The CNS contains the brain and spinal cord from
which the peripheral nerves branch and is safeguarded by the spinal
cord, which encompasses the meninges, cerebrospinal fluid and
spine. The spinal cord exerts important functions, including the
regulation of motor and sensory functions (1,2).
Spinal cord injury (SCI) is the most common disabling spinal
injury; For the last 30 years, its global prevalence has increased
from 236 to 1,298 cases per million populations. The estimated
global rate of SCI falls between 250,000 and 500,000 individuals
every year.
It can damage the normal anatomy of the spinal cord,
leading to axonal rupture, neuronal degeneration and necrosis,
inflammatory response and demyelination, ultimately leading to
severe neurological dysfunction (3,4). SCI
frequently results in sensorimotor disorders, autonomic changes and
intractable pain; Spinal cord injury can also affect respiratory,
urinary, and gastrointestinal functions and is one of the factors
leading to the development of infection. After spinal cord injury,
a large number of inflammatory substances are released into the
blood and cause inflammation throughout the body. Thus seriously
affecting the quality of life of patients (5). SCI is categorized into two types:
Traumatic and non-traumatic. The former is more common and mainly
caused by external physical impacts, such as vehicle accidents,
violence and falls (1,6), whereas the latter is usually caused
by compression of a tumor; the enlargement of some tumors can
compress the spinal cord tissue, resulting in the destruction of
the spinal cord tissue, resulting in clinical symptoms, vascular
ischemia or congenital disease such as Spinal Bifida (7). The current review mainly focused on
traumatic SCI. Following spinal cord injury, axons of the CNS fail
to regenerate. By contrast, peripheral nervous system axons
regenerate after injury and show restored function. The lack of CNS
regeneration after injury may be associated with abnormal
expression of specific molecules in myelin and glial scars in the
CNS, including Nogo, oligodendroglia-myelin glycoproteins and
myelin-associated glycoproteins (8). A previous study reported that these
molecules induce the activity of the Rho-Rho-associated protein
containing kinase 2 (ROCKII) and glycogen synthase kinase-3β
(GSK-3β) signaling pathways, leading to inhibition of axonal
regeneration in the CNS (9). Thus,
the Rho-ROCKII and/or GSK-3β signaling pathways may be targets for
restoring axon regeneration.
The CNS is composed of neurons and glial cells;
glial cells include astrocytes, microglia, oligodendrocytes and
Schwann cells, and are crucial for proper CNS development and
function (10). The interaction
between neurons and glial cells plays an important role in the
physiological processes of the central nervous system. The
dysfunction of neurons and glial cells is one of the pathogenesis
of neuro developmental disorders (11). Glial cells, mainly astrocytes,
collaborate with neurons and vasculature to harvest nutrients from
the bloodstream, thus providing metabolic sustenance to neurons
(12). The myelinating glia of the
CNS and the peripheral nervous system, oligodendrocytes and Schwann
cells, respectively, contribute to the electrical insulation of
axons, thus enabling swift signal transmission (13). Microglial cells are innate immune
cells that reside in the CNS; they dynamically monitor the
microenvironment of the CNS and contribute to the CNS homeostasis
in physiological conditions, and are closely associated with
neuroinflammation in pathological conditions (14).
The pathophysiological process of SCI is quite
complex, involving the dysfunction of neurons and glial cells,
which includes vascular responses, abnormal neuroinflammation,
neuronal loss and demyelination (15,16).
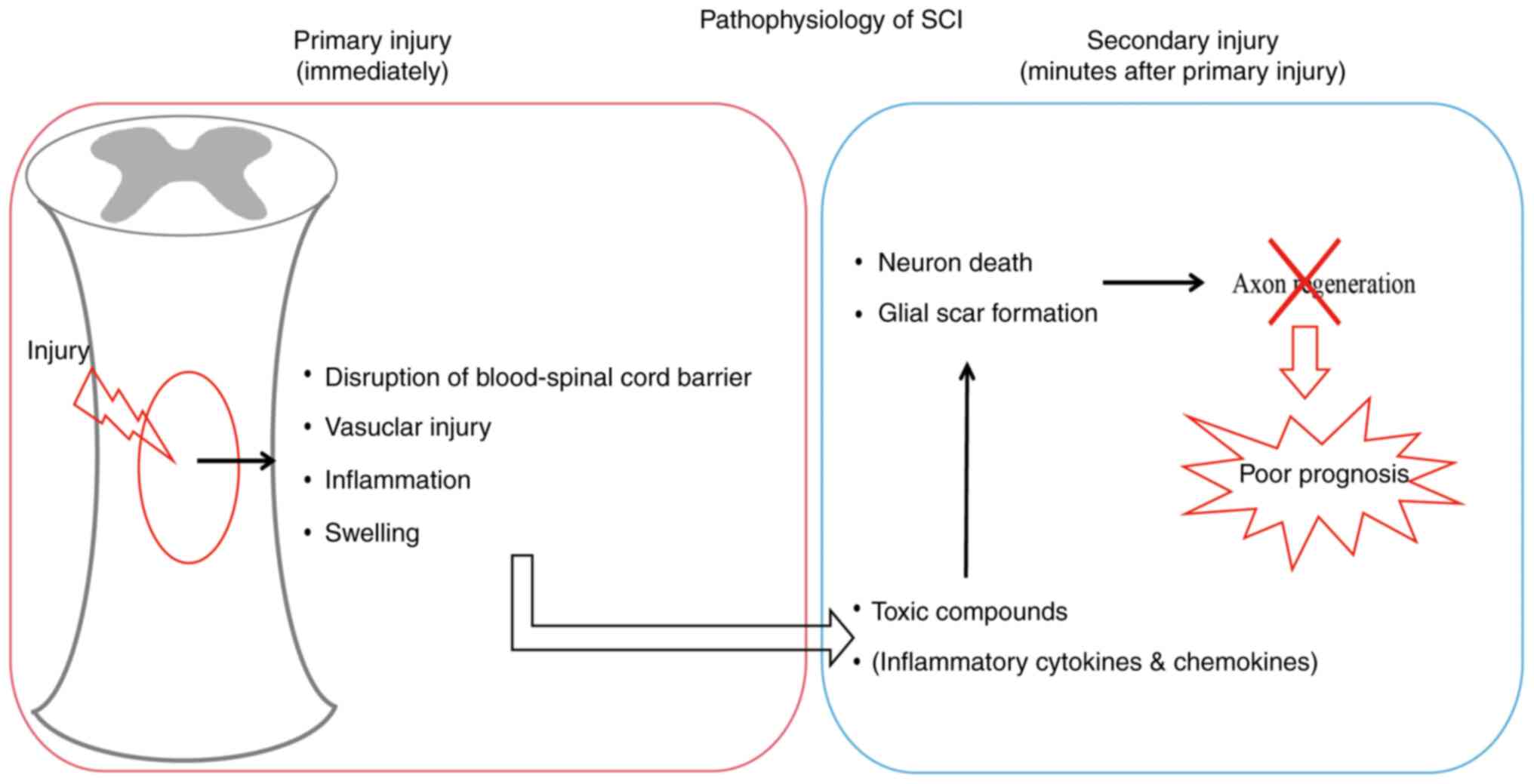
In addition, traumatic SCI can be divided into two phases: i)
Irreversible primary injury, which happens at the moment of injury,
and ii) secondary injury, which occurs within minutes following the
primary injury (17). Spinal cord
compression is the most common pathogenesis of spinal cord injury
and persists after injury (18).
Bleeding can occur in the early stages of an SCI, followed by
disruption of the blood supply. The most common clinical
manifestations immediately following injury are disruption of the
spinal vascular supply and hypotension/hypoperfusion, resulting in
hypovolemia, neurogenic shock and bradycardia due to spinal cord
ischemia (19). Disturbance of
blood flow following SCI leads to hypoxia and ischemic infarction.
