suggest that Nrg1may contributes independently for BBB protection
in LPS-stimulated hBMVECs.
Discussion
To the best of our knowledge, the present study is
the first to report that Tβ4 has the potential to prevent the BBB
damage induced by LPS treatment. The present study also
demonstrated that Tβ4 decreased the inflammatory response during
hBMVEC remodeling. In addition, the present study found Nrg1 to be
a possible target for Tβ4.
TBI has been previously reported to instigate
several secondary injuries, including inflammation and disruption
of the BBB (43). The BBB has a
pivotal role in the removal of waste materials and provides further
protection by preventing entry of pathogenic agents through solute
permeability (44). Maintaining
the integrity of the BBB is therefore necessary for CNS
homeostasis. Permeability of the BBB is typically controlled by
several TJ proteins embedded in between hBMVECs, including ZO1,
ZO2, occludin and claudin 5 (45-47).
The present study demonstrated that the mRNA expression levels of
ZO1, ZO2, and occludin were all reduced after LPS stimulation,
however, this effect was prevented by Tβ4 pre-treatment, with the
exception of ZO1. In addition, the western blotting results using
occludin as a representative marker of TJ proteins revealed
moderate restoration of occludin protein in response to LPS
challenge. This observation was supported by the immunofluorescence
analyses of ZO1, ZO3 and occludin protein expression in the
LPS-stimulated hBMVECs. It is known that dysfunctional TJ proteins
can result in endothelial cell damage, leading to increased BBB
permeability (46). The present
study also found that LPS stimulation increased hBMVEC
permeability, when using an in vitro assay. In addition, the
Tβ4-pretreated cells demonstrated significantly reduced
permeability compared with that in LPS-treated cells, suggesting
its potential therapeutic application for BBB damage. However, the
mechanism underlying the restoration of TJ protein function by Tβ4
remains poorly understood. Tβ4 can become internalized by cells
(48), but the cell surface
receptors remain unknown. A hypothesized mechanism is through
transcription factor-mediated active transport of positively
charged amino acids (48). In
addition, nuclear localization of actin and chromatin remodeling
have been suggested (49).
BBB disruption allows inflammatory molecules to
enter the CNS, thus, triggering the neuroinflammatory response
(50). IL-6 is a major
inflammatory factor that can induce the expression of adhesion
molecules in activated endothelial cells (51). The present study showed that the
LPS-stimulation of IL-6 expression was significantly prevented by
Tβ4 pre-treatment. Similar observations were found regarding IL-1β
and TNFα expression in LPS-stimulated hBMVECs, suggesting an
anti-inflammatory role of Tβ4. These findings are consistent with
previous studies under various physiological and pathological
settings (52-57).
Together, these results suggest an anti-inflammatory role for Tβ4
in hBMVEC remodeling in the neurovascular unit.
Cellular adhesion was found to be disrupted by LPS
in the present study, as demonstrated by the increased ICAM1 and
VCAM1 expression observed following LPS treatment of hBMVECs. This
increase in ICAM1 and VCAM1 expression was significantly prevented
by Tβ4 pre-treatment, indicating a potential role of vascular gene
regulation during BBB disruption. This observation further
suggested that this reduction of vascular gene expression and BBB
permeability by Tβ4 may attenuate the adhesion and migration of
inflammatory cells.
The BBB becomes damaged after ischemic insult or TBI
(7,58). Nrg1 is a growth factor with diverse
functions in the CNS and has been shown to mediate protective
effects in a focal brain ischemic rat model (59). Nrg1 functions by activating ErbB
receptor kinases, specifically ErbB4 (60,61).
It has been previously demonstrated that Nrg1 expression is reduced
in an ischemic stroke model, whereby treatment with Nrg1 prior to
brain injury induction provided neuroprotection (62,63).
The present study found a reduction of Nrg1 mRNA expression in
LPS-stimulated hBMVECs, which was prevented by Tβ4 pre-treatment.
In addition, cells pre-treated with the Nrg1 antibody prevented the
loss of ZO3 and occludin proteins after LPS-stimulation, suggesting
Nrg1 is pivotal in hBMVECs remodeling. Together, to the best of our
knowledge, these findings demonstrated for the first time that Nrg1
may be a possible target for Tβ4. Although Tβ4 has been shown to be
protective against TBI (36,37),
its role in the BBB remains unknown. The present study indicates a
possible therapeutic use of Nrg1 for BBB restoration following TBI
or ischemic stroke. However, this association between Tβ4 and Nrg1
requires further investigation in a BBB model. Furthermore, this
observation also requires verification in animal models of TBI or
ischemic stroke.
