Introduction
Congestive heart failure (CHF) refers to a series of
clinical syndromes in which the systolic and diastolic function of
the heart is seriously impaired by various pathogenic factors,
resulting in a decline in the pumping function of the heart and the
inability to expel blood to meet the metabolic needs of the body
(1). The clinical incidence of CHF
is high with a frequency of 1-2% of the population (2) and it poses a serious threat to the
health of patients. Thus, there is an urgent need to identify novel
therapeutic drugs.
Aconite was widely used in ancient China to treat
similar symptoms of heart failure (3). A previous study showed that the
combination of water-soluble alkaloids of aconite and total
ginsenosides had a therapeutic effect in a rat model of acute heart
rats (4). It can also inhibit
apoptosis in rats with chronic heart failure (5). Salsolinol is the primary
water-soluble heart-stimulating alkaloid of aconite and has a
heart-stimulating strengthening effect similar to that of
β-adrenalin. A previous study showed that salsolinol has analgesic,
anti-inflammatory and heart-strengthening effects (6). Salsolinol alleviates
doxorubicin-induced chronic heart failure in rats and improves
mitochondrial function of H9C2 cardiomyocytes (7). However, the role of salsolinol in
myocardial fibrosis and its mechanism have not been reported thus
far. According to SwissTargetPrediction database (http://www.swisstargetprediction.ch/),
lysine-specific demethylase 1 (LSD1; also known as KDM1A) is a
potential target of salsolinol. LSD1, the first identified histone
demethylase, serves an important role in embryonic development,
epithelial-interstitial transformation, cell differentiation and
tumor proliferation, invasion and metastasis (8). Inhibition of LSD1 in pregnant mice or
neonatal mice prevents cardiomyopathy and LSD1 may be a therapeutic
target for the prevention or treatment of dilated cardiomyopathy
complicated with laminopathy (9).
After 20 weeks of transverse aortic contraction, LSD1 expression
increases not only in human dilated cardiomyopathic hearts but also
in wild-type mouse heart homogenates and isolated cardiac
fibroblasts (10). In addition,
upregulation of LSD1 is also observed in angiotensin II (Ang
II)-treated neonatal rat myocardial fibroblasts and in vivo
myoblast-specific LSD1 knockout significantly alleviated systolic
dysfunction, myocardial hypertrophy and fibrosis at 6 and 20 weeks
after transaortic contraction (10), which indicated that LSD1 might be a
potential target for the treatment of heart failure. However,
whether salsolinol serves a regulatory role in myocardial fibrosis
through LSD1 has not been reported in the literature, to the best
of the authors' knowledge.
The aim of the present study was to determine the
effect of salsolinol in angiotensin II-induced myocardial fibrosis
and ascertain the underlying mechanism, to provide a strong
theoretical basis for the clinical treatment of heart failure with
salsolinol.
Materials and methods
Bioinformatics tools
SwissTargetPrediction database (www.swisstargetprediction.ch) predicted that LSD1
was a potential target of salsolinol.
Patients and ethics
Serum from 15 patients with CHF (7 males and 8
females; age, 49-67; mean age 57.3±4.9 years) was collected at the
Kunshan Hospital of Integrated Traditional Chinese and Western
Medicine from April 2019 to September 2022. The present study was
approved by the ethics committee of Kunshan Hospital of Integrated
Traditional Chinese and Western Medicine (approval no. KY2021003)
and a written consent form was signed by all participants prior to
the experiments.
Cell culture
Human Cardiac fibroblasts (HCFs; cat. no.
BNCC354381) were purchased from the BeNa Culture Collection and
cultured in DMEM medium (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.)
at 37˚C, in a humidified incubator supplied with 5% CO2.
When the cells grew to 50-60% confluence, the cells were pretreated
with salsolinol (5, 10 or 20 µM; cat. no. 57256-34-5; purity:
99.86%; MedChemExpress) for 2 h and then treated with 100 nM
angiotensin II (AngII; Sigma-Aldrich; Merck KGaA) for 48 h.
Cell proliferation assay
Cell proliferation was measured using a Cell
Counting Kit-8 (CCK-8) assay kit (Beyotime Institute of
Biotechnology) according to the manufacturer's instructions.
Briefly, HCF cells were treated as above, after which 10 µl of
CCK-8 solution was added and cells were incubated for 1 h. The
absorbance at 450 nm was measured using a spectrophotometer.
EdU staining assay
An EdU staining kit (Beyotime Institute of
Biotechnology) was used to analyze cell proliferation according to
the manufacturer's protocol. Briefly, HCF cells were treated and
then incubated with EdU (20 mmol/l) at 37˚C for 2 h. The cells were
fixed with 4% paraformaldehyde for 20 min at room temperature.
Wound healing assay
The treated HCF cells were cultured until they were
~90% confluent in six-well plates and then a scratch was created
using a sterile 200 µl pipette tip. Subsequently, cells were
cultured in serum-free DMEM for 24 h. Images of the wounded area
were taken after 0 and 24 h at the same microscopic cross point
using a light microscope (Olympus Corporation). Wound width was
measured using ImageJ version 1.52q (National Institutes of
Health).
Immunofluorescence (IF) staining
After fixing the cells with 4% paraformaldehyde for
20 min at 4˚C, the cells were washed and blocked for 1 h using 5%
BSA (Gibco; Thermo Fisher Scientific, Inc.). Subsequently, cells
were incubated overnight at 4˚C with the primary α-smooth muscle
actin (SMA) antibody (1:5,00; cat. no. orb311091; Biorbyt, Ltd.),
after which cells were incubated with the secondary antibody for 1
h with a horseradish peroxidase-conjugated goat anti-rabbit
secondary antibody (1:10,000; Abcam) at room temperature, followed
by counterstaining with 5 µg/ml DAPI (Beyotime Institute of
Biotechnology) for 2 min at 22˚C. A fluorescence microscope (BXM1;
Olympus Corporation) was used to visualize staining.
