Introduction
Thoracic aortic dissection (TAD) is a complex
disease that occurs in cardiovascular surgery (1); it is one of the most perilous and
catastrophic aortic syndromes due to its high morbidity rate, acute
onset and rapid progression (2).
TAD is characterized by the tearing of the layers of the arterial
wall, causing blood to flow within the arterial wall and pass into
a ‘false lumen’, forming two lumens inside and outside the vessel,
and producing a dissection in the thoracic aorta (3). The outcomes of surgical repair
following TAD are gradually improving. However, the mortality rates
remains high and thus, early and accurate diagnosis is critical to
determine the appropriate therapy (4).
Vascular smooth muscle cells (SMCs) are the major
cell type within the aortic wall; dysregulation of SMC function
contributes to the development of TAD (5). Under certain stimuli (such as single
gene mutations and abnormal gene expression) (6), SMCs can transcend from a quiescent
and differentiated state to a proliferative, migratory and
remodeling state, which is often characterized by their phenotype
switching from the contractile toward the synthetic state; this is
considered to be a primary driver in the pathogenesis of TAD
(7). In addition, extracellular
matrix (ECM) protein degradation can weaken the aortic wall and
destroy the structural integrity of the aorta, which leaves it
vulnerable to dissection (8).
Matrix metalloproteinases (MMPs) can degrade ECM proteins, playing
an important role in ECM metabolism and aortic tissue remodeling,
which may also be related to TAD progression (9).
Serine peptidase inhibitor Kunitz type 2 (SPINT2),
also known as hepatocyte growth factor activator inhibitor
type-2(10), belongs to the Kunitz
family of serine protease inhibitors. This protein is a potent
inhibitor of hepatocyte growth factor (HGF) activator (HGFA)
(11). HGF, also named scatter
factor (SF), may improve cell viability and invasiveness, stimulate
angiogenesis and function as a tumor progression factor (12). Met tyrosine kinase receptor is the
HGF receptor; the HGF/Met pathway plays a prominent role in cell
migration, survival, growth and cardiovascular remodeling following
tissue injury (13). Notably,
SPINT2 binds to and inactivates HGFA, impairing the conversion of
inactive pro-HGF/SF into bioactive HGF/SF (14). SPINT2 may therefore play an
important role in cancer cell biology by regulating the function of
HGF/SF-activating proteases. Previously, it was reported that
SPINT2 overexpression could suppress pro-HGF activation, ECM
degradation and prostate cancer cell invasion via its inhibitory
effect on the proteolytic activity of these enzymes (15). SPINT2 regulates cell proliferation,
migration and invasion via a reduction in HGFA, leading to
apoptosis and necrosis of uterine leiomyosarcoma cells (16). Moreover, SPINT2 decreases cell
migration and invasion abilities of glioma cells via downregulation
of MMP-2 expression and activity (17) and reduces the levels of vimentin in
endometrial cancer cells (18).
In the present study, dataset GSE52093, including 12
samples (7 TAD and 5 normal ascending tissues) was obtained from
the Gene Expression Omnibus (GEO; https://www.ncbi.nlm.nih.gov/geo/) database and was
analyzed by GEO2R (https://www.ncbi.nlm.nih.gov/geo/geo2r/). It was
observed that SPINT2 expression was downregulated in the arterial
tissues of patients with TAD. Therefore, the present study was
designed to investigate the effects of SPINT2 on TAD progression by
examining the role of SPINT2 in the proliferation, migration and
phenotypic switching of aortic SMCs.
Materials and methods
Tissue specimen collection
Aortic dissection specimens removed from patients
with TAD (n=11) during surgery for TAD were collected. Control
aortic specimens were obtained from patients undergoing coronary
artery bypass grafting (n=4). All specimens were obtained from
January 2022 to June 2022 at the First Affiliated Hospital of Anhui
Medical University (Hefei, China). Patients who underwent computed
tomography scans and ultrasonic examination to confirm the
diagnosis of TAD were enrolled in the present study, whereas
patients with connective tissue defects, such as bicuspid aortic
valve malformation, traumatic aneurysms, luetic aortic aneurysms,
or a family history of aortic diseases were excluded. The specimens
were cleared of adventitia carefully and subsequently used as fresh
samples for western blotting and reverse transcription-quantitative
PCR (RT-qPCR) analyses. And aortic dissection specimens were fixed
with 4% paraformaldehyde for 15 min at room temperature for
immunofluorescence (IF) staining. The present study was performed
in accordance with the Declaration of Helsinki and approved by the
Medical Ethics Committee of the First Affiliated Hospital of Anhui
Medical University (approval no. Quick-PJ 2022-14-49). All
participants signed an informed consent form prior to the present
study.
Primary aortic SMC isolation
Male 6-week-old C57BL/6J mice weighing 18-22 g were
purchased from the Experimental Animal Center of the First
Affiliated Hospital of Anhui Medical University. A total of 8 mice
were used in this experiment. The animals were housed in the
Experimental Animal Center of the First Affiliated Hospital of
Anhui Medical University under controlled conditions (temperature,
22±2˚C; humidity, 40-60%) with a 12/12 h light/dark cycle and were
provided with a normal diet with constant air renewal. The animal
experimental procedures were performed with the approval of The
Experimental Animal Ethics Committee of Anhui Medical University
(approval no. LLSC20220940). The mice were sacrificed by
intraperitoneal injection with sodium pentobarbital (150 mg/kg).
Approximately 5x106 primary aortic SMCs were isolated
from one mouse as described previously (19). Under sterile conditions, the
excised aortic tissues were rinsed with PBS and excess tissues
(such as fascia, all adherent fat and connective tissue) were
removed using a surgical scalpel. The tissue pieces were digested
with collagenase II (Biosharp Life Sciences) for 30 min at 37˚C and
the endothelia were gently removed. Subsequently, the cells were
digested and collected using a mixture of collagenase II and
elastase (Shanghai Aladdin Biochemical Technology Co., Ltd.) for 30
min at 37˚C. The cells were allowed to reach a fusion state, cell
passage was performed and the collected cells were maintained in
smooth muscle cell medium (iCell Bioscience, Inc.) and cultured in
an incubator at 37˚C with 5% CO2. The SMCs used in this
experiment were from the 3rd to 5th generations. SMCs were
identified by IF staining of smooth muscle α-actin (α-SMA) and
smooth muscle protein 22-α (SM22α).
Bioinformatics analysis
The gene expression profile data for ascending aorta
from patients with TAD were downloaded from the GEO database with
the accession number GSE52093. The dataset GSE52093 was based on
the platform GPL10558, including 12 samples (7 TAD and 5 normal
ascending tissues). GEO2R (https://www.ncbi.nlm.nih.gov/geo/geo2r/) and R
software (R Development Core Team, version 4.1.2) were used to
screen the differentially expressed genes (DEGs) between TAD and
normal samples; P<0.05 and |log FC|≥1.5 were used as the
screening thresholds. R packages ‘ggplot2’ and ‘pheatmap’ were
utilized to yield the volcano plot and the heat map, respectively,
for data visualization. The R software ‘cluster Profiler’ package
was used to perform Gene Ontology (GO) functional enrichment, which
was visualized with the chord plot. The Kyoto Encyclopedia of Genes
and Genomes (KEGG) pathway (https://www.genome.jp/kegg/) was analyzed by gene set
enrichment analysis (GSEA) used by R software ‘cluster Profiler’
package.
Cell treatment and adenoviral
infection
The cells were treated with platelet-derived growth
factor BB (PDGF-BB; GenScript) at different concentrations (0, 10,
20 and 40 ng/ml) for 24 h at 37˚C to test SPINT2 expression and SMC
viability. PDGF-BB at 20 ng/ml was selected to induce SMC phenotype
modulation to establish the in vitro cell model. To explore
the function of SPINT2, a 1st generation adenoviral system carrying
the SPINT2 sequence was used to overexpress SPINT2 at a
multiplicity of infection of 100 for 24 h in the isolated SMCs. The
SPINT2 overexpressed sequence was synthesized by General Biological
(Anhui) Co., Ltd. and ligated to the vector RedTrack-CMV
(Fenghuishengwu, Co., Ltd.). The AdMax system was used. 293A cells
(Saibaikang Biotechnology Co., Ltd.; iCell-h086) were used to
package the adenovirus and cultured in DMEM containing 10% FBS at
37˚C in a 5% CO2 incubator. The 293A cells were cultured
in a 10-cm culture dish, with a density of ~60% for transfection.
First, 30 µl transfection reagent Lipofectamine 3000®
(Thermo Fisher Scientific, Inc.) was diluted with 50 µl Opti-MEM
(Thermo Fisher Scientific, Inc.). Next, 20 µg plasmids were diluted
with 30 µl P3000 reagent in 500 µl Opti-MEM. The aforementioned two
solutions were mixed and left at room temperature for 15 min. The
mixed solution was transferred to the culture medium of the 293A
cells, which were mixed and cultured in an incubator with 5%
CO2 at 37˚C. When the plaque was ≥50% (~7 days), the
cells and medium were collected into the centrifuge tube.
To verify the effect of SPINT2 on the activation of
ERK, SMCs infected by adenovirus for 24 h were treated with 100
nmol/l 12-O-tetradecanoylphorbol-13-acetate (TPA; Beijing Solarbio
Science & Technology Co., Ltd.) prior to treatment with 20
ng/ml PDGF-BB treatment.
