Introduction
Ectopic gastric mucosa (EGM) is typically discovered
incidentally and may be asymptomatic or present with nonspecific
gastrointestinal symptoms (1).
Several reports describe the canceration of EGM (2-4).
Thus, active treatment of EGM is necessary to prevent further
complications and deterioration.
EGM can occur in several locations, such as the
esophagus and colon, or in rarer instances in the anus (5) and umbilicus (6). To the best of our knowledge, there
are no incidences of EGM of the stomach that have been reported in
the literature. The present report describes a case of EGM, and the
clinicopathological characteristics and immunohistochemical (IHC)
findings are described.
Case report
Case presentation
A 47-year-old man was admitted to the Sunshine Union
Hospital (Weifang, China) in June 2023 due to acid reflux,
heartburn with abdominal distension, and diarrhea (5-10 times a
day) for >10 years. The patient had not received systematic
medication during this period or experienced abdominal pain,
belching, nausea, vomiting, fever, or noticeable weight change.
However, chronic atrophic gastritis was found in the patient during
a gastroscopy in 2022. Routine blood tests and the laboratory
examination were normal. A 13C-urea breath test showed
no Helicobacter pylori infection.
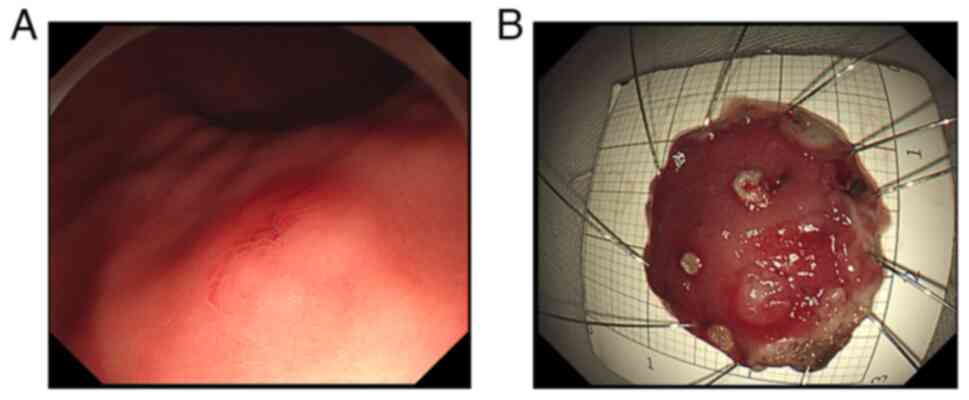
Endoscopic examination revealed a submucosal
eminence, and the biopsy forceps felt slightly hard when touched
(Fig. 1). Based on the endoscopy
results, leiomyoma, ectopic pancreas, gastrointestinal stromal
tumor, and early gastric cancer were considered. The tumor was
excised entirely with endoscopic submucosal dissection (ESD). The
final diagnosis awaited pathological examination.
Pathological findings
Macro-examination. A piece of mucosal tissue
with a 2x2x0.3 cm volume was obtained. The tissue specimens were
fixed in 4% neutral formalin at room temperature for 48 h, followed
by dehydration with alcohol and treatment with xylene.
Subsequently, the specimens were embedded in paraffin at 62˚C and
cooled. Serial sections (4 µm) were prepared and stained at room
temperature with hematoxylin (~5% for 5 min), followed by eosin
[(~1% for 2 min (H&E)] staining. Additionally, IHC staining was
performed using the paraffin-embedded tissues.
