Introduction
Opioids are widely used during general anesthesia.
Among these, remifentanil is an ultra-short-acting µ-opioid
receptor agonist (1). Remifentanil
has a predictable and rapid recovery that is relatively independent
of the dose and duration of infusion; therefore, it can be given in
high doses until skin closure is observed with little risk of
delayed postoperative recovery or respiratory depression. However,
considerable evidence suggests that exposure to high-dose
remifentanil paradoxically enhances pain sensitivity and increases
analgesic requirements (2,3). A previous cohort study reported that
the incidence of postoperative hyperalgesia induced by remifentanil
was 41.8% when cumulative intraoperative infusions of remifentanil
exceeded 30 µg/kg (4). A corollary
of short action is that patients may experience considerable
surgical pain and agitation in the immediate postoperative
period.
The cyclo-oxygenase (COX) inhibitors
flurbiprofen-axetil and nalbuphine have been previously proposed as
adjunctive pre-anesthetics and analgesics for postoperative pain
control. Nalbuphine is a µ-antagonist and a partial κ-agonist for
G-proteins and β-arrestin-2. The role of nalbuphine in the
prevention of acute hyperalgesia may be due to its antagonistic
effect on µ receptors or modulatory action on central κ-receptors
(5). Flurbiprofen-axetil belongs
to the propionic acid derivative class of nonsteroidal
anti-inflammatory drugs (NSAIDs). Similar to other NSAIDs,
flurbiprofen-axetil is a cyclo-oxygenase inhibitor that blocks the
formation of prostaglandins, which are implicated extensively in
inflammatory lesions and certainly involved with inflammatory pain
and connective tissue destruction (6). A previous study reported that spinal
COX inhibition may be of importance in preventing acute
hyperalgesia following surgery (7). The mechanism of inhibition of
hyperalgesia is different in nalbuphine and flurbiprofen-axetil,
whether there is a difference between their preventative and
therapeutic effects, and the effect of combined application remains
to be elucidated. To provide a reference for clinical medication,
the present study compare the effects of nalbuphine and
flurbiprofen-axetil alone or in combination on patients with
remifentanil-induced hyperalgesia (RIH) during laparoscopic
cholecystectomy (LC).
Materials and methods
Study design and participants
A randomized, double-blind, clinical trial was
performed at The Second People's Hospital of Wuhu. The trial plan
was approved by the Ethics Committee of The Second People's
Hospital of Wuhu (approval number: 2021-07) on April 12, 2021. The
study was registered with the Chinese Clinical Trial Registry
(registration no. ChiCTR2100045347) on April 13th, 2021. Written
informed consent was obtained from each patient in the study, which
was performed between April 28th, 2021, and January 25th, 2022. The
study was performed in accordance with the Declaration of
Helsinki.
Patients were 20-65 years old, of any sex, American
Society of Anesthesiologists classification I-II, with a body mass
index of 18-30 kg/m2 (8). All patients were scheduled to undergo
LC. The exclusion criteria were as follows: i) Allergy or
contraindication to the experimental drugs; ii) any serious medical
problems other than the diseased gall bladder or psychiatric
conditions; iii) pregnancy; iv) a history of alcohol or drug abuse,
or chronic use of opioids or sedative drugs; or v) peptic ulcer
disease in the active stage. After randomization, if patients
required open surgery in the abdomen or if the duration of surgery
was >3 h, they were withdrawn from the study.
Randomization and masking
The investigators, who were blinded to the grouping,
prepared the randomized schedule. The randomized numbers generated
by the computer were enclosed in a sealed envelope. The
anesthesiologist received random numbers from the investigators and
divided the patients into four groups. The study drugs were
packaged in containers with the same color and packaging. Patients
were randomly allocated into the flurbiprofen-axetil group (F
group), nalbuphine group (N group), flurbiprofen-axetil combined
with nalbuphine group (FN group) or saline group (S group) in a
1:1:1:1 ratio. Throughout the study, patients, researchers,
anesthesiologists, surgeons, nurses in the post-anesthesia nursing
unit and wards, and perioperative observation index recorders were
all blinded to the allocation of patients to the study groups.
Study treatments
The day before surgery, the baseline mechanical
injury threshold of each patient was assessed. A set of 20
hand-held Von Frey filament (Aesthesio® Precision
Tactile Sensory Evaluator, DanMic Global, LLC) were used at 3, 6
and 9 cm distal to the middle of the non-dominant forearm elbow
crease to evaluate the threshold of mechanical hyperalgesia and
calculate the mean value. Peri incisional mechanical hyperalgesia
threshold was measured on an area 2 cm below the incision of the
infraumbilical trocar at 3 points (both ends and the middle) and
the mean values of hyperalgesia thresholds at these 3 points were
calculated and recorded (9). With
the patient's eyes closed, the investigator pressed the filament of
the Von Frey wire against the skin at a right angle until it bent.
The force was applied for 1 sec and then released. The von Frey
filament application started at 0.4 g and was increased until the
patient felt a pricking sensation. Each measurement was 30 sec
apart to avoid potential error caused by temporal summation. On the
day of LC, a medical monitor (ULTRAVIEW SL® 2700,
Spacelabs Healthcare, Inc.) was used to monitor the pulse, blood
pressure, electrocardiogram, oxygenation and end-tidal carbon
dioxide. Anesthesia was induced by intravenous administration of
remifentanil (1 µg/kg, iv.) and propofol (1-2 mg/kg, iv.). When the
bispectral index score (BIS) dropped to between 40-60, rocuronium
(0.8 mg/kg, iv.) was administered intravenously in all groups.
Maintenance of anesthesia was performed using 0.3
µg/kg/min remifentanil and 1-3% sevoflurane in all groups. The
lowest alveolar concentration was initially set at 2.0% and
adjusted gradually to acceptable hemodynamics including the mean
arterial blood pressure (MAP; -30 to +15%) and the heart rate (HR;
-40 to +15%). Rocuronium (0.2 mg/kg; iv.) was used to maintain
muscle relaxation. Ephedrine (10 mg; iv.) was administered when the
MAP decreased to <60 mmHg. Atropine (0.5 mg; iv.) was
administered when the HR dropped to <45 bpm. Furthermore,
granisetron (3 mg; iv.) was administered during the surgery to
prevent postoperative nausea and vomiting.
Prior to skin incision, patients receive treatment
with placebo (normal saline; 10 ml; iv.) in the S group;
flurbiprofen (flurbiprofen axetil; 50 mg; iv.) in the F group;
nalbuphine (nalbuphine; 0.1 mg/kg; iv.) in the N group; and
flurbiprofen (flurbiprofen axetil; 50 mg; iv.) combined with
nalbuphine (nalbuphine; 0.1 mg/kg; iv.) in the FN group. All drugs
were diluted to a final volume of 10 ml and the injection time did
not exceed 1 min in all groups. The syringe was wrapped in an
opaque sticker, and the anesthesiologist and the recorder were
blinded to the drug administered.