Specifically, these two conditions damage the metabolically higher
gray matter; white matter and gray matter metabolism show different
basic properties, but the responses to neuronal activity are
qualitatively similar. The neurons in the damaged area are
physically broken and the thickness of the myelin sheath is reduced
(20). In addition, edema and
macrophage accumulation in the damaged tissue exacerbate the
deterioration of neuronal transmission. Secondary injuries can be
caused by primary injuries and several pathophysiological
mechanisms can come into play hours or days after an SCI occurs
(21,22). Energy deficiency caused by ischemia
and impaired perfusion at the cellular level is the most
influential factor (23). Key
changes have been identified, such as bleeding, demyelination,
edema, cavity formation with axon and neuron necrosis, and a series
of pathological changes such as neuron death and axon breaking in
nerve tissue following SCI can further increase infarction
(24). Following secondary injury,
increased free radical damage and lipid peroxidation in the cell
membrane, as well as secondary injury signal cascade in the damaged
tissue area, can eventually lead to the death of neurons (25). In addition, during the second
injury, released toxic compounds stimulate the differentiation of
neural stem/progenitor cells into astrocytes, leading to reactive
astrogliosis and the transition from the inflammation phase to
glial scar formation (Fig. 1)
(26).
The poor prognosis of SCI may be, in large part, due
to two critical factors, including glial scar formation and
irreversible neuron loss, which work together to interrupt the
neural pathway and lead to the damage of axon regeneration
(27). In patients with spinal
cord injury and in rodent models, obstruction of axon regeneration
and its functional recovery has been shown to permanently inhibit
regeneration of the spinal cord (28). The central idea of alleviating SCI
is preventing, attenuating and reversing secondary injury and
improving spinal cord neurological functions (1). Common treatments used in clinical
practice include traditional drug therapy (1), surgery (29,30),
cell transplantation (31-34),
tissue engineering (35), cell
therapy and nanomedicine (36).
However, these treatments rarely recover SCIs completely and can
only improve symptoms and reduce complications.
Glial cells of the CNS (mainly astrocytes) are
abundant and their roles in sustaining the dynamic balance of the
neuronal microenvironment and controlling blood flow are
fundamental. Preservation of the blood-brain barrier and the
malleability and purpose of the synapses must be regulated
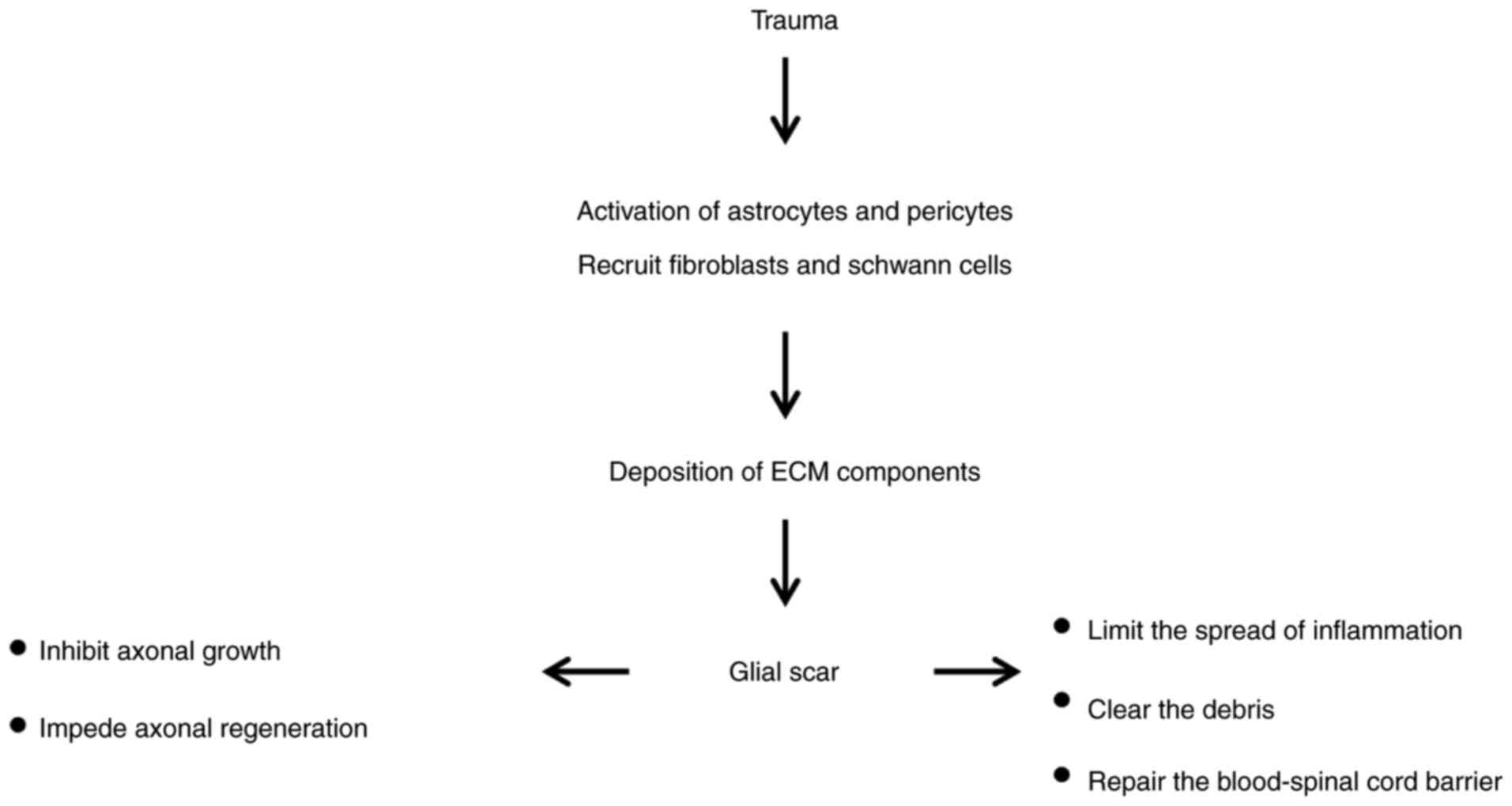
(37). Following SCI, the trauma
activates resident astrocytes and pericytes, and recruits
infiltrating fibroblasts and Schwann cells from the peripheral
nervous system, leading to the formation of glial scars in the
injured spinal cord (38,39). Fibroblasts and Schwann cells
migrate into the epicenter of the lesion and contribute to the
production of extracellular matrix (ECM) proteins, such as nestin,
glial fibrillary acidic protein and proteins transported by the
veins (40,41). The deposition of ECM components and
the accumulation and activation of glial cells contribute to the
formation of a glial scar around the periphery of the lesion. Other
cells such as activated microglia and NG2 glia form a dense
boundary that isolates the damaged area (42). The lesion core includes a mixture
of mononuclear macrophages, activated fibroblasts and ECM proteins
(43,44).
For decades, glial scars have been considered the
main factor against spinal cord regeneration (45). The primary inhibitory ECM molecules
that are produced by reactive astrocytes during glial scar
formation include the chondroitinase enzyme, which acts on
chondroitin. In animal models, treatment with chondroitinase
following SCI exhibited axonal regeneration and functional recovery
(46). In non-mammalian
vertebrates such as zebrafish, a restricted amount of glial
scarring demonstrated the regeneration of the spinal cord,
accompanied by a considerable restoration of motor function
(28,47). However, glial scar formation is
also an essential event during SCI recovery. In the acute phase of
SCI, the formation of a glial scar serves an important role in
restricting the size of the primary injury. The glial scar limits
excessive inflammation from the lesion to normal tissue, clears
debris and repairs the blood-spinal cord barrier, which prevents
the spread of toxic compounds to the surrounding tissue and the
production of neurotrophins (48-50).
In the sub-acute or chronic phase, the glial scar inhibits axonal
regeneration, which has been shown to be harmful to the
regeneration of the spinal cord (45). The dual role of the glial scar
(both harmful and protective) during SCI makes it difficult to
target the glial scar for therapeutic purposes (Fig. 2) (51).
Irreversible neuron loss is another crucial part of
SCI recovery. A combination of multiple causes, such as direct
injury, inflammation, ischemia/reperfusion injury and neurotoxic
cells, can lead to neuron loss (52,53).
The primary sites of active neurogenesis in the adult brain are the
subventricular zone of lateral ventricles and the subgranular zone
of the dentate gyrus, which possess the capacity to generate all
major neuronal phenotypes (54,55).
However, neurons in the spinal cord have low regeneration and
proliferation potential, and the vast majority of the adult spinal
cord is composed of nerve cells, which mainly produce astrocytes
and oligodendrocytes (56).
