Introduction
Among the malignant tumors of the female
reproductive system, the incidence rate of cervical cancer is
second only to breast cancer, which poses a severe threat to
women's health (1). The 5-year
survival rate of patients with cervical cancer in several
underdeveloped countries is <50% (2). With advancements being made in
medicine, cervical cancer treatment has gradually changed from
single surgical treatment to a combination of traditional and
neoadjuvant treatments; however, the prognosis of patients has not
been markedly improved and the mortality rate remains high
(3). Long non-coding RNAs
(lncRNAs) are non-coding RNAs with a length of >200 nt, which
cannot encode proteins but can regulate gene expression through
transcriptional, post-transcriptional and epigenetic mechanisms
(4). Relevant studies demonstrated
that the lncRNA tissue differentiation-inducing non-protein coding
RNA (TINCR) participates in tumor progression and is abnormally
expressed in various tumor tissues (5-8).
TINCR plays an inhibitory role in prostate cancer (5), whereas it plays a promoting role in
other types of tumors including NSCLC, HCC and colon cancer, TINCR
is closely correlation with poor prognosis in NSCLC and colon
cancer and TINCR overexpression also stimulated cancer cells
biological activities including cell proliferation, invasion and
migration (6-8).
However, the expression of lncRNA TINCR in cervical cancer and its
association with the prognosis of patients, as well as its
mechanisms of action in cervical cancer occurrence and development
remain unclear.
It was demonstrated that lncRNAs may play a role in
tumor occurrence and development by regulating the expression of
relevant micro(mi)RNAs (9-12).
miRNAs are endogenous short-chain RNAs, which can specifically bind
to complementary sites in the 3' untranslated region (3'-UTR) of
the target mRNA and inhibit its translation or promote its
degradation, thus regulating target gene level at the
post-transcriptional level. According to a previous study, several
miRNAs are abnormally expressed in cancer (13-15).
For example, the overexpression of miR-17-5p was shown to promote
the malignant proliferation of tumor cells by suppressing E2F
transcription factor 1 expression (14); miR-145 was shown to inhibit tumor
cell growth by targeting MAPK to exert an anti-carcinogenic effect
(15). miRNA-7 is a key member of
the miRNA family (16-18).
Previous studies confirmed that abnormal miRNA-7 level is closely
related to cancer occurrence (16-18).
However, it is not yet clear whether miRNA-7 may play a role in the
regulation of cervical cancer biological activity through
TINCR.
On this basis, in the present study, TINCR was first
detected in cervical cancer and normal para-cancerous tissues using
in situ hybridization (ISH) and reverse
transcription-quantitative PCR (RT-qPCR), and the associations
between the TINCR level and the pathology and prognosis of patients
with cervical cancer were analyzed. Additionally, the effects of
TINCR knockdown on cervical cancer cell biological activities were
examined and the corresponding molecular mechanisms were examined
in vitro.
Materials and methods
Clinical data
A total of 40 patients with cervical cancer
undergoing surgical resection at the Women and Children's Hospital,
Qingdao University (Qingdao, China) from September 2015 to May 2017
were selected as subjects, aged 32-70 years, with an average age of
(54.682±12 27) years. The resected cancer tissues were used as the
experimental group, while the para-cancerous tissues (>2 cm from
the tumor margin) from the same patients were used as the control
group. The inclusion criteria were as follows: i) Confirmed
diagnosis of cervical cancer by a pathological examination; ii)
receiving no radiotherapy or chemotherapy 3 months before surgery;
iii) complete clinical and pathological data; and iv) informed
consent provided by patients and their families. The exclusion
criteria included the following: i) Presence of other malignant
tumors; ii) complications such as hepatic, renal and cardiac
dysfunctions; iii) autoimmune diseases; and iv) pregnant and
lactating women.
Depending on the International Federation of
Gynecology and Obstetrics (FIGO) staging criteria, the subjects
were divided into stage I-II (n=18) and stage III-IV (n=22). There
were 16 patients with adenocarcinoma and 24 with squamous cell
carcinoma. Among all patients, 18 patients presented with and 22
without lymph node metastasis. A total of 17 patients presented
with a myometrial invasion depth >1/2 and 23 with a myometrial
invasion depth ≤1/2. All the patients were followed up for 5 years
from the date of the pathological diagnosis to 1 June 2022, to
record their survival status, without the loss of any patients. The
present study was approved by the Ethics Committee of Women and
Children's Hospital, Qingdao University (approval no.
2015081305).
Sample collection
The cervical cancer tissues resected surgically were
collected as the experimental group. Additionally, para-cancerous
tissues >2 cm from the tumor margin were collected as the
control group. The collected samples were divided into two parts.
One was embedded in paraffin for hematoxylin and eosin (H&E)
staining and ISH, and the other was stored in a refrigerator at
-80˚C for later use.
Measurement of TINCR levels in tissues
using ISH
To measure the lncRNA TINCR levels in cervical
cancer and normal para-cancerous tissues, ISH was performed as per
the instructions provided with the relevant kit (Boster Biological
Technology). Digoxin-labeled lncRNA TINCR probe (Boster Biological
Technology; cat. no. MK10932; 1:400) was added to the
paraffin-embedded tissue sections (5 µm) for incubation at 55˚C for
1 h, followed by washing by PBS. Subsequently, the tissues were
sealed in 0.2xSSC (Sigma-Aldrich; Merck KGaA) solution at 60˚C for
1 h. After removing the reagents, the tissue sections were placed
in TBST containing anti-digoxin antibody (1:200; cat no. ab30512,
Abcam), followed by incubation at 37˚C for 1 h. Finally, the
sections were stained at room temperature with H&E (cat. no.
KGA224; Nanjing KeyGen Biotech Co., Ltd.) for 2 h and observed and
photographed under an optical microscope (CX23; Olympus
Corporation).
Materials and reagents
The HeLa and Siha (lncRNA TINCR expression was most
upregulated in Hela and Siha cell lines; Fig. S1) cells were purchased from The
Cell Bank of Type Culture Collection of the Chinese Academy of
Sciences. FBS, DMEM/F12 (HyClone; Cytiva), TRIzol™ reagent,
Lipofectamine 2000™ (cat. no. 11668-019), RIPA lysate and the BCA
protein quantitative kit were from Invitrogen (Thermo Fisher
Scientific, Inc.). The Annexin V-FITC/PI cell apoptosis kit was
obtained from CapitalBio Technology, Inc. Matrigel was obtained
from BD Biosciences. The MTT assay kit and Transwell chambers were
obtained from MilliporeSigma. The dual-fluorescent enzyme detection
kit was purchased from Promega Corporation. The mTOR (1:500; cat.
no. ab134903), hypoxia-inducible factor 1 subunit α (HIF-1α; 1:500;
cat. no ab.51608), anti-VEGF (1:500; cat. no. ab32152) and
anti-GAPDH (1:500; cat. no. ab8245) antibodies were obtained from
Abcam.
RT-qPCR assay
Total RNA was extracted from cells or tissues using
the TRIzol reagent and reverse transcribed into cDNA with a
SuperScript™ VILO™ cDNA Synthetic reagent kit (Thermo Fisher
Scientific, Inc.; cat. no. 11754050), according to the
manufacturer's instructions. Subsequently, qPCR was then performed
using the SYBR-Green PCR Kit (Takara Biotechnology Co., Ltd.; cat.
no. DRR041A). The RT-PCR conditions were as follows: Initial
denaturation at 95˚C for 10 min, followed by 40 cycles of
denaturation at 95˚C for 5 sec, annealing at 60˚C for 30 sec and
elongation at 72˚C for 32 sec. The primers used for RT-qPCR are
presented in Table I. U6 was used
as the internal reference for miRNA-7 and GAPDH for the other
genes. RNA expression levels were quantified using the
2-ΔΔCq method (19).
 | Table IPrimer sequences used for
quantitative PCR. |
Table I
Primer sequences used for
quantitative PCR.