Following surgery, patients were administered 0.5%
ropivacaine solution percutaneously and subcutaneously (a total of
10 ml including 6 ml to the infraumbilical trocar and 4 ml to the
other two trocar locations). Following the recovery of adequate
spontaneous ventilation and response to verbal commands such as
opening of the eyes, the tracheal tube was removed when BIS values
reached 80. In terms of postoperative analgesia, the visual
analogue scale (VAS, scale 0-10) has been proposed to measure pain
intensity: 0 is no pain and 10 is the most severe pain (10). When initial postoperative pain (VAS
>4) after surgery was primarily managed using sufentanil (5 µg;
iv.) at intervals of 10 min until the VAS score reached <3.
After transfer to the general ward, patients were administered two
doses of flurbiprofen-axetil (50 mg, ivgtt.) per day. Dezocine (5
mg, iv.) was administered as a rescue analgesic upon patient
request or a reported VAS score >4.
Outcomes
The primary outcome was the mechanical hyperalgesia
threshold before and 24 h after the operation. The mechanical
hyperalgesia threshold was defined as the lowest force (g)
necessary to produce a pricking sensation.
The secondary outcomes included VAS and Ramsay
sedation scale (RSS) (11) at 0.5,
1, 4, and 24 h after surgery, perioperative hemodynamics (MAP and
HR), the number of patients using rescue analgesics at 24 h, and
adverse events such as nausea, vomiting, dizziness, headache and
hypoxemia. The MAP and HR were continuously measured and were
recorded immediately before induction of anesthesia (T1), after
induction (T2), after tracheal intubation (T3), after
pneumoperitoneum inflation (T4), after gallbladder removal (T5),
incision closure (T6) and after tracheal extubation (T7). The six
levels of the RSS were used for measurement of the depth of
sedation in patients by an experienced anesthesiologist (12).
Statistical analysis
The sample size was calculated based on VAS at 0.5 h
after surgery from our preliminary trial. The total sample of 116
subjects achieves 90% power (1-β) to detect differences among the
means vs. the alternative of equal means using an F test with a
0.05 significance level (α). The size of the variation in the means
is represented by their standard deviation which is 0.42. The
common standard deviation within a group is assumed to be 1.16.
Considering a possible loss to follow-up, we increased the sample
size by 10% (128 subjects for total sample). A Shapiro-Wilk test
was used to determine whether the sample population was normally
distributed across study parameters (P<0.05). Categorical data
were presented as the frequency (percentage) and were analyzed
using a χ2 test or Fisher's exact test. Continuous
variables were presented as the mean ± SD, or median (interquartile
range) and analyzed using a one-way ANOVA or Kruskal-Wallis H test.
Mechanical hyperalgesia threshold, VAS, RSS and hemodynamic
variables (MAP and HR) were analyzed using a two-way
repeated-measures ANOVA for inter-group comparisons. For multiple
comparisons, P-values were corrected using Bonferroni's correction.
P<0.05 was considered to indicate a statistically significant
difference. Statistical analysis was performed using SPSS version
25 (IBM Corp.).
Results
Patient characteristics
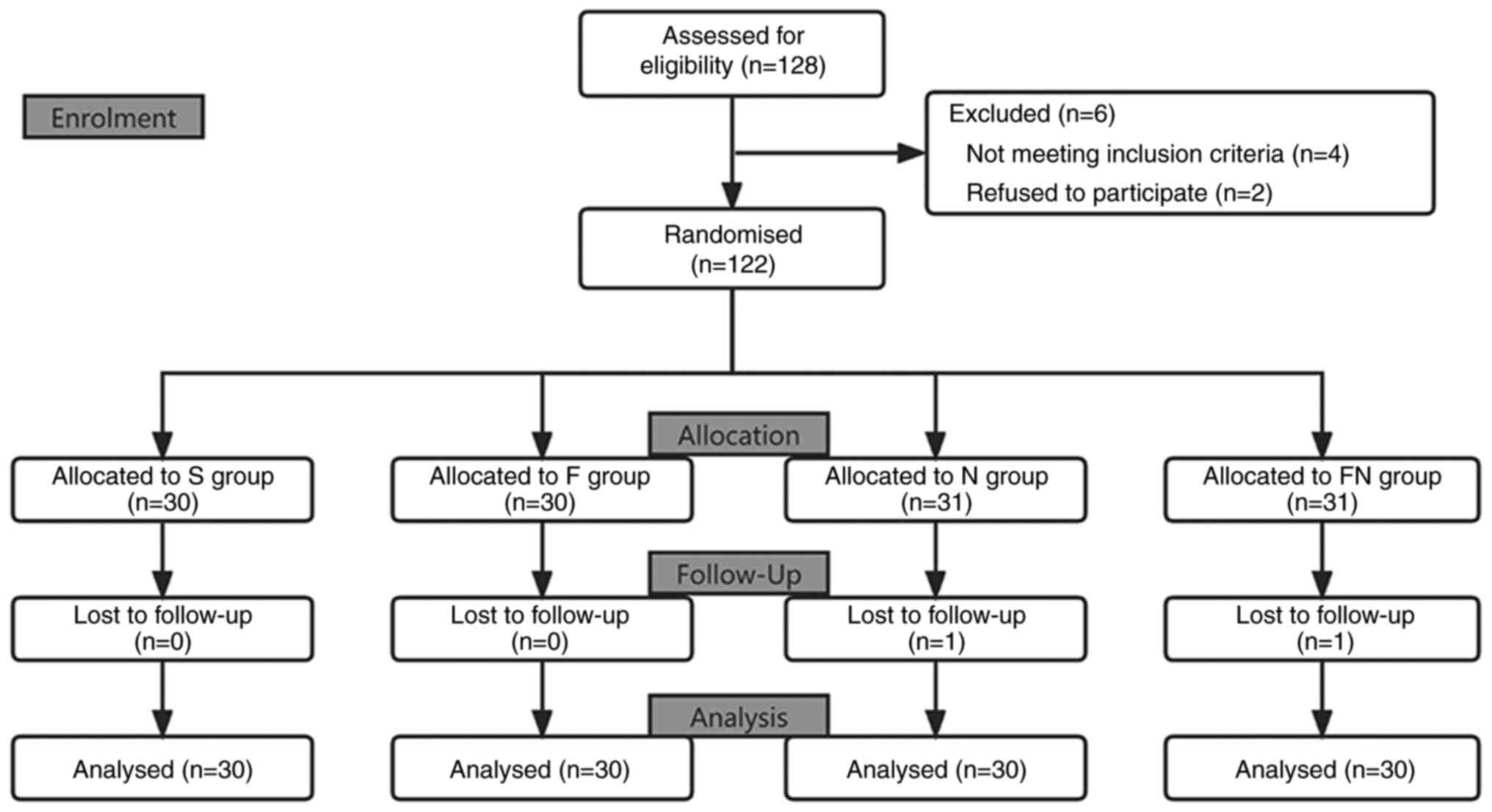
Between April 28, 2021 and January 25, 2022 a total
of 128 patients with LC were enrolled. Of these, 4 patients did not
meet the inclusion criteria, 2 patients refused to participate and
2 patients were ruled out as the surgery duration was >3 h. As
such, 120 patients were evaluated in the present study: 30 in the F
group, 30 in the N group, 30 in the FN group and 30 in the S group
(Fig. 1).