Western blotting
Total proteins were extracted from HCF cells using
RIPA lysis buffer (Beyotime Institute of Biotechnology) and the
protein concentration was quantified with a BCA assay kit (Beyotime
Institute of Biotechnology). Equal amounts of proteins (20 µg per
lane) were loaded on a 10% SDS-gel, resolved using SDS-PAGE and
transferred to PVDF membranes (Invitrogen; Thermo Fisher
Scientific, Inc.), after which, membranes were blocked with 5% BSA
(Gibco; Thermo Fisher Scientific, Inc.) for 2 h at room
temperature. The membranes were incubated with primary antibodies
anti-Collagen I (1:1,000; cat. no. ab138492; Abcam), anti-Collagen
III (1:1,000; cat. no. ab184993; Abcam), anti-α-SMA (1:1,000; cat.
no. orb311091; Biorbyt, Ltd.), anti-LSD1 (1:1,000; cat. no.
ab129195; Abcam), anti-phosphorylated (p-)STAT3 (1:1,000; cat. no.
ab267373; Abcam), anti-STAT3 (1:1,000; cat. no. ab68153; Abcam),
anti-Jagged-1 (1:1,000; cat. no. ab109536; Abcam), anti-NICD
(1:1,000; cat. no. ab52627; Abcam), anti-GAPDH (1:1,000; cat. no.
orb555879; Biorbyt, Ltd.) at 4˚C overnight. The following day,
membranes were incubated with HRP-conjugated anti-rabbit secondary
antibodies (1:5,000; cat. no. ab7090; Abcam) for 1 h at room
temperature. Signals were visualized using enhanced
chemiluminescence reagent (MilliporeSigma) and ImageJ software
1.8.0 (National Institutes of Health) was used for the
semi-quantification of protein density.
ELISA
The treated HCF cells were collected and then the
concentrations of TNF-α (cat. no. H052-1-2) and IL-6 (cat. no.
H007-1-2) were measured by the corresponding ELISA kits (Nanjing
Jiancheng Bioengineering Institute) according to the manufacturer's
instructions.
Detection of reactive oxygen species
(ROS)
The procedures of ROS measurement were according to
manufacturer's instructions. The cells were incubated with 500 µl
PBS containing 50 µM dichlorofluorescein diacetate (DCF-DA; cat.
no. S0033; Beyotime Institute of Biotechnology) for 30 min at 37˚C.
Following three washes with PBS followed by centrifugation at
12,000 x g for 5 min at 4˚C and DCF fluorescence intensity was
detected using a BD FACS Calibur™ flow cytometer (BD
Biosciences) at the excitation and emission wavelengths of 485 and
535 nm. BD CellQuest™ Pro software version 5.1 (BD
Biosciences) was used to analyze ROS levels (11).
Molecular docking
The structure of salsolinol was drawn in the
ChemDraw software (version 18.0) (12) and then imported into OpenBabel
software (version 2.3.1) (13) for
hydrogenation and converted into a mol2 format file. Subsequently,
the structure of LSD1 (PDB ID: 2DW4) was obtained from the RCSB PDB
(https://www.rcsb.org/). Thereafter, the protein
LSD1 file was opened in PyMOL software (version 2.2.0) (14) to remove the excess water molecules,
delete any irrelevant small ligands originally carried and to keep
only the protein structure. As the downloaded protein structure had
ligands, the original ligands were deleted and the original ligand
positions were set as the docking sites. AutoDock (version 1.5.6)
(14) was used to display the
specific docking energy value after running. Finally, the results
were analyzed with the adoption of Protein-Ligand Interaction
Profiler (PLIP; https://plip-tool.biotec.tu-dresden.de/plip-web).
Reverse transcription-quantitative
(RT-q) PCR
Total RNA was extracted from 1x104 HCF
cells using TRIzol® reagent (Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. cDNA was
synthesized from 1 µg total RNA using HIScript-II Q RT SuperMix for
qPCR (Vazyme Biotech Co., Ltd.,) according to the manufacturer's
instructions. qPCR was performed and analyzed using the cDNA and
SYBR Green PCR MasterMix (Nordic Bioscience) according to the
manufacturer's instructions. The qPCR thermocycling conditions
were: 40 cycles of 10 sec at 95˚C and 20 sec at 60˚C. Relative
expression changes were calculated using the 2-ΔΔCq
method (15). LSD1 (KDM1A) forward
and reverse primers were 5'-TGATCTTGGAGCCATGGTGG-3' and
5'-GACAGTGTCAGCTTGTCCGTT-3', GAPDH forward and reverse primers were
5'-AATGGGCAGCCGTTAGGAAA-3' and 5'-GCGCCCAATACGACCAAATC-3'. The
experiments were replicated three times.
Cell transfection
The LSD1 overexpression vector (Ov-LSD1) was
established by inserting the LSD1 gene into the pcDNA3.1 vector
(Shanghai GeneChem Co., Ltd.), whereas an empty vector served as
the negative control (Ov-NC). Then, HCF cells in the 2nd generation
system were inoculated into 6-well plates at a density of
2x105 cells/well and cultured until cell confluence has
reached 80%. After that, a total of 100 nM plasmids were
transfected into HCF cells at 37˚C for 48 h using
Lipofectamine® 2000 transfection reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. HCF cells were infected using a retroviral supernatant
(6x108 TU/ml) for 72 h, then treated with 0.5 µg/ml
puromycin for 2 weeks to obtain stably transfected cells. After
transfection for 48 h, the transfection efficiency was detected
using RT-qPCR and western blotting according to the aforementioned
methods.
Statistical analysis
Data are presented as the mean ± standard deviation
and were analyzed using a one-way ANOVA followed by a Tukey's post
hoc test in SPSS version 16.0 (SPSS, Inc.). The normal distribution
of variables was assessed by the Shapiro-Wilk test. P<0.05 was
considered to indicate a statistically significant difference.