| Gene name | Forward | Reverse |
|---|
| TINCR |
5'-TGTGGCCCAAACTCAGGGATACAT-3' |
5'-AGATGACAGTGGCTGGAGTTGTCA-3' |
| miRNA-7 |
5'-ACGTTGGAAGACTAGTGATTT-3' |
5'-TATGGTTGTTCTGCTCTCTGTCTC-3' |
| mTOR |
5'-CGTCAGCACCATCAACCTCCAA-3' |
5'-TCAGCCGTCTCAGCCATTCCA-3' |
| Hypoxia-inducible
factor 1 subunit α |
5'-GGCGCGAACGACAAGAAAAAG |
5'-CCTTATCAAGATGCGAACTCACA-3' |
| VEGF |
5'-CAGCGCAGCTACTGCCATCCAATCG AGA-3' |
5'-GCTTGTCACATCTGCAAGTACGTTCGT TTA-3' |
| U6 |
5'-TCGCTTCGGCAGCACATATACTAA-3' |
5'-AATATGGAACGCTTCACGAATTTGC-3' |
| GAPDH |
5'-AGGCCGGATGTGTTCGC-3' |
5'-CATGGTTCACACCCATGACG-3' |
Cell culture and transfection
The HeLa and SiHa cells were cultured in DMEM/F12
supplemented with 10% FBS and placed in an incubator a 37˚C with 5%
CO2. When cell confluence reached 70-80%, the cells were
digested with 0.25% trypsin (Sigma-Aldrich; Merck KGaA) and
centrifuged at 800 x g for 5 min at room temperature. After
discarding the supernatant, the cells were resuspended in PBS and
adjusted to a cell density of 5x105/ml. The cell
suspension (1 ml) was collected and seeded in a six-well culture
plate, which was placed overnight in an incubator under the same
conditions as those described above. After the cells had fully
adhered to the wall, the culture medium was removed for transient
transfection. The cervical cancer cells were transfected with small
interfering (si)-negative control (NC) (cat. no. 12935200; Thermo
Fisher Scientific, Inc.), si-TINCR (cat. no. AM16708; Thermo Fisher
Scientific, Inc.), miRNA-7 antisense oligonucleotide (ASO) as
miRNA-7 inhibitor (5'-ACAACAAAAUCACUAGUCUUCCA-3') and ASO-NC
(5'-CAGUACUUUUGUGUAGUACAA-3') at a final concentration of 50
nmol/l, according to the instructions provided with the
Lipofectamine 2000 reagent, and incubated under the aforementioned
conditions. After 4 h, the culture medium was replaced with
DMEM/F12 supplemented with 15% FBS and the transfection efficiency
was examined. The cells were photographed and recorded under an X71
(U-RFL-T) fluorescence microscope (Olympus Corporation).
Lipofectamine 2000 reagent was used to transfect 10 nM vectors into
6x105 cells according to the manufacturer's
instructions. The incubation of the cells with vectors was
performed for 48 h at 37˚C. The transfection rates of lncRNA TINCR
and miR-7 are shown in Fig.
S2.
MTT assay
Following the corresponding treatments for 48 h, the
cells in each group were adjusted to a cell density of
5x103 cells/ml, seeded in a 96-well plate with 200 µl
DMEN/F12 culture medium supplemented with 10% FBS in each well and
then cultured in an incubator at 37˚C with 5% CO2. After
48 h, the cells were collected and 20 µl MTT was added (5
mg/m1/well), followed by incubation at 37˚C for 4 h. After
discarding the original culture medium, the cells were supplemented
with DMSO (150 µl/well) and fully shaken at room temperature for 10
min to completely dissolve the formed purple formazan crystals. The
absorbance at 450 nm was measured using a microplate reader (Thermo
Fisher Scientific, Inc.).
5-ethynyl-2'-deoxyuridine (EdU)
staining
Following the corresponding treatments for 48 h, the
cells in each group were supplemented with 10 µmol/l EdU, according
to the instructions provided with the EdU fluorescent staining cell
proliferation kit (cat. no. KGA337; Nanjing KeyGen Biotech Co.,
Ltd.). Following incubation for 2 h at room temperature, the EdU
not infiltrating the DNA was washed with PBS and 4%
polyformaldehyde was added to fix the cells for 30 min at room
temperature. Subsequently, the fixation solution was washed with
PBS and Apollo dye solution (Sigma-Aldrich; Merck KGaA) was added
to the cells for incubation in the dark at room temperature for 30
min. The dye solution was washed with PBS and the nuclei were
stained with 10 µmol/l DAPI for 5 min at room temperature.
Fluorescence images of five randomly-selected fields were obtained
under an IX73 fluorescence microscope (Olympus Corporation) and the
EdU-positive cells were counted using ImageJ software v1.46
(National Institutes of Health).
Detection of apoptosis using flow
cytometry
Following the corresponding treatments for 48 h, the
cells in each group were routinely digested with 0.25% trypsin,
followed by washing with PBS three times and centrifugation at 800
x g for 5 min at 4˚C. After the supernatant was discarded, the cell
concentration was adjusted to 5x105 cells per sample and
195 µl Annexin V-FITC binding buffer was added to resuspend the
cells. Subsequently, 5 µl Annexin V-FITC and 10 µl PI were added
followed by incubation in the dark at room temperature for 30 min.
Apoptosis analysis was performed using a BD FACScan™ flow cytometer
with BD CellQuest(TM) software version 5.1 (BD Biosciences).
TUNEL staining
Following the corresponding treatments for 48 h, the
cells in each group were fixed with 4% paraformaldehyde (Nanjing
KeyGen Biotech Co., Ltd.) at room temperature for 30 min and then
incubated at room temperature for 5 min with enhanced
immunostaining permeabilization buffer (Nanjing KeyGen Biotech Co.,
Ltd., cat. no. KGIHC010). The cells were then stained with TUNEL
(cat. no. KGA702-1; Nanjing KeyGen Biotech Co., Ltd.) and incubated
in the dark at 37˚C for 60 min to observe cell apoptosis under an
Olympus IX71 fluorescence microscope (Olympus Corporation). Red
fluorescence indicated TUNEL-positive cells.
Transwell assay for cell invasion
Following the corresponding treatments for 48 h, the
cells in each group were digested with trypsin and centrifuged (800
x g at 4˚C for 30 sec). The cells were collected and counted using
a cell counter (Beckman Coulter). After adjusting the cell count
with serum-free medium, Precoat Matrigel at room temperature for 1
h, the cells were seeded into the upper chamber of Transwell plates
at 5x104 cells/well (200 µl/well). The lower chamber was
supplemented with 700 µl complete medium to induce the migration of
tumor cells and placed in an incubator for 24 h at room
temperature. Subsequently, the upper chamber was removed and washed
with PBS twice after the supernatant was discarded; the cells that
did not pass through the inner side of the upper chamber were wiped
off using a cotton swab. Following fixation with 4%
polyformaldehyde for 15 min at room temperature, the chambers were
rinsed with PBS twice and the cells were stained with crystal
violet (Nanjing KeyGen Biotech Co., Ltd.) staining solution for 15
min at room temperature, followed by rinsing with PBS five times.
After drying, the cells were observed and photographed under an X71
(U-RFL-T) fluorescence microscope (Olympus Corporation;
magnification, x200) and five randomly-selected fields were used to
count the number of migrating cells.
Wound healing assay for cell
migration
Following the corresponding treatments for 48 h, the
cells in each group were digested with trypsin and centrifuged (800
x g at 4˚C for 30 sec). The cells were then resuspended in culture
medium supplemented with 5% (w/w) FBS and the cell concentration
was adjusted using a cell counter by serum-free medium.
Subsequently, the cells were seeded in a six-well plate at
2x105 cells/well (2 ml/well). When the cell density
reached 80-90%, the culture medium in the plate was discarded and a
vertical line was scratched using a 20 µl pipette tip under a X71
(U-RFL-T) fluorescence microscope (Olympus Corporation;
magnification, x100). The cells were then rinsed with PBS twice to
remove any residual cells. The changes in cell migration were
observed at 0, 24 and 48 h in each group and photographed under an
inverted X71 (U-RFL-T) fluorescence microscope (Olympus
Corporation; magnification, x100), and the average wound healing
rate was analyzed and calculated.
Western blot analysis for relative
protein expression in cells
Following the corresponding treatments for 48 h, the
cells in each group were collected and centrifuged (800 x g at 4˚C
for 30 sec). After the supernatant was discarded, the cells were
lysed with radioimmunoprecipitation buffer (Nanjing KeyGen Biotech
Co., Ltd.). Following centrifugation (1,000 x g at 4˚C for 45 sec),
the supernatant was collected and the total protein content in the
supernatant was determined using the BCA method. Proteins were
separated by SDS-PAGE on 12% gel and transferred to a PVDF membrane
using the wet transfer method. Subsequently, the membranes were
blocked using 5% skimmed milk powder at room temperature for 2 h
and washed with TBST five times for 10 min each time. The membrane
was then incubated with primary antibodies (all from Abcam) against
mTOR (1:500; cat. no. ab134903), HIF-1α (1:500; cat. no ab.51608;),
anti-VEGF (1:500; cat. no. ab32152) and anti-GAPDH (1:500; cat. no.
ab8245) overnight at 4˚C. Following primary incubation, the
membrane were washed five times with TBST, 10 min each time and
incubated with HRP-conjugated secondary antibodies (1:10,000;
Nanjing KeyGen Biotech Co., Ltd., cat. no. KGP1201) at room
temperature for 2 h. The membrane was then washed with TBST five
times, 10 min each time. Protein bands were visualized using an ECL
reagent (Nanjing KeyGen Biotech Co., Ltd.) and a gel imaging system
(SYNGENE G:BOX Chemi XR5; Syngene), the gray value of the bands was
quantified using ImageJ software v1.46 (National Institutes of
Health) and the protein expression levels were normalized to the
GAPDH reference gene.
