Introduction
The urachus, an embryological remnant, is a midline
tubular structure connecting the anterior dome of the bladder and
umbilicus, which is closed and degenerated before birth or in early
infancy to form the median umbilical ligament (1). If the urachus persists after birth,
tumors of various morphologies and biological behaviors may form;
however, non-cystic adenocarcinoma is the most common, being
reported in 83% of urachal epithelial neoplasms (2). Mucinous cystic tumors are rare and
account for 17%, ranging from benign mucinous cystadenoma to low
malignant potential mucinous cystic tumors and aggressive mucinous
cystadenocarcinoma (2,3). Mucinous cystic tumors of low
malignant potential (MCTLMP) have areas of mild epithelial
proliferation (including flat, tufted, pseudopapillary, villous or
tubule villous patterns), mild to moderate atypia, rare mitoses and
stomal invasion. Intraepithelial carcinoma may arise from MCTLMP
and is characterized by marked epithelial stratification, severe
cellular atypia and abundant mitosis. MCTLMP is rare and only ~40
cases have been reported in the literature (4-10).
MCTLMP not only has the potential for invasion and malignancy but
can also lead to complications, such as pseudomyxoma peritonei
(PMP). PMP is a rare clinical disease defined by an extensive
intraperitoneal spread of mucus associated with a variety of
mucinous tumors of different biological behaviors (11). The primary site of PMP in the
majority of cases is the appendix, but other sites include the
ovary, small bowel, stomach and pancreas. Its incidence is
low(occurring in ~1-2 individuals per million), and disease
progression is slow (12).
Although PMP growth tends to remain confined to the abdomen for
many years and hematogenous lymph node metastasis is uncommon, PMP
demonstrates a tendency for local recurrence, often leading to
multiple operations (11-14).
The urinary tract can also be an uncommon origin of PMP. PMP caused
by mucinous cystadenocarcinoma has been reported previously
(14,15). Urachal MCTLMP can also cause PMP in
cases of tumor rupture and although rare, it should not be ignored.
Currently, only 3 cases of MCTLMP combined with PMP have been
reported (15-17),
and the diagnosis and treatment of this tumor is limited. The aim
of the present case report was to examine a fourth case of MCTLMP
with extensive PMP, analyze its clinicopathological features,
biological behavior, differential diagnosis and prognosis, and
review the literature to enhance the understanding of this
tumor.
Case report
A 74-year-old male patient visited the Department of
Emergency Surgery of Xiaoshan Affiliated Hospital of Wenzhou
Medical University (Hangzhou, China) on 24th June 2022, because of
