Introduction
The skin is the largest multi-functional organ in
the human body, with barrier properties that are essential for
maintaining body homeostasis. The skin is typically structured into
the following three layers: Epidermis, dermis and the subcutaneous
fat tissue (1,2). The stratum corneum makes up the
outermost layer of the epidermis of the skin that serves two major
functions as a physical barrier (1,2). It
serves a water-retention function by preventing the evaporation of
water, which would otherwise result in dry skin. It also serves a
barrier function, preventing the invasion of pathogens whilst
fending off various chemical assaults (1,2). The
stratum corneum consists of ceramides, cholesterol, and free fatty
acids. These lipids are normally synthesized in keratinocytes
before being extruded into the extracellular domains, forming
lipid-rich extracellular layers (3). In particular, natural moisturizing
factors (NMF) are a major contributor to the moisturizing function
of the stratum corneum. NMF include free amino acids and their
derivatives, organic acids, and mineral salts (4). Furthermore, the amino acids that
account for the majority of NMF are derived from the hydrolysis of
a protein called filaggrin (5). In
addition to the stratum corneum, tight junction structures present
in the stratum granulosum also serve an important role as a barrier
in the skin (6,7). Claudin and occludin serve a central
role in the intercellular barrier at tight junctions and are
essential proteins for the maintenance of homeostasis in living
organisms (6,7). Dysfunction of these tight junctions
has been reported to be associated with the development of skin
disorders, such as atopic dermatitis (8). By contrast, the dermis is a tissue
that is rich in extracellular matrix (ECM), which consists of
collagen and elastic fibers produced by fibroblasts. In addition,
hyaluronic acid (HA), produced by fibroblasts, exists to fill the
area around these fibers and holds the water in the dermis
(9). During the aging process, the
dermis undergoes significant changes. Skin aging is accompanied
with reduced elasticity and wrinkle formation, which is the result
of reductions in the quantity of ECM in the dermis (10).
Taurine is a sulfur-containing amino acid derivative
that is present in the majority of mammalian tissues (11). Taurine is distributed throughout
the body of living organisms, including humans, which serves roles
in maintaining cell homeostasis through osmoregulatory,
antioxidant, anti-inflammatory, protein-stabilizing and
calcium-regulating actions (11).
In the skin, taurine is mainly localized in the epidermis and is
involved in modulating the skin moisture content (12,13).
The distribution of taurine and taurine transporters in the skin
was previously found to be in proximity with the location of tight
junctions in the epidermis, suggesting that taurine may have a part
in regulating tight junction function (14). A previous study has also reported
that oral taurine supplementation can ameliorate ultraviolet (UV)
beam-induced wrinkle formation in hairless mice (15). Since taurine is known to be an
organic osmolyte in the body, modulation of osmotic pressure and
maintenance of cell volume are possible mechanisms responsible for
the anti-wrinkle action of taurine (15). Considering the diverse array of
functions taurine can serve, other mechanisms may be involved in
the suppression of wrinkle formation. Taurine has been reported to
stimulate wound healing by increasing skin collagen synthesis in
mice (16), in addition to
stimulating collagen synthesis in osteoblast-like UMR-106 cells
(17). These aforementioned
observations suggest that taurine is able to exert functions on the
ECM, including collagen, in the skin. Despite the various reported
beneficial actions of taurine on skin function, the underlying
mechanisms remained elusive.
Therefore, the present study was undertaken to
determine the mechanisms by which taurine regulates skin function
using three-dimensionally (3D) cultured human epidermis and human
dermal fibroblasts.
Materials and methods
3D epidermis culture
3D epidermis culture specimens and assay medium
(401124E6; LabCyte EPI-MODEL24 6D) were purchased from Japan Tissue
Engineering Co., Ltd. The 3D epidermis cultures were maintained at
37˚C with 5% CO2 in a defined assay medium (EPI-MODEL)
or assay medium containing 3 or 30 mM taurine (Fujifilm Wako Pure
Chemical Corporation) (18). The
medium was changed daily for 7 days. After 6 days of incubation at
37˚C with 5% CO2, 100 µl 100% acetone was added to the
3D epidermal culture from the stratum corneum side. After 5 min,
the acetone was removed by suction with a pipette at room
temperature. The acetone addition and removal procedure was
repeated twice. After 7 days, the transepidermal water loss (TEWL)
of the 3D epidermis culture was measured. Thereafter, only the
epidermis was collected from the 3D culture cups using RNAlater™
Stabilization Solution (Invitrogen; Thermo Fisher Scientific, Inc.)
and the samples were stored at 4˚C until further use.
Measurement of TEWL
On the 7th day of culture, the 3D epidermis culture
specimens were transferred to a new 24-well plate and allowed to
stand at room temperature for 20 min with the plate lid open under
aseptic conditions. TEWL was measured by placing a Tewameter
(TM300; Courage + Khazaka Electronic GmbH) directly on top of the
cup of the 3D epidermis culture.
Normal human dermal fibroblast (NHDFs)
culture
NHDFs were purchased from Kurabo Bio-Medical
Department (KF-4009; Kurabo Industries, Ltd.) and maintained at
37˚C with 5% CO2 in a defined FibroLife S2 Comp Kit
medium (Kurabo Industries, Ltd.; Lifeline Cell Technology). These
cells were used for experiments examining collagen and MMP-1. For
the experiments examining HA, NHDFs purchased from RIKEN
BioResource Center (NB1RGB, cell no. RCB0222) were used and
maintained at 37˚C with 5% CO2 in DMEM (Sigma-Aldrich;
Merck KGaA) supplemented with 10% FCS (Sigma-Aldrich; Merck KGaA),
100 U/ml penicillin, and 100 µg/ml streptomycin (Sigma-Aldrich;
Merck KGaA). NHDFs with two to four cell passages were used for the
experiments. The NHDFs were then incubated with 3-50 mM taurine and
IL-1α (PeproTech, Inc.) for 24 or 48 h at 37˚C. NHDFs cultured in
medium without IL-1α and taurine were used as the control. Cells
and their culture supernatant were then collected. The total RNA of
the cells was extracted for reverse transcription-quantitative PCR
(RT-qPCR), whereas the culture supernatant was collected for
ELISA.