Bioinformatics analysis and
dual-luciferase reporter gene assay
Bioinformatics analysis was performed using
TargetScan version 7.1 (www.targetscan.org). To clarify the association
between TINCR and miRNA-7, the mutant miRNA-7 binding site of TINCR
was generated using the quick-change site-directed mutagenesis kit
(Stratagene; Agilent Technologies, Inc.). The wild-type (WT) or
mutant-type (MT) TINCR was subcloned into the dual-luciferase
target vector by Lipofectamine 2000™ (Promega Corporation) to
produce the WT or MT TINCR luciferase report plasmid, respectively.
To clarify the association between miRNA-7 and mTOR, the MT miR-7
binding site of the mTOR 3'-UTR was generated using the
quick-change site-directed mutagenesis kit (Stratagene; Agilent
Technologies, Inc.). The WT, MT or luciferase report plasmid was
produced. As aforementioned, the cells were co-transfected with
miRNA-7 mimics (10 ng/ml) (5'-UGGAAGACUAGUGAUUUUGUUGU-3') or its NC
(miRNA-NC) (10 ng/ml; 5'-CAGUACUUUUGUGUAGUACAA-3'), WT (or MT)
TINCR reporter plasmid, WT (or MT) MT or 3'-UTR reporter plasmid
using the Lipofectamine 2000 reagent. Following cell transfection
for 48 h at room temperature, the luciferase activity was detected
using a dual-luciferase report assay system (Promega Corporation).
Firefly luciferase activity was normalized to Renilla
luciferase activity.
Statistical analysis
All data were analyzed using SPSS 24.0 software (IBM
Corp.). Continuous data are presented as the mean ± standard
deviation and were analyzed using one-way analysis of variance with
Tukey's post hoc test. Enumeration data are presented as number and
were analyzed using chi-square or Fisher's exact test. A
Kaplan-Meier curve was used to draw the survival curve of patients
with cervical cancer and Cox multivariate analysis was used to
analyze risk factors affecting cervical cancer. All statistical
tests were two-tailed and P<0.05 was considered to indicate a
statistically significant difference. All the experiments were
performed in triplicate.
Results
Pathological changes and TINCR
expression in tissues
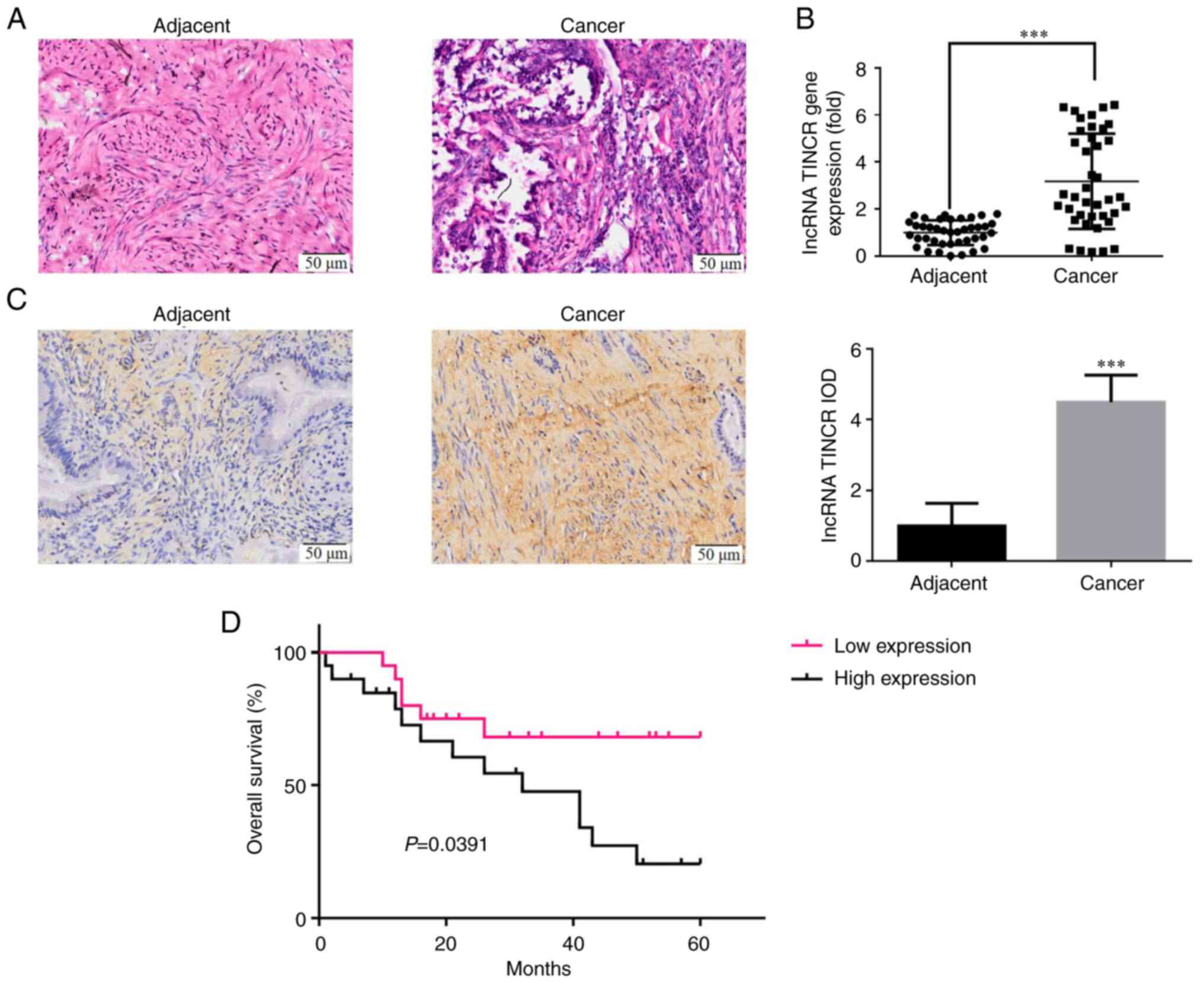
H&E staining revealed the pathological
differences in the cervical cancer tissues were blur, with tissue
infiltration (Fig. 1A). RT-qPCR
(Fig. 1B) and ISH (Fig. 1C) revealed that the expression
level of lncRNA TINCR in the cervical cancer tissues was
significantly higher compared with that in normal tissues
(P<0.001).
Association between the lncRNA TINCR
level and the clinicopathological characteristics of patients with
cervical cancer
The 40 patients with cervical cancer included in the
present study were divided into the high and low expression groups
using the median as cut-off value. The lncRNA TINCR level in the
cervical cancer tissues was not significantly associated with age,
tumor diameter, pathological type and degree of differentiation
(Table II); however, it was
correlated with lymph node metastasis, myometrial invasion depth
and FIGO stage (P<0.05 for all; Table II).
 | Table IIThe relationship between the
expression of lncRNA TINCR and clinicopathological characteristics
in cervical cancer. |
Table II
The relationship between the
expression of lncRNA TINCR and clinicopathological characteristics
in cervical cancer.
| | lncRNA TINCR | |
|---|
| Clinicopathological
parameters | N | High expression
(n=20) | Low expression
(n=20) | χ2 | P-value |
|---|
| Age, years | | | | 0.004 | 0.948 |
|
≤55 | 19 | 9 | 10 | | |
|
>55 | 21 | 10 | 11 | | |
| Tumor diameter
(cm) | | | | 0.110 | 0.740 |
|
≤4 | 16 | 9 | 7 | | |
|
>4 | 24 | 11 | 13 | | |
| Pathological
type | | | | 0.014 | 0.906 |
|
Adenocarcinoma | 9 | 5 | 4 | | |
|
Squamous
cell carcinoma | 31 | 15 | 16 | | |
| Differentiation
degree | | | | 0.043 | 0.835 |
|
Moderately
and well | 21 | 11 | 10 | | |
|
Poor | 19 | 9 | 10 | | |
| Lymph node
metastasis | | | | 12.546 | 0.000 |
|
Yes | 18 | 14 | 4 | | |
|
No | 22 | 6 | 16 | | |
| Myometrial
infiltration depth | | | | 8.154 | 0.001 |
|
>1/2 | 17 | 13 | 4 | | |
|
≤1/2 | 23 | 7 | 16 | | |
| International
Federation of Gynecology and Obstetrics stage | | | | 12.016 | 0.000 |
|
I-II | 18 | 2 | 16 | | |
|
III-IV | 22 | 18 | 4 | | |
Association between the lncRNA TINCR
level and the prognosis of patients with cervical cancer
The 40 patients with cervical cancer were
followed-up for 60 months. The overall 5-year survival rate of
patients with a high expression of lncRNA TINCR was significantly
lower than that of patients with a low expression of lncRNA TINCR
(P=0.0391; Fig. 1D).