right lower abdominal pain. The patient had persistent distension
pain in the right lower abdomen 1 day prior to paroxysmal
aggravation. An abdominal computed tomography (CT) scan indicated
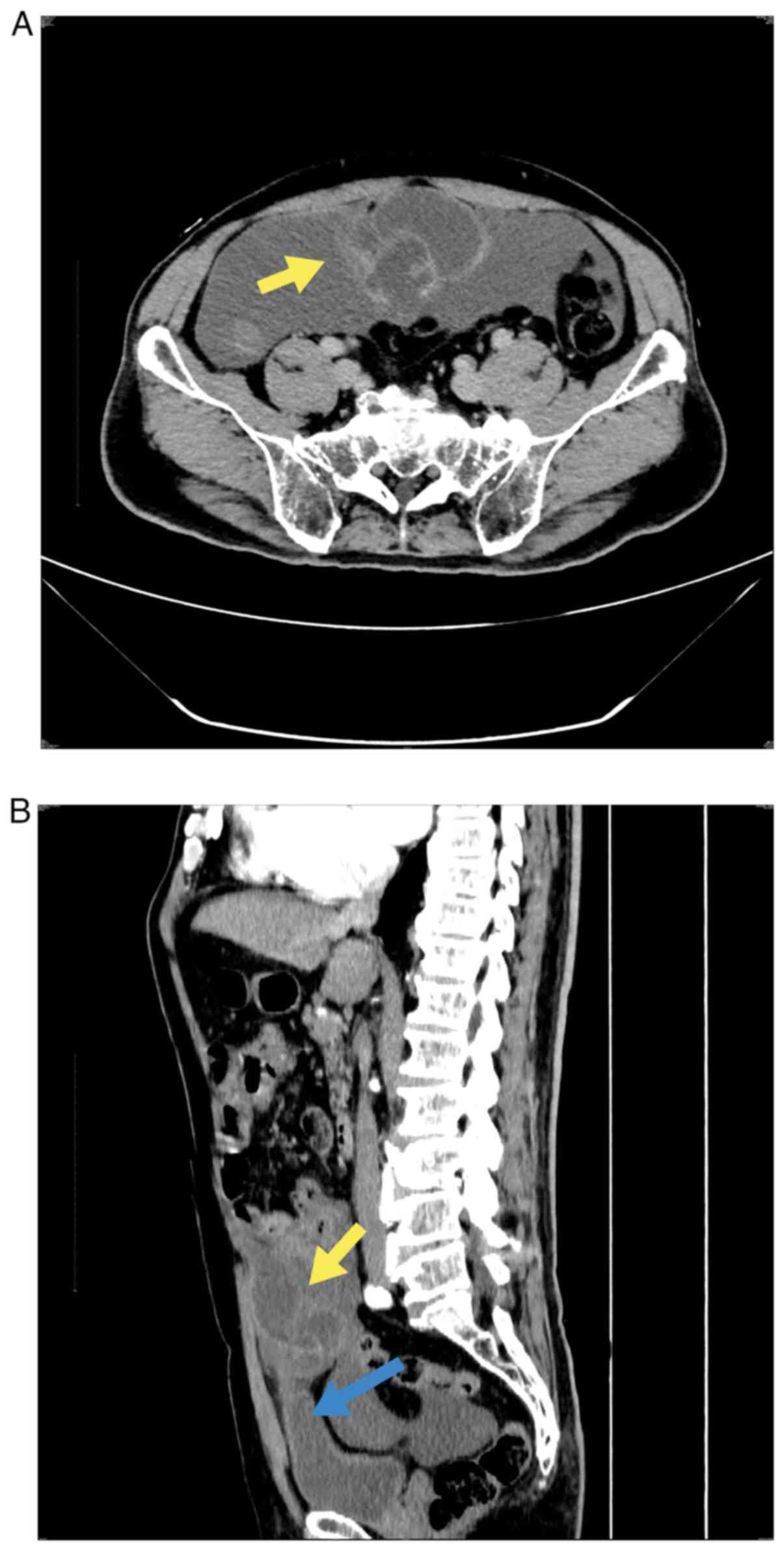
multilocular cystic lesions around the urachal duct (Fig. 1A) at the anterior upper margin of
the bladder (Fig. 1B). The wall of
the capsule was significantly enhanced, accompanied by ascites and
extensive exudation in the abdomen and pelvic cavity. Malignant
urachus tumors with peripheral dissemination were also considered.
A physical examination of the abdomen and routine blood (white
blood cell series, red blood cell series and platelet series) and
biochemical tests (blood glucose, lipids and transaminases) all
revealed no abnormalities. Hematologic tumor markers demonstrated
that carbohydrate antigen 19-9 (CA19-9) was normal, while
carcinoembryonic antigen (CEA) was increased at 13.95 µg/l
(reference value, 0-5 µg/l) and carbohydrate antigen 125 (CA125)
was increased at 41.3 kU/l (reference value, <35.0 kU/l). The
medical history of the patient revealed that the patient had
undergone lithotripsy for left ureteral calculi in 2017 and 2019. A
preoperative CT scan of the urinary system revealed pelvic cystic
lesions in both cases. A urachal cyst with calculi was considered
but was not treated, because the patient had no discomfort. In
addition, the patient had hypertension and diabetes for >10
years, but the blood pressure and sugar levels of the patient were
well controlled. Exploratory laparotomy was performed by a
urological surgeon on 5th July 2022. During surgery, a mass with a
diameter of ~10 cm was revealed in the urachus, which adhered to
the peritoneum below the umbilical tract and was connected to the
top of the bladder below the mass. There were two small lacerations
~1 cm long, and a large amount of yellow jelly-like mucus was
observed leaking out of the mass; the majority of the intestine was
coated in the mucus. Rapid pathological examination of the excised
mass during the operation suggested a mucinous tumor. Mucinous
adenocarcinoma could not be excluded because of the formation of a
large number of mucous lakes and the presence of cell atypia
(although only mild). Therefore, radical resection of the urachal
tumor and release of the intestinal adhesions were performed.
During the operation, the mass, adhesion peritoneum and part of the
bladder wall, ~1 cm from the mass, were completely removed. The
appendix was normal in size and shape. After the bladder was rinsed
with normal saline, the incision was sutured, and the abdominal
cavity and intestines were rinsed with a large amount of sterilized
water by injection to remove the jelly-like mucus.