RT-qPCR
Total RNA was extracted from the 3D epidermis
culture or NHDF cells using QI-Azol lysis reagent and an RNeasy
Mini kit (both Qiagen GmbH). For the 3D epidermis culture, a tissue
lysis solution was prepared using a high-speed cell disruption
system (Precellys® 24; Bertin Technologies) to extract
RNA from cells. Complementary DNA was synthesized from the RNA by
reverse transcription (37˚C for 15 min and 85˚C for 5 sec) using
the PrimeScript® RT Master Mix (Takara Bio, Inc.) and
subsequent qPCR was performed using the Fast SYBR® Green
Master Mix (Invitrogen; Thermo Fisher Scientific, Inc.) in a
StepOnePlus® Real-Time PCR system (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The following amplification
profile was used: 20 sec at 95˚C, followed by 40 cycles of 3 sec at
95˚C and 30 sec at 60˚C. The primer sequences are indicated in
Table I. Amplification was
normalized to the housekeeping gene GAPDH. The gene expression
level was quantified using the standard curve method (19).
 | Table ISequences of the primers used for
reverse transcription-quantitative PCR. |
Table I
Sequences of the primers used for
reverse transcription-quantitative PCR.
| Primers | Sequence
(5'-3') |
|---|
| IL-1α | Forward:
CTCAATTGTATGTGACTGCCCAAGA |
| | Reverse:
AACAAGTTTGGATGGGCAACTGA |
| IL-1β | Forward:
GCTGATGGCCCTAAACAGATGAA |
| | Reverse:
TCCATGGCCACAACAACTGAC |
| IL-1RN | Forward:
CTGTCCTGTGTCAAGTCTGGTG |
| | Reverse:
TCTCGCTCAGGTCAGTGATGTTA |
| FLG | Forward:
CATGGCAGCTATGGTAGTGCAGA |
| | Reverse:
ACCAAACGCACTTGCTTTACAGA |
| SPTLC1 | Forward:
AGACCATCCTGCTCTCAACTACAA |
| | Reverse:
CTAGGGTTATCCAACAATCCAAGAA |
| SMPD1 | Forward:
TCTATGAAGCGATGGCCAAG |
| | Reverse:
GATCCGTGGAGTTGATCAAGAG |
| CERS2 | Forward:
CATCCGAGCTGGGACTCTAATCA |
| | Reverse:
GGGTACACCAGGGTGCAATG |
| CERS4 | Forward:
ATGGCTGTGGGCACCAGTAA |
| | Reverse:
GAGGTAGAAACCCAGCTCCAAGA |
| COL1A1 | Forward:
GCTTGGTCCACTTGCTTGAAGA |
| | Reverse:
GAGCATTGCCTTTGATTGCTG |
| COL3A1 | Forward:
ATGAAGGTGAATTCAAGGCTGAAG |
| | Reverse:
CCACCAATGTCATAGGGTGCAATA |
| COL4A1 | Forward:
CAGCCGCTGCCAAGTCTGTA |
| | Reverse:
AGGTCAATGAAGCAGGGTGTGTTAG |
| COL7A1 | Forward:
AGAAGGGAGAAGCTGCACTGA |
| | Reverse:
GCAGTGTCTGCAGCATAACTAGG |
| MMP-1 | Forward:
CCAAATGGGCTTGAAGCTG |
| | Reverse:
GGTATCCGTGTAGCACATTCTGTC |
| HAS-2 | Forward:
GACAGGCATCTCACGAACCG |
| | Reverse:
CAACGGGTCTGCTGGTTTAGC |
| GAPDH | Forward:
GCACCGTCAAGGCTGAGAAC |
| | Reverse:
TGGTGAAGACGCCAGTGGA |
ELISA
The levels of HA and MMP-1 were measured in the
culture supernatant using ELISA kits for HA (Seikagaku Corporation)
and MMP-1 (cat. no. ab100604; Abcam), respectively, according to
the manufacturer's protocol. The protein concentration in the cell
lysate was measured using a BCA protein assay kit (Thermo Fisher
Scientific, Inc.). Protein levels of MMPs are presented as amounts
per cell protein. Cellular protein concentrations were measured in
cell lysates. MMP-1 was measured in the culture supernatant, which
was applied to the ELISA plate.