Microglia are resident macrophages of the CNS and are essential in
the control of damage repair, brain development and the upkeep of
neuronal networks (57). Microglia
activation is strongly associated with delayed neuronal loss in the
peri-infarct area (58,59). Microglia are found only in the
brain, retina and spinal cord (60). They are cells specialized in the
phagocytosis and digestion of extracellular matter, including other
cells. In normal tissues, microglia are highly differentiated, with
elongated processes capable of engulfing smaller objects, such as
synapses and fragments, but not larger objects such as neurons
(61). However, when microglia are
activated by inflammatory stimuli, they increase the expression of
opsonins, lysosomes and phagocytic receptors; in addition, the
microglia process is retracted, thus producing a large moving cell
body capable of phagocytosing neurons (62).
Insufficient neurogenesis in the adult spinal cord
is a key challenge in reconstructing original neuronal networks; as
such, neural repair and neuroregeneration after nerve repair is a
key step in tissue repair following SCI. Various types of
stem/progenitor cell therapy have been shown to have great
development potential (63,64).
Transplantation of cells is considered to be one of the most
promising therapies for neuronal regeneration following SCI; this
process includes direct injection/transplantation of olfactory
ensheathing cells (65),
intramedullary Schwann cell (66),
embryonic (67) and mesenchymal
stem cells (64,68). Although these therapies have
demonstrated good therapeutic effects in several preclinical
studies, some adverse reactions were found during clinical
application. For instance, direct injection of olfactory
ensheathing cells had serious side effects, such as syrinx
formation, myelomalacia and perioperative morbidity, which limited
its clinical application (69); in
addition, intramedullary transplantation of Schwann cells can
induce unsatisfactory motor and functional improvement (66), and the transplantation of embryonic
stem cells also had severe risks such as the formation of teratomas
(67), whereas mesenchymal stem
cell transplantation could induce tumor formation (70,71).
Neuronal reprogramming is a novel technology that can regenerate
functional neurons from glial cells by overexpressing neurogenic
transcription factors (such as NeuroD1) in several
neurodegenerative disorders, including Huntington's and Alzheimer's
diseases (72-75).
Here, an adeno-associated virus is used to overexpress NeuroD1 to
the convert reactive astrocytes into neurons in the dorsal horn of
the injured spinal cord, thus providing a novel possibility for the
treatment of SCI.
GSK-1, GSK-2 and GSK-3 are highly conserved
serine/threonine kinases in the GSK protein family; they were
initially identified as negative regulators of glycogen metabolism
(76). Among them, GSK-3 is the
most studied as it has pivotal roles in numerous cellular
functions, including regulating gene expression, cell survival and
neuronal polarity (77). GSK-3 has
two isoforms, GSK-3α and GSK-3β, and one splice variant (GSK-3β2),
which is expressed specifically in the nervous system (78). These two isoforms share ~95% amino
acid identity, thus, GSK-3α and GSK-3β have unique and overlapping
functions (79). GSK-3 has a large
number of interacting substrates, including CREB (80), the Nfat family of proteins
(81), neurogenin 2(82), SMAD1(83) and β-catenin (84), all of which are part of the cyclic
AMP response element-binding protein family. Among the two
isoforms, GSK-3β may have more predicted substrates than GSK-3α, so
GSK-3β has traditionally received more attention (85).
GSK-3 is mainly localized in the cytoplasm where it
regulates transcription factors by regulating their protein
concentrations, DNA attachment capabilities and/or nuclear
positioning (86). Most kinases
are inactive in resting cells and become active after
phosphorylation. In contrast with other kinases, GSK-3 is highly
active in unstimulated cells and it is rendered inactive after
phosphorylation following stimulation from various sources,
including growth factors (87).
Growth factor-mediated phosphorylation of GSK-3 inhibits its
activation and leads to the activation of its downstream
substrates.
GSK-3 is ubiquitously expressed in the human body,
and its dysfunction has been confirmed in several disorders such as
cancer, cardiovascular diseases, diabetes and inflammatory
conditions. GSK-3 is also expressed in the CNS and participates in
several physiological and pathological functions (88). There is evidence of a close
association between the disruption of GSK-3 signaling and the
emergence of neuroinflammation, neurodegenerative illnesses and
psychiatric disorders. For example, GSK-3 is a key role in the
pathogenesis of Alzheimer's disease, as it participates in the
abnormal phosphorylation of τ protein and the production of
amyloid-β (89-92).
Dysfunction of the GSK-3β signaling pathway has also been
demonstrated in neuropsychiatric disorders, such as schizophrenia
(93). In postmortem tissues of
patients with schizophrenia, GSK-3β mRNA expression was reduced in
the active frontal cortex and dorsolateral prefrontal cortex,
although there was no difference in occipital cortical protein
expression (93,94). GSK-3 also regulates rhythms in
hippocampal clock gene expression and synaptic plasticity (95). During brain development, GSK-3 and
its upstream and downstream regulators serve key roles in the
fundamental processes of neurodevelopment, and the disruption of
GSK-3 signaling is associated with several neurodevelopmental
disorders such as delayed development and intellectual disability
(78).
Along with its role in neurodegenerative and
neurodevelopmental diseases, GSK-3 also serves an important role in
neurogenesis. Behavioral deficits and neuroprogenitor cell
proliferation in schizophrenia are regulated by the GSK-3/β-catenin
signaling pathway (96). The
hippocampal neurons of adults display heightened neurogenesis, as
well as migration, differentiation, proliferation and
neurophenotypic formation, which are linked to the inhibition of
GSK-3 in rats (97). A correlation
between GSK-3 inhibition and an increase in neurogenesis was
established in vitro and in vivo in adult mouse
neural progenitors (97-99).
Neurogenesis in the dentate gyrus of the hippocampus of adult rats
can be induced by the small molecule NP03112 or lithium-induced
inhibition of GSK-3 (97,100). Conditional deletion of GSK-3 in
mouse neural progenitors increases proliferation (101). Considering the close relationship
between GSK-3 and neurogenesis, the role of GSK-3 signaling pathway
in SCI is further discussed below.
SCI decreases the ratio of p-GSK-3β/t-GSK-3β and
increases the number of apoptotic cells in the spinal dorsal horn.
Increasing this ratio may be a useful strategy for reducing
apoptosis and subsequent neuropathic pain associated with SCI
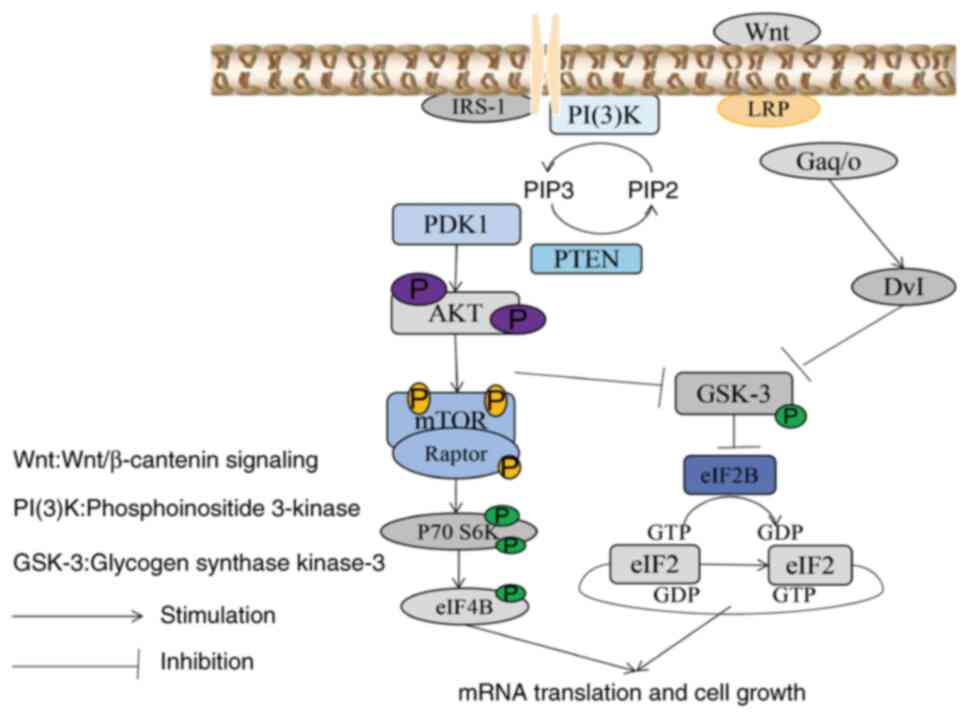
(102). PI3K-mediated activation
of GSK-3β can reduce dorsal root ganglia neurite outgrowth
associated with excitotoxic spinal cord injury dysesthesias
(103). The development of GSK-3
signaling pathway in spinal cord injury is shown in Fig. 3.
