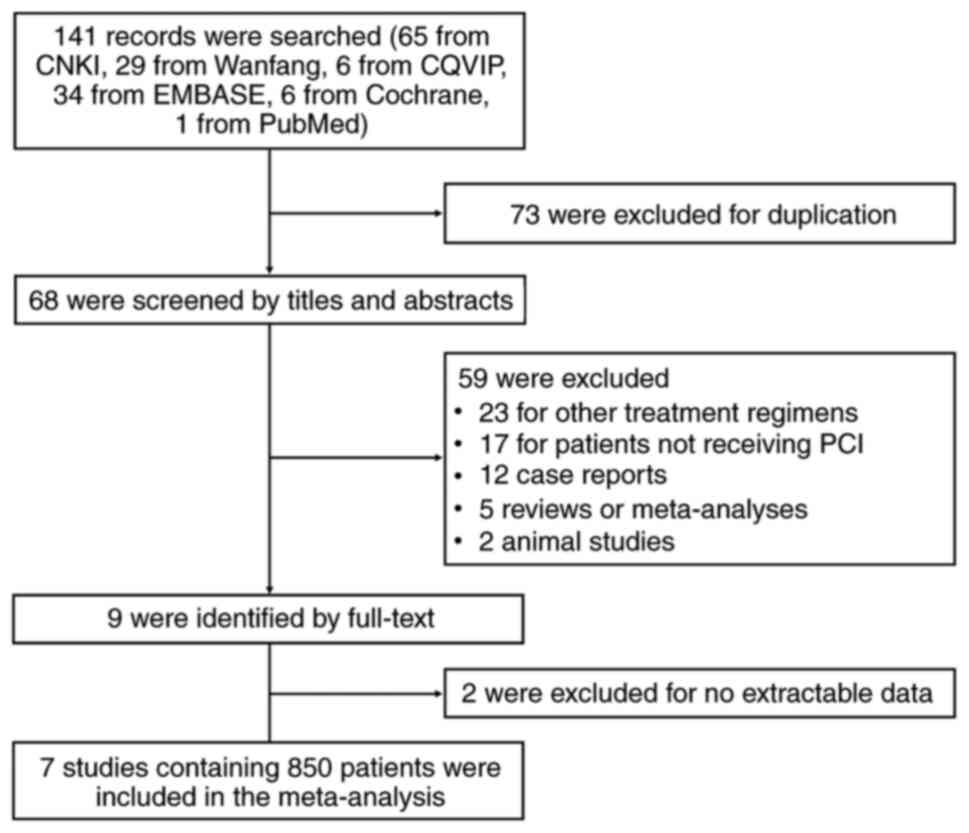
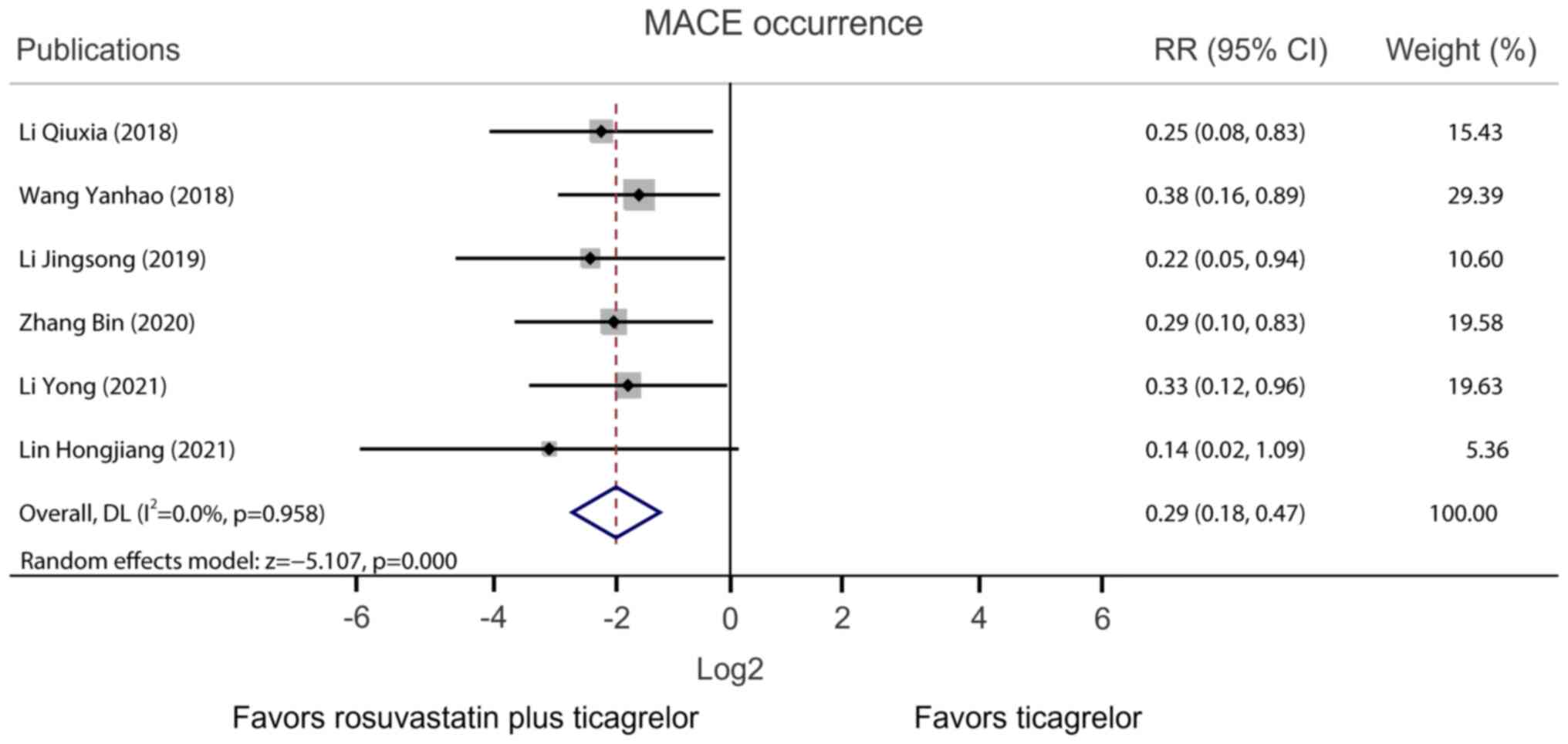
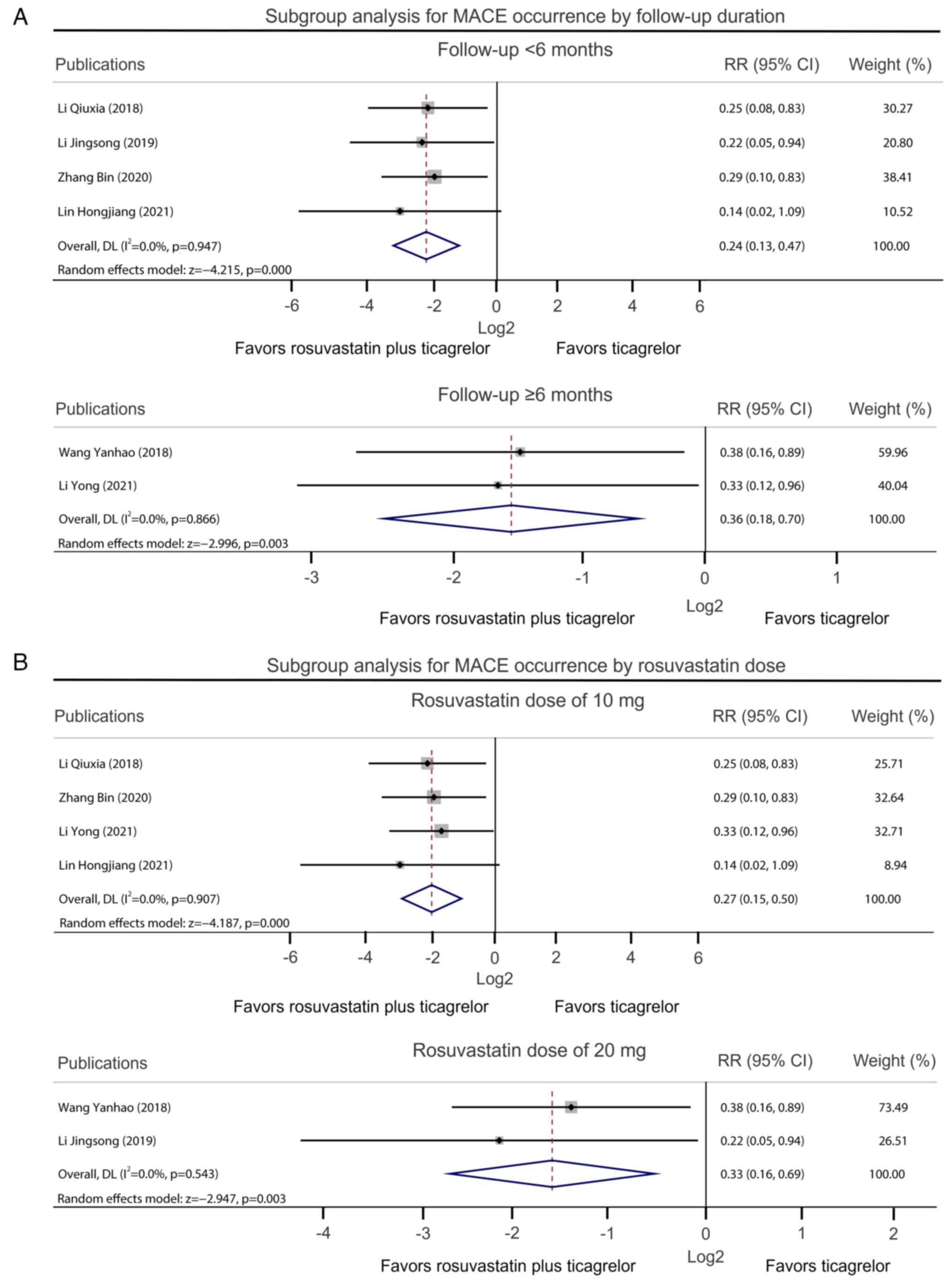
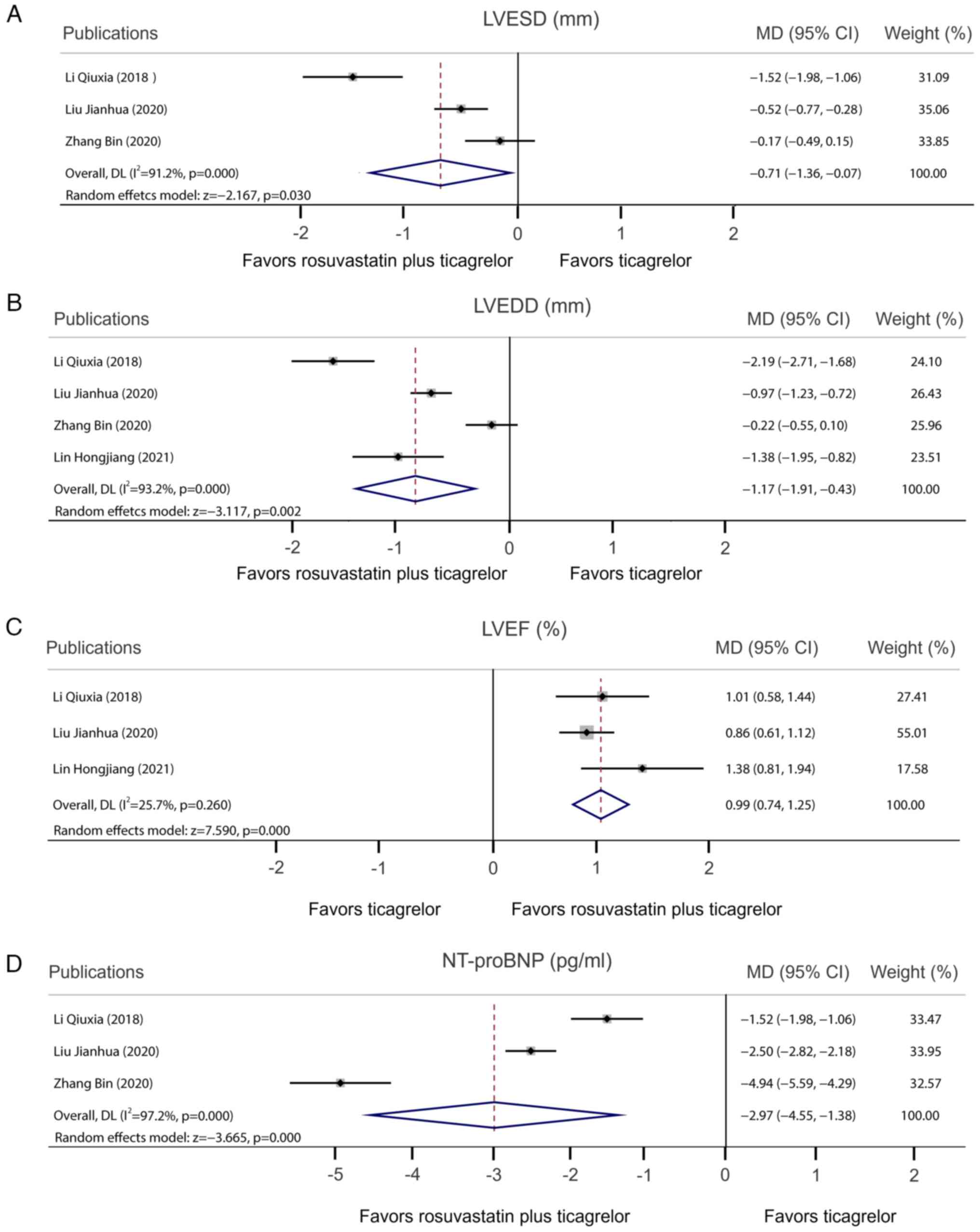
|
1
|
Hoole SP and Bambrough P: Recent advances
in percutaneous coronary intervention. Heart. 106:1380–1386.
2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Akbari T and Al-Lamee R: Percutaneous
coronary intervention in multi-vessel disease. Cardiovasc Revasc
Med. 44:80–91. 2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hirai T and Grantham JA: Indications and
patient selection for percutaneous coronary intervention of chronic
total occlusions. Interv Cardiol Clin. 10:1–5. 2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ybarra LF and Rinfret S: Access selection