Analysis of risk factors affecting the
prognosis of patients with cervical cancer
Univariate analysis demonstrated that lncRNA TINCR
expression, lymph node metastasis, myometrial invasion depth and
FIGO stage were all risk factors affecting the prognosis of
patients with cervical cancer. Multivariate analysis demonstrated
that lncRNA TINCR expression [hazard ratio (HR), 2.58; 95% CI,
1.51-4.38; P<0.05] was an independent risk factor affecting the
prognosis of patients with cervical cancer (Table III).
 | Table IIIAnalysis of risk factors affecting
the prognosis of cervical cancer patients. |
Table III
Analysis of risk factors affecting
the prognosis of cervical cancer patients.
| | Single factor
analysis | Multiple factor
analysis |
|---|
| Variable | Hazard ratio | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| lncRNA TINCR
expression | 2.58 | 1.51-4.38 | 0.000 | 2.51 | 1.67-3.81 | 0.000 |
| Lymph node
metastasis | 2.14 | 1.22-3.68 | 0.004 | 1.55 | 0.92-2.57 | 0.112 |
| Depth of myometrial
infiltration | 3.46 | 2.19-5.12 | 0.002 | 1.6 | 0.75-3.55 | 0.092 |
| International
Federation of Gynecology and Obstetrics stage | 2.75 | 1.77-4.28 | 0.001 | 1.69 | 0.84-3.45 | 0.223 |
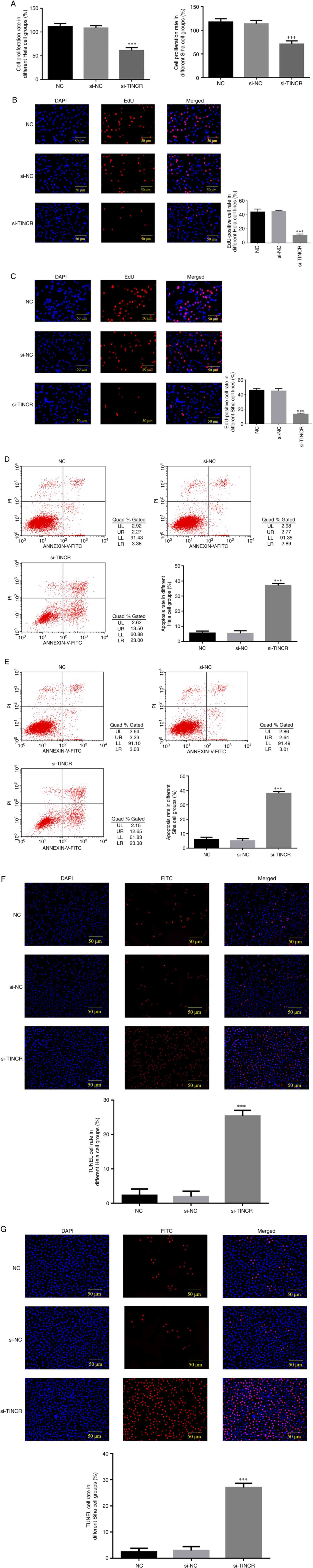
Effect of lncRNA TINCR knockdown on
the proliferation of cervical cancer cells
MTT assay revealed that the proliferation rate of
HeLa and SiHa cells in the si-TINCR group decreased significantly
following lncRNA TINCR knockdown (P<0.001 for both; Fig. 2A). EdU staining revealed that the
