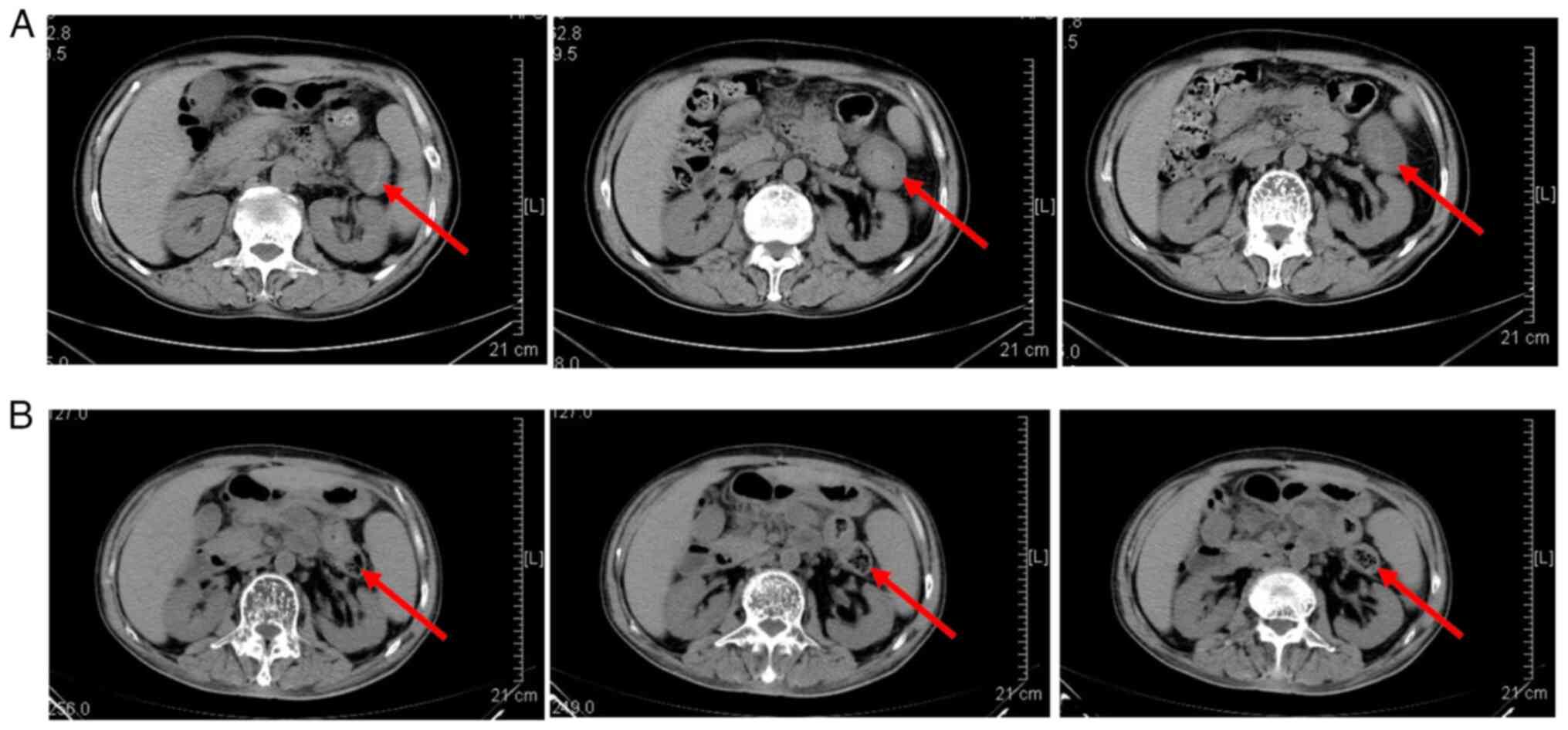
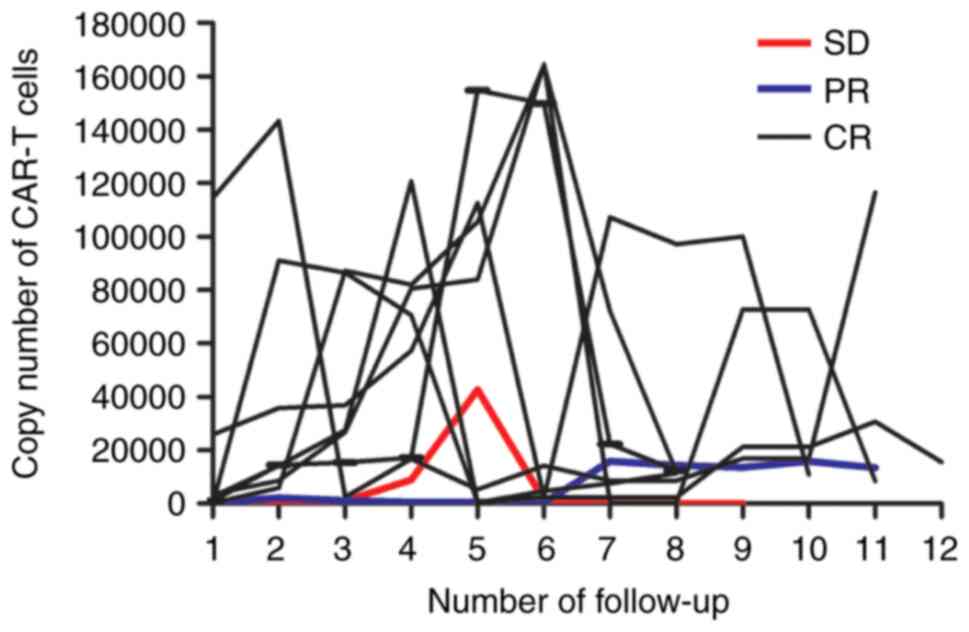
|
1
|
Cowan AJ, Allen C, Barac A, Basaleem H,
Bensenor I, Curado MP, Foreman K, Gupta R, Harvey J, Hosgood HD, et
al: Global burden of multiple myeloma: A systematic analysis for
the global burden of disease study 2016. JAMA Oncol. 4:1221–1227.
2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
van Beurden-Tan CHY, Franken MG,
Blommestein HM, Uyl-de Groot CA and Sonneveld P: Systematic
literature review and network meta-analysis of treatment outcomes
in relapsed and/or refractory multiple myeloma. J Clin Oncol.
35:1312–1319. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Furukawa Y and Kikuchi J: Molecular basis
of clonal evolution in multiple myeloma. Int J Hematol.
111:496–511. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Dai H, Wu Z, Jia H, Tong C, Guo Y, Ti D,
Han X, Liu Y, Zhang W, Wang C, et al: Correction to: Bispecific
CAR-T cells targeting both CD19 and CD22 for therapy of adults with
relapsed or refractory B cell acute lymphoblastic leukemia. J
Hematol Oncol. 13(53)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Finney OC, Brakke HM, Rawlings-Rhea S,
Hicks R, Doolittle D, Lopez M, Futrell RB, Orentas RJ, Li D,
Gardner RA and Jensen MC: CD19 CAR T cell product and disease
attributes predict leukemia remission durability. J Clin Invest.
129:2123–2132. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Abramson JS: Anti-CD19 CAR T-cell therapy
for B-cell non-hodgkin lymphoma. Transfus Med Rev. 34:29–33.
2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
van de Donk NWCJ, Usmani SZ and Yong K:
CAR T-cell therapy for multiple myeloma: State of the art and
prospects. Lancet Haematol. 8:e446–e461. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Cho SF, Anderson KC and Tai YT: Targeting