Microscopic observation. H&E and IHC
staining were examined using an Olympus BX53 light microscope
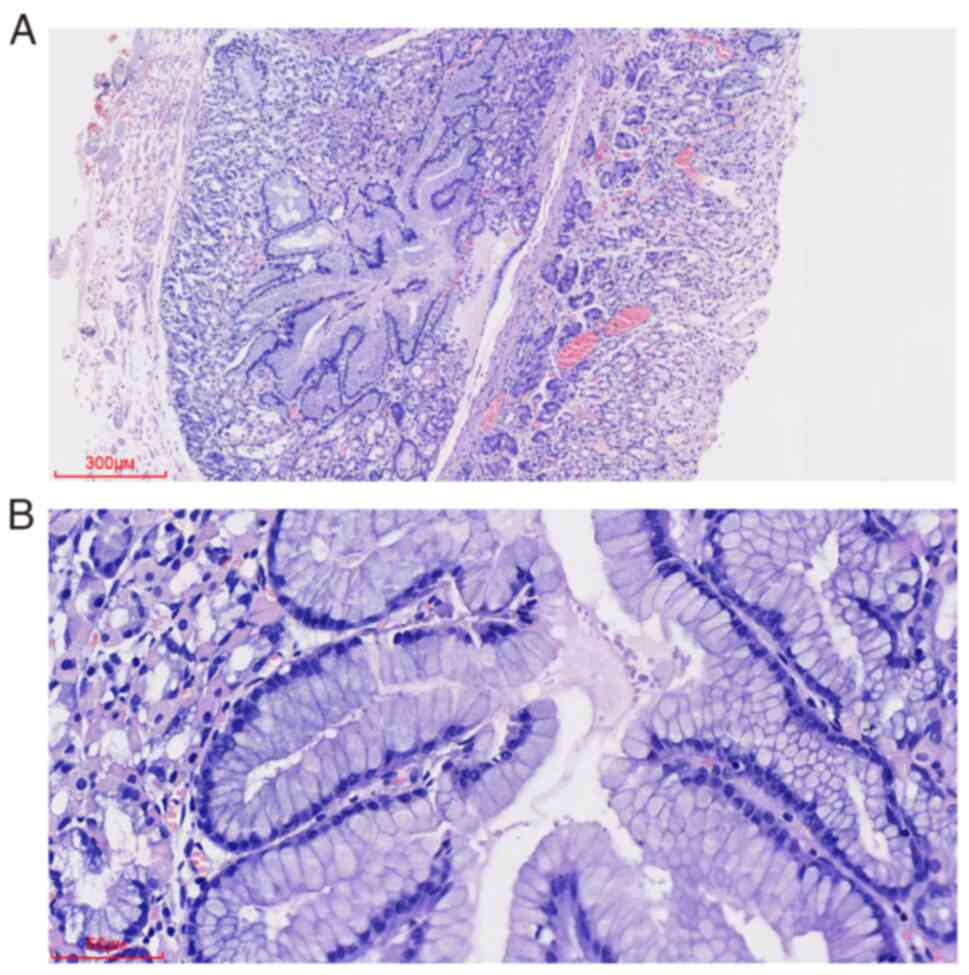
(Olympus Corporation). H&E staining (Fig. 2) showed normal gastric pits,
gastric fundus glands, and mucosal muscles in the gastric body. EGM
components were located below the mucosal muscles between the
muscularis propria. The components demonstrated a well-defined
nodular shape without connecting with the glands of the lamina
propria. EGM composed of surface mucous, main, and parietal cells
that formed a structure similar to gastric pits and gastric fundus
glands, exhibited no prominent structural atypia, cell atypia, or
glandular expansion.
IHC staining (Fig.
3) was performed overnight at 4˚C using the following primary
antibodies (prediluted by the manufacturer; Guangzhou ABP Medicine
Science & Technology Co., Ltd.): anti-mucin-5AC (MUC-5AC, cat.
no. IM109), anti-mucin-6 (MUC-6, cat. no. IM398), anti-mucin-2
(MUC-2, cat. no. IM108), anti-synaptin (Syn, cat. no. IM136),
anti-smooth muscle actin (SMA, cat. no. IM005), and anti-Ki-67
(cat. no. IR098). For IHC, tissue sections (3 µm) were fixed in 4%
formalin at room temperature for 48 h before paraffin embedding.
The sections were rehydrated in a descending alcohol series
(xylene, 100% ethanol, 95% ethanol, 85% ethanol, and ethanol-free
water) and underwent antigen retrieval with EDTA antigen retrieval
treatment (EnVision FLEX Target Retrieval Solution, High pH; cat.