The aforementioned hypothesis, that GSK-3 regulates
SCI, can first be demonstrated using GSK-3 inhibitors. The function
of several GSK-3 inhibitors in spinal cord injury has been
extensively studied. For example, GSK-3 inhibitor Ro3303544 was
demonstrated to stimulate neurogenesis in cultured multipotent stem
cells and in SCI rat model (104), as also demonstrated using
4-benzyl-2-methyl-1,2,4-thiadiazolidine-3 (TDZD-8). GSK-3 is most
effectively and precisely inhibited by a 5-dione non-ATP inhibitor.
Treatment with TDZD-8, one of these inhibitors, following SCI could
significantly inhibit neuronal apoptosis and increases the density
of cortical spinal tract fibers around the injured area (105). Combination therapy with TDZD-8
and Y27632 (a Rho-associated coiled-coil kinase 2 inhibitor) could
improve the protective effect on axonal regeneration in a rat SCI
model (106). Lithium, a
traditional inhibitor of GSK-3β, has been extensively utilized in
the treatment of mood disorders, particularly manic depression
(107). Neurotrophic factors,
such as nerve growth factor, neurotrophic factor-3, brain-derived
neurotrophic factor (BDNF) and receptors in the brain are all
involved in the increase in the concentration and amount of lithium
in animals (108). Lithium also
stimulates stem cells proliferation, including neural stem cells in
the subventricular area, striatum, spinal cord and forebrain
(103). Animal models of stroke
and brain injury, as well as Huntington's, Alzheimer's, Parkinson's
and amyotrophic lateral sclerosis diseases, show that lithium
(107) increases the incidence of
these diseases. Li et al (109) showed that in spinal cord neurons,
lithium inhibits GSK-3 activity through two different signaling
pathways; lithium activates phosphorylation of AKT in the acute
phase and upregulates the expression of
Na+/K+-ATPase α1 in the chronic phase
(109). A hypoxic environment is
often generated around the SCI tissue, so that single therapy with
gene or stem cells becomes inefficient. Combination treatment with
the GSK-3 inhibitor, CHIR99021, and a histone deacetylase
inhibitor, such as valproic acid, can significantly boost gene
expression through hypoxia/neuron-inducible gene expression system
and human-induced neural therapy such as additive stimulus
induction. SCI tends to damage nerve tissue and create a hypoxic
environment (110). A previous
study (56) confirmed that gene or
stem cell therapy alone is inefficient, but studies of combination
stem cell and gene therapy to treat tissue damage have begun to
overcome associated limitations, including inefficient gene
delivery and poor treatment effectiveness. Therefore, the
combination of stem cells, gene therapy and hypoxia-specific
systems may contribute to the reconstruction of SCI (104). Endoplasmic reticulum (ER)
stress-induced apoptosis serves an important role in SCI. The
AKT/GSK-3β signaling pathway was demonstrated to be able to reduce
ER stress-induced apoptosis in SH-SY5Y cells when valproate, a
well-known medication for treating epilepsy and mania in clinics,
is administered (111). Table I outlines the dosage and effects of
GSK-3 inhibitors.
Alongside the inhibitors, the therapeutic effects of
several other treatments in SCI that also target GSK-3 signaling
pathways have been investigated. Basic fibroblast growth factor
(bFGF) is a potential neuroprotective factor that can promote
regeneration and repair of SCI, especially in the early stage of
the injury (112-114).
Adrenomedullin (AM) is highly expressed in the spinal cord; it can
increase p-AKT, p-GSK-3β, p-CREB and BDNF expression levels and
promote cAMP accumulation in dorsal root ganglion, which indicates
the possible beneficial role of AM in the protection, survival and
regeneration of sensory neurons during SCI (115). The potential neuroprotective
effects of astaxanthin, a powerful antioxidant and
anti-inflammatory agent, on spinal cord ischemia-reperfusion injury
may be due to activation of the PI3K/Akt/GSK-3β pathway (116), although the mechanism remains to
be elucidated. Loureirin B is a constituent of Traditional Chinese
Medicine that is extracted from Dragon's blood tree and has been
shown to affect insulin secretion stimulation, blood glucose
reduction and immune suppression (117,118). In addition to these functions,
Loureirin B also promotes neuron polarization and axon regeneration
by regulating the Akt/GSK-3β pathway following SCI (119). Analysis of gene expression
profiles can reveal several essential pathways and genes linked to
neuropathic pain in those suffering from spinal cord injury. Among
them, GSK-3β is identified in human umbilical cord-derived
mesenchymal stem cell (HUCMSC) transplantation has been confirmed
to be an effective therapy to alleviate the symptoms of neuropathic
pain and to improve motor recovery following SCI (120). Stable bFGF-overexpressing HUCMSC
transplantation exhibited improved therapeutic outcomes, such as
reduction of glial scar formation, improvement of nerve
regeneration and proliferation of endogenous neural stem cells and
increased locomotion functional recovery of posterior limbs in a
mouse SCI model (121). In
addition, the promotion of the proliferation and neuronal
differentiation of neural stem cells was demonstrated to operate
through the PI3K-Akt-GSK-3β pathway (116). Neuropathic pain is a common
complication following SCI experienced by 75-80% of patients with
SCI (121,122). GSK-3B protein is in the
protein-protein interaction network (123). Furthermore, the signaling
pathways of GSK-3β have been reported to closely participate in
nerve injuries, such as neurodegenerative diseases, inflammation
and neuropathic pain (102).
Therefore, GSK-3 signaling pathways may also participate in the
pathological process of neuropathic pain following SCI.
Intrathecal injection of ghrelin can significantly
suppress the activation of GSK-3β in the spinal dorsal horn and
alleviate neuropathic pain (124). Activation of the GSK-3 signaling
pathway significantly enhances motor function, as well as reducing
SCI-induced allodynia and hyperalgesia when laser treatment and
human adipose-derived stem cell transplantation are combined
(125). Intrathecal injection of
SB216763, a selective GSK-3β inhibitor, has been shown to increase
the level of p-GSK-3β in the dorsal lumbar sections of the spinal
cord and to completely inhibit the tolerance to morphine analgesia
in rats (126).
Neuroinflammation has been identified to be crucial
in the development of neuropathic pain (127). Chemokine CXCL5, which
participates in the inflammatory process of CNS, regulates
neuropathic pain after injury by modulating GSK-3β phosphorylation
and activity in rats (128).
Valproate can inhibit pAKT/pGSK-3β-mediated neuronal death induced
by neuropathic pain (129).
Spinal nerve ligation could induce mechanical allodynia and thermal
hyperalgesia (130). The
administration of GSK-3β selective inhibitor AR-014418 decreased
mechanical allodynia by increasing the p-/t-GSK-3β ratio and
decreasing apoptosis in spinal nerve ligation model rats; however,
it did not affect thermal hyperalgesia (101). However, there are also reports
(98) showing that GSK-3β activity
was enhanced in the hippocampus but reduced in the spinal dorsal
horn following spared nerve injury. Induced neuropathic pain can
cause short-term memory deficits and treatment with selective
GSK-3β inhibitors, such as SB216763 and AR-A014418, can prevent
short-term memory deficits but does not affect neuropathic pain
(131). These discrepancies may
be due to the use of different animal models, although they all
lead to neuropathic pain.
The pathophysiological process of SCI is quite
complex; nonetheless, the poor prognosis of patients with SCI may
mainly be due to glial scar formation and irreversible neuron loss.
Glial scar formation and concomitant inflammatory responses, on the
one hand, inhibit the spread of lesions; on the other hand, they
limit the injury repair. The dual role of glial scars makes it
difficult to be used as a therapeutic target (46). In addition, irreversible neuron
loss is another critical part of SCI recovery. The importance of
neuron loss has led researchers to develop corresponding
treatments; therefore, several stem/progenitor cells therapies have
been developed (57-59).