In conclusion, the present study demonstrated that
Tβ4 can protect against LPS-induced hBMVEC remodeling by reducing
inflammation, whilst restoring TJ protein and Nrg1 expression
levels. The present study may offer a novel therapeutic platform
for treating BBB damage caused by injury or trauma with Tβ4
potentially serve as a new therapeutic tool for BBB protection,
where inflammation and TJ proteins serve a critical role.
There are many cell lines that can be used in a BBB
study. However, hBMVECs were chosen as the model system in the
present study as it is one of the pivotal cells in the
neurovascular unit. Therefore, the use of only hBMVECs is a
limitation of the present study. In addition, the control cell line
used is a non-TBI cell line, which is also considered a limitation
of the present study.
The present findings indicated that the role of Tβ4
in the restoration of TJ proteins and Nrg1 in hBMVECs may be of
clinical relevance. Nrg1 may be an important component in
neurovascular remodeling and the use of Tβ4 as a therapeutic
molecule for neuronal protection may prove to be instrumental.
Supplementary Material
Dose response of Tβ4 in a permeability
assay. The hBMVECs permeability was performed using an Endothelial
Trans well Permeability Assay Kit. The cells were treated with
three different doses of Tβ4, 250, 500 and 1,000 ng/ml. Data from
this assay were expressed as absorption readings made at 450 nm,
which were considered the relative permeability.
CD31-positivity in hBMVECs. The
hBMVECs were seeded onto the 6-well attachment factor coated cover
glass. Dual-staining immunofluorescence analysis of CD31 (red) is
shown in the left panel. The middle panel shows DAPI (blue)
staining for nucleus. The right panel is the merged image. hBMVECs,
human microvascular endothelial cells.
Acknowledgements
The authors would like to thank Dr Richard W. Seim,
Director of the VISN 17 Center of Excellence for Research on
Returning War Veterans, (Waco, USA) for all required support
including personnel, laboratory space and equipment, to complete
the study.
Funding
Funding: The present study was supported by VISN17 Center of
Excellence's internal funds to Biomarkers & Genetics Core.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SG conceived the project. SG and RSG designed the
experiments. SG wrote the manuscript. RSG critically read the
manuscript and provided constructive feedback. WS, CH and SG
performed the experiments. SG and RSG analyzed the data. WS and CH
cultured cells and performed treatments and maintenance of the cell
passages for all experiments. WS performed the western blot and
RT-qPCR analyses. CH performed RT-qPCR, immunofluorescence
experiments and captured images. SG and WS performed the
permeability assay. SG, WS and CH confirm the authenticity of all
the raw data. All authors have read and approved the final version
of the manuscript.
Ethics approval and consent to
participate
The VISN 17 Center of Excellence for Research on
Returning War Veterans (Waco, USA) comes under the Central Texas
Veterans Health Care System (CTVHCS; Waco, TX, USA), which
regulates its research operations. All required approvals to
conduct the present study were attained through the various CTVHCS
research committees, including ethical approval from the
Institutional Review Board (approval no. 00711), which is the human
research ethics oversight committee of our institute.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
McDonald SD, Walker WC, Cusack SE,
Yoash-Gantz RE, Pickett TC, Cifu DX, Mid-Atlantic Mirecc Workgroup
V and Tupler LA: Health symptoms after war zone deployment-related
mild traumatic brain injury: Contributions of mental disorders and
lifetime brain injuries. Brain Inj. 35:1338–1348. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Otero MC, Rau HK, Shofer JB, Peskin ER and
Pagulayan KF: Self-perceived irritability among OEF/OIF/OND
veterans with a history of deployment-related mTBI: Associations
with prospective memory and quality of life. Clin Neuropsychol.
36:1384–1404. 2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Alam A, Thelin EP, Tajsic T, Khan DZ,
Khellaf A, Patani R and Helmy A: Cellular infiltration in traumatic
brain injury. J neuroinflammation. 17(328)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Abrahamson EE and Ikonomovic MD: Brain
injury-induced dysfunction of the blood brain barrier as a risk for
dementia. Exp Neurol. 328(113257)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Campbell M, Hanrahan F, Gobbo OL, Kelly
ME, Kiang AS, Humphries MM, Nguyen AT, Ozaki E, Keaney J, Blau CW,
et al: Targeted suppression of claudin-5 decreases cerebral oedema
and improves cognitive outcome following traumatic brain injury.