A piece of gray and red nodular tissue from the
resected tumor was examined. Sectioning of the tissue revealed a
multilocular cyst, and the local cyst wall was broken and
indistinct from the surrounding tissue. The cyst measured ~8x6x6
cm, and a jelly-like substance was detected in the cyst.
The tissue was fixed with 4% neutral formalin (24 h
at 25˚C) and embedded in paraffin, and 4-µm serial sections were
prepared that were subjected to hematoxylin and eosin staining (8 h
at 25˚C). The sections were reviewed using a light microscope.
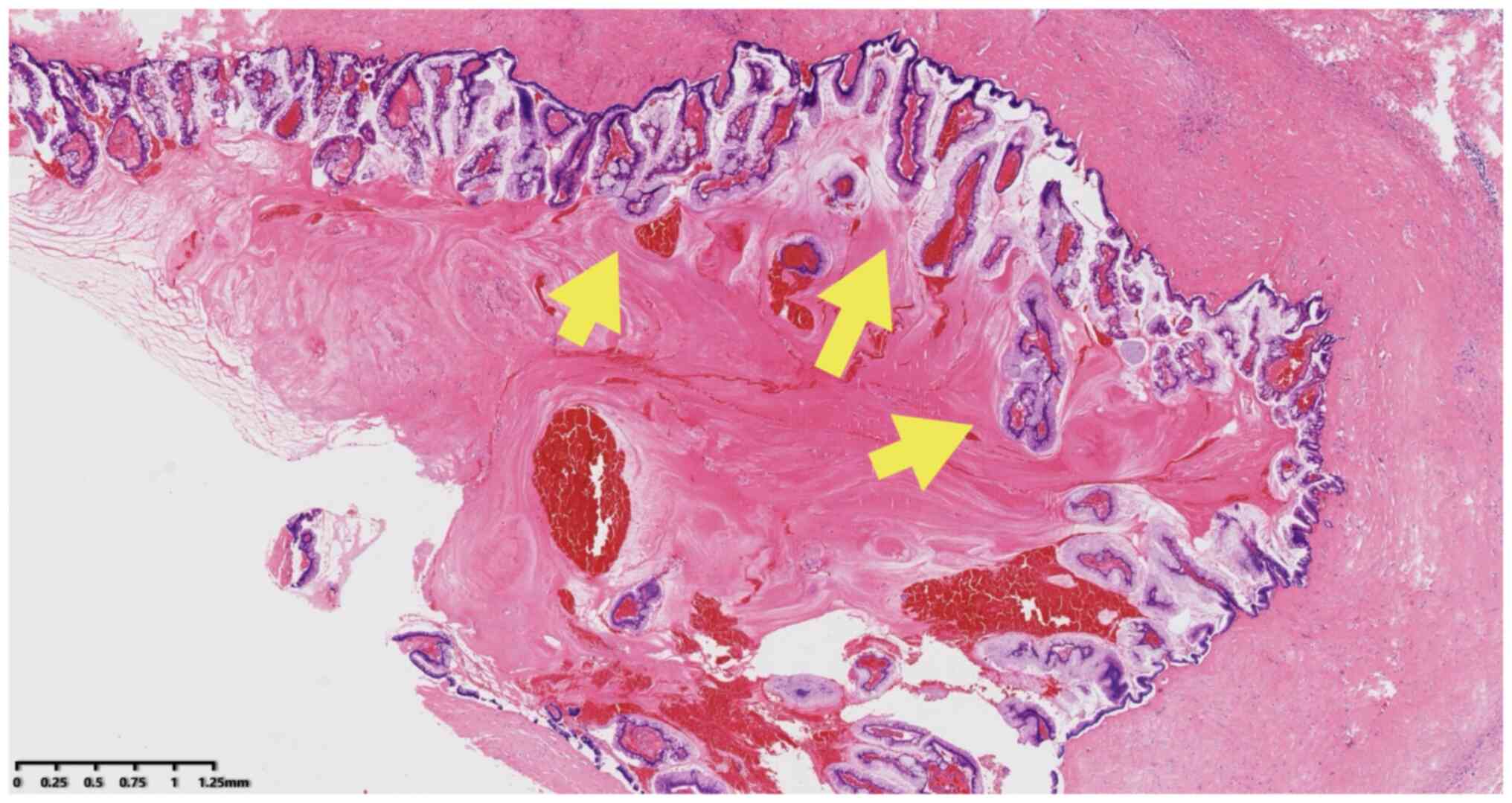
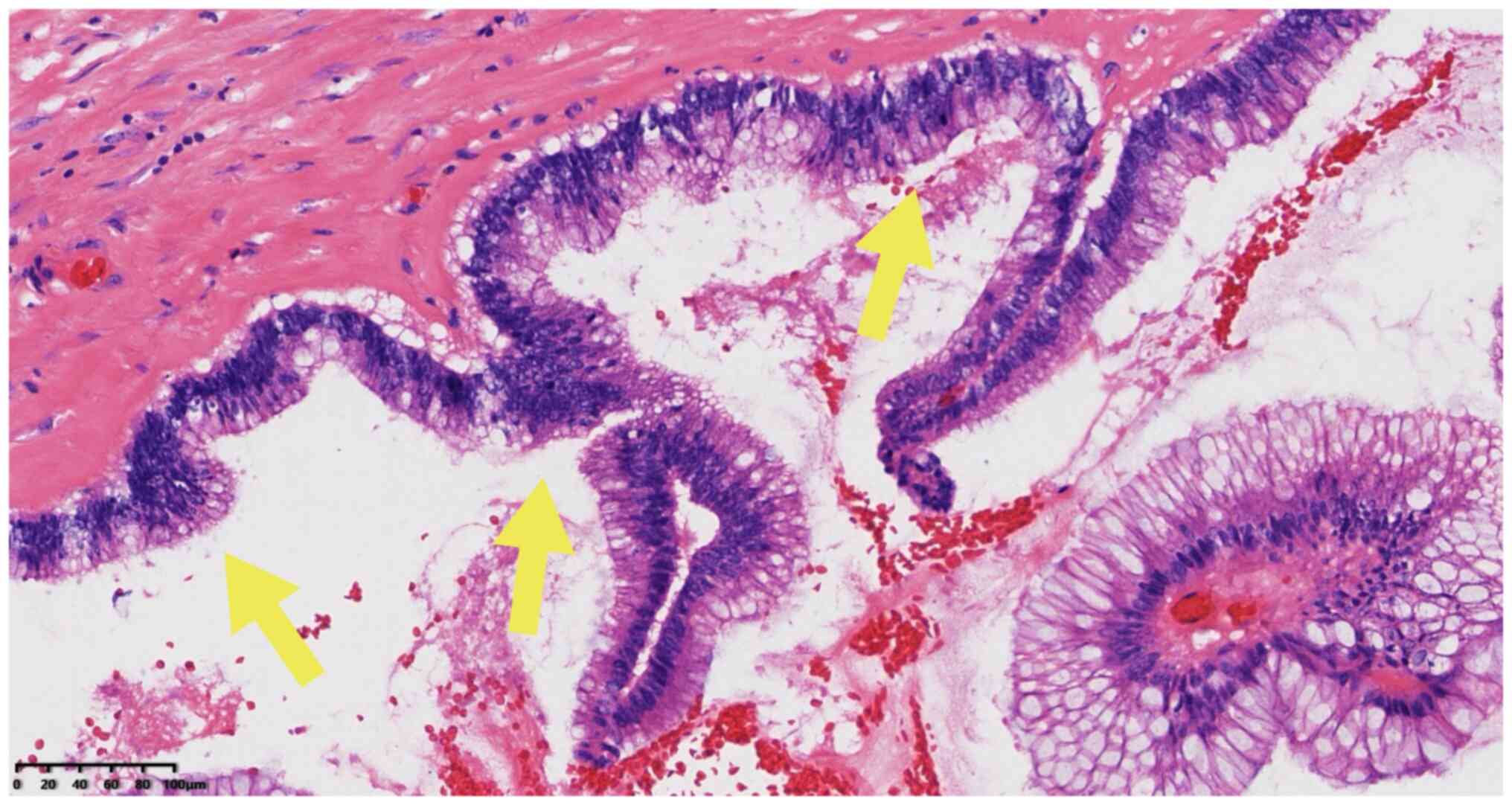
Microscopically, cystic lesions lined with mucous columnar