Statistical analysis
All data are expressed as the mean ± standard
deviation (n=3-6). The statistical analyses were performed using
the SAS preclinical package software (version 5.0; SAS Institute
Japan Co., Ltd.). Statistical analysis was performed using one-way
ANOVA followed by Dunnett's test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of taurine on TEWL and skin
inflammation in 3D cultured epidermis
The effects of taurine on TEWL in the skin and the
expression of inflammatory cytokines were investigated using 3D
cultured epidermis. The addition of acetone to the cultured
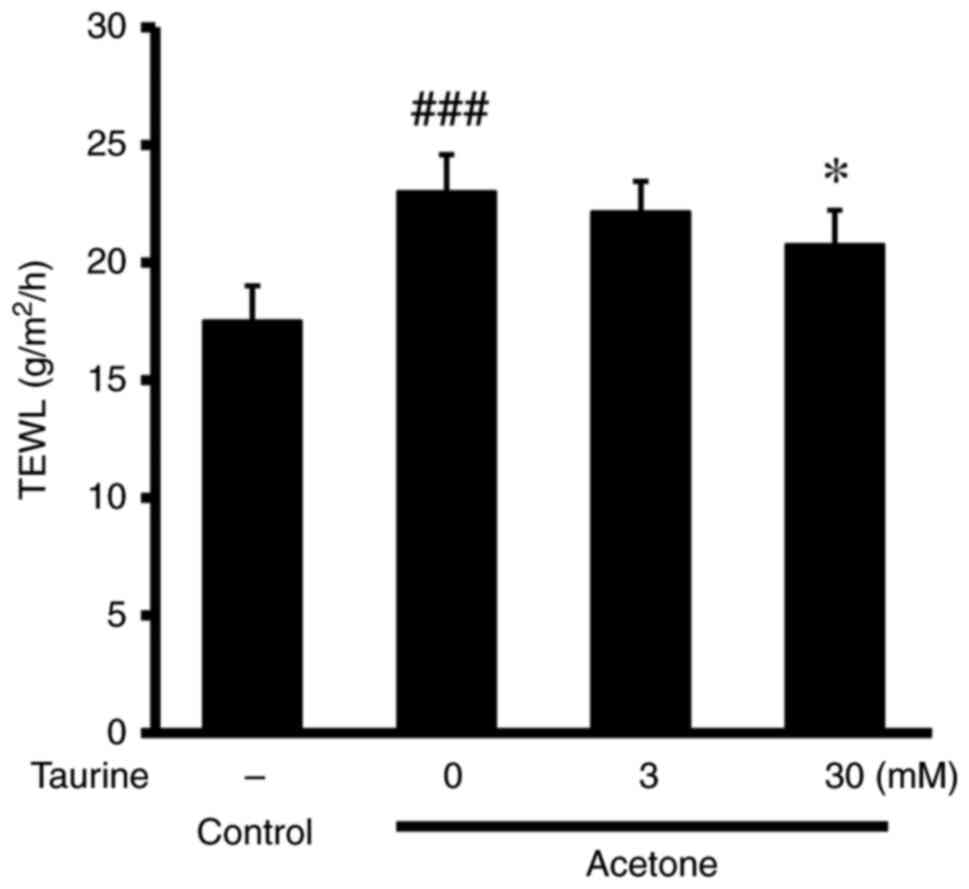
epidermis significantly increased TEWL, whereas this effect was
significantly reversed by treatment with 30 mM taurine (Fig. 1). Acetone also significantly
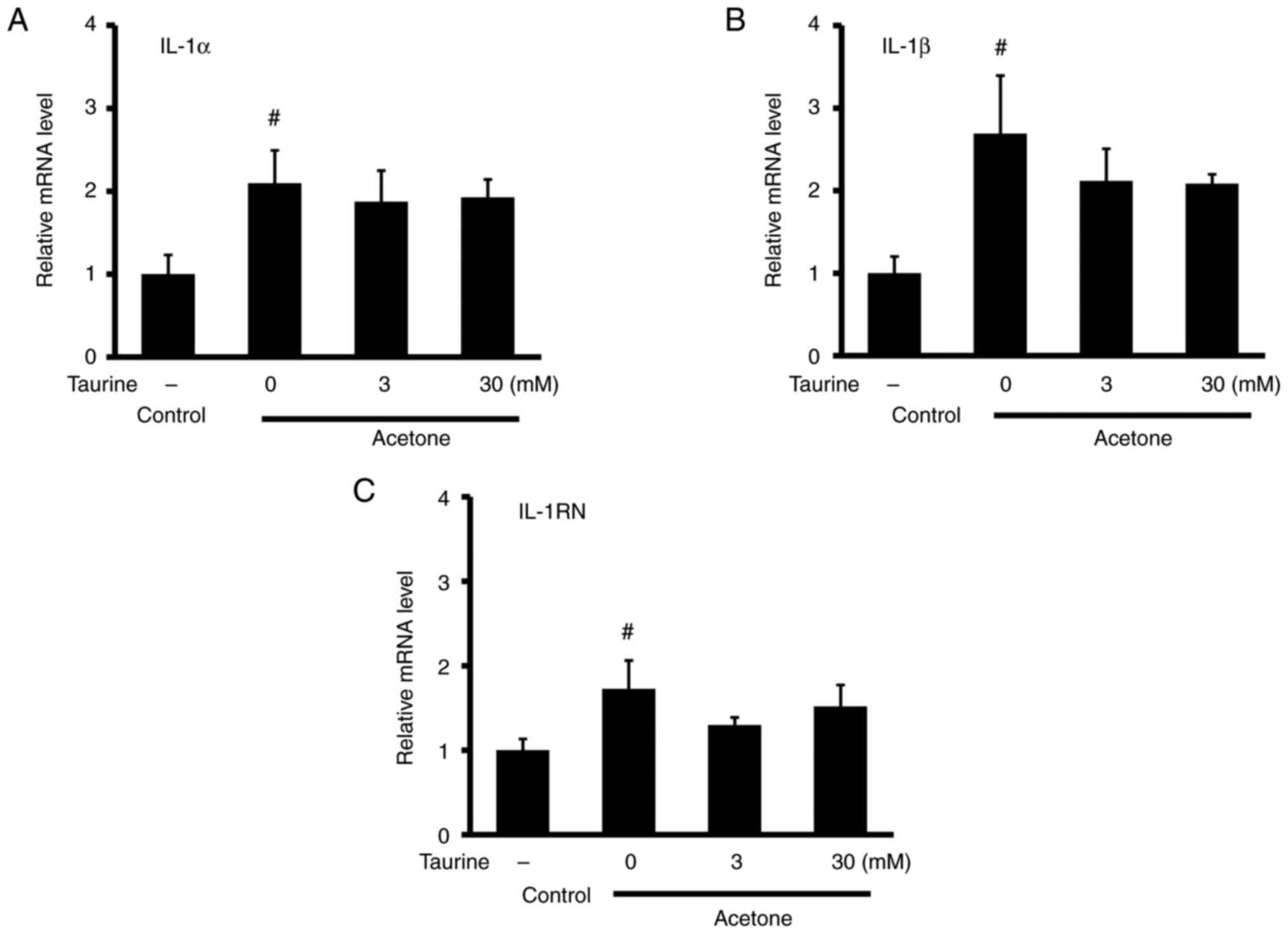
increased the mRNA expression of inflammatory cytokines IL-1α,