number of EdU-positive HeLa and SiHa cells was significantly lower
in the cells in which lncRNA TINCR was knocked down (P<0.001 for
all; Fig. 2B and C).
LncRNA TINCR knockdown promotes the
apoptosis of cervical cancer cells
Flow cytometry revealed that the apoptotic rate of
HeLa and SiHa cells in the si-TINCR group increased significantly
following lncRNA TINCR knockdown (P<0.001 for both; Fig. 2D and E). In addition, TUNEL assay confirmed
that compared with that in the NC group, the number of apoptotic
cells in the si-TINCR group increased significantly (P<0.001 for
both; Fig. 2F and G).
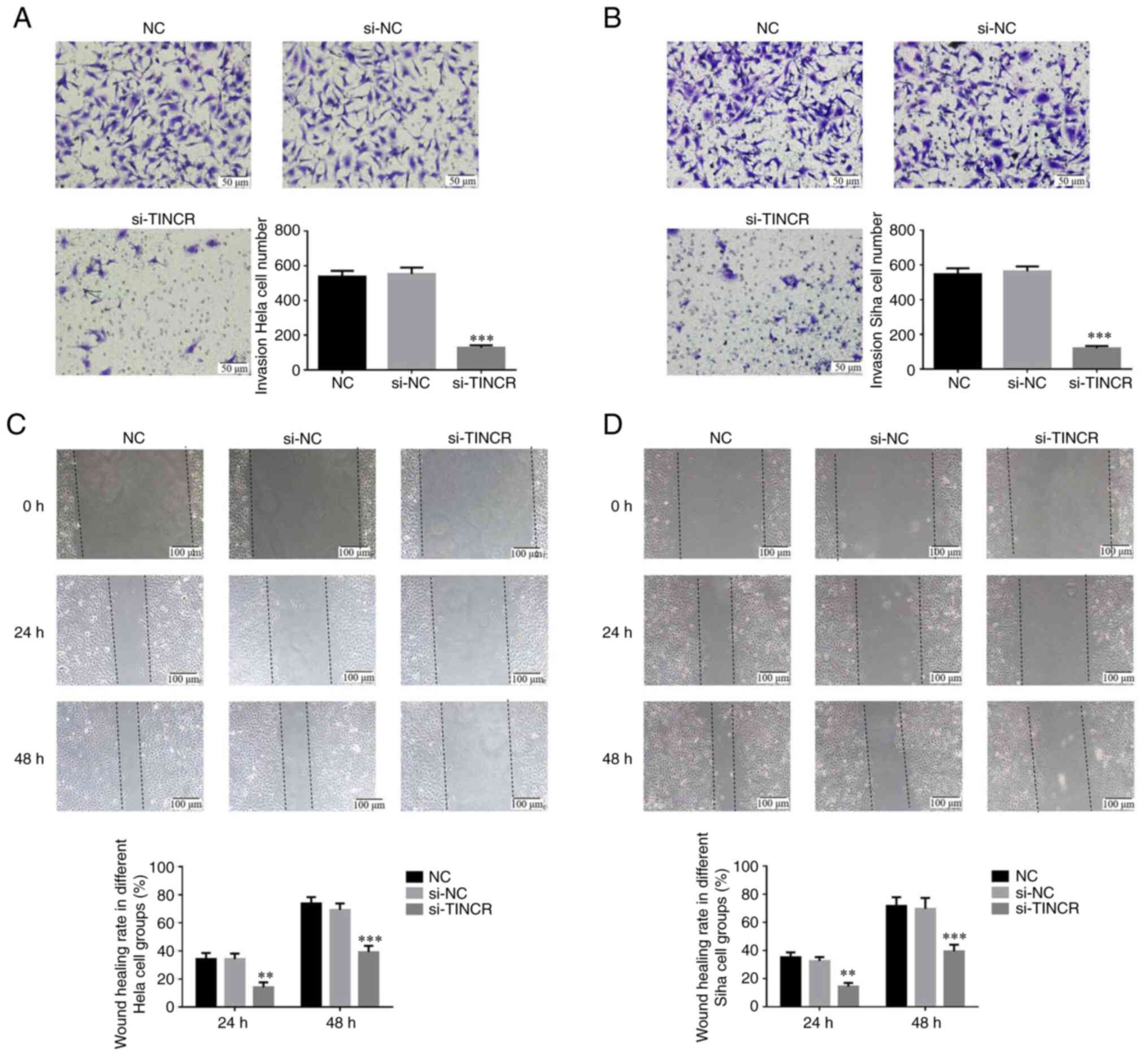
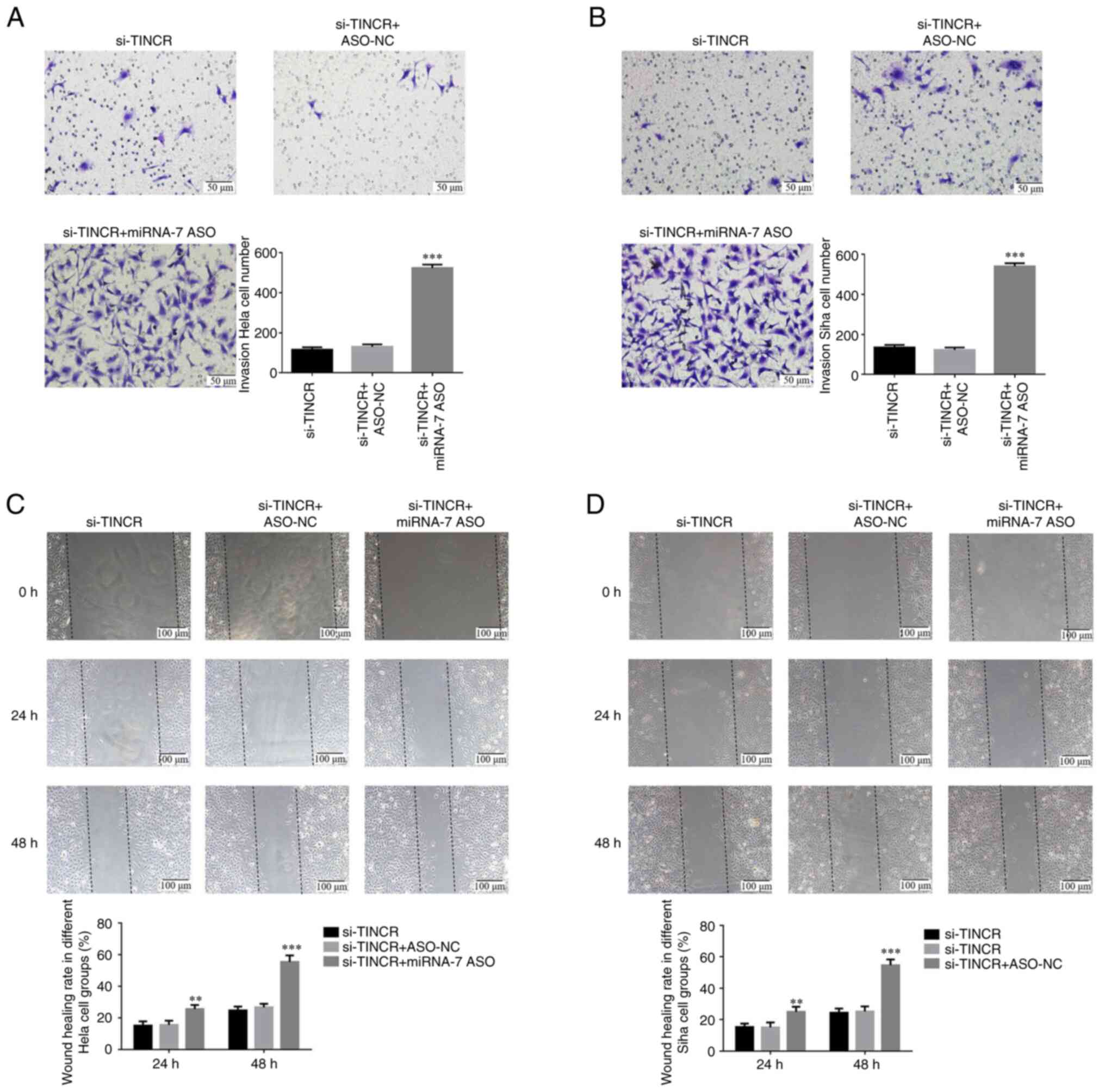
lncRNA TINCR knockdown suppresses the
invasion and migration of cervical cancer cells
Transwell assay confirmed that the number of
invasive HeLa and SiHa cells in the si-TINCR group was
significantly lower than that in the control group (P<0.001 for
both; Fig. 3A and B). Wound healing assay also revealed that
the wound healing rate of HeLa and SiHa cells in the si-TINCR group
at 24 and 48 h was significantly decreased (P<0.01 for both;
Fig. 3C and D).
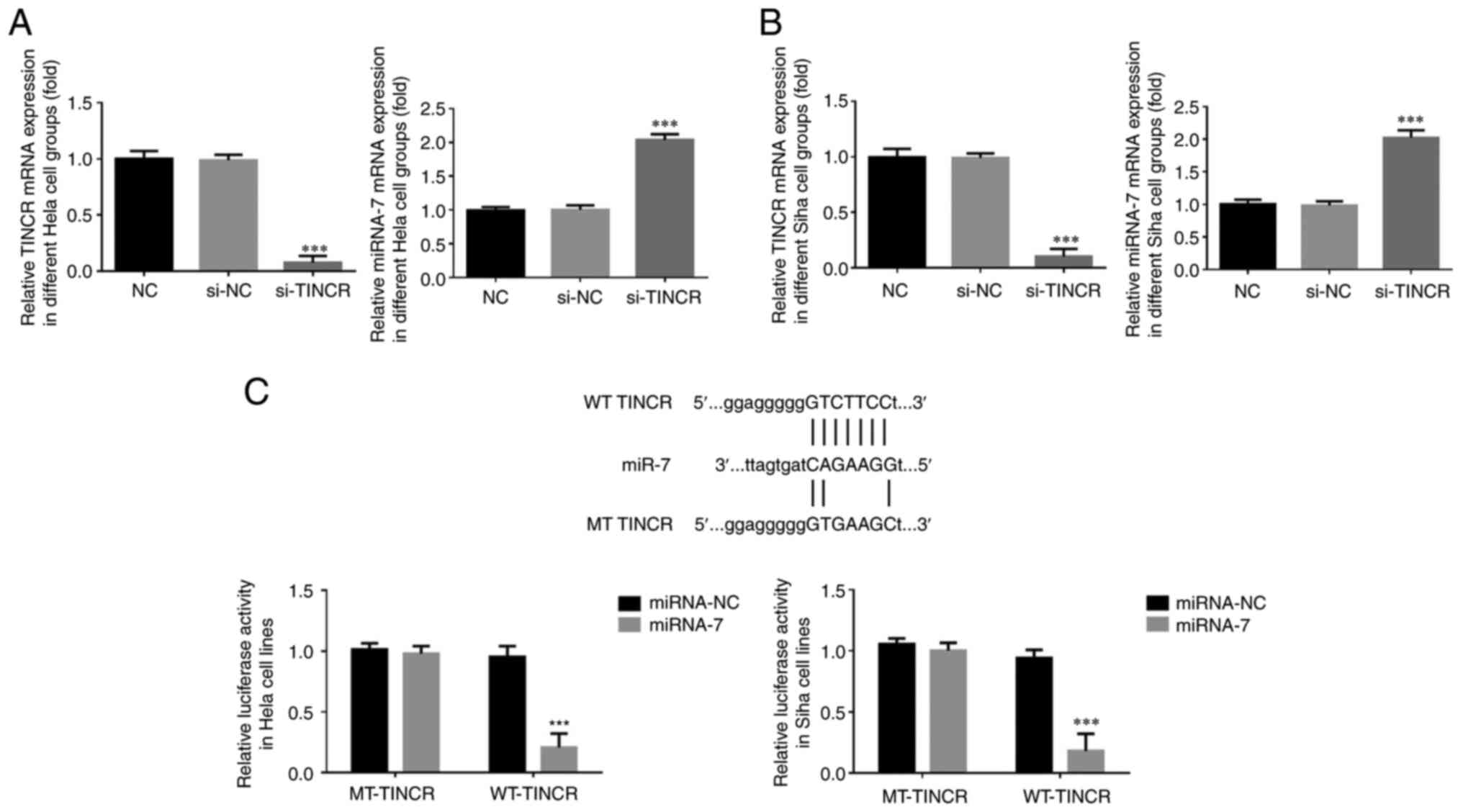
mRNA expression and association
between lncRNA TINCR and miRNA-7
Using RT-qPCR, it was found that compared with that
in the NC group, in the HeLa and SiHa cells transfected with
si-TINCR, lncRNA TINCR mRNA expression was significantly decreased
and the miRNA-7 mRNA level was significantly increased (P<0.001
for both; Fig. 4 and B).