Results
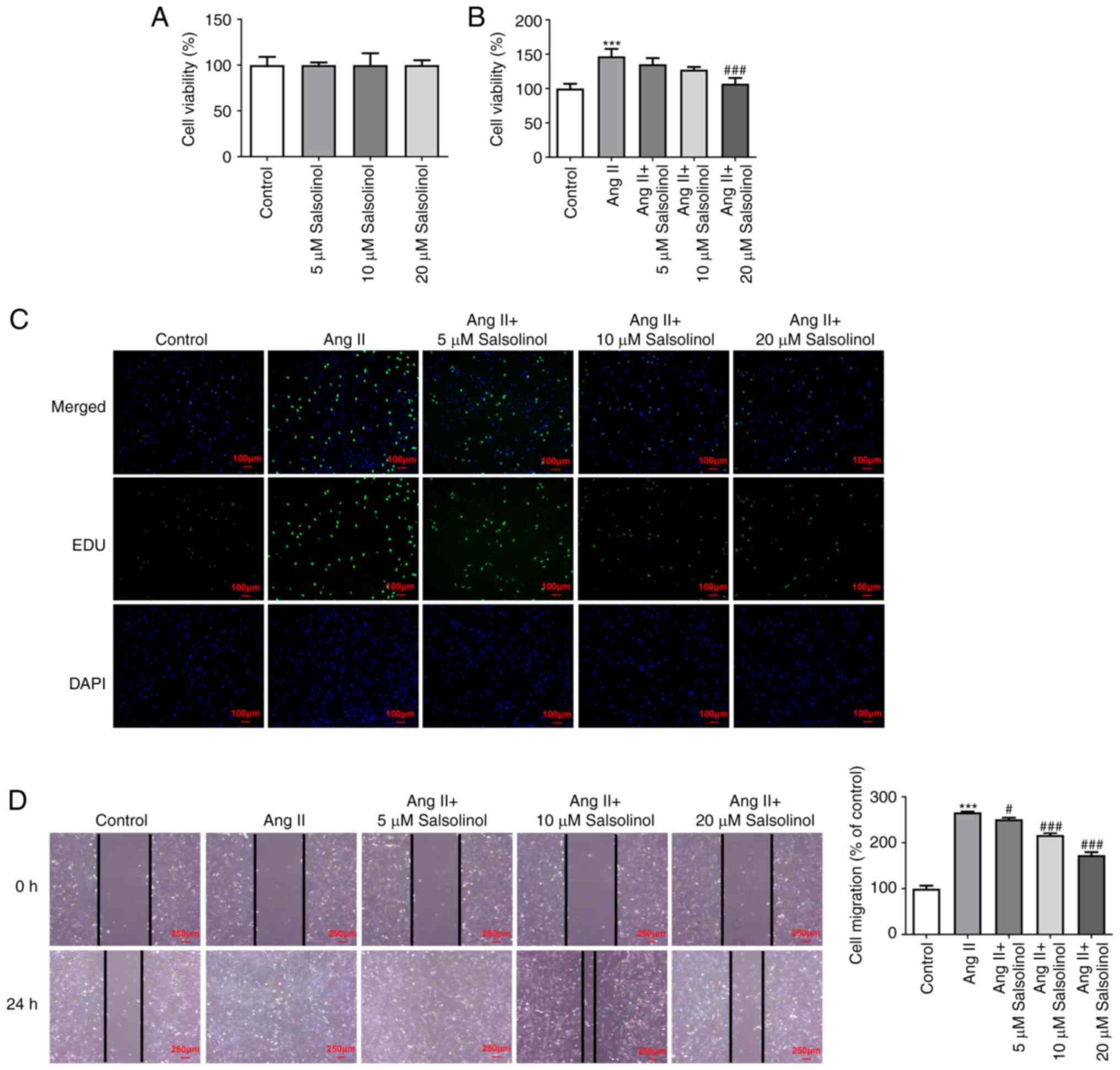
Salsolinol inhibits the proliferation
and migration of AngII-induced HCFs
Different concentrations (5, 10, or 20 µM) of
salsolinol were used to treat HCFs and a CCK-8 assay was used to
detect cell viability. The results showed that 5, 10 and 20 µM
salsolinol did not have a noticeable toxic effect (Fig. 1A). The cells were then divided into
a control, AngII, AngII + 5 µM, AngII + 10 µM and AngII + 20 µM
groups. The results of the CCK-8 and EdU staining assays showed
increased cell proliferation in the AngII group compared with the
control group. However, different concentrations of salsolinol
reduced cell viability in a concentration-dependent manner
(Fig. 1B and C). The cell migratory ability was
detected using a wound healing assay and the results showed that
the migratory ability was significantly increased following AngII
induction compared with the control group. Salsolinol significantly
inhibited HCF migration (Fig.
1D).
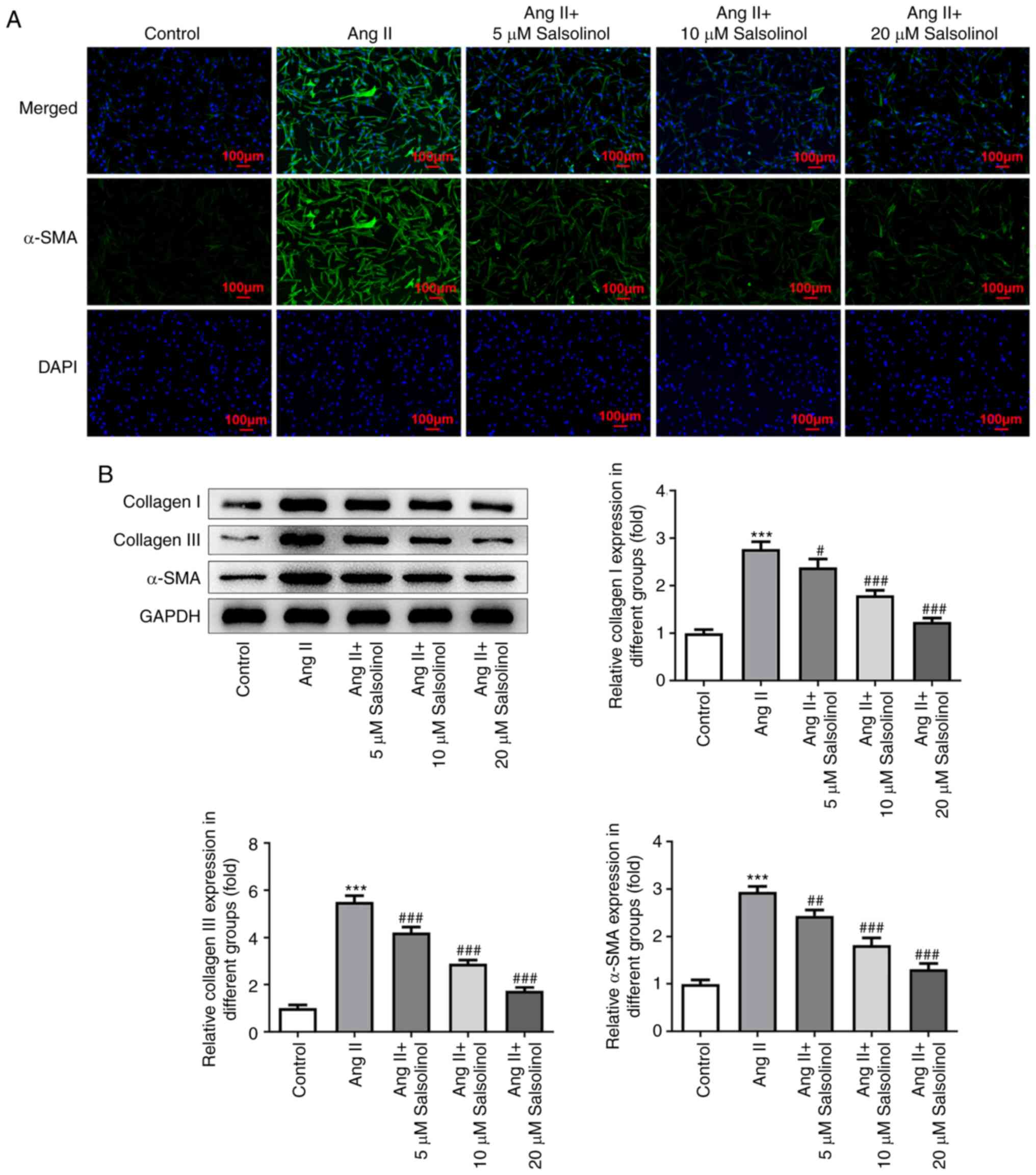
Salsolinol inhibits AngII-induced HCF
fibrosis
The expression of α-SMA was detected using an IF
assay. The results showed that α-SMA expression in the AngII group
was significantly higher than that in the control group; salsolinol
inhibited this increase in a dose-dependent manner (Fig. 2A). Western blotting was used to
detect the expression of fibrosis-related proteins α-SMA, Collagen
I and Collagen III and the results showed that AngII significantly
increased the expression of these proteins in cells. Salsolinol
inhibited this increase in a dose-dependent manner (Fig. 2B).