Unfortunately, only a few therapies reach the clinical trial stage,
and their therapeutic effects are debatable. Exploring the key
mechanism of SCI is crucial for finding improved treatments.
The dysfunction of GSK-3 signaling pathway during
SCI has been widely investigated. SCI decreases the ratio of
p-/t-GSK-3β. Treatment with GSK-3 inhibitors can promote
neurogenesis; in addition, several therapies for the treatment of
SCI also act through GSK-3 signaling pathways. In addition, GSK-3
signaling pathways also participate in the pathological process of
neuropathic pain, which is one of the common complications of SCI.
Based on the current body of evidence, GSK-3 signaling can be
considered a potential therapeutic target for SCI. However, the
data of GSK-3 inhibitors promoting neurogenesis in SCI are mainly
generated from in vitro experiments. The development of
therapies based on GSK-3 still needs further study. Nonetheless,
the present review summarized the participation of GSK-3 signaling
in SCI and may help understand the role GSK-3 signaling during the
pathological processes of SCI.
Not applicable.
Funding: The present study was supported by The Project of
Nantong Municipal Health Commission, Project of Nantong First
People's Hospital (grant nos. MS2022016 and YPYJJZD008),
Postgraduate Research & Practice Innovation Program of Jiangsu
Province (grant no. KYCX21_3107) and Project of Jiangsu
Administration of Traditional Chinese Medicine (grant no.
MS2022090).
Not applicable.
XD and HH were responsible for the literature search
and discussion. ZC made substantial contributions to conception and
design, conducted a thorough review of the manuscript for its
significant intellectual content and gave his approval to the final
version. Data authentication is not applicable. All authors read
and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Ahuja CS, Wilson JR, Nori S, Kotter MRN,
Druschel C, Curt A and Fehlings MG: Traumatic spinal cord injury.
Nat Rev Dis Primers. 3(17018)2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Silva NA, Sousa N, Reis RL and Salgado AJ:
From basics to clinical: A comprehensive review on spinal cord
injury. Prog Neurobiol. 114:25–57. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Vismara I, Papa S, Veneruso V, Mauri E,
Mariani A, De Paola M, Affatato R, Rossetti A, Sponchioni M,
Moscatelli D, et al: Selective modulation of A1 astrocytes by
drug-loaded nano-structured gel in spinal cord injury. ACS Nano.
14:360–371. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Huang X, Gu YK, Cheng XY and Su ZD:
Astrocytes as therapeutic targets after spinal cord injury. Sheng
Li Xue Bao. 69:794–804. 2017.PubMed/NCBI(In Chinese).
|
|
5
|
Erlich S, Alexandrovich A, Shohami E and
Pinkas-Kramarski R: Rapamycin is a neuroprotective treatment for
traumatic brain injury. Neurobiol Dis. 26:86–93. 2007.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wu Q, Li YL, Ning GZ, Feng SQ, Chu TC, Li
Y, Hao Y and Wu QL: Epidemiology of traumatic cervical spinal cord
injury in Tianjin, China. Spinal Cord. 50:740–744. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
McKinley WO, Seel RT and Hardman JT:
Nontraumatic spinal cord injury: Incidence, epidemiology, and
functional outcome. Arch Phys Med Rehabil. 80:619–623.
1999.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lu P, Wang Y, Graham L, McHale K, Gao M,
Wu D, Brock J, Blesch A, Rosenzweig ES, Havton LA, et al:
Long-distance growth and connectivity of neural stem cells after
severe spinal cord injury. Cell. 150:1264–1273. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mar FM, Simões AR, Rodrigo IS and Sousa
MM: Inhibitory injury signaling represses axon regeneration after
dorsal root injury. Mol Neurobiol. 53:4596–4605. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Prinz M and Priller J: The role of
peripheral immune cells in the CNS in steady state and disease. Nat
Neurosci. 20:136–144. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Kim YS, Choi J and Yoon BE: Neuron-glia
interactions in neurodevelopmental disorders. Cells.
9(2176)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Pellerin L, Bouzier-Sore AK, Aubert A,
Serres S, Merle M, Costalat R and Magistretti PJ:
Activity-dependent regulation of energy metabolism by astrocytes:
An update. Glia. 55:1251–1262. 2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Taveggia C: Schwann cells-axon interaction
in myelination. Curr Opin Neurobiol. 39:24–29. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Casano AM and Peri F: Microglia:
Multitasking specialists of the brain. Dev Cell. 32:469–477.
2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Norenberg MD, Smith J and Marcillo A: The
pathology of human spinal cord injury: Defining the problems. J
Neurotrauma. 21:429–440. 2004.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sofroniew MV: Molecular dissection of
reactive astrogliosis and glial scar formation. Trends Neurosci.
32:638–647. 2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Alizadeh A, Dyck SM and Karimi-Abdolrezaee
S: Traumatic spinal cord injury: An overview of pathophysiology,
models and acute injury mechanisms. Front Neurol.
10(282)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wasner G, Naleschinski D and Baron R: A
role for peripheral afferents in the pathophysiology and treatment
of at-level neuropathic pain in spinal cord injury? A case report.
Pain. 131:219–225. 2007.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Anjum A, Yazid MD, Fauzi Daud M, Idris J,
Ng AMH, Selvi Naicker A, Ismail OHR, Athi Kumar RK and Lokanathan
Y: Spinal cord injury: Pathophysiology, multimolecular
interactions, and underlying recovery mechanisms. Int J Mol Sci.
21(7533)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Nickel M and Gu C: Regulation of central
nervous system myelination in higher brain functions. Neural Plast.
2018(6436453)2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Schwartz G and Fehlings MG: Secondary
injury mechanisms of spinal cord trauma: A novel therapeutic
approach for the management of secondary pathophysiology with the
sodium channel blocker riluzole. Prog Brain Res. 137:177–190.
2002.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhang Y, Al Mamun A, Yuan Y, Lu Q, Xiong
J, Yang S, Wu C, Wu Y and Wang J: Acute spinal cord injury:
Pathophysiology and pharmacological intervention (Review). Mol Med
Rep. 23(417)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sharma HS, Patnaik R, Sharma A, Sjöquist
PO and Lafuente JV: Silicon dioxide nanoparticles (SiO2, 40-50 nm)
exacerbate pathophysiology of traumatic spinal cord injury and
deteriorate functional outcome in the rat. An experimental study
using pharmacological and morphological approaches. J Nanosci
Nanotechnol. 9:4970–4980. 2009.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Dimitrijevic MR, Danner SM and Mayr W:
Neurocontrol of movement in humans with spinal cord injury. Artif
Organs. 39:823–833. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhang JX, Wang R, Xi J, Shen L, Zhu AY, Qi
Q, Wang QY, Zhang LJ, Wang FC, Lü HZ and Hu JG: Morroniside
protects SK-N-SH human neuroblastoma cells against H2O2-induced
damage. Int J Mol Med. 39:603–612. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Leal-Filho MB: Spinal cord injury: From
inflammation to glial scar. Surg Neurol Int. 2(112)2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Yang T, Xing L, Yu W, Cai Y, Cui S and
Chen G: Astrocytic reprogramming combined with rehabilitation
strategy improves recovery from spinal cord injury. FASEB J.
34:15504–15515. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lee-Liu D, Edwards-Faret G, Tapia VS and
Larraín J: Spinal cord regeneration: Lessons for mammals from
non-mammalian vertebrates. Genesis. 51:529–544. 2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yılmaz T and Kaptanoğlu E: Current and
future medical therapeutic strategies for the functional repair of
spinal cord injury. World J Orthop. 6:42–55. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ahuja CS, Nori S, Tetreault L, Wilson J,
Kwon B, Harrop J, Choi D and Fehlings MG: Traumatic spinal cord
injury-repair and regeneration. Neurosurgery. 80 (3S):S9–S22.
2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Sabapathy V, Tharion G and Kumar S: Cell
therapy augments functional recovery subsequent to spinal cord
injury under experimental conditions. Stem Cells Int.