B cell maturation antigen (BCMA) in multiple myeloma: Potential
uses of BCMA-Based immunotherapy. Front Immunol.
9(1821)2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Friedman KM, Garrett TE, Evans JW, Horton
HM, Latimer HJ, Seidel SL, Horvath CJ and Morgan RA: Effective
targeting of multiple B-Cell maturation antigen-expressing
hematological malignances by Anti-B-Cell maturation antigen
chimeric antigen receptor T cells. Hum Gene Ther. 29:585–601.
2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Mei H, Li C, Jiang H, Zhao X, Huang Z, Jin
D, Guo T, Kou H, Liu L, Tang L, et al: A bispecific CAR-T cell
therapy targeting BCMA and CD38 in relapsed or refractory multiple
myeloma. J Hematol Oncol. 14(161)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Rajkumar SV, Dimopoulos MA, Palumbo A,
Blade J, Merlini G, Mateos MV, Kumar S, Hillengass J, Kastritis E,
Richardson P, et al: International Myeloma Working Group updated
criteria for the diagnosis of multiple myeloma. Lancet Oncol.
15:e538–e548. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lee DW, Gardner R, Porter DL, Louis CU,
Ahmed N, Jensen M, Grupp SA and Mackall CL: Current concepts in the
diagnosis and management of cytokine release syndrome. Blood.
124:188–195. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Bao L, Bo XC, Cao HW, Qian C, Wang Z and
Li B: Engineered T cells and their therapeutic applications in
autoimmune diseases. Zool Res. 43:150–165. 2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
June CH and Sadelain M: Chimeric antigen
receptor therapy. N Engl J Med. 379:64–73. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Brudno JN, Lam N, Vanasse D, Shen YW, Rose
JJ, Rossi J, Xue A, Bot A, Scholler N, Mikkilineni L, et al: Safety
and feasibility of anti-CD19 CAR T cells with fully human binding
domains in patients with B-cell lymphoma. Nat Med. 26:270–280.