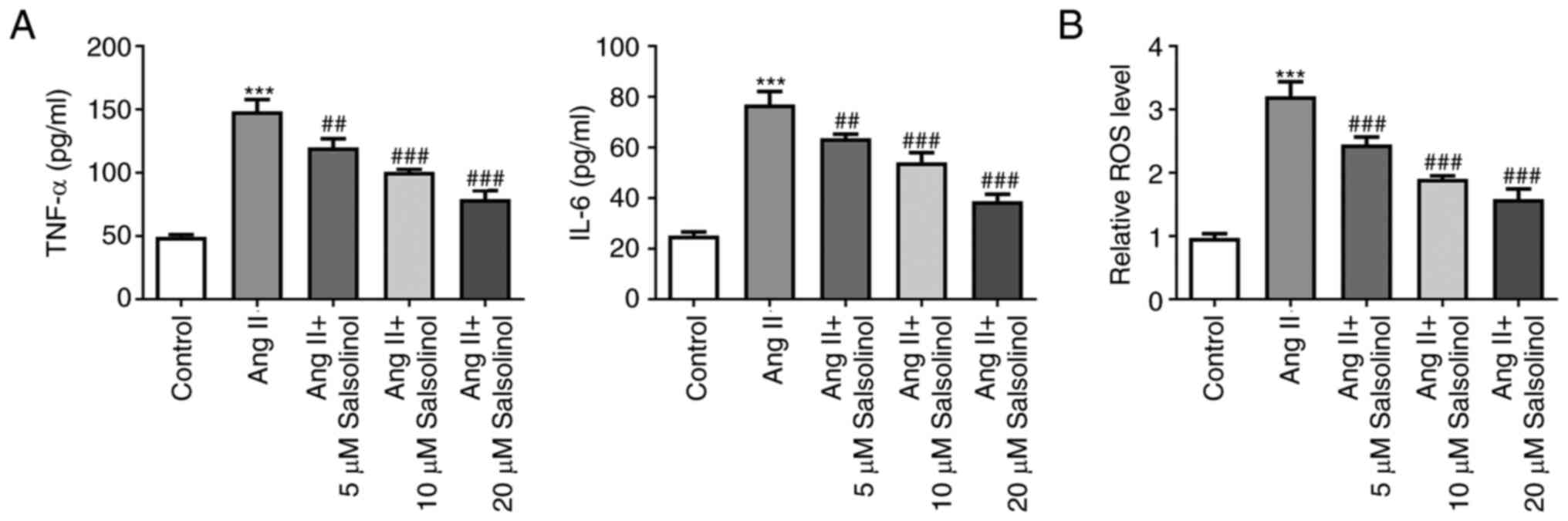
Salsolinol inhibits inflammation and
ROS production in AngII-induced HCFs
Subsequently, the expression of cytokines related to
inflammation was detected and the results of ELISA showed that
compared with the control group, the expression of TNF-α and IL-6
were significantly increased in the AngII group, while salsolinol
dose-dependently reduced the expression of TNF-α and IL-6 compared
with the AngII group (Fig. 3A).
AngII induced an increase in ROS expression in the cells, while
salsolinol inhibited this increase in a dose-dependent manner
(Fig. 3B).