IL-1β and IL-1 receptor antagonist (IL-1RN) (Fig. 2). However, taurine did not affect
the mRNA expression of these aforementioned cytokines. The
potential effects of taurine on the mRNA expression of
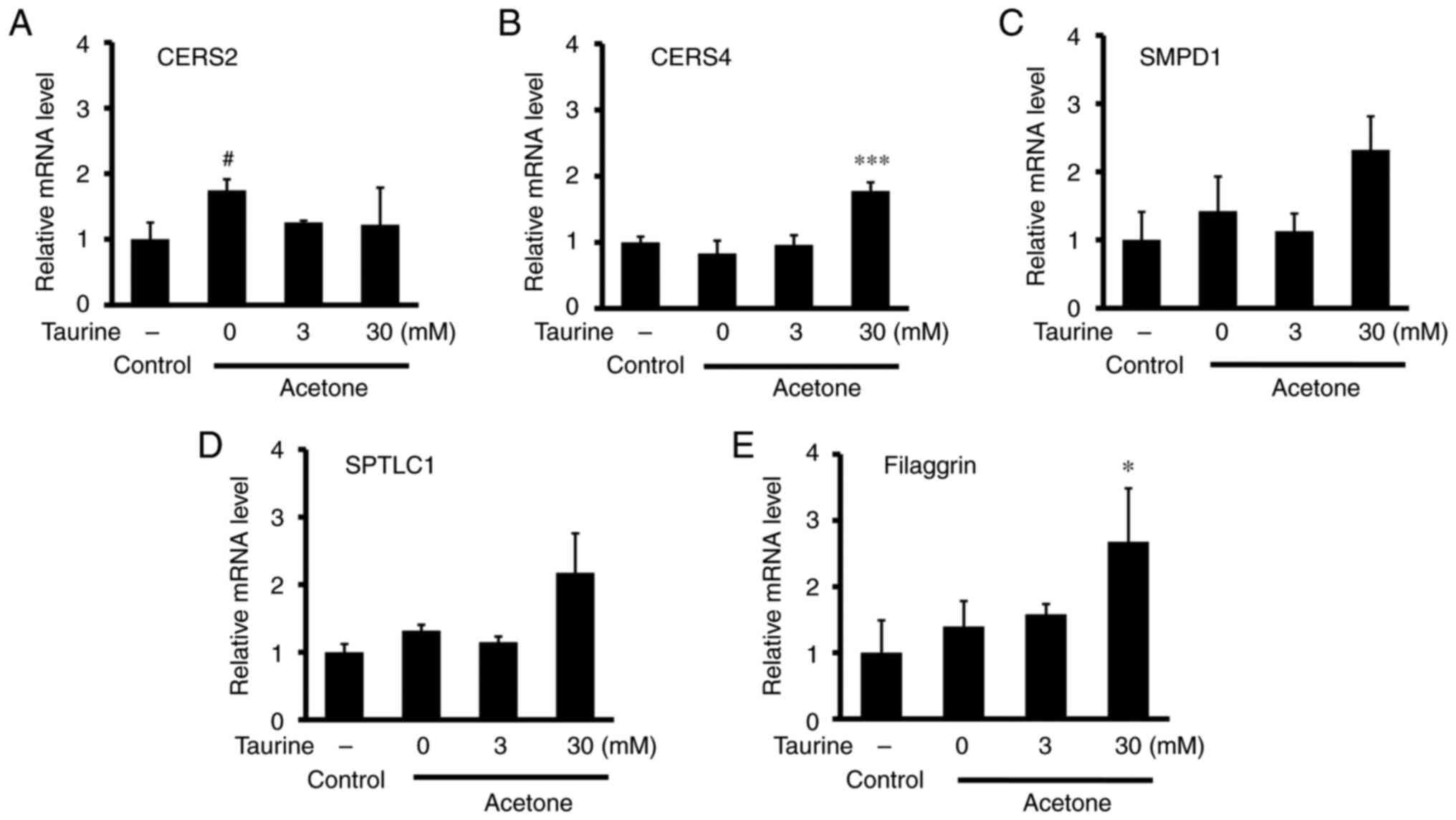
ceramide-related molecules were next examined in 3D-cultured cells.
The mRNA expression of ceramide synthase 2 (CERS2) was found to be
significantly increased by acetone treatment (Fig. 3A). However, the acetone-induced
changes in the mRNA expression of other ceramide-related enzymes
CERS4 (Fig. 3B), sphingomyelin
phosphodiesterase 1 (Fig. 3C) and
serine palmitoyltransferase long chain base subunit 1 (Fig. 3D) were not observed to be
significant. Treatment with 30 mM taurine did significantly
increase the mRNA expression of CERS4 and filaggrin compared with
that in the acetone-only group (Fig.
3B and E).