No significant differences were observed in the
demographics of the four groups. No significant differences were
observed in the intraoperative variables in four groups in terms of
duration of surgery, duration of anesthesia and medication
administered during surgery. There was no significant difference in
the pain threshold of the forearm or incision betweeN groups before
the operation (Table I). HR and
MAP did not significantly differ between the four groups at any of
the time points (Fig. 2).
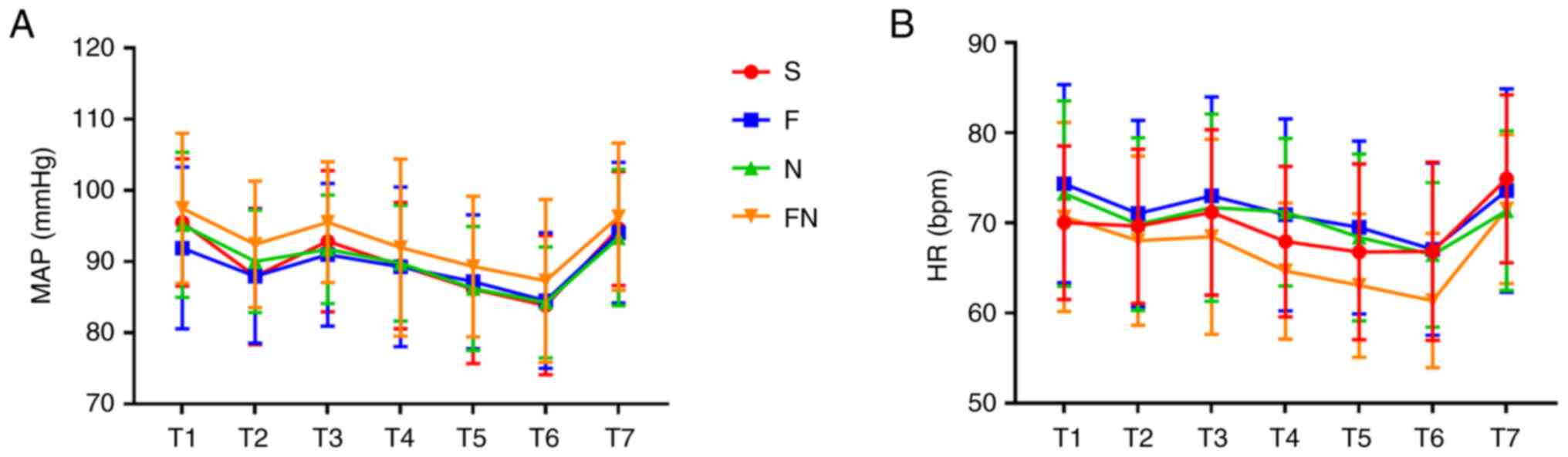 | Figure 2MAP and MAP were assessed during
surgery. (A) MAP and (B) HR at different time points during
surgery. Data are presented as mean ± standard deviation. MAP, mean
arterial pressure; HR, heart rate; S group, 0.3 ug kg-1
min-1 of remifentanil with saline group; F group, 0.3 ug
kg-1 min-1 of remifentanil with 50 mg of
flurbiprofen-axetil group; N group, 0.3 ug kg-1
min-1 of remifentanil with 0.1 mg kg-1 of
nalbuphine group; FN group, 0.3 ug kg-1 min-1
of remifentanil with flurbiprofen-axetil combined with nalbuphine
pretreatment; T1, before induction of anesthesia; T2, immediately
after induction; T3, immediately after tracheal intubation; T4,
immediately after pneumoperitoneum inflation; T5, at gallbladder
removal; T6, incision closure; T7, after tracheal extubation. |
 | Table IPatient baseline characteristics and
intraoperative variables. |
Table I
Patient baseline characteristics and
intraoperative variables.
