1. Introduction
Cervical cancer (CC), as one of the most common
causes of female mortality, poses a serious threat to women's lives
and health. Globally, in 2020, there were an estimated 604,127 CC
cases and 341,831 related deaths, with a corresponding
age-standardized incidence of 13.3 cases per 100,000 women-years
and a mortality rate of 7.2 deaths per 100,000 women-years
(1). Lymph node metastasis (LNM)
is the most common type of CC metastasis and is closely related to
prognosis. The more extensive the LNM is, the worse the prognosis
of patients. Studies have confirmed that the overall 5-year
survival rates of CC patients with 0, 1-2, 3-9 and 10 or more
metastatic lymph nodes are 90, 69, 57 and 35%, respectively
(2). According to the 2009 FIGO
staging principle, LNM does not affect the International Federation
of Obstetrics and Gynecology (FIGO) CC staging. However, the FIGO
staging system released in 2018 clearly states that once CC
patients are diagnosed with LNM, they can be directly diagnosed
with stage IIIC or above CC, which fully demonstrates the important
role of LNM in the progression of CC (3). Unfortunately, little is known
regarding the LNM mechanism in CC, which remains one of the biggest
challenges in treating CC (4).
The tumor microenvironment (TME) is primarily
composed of fibroblasts, endothelial cells, different subsets of
infiltrating immune cells (IICs), bone marrow-derived progenitor
cells, platelets, and inflammatory cytokines (5). Previous studies have confirmed that
tumor cells or host-derived cells (immune cells and fibroblasts,
amongst others) in the TME can release various lymphatic angiogenic
factors, such as vascular endothelial growth factor (VEGF)-A, C and
D, lymphatic vascular factor angiogenin-2, and hepatocyte growth
factor, which can stimulate angiogenesis and lymphangiogenesis
(6). Tumor cells, tumor stromal
cells, and infiltrating white blood cells release chemokines to
recruit different immune cell types into the TME. Chemokines can be
grouped into four main classes, depending on the location of the
first two cysteine (C) residues of their primary protein structure,
namely, the C, CC, CXC and CX3C chemokines. All chemokines signal
by binding to cognate heterotrimeric G protein-coupled receptors
(GPCRs) of the rhodopsin-like family found on migratory cells
(7). According to the special
needs of migration in each environment, chemokines can act as tumor
angiogenesis media, directly interact with chemokine receptors on
endothelial cells, and induce tumors to promote the release of
growth factors. These growth factors can promote tumor growth in a
paracrine signaling manner, thus improving migration,
proliferation, and endothelial cell survival. In addition,
chemokines can also cooperate with other angiogenesis promoters.
For example, VEGF-, CXCL8- and CXCL12-induced upregulation of VEGF
expression produces a positive feedback effect, and VEGF further
stimulates the production of angiogenic chemokines (8). In addition, lymphatic endothelial
cells (LECs) in tumor-draining lymph nodes have been proven to
proliferate, leading to the expansion of the lymphatic sinus
(9). Hypoxia can stimulate the
formation of lymphatic vessels (lymphangiogenesis) and blood
(angiogenesis), such that cancer cells can escape from the
unfavorable tumor microenvironment and spread to an environment
conducive to its survival, ultimately leading to metastatic
diseases and mortality (10).
Currently, research has confirmed that hypoxia promotes lymphatic
metastasis primarily through HIF-1α promoting VEGF-A/-C/-D, TGF-β
transcriptional activation of lymphatic vessel generation mediated
by signal cascades such as Prox-1. Multiple factors, such as ET-1,
C/EBP- δ, EGR-1, AP-1, MIF and NF-κB, can also promote lymphatic
metastasis by promoting the proliferation and migration of LECs
(11).
In the present review, the known mechanisms of
hypoxia, and the involvement of stromal components and immune
inflammatory cells in the tumor microenvironment in lymphatic
metastasis of CC is discussed, and a summary of the clinical trials
for strategies targeting the tumor microenvironment for the
treatment of CC is provided.
2. Mechanism of lymphatic metastasis in
cancer
Tumor cell entry into the lymphatic vasculature is
the first step of metastasis. The lymphatic system primarily
regulates fluid homeostasis and the immune response. Lymphatic
metastasis plays an active role in the spread and metastasis of
primary tumors (12). Similar to
angiogenesis, lymphangiogenesis is a multi-step process. On the one
hand, activated LECs proliferate and migrate under specific stimuli
to form new blood vessels; on the other hand, cancer cells invading
the afferent lymphatic vessels spread to the tumor-draining lymph
nodes, which are an important hub for the stagnation and growth of
metastatic cells, immune regulation, and secondary dissemination to
distant sites (13). The process
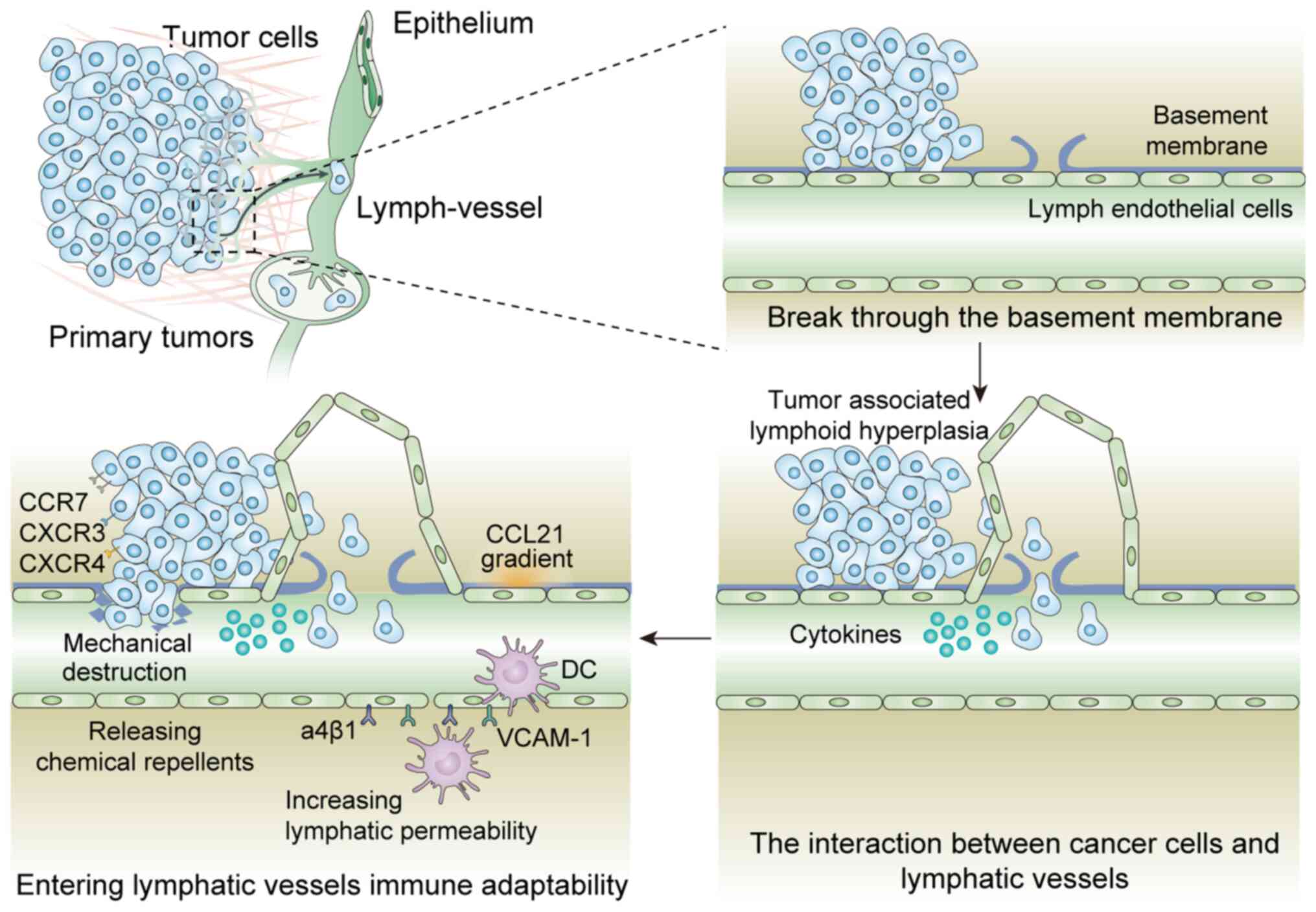
of tumor cells entering lymphatic vessels primarily includes the
following steps: i) Cancer cell invasion through the basement
membrane; ii) tumor-associated lymphoid hyperplasia; iii) tumor
cell recruitment and clustering around lymphatic vessels; iv)
secretion of cytokines by lymph endothelial cells to change the
microenvironment and cause immune escape; and v) tumor cell entry
into lymphatic vessels. The primary mechanisms of the last method
include: i) Mechanical destruction of the lymphatic endothelial
wall; ii) infiltration dependent on the CCL21 concentration
gradient and via the lymphatic endothelial valve; iii) increased
lymphatic permeability induced by mechanisms such as upregulation
of α4β1 integrin and its ligand VCAM-1 in LECs; iv) release of
chemicals to induce contraction of LECs to form an invasion site.
The specific mechanisms are shown in Fig. 1.
Cancer cell invasion through the
basement membrane
Tumor cells at the invasion front usually show
infiltrative behaviors and penetrate into surrounding tissues in
the form of cell stripes or clusters or individual cells, and this
process is primarily mediated by epithelial to mesenchymal
transformation (EMT) in cancer cells, which is an evolutionarily
conserved cell process and is critical to embryogenesis and
pathological reactions (such as wound healing or tissue repair)
(14). EMT mainly occurs by
activating Wnt or TGF-β signal transduction, hypoxia, and
inflammation-related pathways, further inducing the expression of
several key transcription factors of the twist, Snai1 and Zeb
families in the TME (15). In
turn, these transcription factors mediate several phenotypic
changes in cancer cells; they mediate the downregulation of
epithelial traits (including cell polarity and cell connectivity)
and induction of mesenchymal characteristics, such as cytoskeleton
remodeling and the expression of extracellular matrix
(ECM)-degrading proteases, allowing cells to invade surrounding
tissue effortlessly. It is worth noting that EMT is a gradual
process and may occur throughout the entire process of tumor
progression (16).
Tumor-associated
lymphangiogenesis
Research has revealed that increased expression of
the lymphatic angiogenic factors VEGF-C and VEGF-D can
significantly promote LNM in esophageal squamous cell carcinoma
(17). Compared with nonmetastatic
tumors, metastatic melanoma is characterized by increased
lymphangiogenesis, and the degree of tumor lymphangiogenesis is an
important indicator for predicting the overall survival rate and
LNM in patients (18). Tumor
lymphangiogenesis and VEGF-C expression can serve as indicators of
sentinel LNM during surgical resection of primary melanoma
(19). In addition to increasing
the quality of tumor-related lymphatic vessels, lymphangiogenic
factors can also increase the expression of chemokines or adhesion
molecules and receptors involved in tumor cell-LEC interactions by
activating lymphatic endothelial cells, thus actively promoting
cancer dissemination (20). In
general, lymphatic hyperplasia occurs first in tumor LNM. The
increased number and size of peripheral lymphatic vessels may
provide more opportunities for cancer cells to infiltrate (lymph
infiltration), but tumor drainage lymphatic vessels may also
promote tumor proliferation by increasing lymphatic flow and
drainage mediated by VEGF-C (21).