Nat Commun. 3(849)2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Jha RM, Kochanek PM and Simard JM:
Pathophysiology and treatment of cerebral edema in traumatic brain
injury. Neuropharmacology. 145:230–246. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Cash A and Theus MH: Mechanisms of
blood-brain barrier dysfunction in traumatic brain injury. Int J
Mol Sci. 21(3344)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
O'Keeffe E, Kelly E, Liu Y, Giordano C,
Wallace E, Hynes M, Tiernan S, Meagher A, Greene C, Hughes S, et
al: dynamic blood-brain barrier regulation in mild traumatic brain
injury. J Neurotrauma. 37:347–356. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Delaney C and Campbell M: The blood brain
barrier: Insights from development and ageing. Tissue Barriers.
5(e1373897)2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Muoio V, Persson PB and Sendeski MM: The
neurovascular unit-concept review. Acta Physiool (Oxf). 10:790–798.
2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Prakash R and Carmichael ST: Blood-brain
barrier breakdown and neovascularization processes after stroke and
traumatic brain injury. Curr Opin Neurol. 28:556–564.
2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Logsdon AF, Lucke-Wold BP, Turner RC,
Huber JD, Rosen CL and Simpkins JW: Role of microvascular
disruption in brain damage from traumatic brain injury. Compr
Physiol. 5:1147–1160. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Xu L, Nirwane A and Yao Y: Basement
membrane and blood-brain barrier. Stroke Vasc Neurol. 4:78–82.
2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Toyama K, Spin JM and Tsao PS: Role of
microRNAs on blood brain barrier dysfunction in vascular cognitive
impairment. Curr Drug Deliv. 14:744–757. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ballabh P, Braun A and Nedergaard M: The
blood-brain barrier: An overview: Structure, regulation, and
clinical implications. Neurobiol Dis. 16:1–13. 2004.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Varatharaj A and Galea I: The blood-brain
barrier in systemic inflammation. Brain Behav Immun. 60:1–12.
2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Banks WA, Gray AM, Erickson MA, Salameh
TS, Damodarasamy M, Sheibani N, Meabon JS, Wing EE, Morofuji Y,
Cook DG and Reed MJ: Lipopolysaccharide-induced blood-brain barrier
disruption: Roles of cyclooxygenase, oxidative stress,
neuroinflammation, and elements of the neurovascular unit. J
Neuroinflammation. 12(223)2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Corrigan F, Mander KA, Leonard AV and Vink
R: Neurogenic inflammation after traumatic brain injury and its
potentiation of classical inflammation. J Neuroinflammation.
13(264)2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Thal S and Neuhaus W: The blood-brain
barrier as a target in traumatic brain injury treatment. Arch Med
Res. 45:698–710. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Menge T, Zhao Y, Zhao J, Wataha K, Gerber
M, Zhang J, Letourmeau P, Redell J, Shen L, Wang J, et al:
Mesenchymal stem cells regulate blood-brain barrier integrity
through TIMP3 release after traumatic brain injury. Sci Transl Med.
4(161ra150)2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Tang G, Liu Y, Zhang Z, Lu Y, Wang Y,
Huang J, Li Y, Chen X, Gu X, Wang Y and Yang GY: Mesenchymal stem
cells maintain blood-brain barrier integrity by inhibiting
aquaporin-4 upregulation after cerebral ischemia. Stem Cells.
32:3150–3162. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lok J, Sardi SP, Guo S, Besancon E, Ha DM,
Rosell A, Kim WJ, Corfas G and Lo EH: Neuregulin-1 signaling in
brain endothelial cells. J Cereb Blood Flow Metab. 29:39–43.
2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Xu Z, Ford GD, Croslan DR, Jiang J, Gates
A, Allen R and Ford BD: Neuroprotection by neuregulin-1 following
focal stroke is associated with the attenuation of ischemia-induced
pro-inflammatory and stress gene expression. Neurobiol Dis.
19:461–470. 2005.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Guo WP, Wang J, Li RX and Peng YW:
Neuroprotective effects of neuregulin-1 in rat models of focal
cerebral ischemia. Brain Res. 1087:180–185. 2006.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Simmons LJ, Surles-Zeigler MC, Li Y, Ford
GD, Newman GD and Ford BD: Regulation of inflammatory responses by
neuregulin-1 in brain ischemia and microglial cells in vitro
involves the NF-kappa B pathway J. Neuroinflammation.
13(237)2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Citri A and Yarden Y: EGF-ERBB signaling:
Towards the systems level. Nat Rev Mol Cell Biol. 7:505–516.
2006.PubMed/NCBI View
Article : Google Scholar
|
|
27
|
Sun Y, Ikrar T, Davis MF, Gong N, Zheng X,
Luo ZD, Lai C, Mei L, Holmes TC, Gandhi SP and Xu X: Neuregulin-1
(NRG1)/ErbB4 signaling regulates visual cortical plasticity.