| Characteristics and
intraoperative variables | S group (n=30) | F group (n=30) | N group (n=30) | FN group
(n=30) | P-value |
|---|
| Mean age ± SD,
years | 45.9±11.7 | 48.9±12.7 | 51.2±9.5 | 47.0±12.3 | 0.310a |
| Sex, no. (%) | | | | | 0.825b |
|
Female | 20 (66.7) | 18 (60.0) | 21 (70.0) | 21 (70.0) | |
|
Male | 10 (33.3) | 12 (40.0) | 9 (30.0) | 9 (30.0) | |
| Mean BMI ± SD,
kg/m2 | 25.0±2.2 | 24.8±3.0 | 25.0±2.4 | 25.9±2.4 | 0.355a |
| Median mechanical
pain threshold (IQR), g | | | | | |
|
Inner
forearm | 100.0 (60.0,
100.0) | 100.0 (60.0,
100.0) | 100.0 (60.0,
100.0) | 100.0 (60.0,
100.0) | 0.579c |
|
Surgical
incision area | 80.0 (60.0,
100.0) | 80.0 (60.0,
100.0) | 100.0 (60.0,
100.0) | 100.0 (60.0,
100.0) | 0.742c |
| Median duration of
anesthesia (IQR), min | 77.0 (67.3,
97.5) | 77.5 (63.8,
91.8) | 73.5 (65.0,
89.8) | 79.0 (65.0,
92.8) | 0.697c |
| Median duration of
surgery (IQR), min | 45.5 (34.8,
65.0) | 41.5 (34.8,
65.5) | 40.5 (30.0,
54.0) | 42.5 (33.5,
65.0) | 0.722c |
| Medication
administered during surgery | | | | | |
|
Median
remifentanil (IQR), µg | 991.5 (660.8,
1382.8) | 837.5 (618.3,
1344.5) | 808.5 (605.3,
1044.0) | 886.5 (630.0,
1313.3) | 0.748c |
|
Median
rocuronium (IQR), mg | 55.0 (45.0,
60.0) | 50.0 (45.0,
65.0) | 50.0 (45.0,
55.0) | 50.0 (50.0,
60.0) | 0.540c |
|
Median mean
sevoflurane (IQR), % | 2.8 (2.5, 3.0) | 2.8 (2.4, 3.0) | 2.9 (2.5, 3.0) | 2.8 (2.5, 3.0) | 0.427c |
|
Median
lactated Ringer's solution (IQR), ml | 760 (650, 885) | 695 (588, 813) | 740 (598, 863) | 780 (688, 850) | 0.407c |
Outcomes
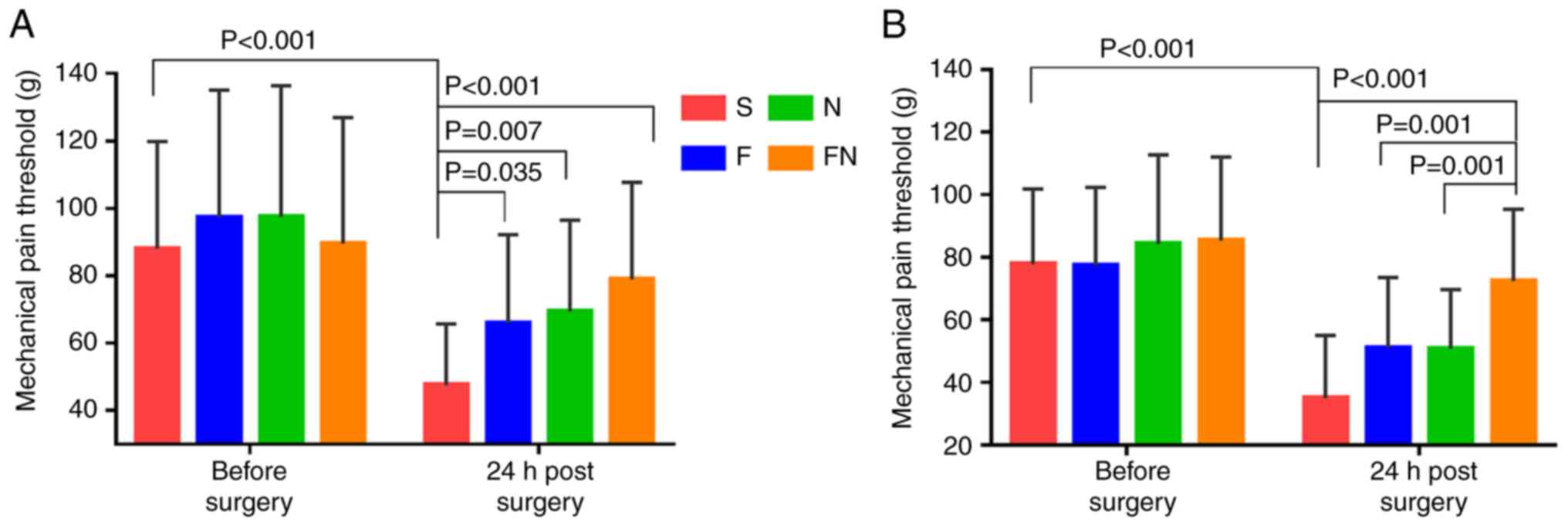
Preoperatively, the mechanical pain threshold on the
inner medial forearm was similar in all groups (P>0.05). A total
of 24 h after the operation, the pain threshold in the S group was
significantly lower compared with that prior to the operation.
Furthermore, the pain threshold was significantly lower in the S
group compared with the F, N and FN groups 24 h after surgery. The
pain threshold in the FN group did not differ significantly
compared with the F group and N group. There was no significant
difference in the pain threshold between the F and N groups
(Fig. 3A).
Preoperatively, the mechanical pain threshold was
similar in the peri-incisional area in all groups. The mechanical
hyperalgesia thresholds 24 h after surgery were significantly lower
in the S group compared with that prior to the operation.
Furthermore, the mechanical pain threshold in the S group was
significantly lower than that in the F, N and FN groups 24 h after
surgery. There was no significant difference in the pain threshold
between the F and N groups. At 24 h post-surgery, the mechanical
pain threshold in the FN group was significantly higher than that
in the F and N groups (Fig.
3B)
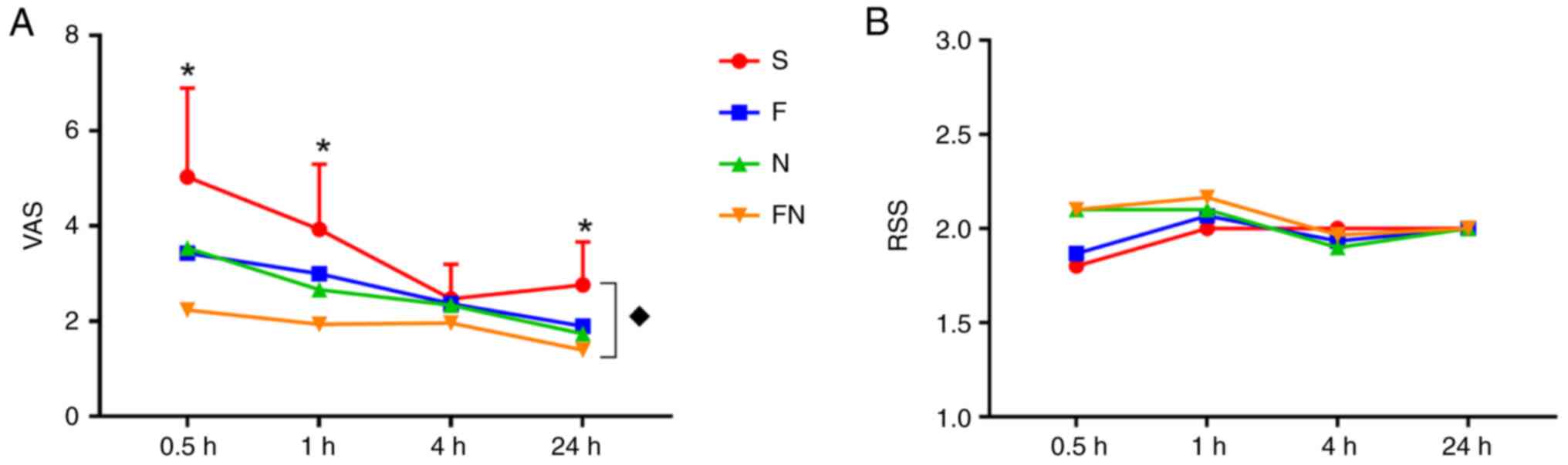
The VAS decreased gradually over time in all groups.