Tumor cells are attracted to and
cluster around lymphatic vessels
Lymphatic hyperplasia serves as a prerequisite for
LNM, which primarily involves cancer cell migration to lymphatic
vessels and recruitment of cancer cells and supporting cells to the
lymph nodes, providing a very conducive tumor microenvironment for
cancer stem cells; this microenvironment regulates the antitumor
immune response at the level of primary tumors and metastatic lymph
nodes. In most normal tissues, lymphatic vessels can secrete a
large amount of the chemokine CCL21, which can enter the lymphatic
system by binding to the activated CCR7 receptor on DCs and can
ultimately be excreted from lymph nodes to initiate an in
vivo immune response (22).
In vitro studies have confirmed that enhanced lymphatic flow
can increase CCL21 production in the lymphatic endothelium
(23). Importantly, when CCR7 was
overexpressed in transplanted melanoma cells, LNM was greatly
enhanced in an experimental tumor model, which indicates that LECs
can serve as guiding clues for metastatic cancer cells in the
physiological function of the immune system (24).
Chemokine CXCL12 (matrix-derived factor 1) was
reported for the first time as a dependent factor involved in the
in vivo lymphatic metastasis of Paget's disease (25). CXCL12 is upregulated in the
lymphatic vascular endothelium of the subcapsular sinus of
tumor-draining lymph nodes and tumor-associated lymphatic
endothelial cells, while its receptor CXCR4 is expressed by
invading tumor cells (26). In
addition, the expression of CXCR3 in tumor cells is also associated
with an increased LNM rate (27).
CCL1 chemokines produced by the lymphatic sinuses mediate the entry
of melanoma cells expressing CCR8 into the lymph nodes while
blocking CCR8 suppresses LN metastasis. In particular, CCR8
inhibition leads to the stagnation of tumor cells in clusters of
lymphatic vessels at the junction with the subcapsular LN sinus
(28).
Tumor cell intravasation into
lymphatic vessels
Previous studies have confirmed that tumor cells
enter lymphatic vessels primarily via one of four ways: i)
Mechanical injury or destruction of the lymphatic endothelial wall;
ii) by detection of a CCL21 gradient and infiltrating through the
junctions of the lymphatic endothelial valves; iii) by increasing
lymphatic permeability, such as through cell growth factor
(TGF-β1)-induced upregulation of α4β1 integrin and its ligand
VCAM-1 in LECs; and iv) LEC contraction and formation of the
invasion gate (CCID) induced by the release of chemical repellents
[such as 12 (s)-hete] (29).
Additionally, research has confirmed that CCR7+ cancer cells can
escape the primary tumor and drift to the tumor-draining lymph
nodes by increasing the expression of CCL21 in tumor-related
lymphatic vessels via VEGF-C (30).
Lymph endothelial cells secrete
cytokines to alter the microenvironment to facilitate immune
escape
Lymph endothelium can not only provide a stable
microenvironment for a cancer with stem cell-like characteristics
but also provides a protective environment for long-term survival
and subsequent metastasis of tumor cells. In addition, tumor cells
may remain dormant in draining lymph nodes for a long period of
time after primary tumor resection (31). The clinical observation of
so-called ‘transit metastasis’ (that is, a metastatic tumor that
has developed in the lymphatic vessel between the draining LN and
the primary tumor) revealed that CXCR4 melanoma cells (CD133+
melanoma cells) were located near the lymphatic vessel-producing
CXCL12 in the metastatic lymph nodes and lungs. The metastatic
activity of CXCR4+/CD133+ cells was higher than that of
CXCR4-/CD13- cells. The study also confirmed that the combined use
of the alkylating agent dacarbazine and CXCR4 blocker, which are
widely used to treat human melanoma, is significantly better than
the single use of dacarbazine in inhibiting the growth, migration,
and metastasis of melanoma (32).
In addition to these direct effects of LECs on the survival of
cancer cells, lymphatic vessels may also provide an
immunoprotective microenvironment through the secretion of
chemokines (33). Studies have
shown that CCL21 may transform the host immune response from
immunogenicity to tolerance, which may potentially promote tumor
progression (34). It has been
reported that LN lymphatic vessels activated by VEGF-C not only
promote tumor metastasis of melanoma but also induce immune
tolerance, which increases the difficulty of treatment (35). Recently, study revealed that LECs
induce tolerance via programmed cell death 1 (PD1) ligand 1 (PD-L1)
and lack of costimulation leading to high-level PD-1 expression on
CD8 T cells (36). Therefore, the
activities and interactions of various cells in the TME play a
decisive role in lymphatic metastasis.
3. Role of the tumor microenvironment in the
lymphatic metastasis of CC
Characteristics of the tumor
microenvironment in CC
The unique tumor microenvironment of CC is primarily
estrogen receptor α (ERα) matrix activation, sustained high-risk
human papillomavirus infection, hypoxia, and matrix and immune
inflammatory cells (mainly including CAFs, TAMs and MDSCs), T
cells, and neutrophils, which ultimately promote angiogenesis and
inflammation (37,38). First, 17-β-estradiol (E2) interacts
with the matrix ERα on the surface of myofibroblasts and
fibroblasts and further induces an increase in the secretion of
anti-apoptotic factors, inflammatory chemokines, extracellular
matrix enzymes, and proangiogenic factors. In addition, epithelial
cells with persistent high-risk HPV infection can attract monocytes
(monocyte chemoattractant protein-1 and macrophage inflammatory
protein-3α), natural killer cells, and Th17 lymphocytes. Positive
expression of cancer proteins (E6 and E7) in high-risk HPV strains
can trigger a series of events to promote CC metastasis through
their host cervical epithelial cells. In short, in contrast to the
matrix progesterone cascade mediated by the progesterone receptor
(PR), the synergistic effect between the activity of stromal ERα
and high-risk HPV oncoproteins induces CC proliferation and
promotes inflammation and angiogenesis, participating in
mesenchymal-epithelial and EMT changes (39,40).
In CC, the lymphatic vessels in the diffusion area
of tumor cells are in a dynamic process of change, and the
lymphatic changes that promote tumor metastasis play a leading role
in solid tumor metastasis (41).
The biggest obstacle for tumor cells to invade lymphatic vessels is
the intact lymphatic vessel structure, which makes it difficult for
them to break through. The integrity of lymphatic vessels is mainly
related to proteins in the adhesion links between LECs (42). In addition, the complete lymphatic
endothelial barrier and sound repair function also depend on the
interstitial cells and their secreted cytokines in the surrounding
environment (43). The stromal
cells and immunoinflammatory cells in the TME can secrete certain
cytokines, such as VEGF-C/D, to promote the formation of lymphatic
vessels, induce the inactivation of CD4+ and CD8+ T cells, help
tumor cells escape immune surveillance, induce tumor cell EMT and
enhance tumor cell invasive ability, finally leading to lymphatic
metastasis of CC (44). Studies
have confirmed that hypoxia can induce the enhancement of the
invasive capacity of CC cells and foster a tumor-supporting
environment through increased CCL8 secretion and TAM recruitment to
promote lymphatic vessel entry and angiogenesis (38). In this review, the mechanisms of
hypoxia, and the involvement of stromal components and immune
inflammatory cells in the tumor microenvironment in the lymphatic
metastasis of CC is summarized.
The role of hypoxia in the lymphatic
metastasis of CC
Hypoxia is a key factor that has been identified to
lead to a poor prognosis in patients with prostate cancer,
pancreatic cancer, breast cancer, head and neck cancer, and other
types of tumors. Targeting hypoxia is one of the directions of LNM
imaging and staging. Therefore, addressing tumor regional hypoxia
is an urgent problem to improve cancer prognosis. The main reasons
for the formation of a hypoxic tumor microenvironment are
imbalances in blood supply and abnormal tumor metabolism. Once the
hypoxic microenvironment is formed, the tumor invasive and
metastatic abilities are enhanced, and the tumor cells can present
features of a dormant state to avoid immune surveillance, resulting
in the failure of immunotherapy. In addition, the activation of HIF
can induce EMT, which is the first step for tumor cells to break
through the basement membrane and metastasize to a distant location
(45). In addition, hypoxia has
been proven to stimulate molecular changes in cancer cells to
facilitate a state of mitotic arrest to make the cells appear
dormant. Increasing evidence shows that dormancy is crucial for
cancer cell survival; the cells need to delay their invasive
behaviors until the distant ‘hostile’ microenvironment is
transformed to enable them to escape from immune surveillance upon
treatment (46). However, the
underlying mechanisms of cancer cell EMT, progression and escape
from apoptosis/necrosis in hypoxic environments remain unclear. Ju
et al (47) found that
CSN8, as a key factor in hypoxia-induced cell dormancy and EMT
occurrence, can promote colorectal cancer cells to evade immune
surveillance and attack, significantly improving their invasive and
metastatic ability. Hsin et al (48) found that upregulation of carbonic
anhydrase IX can promote EMT, and this phenotype combined with
upregulation of PFKFB4 ultimately improves the migration and
metastasis of CC cells. Hypoxia induces EMT in CC to facilitate
further lymphatic metastasis.
The difficulty in early prediction and research of
LNM lies in the lack of highly specific molecular markers,
especially markers of lymphatic vessels and blood vessels around
tumors, which has led to extremely slow progress in research on LNM
for decades. Breakthroughs have been made following the discovery
of VEGF-C/D and its specific receptor VEGFR-3; thus, research on
lymphatic metastasis is gradually increasing. Sugiura et al
(49) found that in oral tumors,
hypoxia in the surgical area can lead to an increase in local
lymphangiogenesis, accompanied by a significant increase in CD11b+
cell infiltration and LNM. In CC, Cairns and Hill (50) found that acute hypoxia can reduce
the volume of the primary tumor lesion in nude mice but
significantly increases the number of LNMs. Chaudary et al
(51) found that hypoxic treatment
of CC cells can significantly upregulate VEGFR3 and promote
lymphatic metastasis. Downregulation of VEGFR3/VEGFC or the use of
VEGFR3/VEGFC blockers can significantly reduce hypoxia-induced
tumor lymphatic proliferation and metastasis. These studies further
confirm that hypoxic conditions are more conducive to the increase
in VEGFR3/VEGFC and promote the lymphatic metastasis of CC. Lee
et al (52) found that the
prognosis of CC patients with LNM is poor. Identifying novel
treatment methods based on the expression of CA9 to prevent LNM is
expected to significantly improve the prognosis of CC patients. CA9
is currently recognized as one of the most commonly used markers of
hypoxia (53,54). Kim et al (55) found that extended-field irradiation
(EFI) has a significant inhibitory effect on the recurrence of
para-aortic lymph nodes in CA9-positive tumor patients, but it is
not significant for improving long-term survival. The reason may be
related to increased local and distant metastasis rates. Hypoxia
promotes LNM by inducing the release of lymphangiogenic factors,
such as VEGF-C, from malignant tumor cells to induce lymphatic
dilation (lymphangiogenesis) of the primary tumor and draining
sentinel LN.
Hypoxia can also lead to lymphatic metastasis
through the release of lymphangiogenic active factors and other
factors that recruit TAMs to accumulate in lymphatic vessels and
form metastatic areas. Chen et al (56) found that high expression of ZEB1
under hypoxic conditions can promote tumor metastasis by increasing
TAM recruitment and CCL8 secretion. Targeting ZEB1 can improve the
prognosis of patients with metastatic tumors by destroying the
hypoxic microenvironment. They also showed that hypoxic TAMs near
lymphatic vessels are the primary cells producing IL-10, and a
sharp increase in IL-10 concentration induces upregulation of Sp1
expression in LECs, promoting lymphatic angiogenesis in tumors
(57). They further confirmed that
macrophages recruited to the hypoxic microenvironment of CC tend to
transform into the M2 phenotype and induce an increase in Nrp-1 in
CC. This suggests that reversing the polarization of TAM towards an
M2 phenotype and interfering with Nrp-1 represents a novel strategy
for improving the hypoxic microenvironment of CC (58). As an important feature of solid
tumors, hypoxia accompanies almost the entire process of tumor
metastasis. The hypoxia of the tumor increases invasion and
metastasis and helps disguise tumor cells as cells in a dormant
state to avoid immune monitoring and attack, increasing the risk of
chemotherapy and immunotherapy resistance. Targeted hypoxia therapy
is an important direction for improving patient prognosis in the
future.