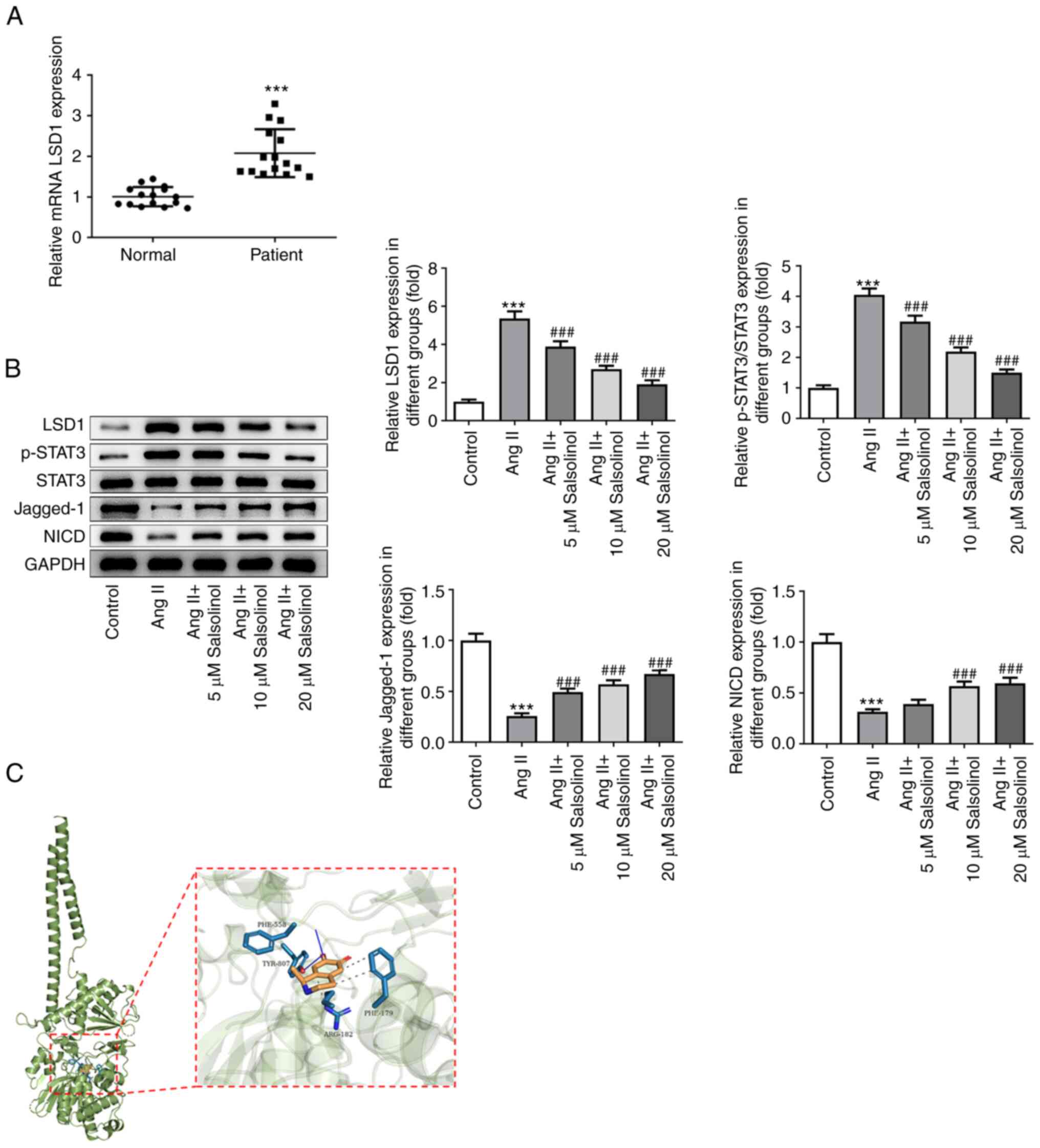
Salsolinol inhibits LSD1 expression
and regulates the STAT3/Notch-1 signaling pathway
As predicted by SwissTargetPrediction database, LSD1
was a potential target of salsolinol. Using serum from patients
with CHF, it was found that LSD1 expression was significantly
increased in patients with CHF compared with the control group
(Fig. 4A). In the in vitro
experiments, western blotting was used to detect the expression of
LSD1 and proteins associated with the STAT3/Notch-1 signaling
pathway. Compared with the control group, the expression of LSD1
was increased in the AngII group. The expression of p-STAT3 was
increased, while the expression of Jagged-1 and NICD proteins was
significantly decreased in the AngII group. Administration of
salsolinol reversed the changes in the expression of these proteins
in a dose-dependent manner (Fig.
4B). Molecular docking analysis showed that salsolinol could
target and regulate the expression of LSD1 (Fig. 4C). Therefore, it was concluded that
salsolinol inhibited LSD1 and regulated the STAT3/Notch-1 signaling
pathway.
Upregulation of LSD1 reverses the
effect of salsolinol on AngII-induced HCFs
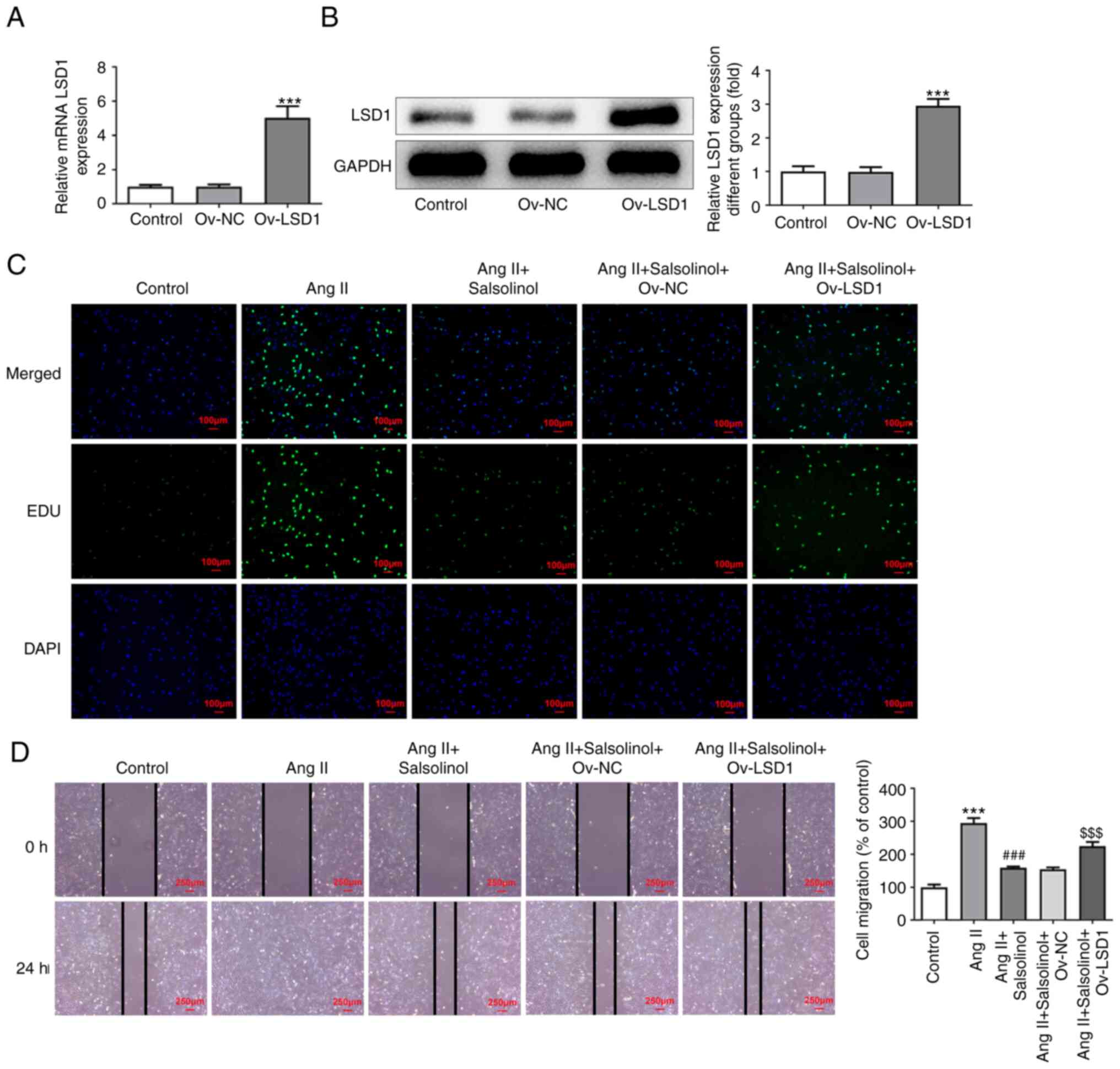
An LSD1 overexpression cell line was constructed and
transfection efficiency was detected using RT-qPCR and western
blotting (Fig. 5A and B); 20 µM salsolinol was selected for
follow-up experiments. Cells were divided into a control, AngII,
AngII + salsolinol, AngII + salsolinol + Ov-NC and AngII +
salsolinol + Ov-LSD1 groups. EdU staining results showed that
compared with the AngII + salsolinol + Ov-NC group, the
proliferative ability of AngII + salsolinol + Ov-LSD1 group was
increased (Fig. 5C). The results
of the wound healing assay showed that overexpression of LSD1