At 0.5, 1 and 24 h after surgery, there were statistically
significant differences between the S group and the other three
groups. There was no significant difference in the VAS between the
F and N groups at any of the time points assessed. The VAS of the
FN group was significantly lower than that in S groups 0.5, 1 and
24 h after surgery (Fig. 4A). No
significant differences were observed in the RSS (Fig. 4B).
There was a statistically significant difference in
the number of patients who required sufentanil amongst the four
groups. The length of time until sufentanil administration was
required in the S group was significantly shorter than that in the
other three groups. There was no significant difference in this
aspect amongst the F, N and FN groups. The total number of patients
taking analgesics within 0.5 h after surgery in the S group was
significantly higher than that in the other three groups. There was
no significant difference in the postoperative side effects amongst
the four groups (Table II).
 | Table IIPostoperative side effects. |
Table II
Postoperative side effects.
| Adverse events | S group (n=30) | F group (n=30) | N group (n=30) | FN (n=30) | P-value |
|---|
| Nausea, n (%) | 10 (33.3) | 4 (13.3) | 5 (16.7) | 3 (10.0) | 0.091a |
| Vomiting, n
(%) | 5 (16.7) | 2 (6.7) | 4 (13.3) | 3 (10.0) | 0.655a |
| Headache, n
(%) | 1 (3.3) | 0 | 1 (3.3) | 1 (3.3) | 0.795a |
| Dizziness, n
(%) | 5 (16.7) | 3 (10.0) | 3 (10.0) | 1 (3.3) | 0.397a |
| Respiratory
depression, no (%) | 0 | 0 | 0 | 0 | - |
| Use of rescue
analgesics, n (%) | | | | | |
|
0.5 h
postoperative period | 8 (26.7) | 2 (6.7) | 3 (10.0) | 1 (3.3) | 0.045a |
|
24 h
postoperative period | 11 (36.7) | 4 (13.3) | 5 (16.7) | 4 (13.3) | 0.069a |
Discussion
Remifentanil is an ultra-short half-life opioid,
that is rapidly metabolized by blood and tissue esterases, works
fast, has an excellent analgesic effect and long-term infusion does
not result in accumulation in the body, thus reducing the
occurrence of delayed awakening (13). However, due to its rapid metabolism
and lack of accumulation, its analgesic effects disappear rapidly
following withdrawal, inducing opioid-induced hyperalgesia (OIH)
and increasing the consumption of analgesic drugs (14,15).
Although OIH does not occur as frequently as other adverse
reactions associated with opioids, it leads to decreased patient
satisfaction. In addition, the incidence of adverse reactions is
higher following the addition of opiates, up to 6.2-10.2% (16,17).
The pathogenesis of RIH has been studied previously,
with reports including the activation of the central glutamate
pathway and the release of excitatory neurotransmitters such as
glutamate and substance P; downregulation of GABA type A receptor
(GABAAα2R) and
K+-cl-cotransporter-2 (KCC2); activation of
dynorphin; activation of n-methyl-d-L-aspartic acid receptor in
central sensitization of the posterior horn of the spinal cord;
changes in opioid receptor signaling, transient receptor potential
channels, increase in cytokines, neurokinin-1 receptors, serotonin
antagonist type 3, cholecystokinin and long-term potentiation (LTP)
and other transcriptional mechanisms (18-20).
Due to the complexity of the mechanism of RIH, it may be necessary
to use several drugs with different mechanisms to achieve a
suitable effect for the prevention of hyperalgesia. Numerous
strategies to alleviate RIH have been previously reported,
including the use of minimal doses of remifentanil, gradual
withdrawal of remifentanil infusion (21,22),
multimodal analgesia, as well as alternative therapy, such as
propofol (23), ketamine (24-26),
dexmedetomidine (27),
N2O (28) and COX
inhibitors (29).
Nalbuphine is a novel synthetic lipophilic opioid
receptor agonist, which acts primarily on the κ receptors at the
level of the spinal cord and thus acts as a spinal analgesic. It
has a long duration of action and can reduce the incidence of
adverse reactions (30). Hu et
al (5) reported that
preemptive nalbuphine can reduce postoperative hyperalgesia induced
by high-dose remifentanil and could reduce postoperative pain and
rescue analgesic consumption in patients undergoing laparoscopic
cholecystectomy. Flurbiprofen-axetil is an NSAID, which has
anti-inflammatory and analgesic effects via inhibition of
prostaglandin synthesis, which reduces the production of
inflammatory mediators, and reduces the inflammatory reaction and
tissue edema caused by surgical trauma. In a study of healthy
volunteers, Lenz et al (7)
reported that pre-intravenous administration of the COX inhibitor
ietorolac inhibited the biosynthesis of prostaglandin E (PGE) by
blocking the biological activity of COX, thus inhibiting the
activity of N-methyl-D-aspartate (NMDA) receptors, increasing the
pain threshold of volunteers, and reducing the central and
peripheral pain sensitivity. The present study evaluated whether
flurbiprofen-axetil and nalbuphine alone or in combination could
prevent RIH and whether the combination was more effective than
either treatment alone.
Patients who underwent abdominal surgery have
reported more moderate to severe pain in the chest and
musculoskeletal sites compared with patients who underwent surgery
of the skin and connective tissue (21). A previous study also reported a
higher incidence of pain after laparoscopy surgery (31). In a previous review, Yu et
al (32) reported that an
infusion of remifentanil (>0.2 µg/kg/min) was associated with
OIH. A study by Schmidt et al (33) compared low doses of remifentanil
(0.1 µg/kg/min) with high doses (0.4 µg/kg/min) and reported that
the mechanical pain threshold decreased more in the high-dose
group, and that the low dose of remifentanil (≥0.1 µg/kg/min or
≥12.7 ng/ml) appeared to be sufficient to induce hypersensitivity.
The incidence of OIH was reported to be significantly increased at
an infusion rate of 0.3±0.2 µg/kg/min, which indicated that
high-dose remifentanil was more likely to induce OIH. Therefore,
intraoperative remifentanil maintenance was controlled at 0.3
µg/kg/min. Propofol is the most commonly used intravenous
anesthetic and there is evidence that propofol may have a
modulatory effect on nociceptive processing and perception, and may
reduce the hyperalgesia induced by high-dose remifentanil during
intravenous anesthesia (23,34),
which may improve postoperative outcomes and analgesic drug
consumption. Therefore, in the present study, inhalation of
volatile agents was used to maintain anesthesia (to reduce the
interference of propofol in the results of the experiment) with the
concentration adjusted according to BIS value and vital signs
during surgery. The circulation of patients in all four groups was
stable during surgery.