Bioinformatics analysis revealed that TINCR could
target and regulate miRNA-7 (Fig.
4C). Dual-luciferase reporter assay demonstrated that following
the transfection of miRNA-7 mimic into HeLa and SiHa cells in the
WT-TINCR and MT-TINCR groups, the fluorescence intensity of lncRNA
TINCR was significantly decreased (P<0.001 for both; Fig. 4C).
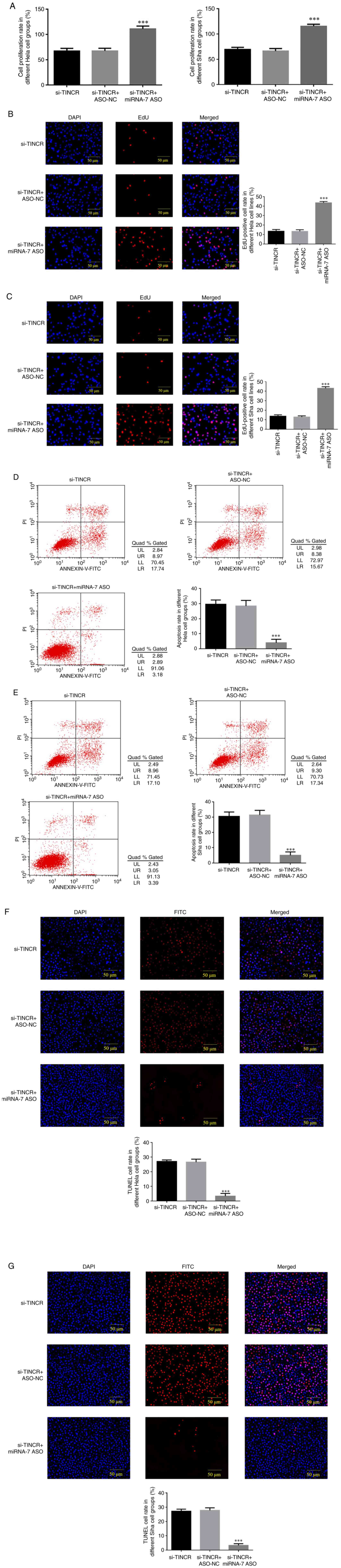
Role of miRNA-7 in the inhibition of
cervical cancer cell proliferation following TINCR knockdown
MTT assay revealed that the cell proliferation rate
in the si-TINCR + miRNA-7 ASO group was significantly increased
(P<0.001 for both; Fig. 5A)
compared with that in the si-TINCR group. EdU staining demonstrated
that after si-TINCR and miRNA-7 ASO were co-transfected into HeLa
and SiHa cells, the EdU-positive HeLa and SiHa cell number was
significantly increased in the si-TINCR + miRNA-7 ASO group
(P<0.001 for both; Fig. 5B and
C).
Role of miRNA-7 in the promotion of
cervical cancer cell apoptosis following TINCR knockdown
Flow cytometry revealed that after si-TINCR and
miRNA-7 ASO were co-transfected into HeLa and SiHa cells, the
apoptotic rate of the si-TINCR + miRNA-7 ASO group was
significantly lower than that of the si-TINCR group (P<0.001 for
both; Fig. 5D and E). TUNEL assay revealed that after
si-TINCR and miRNA-7 ASO were co-transfected into HeLa and SiHa
cells, the positive apoptotic cell rate of the si-TINCR + miRNA-7
ASO group was significantly decreased (P<0.001 for both;
Fig. 5F and G).
Role of miRNA-7 in the inhibition of
cervical cancer cell invasion and migration following TINCR
knockdown
Transwell assay confirmed that after si-TINCR and
miRNA-7 ASO were co-transfected into HeLa and SiHa cells, the
number of invasive cells in the si-TINCR + si-miRNA group
significantly increased compared with that in the si-TINCR group
(P<0.001 for both; Fig. 6A and
B). Wound healing assay revealed
that after si-TINCR and miRNA-7 ASO were co-transfected into HeLa
and SiHa cells, the wound healing rate of the si-TINCR + si-miRNA
group increased significantly at 24 and 48 h (P<0.001 for both;
Fig. 6C and D).
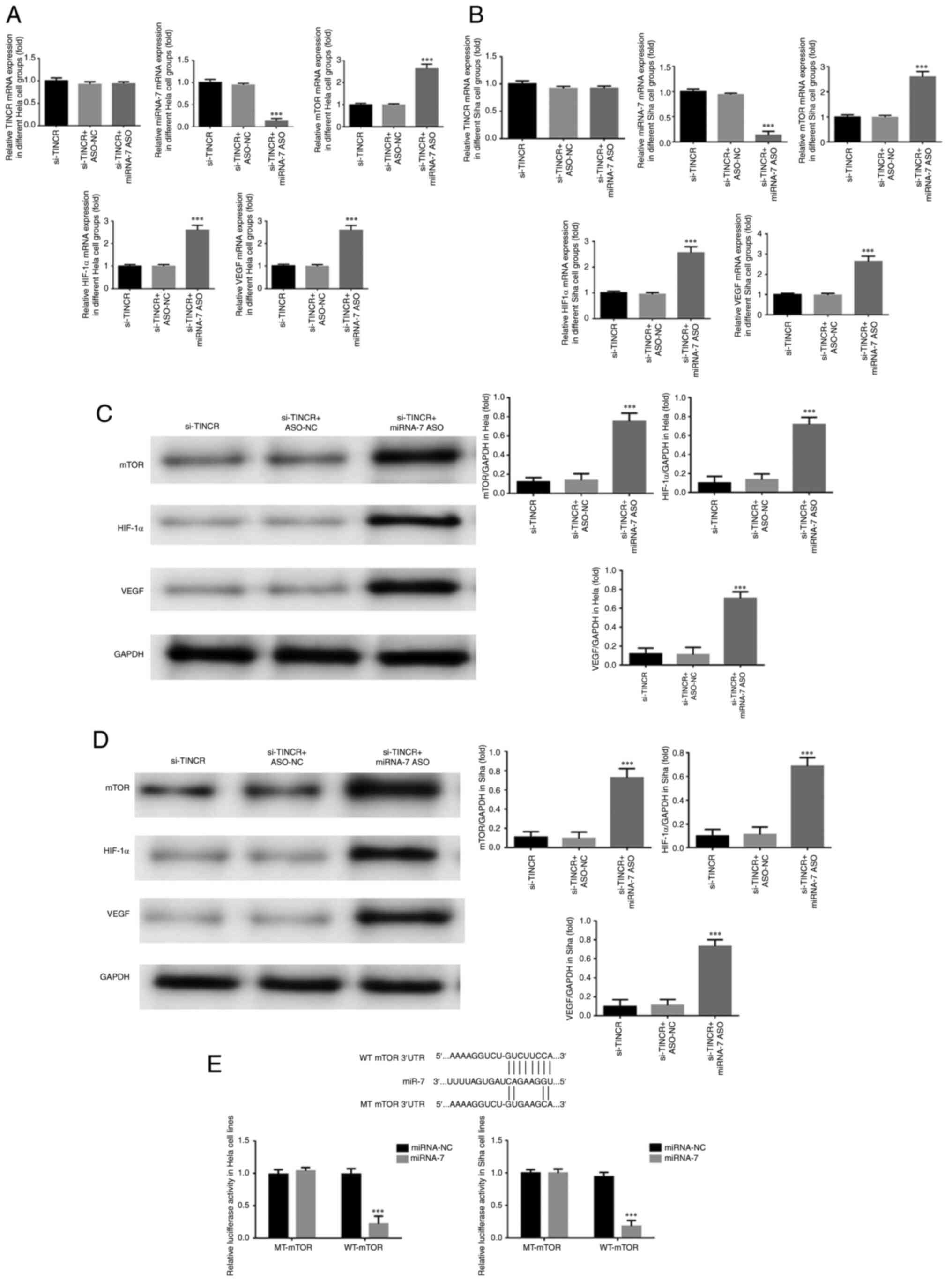
Expression of related genes
RT-qPCR demonstrated that after si-TINCR and miRNA-7
ASO were co-transfected into HeLa and SiHa cells, the level of
miRNA-7 in the si-TINCR + miRNA-7 ASO group was significantly lower
than that in the si-TINCR group, while the mRNA levels of mTOR,
HIF-1α and VEGF increased significantly (P<0.001 for all;
Fig. 7A and B). No significant differences were found
in the mRNA level of TINCR among the three groups (P>0.05;
Fig. 7A and B).