epithelial cells were observed with mucous secretions; a number of
epithelia indicated pseudo-lamellar hyperplasia, while other areas
indicated papillary structure, mild cell atypia, rare mitosis and
cell morphology that was consistent with low-grade intraepithelial
neoplasia (Figs. 2 and 3). Stromal infiltration of tumor cells
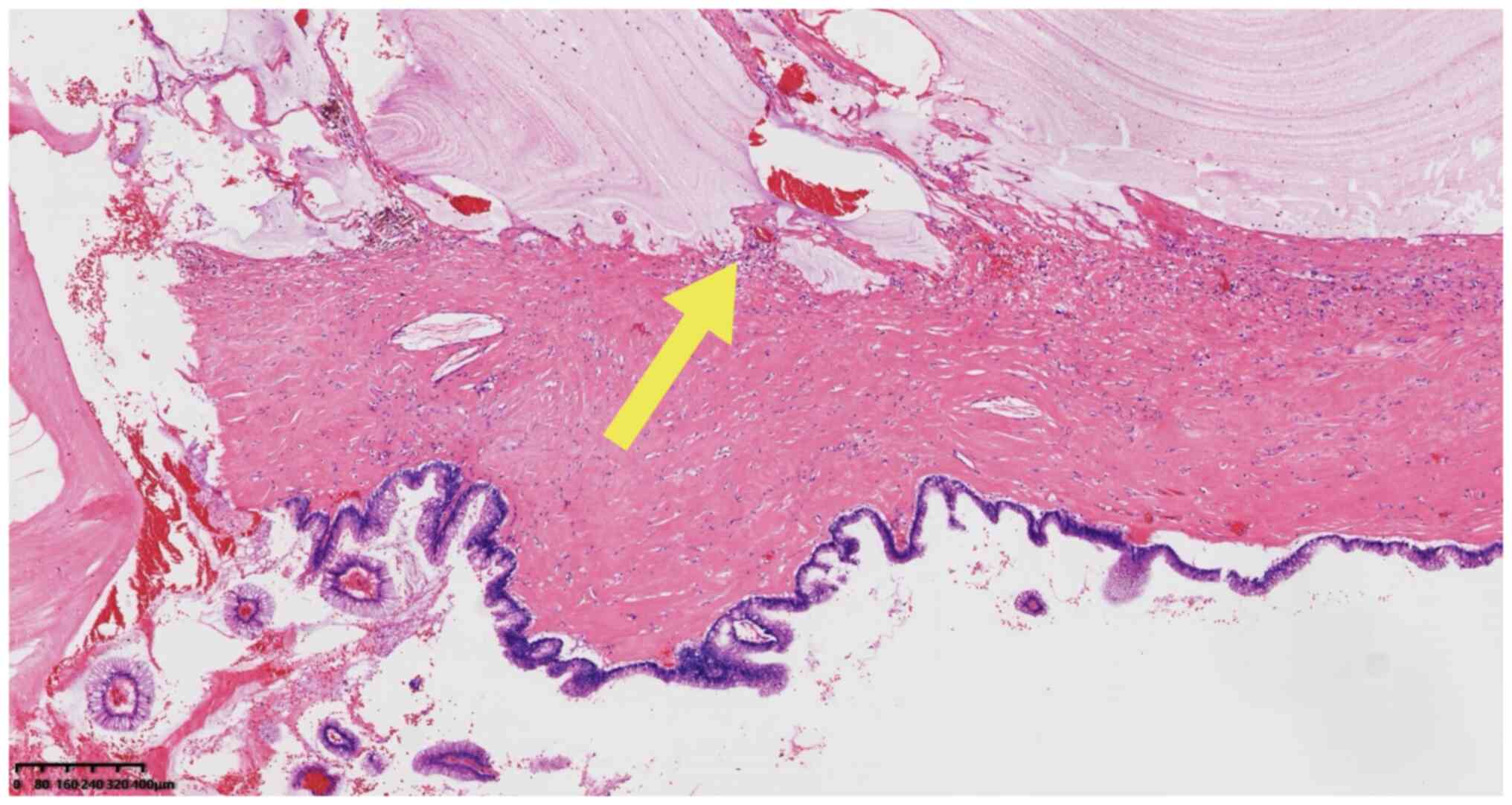
was not observed. A large mucous lake without a cell lining could
be observed in the interstitium (Fig.
4), composed of the surrounding fibrous tissue, with only a
small amount of mucous epithelium floating locally.
Immunohistochemical staining was performed using the
EnVision Systems method as follows: Unstained slides were heated at
60˚C for 120 min, followed by dewaxing in xylene I, II and III for
10 min per cylinder at room temperature. The slides were rehydrated
in 100% ethanol I, 100% ethanol II and 95% ethanol for 3 min each
and, 85 and 75% ethanol for 1 min each, then rinsed with distilled
water. The slides were placed in EDTA repair solution (1:50, pH
9.0, cat. no. MVS-0099 Fuzhou Maixin Biotechnology Development Co.,
Ltd.) at 100˚C for 20 min for antigen repair, washed with water
after natural cooling, treated with 3% hydrogen peroxide solution
for 10 min at room temperature and then rinsed with PBS.