The role of CAFs, TAMs, MDSCs, and
immunoinflammatory cells in the lymphatic metastasis of CC
In addition to hypoxia, stromal components and
inflammatory immune cells also play an essential role in the
process of lymphatic metastasis of CC. These cells will change in
morphology and function with tumor progression to promote tumor
invasion, migration, lymphatic metastasis, and hematogenous
migration. Here, a focus on the role of CAFs, TAMs, MDSCs, and
immune cells in the lymphatic metastasis of CC is discussed.
CAFs
One of the primary obstacles in tumor cell
infiltration through the lymphatic system is the integrity of the
lymphatic endothelium, which is closely related to the protein
complexes that make up junctions between endothelial cells. In
addition, cell homeostasis, and cytokine dynamic balance in the
microenvironment around lymphatic vessels are the basis for
maintaining the structural integrity and barrier function of the
lymphatic endothelium. CAFs have important physiological functions
in maintaining the stability and integrity of most tissues. This
function is realized during the progression of metastasis and can
induce the formation of a metastasis-promoting microenvironment
(43).
Activated CAFs can change the components of the ECM
to reshape the tumor microenvironment, promote local angiogenesis,
tumor cell proliferation, and metastasis and play a role in the
formation of chemotherapy resistance by activating multiple
signaling pathways and secreting activated cytokines. The signaling
of CAFs and tumor cells plays an important role in tumor
progression and treatment. The current view is that CAFs and the
tumor immune microenvironment (TIME) mainly promote tumor
progression. The antitumor components in the TIME and TME are in
dynamic balance and are primarily composed of immune cell
populations in tumors. CAFs interact with tumor-infiltrating immune
cells to form a tumor-immune suppressive microenvironment by
secreting growth factors, cytokines, exosomes, chemokines, and
other effectors, assisting tumor cell escape from immune
surveillance and attack and promoting tumor cell proliferation and
distant metastasis (59). Deep
investigation of the mechanisms and interactions between CAFs and
tumor cells, as well as between CAFs and other immune cells, may
provide novel ideas for immunotherapy.
Previous studies have confirmed that
TGF-β1-activated CAFs promote breast cancer EMT, invasion, and
metastasis by overexpression of FAP-α, and autophagy in breast
cancer. Treatment with both the autophagy inhibitor 3-methyladenine
and FAP-α knockdown can reverse EMT and eliminate lung metastasis
and invasion caused by CAFs, indicating that autophagy and FAP-α in
CAFs are prerequisites for the metastasis of breast cancer to the
lungs (60). Wang et al
(61) revealed that epiregulin
reprograms CAFs via the JAK2/STAT3 pathway to facilitate oral
squamous cell carcinoma invasion. Zhou et al (62) found that CAFs induce EMT functional
changes in tongue squamous cell carcinoma. In CC, Murata et
al (63) reconstituted a
metastatic TME by co-transplanting CAFs and cancer cells into nude
mice to reconstruct the microenvironment of tumor metastasis. It
was surprising to find that 40% of nude mice co-transplanted with
two kinds of cells had LNMs, while nude mice transplanted with a
single cancer cell had no LNMs. They also showed that CC CAFs
secreted large quantities of heparin-bound epidermal growth factor
(HB-EGF), and the platelet-derived growth factor produced by ME180
cells enhances the expression of CAF HB-EGF, which in turn can
significantly promote the proliferation of ME180 cells (64). Xiao et al (65) found that overexpression of TGF-β1
and SDF-1 in CAFs promoted colony formation, growth, and invasion
of CC cells, while when cocultured with TGF-β1 and SDF-1
neutralizing antibodies, these phenomena were reversed. CAFs
secrete a series of growth factors through interaction with tumor
cells, causing tumor cell invasion, migration, and EMT, eventually
leading to LNM of CC, as confirmed by in vivo
experiments.
CAFs, as matrix-supporting cells around LECs, play a
supportive role in maintaining the lymphatic endothelial barrier.
Before lymphatic metastasis, CAFs can induce LNM by damaging the
lymphatic endothelial barrier. Wei et al (66) found a new subgroup of CAFs, namely,
periostin+ CAFs, which significantly increased infiltration in
patients with CC LNM, and the more infiltration there was, the
poorer the prognosis was. Further mechanistic research confirmed
that periostin+ CAFs activated the integrin FAK/Src-VE cadherin
signaling pathway in LECs, disrupted the lymphatic endothelial
barrier, allowed cancer cells to enter the lymphatic vessels, and
ultimately cause CC LNM. If the FAK/Src-VE cadherin signaling
pathway was inhibited, the effect of periostin+ CAFs was weakened.
This study highlights a novel approach to the treatment of CC LNMs,
identifying potential targets for blocking CAF-dependent metastasis
that destroys the lymphatic endothelial barrier and strengthening
and consolidating the integrity and stability of the lymphatic
endothelial barrier is essential for blocking CAF-dependent LNM.
These studies comprehensively show that CAFs can play a key role in
CC lymphatic metastasis by impairing lymphatic endothelial barrier
function and secreting growth factors and activating related
signaling pathways to promote tumor invasion. Targeting CAFs is
thus a relatively novel method for preventing CC lymphatic
metastasis.
TAMs
TAMs are involved in almost the entire process of
tumor occurrence and development. In the early stages of tumor
development, the immune system quickly responds, summoning T cells
and macrophages to attack and clear tumor cells through killing and
phagocytic functions. However, under certain conditions,
macrophages can be easily educated by tumors and converted into
TAMs, which can actually promote tumor progression and assist tumor
cell metastasis. TAMs can also assist in local infiltration and
distant dissemination of tumor cells by participating in
lymphangiogenesis and angiogenesis. To a certain extent, TAMs can
aggregate and even play a leading role in tumor cell metastasis.
Previous studies have confirmed that depletion of TAMs is
equivalent to turning off the switch on angiogenesis, and
eliminating Tie2 TAMs can inhibit angiogenesis in mouse glioma
(67,68).
Hypoxia is a common phenomenon in most solid tumors,
as there are several novel blood vessels in the hypoxic area due to
low oxygenation. Therefore, hypoxia is considered the primary
driving force for angiogenesis. Research has revealed that hypoxic
areas often accompany the aggregation of TAMs, which is primarily
caused by hypoxia stimulating the production of a series of
chemotactic and active factors, such as CCL-2, CXCL4 and VEGF, in
the tumor and interstitial cells. In addition, TAMs can respond to
hypoxia by upregulating the expression of HIF and downstream
proangiogenic factors (69).
Therefore, as the cells at the leading edge of the invading tumor,
TAMs are also known as the cells that aggregate to form the
premetastatic niche; thus, these cells can be used to identify the
direction of tumor cell metastasis to blood and lymphatic vessels
and assist in the prevention of metastasis.
The evidence that TAMs are directly involved in
lymphangiogenesis is that TAM depletion can significantly weaken
VEGFC and VEGFR3 signaling in LECs, weakening lymphatic vessel
formation in early-stage tongue SCC (70,71).
Hosono et al (72) found
that in esophageal squamous cell carcinoma (ESCC), TAMs can release
CXCL8 and bind to CXCR1/2 (known as CXCL8 receptors) of ESCC cell
lines, promoting ESCC invasion by suppressing Akt and Erk1/2
phosphorylation. Neutralizing antibodies against CXCL8, CXCR1 and
CXCR2 can inhibit these effects. Clinical analysis confirmed that
CXCL8+ TAMs are associated with LNM in esophageal cancer. Chen
et al (57) uncovered a new
LNM model for CC in the hypoxic TME: ZEB1 increases CCL8 secretion
and recruits TAMs to encapsulate the lymphatic vessel to form
network centers, promoting CC LNM.
There are several studies on M2-like macrophages in
the invasion and LNM of CC. Guo et al (73) found that the infiltration of CD68+
TAMs and CD163+ M2 TAMs is correlated with tumor progression.
CD163+ M2 TAM infiltration is correlated with LNM and an advanced
FIGO stage. Tan et al (74)
found that activated T-cell nuclear factor 1+ TAMs, as the M2 TAM
subtype, are significantly more abundant in CC tissues and can
promote CC cell proliferation and metastasis by activating the
c-Myc/PKM2 pathway. Jiang et al (75) found that during the progression
from CIN I-III to stage I-IV CC, the levels of TAM aggregation and
neovascularization in the TME increased synchronously, which fully
confirmed that TAMs and tumor angiogenesis play a key role. Li
et al (76) found that the
number of M2-TAMs in CC was higher than that in the surrounding
tissues, and the number of M2-TAMs in the diffusion infiltration
pattern (DIP) was higher than that in a pushing border pattern
(PBP), indicating a strong relationship between M2-TAMs and the
invasive behavior of CC. The above research comprehensively showed
that TAMs promote the LNM of CC. The identification of its
regulatory mechanism not only provides a novel target for the
development of therapies to counter metastasis but also provides a
basis for selecting specific patient cohorts that may benefit from
certain molecular-targeted drugs.
MDSCs
MDSCs are a group of heterogeneous immature myocytes
that are blocked from maturing in cancer. They are one of the
primary driving forces behind the immunosuppressive TME. According
to phenotypic and morphological differences, these cells can be
divided into two subgroups: Granulocytic MDSCs (G-MDSCs), which are
similar to neutrophils, and monocytic MDSCs (Mo-MDSCs) (77). The increase in the number of MDSCs
in CC patients was first confirmed in 2014 and the number of MDSCs,
especially G-MDSCs, significantly increased in the tumor tissue of
CC patients. MDSCs are important immune components in the TME and
are considered to mediate the immunosuppression of tumor-bearing
mice and cancer patients (78). In
addition to enhancing immunosuppression, MDSCs have also been shown
to enhance tumor progression by stimulating cancer cell invasion,
metastasis, and tumor angiogenesis (79). In the TME of CC, tumor cells
secrete various molecules to recruit MDSCs from immature myocytes,
including granulocyte colony-stimulating factor (G-CSF) (80), IL-6(81), and highly expressed C-X-C chemokine
receptor 2 (CXCR2) chemokines, such as CXCL1, CXCL2, CXCL3, CXCL5
and CXCL8(82). Mo-MDSCs enhance
the stemness of pancreatic cancer cells by producing IL-6 and
subsequently activating STAT3 activator in cancer cells (83). Peng et al (84) recently showed that MDSCs enhance
the dryness of breast cancer cells by producing IL-6 and nitric
oxide and subsequently activating the STAT3 and Notch signaling
pathways, respectively. Kuroda et al (85) found that G-CSF-induced MDSCs
enhance the dryness of CC cells by producing prostaglandin E2
(PGE2). It was also demonstrated that the inhibition of MDSCs or
PGE2 effectively inhibits the induction of CSCs and enhances the
efficacy of cisplatin in CC. Ni et al (86) revealed that patients with high
levels of METTL3 and CD33 expression in MDSCs in tumor tissues have
a poor prognosis, and these phenotypes are independent risk factors
for the prognosis of CC patients. Heeren et al (87) found that elevated MDSC levels
increase CC LNM and weaken sensitivity to radiotherapy and
chemotherapy. Rodríguez and Ochoa (88) further confirmed that MDSC-mediated
niche formation before LNM can induce FDG uptake during FDG
positron emission tomography/CT scans and leads to false-positive
detection of an LNM. Using the HPV-mediated CC mouse model,
researchers have demonstrated that MDSCs mediate immunosuppressive
activity through IL-6/JAK/STAT3 signaling. The activation of STAT6
mediated by the proinflammatory cytokine IL-3 may be the reason for
the expansion of MDSCs, which then accelerates tumor growth
(89). In addition, MDSCs interact
with B lymphocytes in the TME of CC through B-cell activating
factor (BAFF) expressed on the surface of MDSCs. MDSCs induce B
cells to differentiate into Breg cells by acting on BAFF receptors
expressed in B cells. In addition, IL-10 secreted by Breg cells can
promote STAT3 phosphorylation and activate MDSCs, thereby
establishing a positive feedback loop. The continuous
differentiation of Breg cells and the activation of MDSCs induce
immunosuppressive states and lead to tumor immune escape in CC
patients (90). MDSCs also
stimulate tumor angiogenesis by secreting Bv8 (a proangiogenic
molecule), which increases the expression of tumor G-CSF and MDSCs.