2015(132172)2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yousefifard M, Rahimi-Movaghar V,
Nasirinezhad F, Baikpour M, Safari S, Saadat S, Moghadas Jafari A,
Asady H, Razavi Tousi SM and Hosseini M: Neural stem/progenitor
cell transplantation for spinal cord injury treatment; A systematic
review and meta-analysis. Neuroscience. 322:377–397.
2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ide C and Kanekiyo K: Points regarding
cell transplantation for the treatment of spinal cord injury.
Neural Regen Res. 11:1046–1049. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lin XY, Lai BQ, Zeng X, Che MT, Ling EA,
Wu W and Zeng YS: Cell transplantation and neuroengineering
approach for spinal cord injury treatment: A summary of current
laboratory findings and review of literature. Cell Transplant.
25:1425–1438. 2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Dumont CM, Margul DJ and Shea LD: Tissue
engineering approaches to modulate the inflammatory milieu
following spinal cord injury. Cells Tissues Organs. 202:52–66.
2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Raspa A, Pugliese R, Maleki M and Gelain
F: Recent therapeutic approaches for spinal cord injury. Biotechnol
Bioeng. 113:253–259. 2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Gradišnik L, Bošnjak R, Maver T and Velnar
T: Advanced bio-based polymers for astrocyte cell models. Materials
(Basel). 14(3664)2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Orr MB and Gensel JC: Spinal cord injury
scarring and inflammation: Therapies targeting glial and
inflammatory responses. Neurotherapeutics. 15:541–553.
2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Beck KD, Nguyen HX, Galvan MD, Salazar DL,
Woodruff TM and Anderson AJ: Quantitative analysis of cellular
inflammation after traumatic spinal cord injury: Evidence for a
multiphasic inflammatory response in the acute to chronic
environment. Brain. 133:433–447. 2010.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Buss A, Pech K, Kakulas BA, Martin D,
Schoenen J, Noth J and Brook GA: Growth-modulating molecules are
associated with invading Schwann cells and not astrocytes in human
traumatic spinal cord injury. Brain. 130:940–953. 2007.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Zhang SX, Huang F, Gates M and Holmberg
EG: Role of endogenous Schwann cells in tissue repair after spinal
cord injury. Neural Regen Res. 8:177–185. 2013.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Tran AP, Warren PM and Silver J: The
biology of regeneration failure and success after spinal cord
injury. Physiol Rev. 98:881–917. 2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Adams KL and Gallo V: The diversity and
disparity of the glial scar. Nat Neurosci. 21:9–15. 2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Pang QM, Chen SY, Xu QJ, Fu SP, Yang YC,
Zou WH, Zhang M, Liu J, Wan WH, Peng JC and Zhang T:
Neuroinflammation and scarring after spinal cord injury:
Therapeutic roles of MSCs on inflammation and glial scar. Front
Immunol. 12(751021)2021.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Silver J and Miller JH: Regeneration
beyond the glial scar. Nat Rev Neurosci. 5:146–156. 2004.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Bradbury EJ, Moon LD, Popat RJ, King VR,
Bennett GS, Patel PN, Fawcett JW and McMahon SB: Chondroitinase ABC
promotes functional recovery after spinal cord injury. Nature.
416:636–640. 2002.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Diaz Quiroz JF and Echeverri K: Spinal
cord regeneration: Where fish, frogs and salamanders lead the way,
can we follow? Biochem J. 451:353–364. 2013.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Okada S, Nakamura M, Katoh H, Miyao T,
Shimazaki T, Ishii K, Yamane J, Yoshimura A, Iwamoto Y, Toyama Y
and Okano H: Conditional ablation of Stat3 or Socs3 discloses a
dual role for reactive astrocytes after spinal cord injury. Nat
Med. 12:829–834. 2006.PubMed/NCBI View
Article : Google Scholar
|
|
49
|
Herrmann JE, Imura T, Song B, Qi J, Ao Y,
Nguyen TK, Korsak RA, Takeda K, Akira S and Sofroniew MV: STAT3 is
a critical regulator of astrogliosis and scar formation after
spinal cord injury. J Neurosci. 28:7231–7243. 2008.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Sofroniew MV: Astrocyte barriers to
neurotoxic inflammation. Nat Rev Neurosci. 16:249–263.
2015.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Yang T, Dai Y, Chen G and Cui S:
Dissecting the dual role of the glial scar and scar-forming
astrocytes in spinal cord injury. Front Cell Neurosci.
14(78)2020.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Sofroniew MV: Dissecting spinal cord
regeneration. Nature. 557:343–350. 2018.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Wang L, Pei S, Han L, Guo B, Li Y, Duan R,
Yao Y, Xue B, Chen X and Jia Y: Mesenchymal stem cell-derived
exosomes reduce A1 astrocytes via downregulation of phosphorylated
NFκB P65 subunit in spinal cord injury. Cell Physiol Biochem.
50:1535–1559. 2018.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Alvarez-Buylla A and Lim DA: For the long
run: Maintaining germinal niches in the adult brain. Neuron.
41:683–686. 2004.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Ming GL and Song H: Adult neurogenesis in
the mammalian brain: Significant answers and significant questions.
Neuron. 70:687–702. 2011.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Horner PJ, Power AE, Kempermann G, Kuhn
HG, Palmer TD, Winkler J, Thal LJ and Gage FH: Proliferation and
differentiation of progenitor cells throughout the intact adult rat
spinal cord. J Neurosci. 20:2218–2228. 2000.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Borst K, Dumas AA and Prinz M: Microglia:
Immune and non-immune functions. Immunity. 54:2194–2208.
2021.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Park JH, Cho JH, Ahn JH, Choi SY, Lee TK,
Lee JC, Shin BN, Hong S, Jeon YH, Kim YM, et al: Neuronal loss and
gliosis in the rat striatum subjected to 15 and 30 min of middle
cerebral artery occlusion. Metab Brain Dis. 33:775–784.
2018.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Lee Y, Lee SR, Choi SS, Yeo HG, Chang KT
and Lee HJ: Therapeutically targeting neuroinflammation and
microglia after acute ischemic stroke. Biomed Res Int.
2014(297241)2014.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Wolf SA, Boddeke HWGM and Kettenmann H:
Microglia in physiology and disease. Annu Rev Physiol. 79:619–643.
2017.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Savage JC, Carrier M and Tremblay MÈ:
Morphology of microglia across contexts of health and disease.
Methods Mol Biol. 2034:13–26. 2019.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Brown GC: Neuronal loss after stroke due
to microglial phagocytosis of stressed neurons. Int J Mol Sci.
22(13442)2021.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Mothe AJ and Tator CH: Proliferation,
migration, and differentiation of endogenous ependymal region
stem/progenitor cells following minimal spinal cord injury in the
adult rat. Neuroscience. 131:177–187. 2005.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Park JH, Kim DY, Sung IY, Choi GH, Jeon
MH, Kim KK and Jeon SR: Long-term results of spinal cord injury
therapy using mesenchymal stem cells derived from bone marrow in
humans. Neurosurgery. 70:1238–1247. 2012.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Lima C, Escada P, Pratas-Vital J, Branco
C, Arcangeli CA, Lazzeri G, Maia CA, Capucho C, Hasse-Ferreira A
and Peduzzi JD: Olfactory mucosal autografts and rehabilitation for
chronic traumatic spinal cord injury. Neurorehabil Neural Repair.
24:10–22. 2010.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Saberi H, Firouzi M, Habibi Z, Moshayedi
P, Aghayan HR, Arjmand B, Hosseini K, Razavi HE and Yekaninejad MS:
Safety of intramedullary Schwann cell transplantation for
postrehabilitation spinal cord injuries: 2-Year follow-up of 33
cases. J Neurosurg Spine. 15:515–525. 2011.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Ronaghi M, Erceg S, Moreno-Manzano V and
Stojkovic M: Challenges of stem cell therapy for spinal cord
injury: Human embryonic stem cells, endogenous neural stem cells,
or induced pluripotent stem cells? Stem Cells. 28:93–99.
2010.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Osaka M, Honmou O, Murakami T, Nonaka T,
Houkin K, Hamada H and Kocsis JD: Intravenous administration of
mesenchymal stem cells derived from bone marrow after contusive
spinal cord injury improves functional outcome. Brain Res.