for chronic total occlusion percutaneous coronary intervention and
complication management. Interv Cardiol Clin. 10:109–120.
2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Simsek B, Kostantinis S, Karacsonyi J,
Hall A, Rangan BV, Croce KJ, Azzalini L, McEntegart M, Shishehbor
M, Egred M, et al: International percutaneous coronary intervention
complication survey. Catheter Cardiovasc Interv. 99:1733–1740.
2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lin YJ, Jiao KL, Liu B, Fang L and Meng S:
Antiplatelet and myocardial protective effect of Shexiang Tongxin
dropping pill in patients undergoing percutaneous coronary
intervention: A randomized controlled trial. J Integr Med.
20:126–134. 2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lamb YN: Rosuvastatin/Ezetimibe: A review
in hypercholesterolemia. Am J Cardiovasc Drugs. 20:381–392.
2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kabil MF, Dena AS and El-Sherbiny IM:
Ticagrelor. Profiles Drug Subst Excip Relat Methodol. 47:91–111.
2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wallentin L, Becker RC, Budaj A, Cannon
CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, et
al: Ticagrelor versus clopidogrel in patients with acute coronary
syndromes. N Engl J Med. 361:1045–1057. 2009.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Koshy AN, Giustino G, Sartori S, Kyaw H,
Yadav M, Zhang Z, Hooda A, Farooq A, Krishnamoorthy P, Sweeny JM,
et al: Ticagrelor vs prasugrel in a contemporary real-world cohort
undergoing percutaneous coronary intervention. JACC Cardiovasc
Interv. 15:2270–2280. 2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Jiao Y, Hu F, Zhang Z, Gong K, Sun X, Li A
and Liu N: Efficacy and safety of loading-dose rosuvastatin therapy
in elderly patients with acute coronary syndromes undergoing
elective percutaneous coronary intervention. Clin Drug Investig.
35:777–784. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Li Q, Sun Y and Mu C: Effect of
Rosuvastatin combined with Ticagrelor in the treatment of acute
coronary syndrome. Henan Med Res. 27:4505–4506. 2018.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
13
|
Wang Y: Effect of rosuvastatin combined
with tegretol on lipid levels and incidence of MACE after PCI in
patients with acute coronary syndrome. J Math Medi. 31:1847–1848.