CC patients have poor survival rates, and G-CSF-producing CC is
sensitive to cisplatin after splenectomy or administration of
anti-Gr-1 antibodies, leading to the depletion of MDSCs (91). In summary, MDSCs utilize multiple
mechanisms to enhance the proliferation and metastasis of CC. After
being recruited to the TME, they mainly exert strong
immunosuppressive effects by inhibiting T-cell function.
Immune and inflammatory cells
During the immune escape process of tumors,
regulatory T cells (Tregs) secrete large quantities of IL-10,
TGF-β, and IL-35, which inhibits antigen presentation by dendritic
cells and CD4 helper T-cell function, regulates the expression of
inhibitory receptors and CD8-tumor-infiltrating lymphocyte (TIL)
depletion-related transcriptome characteristics, promote T-cell
depletion in tumors, downregulate antitumor immunity, and produce
tumor-specific CD8 cytotoxic T lymphocytes (92). As the role of adaptive immune cells
in the tumor microenvironment has been elucidated, the first
treatment scheme that interferes with the function of T cells has
been successfully approved by the U.S. FDA for antibody-based
treatment of patients with advanced cancers, such as Nivolumab,
Balstilimab and Zalifllimab. Wu et al (93) showed that a significant number of
CD4+ CD25+ FOXP3+ Treg cells accumulate around tumor cells and that
the proportion of FOXP3+ T cells in CC is higher than that in
cervical intraepithelial neoplasia. Moreover, the proportion of
FOXP3+ T cells in CC with LNM is significantly higher than that in
CC without LNM. Nakamura et al (94) found that the higher the Foxp3+/CD4+
Treg cell ratio was, the greater the rate of LNM was. Foxp3+ Treg
cells contact the immunoregulatory enzyme indoleamine
2,3-dioxygenase (IDO)+ APC. Foxp3+ Treg cells form a premetastatic
microecology and establish a network with IDO to induce immune
escape. Compared with that in the satellite lymph nodes without
tumors, the proportion of Foxp3 Treg cells is significantly
increased in lymph node metastases (94). There is a high incidence of LNMs at
Foxp3 Treg cell aggregation sites. Foxp3+ Treg cells can directly
contact IDO APCs in the context of LNM. In the metastatic
microenvironment of CC, Treg cells first infiltrate and accumulate
to form an immunosuppressive microenvironment to attract cancer
cells to the site of metastasis. These studies provide evidence
that the recruitment of Foxp3+ Tregs can promote cancer cell
migration.
Previous studies have noted that based on flow
cytometry analysis, CC patients with a low CD8 T-cell/Treg ratio,
high Treg level, and high levels of PD-L1 and major
histocompatibility complex, class II, DR (HLA)-DR myeloid cells are
more likely to have LNMs; therefore, this environment can also be
considered an immunosuppressive microenvironment. Heeren et
al (95) found that delineated
fields of Treg-associated immune suppression in anatomically
colocalized tumor-draining lymph nodes primarily form a
micro-transfer microenvironment to further integrate and expand to
further regions, which provides a basis for early surgical
intervention. In another study by the same lab, it was found that a
higher number of CD8+ T cells significantly reduced the frequency
of CD4+ cells, increased the expression of the memory marker CD45RO
and activation markers [PD-1, inducible T cell costimulator, HLA-DR
and cytotoxic T-lymphocyte-associated protein (CTLA-4)], and
significantly promoted LNM. Furthermore, they discovered that in
metastatic lymphoid tissue, the proportions of the FoxP3+ Tregs and
regulatory antigen-presenting cells (APCs) [PD-L1+ CD11c(hi),
CD14+], APC/myeloid suppressor cell subpopulations increased
significantly. After treatment with different Toll-like receptor
ligands, the expression of IFN-γ in metastatic lymphoid tissue
significantly decreased, but IL-10, IL-6, and TNF-α expression
significantly increased (87).
Wang et al (96) used 18F
fluorodeoxyglucose microPET/CT and bioluminescence imaging analysis
of mouse neck xenograft tumors to confirm that PD-L1 overexpression
promoted tumor glucose uptake by activating the ITGB4/SNAI1/SIRT3
signaling pathway, ultimately leading to LNM. At present, it is
suggested that targeting PD-L1 and CTLA4 is a potential approach
for the treatment of LNM in CC.
Research on the relationship between tumors and
inflammation is developing constantly. Tumor-induced inflammation
can result in DNA damage and micrometastases. Systemic inflammatory
responses can aggravate malnutrition in patients. Inflammatory
indicators have certain reference values in the diagnosis and
prognosis of various malignant tumors, and research has confirmed
that inflammation may promote tumor progression (97). Zhang et al (98) found that the
neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte
ratio (PLR) were valuable for predicting LNM in gastric cancer. The
NLR is superior to the PLR in predicting the gastric cancer
survival rate. Ayhan et al (99) found that a high NLR and PLR was
significantly correlated with tumor volume (>2 cm) and deep
muscle interstitial infiltration. A high monocyte-to-lymphocyte
ratio was significantly correlated with abdominal aortic LNM,
pelvic LNM, and locally advanced CC (IB3-IIIC2). Lee et al
(100) confirmed that an NLR ≥3.1
was indicative of a shift in the immune response from tumor
suppression to cancer promotion, often accompanied by radiation
resistance, rapid tumor progression, and LNM, which is associated
with a poor prognosis in CC patients. The use of inflammatory
indices in predicting the clinical outcomes of patients with
lymphatic metastasis of CC is deserved of further attention in
light of the convenient and low-cost means of access to the
data.
4. Therapeutic strategies for targeting the
TME of CC
The standard method of treatment for advanced CC
includes radiotherapy and chemotherapy; however, the patient
survival rate is very low (101).
In recent years, the gradual promotion and application of
immunotherapy has provided renewed hope. The role of the TME in
tumor metastasis has been confirmed; cancer cells can recruit
Tregs, downregulate cancer cell surface antigens, induce T-cell
apoptosis, secrete immunosuppressive factors, and evade immune
surveillance and attack, eventually forming the immunosuppressive
TME on which they rely for survival (102). The primary purpose of
immunotherapy is to reactivate the antitumor immune response or
reshape the immunosuppressive microenvironment. The US Food and
Drug Administration (FDA) has approved three immunotherapy-based
drugs for the treatment of CC: Pembrolizumab, Tisotumab Vedotin and
Nivolumab. Other immunotherapeutic strategies for the TME are still
in the exploratory stage, and the progress of TME treatment
strategies for CC is briefly summarized below.
Targeting hypoxia
Hypoxia increases the risk of local invasion,
metastasis, and treatment failure in CC. Hypoxia represents an
attractive therapeutic target, and a number of strategies have been
researched. The known studies on hypoxia-targeted strategies are as
follows: i) Increasing intratumoral oxygen: A phase II clinical
trial of 139 patients with locally advanced CC concluded that the
addition of carbogen and nicotinamide hypoxia modification to
standard therapy was feasible and safe (103). ii) Decreasing tumor oxygen
consumption: Metformin, an antidiabetic agent, reduces cancer
incidence in patients with diabetes (104). Subsequent experiments highlight a
complex interplay with hypoxia-associated molecular pathways,
possibly through the inhibition of the mammalian target of the
rapamycin-HIF-1α axis (105). Two
phase II randomized clinical trials, NCT02394652 and NCT04275713,
are currently investigating the use of metformin as a
hypoxia-modifying therapy for locally advanced CCs. These trials
will assess metformin-induced changes in tumor hypoxia using
imaging and gene expression biomarkers (3). Hypoxia-specific radiosensitizers and
cytotoxins: There are several classes of hypoxia-specific
cytotoxins. Quinone-based agents selectively activate hypoxia
through a reductive mechanism and induce DNA alkylation-mediated
cytotoxicity. Sharma et al (106) performed a study with 160 patients
with locally advanced squamous cell carcinoma of the uterine cervix
that participated in a multicenter phase III trial that randomized
participants to receive radiotherapy alone or radiotherapy with
concomitant mitomycin C. Despite improved 4-year disease-free
survival rates in the intervention group, the study failed to show
a significant benefit in overall survival or local recurrence
rates. Discovered ~35 years ago by Zeman et al (107) and Brown (108), tirapazamine was the first purely
hypoxic cytotoxin and is one of the most advanced bioreductive
drugs in clinical trials. The best-known aromatic N-oxide is used
as an anticancer drug that undergoes enzymatic one-electron
reduction and converts to an electron-donating mono-N-oxide
metabolite (tirapazamine radical). Murine model experiments showed
considerably more tumor cell death was observed when tirapazamine
was combined with radiotherapy or cisplatin chemotherapy compared
with monotherapy (108), but
unfortunately, the follow-up clinical trial of DiSilvestro et
al (109) failed to show a
clinically meaningful result. iv) Hyperthermia: A strategy that
encompasses a variety of hypoxia-targeting mechanisms is
hyperthermia. It is assumed to improve oxygenation by causing
vasodilatation, direct cellular damage, immune-mediated killing of
tumor cells, and inhibition of DNA repair. In CC, hyperthermia has
been used to sensitize tumors to radiotherapy, and the evidence
suggests that combining radiotherapy with hyperthermia results in
improved locoregional control when compared with using radiotherapy
alone (77 vs. 52%) (38).
Effectively decreasing hypoxia would clearly improve the response
to therapy and reduce the likelihood of metastatic spread in
CC.
Targeting immune checkpoint
molecules
The tumor microenvironment plays a key role in tumor
metastasis. Cancer cells can recruit Tregs, downregulate tumor
antigen expression, induce T-cell tolerance and/or apoptosis, and
generate immunosuppressive cytokines to stimulate immunosuppressive
immune checkpoints, which leads to a unique and highly
immunosuppressive tumor microenvironment (TME) (102). To overcome these
immunosuppressive conditions, immune checkpoints may be modulated
by either agonist or antagonist monoclonal antibodies used to
enhance T-cell activation and eliminate inhibition of T-cell
activation, respectively, to reactivate T cells to attack tumors
(110). At present, immune
checkpoint inhibitors in CC primarily include the following: i)
Programmed death ligand 1 (PD-L1): PD-L1 is an immunomodulator that
is expressed on antigen-presenting cells (APCs) and 20-50% of human
cancer cells. Tumor-induced PD-L1 inhibits T-cell function and
induces immune tolerance but also induces T-cell apoptosis. By
contrast, PD-L1 induces expansion registration of T cells.