The effect of RIH appeared to be greatest in the
early postoperative period (32).
Postoperative pain usually occurs between 24 and 72 h after surgery
(35,36). Most patients who undergo
laparoscopic cholecystectomy are discharged on the day of surgery
or the second day after surgery (37). It is more difficult to evaluate and
collect data for such patients at 48 h and beyond post-surgery. Hu
et al (5) reported that the
pain threshold of remifentanil induced postoperative hyperalgesia
in the control group was significantly lower than the preoperative
baseline 24 h after surgery, but there was no significant
difference 48 h after surgery. Therefore, the appropriate
indicators measured for 24 h after surgery was used to determine
whether there was an abnormal decrease in pain threshold following
opioid administration, characterized by increased perception of
pain following opioid-based anesthesia and surgery. The primary
measured outcome of the present study was the use of von Frey
filaments to assess the mechanical pain threshold. As pain is a
subjective and complex proprioceptive sensation, the mechanical
pain field was produced by the stimulation of a δ fiber in the
skin, which is safe, reliable and easy to use method. In addition,
given the complexity of the clinical situation, the pain threshold
was measured and the VAS was recorded at each time point after
surgery to better evaluate the effect of analgesia.
At 24 h post-surgery, the forearm and incision pain
thresholds in all four groups were markedly lower than those prior
to surgery, which indicated that continuous infusion of high-dose
remifentanil could induce marked hyperalgesia, this was in line
with a study by Angst et al (38) on the effect of remifentanil on
hyperalgesia in volunteers. There were significant differences in
the pain threshold in the forearm and incision among the four
groups. After surgery, the pain threshold of the forearm and
incision was significantly higher in the F group compared with that
in the S group, which was consistent with the results of a study by
Zhang et al (39). The
mechanism may be related to the inhibition of TNF-α and 5-HT
release, the alleviation of pain caused by Kinin and cytokines, and
the interaction with endogenous opioid peptides. However, the
mechanism could also be related to the inhibitory effect of COX-2
inhibitors on the activity of central NMDA receptors. Shimoyama
et al (40) reported the
role of NMDA receptors in the development of opioid tolerance and
hyperalgesia in a rat model by administering 18-polyphosphate
antisense oligodeoxynucleotides to interrupt the upregulation of
NMDA receptors. As to the mechanism of COX inhibitors acting on
RIH, it has been reported that COX inhibitors antagonize the
activation of NMDA receptors and COX inhibition in the spinal cord
serves an important role in decreasing hypersensitivity (7). This may be the reason why COX
inhibitors serve an important role in the prevention of
postoperative acute hyperalgesia. The pain threshold 24 h after
surgery in the N group was significantly higher than that in the S
group, consistent with the findings of Hu et al (5). The specific mechanism of nalbuphine
to prevent RIH is not clear; however, it may be related to its
activation of κ-receptors and its promotion of spinal analgesia
(30). The brain and spinal cord
are the primary sites of κ-receptors and the activation of
κ-receptors has a strong analgesic effect; therefore, nalbuphine
may promote the reduction of postoperative hyperalgesia. Another
possibility is that the activation of opioid receptors in the
dorsal root of the spinal nerve promotes the release of excitatory
neuropeptides. Blocking these opioid receptors in advance could
achieve the goal of preemptive analgesia (41). One of the mechanisms of RIH is the
release of Dynorphin, Dynorphin is a κ-opioid receptor-specific
ligand with endogenous anti-opioid effects (42). However, nalbuphine is also a
κ-opioid receptor agonist; therefore, it was hypothesized that
nalbuphine competes with Dynorphin for the κ-opioid receptor and
has an inhibitory effect on hyperalgesia; however, this needs to be
further assessed in future studies.
The forearm and incision pain thresholds 24 h after
surgery in the FN group were significantly higher than those in the
S group, which supported the aforementioned hypothesis that
Flurbiprofen-axetil combined with nalbuphine can prevented RIH
effectively. The pain threshold in the incision of the FN group was
significantly higher than that in the F and N groups; however, the
pain threshold in the forearm of the FN group did not differ
significantly from those of the F and N groups. The pain threshold
can be induced either by drugs, such as remifentanil, or as a
surgical nociceptor, a consequence of tissue and nerve trauma
(43). Therefore, incision pain
threshold may be a better indicator of RIH in patients with
trauma.
Shortly after the cessation of remifentanil
infusion, the pain grades in the F group, N group, and FN group
were all markedly lower than those in the control group. The
analgesic effect was greatest 30 min after cessation of infusion,
and the analgesic demand in the S group was significantly higher
than that in the other three groups 30 min after surgery, although
there was no significant difference in the total demand among the
four groups, which indicated that patients in the S group
experienced moderate and severe pain relatively sooner after
surgery compared with the other drugs. At 0.5 h after surgery, 8
patients (26.7%) in S group used rescue analgesics within the PACU,
which was more than that in the other three groups, but the total
number of patients requiring rescue analgesics within 24 h did not
differ significantly among the four groups.. The VAS gradually
decreased over time in all groups, but compared with the other
three groups, the S group still maintained a markedly higher VAS at
0.5, 1 and 24 h after surgery. At 4 h after surgery, there was no
significant different between the four groups. This may be related
to the gradual improvement of the inflammatory response or the
gradual activation of NMDA receptors by postoperative
flurbiprofen-axetil.
Previous studies have reported a poor association
between opioid-induced hyperalgesia and postoperative pain
(44,45). However, a correlation between pain
intensity or relief analgesic consumption and pain threshold was
demonstrated in the present study. This may be due to a
multifactorial modulation of clinical pain. In addition, there was
no significant difference in adverse events between the four
groups, which may be related to the high dose of remifentanil
activating the µ-opioid receptor (46), and the need for postoperative
analgesic drugs, such as sufentanil and dezocine. Nalbuphine can
reduce the incidence of opioid-associated adverse reactions such as
nausea, vomiting and skin pruritus related to G-protein
interactions (47). Nalbuphine can
even reverse the opioid-induced respiratory depression (42). However, in the present study, the
incidence of nausea and vomiting in the N group was not
significantly lower than that in the S group. This may be due to
the small sample size in the present study and further expansion of
the sample size is needed properly assess the effects of nalbuphine
in this respect. Adverse events associated with NSAID were
generally rare, with occasional alimentary tract adverse reactions
(48).