no. K8000; Agilent Technologies, Inc.) in a microwave for 3 min at
high heat (wattage, 700 W), followed by incubation at room
temperature for 8 min. Endogenous peroxidase activity was quenched
using 3% hydrogen peroxide in methanol before incubation with the
primary antibodies. The secondary antibody from EnVision FLEX/HRP
(prediluted by the manufacturer; cat. no. K8000; Agilent
Technologies, Inc.) was used to treat the sections at room
temperature for 25 min. Subsequently, a chromogen detection reagent
was used according to the manufacturer's protocol (EnVision FLEX
DAB+ Chromogen; cat. no. K8000; Agilent Technologies, Inc.). IHC
revealed that MUC-5AC was positively expressed in the EGM surface
mucus cells and MUC-6 in the EGM mucous neck cells. SMA was
expressed in the mucosal muscle and was used to determine the
integrity of the mucosal muscle. The Ki-67 proliferating index was
<1% of EGM. Additionally, Syn was positive in scattered
neuroendocrine cells near the basal region of the gland, consistent
with the positive pattern of neuroendocrine cells in the glands of
the lamina propria. Finally, MUC-2 was negatively expressed in EGM
and normal gastric mucosa.
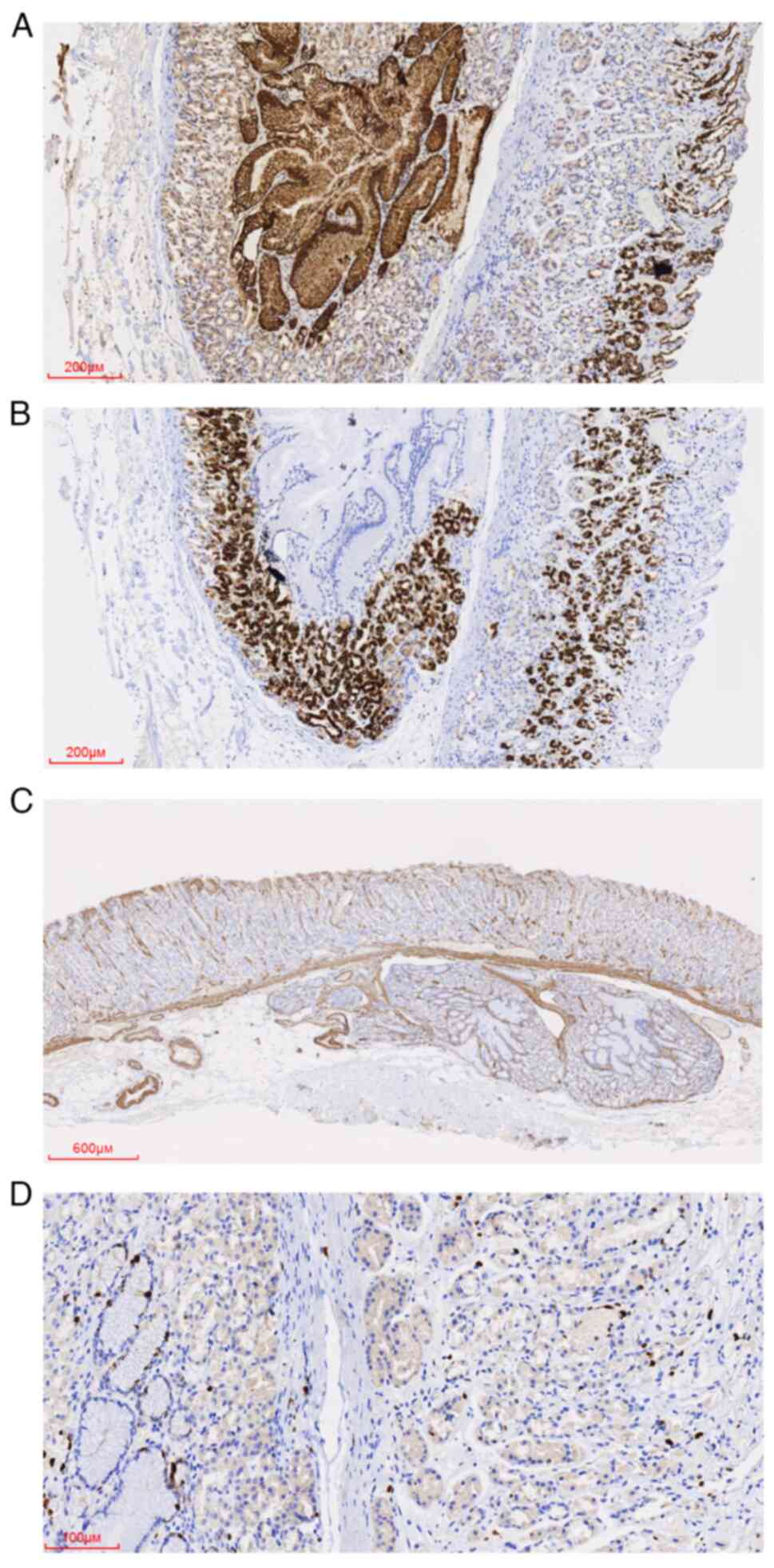 | Figure 3Immunohistochemical staining of the
ectopic gastric mucosa. (A) MUC-5AC was expressed in the surface
mucus cells of normal and ectopic gastric mucosa. Magnification,
x50; scale bar, 200 µm. (B) MUC-6 was expressed in the mucous neck
cells of normal and ectopic gastric mucosa. Magnification, x50,
scale bar, 200 µm. (C) SMA is expressed in the mucosal muscle and
interstitial vascular wall. Magnification, x20, scale bar, 600 µm.