Ready-to-use endogenous peroxidase blocker included in the DAB
Detection Kit (Polymer) (MaxVision DAB; cat. no. kit-0014; Fuzhou
Maixin Biotechnology Development Co., Ltd.) was used according to
the manufacturers protocol to block endogenous peroxidase activity.
Primary antibodies were added and incubated at room temperature for
40 min, then washed with PBS three times. Membranes were then
incubated with sheep anti-rat/rabbit IgG polymer horseradish
peroxidase labeled (cat. no. PV8000D; Beijing Zhongshan Jinqiao
Biotechnology Co., LTD.) secondary antibodies at room temperature
for 15 min, and then washed with PBS 3 times. DAB color developing
solution (Fuzhou Maixin Biotechnology Development Co., Ltd.) was
used to visualize the staining and the slides were counterstained
with hematoxylin for 1 min at room temperature. Slides were sealed
with neutral gum and observed under a light microscope. Primary
antibodies against cytokeratin (CK) 20 (1:200; cat. no. ZA-0574),
CDX2 (ready-to-use; cat. no. ZA-0520), special AT-rich
sequence-binding protein 2 (ready-to-use; cat. no. ZM-0163), GATA-3
(ready-to-use; cat. no. ZA-0661), Spalt like transcription factor 4
(ready-to-use; cat. no. ZM-0393), prostate-specific antigen
(ready-to-use; cat. no. ZM-0218), α-methylacyl-CoA racemase
(ready-to-use; cat. clone 13H4 no.ZA-0227) and Ki-67 (1:200; cat.
clone UMAB107 no. ZM-0166) were from OriGene Technologies, Inc. and
those against Villin (ready-to-use; cat. no. MAB-0540), β-catenin
(ready-to-use; cat. no. MAB-0754), p53 (1:100; cat. no. MAB-0674),
MLH1 (ready-to-use; cat. no. MAB-0789), MutS homolog 2
(ready-to-use; cat. no. MAB-0836), MutS Homolog 6 (ready-to-use;
cat. no. MAB-0831), PMS1 homolog 2 (ready-to-use; cat. no.
RMA-0775) and CK7 (ready-to-use; cat. no. KIT-0021) were from
Fuzhou Maixin Biotechnology Development Co., Ltd. The
immunohistochemical results revealed that cells were: Positive for
CK20 (Fig. S1), CDX2 (Fig. S2), Villin (Fig. S3), β-catenin (Fig. S4), wild-type p53 (Fig. S5), special AT-rich
sequence-binding protein 2 (Fig.
S6), DNA mismatch repair protein MLH1 (Fig. S7), MutS homolog 2 (Fig. S8), MutS Homolog 6 (Fig. S9) and PMS1 homolog 2, mismatch
repair system component (Fig.
S10); negative for CK7 (Fig.
S11), GATA-3 (Fig. S12),
Spalt like transcription factor 4 (Fig. S13), prostate-specific antigen
(Fig. S14) and α-methylacyl-CoA
racemase (Fig. S15); and
slightly positive for the proliferation marker Ki-67 (Fig. S16). The results were assessed
using a digital slice scanner (Ningbo Jiangfeng Biological
Information Technology Co., Ltd.).
On the basis of the location of the mucinous cyst in
the urachus region, cell dysplasia and non-invasion of stroma, the
pathological diagnosis was made as urachal mucinous cystic tumor of
low malignant potential with peritoneal pseudomyxoma. A one off
intraperitoneal infusion of 1,000 mg 5-fluorouracil was performed
after surgery. The patient recovered well and had no other
discomfort after an 8-month follow-up period and continues to
undergo a regular review every three months.