significantly reversed the increase in cell migration induced by
salsolinol (Fig. 5D). The results
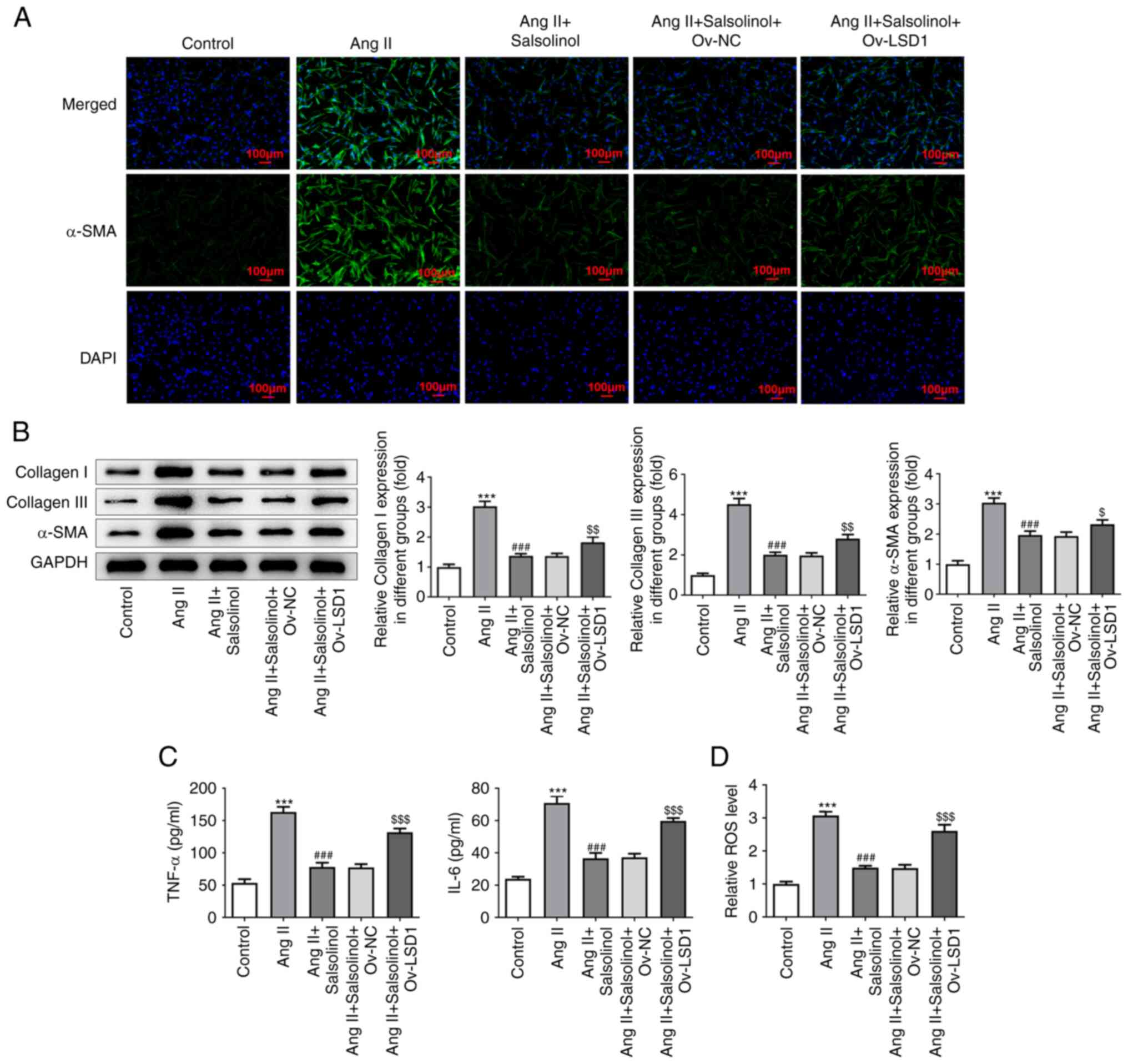
of IF and western blotting showed that compared with the AngII +
salsolinol + Ov-NC group, the expression of α-SMA, Collagen I and
Collagen III in the AngII + Salsolinol + Ov-LSD1 group was
increased (Fig. 6A and B). The results of ELISA showed that
overexpression of LSD1 significantly reversed the inhibition of
TNF-α, IL-6 and ROS by salsolinol (Fig. 6C and D).
Discussion
Patients with CHF have relatively poor cardiac
function and the myocardial interstitium also presents in an
abnormal state during the occurrence and development of the
disease, leading to the relative disorder of the myocardial
structure (16). Several studies
have shown that the above factors can lead to myocardial fibrosis
and thus lead to abnormal cardiac function (17,18).
In addition, myocardial fibrosis is a common pathological
manifestation of the majority of heart diseases, which leads to
heart failure and arrhythmia caused by changes in the electrical
conduction of the heart. Therefore, it is necessary to study the
changes of myocardial fibrosis in these patients.
Cardiac fibroblasts are the most abundant type of
cells in the heart that respectively account for ~27, ~64 and ~72%
of the heart mass in mice, rats and humans and are the key cell
type responsible for maintaining the structural integrity of the
heart (19). In a pathological
state, fibroblasts are activated to transform into myofibroblasts,
which have higher contractility and mobility, stronger ability to
synthesize extracellular matrix proteins and the expression of
several marker proteins such as α-SMA in these cells is
significantly upregulated (20,21).