(D) The Ki-67 proliferating index in normal and ectopic gastric
mucosa was low; EGM on the left and normal mucosa on the right.
Magnification, x200, scale bar, 100 µm. MUC, mucin; SMA, smooth
muscle actin. |
Pathological diagnosis. The patient was
diagnosed with (gastric body) EGM in the submucosa.
Follow-up. ESD removed the tumor completely,
and postoperative recovery was good. No recurrence was observed
during the 5 week follow-up.
Discussion
Two theories currently explain the mechanism of EGM
in the stomach. The most widely accepted theory is that the EGM is
an embryological remnant. The second theory is that EGM is the
product of abnormal inflammation-related proliferation (7). In the present report, the 47-year-old
patient was hospitalized due to several atypical symptoms,
including chronic atrophic gastritis, for ~10 years. For the
‘congenital malformation’ theory to apply to this patient, EGM
could not have caused the symptoms. According to the second theory,
the symptoms were potentially caused by EGM. Alternatively, the
symptoms could have been the result of chronic colitis. There is no
direct evidence to support whether EGM caused these symptoms.
To the best of our understanding, few reports exist
about EGM in the stomach, and EGM before pathological examination
has not been considered. The collected literature (Table I) was reviewed (7-10),
and found that all the patients were males aged 23-72 years old
with primary complaints of nonspecific abdominal symptoms. The
diseased sites in the stomach varied (for example the pylorus,
gastric fundus, lesser curvature, and gastric body). There were no
records of death in the cases with the follow-up data. Notably, all
patients were Eastern Asians (for example Chinese and Japanese).
Eastern Asian countries have the highest incidence of gastritis and
gastric cancer in the world (11,12).
With the results of the present study, it is suggested that
regardless of severity, gastric diseases in Eastern Asians should
be treated cautiously to prevent the disease from progressing.
Additionally, regular physical examinations are recommended for the
early detection and timely management of any gastric diseases.
 | Table IOverview of the literature on ectopic
gastric mucosa in the stomach. |
Table I
Overview of the literature on ectopic
gastric mucosa in the stomach.