Effects of taurine on collagen HA in
human dermal fibroblasts
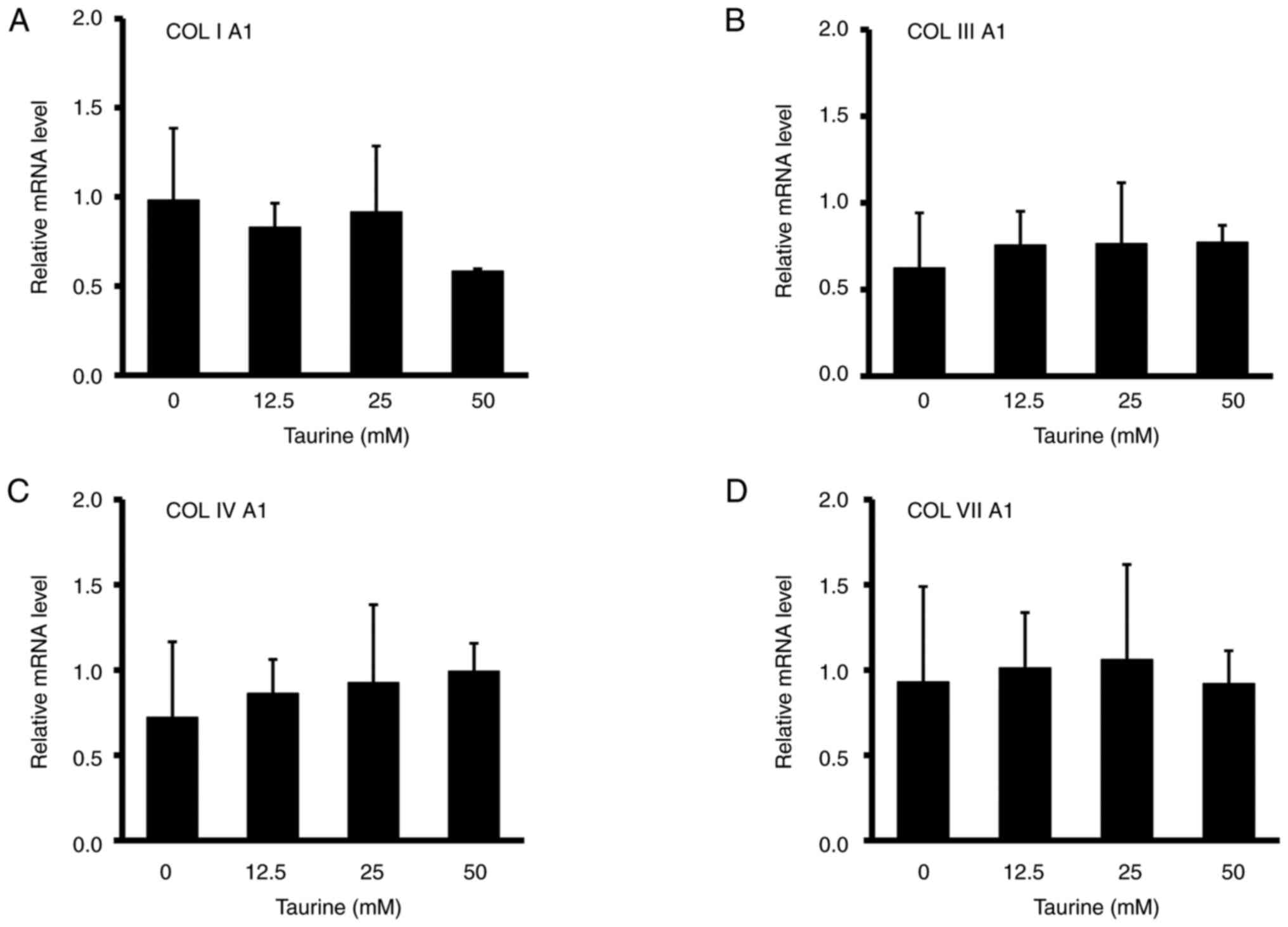
The addition of 12.5-50 mM taurine to the dermal
fibroblasts did not significantly affect the mRNA expression of
collagens collagen (COL)ⅠA1 (Fig.
4A), COLIIIA1 (Fig. 4B),
COLⅣA1 (Fig. 4C) and COLⅦA1
(Fig. 4D). The possible effects of
taurine on the degradation of collagen were then evaluated in
IL-1α-stimulated human dermal fibroblasts by measuring the mRNA and
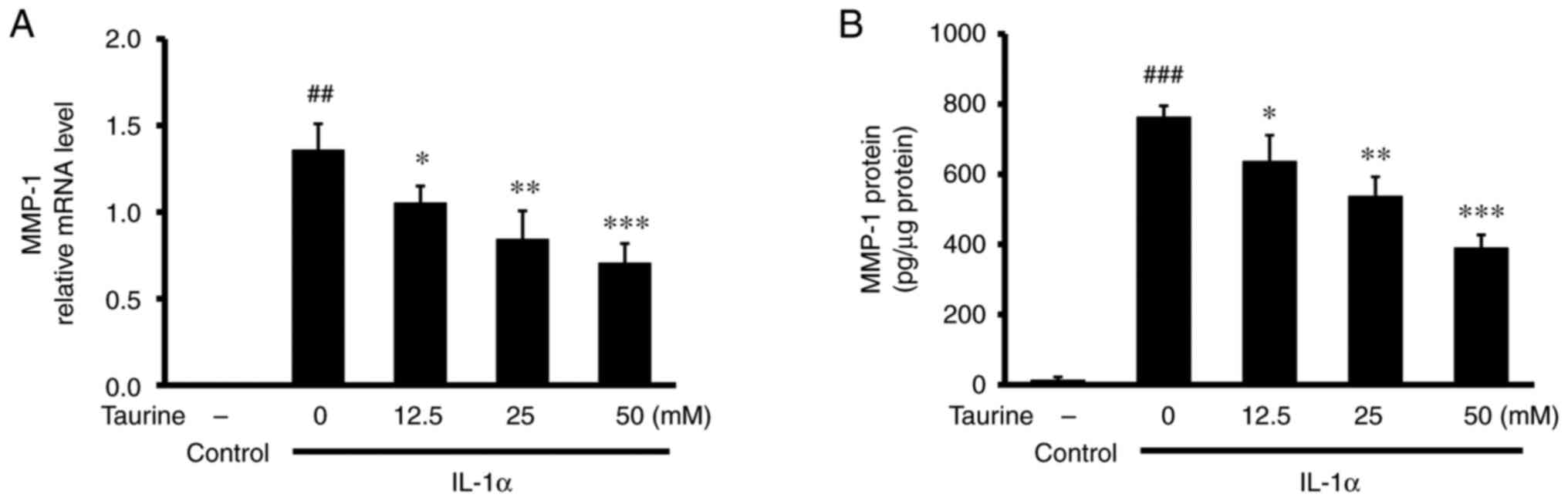
protein expression of MMP-1. Although IL-1α significantly increased
the mRNA expression and protein expression levels of MMP-1, further
taurine treatment significantly counteracted this effect of IL-1α,
in a dose-dependent manner (Fig.