RT-qPCR
Total RNA was extracted from tissue specimens and
SMCs using the TRIpure reagent (BioTeke Corporation). Subsequently,
cDNA was synthesized using M-murine leukemia virus reverse
transcriptase (Beyotime Institute of Biotechnology) (incubate with
4 µl of RT buffer and 2 µl of dNTP for 50 min at 42˚C, then
terminate the reaction by heating at 80˚C for 10 min). The PCR
amplification was performed using SYBR Green reagent (Beijing
Solarbio Science & Technology Co., Ltd.) on an Exicycler™ 96
Real-Time Quantitative PCR instrument (Bioneer Corporation).
Thermocycling conditions were as follows: Initial denaturation for
5 min at 95˚C, followed by 40 cycles of 10 sec at 95˚C and 10 sec
at 60˚C, and extension at 72˚C for 30 sec. The relative expression
levels of SPINT2 mRNA were analyzed using the 2-ΔΔCq
method (20). The following primer
sequences were used for analysis of the tissue specimens:
SPINT2 forward (F), 5'-AACGCAGCATCCACGACT-3' and reverse
(R), 5'-GGCACATTTCTTGAGGCACT-3'. The following sequences were used
for the analysis of SMCs: SPINT2 F,
5'-GTGAAGGCAATGGCAATAAC-3' and R, 5'-ATAGTACCAGCGAGGGAAGG-3'.
Western blotting analysis
Aorta tissues or SMCs were lysed in RIPA lysis
buffer (Beijing Solarbio Science & Technology Co., Ltd.) and
sonicated for 5 min at 4˚C at 14,000 rpm to extract the total
proteins. The protein concentrations were quantified using the
bicinchoninic protein assay kit (Beijing Solarbio Science &
Technology Co., Ltd.). A total of 20 µg/lane protein samples were
separated by SDS-PAGE (Beijing Solarbio Science & Technology
Co., Ltd.) on 10% gels and subsequently transferred onto
polyvinylidene fluoride membranes (MilliporeSigma) by
electroblotting. The membranes were blocked in 5% (M/V) non-fat
milk powder for 1 h at room temperature and incubated with the
primary antibodies overnight at 4˚C. Subsequently, the horseradish
peroxidase-labeled secondary antibody (1:3,000; cat. no.
SE131/SE134; Beijing Solarbio Science & Technology Co., Ltd.)
was incubated with the membranes at 37˚C for 1 h. The bands on the
membranes were visualized using an enhanced chemiluminescence
reagent (Beijing Solarbio Science & Technology Co., Ltd.). The
gray values of the target bands were analyzed using Tanon-5200
Image Analysis System (Tanon Science and Technology Co., Ltd.). The
primary antibodies included anti-vimentin (1:1,000; cat. no.
AF7013), anti-collagen I (1:1,000; cat. no. AF7001), anti-α-SMA
(1:1,000; cat. no. AF1032), anti-SM22α (1:1,000; cat. no. AF9266),
anti-MMP-9 (1:1,000; cat. no. AF5228), anti-p-ERK (1:500; cat. no.
AF1015) and anti-ERK (1:500; cat. no. AF0155) antibodies (all
Affinity Biosciences). Additionally, anti-MMP-2 (1:2,000; cat. no.
10373-2-AP; Proteintech Group, Inc.) and anti-SPINT2 (1:500; cat.
no. sc-398119; Santa Cruz Biotechnology, Inc.) antibodies were
used, and GAPDH (1:20,000; cat. no. 60004-1-Ig; ProteinTech Group,
Inc.) was the internal control.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
SMCs were seeded into 96-well plates at a density of
4x103 cells/well. Following incubation overnight, SMCs
were treated with different concentrations of PDGF-BB (0, 10, 20
and 40 ng/ml) for 24 h. Subsequently, 10 µl MTT reagent (Beyotime
Institute of Biotechnology) was added into each well and incubated
for an additional 4 h at 37˚C. Dimethyl sulfoxide (100 µl) was
added to each well to terminate the reaction, until it was observed
under an ordinary optical microscope that the formazan was
completely dissolved. Similarly, 20 ng/ml PDGF-BB or 20 ng/ml
PDGF-BB + 100 nmol/l TPA was used to treat SMCs for 0, 24, 48 and
72 h, and MTT reagent was used to detect cell proliferation.
Finally, the optical density values at 570 nm were measured with a
microplate reader (BioTek Instruments, Inc.).
Wound healing assay
When the cells reached 90% confluence, the cell
medium was altered to serum-free medium and 10 µg/ml mitomycin C
(Merck KGaA). SMCs were scratched using 200-µl pipette tips. The
cell surface was washed once with serum-free medium to remove cell
debris. Subsequently, the cells of each group were incubated for 0
and 24 h in an incubator at 37˚C with 5% CO2 and the
images were captured using an inverted microscope (Olympus
Corporation) at a magnification of x100. Finally, the wound healing
distance of SMCs was calculated to measure the cell migratory
ability. Cell migration rate=(0 h scratch width-24 h scratch
width)/0 h scratch width x100.
ELISA
The cell supernatants were centrifuged at 4˚C for 10
min at 300 x g to remove the precipitate for detection. The mouse
MMP-2 ELISA Kit (cat. no. EM0142; Wuhan Fine Biotech, Co., Ltd.)
and the MMP-9 ELISA Kit [cat. no. EK2M09; Multisciences (Lianke)
Biotech Co., Ltd.] were used to evaluate the contents of MMP-2 and
MMP-9, respectively according to the manufacturer's
instructions.
IF staining
The sections (5 µm) of the TAD specimens were
incubated overnight at 4˚C with the following goat anti-mouse
primary antibodies: SPINT2 (1:50; cat. no. sc-398119; Santa Cruz
Biotechnology, Inc.) and α-SMA (1:200; cat. no. AF1032; Affinity
Biosciences). Subsequently, they were incubated with fluorescein
isothiocyanate (FITC)-labeled goat anti-mouse IgG (1:200; cat. no.
ab6785; Abcam) and Cy3-labeled goat anti-rabbit IgG (1:200; cat.
no. ab6939; Abcam) secondary antibodies, respectively, for 90 min
at room temperature. SMCs were fixed in 4% paraformaldehyde for 15
min at room temperature and permeabilized with 0.1% Triton X-100
(Beyotime Institute of Biotechnology). Subsequently, the cells were
blocked in 1% BSA (Sangon Biotech Co., Ltd.) at room temperature
for 15 min and incubated with the following diluted primary
antibodies: α-SMA (1:200; cat. no. AF1032; Affinity Biosciences),
SM22α (1:100; cat. no. AF9266; Affinity Biosciences), Ki-67 (1:100;
cat. no. AF0198; Affinity Biosciences) and vimentin (1:200; cat.
no. AF7013; Affinity Biosciences) overnight at 4˚C. Subsequently,
the samples were incubated with the secondary antibody,
FITC-labeled goat anti-rabbit IgG (1:200; cat. no. ab6717; Abcam),
at room temperature for 60 min. 4',6-diamidino-2-phenylindole
(Shanghai Aladdin Biochemical Technology Co., Ltd.) was used to
counterstain the nuclei at room temperature for 5 min. The images
were observed under a fluorescence microscope (Olympus Corporation)
and imaged at x200 magnification.
Statistical analysis
GraphPad Prism 8 software (GraphPad Software;
Dotmatics) was used for statistical analysis. All experiments were
repeated at least three times. Quantitative values are presented as
the mean ± standard deviation (SD). Comparisons between two groups
were evaluated using unpaired Student's t-test. The comparisons of
statistical significance among multiple groups were assessed using
one-way analysis of variance, with Tukey's multiple comparison test
as the post-hoc analysis. P<0.05 was considered to indicate a
statistically significant difference. All experiments were repeated
at least three times.