| First author,
year | Language | Age, years | Chief complaint | Race | Examination | Diagnosis before
pathological examination | Ectopic site | Cancerous | Operation | Follow-up | (Refs.) |
|---|
| Wang et al,
2019 | English | 30 | Abdominal distension
of a six-month duration | Chinese | Gastroscopy | - | The cardia of the
gastric fundus, located between the muscularis mucosae and
submucosa | No | ESD | Alive after 1
year | (7) |
| Zhou et al,
2002 | Chinese | 48 | Paroxysmal upper
abdominal pain for more than 2 years | Chinese | Barium meal
examination | Leiomyoma, GC | Pyloris, muscular
layer | No | Subtotal
gastrectomy | - | (8) |
| Gu et al,
2002 | Chinese | 23 | Pain in stomach a
month ago | Chinese | Gastroscopy | Leiomyoma, ectopic
pancreas | Pyloris, muscular
layer | No | Locally surgical
resection | - | (9) |
| Nakano et al,
1987 | Japanese | 72 | Anorexia and hungry
epigastric pain | Japanese | Gastroscopy and upper
gastrointestinal series examination | GC | Anterior and
posterior walls of the lesser curvature | Yes | Total
gastrectomy | - | (10) |
| Present report | English | 47 | Acid reflux,
heartburn with abdominal distension and diarrhea (5-10 times a day)
for more than 10 years | Chinese | Gastroscopy | Leiomyoma, ectopic
pancreas, gastrointestinal stromal tumor, early GC | Gastric body | No | ESD | Alive after 5
weeks | - |
EGM is distinct from other diseases, including
gastric adenocarcinoma of the fundic gland, neuroendocrine tumor
grade G1, ectopic pancreas, gastritis cystica profunda, and
inverted hyperplastic polyp (IHP). First, in gastric adenocarcinoma
of the fundic gland type, the gland is similar to a normal gastric
fundus gland, with several cell types such as main and parietal
cells. Typically, under the microscope, the cell atypia is
unapparent or mild (13), and
mitotic images are rare. The gastric pit epithelial cells on the
atypical gland surface are normal. The glands with structural
dysplasia are deep in the lamina propria. The glands are dilated
and irregular; some may be sieve-shaped. The disease is diagnosed
when the glands with slight cell atypia but prominent structural
atypia invade the submucosa. In the present case, no cell atypia or
structural atypia was observed, neither in the glands of the mucosa
lamina propria nor the ectopic submucosal gland. Thus, the present
case was distinct from gastric adenocarcinoma of the fundic gland
type.
Second, EGM is distinguished from neuroendocrine
tumor grade G1. In neuroendocrine tumor grade G1, the tumor cells
are uniform in size and shape, with a round, oval, or short spindle
shape, light to moderate nuclear dysplasia, granular nuclear
chromatin, rare mitotic images, and tumor cells sometimes arranged
in nests and chordates, Ki-67 proliferating index is low (≤1%)
(14). In the present case, IHC
staining revealed positive expression of Syn, CD56, and CgA; the
mucous, main, and parietal cells were the primary components,
whereas the neuroendocrine cells were scattered in ectopic glands.
Thus, the present case was distinct from neuroendocrine tumor grade
G1.
Third, EGM is distinguished from gastritis cystica
profunda, where the glands are composed of similar, normal gastric
pits and glands of the lamina propria. The gastritis cystica
profunda is usually continuous with the glands of the lamina
propria, and the glands are expanded to varying degrees (15). Lymphocytes may be present gathered
in the stroma around the glands. The present case lacked these
manifestations.
Fourth, EGM is separate from ectopic pancreas. An
ectopic pancreas is typically comprised of one or more components
of the pancreatic acinus, duct, and islet (16). IHC staining of the pancreatic
acinar components was positive for lipase, trypsinogen, and
amylase, additionally, neuroendocrine markers of islet components
were positive. The case comprised gastric pits rich in mucus, main,
and parietal cells, with notably no pancreatic component.
Finally, EGM is distinct from inverted hyperplastic
polyp (IHP). IHP is rare and was considered heterotopic or
hamartomatous until the 1990s. It is characterized by inverted
growth of the hyperplastic mucosa under the normal mucosa and can
be pedicled (17). Most of the
glands in IHP are cystic dilatation, a key distinguishing feature
of EGM.
As EGM can become cancerous, it must be completely
removed as soon as reasonably possible when something suspicious is
found. It is vital to examine the infiltration depth of malignant
components, vascular invasion, and the incised edge to determine
whether additional surgery is required. According to the literature
review performed for the present report, the prognosis of patients
is often very good. However, follow-up is also essential according
to the existing data, as no evidence exists that EGM will not
recur.
In summary, EGM is a rare lesion with unique
morphological features. As it can become cancerous, efforts should
be made to avoid its misdiagnosis. Complete resection is required
during treatment, and patients are advised to have regular
follow-ups.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Research
Projects of Weifang Municipal Health Committee (grant no.
WFWSJK-2022-239) and the Sunshine Union Hospital Research Project
(grant no. 2022YGRH043).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JHX and XJY drafted the manuscript and conceived the
study. JHX, NYS and WJG performed the research and analyzed the
data. JHX wrote the manuscript. XJY and NYS revised the manuscript.
XJY and WJG confirm the authenticity of all the raw data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Sunshine Union Hospital (approval no. 2023-06-0008).