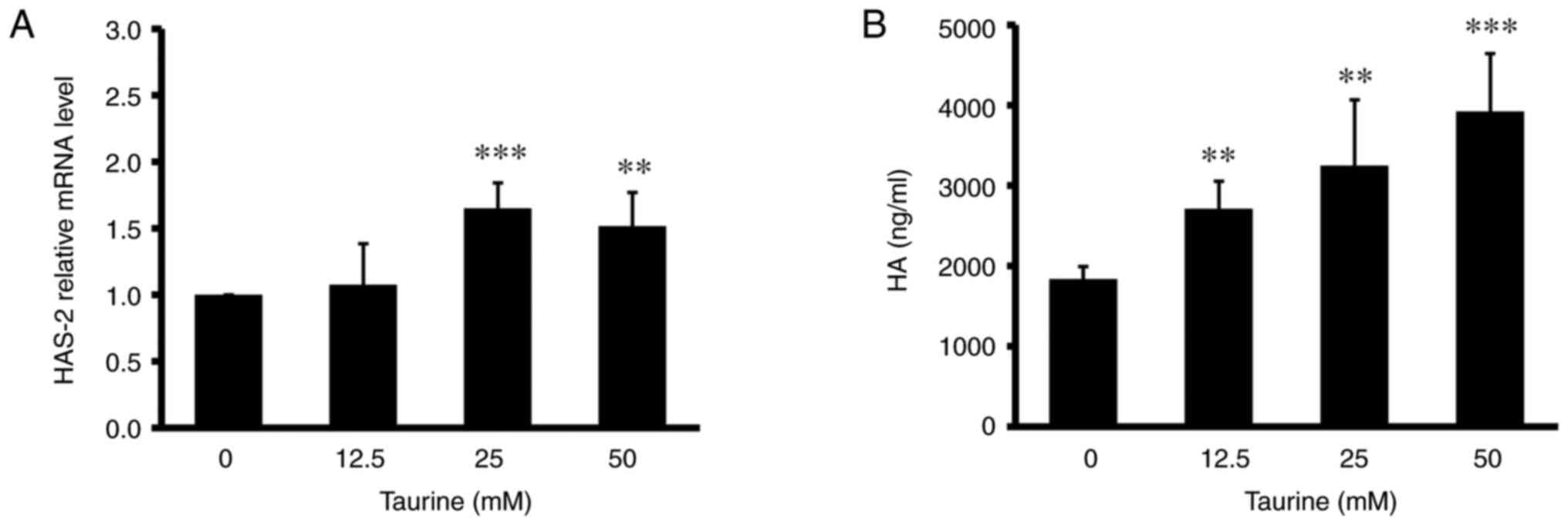
5A and B). In addition, higher
doses of taurine (25 and 50 mM) significantly increased the HA
content in the culture medium and the mRNA expression of hyaluronan
synthase 2 (HAS-2), an HA synthetic enzyme, in the skin dermal
fibroblasts (Fig. 6A and B).
Discussion
The present study revealed that taurine can modulate
components of the epidermal barrier and dermal ECM in vitro.
These effects can potentially contribute to the modulation of the
homeostatic action in the skin exerted by taurine. Taurine is
distributed in the skin and has a pivotal role in maintaining skin
homeostasis (12,13). The effects of taurine
administration on skin function have been evaluated in animals,
humans and cultured cells (12,15,20).
It was indicated that the topical application of taurine attenuated
TEWL in surfactant-treated human skin (20). A previous study also showed that
the oral ingestion of taurine decreased UV beam-induced TEWL and
suppressed the development of wrinkles in the skin of hairless mice
(15). Furthermore, other previous
animal experiments have demonstrated that oral taurine
administration can restore the age-induced or UV-induced decline in
the skin taurine content (15,21).
Therefore, taurine supplementation may be beneficial for
maintaining taurine levels and normal skin function. However, the
mechanisms underlying the protective effects of taurine on the skin
remain to be fully elucidated.
In the present study, TEWL, the mRNA expression
levels of inflammatory cytokines and ceramide synthases were
evaluated using an acetone-induced 3D cultured epidermis model.
Acetone is known to disrupt skin barrier function by altering the
composition of intracellular lipids, thereby increasing TEWL
(22). Consistent with the results
from previous animal and human studies (19,20),
taurine was found to suppress the acetone-induced elevation of TEWL
in the 3D cultured epidermis in the present study. When skin is
irradiated with UV, the inflammatory cytokine IL-1α is produced
(23). IL-1α acts on dermal
fibroblasts to increase the production of MMP-1(24). Although taurine treatment did not
affect the acetone-induced inflammatory response as evidenced by
the lack of changes in the mRNA expression of IL-1α, IL-1β and
IL-1RN, it did increase the mRNA expression of the ceramide
synthetic enzyme CERS4. Ceramides are fatty acids in the skin that
facilitate the maintenance of the skin barrier and to retain
moisture (25). Taurine was also
found to increase the mRNA expression of filaggrin in the
acetone-induced 3D cultured epidermis. Filaggrin is a major
structural protein in the stratum corneum, which provides NMF to
hydrate the skin and preserve barrier functions (4,26).
The effects of taurine on ceramides have been previously reported.
In the reconstructed epidermis, taurine was found to stimulate the
synthesis of barrier lipids, including ceramides, cholesterol and
fatty acids (20). Recently,
taurine was reported to enhance the barrier function of the skin by
stimulating the expression of tight junction proteins, including
claudin 1, claudin 4 and occludin, in cultured human keratinocytes
(27). These findings suggest that
the moisture retention effects of taurine may be partially
associated with the potentiation of barrier structure and function
by stimulating tight junction protein expression and barrier lipid
synthesis. Another recent study revealed that the regulation of
osmotic pressure was important for the protective effects of
taurine on the skin (21), since
taurine was indicated to be a major organic osmolyte in living
organisms (28). The present study
raised the possibility that enhancement of barrier function by
increasing ceramide and filaggrin synthesis may also be important
for keratinocyte hydration induced by taurine.