Therefore, blocking this ligand on tumor cells and APCs can improve
tumor defense, and T cells with anticancer properties can restore
their effector functions (111).
ii) Anti-CTLA4 antibody: Under physiological conditions, T cells
are stimulated by CD28, and CD28 interacts with B7-1 and B7-2 on
dendritic cells. In addition to the ‘key’ CD28, T cells also
express CTLA4, which can be regarded as ‘key off’. CTLA4 acts as a
symbiotic factor on activated T cells to regulate their immune
response (112). The application
of immune checkpoint inhibitor targeting in CC is summarized in
Table I.
 | Table ISummary of clinical trials targeting
immune checkpoint molecules in cervical cancer. |
Table I
Summary of clinical trials targeting
immune checkpoint molecules in cervical cancer.
| First author,
year | NCT Number/phase of
clinical trial | Immunotherapeutic
regimen | Additional
therapy | Patient population
(n) | Trial
status/clinical efficacy | (Refs.) |
|---|
| Naumann, 2019 | NCT02488759/Phase
2 | Nivolumab
(anti-PD-1 antibody) | |
Recurrent/metastatic cervical, vaginal or
vulvar cancer (24 participants) | Completed/ORR,
26.3%; MOS, 21.9 months | (113) |
| O'Malley, 2022 | NCT03495882/Phase
2 | Balstilimab
(anti-PD-1 antibody) and Zalifrelimab (anti-CTLA-4 antibody) | | Recurrent and/or
metastatic cervical cancer (155 participants) | Completed/ORR,
25.6%; PDL-1+, 32.8%; PDL-1(-): 9.1%. | (114) |
| Santin, 2020 | NCT02257528/Phase
2 | Nivolumab
(anti-PD-1 antibody) | | Persistent,
recurrent, metastatic cervical cancer (26 participants) | Completed/PFS and
OS at 6 months were 16 and 78.4%, respectively. | (115) |
| Frenel, 2017 | NCT02054806/Phase
1 | Pembrolizumab
(anti-PD-1 antibody) | | Advanced cervical
cancer (42 participants) | Completed/ORR,
17% | (116) |
| Chung, 2019 | NCT02628067/Phase
2 | Pembrolizumab
(anti-PD-1 antibody) | | Advanced cervical
cancer (46 participants) | Completed/ORR,
12.2%; PD-L1+ ORR, 14.6% | (117) |
| De Jaeghere,
2023 | NCT03192059/Phase
2 | Pembrolizumab
(anti-PD-1 antibody) | Radiotherapy,
vitamin D, Aspirin, Lansoprazole, Cyclophosphamide, and
Curcumin | Refractory or
persistent endometrial, cervical, or uterine cancer patients (43
participants) | Completed/ORR,
11.1%; MOS, 39.6 weeks | (118) |
Early data suggested that immune cells, in
particular CD8+ T cells, play a key role in tumor cell death within
a radiation field (119).
Radiation therapy causes migration of dendritic cells and
cross-penetration of tumor antigens, which can result in T-cell
activation and proliferation. Furthermore, radiation therapy
increases the density of TILs within a tumor, likely via
extravasation of TILs within the vasculature of tumors and
chemokine activation (120). It
is known that radiation therapy alters the T-cell receptor
repertoire of peripheral T-cell clones (121). Thus, there is a strong rationale
for the combination of radiation with immune checkpoint blockade.
There are limited data surrounding the optimal dose and
fractionation needed to provoke an ideal immune response when
combining immunotherapy with radiation in CC. Sequencing of CTLA-4
blockade with immunotherapy in preclinical models demonstrates that
when anti-CTLA-4 is delivered prior to radiotherapy, there is
increased efficacy compared to delivery after radiotherapy
(122). Studies have also
demonstrated that radiotherapy increases PD-L1 expression, which
may act as a negative feedback mechanism preventing T-cell-mediated
tumor rejection (123).
Radiotherapy and chemotherapy combined with PD-1 and CTLA-4 immune
checkpoint blockades provide a more effective scheme than
monotherapy for the treatment of advanced and recurrent cervical
cancer.
Targeting suppressive immune
cells
Suppressive immune cells, such as Tregs, MDSCs, and
type 2 macrophages, form an immunosuppressive microenvironment to
assist tumor cell escape from immune surveillance. Research has
shown that practical CXCR2 antagonist therapy can weaken the
proliferation and migration of CC cells (124). In addition, the method of
targeting the CSF-1/CSF-1R axis of TAMs is being assessed in a
mouse model. CSF-1R inhibition weakened the turnover rate of TAMs
and increased the number of CD8 T cells infiltrating tumor tissue
(125). The antitumor effect of
anti-PD-1 therapy is enhanced by inhibiting CXCR2, the primary
chemokine receptor for MDSC recruitment in human pancreatic cancer
(126). However, these studies
are still limited to in vitro and in vivo
experiments, although they provide novel ideas for future clinical
trials. In addition, metabolites targeting suppressive immune
cells, such as Arg-1 and IDO, are also novel avenues for targeting
suppressive immune cells. The arginase inhibitor INCB001158 is
being used to treat metastatic solid tumors (NCT02903914).
Treatment of IL-6 knockout mice with IDO inhibitors has been proven
to inhibit the expression of IDO. In addition, combination therapy
with therapeutic vaccines leads to a decrease in polymorphonuclear
MDSCs and Treg cells in tumors, supporting IL-6 and IDO as
immunometabolic adjuvants for immunotherapy against CC (127). Combination therapy targeting
inhibitory immune cells and metabolites within the TME represents a
new strategy for antitumor therapy.
Anti-lymphangiogenesis and
anti-inflammatory therapy
Since lymphatic vessels and lymphatic remodeling
play a key role in lymphatic metastasis, targeting lymphatic
vessels is the key to the treatment of metastatic CC. The
VEGF-C/VEGFR-3 signaling axis induces tumor lymphatic vessel
formation. In an experimental model, blocking VEGF-C/VEGFR-3 has
been proven to reduce tumor lymphatic vessel formation and
metastasis (128). However, this
study has not yet entered a clinical trial stage. Inflammation
plays a certain role in tumor metastasis. Nonsteroidal
anti-inflammatory drugs combined with chemotherapy and radiotherapy
can increase the sensitivity of patients with locally advanced
cervical cancer. Studies have confirmed that blocking the
inflammatory signaling pathway (COX/PGE2) and regulating the immune
response against HPV and targeting the virus are the best choices
for antitumor treatment of cervical cancer (129). The interaction of various cells
in the TME is very complex, and the effect of any single therapy is
limited. Combination therapy may provide a breakthrough for
improving the prognosis of patients with recurrent and metastatic
CC in the future.
5. Conclusions and future perspectives
Lymphatic metastasis is a key factor affecting the
prognosis of patients with cervical cancer. CAFs, TAMs, and immune
and inflammatory cells (primarily T cells and neutrophils) in the
tumor microenvironment promote lymphatic metastasis by releasing a
series of cytokines (such as VEGF-A/C/D and TGF-β) to induce tumor
cell EMT, lymphatic vessel proliferation, and immune evasion, which
ultimately leads to lymphatic metastasis. The above effects are
enhanced under hypoxic conditions through hypoxia-related signaling
pathways and transcription factors (such as HIFs). Although
progress has been made in clinical trials on hypoxia-targeting
strategies, and PD-1 and CTLA-4 immune checkpoint blockades in
advanced CC, there are few clinical trials and drugs that
specifically target markers for predicting and treating lymphatic
metastasis in CC (130). A few
clinical trials have shown that simultaneous radiotherapy combined
with immunotherapy is more effective than monotherapy, but the
specific mechanism remains unclear, and it is meaningful to expand
the population to further study the mechanism of action to guide
clinical treatment. The tumor heterogeneity of individual CC
patients increases the complexity of treatment and leads to
differences in the involvement of factors related to lymphatic
metastasis among patients. Hypoxia can allow tumor cells to appear
dormant, increase the difficulty of treatment, and recruit more
TAMs as a lymphatic angiogenesis switch. TAMs directly participate
in lymphatic angiogenesis. CAFs damage the lymphatic endothelial
barrier and destroy the integrity of the lymphatic endothelium, and
immune-inflammatory cells to create an immunosuppressive
microenvironment. These complex and orderly steps involved in tumor
microenvironment formation eventually leading to LNM. However, the
mechanism is still unclear. There remain several aspects that need
to be studied and explored in the future to understand and reduce
the incidence of lymphatic metastasis of CC and improve the
survival rate. Development of individualized treatments based on
the tumor microenvironment is an important direction that is
expected to be an important strategy for treating lymphatic
metastasis of CC in the future.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by the National Natural Science
Foundation of China (grant no. 81902140).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LFW and JC conceived the concept of the review and
revised the manuscript. LFW, SYY, YT, and WHL drafted the
manuscript. LFW and WHL prepared the figures. Data authentication
is not applicable. All authors have read and approved the final
manuscript.
Ethical approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Singh D, Vignat J, Lorenzoni V, Eslahi M,
Ginsburg O, Lauby-Secretan B, Arbyn M, Basu P, Bray F and
Vaccarella S: Global estimates of incidence and mortality of
cervical cancer in 2020: A baseline analysis of the WHO global
cervical cancer elimination initiative. Lancet Glob Health.
11:e197–e206. 2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Tax C, Rovers MM, de Graaf C, Zusterzeel
PL and Bekkers RL: The sentinel node procedure in early stage
cervical cancer, taking the next step; a diagnostic review. Gynecol
Oncol. 139:559–567. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bhatla N, Aoki D, Sharma DN and
Sankaranarayanan R: Cancer of the cervix uteri. Int J Gynaecol
Obstet. 143 (Suppl 2):S22–S36. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Jürgenliemk-Schulz IM, Beriwal S, de Leeuw
AAC, Lindegaard JC, Nomden CN, Pötter R, Tanderup K, Viswanathan AN
and Erickson B: Management of nodal disease in advanced cervical
cancer. Semin Radiat Oncol. 29:158–165. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Dadafarin S, Carnazza M, Islam HK,
Moscatello A, Tiwari RK and Geliebter J: Noncoding RNAs in
papillary thyroid cancer: Interaction with cancer-associated
fibroblasts (CAFs) in the tumor microenvironment (TME) and
regulators of differentiation and lymph node metastasis. Adv Exp
Med Biol. 1350:145–155. 2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Solis-Castillo LA, Garcia-Romo GS,
Diaz-Rodriguez A, Reyes-Hernandez D, Tellez-Rivera E,
Rosales-Garcia VH, Mendez-Cruz AR, Jimenez-Flores JR,
Villafana-Vazquez VH and Pedroza-Gonzalez A: Tumor-infiltrating
regulatory T cells, CD8/Treg ratio, and cancer stem cells are
correlated with lymph node metastasis in patients with early breast
cancer. Breast Cancer. 27:837–849. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Griffith JW, Sokol CL and Luster AD:
Chemokines and chemokine receptors: Positioning cells for host
defense and immunity. Annu Rev Immunol. 32:659–702. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Singh S, Sadanandam A and Singh RK:
Chemokines in tumor angiogenesis and metastasis. Cancer Metastasis
Rev. 26:453–467. 2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
He M, He Q, Cai X, Chen Z, Lao S, Deng H,
Liu X, Zheng Y, Liu X, Liu J, et al: Role of lymphatic endothelial
cells in the tumor microenvironment-a narrative review of recent
advances. Transl Lung Cancer Res. 10:2252–2277. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Schito L: Hypoxia-dependent angiogenesis
and lymphangiogenesis in cancer. Adv Exp Med Biol. 1136:71–85.