Results
SPINT2 expression is downregulated in
TAD, and SMC proliferation, migration and differentiation are
related to TAD progression
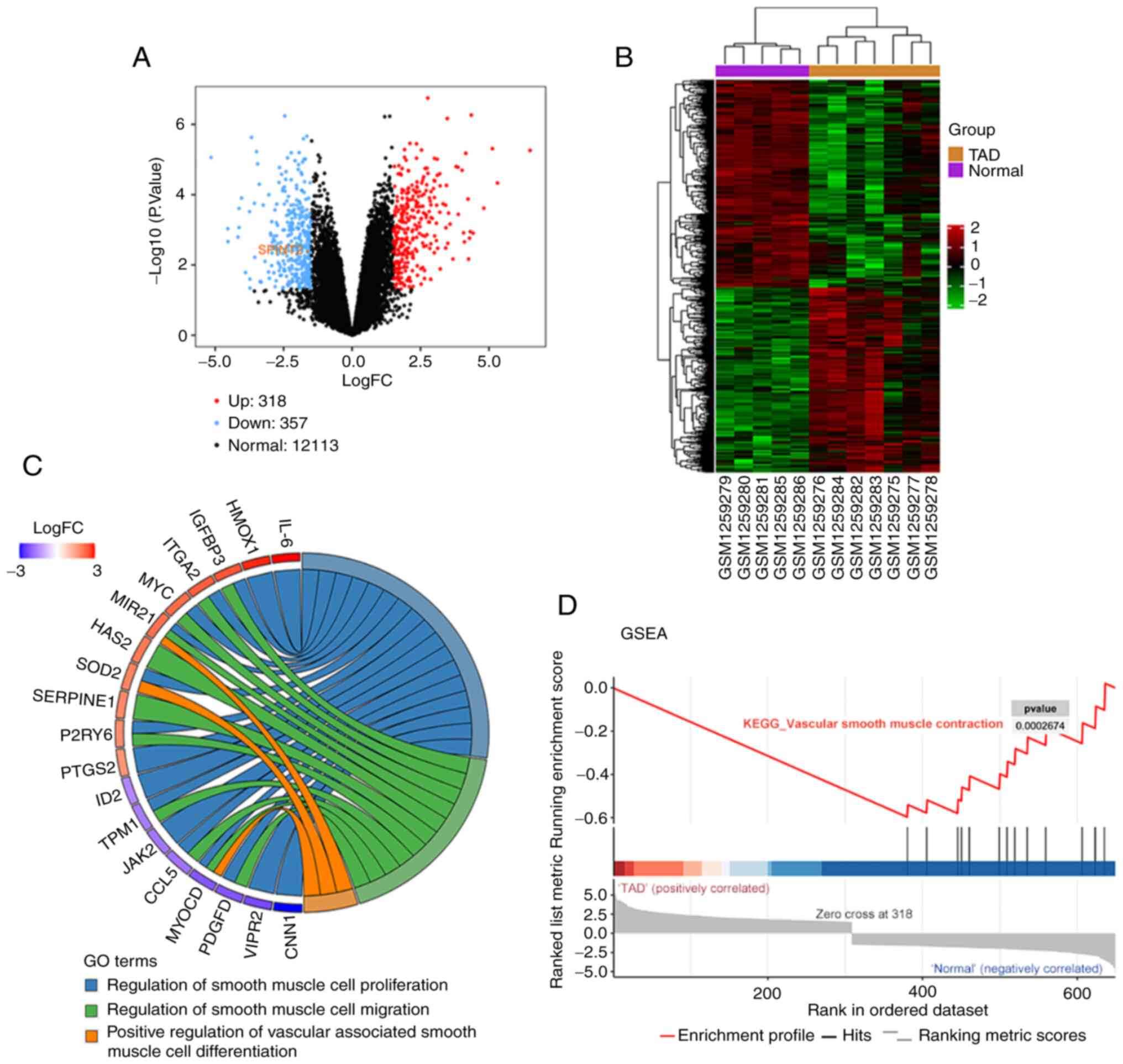
A total of 318 upregulated genes (red dots) and 357
downregulated genes (blue dots) were identified from the GSE52093
dataset in ascending aorta samples from patients with TAD compared
with the tissues derived from patients devoid of TAD. The
differentially expressed SPINT2 was prominently decreased in TAD
(Fig. 1A and B). In the GO chord plot, the top 19 GO
terms of DEGs indicated that several biological processes,
including ‘regulation of smooth muscle cell proliferation’,
‘regulation of smooth muscle cell migration’ and ‘positive
regulation of vascular associated smooth muscle cell
differentiation’ were enriched in TAD (Fig. 1C). The GSEA result also showed the
negative value of the enrichment score, which indicated that TAD
progression was negatively associated with ‘vascular smooth muscle
contraction’ (Fig. 1D).
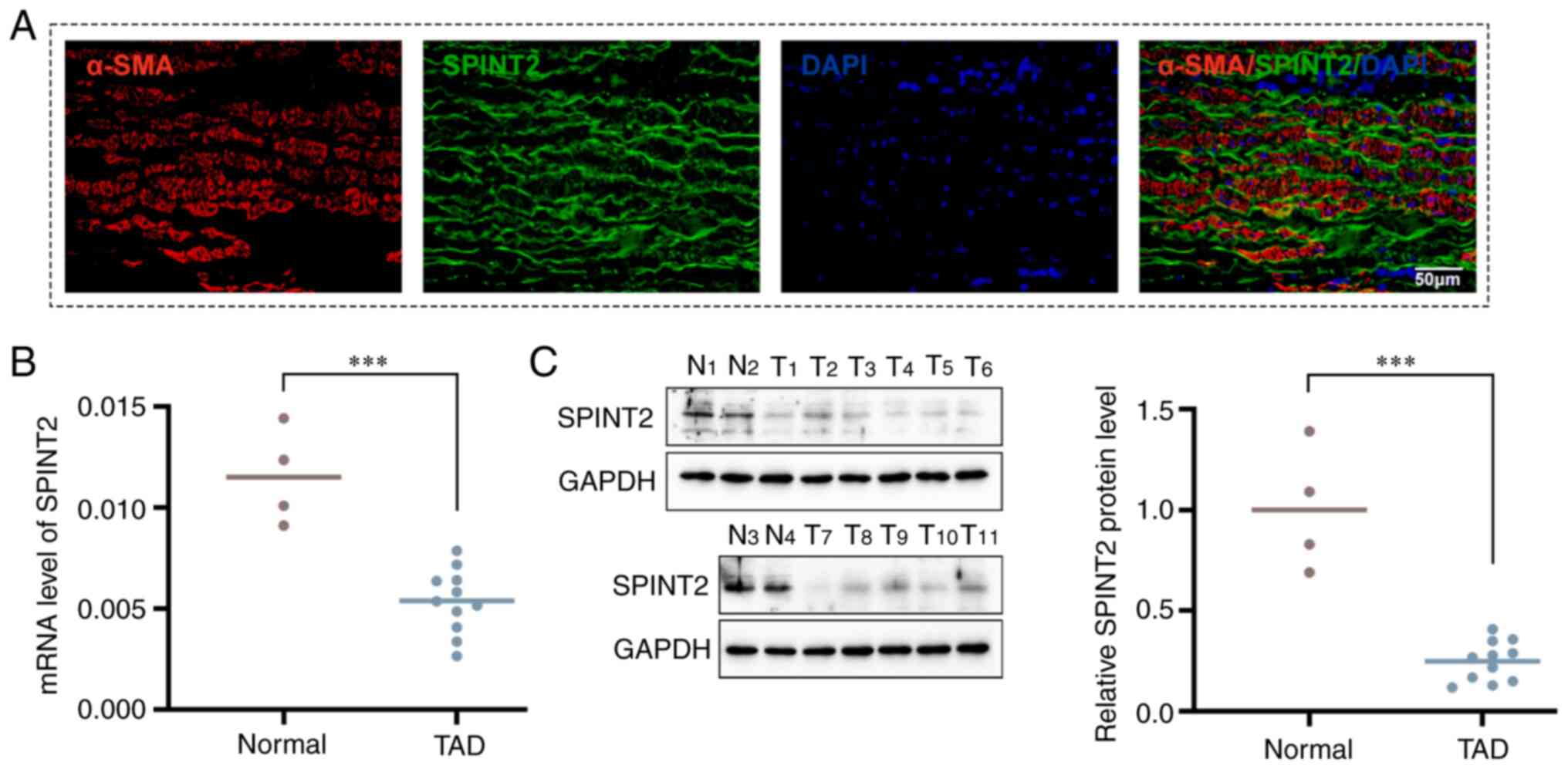
SPINT2 is expressed at low levels in
aorta tissues of TAD specimens
In the present study, the location of SPINT2 was
identified in aortic dissection specimens by co-staining with
α-SMA. It was found that SPINT2 was mainly localized in the aortic
SMCs (Fig. 2A). The expression
levels of SPINT2 mRNA and protein in aortic dissection specimens
and normal aorta tissues were measured using RT-qPCR and western
blotting analyses, respectively. The results further revealed that
SPINT2 expression was significantly lower in TAD tissues than that
noted in normal aorta tissues (P<0.001; Fig. 2B and C).
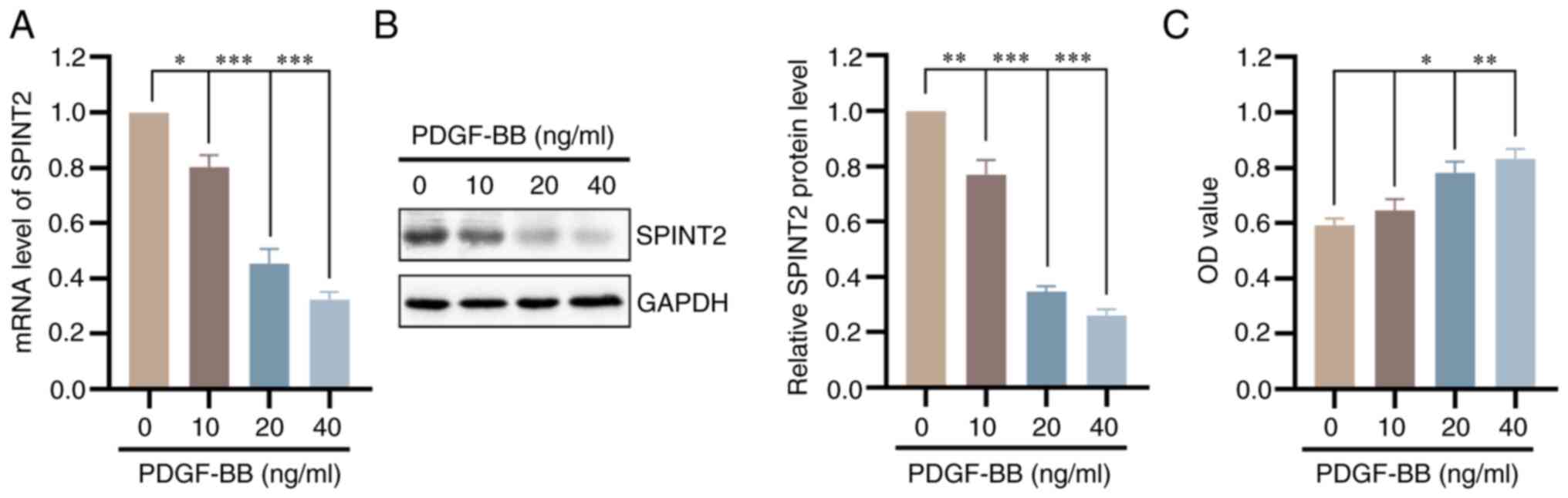
SPINT2 expression is decreased in
PDGF-BB-cultured SMCs
As shown in Fig.