HA is a major component of the ECM. In the skin,
large quantities of HA reside in the dermal connective tissue,
where they can regulate water balance (9). Reduction in the HA content is a major
factor responsible for wrinkle formation and loss of skin
elasticity (29). HA is
synthesized by HASs, whereby three different HAS isoforms have been
identified to date (30). Since
HAS-2 is a critical isoform responsible for HA synthesis in dermal
fibroblasts (31), the present
study investigated the effects of taurine on the mRNA expression of
HAS-2 in skin dermal fibroblasts. Taurine was observed to stimulate
HAS-2 mRNA expression and increase the secretion of HA into the
medium. These findings suggest that taurine can promote HA
synthesis by upregulating HAS-2 mRNA expression. Considering the
important role of HA in maintaining skin integrity, architecture
and water balance, an increase in HA synthesis may be associated
with the previously reported anti-wrinkle effect of taurine
(15).
The dermis predominantly consists of ECM components,
such as collagen, which is produced by fibroblasts (32). Collagen is the principal component
of the dermis (32). Specifically,
type I and III collagen are found in abundance (32). Fibroblasts form the primary cell
type found within the dermis, where they serve important roles
maintaining the normal structure and function (33,34).
When the structure of the ECM becomes impaired with age or UV
irradiation, collagen production also becomes impaired (35). MMPs are a family of zinc-containing
proteinases that are involved in the degradation of ECM proteins.
MMP gene expression was reported to be upregulated by UV radiation,
aging and inflammatory cytokines (36,37).
In human skin, MMP-1 is the major protease involved in the
fragmentation of native collagens (38). In the present study, addition of
IL-1α to skin dermal fibroblasts increased the mRNA expression of
MMP-1, whilst taurine treatment suppressed the increase in a
dose-dependent manner. Although the possibility that taurine can
affect collagen secretion could not be ruled out, the present
results suggest that taurine can at least exert an effect on
collagen degradation.
Collagen degradation products and collagen fragments
were reported to inhibit the expression of HAS-2 mRNA and HA
synthesis in human skin fibroblasts (39). Collagen fragments are also known to
inhibit collagen synthesis (40).
In the present study, taurine suppressed the degradation of
collagen by inhibiting MMP-1, whilst taurine also increased the
expression of HAS-2 mRNA. These findings suggest that the
stimulation of HAS-2 mRNA expression by taurine is associated with
the inhibition of the MMP-1 expression and the subsequent
suppression of collagen degradation.
In summary, the present study using cultured cells
revealed that taurine stimulated the expression of the skin barrier
components, including CERS4 and filaggrin. Furthermore, taurine
upregulated HA synthesis. These findings suggest that enhancement
of the epidermal barrier function, dermal water retention and
elasticity may contribute to the beneficial effects of taurine on
the skin. Since the present study was conducted using cultured skin
cells, it is necessary to confirm whether these mechanisms of
action are also in place in vivo. In addition, taurine has
an important role in the skin as an organic osmolyte. Therefore,
its relevance to the results of the present study requires
verification.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TY, TS, TN and SM conceived and designed the
experiments. TY, CM, JIN and TS performed the experiments and
analyzed the data. TY and SM wrote the manuscript. TY and SM
confirm the authenticity of all the raw data. All authors have read
and approved the final version of the manuscript.
Ethics approved and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bouwstra JA and Ponec M: The skin barrier
in healthy and diseased state. Biochim Biophys Acta.
1758:2080–2095. 2006.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Proksch E, Brandner JM and Jensen JM: The
skin: an indispensable barrier. Exp Dermatol. 17:1063–1072.
2008.PubMed/NCBI View Article : Google Scholar
|
|
3
|
van Smeden J, Janssens M, Gooris GS and
Bouwstra JA: The important role of stratum corneum lipids for the
cutaneous barrier function. Biochim Biophys Acta. 1841:295–313.
2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Rawlings AV and Harding CR: Moisturization
and skin barrier function. Dermatol Ther. 17 (Suppl 1):S43–S48.
2004.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kezic S and Jakasa I: Filaggrin and skin
barrier function. Curr Probl Dermatol. 49:1–7. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kirschner N and Brandner JM: Barriers and
more: Functions of tight junction proteins in the skin. Ann N Y
Acad Sci. 1257:158–166. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Brandner JM: Importance of tight junctions
in relation to skin barrier function. Curr Probl Dermatol.
49:27–37. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Boguniewicz M and Leung DY: Atopic
dermatitis: A disease of altered skin barrier and immune
dysregulation. Immunol Rev. 242:233–246. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ruiz Martínez MA, Peralta Galisteo S,
Castán H and Morales*Hernández ME: Role of proteoglycans on skin
ageing: A review. Int J Cosmet Sci. 42:529–535. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Baumann L: Skin ageing and its treatment.
J Pathol. 211:241–251. 2007.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Huxtable RJ: Physiological actions of
taurine. Physiol Rev. 72:101–163. 1992.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Janeke G, Siefken W, Carstensen S,
Springmann G, Bleck O, Steinhart H, Höger P, Wittern KP, Wenck H,
Stäb F, et al: Role of taurine accumulation in keratinocyte
hydration. J Invest Dermatol. 121:354–361. 2003.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Warskulat U, Reinen A, Grether-Beck S,
Krutmann J and Häussinger D: The osmolyte strategy of normal human
keratinocytes in maintaining cell homeostasis. J Invest Dermatol.
123:516–521. 2004.PubMed/NCBI View Article : Google Scholar
|
|
14
|
El-Chami C, Haslam IS, Steward MC and
O'Neill CA: Organic osmolytes preserve the function of the
developing tight junction in ultraviolet B-irradiated rat epidermal
keratinocytes. Sci Rep. 26(5167)2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yoshimura T, Manabe C, Inokuchi Y, Mutou
C, Nagahama T and Murakami S: Protective effect of taurine on
UVB-induced skin aging in hairless mice. Biomed Pharmacother.