2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ji RC: Hypoxia and lymphangiogenesis in
tumor microenvironment and metastasis. Cancer Lett. 346:6–16.
2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Dieterich LC, Tacconi C, Ducoli L and
Detmar M: Lymphatic vessels in cancer. Physiol Rev. 102:1837–1879.
2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chen JM, Luo B, Ma R, Luo XX, Chen YS and
Li Y: Lymphatic endothelial markers and tumor lymphangiogenesis
assessment in human breast cancer. Diagnostics (Basel).
12(4)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lambert AW and Weinberg RA: Linking EMT
programmes to normal and neoplastic epithelial stem cells. Nat Rev
Cancer. 21:325–338. 2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Bakir B, Chiarella AM, Pitarresi JR and
Rustgi AK: EMT, MET, plasticity, and tumor metastasis. Trends Cell
Biol. 30:764–776. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sinha D, Saha P, Samanta A and Bishayee A:
Emerging concepts of hybrid epithelial-to-mesenchymal transition in
cancer progression. Biomolecules. 10(1561)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kumagai Y, Tachikawa T, Higashi M,
Sobajima J, Takahashi A, Amano K, Fukuchi M, Ishibashi K, Mochiki
E, Yakabi K, et al: Vascular endothelial growth factors C and D and
lymphangiogenesis at the early stage of esophageal squamous cell
carcinoma progression. Dis Esophagus. 31:2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
García-Silva S, Benito-Martín A, Nogués L,
Hernández-Barranco A, Mazariegos MS, Santos V, Hergueta-Redondo M,
Ximénez-Embún P, Kataru RP, Lopez AA, et al: Melanoma-derived small
extracellular vesicles induce lymphangiogenesis and metastasis
through an NGFR-dependent mechanism. Nat Cancer. 2:1387–1405.
2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Dadras SS, Lange-Asschenfeldt B, Velasco
P, Nguyen L, Vora A, Muzikansky A, Jahnke K, Hauschild A, Hirakawa
S, Mihm MC and Detmar M: Tumor lymphangiogenesis predicts melanoma
metastasis to sentinel lymph nodes. Mod Pathol. 18:1232–1242.
2005.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Roy S, Kumaravel S, Banerjee P, White TK,
O'Brien A, Seelig C, Chauhan R, Ekser B, Bayless KJ, Alpini G, et
al: Tumor lymphatic interactions induce CXCR2-CXCL5 axis and alter
cellular metabolism and lymphangiogenic pathways to promote
cholangiocarcinoma. Cells. 10(3093)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Gogineni A, Maresa C, Ailey C, Lee CR, Fuh
G, van Bruggen N, Ye W and Weimer RM: Inhibition of VEGF-C
modulates distal lymphatic remodeling and secondary metastasis.
PLoS One. 8(e68755)2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Aebischer D, Iolyeva M and Halin C: The
inflammatory response of lymphatic endothelium. Angiogenesis.
17:383–393. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Miteva DO, Rutkowski JM, Dixon JB,
Kilarski W, Shields JD and Swartz MA: Transmural flow modulates
cell and fluid transport functions of lymphatic endothelium. Circ
Res. 106:920–931. 2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wiley HE, Gonzalez EB, Maki W, Wu MT and
Wang ST: Expression of CC chemokine receptor-7 and regional lymph
node metastasis of B16 murine melanoma. J Nat Cancer Inst.
93:1638–1643. 2001.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Mezzapelle R, Leo M, Caprioglio F, Colley
LS, Lamarca A, Sabatino L, Colantuoni V, Crippa MP and Bianchi ME:
CXCR4/CXCL12 activities in the tumor microenvironment and
implications for tumor immunotherapy. Cancers (Basel).
14(2314)2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hirakawa S, Detmar M, Kerjaschki D,
Nagamatsu S, Matsuo K, Tanemura A, Kamata N, Higashikawa K, Okazaki
H, Kameda K, et al: Nodal lymphangiogenesis and metastasis: Role of
tumor-induced lymphatic vessel activation in extramammary Paget's
disease. Am J Pathol. 175:2235–2248. 2009.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kawada K, Hosogi H, Sonoshita M, Sakashita
H, Manabe T, Shimahara Y, Sakai Y, Takabayashi A, Oshima M and
Taketo MM: Chemokine receptor CXCR3 promotes colon cancer
metastasis to lymph nodes. Oncogene. 26:4679–4688. 2007.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Das S, Sarrou E, Podgrabinska S, Cassella
M, Mungamuri SK, Feirt N, Gordon R, Nagi CS, Wang Y, Entenberg D,
et al: Tumor cell entry into the lymph node is controlled by CCL1
chemokine expressed by lymph node lymphatic sinuses. J Exp Med.
210:1509–1528. 2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Fujimoto N and Dieterich LC: Mechanisms
and clinical significance of tumor lymphatic invasion. Cells.
10(2585)2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Issa A, Le TX, Shoushtari AN, Shields JD
and Swartz MA: Vascular endothelial growth factor-C and C-C
chemokine receptor 7 in tumor cell-lymphatic cross-talk promote
invasive phenotype. Cancer Res. 69:349–357. 2009.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Meier F, Will S, Ellwanger U,
Schlagenhauff B, Schittek B, Rassner G and Garbe C: Metastatic
pathways and time courses in the orderly progression of cutaneous
melanoma. Br J Dermatol. 147:62–70. 2002.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kim M, Koh YJ, Kim KE, Koh BI, Nam DH,
Alitalo K, Kim I and Koh GY: CXCR4 signaling regulates metastasis
of chemoresistant melanoma cells by a lymphatic metastatic niche.
Cancer Res. 70:10411–10421. 2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Farnsworth RH, Karnezis T, Maciburko SJ,
Mueller SN and Stacker SA: The interplay between lymphatic vessels
and chemokines. Front Immunol. 10(518)2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Shields JD, Kourtis IC, Tomei AA, Roberts
JM and Swartz MA: Induction of lymphoidlike stroma and immune
escape by tumors that express the chemokine CCL21. Science.
328:749–752. 2010.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lund AW, Duraes FV, Hirosue S, Raghavan
VR, Nembrini C, Thomas S, Issa A, Hugues S and Swartz MA: VEGF-C
promotes immune tolerance in B16 melanomas and cross-presentation
of tumor antigen by lymph node lymphatics. Cell Rep. 1:191–199.
2012.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Tewalt EF, Cohen JN, Rouhani SJ, Guidi CJ,
Qiao H, Fahl SP, Conaway MR, Bender TP, Tung KS, Vella AT, et al:
Lymphatic endothelial cells induce tolerance via PD-L1 and lack of
costimulation leading to high-level PD-1 expression on CD8 T cells.
Blood. 120:4772–4782. 2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
De Nola R, Loizzi V, Cicinelli E and
Cormio G: Dynamic crosstalk within the tumor microenvironment of
uterine cervical carcinoma: Baseline network, iatrogenic
alterations, and translational implications. Crit Rev Oncol
Hematol. 162(103343)2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Datta A, West C, O'Connor JPB, Choudhury A
and Hoskin P: Impact of hypoxia on cervical cancer outcomes. Int J
Gynecol Cancer. 31:1459–1470. 2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Rojo-León V, García C, Valencia C, Méndez
MA, Wood C and Covarrubias L: The E6/E7 oncogenes of human
papilloma virus and estradiol regulate hedgehog signaling activity
in a murine model of cervical cancer. Exp Cell Res. 381:311–322.
2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
De Nola R, Menga A, Castegna A, Loizzi V,
Ranieri G, Cicinelli E and Cormio G: The crowded crosstalk between
cancer cells and stromal microenvironment in gynecological
malignancies: Biological pathways and therapeutic implication. Int
J Mol Sci. 20(2401)2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Lea JS and Lin KY: Cervical cancer. Obstet
Gynecol Clin North Am. 39:233–253. 2012.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Baluk P, Fuxe J, Hashizume H, Romano T,
Lashnits E, Butz S, Vestweber D, Corada M, Molendini C, Dejana E
and McDonald DM: Functionally specialized junctions between
endothelial cells of lymphatic vessels. J Exp Med. 204:2349–2362.
2007.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Tacconi C, Correale C, Gandelli A,
Spinelli A, Dejana E, D'Alessio S and Danese S: Vascular
endothelial growth factor C disrupts the endothelial lymphatic
barrier to promote colorectal cancer invasion. Gastroenterology.
148:1438–1451.e8. 2015.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Chen C, Shen N, Chen Y, Jiang P, Sun W,
Wang Q, Wang Z, Wang Y, Cheng W, Fu S and Wang S: LncCCLM inhibits
lymphatic metastasis of cervical cancer by promoting STAU1-mediated
IGF-1 mRNA degradation. Cancer Lett. 518:169–179. 2021.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Alavi A, Carlin SD, Werner TJ and Zaghal
AA: Suboptimal sensitivity and specificity of PET and other gross
imaging techniques in assessing lymph node metastasis. Mol Imaging
Biol. 21:808–811. 2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Phan TG and Croucher PI: The dormant
cancer cell life cycle. Nat Rev Cancer. 20:398–411. 2020.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Ju S, Wang F, Wang Y and Ju S: CSN8 is a
key regulator in hypoxia-induced epithelial-mesenchymal transition
and dormancy of colorectal cancer cells. Mol Cancer.
19(168)2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Hsin MC, Hsieh YH, Hsiao YH, Chen PN, Wang
PH and Yang SF: Carbonic anhydrase IX promotes human cervical
cancer cell motility by regulating PFKFB4 expression. Cancers
(Basel). 13(1174)2021.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Sugiura K, Nakajima S, Kato I, Okubo-Sato
M, Nakazawa Y, Mitsudo K and Kioi M: Hypoxia and CD11b+ cell influx
are strongly associated with lymph node metastasis of oral cancer.
Anticancer Res. 40:6845–6852. 2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Cairns RA and Hill RP: Acute hypoxia
enhances spontaneous lymph node metastasis in an orthotopic murine
model of human cervical carcinoma. Cancer Res. 64:2054–2061.
2004.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Chaudary N, Milosevic M and Hill RP:
Suppression of vascular endothelial growth factor receptor 3
(VEGFR3) and vascular endothelial growth factor C (VEGFC) inhibits
hypoxia-induced lymph node metastases in cervix cancer. Gynecol
Oncol. 123:393–400. 2011.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Lee S, Shin HJ, Han IO, Hong EK, Park SY,
Roh JW, Shin KH, Kim TH and Kim JY: Tumor carbonic anhydrase 9
expression is associated with the presence of lymph node metastases
in uterine cervical cancer. Cancer Sci. 98:329–333. 2007.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Li Z, Jiang L, Chew SH, Hirayama T, Sekido
Y and Toyokuni S: Carbonic anhydrase 9 confers resistance to
ferroptosis/apoptosis in malignant mesothelioma under hypoxia.
Redox Biol. 26(101297)2019.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Hu HM, Mao MH, Hu YH, Zhou XC, Li S, Chen
CF, Li CN, Yuan QL and Li W: Artemisinin protects DPSC from hypoxia
and TNF-α mediated osteogenesis impairments through CA9 and Wnt
signaling pathway. Life Sci. 277(119471)2021.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Kim JH, Kim JY, Yoon MS, Kim YS, Lee JH,
Kim HJ, Kim H, Kim YJ, Yoo CW, Nam BH, et al: Prophylactic
irradiation of para-aortic lymph nodes for patients with locally
advanced cervical cancers with and without high CA9 expression
(KROG 07-01): A randomized, open-label, multicenter, phase 2 trial.