The present study has several limitations. Firstly,
the present study was a single-center study, and only one surgical
method was used as the research background, which may have resulted
in a selection bias. Secondly, hyperalgesia was measured using a
specific instrument (von Frey filament) and there may be errors in
this manual measurement. The analgesic flurbiprofen-axetil was
routinely administered postoperatively, and if the VAS was >4,
10 mg dezocine was administered intramuscularly, the administration
of these drugs may be a confounding factor in the results of the
present study; however, the findings of the present study may apply
to real world clinical settings. The criteria for intraoperative
drug administration require further study to compare the
differences between single or multiple-dose regimens, or alternate
continuous-infusion regimens. Finally, the present study, only
followed patients for 24 h after surgery, and did not show any
effect of preemptive analgesia on chronic pain due to hyperalgesia;
therefore, further studies are required to evaluate these clinical
results to guide the management of chronic pain, given that the
management of chronic postoperative pain remains a challenge for
anesthesiologists and surgeons.
In conclusion, both flurbiprofen-axetil alone and
nalbuphine alone effectively prevented RIH 24 h after surgery in
LC. The combination of two analgesic drugs with different
mechanisms of action was not superior to monotherapy.
Acknowledgements
The authors would like to thank Dr Chengyang Hu
(Department of Epidemiology and Biostatistics, School of Public
Health, Anhui Medical University, Hefei, China) for his help in
data analysis.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets generated during and/or analyzed during
the current study are available from the corresponding author on
reasonable request.
Author contributions
YZ and HM designed the study and wrote the first
draft of the manuscript. YZ, HM and JZ collected the clinical data.
YL designed the study and revised the manuscript. YZ and HM confirm
the authenticity of all the raw data. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the ethics
committee of The Second People's Hospital of Wuhu (approval number:
2021-07) on April 12, 2021. Written informed consent was obtained
from each patient.
Patient consent for publication
Written informed consent for publication was
obtained from all the patients.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Stroumpos C, Manolaraki M and Paspatis GA:
Remifentanil, a different opioid: Potential clinical applications
and safety aspects. Expert Opin Drug Saf. 9:355–364.
2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Su X, Zhu W, Tian Y, Tan L, Wu H and Wu L:
Regulatory effects of propofol on high-dose remifentanil-induced
hyperalgesia. Physiol Res. 69:157–164. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Song YK, Lee C, Seo DH, Park SN, Moon SY
and Park CH: Interaction between postoperative shivering and
hyperalgesia caused by high-dose remifentanil. Korean J
Anesthesiol. 66:44–51. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ma JF, Huang ZL, Li J, Hu SJ and Lian QQ:
Cohort study of remifentanil-induced hyperalgesia in postoperative
patients. Zhonghua Yi Xue Za Zhi. 91:977–979. 2011.PubMed/NCBI(In Chinese).
|
|
5
|
Hu J, Chen S, Zhu M, Wu Y, Wang P, Chen J
and Zhang Y: Preemptive nalbuphine attenuates remifentanil-induced
postoperative hyperalgesia after laparoscopic cholecystectomy: A
prospective randomized double-blind clinical trial. J Pain Res.
13:1915–1924. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wang RD, Sheng XR, Guan WX, Wang M, Peng
C, Yang YY, Huang HG and Ning-Li and Jia WD: Flurbiprofen axetil
for postoperative analgesia in upper abdominal surgery: A
randomized, parallel controlled, double-blind, multicenter clinical
study. Surg Today. 50:749–756. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lenz H, Raeder J, Draegni T, Heyerdahl F,
Schmelz M and Stubhaug A: Effects of COX inhibition on experimental
pain and hyperalgesia during and after remifentanil infusion in
humans. Pain. 152:1289–1297. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Horvath B, Kloesel B, Todd MM, Cole DJ and
Prielipp RC: The evolution, current value, and future of the
American society of anesthesiologists physical status
classification system. Anesthesiology. 135:904–919. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Subramaniam K, Ibarra A, Ruppert K,
Mallikarjun K and Orebaugh S: Intraoperative remifentanil infusion
and postoperative pain outcomes after cardiac surgery-results from
secondary analysis of a randomized, open-label clinical trial. J
Cardiothorac Vasc Anesth. 35:458–466. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Reed MD and Van Nostran W: Assessing pain
intensity with the visual analog scale: A plea for uniformity. J
Clin Pharmacol. 54:241–244. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Ramsay MA, Savege TM, Simpson BR and
Goodwin R: Controlled sedation with alphaxalone-alphadolone. Br Med
J. 2:656–659. 1974.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Allen SM and Madrio ME: Ramsay sedation
scale project: Small, easy changes for a big effect on patient
safety. Crit Care Nurse. 39:64–66. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Angst MS: Intraoperative use of
remifentanil for TIVA: Postoperative pain, acute tolerance, and
opioid-induced hyperalgesia. J Cardiothorac Vasc Anesth. 29 (Suppl
1):S16–S22. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Shu RC, Zhang LL, Wang CY, Li N, Wang HY,
Xie KL, Yu YH and Wang GL: Spinal peroxynitrite contributes to
remifentanil-induced postoperative hyperalgesia via enhancement of
divalent metal transporter 1 without iron-responsive
element-mediated iron accumulation in rats. Anesthesiology.
122:908–920. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Roeckel LA, Le Coz GM, Gavériaux-Ruff C
and Simonin F: Opioid-induced hyperalgesia: Cellular and molecular
mechanisms. Neuroscience. 338:160–182. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ing Lorenzini K, Wainstein L, Spechbach H,
Sarasin F, Ramlawi M, Desmeules J and Piguet V: Opioid-related
adverse drug reactions in patients visiting the emergency division
of a tertiary hospital. Pharmacol Res Perspect.
10(e01033)2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yiu CH, Gnjidic D, Patanwala A, Fong I,
Begley D, Khor KE, Rimington J, Bugeja B and Penm J: Opioid-related
adverse drug events in surgical patients: Risk factors and
association with clinical outcomes. Expert Opin Drug Saf.
21:1211–1223. 2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Gao Y, Zhan W, Jin Y, Chen X, Cai J, Zhou
X, Huang X, Zhao Q, Wang W and Sun J: KCC2 receptor upregulation
potentiates antinociceptive effect of GABAAR agonist on
remifentanil-induced hyperalgesia. Mol Pain.
18(17448069221082880)2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Fujii T and Nishiwaki K: Ketamine reduces
the dose of remifentanil required during prolonged head and neck
surgery: A propensity-matched analysis. Nagoya J Med Sci. 84:1–6.