Discussion
Urachal-derived tumors are usually difficult to
detect due to their location and lack of characteristic clinical
manifestations; therefore, they are not commonly diagnosed and
treated in clinical settings. Histologically, the lumen of the
urachal duct is lined with the urothelium, and epithelial tumors
(such as adenoma, adenocarcinoma, non-adenocarcinoma and mixed
tumors) can occur through metaplasia, while malignant non-cystic
adenocarcinomas are more common (2). Mucous cystic tumors of the urachus
are rare. Consequently, there was a lack of a uniform
classification for this type of tumor until recently. In
particular, the distinction between non-invasive mucinous cystic
tumors with mild structural abnormalities, nuclear atypia and
benign or invasive lesions was ambiguous, and the names were
confusing, as evidenced from the multiple names used in the
literature, such as mucinous tumor of uncertain malignant potential
(5), urachal borderline mucinous
cystadenoma (6) and urachal
adenocarcinoma in situ (16). In
2016, the World Health Organization adopted the nomenclature
proposed by Paner et al (18) and classified mucinous cystic tumors
of the urachus into mucinous cystadenoma, invasive mucinous
cystadenocarcinoma and MCTLMP. MCTLMP is a borderline mucinous
cystic tumor that accounts for ~65% of urachal cystic tumors
(18). Generally, MCTLMP appears
as a mucus-rich multilocular cystic mass. Histologically, the cyst
wall is lined with a mucous columnar epithelium. The presence of
low-grade epithelial hyperplasia, including flat, tufted,
pseudopapillary, villous and tubular villous structures, is the
basis for diagnosis of low malignant potential (3). Cells demonstrating malignant features
without stromal infiltration are referred to as MCTLMP with
intraepithelial carcinoma (3). To
date, to the best of our knowledge, 40 cases of MCTLMP have been
reported in the literature, the majority of which are individual
cases. The present study reviewed all the reported cases in the
literature (including the present case), which consisted of 23 male
and 18 female patients. The age distribution ranged from 26-80
years, with a median age of 50 years. The majority of cases
occurred between the ages of 50 and 60 years. Tumor sizes ranged
from 0.8-15.5 cm, with a mean size of 5.9 cm. Because of its
location, MCTLMP often has no specific symptoms in its early
stages, but the following symptoms may appear with enlargement of
the tumor: i) Mucusuria, hematuria and frequent urination may occur
when it connects with the bladder; ii) repeated mucus extravasation
occurs when it connects with the umbilicus; and iii) if the tumor
is disconnected from the bladder, and omphalus mucus retention
increases gradually, resulting in an abdominal mass (8). Clinically, MCTLMP was mostly
identified by accident through medical examinations for other
disease without clear symptoms and then diagnosed after surgery (14
cases), followed by the clinical manifestations of abdominal pain
(10 cases) and umbilical mass presentation (7 cases). The rarer
clinical manifestations of all cases were hematuria (4 cases,
including 1case with microscopic hematuria), mucusuria (3 cases)
and umbilical mucus exudation (2 cases). Although these symptoms
can also appear in patients with invasive adenocarcinoma, it has
been reported that the latter more often manifests with hematuria
(4), which may be associated with
its invasive and destructive characteristics. It can be
hypothesized that histologically, in addition to areas similar to
those of mucinous cystadenomas, low-grade cellular or structural
changes occur in the epithelium of the cyst wall. Atypical columnar
epithelial hyperplasia usually contains one to three layers of
cells, ranging from flat to tufted, pseudopapillary and villous,
but without stromal infiltration.
Among the cases examined, 4cases were reported to be
accompanied by PMP (including the present case). Their
characteristics are described in Table
I. As the tumor ruptures during growth, mucus enters the
abdominal cavity and spreads widely to form PMP, as evidenced by
the present case. All 4 patients with MCTLMP complicated by PMP
were male, and 3 of them had abdominal pain as the first symptom,
experiencing tumors >8 cm in size. In addition, the levels of
the blood tumor markers CEA and CA125 were increased compared with
the aforementioned reference values. Regarding the association
between tumor markers and PMP, Agrawal et al (15) reported that ~42% of the mucinous
tumors in the urachus complicated by PMP had increased levels of
serum CEA and carbohydrate antigen 19-9 (CA19-9). In the present
case, the CA19-9 levels were normal, while increased level of tumor
markers was not indicated in the other 3 cases. Although serum CEA
and CA19-9 are not disease specific markers, they are still useful
and recommended for the initial diagnosis, evaluation of surgical
effects and postoperative follow-up (19-24).