S1, the cells isolated from mice were identified as SMCs by
positive α-SMA and SM22α staining. Subsequently, SMCs were cultured
with different concentrations of PDGF-BB for 24 h and SPINT2
expression was detected. Both the mRNA and protein levels of SPINT2
were significantly decreased following PDGF-BB stimulation in a
concentration-dependent manner (P<0.05; Fig. 3A and B). The MTT assay confirmed that the cell
viability of SMCs was increased in the presence of PDGF-BB
(P<0.05; Fig. 3C).
Overexpression of SPINT2 suppresses
viability and proliferation of PDGF-BB-treated SMCs
Initially, the SMCs were infected with the
adenoviral vector, Ad-SPINT2, to construct SPINT2-overexpressed
cell lines. The mRNA and protein expression levels of SPINT2 were
significantly increased in the Ad-SPINT2 group compared with those
in the control group, which confirmed the successful construction
of the adenoviral model overexpressing SPINT2 (Fig. S2). To explore the effect of SPINT2
on SMC viability and phenotypic switching without PDGF-BB, an MTT
assay was conducted and the expression levels of SMC synthetic
markers (vimentin and collagen I) and contractile markers (α-SMA
and SM22α) were measured using western blotting. As shown in
Fig. S3, SPINT2 overexpression
has no marked effects on SMC viability or phenotypic switching.
After treatment with 20 ng/ml PDGF-BB for 24 h, the expression of
SPINT2 was markedly increased in SMCs infected with an adenovirus
overexpressing SPINT2. The PDGF-BB-induced decrease in SPINT2 was
also reversed following infection of the cells with SPINT2
overexpression adenovirus (P<0.001; Fig. 4A and B). PDGF receptor β (PDGFRβ) protein
levels were determined using western blotting as shown in Fig. S4. PDGFRβ expression was higher in
the presence of PDGF-BB compared with the control group. The
PDGF-BB-induced increase in PDGFRβ was also reversed following
infection of the cells with SPINT2 overexpression adenovirus. To
investigate the roles of SPINT2 in SMC viability, an MTT assay was
conducted, which confirmed that SPINT2 inhibited the high viability
of SMCs in the presence of PDGF-BB (Fig. 4C). SMC proliferation was detected
via the presence of Ki-67-positive cells (IF staining). PDGF-BB
stimulation increased the number of Ki-67-positive cells. However,
SPINT2 overexpression decreased the number of Ki-67-positive cells
(Fig. 4D). The data confirmed the
inhibitory effects of SPINT2 on SMC viability and
proliferation.
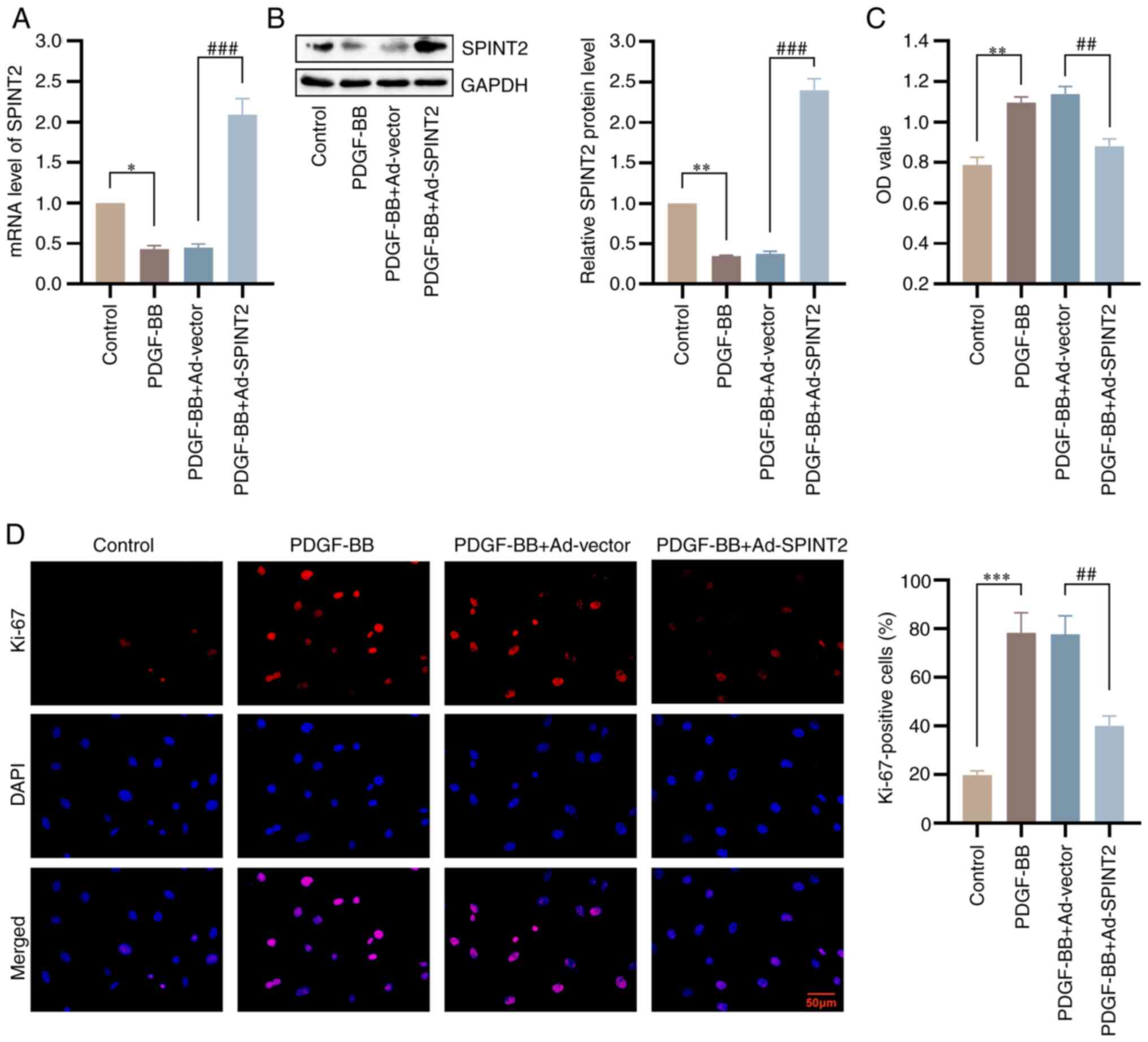 | Figure 4Overexpression of SPINT2 suppresses
viability and proliferation of PDGF-BB-treated SMCs. SMCs were
infected with NC or SPINT2-overexpression adenovirus for 24 h and
subsequently cultured with 20 ng/ml PDGF-BB for 24 h. (A and B) The
mRNA and protein levels of SPINT2 were verified by reverse
transcription-quantitative PCR and western blotting following
PDGF-BB treatment for 24 h. (C) The SMC viability at 48 h following
PDGF-BB treatment was detected using a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay.
(D) The expression levels of Ki-67 were determined by
immunofluorescence staining to evaluate the SMC proliferation
(scale bar, 50 µm). *P<0.05, **P<0.01
and ***P<0.001 compared with control group.