Expression of related proteins
Western blot analysis revealed that after si-TINCR
and miRNA-7 ASO were co-transfected into HeLa and Siha cells, the
protein expression of mTOR, HIF-1α and VEGF increased significantly
in the si-TINCR + miRNA-7 ASO group (P<0.001 for all; Fig. 7C and D).
Targeted regulation of mTOR by
miRNA-7
Bioinformatics analysis revealed that miRNA-7 could
target and regulate mTOR (Fig.
7E). Dual-luciferase reporter assay confirmed that in the WT
mTOR group of HeLa and SiHa cells, the fluorescence intensity of
the miRNA-7 group decreased significantly following the
transfection of miRNA-7 into HeLa and SiHa cells (P<0.001 for
both; Fig. 7E). In the MT mTOR
group, there was no significant difference between the miRNA-NC and
miRNA-7 groups (P>0.05).
Discussion
In the human genome, only 2% of genes can encode
proteins, and non-coding RNAs account for ~98%. lncRNAs are a class
of relatively long non-coding RNAs, which can regulate cell
proliferation, migration, invasion and apoptosis through a variety
of mechanisms, and are closely related to cancer occurrence and
development (20). The majority of
lncRNAs have highly conserved secondary and tertiary structures,
which indicate that they can play a role in a variety of biological
processes (21). lncRNAs in
cervical cancer have been widely reported. TINCR has been shown to
promote cell proliferation, and is involved in tumorigenesis,
although it has different effects in different tumors (5,7). The
expression of TINCR is upregulated in lung cancer and it is related
to the clinicopathological characteristics of patients; the
suppression of the TINCR level can inhibit the malignant metastasis
potential of tumor cells (6).
However, TINCR functions as a tumor suppressor in prostate cancer
and can inhibit tumor metastasis (5). TINCR is highly expressed in liver
cancer, and TINCR may be a promoter of liver cancer progression
(7). The current study showed that
the level of lncRNA TINCR was significantly higher in cervical
cancer tissues, suggesting that lncRNA TINCR may be involved in the
occurrence and development of cervical cancer. The previous studies
found lncRNA TINCR as a suppressor factor in prostate cancer
(5) and squamous cell carcinoma
(22) and as an oncogenic factor
in colorectal cancer (23).
Meanwhile, the present research also found lncRNA TINCR was
significantly upregulated in cervical cancer tissues and closely
correlated with poor prognosis in cervical cancer like colorectal
cancer (23); however, the
mechanism was different, based on the present results, with lncRNA
TINCR knockdown, miRNA-7 increasing and mTOR, HIF-11α and VEGF
expression decreasing. The expression of lncRNA TINCR in cervical
cancer tissues was associated with lymph node metastasis,
myometrial invasion depth and FIGO stage, indicating that the
expression of lncRNA TINCR may affect the progression of cervical
cancer. In addition, the survival curves of patients with high and
low lncRNA TINCR expression at 1-60 months following surgery were
drawn. The 5-year survival rate of patients with a high lncRNA
TINCR expression was significantly lower than that of those with a
low lncRNA TINCR expression, suggesting that a high lncRNA TINCR
expression has a detrimental effect on the prognosis of patients
with cervical cancer. Moreover, Cox regression analysis of lncRNA
TINCR expression, lymph node metastasis, myometrial invasion depth
and FIGO stage revealed that lncRNA TINCR expression, lymph node
metastasis, myometrial invasion depth and FIGO stage were all risk
factors affecting the prognosis of patients with cervical cancer,
among which lncRNA TINCR expression was an independent risk factor
affecting the prognosis of patients with cervical cancer; this
suggests that the inhibition of lncRNA TINCR expression may improve
the prognosis of patients with cervical cancer. To further explore
the mechanisms of lncRNA TINCR in cervical cancer, lncRNA TINCR was
knocked down using in vitro experiments, revealing that the
biological activities (proliferation, invasion and migration) of
cervical cancer cell lines Siha and Hela were significantly
inhibited. The aforementioned results suggested that TINCR may play
a promoting role in the progression of cervical cancer, and its
knockdown can effectively inhibit the occurrence and progression of
colon cancer.
Research on the mechanisms of lncRNAs revealed that
lncRNAs could play different biological roles by targeting and
regulating the expression of downstream genes, and the targeting
and regulatory role of lncRNAs in different tissues and
pathological processes may differ (24). In the present study, it was found
that TINCR targeted and regulated the expression of miRNA-7 in
cervical cancer cell lines. miRNA-7 is a downregulated miRNA
molecule in cancer tissues, and its overexpression can inhibit the
biological activities of cancer cell lines (16). Additionally, it was observed that
TINCR targeted and regulated the expression of miRNA-7 in cervical
cancer cell lines, and the silencing of miRNA-7 reversed the
inhibitory effects of TINCR knockdown on the invasion and migration
of cervical cancer cells; this indicates that the inhibitory
effects of TINCR knockdown on the invasion and migration of
cervical cancer cells are related to the targeted regulation of
miRNA-7.
Through bioinformatics analysis, it was found that
miRNA-7 could target and regulate mTOR, playing a role in
inhibiting the biological activities of cervical cancer cells. mTOR
is an atypical serine/threonine kinase composed of 2,549 amino
acids, which is a member of the phosphatidylinositol
3-kinase-related protein kinase family (25). The mTOR signaling pathway regulates
cell membrane transport, protein degradation, nutritional
metabolism and tumor occurrence through phosphorylation, and plays
a critical regulatory role in physiological processes, including
cell growth, proliferation, differentiation and apoptosis (26), it is also widely involved in
biological processes, such as gene transcription, protein
translation and ribosome biogenesis (27). HIF-1α is a DNA-binding protein,
which is widely involved in the specific response induced by
hypoxia in mammalian cells, and plays a key role in the regulation
of gene expression induced by hypoxia (28). Ryan et al (29) first proved in 1998 that HIF-1α
deletion can inhibit tumor growth and reduce the ability of tumor
cell invasion and metastasis. The increased expression of HIF-1α
can lead to the upregulation of numerous target genes, such as
HVEGF, erythropoietin, glucose-encoding transporter, glycolytic
enzyme, etc. Its activity plays a key role in maintaining the
energy metabolism of tumor cells, angiogenesis, and promoting tumor
proliferation and metastasis (30). EGF is a heparin-binding growth
factor specific to vascular endothelial cells, with a classical
signal sequence (31). VEGF can
cause a series of signal transduction mechanisms by binding with
receptors, promoting the release of various growth factors and
cytokines, and promoting the proliferation and migration of
endothelial cells, thus inducing angiogenesis in vivo
(32). Therefore, the expression
and secretion level of VEGF is one of the key indicators for the
evaluation of angiogenesis. Tumor cells can overexpress VEGF,
thereby accelerating angiogenesis and promoting tumor invasion and
metastasis (33). The expression
of EGF is regulated by the transcription factor, HIF-1α, and HIF-1α
activity is regulated by mTOR; thus, mTOR is the key signaling
pathway regulating VEGF expression (34). HIF-1α and VEGF are considered
downstream targets of mTOR. A previous study (35) suggested that the mTOR signaling
pathway can be inhibited by directly inhibiting the growth of
vascular endothelial cells stimulated by VEGF. In the present
study, it was found that following the knockdown of lncRNA TINCR,
with the increase in miRNA-7 expression, the mTOR/HIF-1α/VEGF
signaling pathway was significantly inhibited, indicating that the
cancer-promoting effect of lncRNA TINCR in cervical cancer may be
related to the activation of the mTOR/HIF-1α/VEGF signaling pathway
caused by the decreased expression of miRNA-7.
In conclusion, the present study demonstrates that
the abnormally high expression of lncRNA TINCR plays a critical
role in the occurrence and development of cervical cancer. The
mechanism of lncRNA TINCR in promoting carcinogenesis may be
closely related to the miRNA-7/mTOR axis and the mTOR/IF-1α/VEGF
signaling pathway.