Patient consent for publication
The patient provided written informed consent for
the case study to be published.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wlaź J, Mądro A, Kaźmierak W, Celiński K
and Słomka M: Pancreatic and gastric heterotopy in the
gastrointestinal tract. Postepy Hig Med Dosw (Online).
68:1069–1075. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Martins FP, Artigiani Neto R, Oshima CT,
Costa PP, N M F and Ferrari AP: Over-expression of cyclooxygenase-2
in endoscopic biopsies of ectopic gastric mucosa. Braz J Med Biol
Res. 40:1447–1454. 2007.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kitasaki N, Hamai Y, Yoshikawa T, Emi M,
Kurokawa T, Hirohata R, Ohsawa M and Okada M: Recurrent esophageal
adenocarcinoma derived from ectopic gastric mucosa: A case report.
Thorac Cancer. 13:876–879. 2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Nobumoto D, Oda K, Shimizu Y, Tonouchi A,
Fujino M, Ando K and Kubosawa H: A case of adenocarcinoma arising
from ectopic gastric mucosa in Meckel's diverticulum with abdominal
wall abscess. Gan To Kagaku Ryoho. 47:2332–2334. 2020.PubMed/NCBI(In Japanese).
|
|
5
|
Steele SR, Mullenix PS, Martin MJ, Ormseth
E, Weppler E, Graham J and Place RJ: Heterotopic gastric mucosa of
the anus: A case report and review of the literature. Am Surg.
70:715–719. 2004.PubMed/NCBI
|
|
6
|
Iwasaki M, Taira K, Kobayashi H and Saiga
T: Umbilical cyst containing ectopic gastric mucosa originating
from an omphalomesenteric duct remnant. J Pediatr Surg.
44:2399–2401. 2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wang H, Tan Y and Liu D: A rare
heterotopic gastric mucosa appearing between the muscularis mucosae
and submucosa. Rev Esp Enferm Dig. 111:712–713. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhou HB, Ge HJ and Qu XH: Diagnosis and
treatment of a case of ectopic gastric mucosa in gastric muscle
layer. J Shandong Med. 2(71)2002.(In Chinese).
|
|
9
|
Gu YH: Ectopic gastric mucosa: A case
report. J Shenyang Med Coll. 1(40)2002.(In Chinese).
|
|
10
|
Nakano H, Nakahara Y, Mizumoto K, Yoshioka
Y, Tamura Y, Tanabe M and Ogino T: Early gastric cancer associated
with ectopic gastric mucosa (submucosal cysts). Nihon Geka Gakkai
Zasshi. 88:1024–1030. 1987.PubMed/NCBI(In Japanese).
|
|
11
|
Suzuki H and Mori H: Different
pathophysiology of gastritis between east and west? An asian
perspective. Inflamm Intest Dis. 1:123–128. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Natsume H, Szczepaniak K, Yamada H,
Iwashita Y, Gędek M, Šuto J, Ishino K, Kasajima R, Matsuda T,
Manirakiza F, et al: Non-CpG sites preference in G:C > A:T
transition of TP53 in gastric cancer of Eastern Europe (Poland,
Romania and Hungary) compared to East Asian countries (China and
Japan). Genes Environ. 45(1)2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kai K, Satake M and Tokunaga O: Gastric
adenocarcinoma of fundic gland type with signet-ring cell carcinoma
component: A case report and review of the literature. World J
Gastroenterol. 24:2915–2920. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Uppin MS, Uppin SG, Sunil CS, Hui M, Paul
TR and Bheerappa N: Clinicopathologic study of neuroendocrine
tumors of gastroenteropancreatic tract: A single institutional
experience. J Gastrointest Oncol. 8:139–147. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Du Y, Zhang W, Ma Y and Qiu Z: Gastritis
cystica profunda: A case report and literature review. Ann Palliat
Med. 9:3668–3677. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Xiang S, Zhang F and Xu G: Ectopic
pancreas in the ileum: An unusual condition and our experience.
Medicine (Baltimore). 98(e17691)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ma Q, Gao L, Sun N, Chen Y, Liu L, Liu L,
Guo W and Yang X: Gastric inverted hyperplastic polyp: A case
report. Exp Ther Med. 25(6)2022.PubMed/NCBI View Article : Google Scholar
|