Neuron. 92:160–173. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lemmens K, Doggen K and De Keulenaer GW:
Role of neuregulin-1/ErbB signaling in cardiovascular physiology
and disease: Implications for therapy of heart failure.
Circulation. 116:954–960. 2007.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Goldstein AL, Hannappel E and Kleinman HK:
Thymosin beta4: Actin-sequestering protein moonlights to repair
injured tissues. Trends Mol Med. 11:421–429. 2005.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Crockford D, Turjman N, Allan C and Angel
J: Thymosin beta4: Structure, function, and biological properties
supporting current and future clinical applications. Ann N Y Acad
Sci. 1194:179–189. 2010.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Bock-Marquette I, Saxena A, White MD,
Dimaio JM and Srivastava D: Thymosin beta4 activates
integrin-linked kinase and promotes cardiac cell migration,
survival and cardiac repair. Nature. 432:466–472. 2004.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Sopko N, Qin Y, Finan A, Dadabayev A,
Chigurupati S, Qin J, Penn MS and Gupta S: Significance of Thymosin
β4 and Implication of PINCH-1-ILK-α-Parvin (PIP) complex in human
dilated cardiomyopathy. PLoS One. 6(e20184)2011.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Sosne G, Szliter EA, Barrett R, Kernacki
KA, Kleinman H and Hazlett LD: Thymosin beta 4 promotes corneal
wound healing and decreases inflammation in vivo following alkali
injury. Exp Eye Res. 74:293–299. 2002.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Chiu LL, Reis LA, Momen A and Radisic M:
Controlled release of thymosin β4 from injected collagen-chitosan
hydrogels promotes angiogenesis and prevents tissue loss after
myocardial infarction. Regen Med. 7:523–533. 2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Yuan H, Ma C, Moinet L, Sato N and
Martins-Green M: Reversal of second-hand cigarette smoke-induced
impairment of corneal wound healing by thymosin beta4 combined with
anti-inflammatory agents. Invest Ophthalmol Vis Sci. 51:2424–2435.
2010.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Malinda KM, Sidhu GS, Mani H, Banaudha K,
Maheshwari RK, Goldstein AL and Kleinman H: Thymosin beta4
accelerates wound healing. J Invest Dermatol. 113:364–368.
1999.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Kumar S and Gupta S: Thymosin beta 4
prevents oxidative stress by targeting antioxidant and
anti-apoptotic genes in cardiac fibroblasts. PLoS One.
6(e26912)2011.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Osei J, Kelly W, Toffolo K, Donahue K,
Levy B, Bard J, Wang J, Levy E, Nowak N and Poulsen D: Thymosin
beta 4 induces significant changes in the plasma miRNA profile
following severe traumatic brain injury in the rat lateral fluid
percussion injury model. Expert Opin Biol Ther. 18(sup1):S159–S164.
2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Xaio H, Banks WA, Niehoff ML and Morley
JE: Effect of LPS on the permeability of the blood-brain barrier to
insulin. Brain Res. 896:36–42. 2001.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Al-Nedawi KN, Czyz M, Bednarek R, Szemraj
J, Swiatkowska M, Cierniewska-Cieslak A, Wyczolkowska J and
Cierniewski CS: Thymosin beta 4 induces the synthesis of
plasminogen activator inhibitor 1 in cultured endothelial cells and
increases its extracellular expression. Blood. 103:1319–1324.
2004.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Su L, Kong X, Loo S, Gao Y, Liu B, Su Z,
Dalan R, Ma J and Ye L: Thymosin beta-4 improves endothelial
function and reparative potency of diabetic endothelial cells
differentiated from patient induced pluripotent stem cells. Stem
Cell Res Ther. 13(13)2022.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Liu M, Solomon W, Cespedes JC, Wilson NO,
Ford B and Stiles JK: Neuregulin-1 attenuates experimental cerebral
malaria (ECM) pathogenesis by regulating ErbB4/AKT/STAT3 signaling.
J Neuroinflammation. 15(104)2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
44
|
van Vliet EA, Ndode-Ekane XE, Lehto LJ,
Gorter JA, Andrade P, Aronica E, Gröhn O and Pitkänen A:
Long-lasting blood-brain barrier dysfunction and neuroinflammation
after traumatic brain injury. Neurobiol. Dis.
145(105080)2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Abbott NJ, Patabendige AK, Dolman DE,
Yusof SR and Begley DJ: Structure and function of the blood-brain
barrier Neurobiol. Dis. 37:13–25. 2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Harhaj NS and Antonett DA: Regulation of
tight junctions and loss of barrier function in pathophysiology.