##P<0.01 and ###P<0.001 compared with
the PDGF-BB + Ad-vector group. SPINT2, serine peptidase inhibitor
Kunitz type 2; PDGF-BB, platelet-derived growth factor BB; SMCs,
smooth muscle cells; NC, PDGF-BB + Ad-vector; DAPI,
4',6-diamidino-2-phenylindole; Ad, adenovirus. |
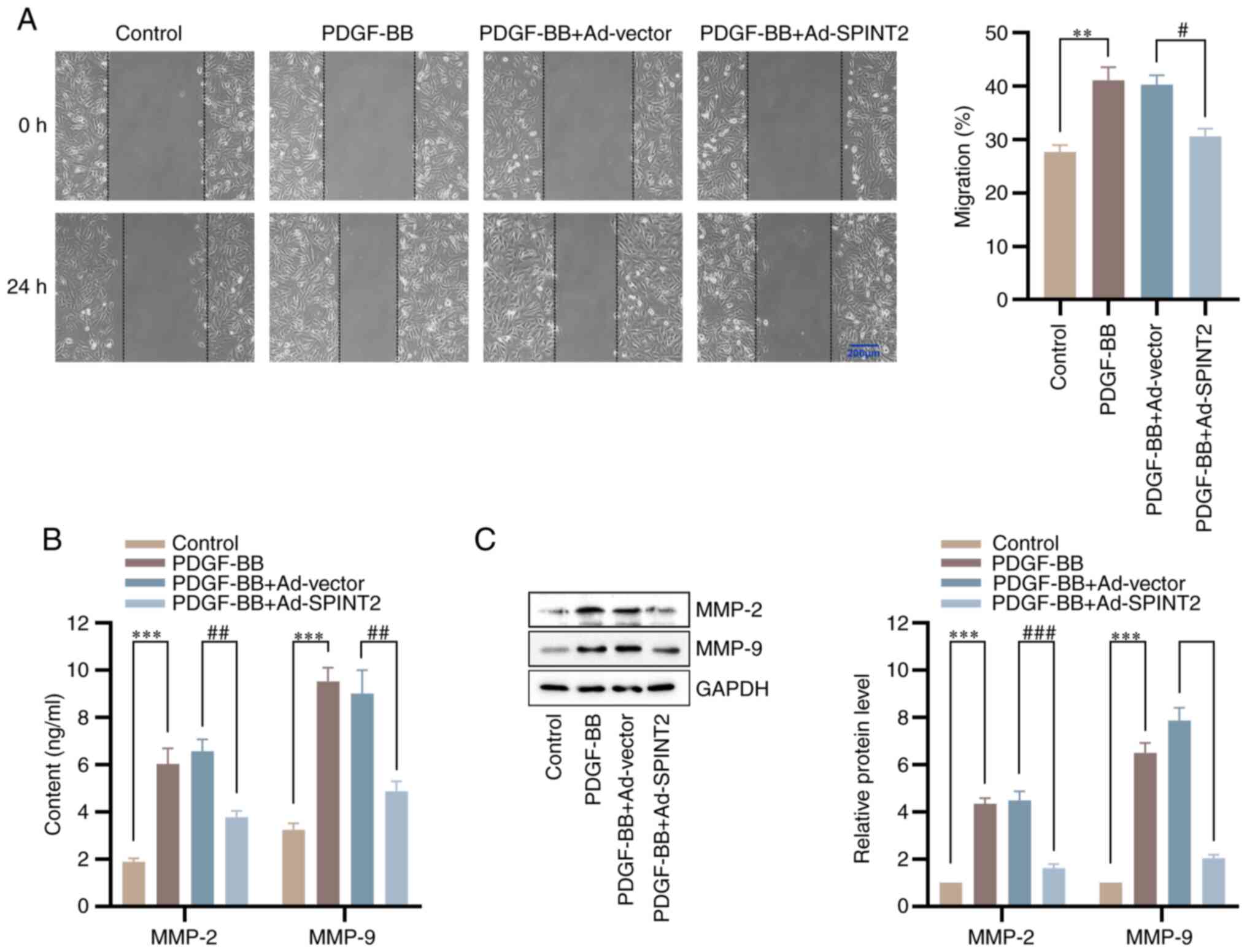
Overexpression of SPINT2 inhibits SMC
migration and expression of MMPs
To explore the effects of SPINT2 on SMC migration, a
wound healing assay was conducted. Following stimulation of the
cells with PDGF-BB for 24 h, the wound healing was increased,
whereas the PDGF-BB-induced SMC migration was reduced by SPINT2
(Fig. 5A). To further determine
the mechanisms of the inhibitory effects of SPINT2 on SMC
migration, ELISA and western blotting assays were used to measure
the content and expression levels of MMP-2 and MMP-9, which are
involved in SMC migration via ECM degradation (21). SPINT2 significantly blocked the
increase in MMP-2 and MMP-9 content and expression in SMCs induced
by PDGF-BB (Fig. 5B and C). These results indicated that SPINT2
exerted inhibitory effects on SMC migration via MMP-2 and
MMP-9.
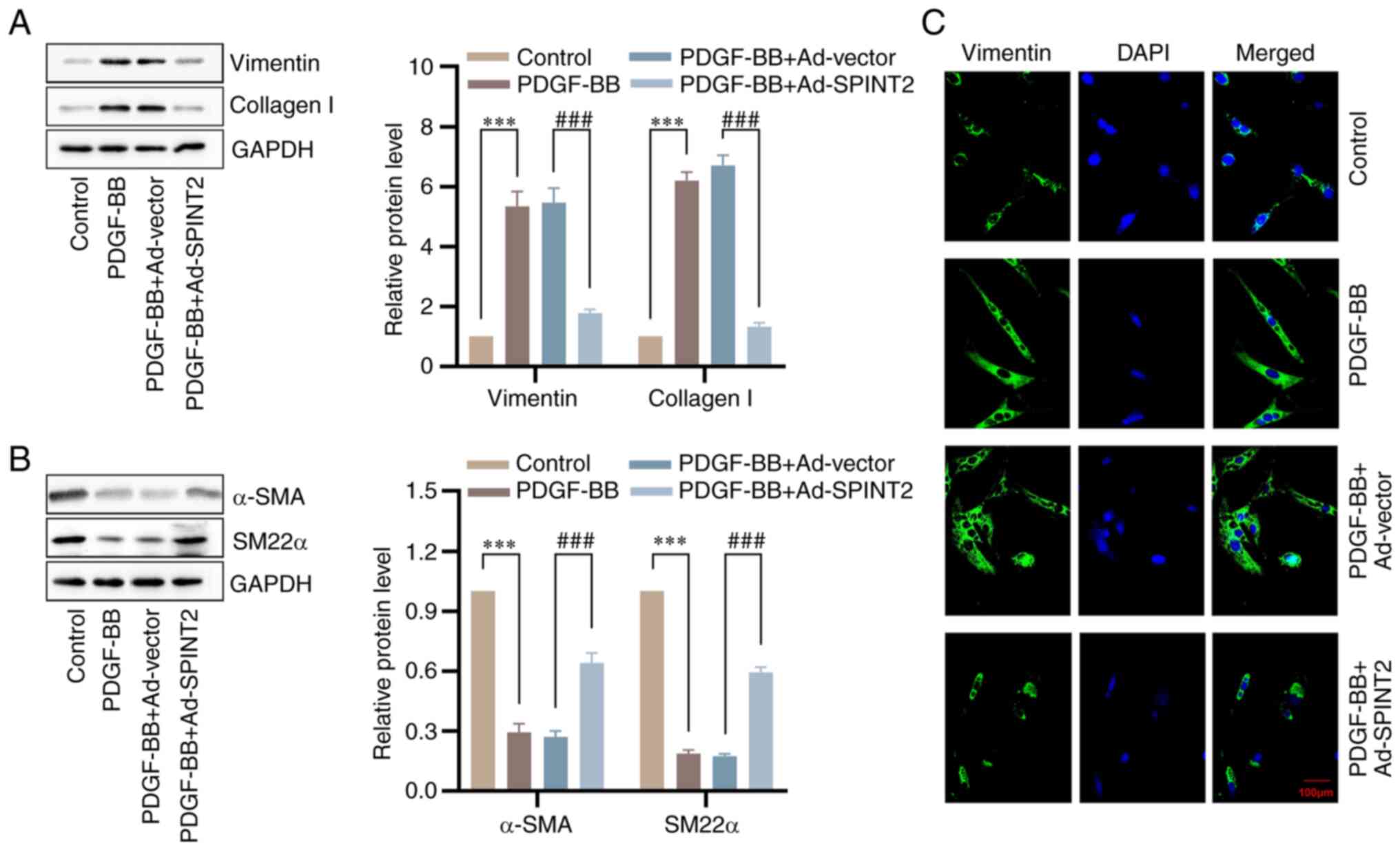
Overexpression of SPINT2 prevents
PDGF-BB-induced SMC phenotypic switching
To confirm the effects of SPINT2 on SMC phenotypic
switching, the expression levels of vimentin, collagen I, α-SMA and
SM22α were measured using western blotting. The results indicated
that PDGF-BB treatment resulted in a significant upregulation in
the protein levels of vimentin and collagen I, and a downregulation
in the protein levels of α-SMA and SM22α. However, the promotional
effects of PDGF-BB on the synthetic markers and the inhibitory
effects on the contractile markers were mitigated by SPINT2
overexpression (Fig. 6A and
B). Meanwhile, the IF assay
demonstrated a similarly altered trend for vimentin in SMCs
(Fig. 6C).
SPINT2 reverses SMC phenotypic
switching induced by the activation of the ERK pathway
To further explore the mechanism by which SPINT2
regulates SMC phenotypic switching, the present study focused on
the effect of SPINT2 overexpression on the activation of ERK.
Western blotting analysis indicated that SPINT2 markedly inhibited
the PDGF-BB-induced increase in p-ERK protein in SMCs, whereas the
expression of ERK was not significantly different before or after
PDGF-BB treatment (Fig. 7A).
Administration of TPA (an ERK agonist) attenuated the
SPINT2-mediated inhibition of viability in PDGF-BB-treated SMCs
(Fig. 7B). In addition, TPA
markedly reversed downregulation of MMP-2 and MMP-9 contents
induced by SPINT2 (Fig. 7C).
Furthermore, TPA significantly blocked the SPINT2-mediated decrease
in the level of vimentin and the increase in the levels of SM22α in
PDGF-BB-treated SMCs (Fig. 7D and
E).