2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Julius D: TRP channels and pain. Annu Rev
Cell Dev Biol. 29:355–384. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Huang YH, Lee MS, Lin YT, Huang NC, Kao J,
Lai HC, Lin BF, Cheng KI and Wu ZF: Postoperative drip-infusion of
remifentanil reduces postoperative pain-A retrospective observative
study. Int J Environ Res Public Health. 18(9225)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Saxena S, Gonsette K, Terram W, Huybrechts
I, Nahrwold DA, Cappello M, Barvais L and Engelman E: Gradual
withdrawal of remifentanil delays initial post-operative analgesic
demand after thyroid surgery; double-blinded, randomized controlled
trial. BMC Anesthesiol. 19(60)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Shin SW, Cho AR, Lee HJ, Kim HJ, Byeon GJ,
Yoon JW, Kim KH and Kwon JY: Maintenance anaesthetics during
remifentanil-based anaesthesia might affect postoperative pain
control after breast cancer surgery. Br J Anaesth. 105:661–667.
2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Choi E, Lee H, Park HS, Lee GY, Kim YJ and
Baik HJ: Effect of intraoperative infusion of ketamine on
remifentanil-induced hyperalgesia. Korean J Anesthesiol.
68:476–480. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ithnin FB, Tan DJA, Xu XL, Tan CH, Sultana
R and Sng BL: Low-dose S+ ketamine in target-controlled intravenous
anaesthesia with remifentanil and propofol for open gynaecological
surgery: A randomised controlled trial. Indian J Anaesth.
63:126–133. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Leal PC, Salomão R, Brunialti MK and
Sakata RK: Evaluation of the effect of ketamine on
remifentanil-induced hyperalgesia: A double-blind, randomized
study. J Clin Anesth. 27:331–337. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wu Z, Yu J, Lin Q, Li H, Zhang T, Tan H,
Lin W and Cao L: Effects of an intraoperative intravenous bolus
dose of dexmedetomidine on remifentanil-induced postinfusion
hyperalgesia in patients undergoing thyroidectomy: A double-blind
randomized controlled trial. Anesth Analg. 132:320–328.
2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wehrfritz A, Bauer M, Noel N, Ramirez-Gil
JF, Ihmsen H, Prottengeier J, Schüttler J and Bessiere B:
Evaluation of antihyperalgesic and analgesic effects of 35% nitrous
oxide when combined with remifentanil: A randomised phase 1 trial
in volunteers. Eur J Anaesthesiol. 38:1230–1241. 2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yalcin N, Uzun ST, Reisli R, Borazan H and
Otelcioglu S: A comparison of ketamine and paracetamol for
preventing remifentanil induced hyperalgesia in patients undergoing
total abdominal hysterectomy. Int J Med Sci. 9:327–333.
2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Gress K, Charipova K, Jung JW, Kaye AD,
Paladini A, Varrassi G, Viswanath O and Urits I: A comprehensive
review of partial opioid agonists for the treatment of chronic
pain. Best Pract Res Clin Anaesthesiol. 34:449–461. 2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
McGrail K, Chapple AG, Stone G, Sutton EF
and Chappell NR: Systematic review and meta-analysis of
perioperative administration of acetazolamide for management of
postoperative pain after laparoscopy. JSLS.
26(e2022.00032)2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yu EH, Tran DH, Lam SW and Irwin MG:
Remifentanil tolerance and hyperalgesia: Short-term gain, long-term
pain? Anaesthesia. 71:1347–1362. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Schmidt S, Bethge C, Förster MH and
Schäfer M: Enhanced postoperative sensitivity to painful pressure
stimulation after intraoperative high dose remifentanil in patients
without significant surgical site pain. Clin J Pain. 23:605–611.
2007.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Santonocito C, Noto A, Crimi C and
Sanfilippo F: Remifentanil-induced postoperative hyperalgesia:
Current perspectives on mechanisms and therapeutic strategies.
Local Reg Anesth. 11:15–23. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Small C and Laycock H: Acute postoperative
pain management. Br J Surg. 107:e70–e80. 2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Jiang B and Ye S: Pharmacotherapeutic pain
management in patients undergoing laparoscopic cholecystectomy: A
review. Adv Clin Exp Med. 31:1275–1288. 2022.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Tang H, Dong A and Yan L: Day surgery vs.
overnight stay laparoscopic cholecystectomy: A systematic review
and meta-analysis. Dig Liver Dis. 47:556–561. 2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Angst MS, Koppert W, Pahl I, Clark DJ and
Schmelz M: Short-term infusion of the mu-opioid agonist
remifentanil in humans causes hyperalgesia during withdrawal. Pain.
106:49–57. 2003.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zhang L, Shu R, Zhao Q, Li Y, Wang C, Wang
H, Yu Y and Wang G: Preoperative but not postoperative flurbiprofen
axetil alleviates remifentanil-induced hyperalgesia after
laparoscopic gynecological surgery: A prospective, randomized,
double-blinded, trial. Clin J Pain. 33:435–442. 2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Shimoyama N, Shimoyama M, Davis AM,
Monaghan DT and Inturrisi CE: An antisense oligonucleotide to the
N-methyl-D-aspartate (NMDA) subunit NMDAR1 attenuates NMDA-induced
nociception, hyperalgesia, and morphine tolerance. J Pharmacol Exp
Ther. 312:834–840. 2005.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Yu P, Zhang J and Wang J: Nalbuphine for
spinal anesthesia: A systematic review and meta-analysis. Pain
Pract. 22:91–106. 2022.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Jannuzzi RG: Nalbuphine for treatment of
opioid-induced pruritus: A systematic review of literature. Clin J
Pain. 32:87–93. 2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Weinbroum AA: Postoperative hyperalgesia-A
clinically applicable narrative review. Pharmacol Res. 120:188–205.
2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Koo CH, Yoon S, Kim BR, Cho YJ, Kim TK,
Jeon Y and Seo JH: Intraoperative naloxone reduces
remifentanil-induced postoperative hyperalgesia but not pain: A
randomized controlled trial. Br J Anaesth. 119:1161–1168.
2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Song JW, Lee YW, Yoon KB, Park SJ and Shim
YH: Magnesium sulfate prevents remifentanil-induced postoperative
hyperalgesia in patients undergoing thyroidectomy. Anesth Analg.
113:390–397. 2011.PubMed/NCBI View Article : Google Scholar
|
|
46
|
de Boer HD, Detriche O and Forget P:
Opioid-related side effects: Postoperative ileus, urinary
retention, nausea and vomiting, and shivering. A review of the
literature. Best Pract Res Clin Anaesthesiol. 31:499–504.
2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Davis MP, Fernandez C, Regel S and
McPherson ML: Does nalbuphine have a niche in managing pain? J
Opioid Manag. 14:143–151. 2018.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Martinez L, Ekman E and Nakhla N:
Perioperative opioid-sparing strategies: Utility of conventional
NSAIDs in adults. Clin Ther. 41:2612–2628. 2019.PubMed/NCBI View Article : Google Scholar
|