Int J Biochem Cell Biol. 36:1206–1237. 2004.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Jiao H, Wang Z, Liu Y, Wang P and Xue Y:
Specific role of tight junction proteins claudin-5, occludin, and
ZO-1 of the blood-brain barrier in a focal cerebral ischemic
insult. J Mol Neurosci. 44:130–139. 2011.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Huff T, Rosorius O, Otto AM, Muller CS,
Ballweber E, Hannappel E and Mannherz HG: Nuclear localisation of
the G-actin sequestering peptide thymosin beta4. J Cell Sci. 117(Pt
22):5333–5341. 2004.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Percipalle P, Zhao J, Pope B, Weeds A,
Lindberg U and Daneholt B: Actin bound to the heterogeneous nuclear
ribonucleoprotein hrp36 is associated with Balbiani ring mRNA from
the gene to polysomes. Cell Biol. 153:229–236. 2001.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Takata F, Nakagawa S, Matsumoto J and
Dohgu S: Blood-Brain barrier dysfunction amplifies the development
of neuroinflammation: Understanding of cellular events in brain
microvascular endothelial cells for prevention and treatment of BBB
dysfunction. Front Cell Neurosci. 15(661838)2021.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Dri E, Lampas E, Lazaros G, Lazarou E,
Theofilis P, Tsioufis C and Tousoulis D: Inflammatory mediators of
endothelial dysfunction. Life (Basel). 13(1420)2023.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Xiong Y, Zhang Y, Mahmood A, Meng Y, Zhang
ZG, Morris DC and Chopp M: Neuroprotective and neurorestorative
effects of thymosin β4 treatment initiated 6 hours after traumatic
brain injury in rats. J Neurosurg. 116:1081–1092. 2012.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Xiong Y, Mahmood A, Meng Y, Zhang Y, Zhang
ZG, Morris DC and Chopp M: Treatment of traumatic brain injury with
thymosin β4 in rats. J Neurosurg. 114:102–115.
2011.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Zhang Y, Zhang ZG, Chopp M, Meng Y, Zhang
L, Mahmood A and Xiong Y: Treatment of traumatic brain injury in
rats with N-acetyl-seryl-aspartyl-lysyl-proline. J Neurosurg.
126:782–795. 2017.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Shah R, Reyes-Gordillo K, Cheng Y,
Varatharajalu R, Ibrahim J and Lakshman MR: Thymosin β4 prevents
oxidative stress, inflammation, and fibrosis in Ethanol- and
LPS-Induced liver injury in mice. Oxid Med Cell Longev.
2018(9630175)2018.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Pardon MC: Anti-inflammatory potential of
thymosin β4 in the central nervous system: Implications for
progressive neurodegenerative diseases. Expert Opin Biol Ther.
18(sup1):S165–S169. 2018.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Gupta S, Kumar S, Sopko N, Qin Y, Wei C
and Kim IK: Thymosin β4 and cardiac protection: Implication in
inflammation and fibrosis. Ann N Y Acad Sci. 1269:84–91.
2012.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Abdullahi W, Tripathi D and Ronaldson PT:
Blood-brain barrier dysfunction in ischemic stroke: Targeting tight
junctions and transporters for vascular protection. Am J Physiol
Cell Physiol. 315:C343–C356. 2018.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Li Y, Xu Z, Ford GD, Croslan DR, Cairobe
T, Li Z and Ford BD: Neuroprotection by neuregulin-1 in a rat model
of permanent focal cerebral ischemia. Brain Res. 1184:277–283.
2007.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Mei L and Nave KA: Neuregulin-ERBB
signaling in the nervous system and neuropsychiatric diseases.
Neuron. 83:27–49. 2014.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Chen YJ, Zhang M, Yin DM, Wen L, Ting A,
Wang P, Lu YS, Zhu XH, Li SJ, Wu CY, et al: ErbB4 in
parvalbumin-positive interneurons is critical for neuregulin 1
regulation of long-term potentiation. Proc Natl Acad Sci USA.
107:21818–21823. 2010.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Xu Z, Jiang J, Ford G and Ford BD:
Neuregulin-1 is neuroprotective and attenuates inflammatory
responses induced by ischemic stroke. Biochem Biophys Res Commun.
322:440–446. 2004.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Noll JM, Li Y, Distel TJ, Ford GD and Ford
BD: Neuroprotection by exogenous and endogenous neuregulin-1 in
mouse models of focal ischemic stroke. J Mol Neurosci. 69:333–342.
2019.PubMed/NCBI View Article : Google Scholar
|