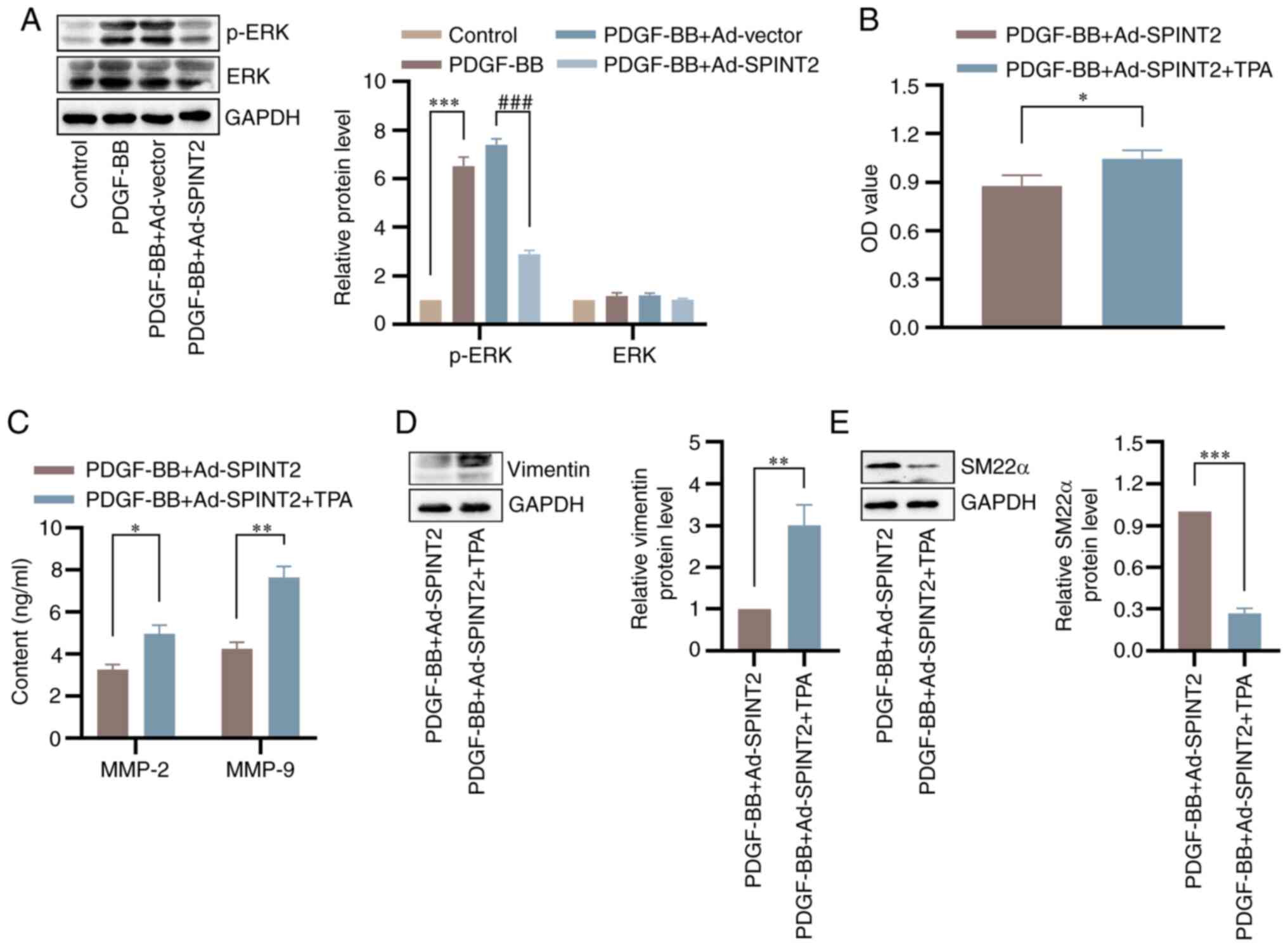 | Figure 7SPINT2 regulates SMC phenotypic
transition via activation of the ERK signaling pathway. (A) SMCs
were incubated with 20 ng/ml PDGF-BB for 24 h following 24 h of
adenoviral infection. The expression levels of p-ERK and ERK in
SMCs were detected by western blotting. ***P<0.001
compared with the control group. ###P<0.001 compared
with the PDGF-BB + Ad-vector group. (B) SMCs were cultured with 100
nmol/l TPA following 24 h of adenoviral infection. SMC
proliferation at 48 h following PDGF-BB treatment was detected
using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide assay. (C) Following 24 h of PDGF-BB treatment, the
expression levels of MMP-2 and MMP-9 were determined using ELISA.
(D and E) The expression levels of vimentin and SM22α proteins were
detected by western blotting. *P<0.05,
**P<0.01 and ***P<0.001 compared with
the PDGF-BB + Ad-SPINT2 group. SPINT2, serine peptidase inhibitor
Kunitz type 2; SMCs, smooth muscle cells; PDGF-BB, platelet-derived
growth factor BB; p, phosphorylated; TPA,
tetradecanoylphorbol-13-acetate; MMP, matrix metalloproteinase;
SM22α, smooth muscle protein 22-α; Ad, adenovirus. |
Discussion
SMCs are the major cellular component of the aortic
wall, and their dysregulation can disturb aortic function and
homeostasis, leading to TAD pathogenesis (22). The results of the present study
revealed a significant decrrease in SPINT2 expression in the aorta
tissues of patients with TAD and in PDGF-BB-induced SMCs,
suggesting that SPINT2 may be related to the development of TAD.
Furthermore, SPINT2 overexpression suppressed PDGF-BB-induced SMC
proliferation, migration and MMP production, and prevented
synthetic phenotype switching of PDGF-BB-induced SMCs via ERK
activation.
The principal function of SMCs in the body is to
regulate blood flow through the contraction and relaxation of the
vessel walls (23). In response to
environmental stress, SMCs undergo phenotype switching from a
differentiated and quiescent ‘contractile’ state to a highly
proliferative and migratory ‘synthetic’ state, which is considered
as a major driver in the pathogenesis of TAD (24). It has been demonstrated that
PDGF-BB is a potent stimulant of SMC proliferation and migration
(25). Both in vitro and
in vivo results indicated that inhibition of SMC
proliferation and migration by downregulation of SPINT2 expression
may suppress vascular-media degeneration and mitigate the loss of
elastic-fiber integrity in TAD (26). However, to the best of our
knowledge the role of SPINT2 in SMC proliferation and migration has
not been investigated to date. It was reported that SPINT2 could
inhibit cell proliferation and migration in diverse cancer cell
lines (27). For example, in a
breast cancer cell line, the elimination of SPINT2 expression
significantly enhanced the proliferative and invasive nature of the
cells (28). Moreover, SPINT2
could inhibit cell proliferation, migration and invasion in
endometrial cancer (EC) cell lines. Therefore, it may be considered
as a therapeutic target and a favorable prognostic marker for EC
(18). Furthermore, SPINT2
suppressed the cellular invasion and metastasis of prostate cancer
via inhibition of transmembrane protease serine 2 expression
(15) and regulation of matriptase
(29). One study verified that
decreased SPINT2 expression was significantly associated with tumor
invasion, metastasis and poor prognosis in patients with non-small
cell lung cancer (30). In lung
cancer cells, SPINT2 repressed cell motility and metastasis as a
novel inhibitor of plasmin (31).
In line with previous reports, the results of the present study
suggested that SPINT2 suppressed PDGF-BB-induced SMC viability and
migration in vitro.
ECM degradation is mainly regarded as a key
pathophysiological feature in TAD formation (32). MMP-2 and MMP-9, which belong to the
MMP family, are important in ECM remodeling (33,34).
In the present study, significantly increased expression levels of
MMP-2 and MMP-9 were noted in SMCs of aorta tissues from patients
with TAD, suggesting that MMP-2 and MMP-9 promote degradation of
proteins associated with fibrosis and causing vulnerability of
aorta via hemodynamic stress, which is involved in TAD formation.
Previous studies verified that PDGF-BB markedly upregulated the
expression levels of MMP-2 and MMP-9 in SMCs (35,36).
By contrast, it was shown that SPINT2 could downregulate MMP-2
expression in high-grade glioma cell lines (17). In the present study, the results
demonstrated that the expression levels of SPINT2 were associated
negatively with those of MMP-2 and MMP-9 in PDGF-BB-induced SMCs,
suggesting that administration of SPINT2 may alleviate
TAD-stimulated ECM remolding and prevent the formation of TAD via
downregulation of MMP-2 and MMP-9 expression in aortic SMCs.
In TAD progression, SMCs usually present with a
prevalent synthetic phenotype as opposed to a contractile
phenotype, causing an impaired contractile function and
endothelial-dependent relaxation of the aortic tissue (37,38).
It was previously reported that SMCs decreased the expression
levels of contractile/differentiated markers, α-SMA and SM22α,
under a synthetic state (39),
along with an increase in the expression levels of the
synthetic/dedifferentiated marker, vimentin (40). PDGF-BB is essential for inducing a
dedifferentiated state of SMCs (41), and the inhibitory effects of SPINT2
on vimentin expression have been verified (18). However, the significance of SPINT2
in the regulation of SMC phenotypic switching requires further
elucidation and thus, the potential effect of SPINT2 on phenotype
switching of SMCs was further studied in vitro in the
present study. Initially, it was found that PDGF-BB promoted SMC
dedifferentiation by conversion from a contractile to a synthetic
state, which was consistent with previous studies (42,43).