2018.(In Chinese).
|
|
14
|
Li J: Effect of Rosuvastatin combined with
Tegretol on percutaneous coronary intervention in patients with
coronary artery disease. Strait Pharm J. 31:193–195. 2019.(In
Chinese).
|
|
15
|
Liu J, Jiang R, Zhou J, Yang W, Guo Y, Dai
W and Lv J: Effect of Rosuvastatin combined with Tegretol on
myocardial fibrosis in elderly patients with acute myocardial
infarction. Chin J Gerontol. 40:4496–4499. 2020.(In Chinese).
|
|
16
|
Zhang B, Sun M, Duan C and Shi Y: Effects
of ticagrelor combined with rosuvastatin on cardiac function and
serological indicators in patients with acute ST elevation
myocardial infarction. Drug Evaluation Res. 43:1805–1808. 2020.(In
Chinese).
|
|
17
|
Yong L and Lei J: Effect of rosuvastatin
calcium + tegretol on carotid plaque area, intima-media thickness
and MACE incidence after PCI in patients with coronary artery
disease. Clin Res. 29:83–84. 2021.(In Chinese).
|
|
18
|
Hongjiang L: Effect of rosuvastatin
combined with ticagrelor in patients undergoing percutaneous
coronary intervention for acute myocardial infarction. Chinese and
Foreign Medical Research. 19:133–135. 2021.(In Chinese).
|
|
19
|
Hutton B, Salanti G, Caldwell DM, Chaimani
A, Schmid CH, Cameron C, Ioannidis JP, Straus S, Thorlund K, Jansen
JP, et al: The PRISMA extension statement for reporting of
systematic reviews incorporating network meta-analyses of health
care interventions: checklist and explanations. Ann Intern Med.
162:777–784. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Higgins JP, Altman DG, Gotzsche PC, Jüni
P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, et
al: The cochrane collaboration's tool for assessing risk of bias in
randomised trials. BMJ. 343(d5928)2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Stang A: Critical evaluation of the
Newcastle-Ottawa scale for the assessment of the quality of
nonrandomized studies in meta-analyses. Eur J Epidemiol.
25:603–605. 2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Luo C, Marks-Anglin A, Duan R, Lin L, Hong
C, Chu H and Chen Y: Accounting for publication bias using a
bivariate trim and fill meta-analysis procedure. Stat Med.
41:3466–3478. 2022.PubMed/NCBI View
Article : Google Scholar
|
|
23
|
Matsumoto I, Moriya S, Kurozumi M, Namba T
and Takagi Y: Relationship between serum uric acid levels and the
incidence of cardiovascular events after percutaneous coronary
intervention. J Cardiol. 78:550–557. 2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Savic L, Mrdovic I, Asanin M, Stankovic S,
Krljanac G and Lasica R: Using the RISK-PCI score in the long-term
prediction of major adverse cardiovascular events and mortality
after primary percutaneous coronary intervention. J Interv Cardiol.
2019(2679791)2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yang Y and Huang Y: Association between
serum hemoglobin and major cardiovascular adverse event in Chinese
patients with ST-segment elevation myocardial infarction after
percutaneous coronary intervention. J Clin Lab Anal.
36(e24126)2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Levine GN, Bates ER, Bittl JA, Brindis RG,
Fihn SD, Fleisher LA, Granger CB, Lange RA, Mack MJ, Mauri L, et
al: 2016 ACC/AHA guideline focused update on duration of dual
antiplatelet therapy in patients with coronary artery disease: A
report of the American college of cardiology/American heart
association task force on clinical practice guidelines: An update
of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary
intervention, 2011 ACCF/AHA guideline for coronary artery bypass
graft surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS guideline for
the diagnosis and management of patients with stable ischemic heart
disease, 2013 ACCF/AHA guideline for the management of ST-elevation
myocardial infarction, 2014 AHA/ACC guideline for the management of
patients with non-ST-elevation acute coronary syndromes, and 2014
ACC/AHA guideline on perioperative cardiovascular evaluation and
management of patients undergoing noncardiac surgery. Circulation.