2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Majzner RG and Mackall CL: Clinical
lessons learned from the first leg of the CAR T cell journey. Nat
Med. 25:1341–1355. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kersten MJ, Spanjaart AM and Thieblemont
C: CD19-directed CAR T-cell therapy in B-cell NHL. Curr Opin Oncol.
32:408–417. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Mikkilineni L and Kochenderfer JN:
Chimeric antigen receptor T-cell therapies for multiple myeloma.
Blood. 130:2594–2602. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Tai YT and Anderson KC: B cell maturation
antigen (BCMA)-based immunotherapy for multiple myeloma. Expert
Opin Biol Ther. 19:1143–1156. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Berdeja JG, Madduri D, Usmani SZ,
Jakubowiak A, Agha M, Cohen AD, Stewart AK, Hari P, Htut M,
Lesokhin A, et al: Ciltacabtagene autoleucel, a B-cell maturation
antigen-directed chimeric antigen receptor T-cell therapy in
patients with relapsed or refractory multiple myeloma
(CARTITUDE-1): A phase 1b/2 open-label study. Lancet. 398:314–324.
2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Bahlis NJ, Dimopoulos MA, White DJ,
Benboubker L, Cook G, Leiba M, Ho PJ, Kim K, Takezako N, Moreau P,
et al: Daratumumab plus lenalidomide and dexamethasone in
relapsed/refractory multiple myeloma: Extended follow-up of POLLUX,
a randomized, open-label, phase 3 study. Leukemia. 34:1875–1884.
2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Usmani SZ, Nahi H, Legiec W, Grosicki S,
Vorobyev V, Spicka I, Hungria V, Korenkova S, Bahlis NJ, Flogegard
M, et al: Final analysis of the phase III non-inferiority COLUMBA
study of subcutaneous versus intravenous daratumumab in patients
with relapsed or refractory multiple myeloma. Haematologica.
107:2408–2417. 2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Stork M, Spicka I, Radocha J, Minarik J,
Jelinek T, Jungova A, Pavlicek P, Pospisilova L, Sedlak F, Straub
J, et al: Daratumumab with lenalidomide and dexamethasone in
relapsed or refractory multiple myeloma patients-real world
evidence analysis. Ann Hematol. 102:1501–1511. 2023.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Brudno JN, Maric I, Hartman SD, Rose JJ,
Wang M, Lam N, Stetler-Stevenson M, Salem D, Yuan C, Pavletic S, et
al: T cells genetically modified to express an Anti-B-Cell
maturation antigen chimeric antigen receptor cause remissions of
poor-prognosis relapsed multiple myeloma. J Clin Oncol.
36:2267–2280. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Raje N, Berdeja J, Lin Y, Siegel D,
Jagannath S, Madduri D, Liedtke M, Rosenblatt J, Maus MV, Turka A,
et al: Anti-BCMA CAR T-Cell Therapy bb2121 in relapsed or
refractory multiple myeloma. N Engl J Med. 380:1726–1737.
2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhao WH, Liu J, Wang BY, Chen YX, Cao XM,
Yang Y, Zhang YL, Wang FX, Zhang PY, Lei B, et al: A phase 1,
open-label study of LCAR-B38M, a chimeric antigen receptor T cell
therapy directed against B cell maturation antigen, in patients
with relapsed or refractory multiple myeloma. J Hematol Oncol.
11(141)2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Maus MV and June CH: Making better
chimeric antigen receptors for adoptive T-cell therapy. Clin Cancer
Res. 22:1875–1884. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Enblad G, Karlsson H, Gammelgard G, Wenthe
J, Lövgren T, Amini RM, Wikstrom KI, Essand M, Savoldo B, Hallböök
H, et al: A Phase I/IIa trial using CD19-Targeted Third-Generation
CAR T cells for lymphoma and leukemia. Clin Cancer Res.
24:6185–6194. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Schultz L and Mackall C: Driving CAR T
cell translation forward. Sci Transl Med.
11(eaaw2127)2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kansal R, Richardson N, Neeli I, Khawaja
S, Chamberlain D, Ghani M, Ghani QU, Balazs L, Beranova-Giorgianni
S, Giorgianni F, et al: Sustained B cell depletion by CD19-targeted
CAR T cells is a highly effective treatment for murine lupus. Sci
Transl Med. 11(eaav1648)2019.PubMed/NCBI View Article : Google Scholar
|