1343:226–235. 2010.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Chhabra HS, Lima C, Sachdeva S, Mittal A,
Nigam V, Chaturvedi D, Arora M, Aggarwal A, Kapur R and Khan TAH:
Autologous olfactory [corrected] mucosal transplant in chronic
spinal cord injury: An Indian pilot study. Spinal Cord. 47:887–895.
2009.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Liu C, Chen Z, Chen Z, Zhang T and Lu Y:
Multiple tumor types may originate from bone marrow-derived cells.
Neoplasia. 8:716–724. 2006.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Tolar J, Nauta AJ, Osborn MJ, Panoskaltsis
Mortari A, McElmurry RT, Bell S, Xia L, Zhou N, Riddle M, Schroeder
TM, et al: Sarcoma derived from cultured mesenchymal stem cells.
Stem Cells. 25:371–379. 2007.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Wu Z, Parry M, Hou XY, Liu MH, Wang H,
Cain R, Pei ZF, Chen YC, Guo ZY, Abhijeet S and Chen G: Gene
therapy conversion of striatal astrocytes into GABAergic neurons in
mouse models of Huntington's disease. Nat Commun.
11(1105)2020.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Chen YC, Ma NX, Pei ZF, Wu Z, Do-Monte FH,
Keefe S, Yellin E, Chen MS, Yin JC, Lee G, et al: A NeuroD1
AAV-based gene therapy for functional brain repair after ischemic
injury through in vivo astrocyte-to-neuron conversion. Mol Ther.
28:217–234. 2020.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Li H and Chen G: In vivo reprogramming for
CNS repair: Regenerating neurons from endogenous glial cells.
Neuron. 91:728–738. 2016.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Guo Z, Zhang L, Wu Z, Chen Y, Wang F and
Chen G: In vivo direct reprogramming of reactive glial cells into
functional neurons after brain injury and in an Alzheimer's disease
model. Cell Stem Cell. 14:188–202. 2014.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Hemmings BA, Yellowlees D, Kernohan JC and
Cohen P: Purification of glycogen synthase kinase 3 from rabbit
skeletal muscle. Copurification with the activating factor (FA) of
the (Mg-ATP) dependent protein phosphatase. Eur J Biochem.
119:443–451. 1981.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Jope RS: Lithium and GSK-3: One inhibitor,
two inhibitory actions, multiple outcomes. Trends Pharmacol Sci.
24:441–443. 2003.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Hur EM and Zhou FQ: GSK3 signalling in
neural development. Nat Rev Neurosci. 11:539–551. 2010.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Force T and Woodgett JR: Unique and
overlapping functions of GSK-3 isoforms in cell differentiation and
proliferation and cardiovascular development. J Biol Chem.
284:9643–9647. 2009.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Grimes CA and Jope RS: CREB DNA binding
activity is inhibited by glycogen synthase kinase-3 beta and
facilitated by lithium. J Neurochem. 78:1219–1232. 2001.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Neal JW and Clipstone NA: Glycogen
synthase kinase-3 inhibits the DNA binding activity of NFATc. J
Biol Chem. 276:3666–3673. 2001.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Ma YC, Song MR, Park JP, Henry Ho HY, Hu
L, Kurtev MV, Zieg J, Ma Q, Pfaff SL and Greenberg ME: Regulation
of motor neuron specification by phosphorylation of neurogenin 2.
Neuron. 58:65–77. 2008.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Fuentealba LC, Eivers E, Ikeda A, Hurtado
C, Kuroda H, Pera EM and De Robertis EM: Integrating patterning
signals: Wnt/GSK3 regulates the duration of the BMP/Smad1 signal.
Cell. 131:980–993. 2007.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Kazi A, Xiang S, Yang H, Delitto D,
Trevino J, Jiang RHY, Ayaz M, Lawrence HR, Kennedy P and Sebti SM:
GSK3 suppression upregulates β-catenin and c-Myc to abrogate
KRas-dependent tumors. Nat Commun. 9(5154)2018.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Linding R, Jensen LJ, Ostheimer GJ, van
Vugt MA, Jørgensen C, Miron IM, Diella F, Colwill K, Taylor L,
Elder K, et al: Systematic discovery of in vivo phosphorylation
networks. Cell. 129:1415–1426. 2007.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Beurel E, Grieco SF and Jope RS: Glycogen
synthase kinase-3 (GSK3): Regulation, actions, and diseases.
Pharmacol Ther. 148:114–131. 2015.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Mancinelli R, Carpino G, Petrungaro S,
Mammola CL, Tomaipitinca L, Filippini A, Facchiano A, Ziparo E and
Giampietri C: Multifaceted roles of GSK-3 in cancer and
autophagy-related diseases. Oxid Med Cell Longev.
2017(4629495)2017.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Jaworski T, Banach-Kasper E and Gralec K:
GSK-3 β at the intersection of neuronal plasticity and
neurodegeneration. Neural Plast. 2019(4209475)2019.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Hernandez F, Lucas JJ and Avila J: GSK3
and tau: Two convergence points in Alzheimer's disease. J
Alzheimers Dis. 33 (Suppl 1):S141–S144. 2013.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Albeely AM, Ryan SD and Perreault ML:
Pathogenic feed-forward mechanisms in Alzheimer's and Parkinson's
disease converge on GSK-3. Brain Plast. 4:151–167. 2018.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Manduca JD, Thériault RK and Perreault ML:
Glycogen synthase kinase-3: The missing link to aberrant circuit
function in disorders of cognitive dysfunction? Pharmacol Res.
157(104819)2020.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Wu YY, Wang X, Tan L, Liu D, Liu XH, Wang
Q, Wang JZ and Zhu LQ: Lithium attenuates scopolamine-induced
memory deficits with inhibition of GSK-3β and preservation of
postsynaptic components. J Alzheimers Dis. 37:515–527.
2013.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Kozlovsky N, Belmaker RH and Agam G: Low
GSK-3 activity in frontal cortex of schizophrenic patients.
Schizophr Res. 52:101–105. 2001.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Kozlovsky N, Shanon-Weickert C,
Tomaskovic-Crook E, Kleinman JE, Belmaker RH and Agam G: Reduced
GSK-3beta mRNA levels in postmortem dorsolateral prefrontal cortex
of schizophrenic patients. J Neural Transm (Vienna). 111:1583–1592.
2004.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Besing RC, Rogers CO, Paul JR, Hablitz LM,
Johnson RL, McMahon LL and Gamble KL: GSK3 activity regulates
rhythms in hippocampal clock gene expression and synaptic
plasticity. Hippocampus. 27:890–898. 2017.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Mao Y, Ge X, Frank CL, Madison JM, Koehler
AN, Doud MK, Tassa C, Berry EM, Soda T, Singh KK, et al: Disrupted
in schizophrenia 1 regulates neuronal progenitor proliferation via
modulation of GSK3beta/beta-catenin signaling. Cell. 136:1017–1031.
2009.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Morales-Garcia JA, Luna-Medina R,
Alonso-Gil S, Sanz-Sancristobal M, Palomo V, Gil C, Santos A,
Martinez A and Perez-Castillo A: Glycogen synthase kinase 3
inhibition promotes adult hippocampal neurogenesis in vitro and in
vivo. ACS Chem Neurosci. 3:963–971. 2012.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Lange C, Mix E, Frahm J, Glass A, Müller
J, Schmitt O, Schmöle AC, Klemm K, Ortinau S, Hübner R, et al:
Small molecule GSK-3 inhibitors increase neurogenesis of human
neural progenitor cells. Neurosci Lett. 488:36–40. 2011.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Lie DC, Colamarino SA, Song HJ, Désiré L,
Mira H, Consiglio A, Lein ES, Jessberger S, Lansford H, Dearie AR
and Gage FH: Wnt signalling regulates adult hippocampal
neurogenesis. Nature. 437:1370–1375. 2005.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Wexler EM, Geschwind DH and Palmer TD:
Lithium regulates adult hippocampal progenitor development through
canonical Wnt pathway activation. Mol Psychiatry. 13:285–292.
2008.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Kim WY, Wang X, Wu Y, Doble BW, Patel S,
Woodgett JR and Snider WD: GSK-3 is a master regulator of neural
progenitor homeostasis. Nat Neurosci. 12:1390–1397. 2009.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Rashvand M, Danyali S and Manaheji H: The
potential role of glycogen synthase kinase-3β in neuropathy-induced
apoptosis in spinal cord. Basic Clin Neurosci. 11:15–30.