134:e123–e155. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ge Z, Baber U, Claessen BE, Farhan S,
Chandrasekhar J, Li SX, Sartori S, Kini AS, Rao SV, Weiss S, et al:
The prevalence, predictors and outcomes of guideline-directed
medical therapy in patients with acute myocardial infarction
undergoing PCI, an analysis from the PROMETHEUS registry. Catheter
Cardiovasc Interv. 93:E112–E119. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Berwanger O, Santucci EV, de Barros ESPGM,
Jesuíno IA, Damiani LP, Barbosa LM, Santos RHN, Laranjeira LN,
Egydio FM, de Oliveira JA, et al: Effect of loading dose of
atorvastatin prior to planned percutaneous coronary intervention on
major adverse cardiovascular events in acute coronary syndrome: The
SECURE-PCI randomized clinical trial. JAMA. 319:1331–1340.
2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
He W, Cao M and Li Z: Effects of different
doses of atorvastatin, rosuvastatin, and simvastatin on elderly
patients with ST-elevation acute myocardial infarction (AMI) after
percutaneous coronary intervention (PCI). Drug Dev Res. 81:551–556.
2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Merla R, Daher IN, Ye Y, Uretsky BF and
Birnbaum Y: Pretreatment with statins may reduce cardiovascular
morbidity and mortality after elective surgery and percutaneous
coronary intervention: Clinical evidence and possible underlying
mechanisms. Am Heart J. 154:391–402. 2007.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chen W, Fan Z, Huang C, Han Z and Liu J:
Enhanced-dose statins for ST-segment elevation myocardial
infarction patients after emergency percutaneous coronary
intervention. Dis Markers. 2022(2751750)2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Cortese F, Gesualdo M, Cortese A,
Carbonara S, Devito F, Zito A, Ricci G, Scicchitano P and Ciccone
MM: Rosuvastatin: Beyond the cholesterol-lowering effect. Pharmacol
Res. 107:1–18. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Xie W, Li P, Wang Z, Chen J, Lin Z, Liang
X and Mo Y: Rosuvastatin may reduce the incidence of cardiovascular
events in patients with acute coronary syndromes receiving
percutaneous coronary intervention by suppressing miR-155/SHIP-1
signaling pathway. Cardiovasc Ther. 32:276–282. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Birnbaum Y, Birnbaum GD, Birnbaum I,
Nylander S and Ye Y: Ticagrelor and rosuvastatin have additive
cardioprotective effects via adenosine. Cardiovasc Drugs Ther.
30:539–550. 2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Chen C, Wu X, Li Y and Peng Y: Study on
the application effect of bisoprolol combined with sacubitril
valsartan sodium tablets in the cardiac rehabilitation of patients
with acute myocardial infarction combined with left heart failure
after percutaneous coronary intervention (PCI). Ann Palliat Med.
10:5455–5461. 2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Guo J, Zhang WZ, Zhao Q, Wo JS and Cai SL:
Study on the effect of different doses of rosuvastatin on
ventricular remodeling in patients with acute coronary syndrome
after emergency percutaneous coronary intervention. Eur Rev Med
Pharmacol Sci. 21:4457–4463. 2017.PubMed/NCBI
|
|
37
|
Lee SA, Kim W, Hong TJ, Ahn Y, Kim MH,
Hong SJ, Kim BS, Kim SY, Chae IH, Kim BJ, et al: Effects of
fixed-dose combination of low-intensity rosuvastatin and ezetimibe
versus moderate-intensity rosuvastatin monotherapy on lipid
profiles in patients with hypercholesterolemia: A randomized,
double-blind, multicenter, phase III study. Clin Ther.
43:1573–1589. 2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Ren G, Zhou Q, Lu M and Wang H:
Rosuvastatin corrects oxidative stress and inflammation induced by
LPS to attenuate cardiac injury by inhibiting the NLRP3/TLR4
pathway. Can J Physiol Pharmacol. 99:964–973. 2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zhong Z, Hou J, Zhang Q, Zhong W, Li B, Li
C, Liu Z, Yang M and Zhao P: Assessment of the LDL-C/HDL-C ratio as
a predictor of one year clinical outcomes in patients with acute
coronary syndromes after percutaneous coronary intervention and
drug-eluting stent implantation. Lipids Health Dis.
18(40)2019.PubMed/NCBI View Article : Google Scholar
|