141(111898)2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Değim Z, Celebi N, Sayan H, Babül A,
Erdoğan D and Take G: An investigation on skin wound healing in
mice with a taurine-chitosan gel formulation. Amino Acids.
22:187–198. 2002.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Park S, Kim H and Kim SJ: Stimulation of
ERK2 by taurine with enhanced alkaline phosphatase activity and
collagen synthesis in osteoblast-like UMR-106 cells. Biochem
Pharmacol. 62:1107–1111. 2001.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kojima H, Ando Y, Idehara K, Katoh M,
Kosaka T, Miyaoka E, Shinoda S, Suzuki T, Yamaguchi Y, Yoshimura I,
et al: Validation study of the in vitro skin irritation test with
the LabCyte EPI-MODEL24. Altern Lab Anim. 40:33–50. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Anderheggen B, Jassoy C, Waldmann-Laue M,
Förster T, Wadle A and Doering T: Taurine improves epidermal
barrier properties stressed by surfactants-a role for osmolytes in
barrier homeostasis. J Cosmet Sci. 57:1–10. 2006.PubMed/NCBI
|
|
21
|
Yoshimura T, Inokuchi Y, Mutou C, Sakurai
T, Nagahama T and Murakami S: Age-related decline in the taurine
content of the skin in rodents. Amino Acids. 53:429–434.
2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Rissmann R, Oudshoorn MH, Hennink WE,
Ponec M and Bouwstra JA: Skin barrier disruption by acetone:
observations in a hairless mouse skin model. Arch Dermatol Res.
301:609–613. 2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Griswold DE, Connor JR, Dalton BJ, Lee JC,
Simon P, Hillegass L, Sieg DJ and Hanna N: Activation of the IL-1
gene in UV-irradiated mouse skin: Association with inflammatory
sequelae and pharmacologic intervention. J Invest Dermatol.
97:1019–1023. 1991.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wang X, Bi Z, Chu W and Wan Y: IL-1
receptor antagonist attenuates MAP kinase/AP-1 activation and MMP1
expression in UVA-irradiated human fibroblasts induced by culture
medium from UVB-irradiated human skin keratinocytes. Int J Mol Med.
16:1117–1124. 2005.PubMed/NCBI
|
|
25
|
Coderch L, López O, de la Maza A and Parra
JL: Ceramides and skin function. Am J Clin Dermatol. 4:107–129.
2003.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kim Y and Lim KM: Skin barrier dysfunction
and filaggrin. Arch Pharm Res. 44:36–48. 2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
El-Chami C, Foster AR, Johnson C, Clausen
RP, Cornwell P, Haslam IS, Steward MC, Watson REB, Young HS and
O'Neill CA: Organic osmolytes increase expression of specific tight
junction proteins in skin and alter barrier function in
keratinocytes. Br J Dermatol. 184:482–494. 2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lambert IH: Regulation of the cellular
content of the organic osmolyte taurine in mammalian cells.
Neurochem Res. 29:27–63. 2004.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Bravo B, Correia P, Gonçalves Junior JE,
Sant'Anna B and Kerob D: Benefits of topical hyaluronic acid for
skin quality and signs of skin aging: From literature review to
clinical evidence. Dermatol Ther. 35(e15903)2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Itano N, Sawai T, Yoshida M, Lenas P,
Yamada Y, Imagawa M, Shinomura T, Hamaguchi M, Yoshida Y, Ohnuki Y,
et al: Three isoforms of mammalian hyaluronan synthases have
distinct enzymatic properties. J Biol Chem. 274:25085–25092.
1999.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Jacobson A, Brinck J, Briskin MJ, Spicer
AP and Heldin P: Expression of human hyaluronan synthases in
response to external stimuli. Biochem J. 348:29–35. 2000.PubMed/NCBI
|
|
32
|
Nyström A and Bruckner-Tuderman L: Matrix
molecules and skin biology. Semin Cell Dev Biol. 89:136–146.
2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Varani J, Dame MK, Rittie L, Fligiel SE,
Kang S, Fisher GJ and Voorhees JJ: Decreased collagen production in
chronologically aged skin: Roles of age-dependent alteration in
fibroblast function and defective mechanical stimulation. Am J
Pathol. 168:1861–1868. 2006.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Cole MA, Quan T, Voorhees JJ and Fisher
GJ: Extracellular matrix regulation of fibroblast function:
redefining our perspective on skin aging. J Cell Commun Signal.
12:35–43. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Nishimori Y, Edwards C, Pearse A,
Matsumoto K, Kawai M and Marks R: Degenerative alterations of
dermal collagen fiber bundles in photodamaged human skin and
UV-irradiated hairless mouse skin: possible effect on decreasing
skin mechanical properties and appearance of wrinkles. J Invest
Dermatol. 117:1458–1463. 2001.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Sárdy M: Role of matrix metalloproteinases
in skin ageing. Connect Tissue Res. 50:132–138. 2009.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Pittayapruek P, Meephansan J, Prapapan O,
Komine M and Ohtsuki M: Role of matrix metalloproteinases in
photoaging and photocarcinogenesis. Int J Mol Sci.
17(868)2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Kim MS, Kim YK, Cho KH and Chung JH:
Regulation of type I procollagen and MMP-1 expression after single
or repeated exposure to infrared radiation in human skin. Mech
Ageing Dev. 127:875–882. 2006.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Röck K, Grandoch M, Majora M, Krutmann J
and Fischer JW: Collagen fragments inhibit hyaluronan synthesis in
skin fibroblasts in response to ultraviolet B (UVB): New insights
into mechanisms of matrix remodeling. J Biol Chem. 286:18268–18276.
2011.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Varani J, Warner RL, Gharaee-Kermani M,
Phan SH, Kang S, Chung JH, Wang ZQ, Datta SC, Fisher GJ and
Voorhees JJ: Vitamin A antagonizes decreased cell growth and
elevated collagen-degrading matrix metalloproteinases and
stimulates collagen accumulation in naturally aged human skin. J
Invest Dermatol. 114:480–486. 2000.PubMed/NCBI View Article : Google Scholar
|