Phenotypic changes of cardiac fibroblasts in cardiovascular
diseases and a series of functional changes following these changes
are the basis of myocardial fibrosis (22). In the present study, HCFs were used
for experimentation and AngII was used to induce HCFs as a model of
cardiac fibrosis.
At present, there are few clinical methods for the
treatment of myocardial fibrosis and those that do exist have
limited effects. Research has found that certain types of
traditional Chinese medicines can improve heart failure through an
anti-cardiac fibrosis effect and this research direction has
gradually garnered increasing interest (1). Research has shown that salsolinol, a
chemical present in the popular prescription Ershen Zhenwu
Decoction (ESZWD) has potential for treating heart failure with
what is termed in traditional Chinese medicine heart-kidney Yang
deficiency syndrome (23). Water
soluble alkaloids of aconitum have a significant therapeutic effect
on acute heart failure rats and salsolinol is a biological
component of the water-soluble alkaloids present in aconitum,
suggesting that salsolinol may have a therapeutic effect on acute
heart failure (4). Salsolinol
regulates angiotensin-converting enzyme, which serves an important
role in improving chronic myocardial ischemia (24). However, whether salsolinol has a
therapeutic effect on myocardial fibrosis in CHF has not been
reported. In the present study, salsolinol was shown to exhibit no
significant cytotoxic effects on HCFs. In addition, it
significantly inhibited the abnormal proliferation and migration of
HCFs in a dose dependent manner. Salsolinol significantly inhibited
the expression of fibrosis-related proteins α-SMA, Collagen I and
Collagen III, the expression of inflammatory factors TNF-α and IL-6
and the generation of oxidative stress factor ROS in a dose
dependent manner. It indicated that salsolinol inhibited fibrotic
effect of HCFs as well as the cellular inflammatory and oxidative
stress responses in a dose-dependent manner. A previous study has
shown that salsolinol has obvious cardiotonic and anti-inflammatory
effects (5). This is consistent
with the results of salsolinol on anti-inflammatory and antioxidant
stress of HCFs in the present study.
Next, the specific regulatory mechanism of the
effects of salsolinol on HFCs was determined. Using molecular
docking analysis, it was found that salsolinol could dock with
LSD1. In addition, it was found that LSD1 expression was abnormally
elevated in the serum of CHF patients and AngII-induced HCFs. A
previous study showed increased expression of LSD1 in the kidney of
mice with unilateral ureteral obstruction and TGF-β1-induced
NRK-52E cells and inhibition of LSD1 using a specific inhibitor,
ORY1001, alleviated renal epithelial-to-mesenchymal transition and
fibrosis (25). In
bleomycin-induced pulmonary fibrosis mice and lung tissues of
TGF-β1-treated lung fibroblasts, LSD1 expression was elevated and
LSD1 activation promoted differentiation and fibrosis of lung
myoblasts by targeting the TGF-β1/Smad3 signaling pathway (26). These results indicated that LSD1
served an important regulatory role in tissue fibrosis. In
addition, LSD1 deficiency in myofibroblasts has been shown to
alleviate heart failure in mice. The results of the present study
showed the that upregulation of LSD1 reversed the effects of
salsolinol on AngII-induced HCF proliferation, migration,
inflammatory response and fibrosis.
A previous study showed that LSD1 induced epithelial
interstitial transformation and promoted renal fibrosis through the
Jagged-1/Notch signaling pathway (27). TRIM72 promoted cardiac fibrosis via
regulation of the STAT3/Notch-1 signaling (28). PKM2 can also promote AngII-induced
cardiac remodeling via activation of the TGF-β/Smad2/3 and
Jak2/Stat3 pathways via oxidative stress (29). Feverolin, an inhibitor of the STAT3
signaling pathway, attenuates AngII-induced left ventricular
hypertrophy via regulation of fibroblast activity (30). Therefore, it is reasonable to
hypothesize that LSD1 can regulate the downstream pathway
STAT3/Notch-1 following salsolinol-mediated regulation of LSD1,
thereby inhibiting myocardial fibrosis. The results of the present
study showed that following AngII induction of HCFs, p-STAT3
expression was activated and Jagged-1 and NICD expression were
inhibited in cells. The expression of the members of the
STAT3/Notch-1 signaling pathway was reversed following salsolinol
treatment of AngII-induced HCFs. Therefore, it was preliminarily
concluded that salsolinol inhibited LSD1 via regulation of the
STAT3/Notch-1 signaling pathway to improve AngII-induced myocardial
fibrosis in vitro. However, whether this mechanism is
observed in vivo remains to be determined and thus serves as
a limitation of the present study. In addition, there is another
limitation to the present study, that is, it only detected the
expression level of LDS1 protein in patients' blood and the
expression level of LDS1 protein in patient tissues will be
detected in the future experiments.
In conclusion, salsolinol inhibited LSD1 via
regulation of the STAT3/Notch-1 signaling pathway to improve
AngII-induced myocardial fibrosis in vitro. The results of
the present study provide a theoretical basis for the clinical
treatment of CHF with salsolinol. However, in vivo
experiments and clinical investigations should be performed to
verify the mechanism of salsolinol pair against specific types of
cancer in future studies.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the 2019 Kunshan Key
Research and Development Plan Social Development Project (grant no.
KS1915), the 2021 Suzhou Science and Technology Development Plan
(grant no. SKJYD2021206), the 2022 Kunshan Key Research and
Development Plan Social Development Project (grant no. KSZ2212) and
the Suzhou Science and Technology Development Plan Project (grant
no. SKYXD2022069).
Availability of data and materials
The data sets generated and/or analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
JW designed and conceived the present study. XZ, ZS,
YN, FC and XY performed the experiments and wrote the manuscript.
JW, XZ and ZS performed the data analysis. YN and FC confirmed the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the ethics
committee of Kunshan Hospital of Integrated Traditional Chinese and
Western Medicine (approval no. KY2021003) and informed consent was
obtained from patients prior to beginning the experiments.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kennelly P, Sapkota R, Azhar M, Cheema FH,
Conway C and Hameed A: Diuretic therapy in congestive heart
failure. Acta Cardiol. 77:97–104. 2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Abdelbasset WK and Alqahtani BA: A
randomized controlled trial on the impact of moderate-intensity
continuous aerobic exercise on the depression status of middle-aged
patients with congestive heart failure. Medicine (Baltimore).