Moreover, SPINT2 blocked PDGF-BB-induced SMC dedifferentiation by
increasing the levels of the contractile proteins and decreasing
the levels of the synthetic markers, indicating that it
participated in the maintenance of SMC contractility.
There is extensive crosstalk of the HGF/MET axis
with numerous other signaling pathways, including growth
factor-dependent pathways (such as PI3K/AKT/mTOR and RAS/RAF/ERK)
(44). ERK signaling has been
implicated as a driver of aortic aneurysm pathogenesis by mediating
contractile-to-synthetic phenotype switching of SMCs (45). For example, Liang et al
(46) indicated that berberine
blocked injury-induced SMC regrowth in vitro via
inactivation of the ERK signaling pathway. In addition, IL-11
caused SMC phenotypic switching to a similar extent with TGFβ1 or
angiotensin II stimulation, via activation of ERK signaling, which
played an important role in aortic pathobiology (47). It had been reported that increased
cell motility that was associated with SPINT2 silencing was
abrogated by treatment with ERK/MAPK inhibitors (10). Therefore, ERK as a MET-downstream
signal was investigated in the present study. However, whether ERK
is involved in the regulation of SPINT2 for SMC phenotypic
switching remains unknown. TPA acts as an activator of the ERK/MAPK
signaling pathway. In transgenic (Eisuke) mice expressing ERK
fluorescence resonance energy transfer biosensors, treatment with
TPA resulted in a gradual increase in ERK activity, peaking at ~6 h
(48). In A549 cells, a human lung
cancer cell line, TPA served as a potent ERK activator that was
observed to induce early, intense and relatively transient
phosphorylation of ERK (49). In
mouse dual specificity phosphatase 5(+/+) embryonic fibroblasts,
the application of TPA led to an elevation in ERK expression levels
(50). MMP-9 secretion induced by
PDGF/IL-1 was mediated via the ERK pathway, which was induced by
TPA specifically (51). Therefore,
TPA was selected as an ERK agonist to explore the effects of SPINT2
overexpression on ERK activation in the present study. The results
of the present study demonstrated that the expression of p-ERK was
downregulated by SPINT2. Activation of ERK using TPA partially
abolished the SPINT2-mediated inhibitory effect on SMC
proliferation and dedifferentiation. Therefore, it was suggested
that SPINT2 reversed SMC phenotypic switching induced by activation
of the ERK pathway.
The present study reported that SPINT2 levels were
significantly downregulated in the aorta tissues of patients with
TAD and that SPINT2 overexpression in a mouse vascular SMC line
could inhibit cell proliferation and migration, MMP-2 and MMP-9
expression, and phenotypic switching from a contractile to a
synthetic type. These findings indicate that SPINT2 protects
against TAD development. However, the present study had several
limitations that should be mentioned. Control aortic specimens
obtained from patients devoid of TAD were used only for RT-qPCR and
western blotting. Therefore, SPINT2 expression levels in dissection
vs. control tissues were not compared by IF or IHC images. In
future studies, if suitable samples are encountered, they will be
collected and IF or IHC staining will be used to detect the SPINT2
expression levels in TAD lesions more intuitively. In addition,
conclusions were drawn from cell experiments only, and it is
crucial to verify the role of SPINT2 in TAD formation in animal
experiments. In vivo experiments will be performed in future
studies.
Supplementary Material
Identity of cultured smooth muscle
cell verified by α-SMA and SM22α IF staining. (A) IF staining of
α-SMA. Scale bar, 100 μm. (B) IF staining of SM22α. Scale
bar, 50 μm. DAPI, 4',6-diamidino-2-phenylindole; α-SMA,
smooth muscle α-actin; SM22α, smooth muscle protein 22-α; IF,
immunofluorescence.
Proof of infection success. SPINT2 (A)
mRNA and (B) protein expression levels in adenovirus-infected cells
were detected to confirm transduction efficiency.
***P<0.001. SPINT2, serine peptidase inhibitor Kunitz
type 2.
SPINT2 overexpression has no
significant effects on SMC viability or phenotypic switching. (A)
The SMC viability at 48 h was detected using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay.
(B) The expression levels of the synthetic proteins (vimentin and
collagen I) were detected by western blotting. (C) The expression
levels of the contractile proteins (α-SMA and SM22α) were detected
by western blotting. α-SMA, smooth muscle α-actin; SM22α, smooth
muscle protein 22-α; SPINT2, serine peptidase inhibitor Kunitz type
2; SMC, smooth muscle cell; Ad, adenovirus.
Effects of SPINT2 on the protein level
of PDGFRβ. **P<0.01 vs. control group.
##P<0.01 vs. PDGF-BB + Ad-vector group. PDGF-BB,
platelet-derived growth factor BB; SPINT2, serine peptidase
inhibitor Kunitz type 2; PDGFR β, PDGF receptor β; Ad,
adenovirus.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CY conceived the project, designed the experiments
and revised the paper. JL performed the experiments and wrote the
original paper. KY, ZC, DX, BZ, WX and HO assisted with the
experiments, data analysis and interpretation. All authors read and
approved the final version of the manuscript. CY and JL confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
This study was approved by The Medical Ethics
Committee of the First Affiliated Hospital of Anhui Medical
University (Hefei, China; approval no. Quick-PJ 2022-14-49). The
animal experimental procedures were performed with the approval of
The Experimental Animal Ethics Committee of Anhui Medical
University (Hefei, China; approval no. LLSC20220940). Written
informed consent regarding the use of specimens was obtained from
all patients prior to participation in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Xu Y, Ye J, Wang M, Wang Y, Ji Q, Huang Y,
Zeng T, Wang Z, Ye D, Jiang H, et al: Increased interleukin-11
levels in thoracic aorta and plasma from patients with acute
thoracic aortic dissection. Clin Chim Acta. 481:193–199.
2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
DeMartino RR, Sen I, Huang Y, Bower TC,
Oderich GS, Pochettino A, Greason K, Kalra M, Johnstone J, Shuja F,
et al: Population-based assessment of the incidence of aortic
dissection, intramural hematoma, and penetrating ulcer, and its
associated mortality from 1995 to 2015. Circ Cardiovasc Qual
Outcomes. 11(e004689)2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Doyle BJ and Norman PE: Computational
biomechanics in thoracic aortic dissection: Today's approaches and
Tomorrow's opportunities. Ann Biomed Eng. 44:71–83. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Fukui T: Management of acute aortic
dissection and thoracic aortic rupture. J Intensive Care.
6(15)2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Rombouts KB, van Merrienboer TAR, Ket JCF,
Bogunovic N, van der Velden J and Yeung KK: The role of vascular
smooth muscle cells in the development of aortic aneurysms and
dissections. Eur J Clin Invest. 52(e13697)2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yuan Y, Wang C, Xu J, Tao J, Xu Z and
Huang S: BRG1 overexpression in smooth muscle cells promotes the
development of thoracic aortic dissection. BMC Cardiovasc Disord.
14(144)2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Rodrigues Bento J, Meester J, Luyckx I,
Peeters S, Verstraeten A and Loeys B: The genetics and typical
traits of thoracic aortic aneurysm and dissection. Annu Rev
Genomics Hum Genet. 23:223–253. 2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhang X, Shen YH and LeMaire SA: Thoracic
aortic dissection: Are matrix metalloproteinases involved?
Vascular. 17:147–157. 2009.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Li T, Lv Z, Jing JJ, Yang J and Yuan Y:
Matrix metalloproteinase family polymorphisms and the risk of
aortic aneurysmal diseases: A systematic review and meta-analysis.
Clin Genet. 93:15–32. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Morris MR, Gentle D, Abdulrahman M, Maina
EN, Gupta K, Banks RE, Wiesener MS, Kishida T, Yao M, Teh B, et al:
Tumor suppressor activity and epigenetic inactivation of hepatocyte
growth factor activator inhibitor type 2/SPINT2 in papillary and
clear cell renal cell carcinoma. Cancer Res. 65:4598–4606.
2005.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kawaguchi T, Qin L, Shimomura T, Kondo J,
Matsumoto K, Denda K and Kitamura N: Purification and cloning of
hepatocyte growth factor activator inhibitor type 2, a Kunitz-type
serine protease inhibitor. J Biol Chem. 272:27558–27564.
1997.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Rosen EM, Lamszus K, Laterra J, Polverini
PJ, Rubin JS and Goldberg ID: HGF/SF in angiogenesis. Ciba Found
Symp. 212:215–229. 1997.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Gallo S, Sala V, Gatti S and Crepaldi T:
Cellular and molecular mechanisms of HGF/Met in the cardiovascular
system. Clin Sci (Lond). 129:1173–1193. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kawaguchi M and Kataoka H: Mechanisms of
hepatocyte growth factor activation in cancer tissues. Cancers
(Basel). 6:1890–1904. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ko CJ, Hsu TW, Wu SR, Lan SW, Hsiao TF,
Lin HY, Lin HH, Tu HF, Lee CF, Huang CC, et al: Inhibition of
TMPRSS2 by HAI-2 reduces prostate cancer cell invasion and
metastasis. Oncogene. 39:5950–5963. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Nakamura K, Abarzua F, Hongo A, Kodama J,
Nasu Y, Kumon H and Hiramatsu Y: Hepatocyte growth factor activator
inhibitors (HAI-1 and HAI-2) are potential targets in uterine
leiomyosarcoma. Int J Oncol. 37:605–614. 2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Pereira MS, Celeiro SP, Costa AM, Pinto F,
Popov S, de Almeida GC, Amorim J, Pires MM, Pinheiro C, Lopes JM,
et al: Loss of SPINT2 expression frequently occurs in glioma,
leading to increased growth and invasion via MMP2. Cell Oncol
(Dordr). 43:107–121. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Nakamura K, Hongo A, Kodama J and
Hiramatsu Y: The role of hepatocyte growth factor activator
inhibitor (HAI)-1 and HAI-2 in endometrial cancer. Int J Cancer.