2020.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Bareiss SK, Dugan E and Brewer KL: PI3K
mediated activation of GSK-3β reduces at-level primary afferent
growth responses associated with excitotoxic spinal cord injury
dysesthesias. Mol Pain. 11(35)2015.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Rodriguez-Jimenez FJ, Vilches A,
Perez-Arago MA, Clemente E, Roman R, Leal J, Castro AA, Fustero S,
Moreno-Manzano V, Jendelova P, et al: Activation of neurogenesis in
multipotent stem cells cultured in vitro and in the spinal cord
tissue after severe injury by inhibition of glycogen synthase
kinase-3. Neurotherapeutics. 18:515–533. 2021.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Lei F, He W, Tian X, Zhou Q, Zheng L, Kang
J, Song Y and Feng D: GSK-3 inhibitor promotes neuronal cell
regeneration and functional recovery in a rat model of spinal cord
injury. Biomed Res Int. 2019(9628065)2019.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Zhang G, Lei F, Zhou Q, Feng D and Bai Y:
Combined application of Rho-ROCKII and GSK-3β inhibitors exerts an
improved protective effect on axonal regeneration in rats with
spinal cord injury. Mol Med Rep. 14:5180–5188. 2016.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Burgess S, Geddes J, Hawton K, Townsend E,
Jamison K and Goodwin G: Lithium for maintenance treatment of mood
disorders. Cochrane Database Syst Rev. (CD003013)2001.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Young W: Review of lithium effects on
brain and blood. Cell Transplant. 18:951–975. 2009.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Li B, Ren J, Yang L, Li X, Sun G and Xia
M: Lithium inhibits GSK3β activity via two different signaling
pathways in neurons after spinal cord injury. Neurochem Res.
43:848–856. 2018.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Pan Z, Oh J, Huang L, Zeng Z, Duan P, Li
Z, Yun Y, Kim J, Ha Y and Cao K: The combination of forskolin and
VPA increases gene expression efficiency to the
hypoxia/neuron-specific system. Ann Transl Med.
8(933)2020.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Li Z, Wu F, Zhang X, Chai Y, Chen D, Yang
Y, Xu K, Yin J, Li R, Shi H, et al: Valproate attenuates
endoplasmic reticulum stress-induced apoptosis in SH-SY5Y cells via
the AKT/GSK3β signaling pathway. Int J Mol Sci.
18(315)2017.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Zhou Y, Wang Z, Li J, Li X and Xiao J:
Fibroblast growth factors in the management of spinal cord injury.
J Cell Mol Med. 22:25–37. 2018.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Rabchevsky AG, Fugaccia I, Turner AF,
Blades DA, Mattson MP and Scheff SW: Basic fibroblast growth factor
(bFGF) enhances functional recovery following severe spinal cord
injury to the rat. Exp Neurol. 164:280–291. 2000.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Rabchevsky AG, Fugaccia I, Fletcher-Turner
A, Blades DA, Mattson MP and Scheff SW: Basic fibroblast growth
factor (bFGF) enhances tissue sparing and functional recovery
following moderate spinal cord injury. J Neurotrauma. 16:817–830.
1999.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Sisakht M, Khoshdel Z, Mahmoodazdeh A,
Shafiee SM and Takhshid MA: Adrenomedullin increases cAMP
accumulation and BDNF expression in rat DRG and spinal motor
neurons. Iran J Basic Med Sci. 24:978–985. 2021.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Fu J, Sun H, Wei H, Dong M, Zhang Y, Xu W,
Fang Y and Zhao J: Astaxanthin alleviates spinal cord
ischemia-reperfusion injury via activation of PI3K/Akt/GSK-3β
pathway in rats. J Orthop Surg Res. 15(275)2020.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Ding Y, Xia S, Fang H, Niu B and Chen Q:
Loureirin B attenuates insulin resistance in HepG2 cells by
regulating gluconeogenesis signaling pathway. Eur J Pharmacol.
910(174481)2021.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Shi S, Zhao Q, Ke C, Long S, Zhang F,
Zhang X, Li Y, Liu X, Hu H and Yin S: Loureirin B exerts its
immunosuppressive effects by inhibiting STIM1/Orai1 and
KV1.3 channels. Front Pharmacol.
12(685092)2021.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Wang Q, Cai H, Hu Z, Wu Y, Guo X, Li J,
Wang H, Liu Y, Liu Y, Xie L, et al: Loureirin B promotes axon
regeneration by inhibiting endoplasmic reticulum stress: Induced
mitochondrial dysfunction and regulating the Akt/GSK-3β pathway
after spinal cord injury. J Neurotrauma. 36:1949–1964.
2019.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Yousefifard M, Nasirinezhad F, Shardi
Manaheji H, Janzadeh A, Hosseini M and Keshavarz M: Human bone
marrow-derived and umbilical cord-derived mesenchymal stem cells
for alleviating neuropathic pain in a spinal cord injury model.
Stem Cell Res Ther. 7(36)2016.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Schieweck R, Schöneweiss EC, Harner M,
Rieger D, Illig C, Saccà B, Popper B and Kiebler MA: Pumilio2
promotes growth of mature neurons. Int J Mol Sci.
22(8998)2021.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Huang F, Gao T, Wang W, Wang L, Xie Y, Tai
C, Liu S, Cui Y and Wang B: Engineered basic fibroblast growth
factor-overexpressing human umbilical cord-derived mesenchymal stem
cells improve the proliferation and neuronal differentiation of
endogenous neural stem cells and functional recovery of spinal cord
injury by activating the PI3K-Akt-GSK-3β signaling pathway. Stem
Cell Res Ther. 12(468)2021.PubMed/NCBI View Article : Google Scholar
|
|
123
|
New PW, Lim TC, Hill ST and Brown DJ: A
survey of pain during rehabilitation after acute spinal cord
injury. Spinal Cord. 35:658–663. 1997.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Störmer S, Gerner HJ, Grüninger W,
Metzmacher K, Föllinger S, Wienke C, Aldinger W, Walker N,
Zimmermann M and Paeslack V: Chronic pain/dysaesthesiae in spinal
cord injury patients: Results of a multicentre study. Spinal Cord.
35:446–455. 1997.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Peng Z, Zha L, Yang M, Li Y, Guo X and
Feng Z: Effects of ghrelin on pGSK-3β and β-catenin expression when
protects against neuropathic pain behavior in rats challenged with
chronic constriction injury. Sci Rep. 9(14664)2019.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Sarveazad A, Janzadeh A, Taheripak G,
Dameni S, Yousefifard M and Nasirinezhad F: Co-administration of
human adipose-derived stem cells and low-level laser to alleviate
neuropathic pain after experimental spinal cord injury. Stem Cell
Res Ther. 10(183)2019.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Parkitna JR, Obara I, Wawrzczak-Bargiela
A, Makuch W, Przewlocka B and Przewlocki R: Effects of glycogen
synthase kinase 3beta and cyclin-dependent kinase 5 inhibitors on
morphine-induced analgesia and tolerance in rats. J Pharmacol Exp
Ther. 319:832–839. 2006.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Wang X, Lin C, Jin S, Wang Y, Peng Y and
Wang X: Cannabidiol alleviates neuroinflammation and attenuates
neuropathic pain via targeting FKBP5. Brain Behav Immun.
111:365–375. 2023.PubMed/NCBI View Article : Google Scholar
|
|
129
|
Xu W, Zhu M, Yuan S and Yu W: Spinal CXCL5
contributes to nerve injury-induced neuropathic pain via modulating
GSK-3β phosphorylation and activity in rats. Neurosci Lett.
634:52–59. 2016.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Chen JY, Chu LW, Cheng KI, Hsieh SL, Juan
YS and Wu BN: Valproate reduces neuroinflammation and neuronal
death in a rat chronic constriction injury model. Sci Rep.
8(16457)2018.PubMed/NCBI View Article : Google Scholar
|
|
131
|
Cheng H, Zhang L, Xia F, Jin L, Liu S, Ren
H, Zhu C, Ji Q and Tang J: Astrocytic NDRG2 is critical in the
maintenance of neuropathic pain. Brain Behav Immun. 89:300–313.
2020.PubMed/NCBI View Article : Google Scholar
|