98(e15344)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wen J, Li M, Zhang W, Wang H, Bai Y, Hao
J, Liu C, Deng K and Zhao Y: Role of higenamine in heart diseases:
A mini-review. Front Pharmacol. 12(798495)2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Liu M, Li Y, Tang Y, Zheng L and Peng C:
Synergistic effect of aconiti lateralis radix praeparata
water-soluble alkaloids and ginseng radix et rhizoma total
ginsenosides compatibility on acute heart failure rats. J
Chromatogr B Analyt Technol Biomed Life Sci.
1137(121935)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Xu X, Xie X, Zhang H, Wang P, Li G, Chen
J, Chen G, Cao X, Xiong L, Peng F and Peng C: Water-soluble
alkaloids extracted from aconiti radix lateralis praeparata protect
against chronic heart failure in rats via a calcium signaling
pathway. Biomed Pharmacother. 135(111184)2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yang Y, Hu P, Zhou X, Wu P, Si X, Lu B,
Zhu Y and Xia Y: Transcriptome analysis of aconitum carmichaelii
and exploration of the salsolinol biosynthetic pathway.
Fitoterapia. 140(104412)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wen J, Zhang L, Liu H, Wang J, Li J, Yang
Y, Wang Y, Cai H, Li R and Zhao Y: Salsolinol attenuates
doxorubicin-induced chronic heart failure in rats and improves
mitochondrial function in H9c2 cardiomyocytes. Front Pharmacol.
10(1135)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhang S, Liu M, Yao Y, Yu B and Liu H:
Targeting LSD1 for acute myeloid leukemia (AML) treatment.
Pharmacol Res. 164(105335)2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Guenantin AC, Jebeniani I, Leschik J,
Watrin E, Bonne G, Vignier N and Pucéat M: Targeting the histone
demethylase LSD1 prevents cardiomyopathy in a mouse model of
laminopathy. J Clin Invest. 131(e136488)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Huo JL, Jiao L, An Q, Chen X, Qi Y, Wei B,
Zheng Y, Shi X, Gao E, Liu HM, et al: Myofibroblast deficiency of
LSD1 alleviates TAC-induced heart failure. Circ Res. 129:400–413.
2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Bao J, Ye C, Zheng Z and Zhou Z: Fmr1
protects cardiomyocytes against lipopolysaccharide-induced
myocardial injury. Exp Ther Med. 16:1825–1833. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Evans DA: History of the harvard ChemDraw
project. Angew Chem Int Ed Engl. 53:11140–11145. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lin Z, Zhang Z, Ye X, Zhu M, Li Z, Chen Y
and Huang S: Based on network pharmacology and molecular docking to
predict the mechanism of Huangqi in the treatment of
castration-resistant prostate cancer. PLoS One.
17(e0263291)2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhang Y, Liu X, Long J, Cheng X, Wang X
and Feng X: Exploring active compounds and mechanisms of angong
niuhuang wan on ischemic stroke based on network pharmacology and
molecular docking. Evid Based Complement Alternat Med.
2022(2443615)2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yu H, Basu S and Hallow KM: Cardiac and
renal function interactions in heart failure with reduced ejection
fraction: A mathematical modeling analysis. PLoS Comput Biol.
16(e1008074)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
González A, Schelbert EB, Diez J and
Butler J: Myocardial interstitial fibrosis in heart failure:
Biological and translational perspectives. J Am Coll Cardiol.
71:1696–1706. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Rao M, Wang X, Guo G, Wang L, Chen S, Yin
P, Chen K, Chen L, Zhang Z, Chen X, et al: Resolving the
intertwining of inflammation and fibrosis in human heart failure at
single-cell level. Basic Res Cardiol. 116(55)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kurose H: Cardiac fibrosis and
fibroblasts. Cells. 10(1716)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Tallquist MD: Cardiac fibroblast
diversity. Annu Rev Physiol. 82:63–78. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Liu M, López de Juan Abad B and Cheng K:
Cardiac fibrosis: Myofibroblast-mediated pathological regulation
and drug delivery strategies. Adv Drug Deliv Rev. 173:504–519.
2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Maruyama K and Imanaka-Yoshida K: The
pathogenesis of cardiac fibrosis: A review of recent progress. Int
J Mol Sci. 23(2617)2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hong LL, Zhao Y, Chen WD, Yang CY, Li GZ,
Wang HS and Cheng XY: Tentative exploration of pharmacodynamic
substances: Pharmacological effects, chemical compositions, and
multi-components pharmacokinetic characteristics of ESZWD in
CHF-HKYd rats. Front Cardiovasc Med. 9(913661)2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Guo S, Li P, Fu B, Chuo W, Gao K, Zhang W,
Wang J, Chen J and Wang W: Systems-biology dissection of mechanisms
and chemical basis of herbal formula in treating chronic myocardial
ischemia. Pharmacol Res. 114:196–208. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhang X, Li LX, Yu C, Nath KA, Zhuang S
and Li X: Targeting lysine-specific demethylase 1A inhibits renal
epithelial-mesenchymal transition and attenuates renal fibrosis.
FASEB J. 36(e22122)2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Pan X, Li J, Tu X, Wu C, Liu H, Luo Y,
Dong X, Li X, Pan LL and Sun J: Lysine-specific demethylase-1
regulates fibroblast activation in pulmonary fibrosis via
TGF-β1/Smad3 pathway. Pharmacol Res. 152(104592)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhang H, Xing J and Zhao L:
Lysine-specific demethylase 1 induced epithelial-mesenchymal
transition and promoted renal fibrosis through Jagged-1/Notch
signaling pathway. Hum Exp Toxicol. 40 (12 Suppl):S203–S214.
2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chen X, Su J, Feng J, Cheng L, Li Q, Qiu C
and Zheng Q: TRIM72 contributes to cardiac fibrosis via regulating
STAT3/Notch-1 signaling. J Cell Physiol. 234:17749–17756.
2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhang X, Zheng C, Gao Z, Wang L, Chen C,
Zheng Y and Meng Y: PKM2 promotes angiotensin-II-induced cardiac
remodelling by activating TGF-β/Smad2/3 and Jak2/Stat3 pathways
through oxidative stress. J Cell Mol Med. 25:10711–10723.
2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Skoumal R, Tóth M, Serpi R, Rysä J,
Leskinen H, Ulvila J, Saiho T, Aro J, Ruskoaho H, Szokodi I and
Kerkelä R: Parthenolide inhibits STAT3 signaling and attenuates
angiotensin II-induced left ventricular hypertrophy via modulation
of fibroblast activity. J Mol Cell Cardiol. 50:634–641.
2011.PubMed/NCBI View Article : Google Scholar
|