128:2613–2624. 2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Golovina VA and Blaustein MP: Preparation
of primary cultured mesenteric artery smooth muscle cells for
fluorescent imaging and physiological studies. Nat Protoc.
1:2681–2687. 2006.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhu N, Xiang Y, Zhao X, Cai C, Chen H,
Jiang W, Wang Y and Zeng C: Thymoquinone suppresses
platelet-derived growth factor-BB-induced vascular smooth muscle
cell proliferation, migration and neointimal formation. J Cell Mol
Med. 23:8482–8492. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Shen YH, LeMaire SA, Webb NR, Cassis LA,
Daugherty A and Lu HS: Aortic aneurysms and dissections series.
Arterioscler Thromb Vasc Biol. 40:e37–e46. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Liu R, Leslie KL and Martin KA: Epigenetic
regulation of smooth muscle cell plasticity. Biochim Biophys Acta.
1849:448–453. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Grond-Ginsbach C, Pjontek R, Aksay SS,
Hyhlik-Durr A, Bockler D and Gross-Weissmann ML: Spontaneous
arterial dissection: Phenotype and molecular pathogenesis. Cell Mol
Life Sci. 67:1799–1815. 2010.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Bornfeldt KE, Raines EW, Graves LM,
Skinner MP, Krebs EG and Ross R: Platelet-derived growth factor.
Distinct signal transduction pathways associated with migration
versus proliferation. Ann N Y Acad Sci. 766:416–430.
1995.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Sun L, Wang C, Yuan Y, Guo Z, He Y, Ma W
and Zhang J: Downregulation of HDAC1 suppresses media degeneration
by inhibiting the migration and phenotypic switch of aortic
vascular smooth muscle cells in aortic dissection. J Cell Physiol.
235:8747–8756. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Roversi FM, Olalla Saad ST and
Machado-Neto JA: Serine peptidase inhibitor Kunitz type 2 (SPINT2)
in cancer development and progression. Biomed Pharmacother.
101:278–286. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Parr C and Jiang WG: Hepatocyte growth
factor activation inhibitors (HAI-1 and HAI-2) regulate HGF-induced
invasion of human breast cancer cells. Int J Cancer. 119:1176–1183.
2006.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Tsai CH, Teng CH, Tu YT, Cheng TS, Wu SR,
Ko CJ, Shyu HY, Lan SW, Huang HP, Tzeng SF, et al: HAI-2 suppresses
the invasive growth and metastasis of prostate cancer through
regulation of matriptase. Oncogene. 33:4643–4652. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ma Z, Liu D, Li W, Di S, Zhang Z, Zhang J,
Xu L, Guo K, Zhu Y, Han J, et al: STYK1 promotes tumor growth and
metastasis by reducing SPINT2/HAI-2 expression in non-small cell
lung cancer. Cell Death Dis. 10(435)2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wu SR, Lin CH, Shih HP, Ko CJ, Lin HY, Lan
SW, Lin HH, Tu HF, Ho CC, Huang HP and Lee MS: HAI-2 as a novel
inhibitor of plasmin represses lung cancer cell invasion and
metastasis. Br J Cancer. 120:499–511. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Maguire EM, Pearce SWA, Xiao R, Oo AY and
Xiao Q: Matrix metalloproteinase in abdominal aortic aneurysm and
aortic dissection. Pharmaceuticals (Basel). 12(118)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Liu O, Li J, Xin Y, Qin Y, Li H, Gong M,
Liu Y, Wang X, Li J and Zhang H: Association of MMP-2 gene
haplotypes with thoracic aortic dissection in Chinese Han
population. BMC Cardiovasc Disord. 16(11)2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Huang H: Matrix Metalloproteinase-9
(MMP-9) as a cancer biomarker and MMP-9 biosensors: Recent
advances. Sensors (Basel). 18(3249)2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Hsuan CF, Lu YC, Tsai IT, Houng JY, Wang
SW, Chang TH, Chen YL and Chang CC: Glossogyne tenuifolia
Attenuates proliferation and migration of vascular smooth muscle
cells. Molecules. 25(5825)2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Jing Y, Gao B, Han Z, Xia L and Xin S: The
protective effect of HOXA5 on carotid atherosclerosis occurs by
modulating the vascular smooth muscle cell phenotype. Mol Cell
Endocrinol. 534(111366)2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Romaniello F, Mazzaglia D, Pellegrino A,
Grego S, Fiorito R, Ferlosio A, Chiariello L and Orlandi A:
Aortopathy in Marfan syndrome: An update. Cardiovasc Pathol.
23:261–266. 2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Milewicz DM, Trybus KM, Guo DC, Sweeney
HL, Regalado E, Kamm K and Stull JT: Altered smooth muscle cell
force generation as a driver of thoracic aortic aneurysms and
dissections. Arterioscler Thromb Vasc Biol. 37:26–34.
2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zhu SB, Zhu J, Zhou ZZ, Xi EP, Wang RP and
Zhang Y: TGF-β1 induces human aortic vascular smooth muscle cell
phenotype switch through PI3K/AKT/ID2 signaling. Am J Transl Res.
7:2764–2774. 2015.PubMed/NCBI
|
|
40
|
Shi X, Ma W, Pan Y, Li Y, Wang H, Pan S,
Tian Y, Xu C and Li L: MiR-126-5p promotes contractile switching of
aortic smooth muscle cells by targeting VEPH1 and alleviates Ang
II-induced abdominal aortic aneurysm in mice. Lab Invest.
100:1564–1574. 2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Kim S and Kang H: miR-15b induced by
platelet-derived growth factor signaling is required for vascular
smooth muscle cell proliferation. BMB Rep. 46:550–554.
2013.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Shi N, Li CX, Cui XB, Tomarev SI and Chen
SY: Olfactomedin 2 regulates smooth muscle phenotypic modulation
and vascular remodeling through mediating runt-related
transcription factor 2 binding to serum response factor.
Arterioscler Thromb Vasc Biol. 37:446–454. 2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Liu H, Chen H, Deng X, Peng Y, Zeng Q,
Song Z, He W, Zhang L, Xiao T, Gao G and Li B: Knockdown of TRIM28
inhibits PDGF-BB-induced vascular smooth muscle cell proliferation
and migration. Chem Biol Interact. 311(108772)2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Fasolo A, Sessa C, Gianni L and Broggini
M: Seminars in clinical pharmacology: An introduction to MET
inhibitors for the medical oncologist. Ann Oncol. 24:14–20.
2013.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Pedroza AJ, Koyano T, Trojan J, Rubin A,
Palmon I, Jaatinen K, Burdon G, Chang P, Tashima Y, Cui JZ, et al:
Divergent effects of canonical and non-canonical TGF-β signalling
on mixed contractile-synthetic smooth muscle cell phenotype in
human Marfan syndrome aortic root aneurysms. J Cell Mol Med.
24:2369–2383. 2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Liang KW, Ting CT, Yin SC, Chen YT, Lin
SJ, Liao JK and Hsu SL: Berberine suppresses MEK/ERK-dependent
Egr-1 signaling pathway and inhibits vascular smooth muscle cell
regrowth after in vitro mechanical injury. Biochem Pharmacol.
71:806–817. 2006.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Lim WW, Corden B, Ng B, Vanezis K,
D'Agostino G, Widjaja AA, Song WH, Xie C, Su L, Kwek XY, et al:
Interleukin-11 is important for vascular smooth muscle phenotypic
switching and aortic inflammation, fibrosis and remodeling in mouse
models. Sci Rep. 10(17853)2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Hiratsuka T, Fujita Y, Naoki H, Aoki K,
Kamioka Y and Matsuda M: Intercellular propagation of extracellular
signal-regulated kinase activation revealed by in vivo imaging of
mouse skin. Elife. 4(e05178)2015.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Refsnes M, Skuland T, Lag M, Schwarze PE
and Ovrevik J: Differential NF-kappaB and MAPK activation underlies
fluoride- and TPA-mediated CXCL8 (IL-8) induction in lung
epithelial cells. J Inflamm Res. 7:169–185. 2014.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Rushworth LK, Kidger AM, Delavaine L,
Stewart G, van Schelven S, Davidson J, Bryant CJ, Caddye E, East P,
Caunt CJ and Keyse SM: Dual-specificity phosphatase 5 regulates
nuclear ERK activity and suppresses skin cancer by inhibiting
mutant Harvey-Ras (HRasQ61L)-driven SerpinB2 expression. Proc Natl
Acad Sci USA. 111:18267–18272. 2014.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Turner NA, O'Regan DJ, Ball SG and Porter
KE: Simvastatin inhibits MMP-9 secretion from human saphenous vein
smooth muscle cells by inhibiting the RhoA/ROCK pathway and
reducing MMP-9 mRNA levels. FASEB J. 19:804–806. 2005.PubMed/NCBI View Article : Google Scholar
|