Radiother Oncol. 120:383–389. 2016.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Chen XJ, Deng YR, Wang ZC, Wei WF, Zhou
CF, Zhang YM, Yan RM, Liang LJ, Zhong M, Liang L, et al:
Hypoxia-induced ZEB1 promotes cervical cancer progression via
CCL8-dependent tumour-associated macrophage recruitment. Cell Death
Dis. 10(508)2019.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Chen XJ, Wei WF, Wang ZC, Wang N, Guo CH,
Zhou CF, Liang LJ, Wu S, Liang L and Wang W: A novel lymphatic
pattern promotes metastasis of cervical cancer in a hypoxic
tumour-associated macrophage-dependent manner. Angiogenesis.
24:549–565. 2021.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Chen XJ, Wu S, Yan RM, Fan LS, Yu L, Zhang
YM, Wei WF, Zhou CF, Wu XG, Zhong M, et al: The role of the
hypoxia-Nrp-1 axis in the activation of M2-like tumor-associated
macrophages in the tumor microenvironment of cervical cancer. Mol
Carcinog. 58:388–397. 2019.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Mao X, Xu J, Wang W, Liang C, Hua J, Liu
J, Zhang B, Meng Q, Yu X and Shi S: Crosstalk between
cancer-associated fibroblasts and immune cells in the tumor
microenvironment: New findings and future perspectives. Mol Cance.
20(131)2021.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Huang M, Fu M, Wang J, Xia C, Zhang H,
Xiong Y, He J, Liu J, Liu B, Pan S and Liu F: TGF-β1-activated
cancer-associated fibroblasts promote breast cancer invasion,
metastasis and epithelial-mesenchymal transition by autophagy or
overexpression of FAP-α. Biochem Pharmacol.
188(114527)2021.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Wang Y, Jing Y, Ding L, Zhang X, Song Y,
Chen S, Zhao X, Huang X, Pu Y, Wang Z, et al: Epiregulin reprograms
cancer-associated fibroblasts and facilitates oral squamous cell
carcinoma invasion via JAK2-STAT3 pathway. J Exp Clin Cancer Res.
38(274)2019.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Zhou B, Chen WL, Wang YY, Lin ZY, Zhang
DM, Fan S and Li JS: A role for cancer-associated fibroblasts in
inducing the epithelial-to-mesenchymal transition in human tongue
squamous cell carcinoma. J Oral Pathol Med. 43:585–592.
2014.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Murata T, Mekada E and Hoffman RM:
Reconstitution of a metastatic-resistant tumor microenvironment
with cancer-associated fibroblasts enables metastasis. Cell Cycle.
16:533–535. 2017.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Murata T, Mizushima H, Chinen I, Moribe H,
Yagi S, Hoffman RM, Kimura T, Yoshino K, Ueda Y, Enomoto T and
Mekada E: HB-EGF and PDGF mediate reciprocal interactions of
carcinoma cells with cancer-associated fibroblasts to support
progression of uterine cervical cancers. Cancer Res. 71:6633–6642.
2011.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Xiao L, Zhu H, Shu J, Gong D, Zheng D and
Gao J: Overexpression of TGF-β1 and SDF-1 in cervical
cancer-associated fibroblasts promotes cell growth, invasion and
migration. Arch Gynecol Obstet. 305:179–192. 2022.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Wei WF, Chen XJ, Liang LJ, Yu L, Wu XG,
Zhou CF, Wang ZC, Fan LS, Hu Z, Liang L and Wang W:
Periostin+ cancer-associated fibroblasts promote lymph
node metastasis by impairing the lymphatic endothelial barriers in
cervical squamous cell carcinoma. Mol Oncol. 15:210–227.
2021.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Nielsen SR and Schmid MC: Macrophages as
key drivers of cancer progression and metastasis. Mediators
Inflamm. 2017(9624760)2017.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Mazzieri R, Pucci F, Moi D, Zonari E,
Ranghetti A, Berti A, Politi LS, Gentner B, Brown JL, Naldini L and
De Palma M: Targeting the ANG2/TIE2 axis inhibits tumor growth and
metastasis by impairing angiogenesis and disabling rebounds of
proangiogenic myeloid cells. Cancer Cell. 19:512–526.
2011.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Yeo EJ, Cassetta L, Qian BZ, Lewkowich I,
Li JF, Stefater JA III, Smith AN, Wiechmann LS, Wang Y, Pollard JW
and Lang RA: Myeloid WNT7b mediates the angiogenic switch and
metastasis in breast cancer. Cancer Res. 74:2962–2973.
2014.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Ji H, Cao R, Yang Y, Zhang Y, Iwamoto H,
Lim S, Nakamura M, Andersson P, Wang J, Sun Y, et al: TNFR1
mediates TNF-α-induced tumour lymphangiogenesis and metastasis by
modulating VEGF-C-VEGFR3 signalling. Nat Commun.
5(4944)2014.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Kimura S, Noguchi H, Nanbu U and Nakayama
T: Macrophage CCL22 expression promotes lymphangiogenesis in
patients with tongue squamous cell carcinoma via IL-4/STAT6 in the
tumor microenvironment. Oncol Lett. 21(383)2021.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Hosono M, Koma YI, Takase N, Urakawa N,
Higashino N, Suemune K, Kodaira H, Nishio M, Shigeoka M, Kakeji Y
and Yokozaki H: CXCL8 derived from tumor-associated macrophages and
esophageal squamous cell carcinomas contributes to tumor
progression by promoting migration and invasion of cancer cells.
Oncotarget. 8:106071–106088. 2017.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Guo F, Kong W, Zhao G, Cheng Z, Ai L, Lv
J, Feng Y and Ma X: The correlation between tumor-associated
macrophage infiltration and progression in cervical carcinoma.
Biosci Rep. 41(BSR20203145)2021.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Tan J, Yang L, Zhao H, Ai Y, Ren L, Zhang
F, Dong W, Shi R, Sun D and Feng Y: The role of
NFATc1/c-myc/PKM2/IL-10 axis in activating cervical cancer
tumor-associated M2 macrophage polarization to promote cervical
cancer progression. Exp Cell Res. 413(113052)2022.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Jiang S, Yang Y, Fang M, Li X and Yuan XJ:
Co-evolution of tumor-associated macrophages and tumor neo-vessels
during cervical cancer invasion. Oncol Lett. 12:2625–2631.
2016.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Li Y, Huang G and Zhang S: Associations
between intratumoral and peritumoral M2 macrophage counts and
cervical squamous cell carcinoma invasion patterns. Int J Gynaecol
Obstet. 139:346–351. 2017.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Dou A and Fang J: Heterogeneous myeloid
cells in tumors. Cancers (Basel). 13(3772)2021.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Mabuchi S, Matsumoto Y, Kawano M, Minami
K, Seo Y, Sasano T, Takahashi R, Kuroda H, Hisamatsu T, Kakigano A,
et al: Uterine cervical cancer displaying tumor-related
leukocytosis: A distinct clinical entity with radioresistant
feature. J Natl Cancer Inst. 106(dju147)2014.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Marvel D and Gabrilovich DI:
Myeloid-derived suppressor cells in the tumor microenvironment:
Expect the unexpected. J Clin Invest. 125:3356–3364.
2015.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Mabuchi S, Komura N, Sasano T, Shimura K,
Yokoi E, Kozasa K, Kuroda H, Takahashi R, Kawano M, Matsumoto Y, et
al: Pretreatment tumor-related leukocytosis misleads positron
emission tomography-computed tomography during lymph node staging
in gynecological malignancies. Nat Commun. 11(1364)2020.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Lee BR, Kwon BE, Hong EH, Shim A, Song JH,
Kim HM, Chang SY, Kim YJ, Kweon MN, Youn JI and Ko HJ:
Interleukin-10 attenuates tumour growth by inhibiting
interleukin-6/signal transducer and activator of transcription 3
signalling in myeloid-derived suppressor cells. Cancer Lett.
381:156–164. 2016.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Kim KH, Sim NS, Chang JS and Kim YB: Tumor
immune microenvironment in cancer patients with leukocytosis.
Cancer Immunol Immunother. 69:1265–1277. 2020.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Panni RZ, Sanford DE, Belt BA, Mitchem JB,
Worley LA, Goetz BD, Mukherjee P, Wang-Gillam A, Link DC, Denardo
DG, et al: Tumor-induced STAT3 activation in monocytic
myeloid-derived suppressor cells enhances stemness and mesenchymal
properties in human pancreatic cancer. Cancer Immunol Immunother.
63:513–528. 2014.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Peng D, Tanikawa T, Li W, Zhao L, Vatan L,
Szeliga W, Wan S, Wei S, Wang Y, Liu Y, et al: Myeloid-derived
suppressor cells endow stem-like qualities to breast cancer cells
through IL6/STAT3 and NO/NOTCH cross-talk signaling. Cancer Res.
76:3156–3165. 2016.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Kuroda H, Mabuchi S, Yokoi E, Komura N,
Kozasa K, Matsumoto Y, Kawano M, Takahashi R, Sasano T, Shimura K,
et al: Prostaglandin E2 produced by myeloid-derived suppressive
cells induces cancer stem cells in uterine cervical cancer.
Oncotarget. 9:36317–36330. 2018.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Ni HH, Zhang L, Huang H, Dai SQ and Li J:
Connecting METTL3 and intratumoural CD33+ MDSCs in
predicting clinical outcome in cervical cancer. J Transl Med.
18(393)2020.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Heeren AM, Koster BD, Samuels S, Ferns DM,
Chondronasiou D, Kenter GG, Jordanova ES and de Gruijl TD: High and
interrelated rates of PD-L1+CD14+ antigen-presenting cells and
regulatory T cells mark the microenvironment of metastatic lymph
nodes from patients with cervical cancer. Cancer Immunol Res.
3:48–58. 2015.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Rodríguez PC and Ochoa AC: Arginine
regulation by myeloid derived suppressor cells and tolerance in
cancer: Mechanisms and therapeutic perspectives. Immunol Rev.
222:180–191. 2008.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Galliverti G, Wullschleger S, Tichet M,
Murugan D, Zangger N, Horton W, Korman AJ, Coussens LM, Swartz MA
and Hanahan D: Myeloid cells orchestrate systemic
immunosuppression, impairing the efficacy of immunotherapy against
HPV+ cancers. Cancer Immunol Res. 8:131–145.
2020.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Jianyi D, Haili G, Bo Y, Meiqin Y, Baoyou
H, Haoran H, Fang L, Qingliang Z and Lingfei H: Myeloid-derived
suppressor cells cross-talk with B10 cells by BAFF/BAFF-R pathway
to promote immunosuppression in cervical cancer. Cancer Immunol
Immunother. 72:87–89. 2023.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Kawano M, Mabuchi S, Matsumoto Y, Sasano
T, Takahashi R, Kuroda H, Kozasa K, Hashimoto K, Isobe A, Sawada K,
et al: The significance of G-CSF expression and myeloid-derived
suppressor cells in the chemoresistance of uterine cervical cancer.
Sci Rep. 5(18217)2015.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Sawant DV, Yano H, Chikina M, Zhang Q,
Liao M, Liu C, Callahan DJ, Sun Z, Sun T, Tabib T, et al: Adaptive
plasticity of IL-10+ and IL-35+
Treg cells cooperatively promotes tumor T cell
exhaustion. Nat Immunol. 20:724–735. 2019.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Wu MY, Kuo TY and Ho HN:
Tumor-infiltrating lymphocytes contain a higher proportion of
FOXP3(+) T lymphocytes in cervical cancer. J Formos Med Assoc.