Supplementary Material
Long non-coding RNA tissue
differentiation-inducing non-protein coding RNA expression in
different cell lines. **P<0.01 and
***P<0.001 vs. HcerEpic cell line.
TINCR and miRNA-7 mRNA level in
different cell groups. (A) LncRNA TINCR and (B) miRNA-7 levels in
different groups. ***P<0.001 vs. si-NC or ASO-NC
group. MiRNA, microRNA; ASO, miRNA-7 inhibitor; NC, negative
control; si-, small interfering RNA; TINCR, tissue
differentiation-inducing non-protein coding RNA.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TZ and XL designed the study and performed the
experiments. XL revised the manuscript for important intellectual
content. XL, CW and QF collected and analyzed the data. XW and QF
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Women and Children's Hospital, Qingdao University
(approval no. 2015081305). The patient or immediate family member
signed an informed consent form.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Small W Jr, Bacon MA, Bajaj A, Chuang LT,
Fisher BJ, Harkenrider MM, Jhingran A, Kitchener HC, Mileshkin LR,
Viswanathan AN and Gaffney DK: Cervical cancer: A global health
crisis. Cancer. 123:2404–2412. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wardak S: Human Papillomavirus (HPV) and
cervical cancer. Med Dosw Mikrobiol. 68:73–84. 2016.PubMed/NCBI
|
|
3
|
Shah CA, Beck T, Liao JB, Giannakopoulos
NV, Veljovich D and Paley P: Surgical and oncologic outcomes after
robotic radical hysterectomy as compared to open radical
hysterectomy in the treatment of early cervical cancer. J Gynecol
Oncol. 28(e82)2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Schmitz SU, Grote P and Herrmann BG:
Mechanisms of long non-coding RNA function in development and
disease. Cell Mol Life Sci. 73:2491–2509. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Dong L, Ding H, Li Y, Xue D and Liu Y:
LncRNA TINCR is associated with clinical progression and serves as
tumor suppressive role in prostate cancer. Cancer Manag Res.
10:2799–2807. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhu ZJ and He JK: TINCR facilitates
non-small cell lung cancer progression through BRAF-activated MAPK
pathway. Biochem Biophys Res Commun. 497:971–977. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tian F, Xu J, Xue F, Guan E and Xu X:
TINCR expression is associated with unfavorable prognosis in
patients with hepatocellular carcinoma. Biosci Rep.
37(BSR20170301)2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Xu J, Zeng W, Liu T, Wan Z, Yang X, Chen J
and Liu F: lncRNA TINCR knockdown inhibits colon cancer cells via
regulation of autophagy. Food Sci Nutr. 11:1965–1981.
2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Luo H, Xu C, Le W, Ge B and Wang T: lncRNA
CASC11 promotes cancer cell proliferation in bladder cancer through
miRNA-150. J Cell Biochem. 120:13487–13493. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Luan X and Wang Y: LncRNA XLOC_006390
facilitates cervical cancer tumorigenesis and metastasis as a ceRNA
against miR-331-3p and miR-338-3p. J Gynecol Oncol.
29(e95)2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhao W, Geng D, Li S, Chen Z and Sun M:
LncRNA HOTAIR influences cell growth, migration, invasion, and
apoptosis via the miR-20a-5p/HMGA2 axis in breast cancer. Cancer
Med. 7:842–855. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Peng CL, Zhao XJ, Wei CC and Wu JW: LncRNA
HOTAIR promotes colon cancer development by down-regulating
miRNA-34a. Eur Rev Med Pharmacol Sci. 23:5752–5761. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Thiel J, Alter C, Luppus S, Eckstein A,
Tan S, Führer D, Pastille E, Westendorf AM, Buer J and Hansen W:
MicroRNA-183 and microRNA-96 are associated with autoimmune
responses by regulating T cell activation. J Autoimmun. 96:94–103.
2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kara M, Yumrutas O, Ozcan O, Celik OI,
Bozgeyik E, Bozgeyik I and Tasdemir S: Differential expressions of
cancer-associated genes and their regulatory miRNAs in colorectal
carcinoma. Gene. 567:81–86. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yang Y, Li XJ, Li P and Guo XT:
MicroRNA-145 regulates the proliferation, migration and invasion of
human primary colon adenocarcinoma cells by targeting MAPK1. Int J
Mol Med. 42:3171–3180. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zeng CY, Zhan YS, Huang J and Chen YX:
MicroRNA-7 suppresses human colon cancer invasion and proliferation
by targeting the expression of focal adhesion kinase. Mol Med Rep.
13:1297–1303. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Xu N, Lian YJ, Dai X and Wang YJ: MiR-7
increases cisplatin sensitivity of gastric cancer cells through
suppressing mTOR. Technol Cancer Res Treat. 16:1022–1030.
2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chen WS, Yen CJ, Chen YJ, Chen JY, Wang
LY, Chiu SJ, Shih WL, Ho CY, Wei TT, Pan HL, et al: MiRNA-7/21/107
contribute to HBx-induced hepatocellular carcinoma progression
through suppression of maspin. Oncotarget. 6:25962–25974.
2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Tang Y, Cheung BB, Atmadibrata B, Marshall
GM, Dinger ME, Liu PY and Liu T: The regulatory role of long
non-coding RNAs in cancer. Cancer Lett. 391:12–19. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
McCabe EM and Rasmussen TP: lncRNA
involvement in cancer stem cell function and epithelial-mesenchymal
transitions. Semin Cancer Biol. 75:38–48. 2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Morgado-Palacin L, Brown JA, Martinez TF,
Garcia-Pedrero JM, Forouhar F, Quinn SA, Reglero C, Vaughan J,
Heydary YH, Donaldson C, et al: The TINCR ubiquitin-like
microprotein is a tumor suppressor in squamous cell carcinoma. Nat
Commun. 14(1328)2023.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhang X, Yao J, Shi H, Gao B and Zhang L:
LncRNA TINCR/microRNA-107/CD36 regulates cell proliferation and
apoptosis in colorectal cancer via PPAR signaling pathway based on
bioinformatics analysis. Biol Chem. 400:663–675. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhu X, Li H, Wu Y, Zhou J, Yang G and Wang
W: LncRNA MEG3 promotes hepatic insulin resistance by serving as a
competing endogenous RNA of miR-214 to regulate ATF4. Int J Mol
Med. 43:345–357. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sehgal SN: Sirolimus: Its discovery,
biological properties, and mechanism of action. Transplant Proc. 35
(3 Suppl):7S–14S. 2003.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wiederrecht GJ, Sabers CJ, Brunn GJ,
Martin MM, Dumont FJ and Abraham RT: Mechanism of action of
rapamycin: New insights into the regulation of G1-phase progression
in eukaryotic cells. Prog Cell Cycle Res. 1:53–71. 1995.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Edinger AL and Thompson CB: Akt maintains
cell size and survival by increasing mTOR-dependent nutrient
uptake. Mol Biol Cell. 13:2276–2288. 2002.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Semenza GL: HIF-1 mediates metabolic
responses to intratumoral hypoxia and oncogenic mutations. J Clin
Invest. 123:3664–3671. 2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ryan HE, Lo J and Johnson RS: HIF-1 alpha
is required for solid tumor formation and embryonic
vascularization. EMBO J. 17:3005–3015. 1998.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Li L, Qu Y, Li J, Xiong Y, Mao M and Mu D:
Relationship between HIF-1alpha expression and neuronal apoptosis
in neonatal rats with hypoxia-ischemia brain injury. Brain Res.
1180:133–139. 2007.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chen Y, Peng GF, Han XZ, Wang W, Zhang GQ
and Li X: Apoptosis prediction via inhibition of AKT signaling
pathway by neogrifolin. Int J Clin Exp Pathol. 8:1154–1164.
2015.PubMed/NCBI
|
|
32
|
Kigure W, Fujii T, Sutoh T, Morita H,
Katoh T, Yajima RN, Yamaguchi S, Tsutsumi S, Asao T and Kuwano H:
The association of VEGF-C expression with tumor lymphatic vessel
density and lymph node metastasis in patients with gastric cancer
and gastrointestinal stromal tumor. Hepatogastroenterology.
60:277–280. 2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lugano R, Ramachandran M and Dimberg A:
Tumor angiogenesis: Causes, consequences, challenges and
opportunities. Cell Mol Life Sci. 77:1745–1770. 2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Li W, Petrimpol M, Molle KD, Hall MN,
Battegay EJ and Humar R: Hypoxia-induced endothelial proliferation
requires both mTORC1 and mTORC2. Circ Res. 100:79–87.
2007.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Tsai SY, Yang LY, Wu CH, Chang SF, Hsu CY,
Wei CP, Leu SJ, Liaw J, Lee YH and Tsai MD: Injury-induced Janus
kinase/protein kinase C-dependent phosphorylation of
growth-associated protein 43 and signal transducer and activator of
transcription 3 for neurite growth in dorsal root ganglion. J
Neurosci Res. 85:321–331. 2007.PubMed/NCBI View Article : Google Scholar
|