110:580–586. 2011.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Nakamura T, Shima T, Saeki A, Hidaka T,
Nakashima A, Takikawa O and Saito S: Expression of indoleamine 2,
3-dioxygenase and the recruitment of Foxp3-expressing regulatory T
cells in the development and progression of uterine cervical
cancer. Cancer Sci. 98:874–881. 2007.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Heeren AM, de Boer E, Bleeker MC, Musters
RJ, Buist MR, Kenter GG, de Gruijl TD and Jordanova ES: Nodal
metastasis in cervical cancer occurs in clearly delineated fields
of immune suppression in the pelvic lymph catchment area.
Oncotarget. 6:32484–32493. 2015.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Wang S, Li J, Xie J, Liu F, Duan Y, Wu Y,
Huang S, He X, Wang Z and Wu X: Programmed death ligand 1 promotes
lymph node metastasis and glucose metabolism in cervical cancer by
activating integrin β4/SNAI1/SIRT3 signaling pathway. Oncogene.
37:4164–4180. 2018.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Stein M and Eckert KA: Impact of
G-quadruplexes and chronic inflammation on genome instability:
Additive effects during carcinogenesis. Genes (Basel).
12(1779)2021.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Zhang LX, Wei ZJ, Xu M and Zang JH: Can
the neutrophil-lymphocyte ratio and platelet-lymphocyte ratio be
beneficial in predicting lymph node metastasis and promising
prognostic markers of gastric cancer patients? Tumor maker
retrospective study. Int J Surg. 56:320–327. 2018.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Ayhan S, Akar S, Kar İ, Turan AT, Türkmen
O, Kiliç F, Aytekin O, Ersak B, Ceylan Ö, Moraloğlu Tekin Ö and
Kimyon Comert G: Prognostic value of systemic inflammatory response
markers in cervical cancer. J Obstet Gynaecol.
42(2411)2022.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Lee WH, Kim GE and Kim YB: Prognostic
factors of dose-response relationship for nodal control in
metastatic lymph nodes of cervical cancer patients undergoing
definitive radiotherapy with concurrent chemotherapy. J Gynecol
Oncol. 33(e59)2022.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Polgár C, Major T and Varga S:
Radiotherapy and radio-chemotherapy of cervical cancer. Magy Onkol.
66:307–314. 2022.PubMed/NCBI(In Hungarian).
|
|
102
|
Vinay DS, Ryan EP, Pawelec G, Talib WH,
Stagg J, Elkord E, Lichtor T, Decker WK, Whelan RL, Kumara HMCS, et
al: Immune evasion in cancer: Mechanistic basis and therapeutic
strategies. Semin Cancer Biol. 35 (Suppl):S185–S198.
2015.PubMed/NCBI View Article : Google Scholar
|
|
103
|
van Weelden WJ, Sekarutami SM, Bekkers RL,
Kaanders JH, Bussink J, Gondhowiardjo S, Leer JW and Span PN: The
effect of carbogen breathing and nicotinamide added to standard
(chemo) radiation treatment of advanced cervical cancer in
Indonesia. Int J Gynecol Cancer. 24:1628–1635. 2014.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Samsuri NAB, Leech M and Marignol L:
Metformin and improved treatment outcomes in radiation therapy-A
review. Cancer Treat Rev. 55:150–162. 2017.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Lin A and Maity A: Molecular pathways: A
novel approach to targeting hypoxia and improving radiotherapy
efficacy via reduction in oxygen demand. Clin Cancer Res.
21:1995–2000. 2015.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Sharma A, Arambula JF, Koo S, Kumar R,
Singh H, Sessler JL and Kim JS: Hypoxia-targeted drug delivery.
Chem Soc Rev. 48:771–813. 2019.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Zeman EM, Brown JM, Lemmon MJ, Hirst VK
and Lee WW: SR-4233: A new bioreductive agent with high selective
toxicity for hypoxic mammalian cells. Int J Radiat Oncol Biol Phys.
12:1239–1242. 1986.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Brown JM: SR 4233 (tirapazamine): A new
anticancer drug exploiting hypoxia in solid tumours. Br J Cancer.
67:1163–1170. 1993.PubMed/NCBI View Article : Google Scholar
|
|
109
|
DiSilvestro PA, Ali S, Craighead PS, Lucci
JA, Lee YC, Cohn DE, Spirtos NM, Tewari KS, Muller C, Gajewski WH,
et al: Phase III randomized trial of weekly cisplatin and
irradiation versus cisplatin and tirapazamine and irradiation in
stages IB2, IIA, IIB, IIIB, and IVA cervical carcinoma limited to
the pelvis: A gynecologic oncology group study. J Clin Oncol.
32:458–464. 2014.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Seidel JA, Otsuka A and Kabashima K:
Anti-PD-1 and anti-CTLA-4 therapies in cancer: Mechanisms of
action, efficacy, and limitations. Front Oncol.
8(86)2018.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Chen DS and Mellman I: Oncology meets
immunology: The cancer-immunity cycle. Immunity. 39:1–10.
2013.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Yao S, Zhu Y and Chen L: Advances in
targeting cell surface signalling molecules for immune modulation.
Nat Rev Drug Discov. 12:130–146. 2013.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Naumann RW, Hollebecque A, Meyer T, Devlin
MJ, Oaknin A, Kerger J, López-Picazo JM, Machiels JP, Delord JP,
Evans TRJ, et al: Safety and efficacy of Nivolumab Monotherapy in
recurrent or metastatic cervical, vaginal, or vulvar carcinoma:
Results from the phase I/II CheckMate 358 trial. J Clin Oncol.
37:2825–2834. 2019.PubMed/NCBI View Article : Google Scholar
|
|
114
|
O'Malley DM, Neffa M, Monk BJ, Melkadze T,
Huang M, Kryzhanivska A, Bulat I, Meniawy TM, Bagameri A, Wang EW,
et al: Dual PD-1 and CTLA-4 checkpoint blockade using Balstilimab
and Zalifrelimab combination as second-line treatment for advanced
cervical cancer: An open-label phase II study. J Clin Oncol.
40:762–771. 2022.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Santin AD, Deng W, Frumovitz M, Buza N,
Bellone S, Huh W, Khleif S, Lankes HA, Ratner ES, O'Cearbhaill RE,
et al: Phase II evaluation of nivolumab in the treatment of
persistent or recurrent cervical cancer (NCT02257528/NRG-GY002).
Gynecol Oncol. 157:161–166. 2020.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Frenel JS, Le Tourneau C, O'Neil B, Ott
PA, Piha-Paul SA, Gomez-Roca C, van Brummelen EMJ, Rugo HS, Thomas
S, Saraf S, et al: Safety and efficacy of Pembrolizumab in
advanced, programmed death ligand 1-positive cervical cancer:
Results from the phase Ib KEYNOTE-028 trial. J Clin Oncol.
35:4035–4041. 2017.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Chung HC, Ros W, Delord JP, Perets R,
Italiano A, Shapira-Frommer R, Manzuk L, Piha-Paul SA, Xu L,
Zeigenfuss S, et al: Efficacy and safety of pembrolizumab in
previously treated advanced cervical cancer: results from the phase
II KEYNOTE-158 study. J Clin Oncol. 37:1470–1478. 2019.PubMed/NCBI View Article : Google Scholar
|
|
118
|
De Jaeghere EA, Tuyaerts S, Van Nuffel
AMT, Belmans A, Bogaerts K, Baiden-Amissah R, Lippens L, Vuylsteke
P, Henry S, Trinh XB, et al: Pembrolizumab, radiotherapy, and an
immunomodulatory five-drug cocktail in pretreated patients with
persistent, recurrent, or metastatic cervical or endometrial
carcinoma: Results of the phase II PRIMMO study. Cancer Immunol
Immunother. 72:475–491. 2023.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Lee Y, Auh SL, Wang Y, Burnette B, Wang Y,
Meng Y, Beckett M, Sharma R, Chin R, Tu T, et al: Therapeutic
effects of ablative radiation on local tumor require CD8+ T cells:
Changing strategies for cancer treatment. Blood. 114:589–595.
2009.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Sharabi AB, Lim M, DeWeese TL and Drake
CG: Radiation and checkpoint blockade immunotherapy:
Radiosensitisation and potential mechanisms of synergy. Lancet
Oncol. 16:e498–e509. 2015.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Twyman-Saint Victor C, Rech AJ, Maity A,
Rengan R, Pauken KE, Stelekati E, Benci JL, Xu B, Dada H, Odorizzi
PM, et al: Radiation and dual checkpoint blockade activate
non-redundant immune mechanisms in cancer. Nature. 520:373–377.
2015.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Young KH, Baird JR, Savage T, Cottam B,
Friedman D, Bambina S, Messenheimer DJ, Fox B, Newell P, Bahjat KS,
et al: Optimizing timing of immunotherapy improves control of
tumors by hypofractionated radiation therapy. PLoS One.
11(e0157164)2016.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Dovedi SJ, Adlard AL, Lipowska-Bhalla G,
McKenna C, Jones S, Cheadle EJ, Stratford IJ, Poon E, Morrow M,
Stewart R, et al: Acquired resistance to fractionated radiotherapy
can be overcome by concurrent PD-L1 blockade. Cancer Res.
74:5458–5468. 2014.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Sun J and Yuan J: Chemokine (C-X-C motif)
ligand 1/chemokine (C-X-C motif) receptor 2 autocrine loop
contributes to cellular proliferation, migration and apoptosis in
cervical cancer. Bioengineered. 13:7579–7591. 2022.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Strachan DC, Ruffell B, Oei Y, Bissell MJ,
Coussens LM, Pryer N and Daniel D: CSF1R inhibition delays cervical
and mammary tumor growth in murine models by attenuating the
turnover of tumor-associated macrophages and enhancing infiltration
by CD8+ T cells. Oncoimmunology.
2(e26968)2013.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Steele CW, Karim SA, Leach JDG, Bailey P,
Upstill-Goddard R, Rishi L, Foth M, Bryson S, McDaid K, Wilson Z,
et al: CXCR2 inhibition profoundly suppresses metastases and
augments immunotherapy in pancreatic ductal adenocarcinoma. Cancer
Cell. 29:832–845. 2016.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Pagni RL, Souza PDC, Pegoraro R, Porchia
BFMM, da Silva JR, Aps LRMM, Silva MO, Rodrigues KB, Sales NS,
Ferreira LCS and Moreno ACR: Interleukin-6 and
indoleamine-2,3-dioxygenase as potential adjuvant targets for
papillomavirus-related tumors immunotherapy. Front Immunol.
13(1005937)2022.PubMed/NCBI View Article : Google Scholar
|
|
128
|
He Y, Kozaki K, Karpanen T, Koshikawa K,
Yla-Herttuala S, Takahashi T and Alitalo K: Suppression of tumor
lymphangiogenesis and lymph node metastasis by blocking vascular
endothelial growth factor receptor 3 signaling. J Natl Cancer Inst.
94:819–825. 2002.PubMed/NCBI View Article : Google Scholar
|
|
129
|
García-Quiroz J, Vázquez-Almazán B,
García-Becerra R, Díaz L and Avila E: The interaction of human
papillomavirus infection and prostaglandin E2 signaling
in carcinogenesis: A focus on cervical cancer therapeutics. Cells.
11(2528)2022.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Peng H, He X and Wang Q: Immune checkpoint
blockades in gynecological cancers: A review of clinical trials.
Acta Obstet Gynecol Scand. 101:941–951. 2022.PubMed/NCBI View Article : Google Scholar
|