The lack of characteristic symptoms and the slow clinical course of
the disease often lead to diagnosis of PMP in the locally advanced
stage (25,26). As observed in the present case, the
presence of a large amount of mucus in the abdominal cavity
enclosing the bowel, the presentation of a large amount of ascites
on the CT image and the increased levels of tumor markers, which
are common in malignant tumors, led both the radiologist and the
clinician to assume preoperatively that the lesion was a highly
aggressive adenocarcinoma. Even in rapid intraoperative
pathological examination, it is difficult to exclude mucinous
cystadenocarcinoma due to a certain degree of cell atypia and the
presence of a large amount of mucus, as well as the limited
sampling; therefore, the presence of visible PMP increases the
difficulty of diagnosis.
 | Table ICases of urachal mucinous tumors of
low malignant potential with PMP reported in the literature. |
Table I
Cases of urachal mucinous tumors of
low malignant potential with PMP reported in the literature.
| First author,
year | Age, years | Sex | Symptoms | Size, cm | Reported
diagnosis | PMP | Treatment | Tumor marker | Follow-up, months
(outcome) | (Refs.) |
|---|
| Stenhouse et
al, 2003 | 54 | Male | Abdominal pain | 14 | Urachal adenocarci
noma in situ | Yes | NA | NA | 6(A) | (16) |
| Shinohara et
al, 2006 | 54 | Male | Incidental finding
with left inguinal hernia | 9 | MCTLMP | Yes | Radical excision,
partial cystectomy, intraperitoneal lavage and
5'-deoxy-5-fluorouridine (1,200 mg/day) taken orally for 4
years. | Normal CEA and
CA19-9 | 84(A) | (17) |
| Agrawal et al,
2014 | 50 | Male | Intermittent lower
abdominal pain radiating to the back | 8 | Low-grade mucinous
neoplasm | Yes | Tumorectomy, partial
cystectomy and extended parietal peritonectomy | Normal CEA and
CA19-9 | NA | (15) |
| Present case | 74 | Male | Abdominal pain | 8 | MCTLMP | Yes | Tumorectomy,
partial cystectomy, extended parietal peritonectomy and peritoneal
irrigation with 5-fluorouracil | Elevated CEA and
CA125 | 8 (A) | NA |
The diagnosis of urachal MCTLMP is primarily based
on histopathological examination. Ultrasonography can be used as a
preliminary screening method but has low specificity (6). CT helps to provide accurate location,
size and presence or absence of infiltration, regardless of PMP;
however, because the morphology of the lining epithelium is not
visible, its value is limited in determining the nature of the
lesion (6,15). The present case was diagnosed as a
urachal cyst on CT scan two times before the onset of symptoms, and
was later suggested as a malignant tumor combined with PMP due to
the presence of ascites. Therefore, we hypothesize that a
combination of imaging and pathological morphology is conducive to
the correct diagnosis. In the pathological diagnosis of MCTLMP,
attention should be paid to differentiating it from mucinous
cystadenoma and mucinous cystadenocarcinoma. Mucinous cystadenomas
are usually lined with a single layer of mucous columnar epithelium
with no cell atypia. Mucinous cystadenocarcinoma cells are
atypically distinct, with small or obvious stromal infiltration
(3). Due to the difference in
prognosis, over-diagnosing low-grade MCTLMP as adenocarcinoma must
be carefully monitored. By contrast, areas of heterogeneous or
invasive cells may be focal, therefore extensive sampling and
careful examination of multilocular cystic tumors is warranted.
This is especially the case with intraepithelial carcinoma, where
further sampling may be needed to avoid missing the focus of
invasive carcinoma, since even a small number of invasive cancer
cells have a potential prognostic impact (4). In addition, since the morphology of
MCTLMP is similar to that of the more common low-grade mucinous
tumors of ovarian and appendiceal origin, attention should be paid
to identification, combined with imaging examination and
intraoperative exploration of the appendices, ovaries and other
organs. In particular, the possibility of the urachus as the origin
should not be ignored when combined with PMP. It has been reported
that the immunohistochemical markers CK7, CK20, CDX2, β-catenin,
estrogen receptor and progesterone receptor can be used to
distinguish primary and metastatic mucous cystic tumors, but their
specificity is not strong (4);
however, CK7 and β-catenin in combination has been suggested to be
helpful in distinguishing MCTLMP from colorectal tumors (18). In the present case report,
immunohistochemical analysis does not seem to add any diagnostic
value.
In terms of treatment, complete surgical excision of
MCTLMP has been suggested as the optimal treatment. Depending on
the size and location of the tumor, tumor resection, urachus
resection or urachus resection combined with partial cystectomy
should be performed to prevent tumor rupture and avoid
complications caused by mucus entering the abdominal cavity during
surgery (4,6,8). In
the present case, radical resection of the urachal tumor, adhesion
peritoneum and part of the bladder wall were performed. There is no
recommended optimal treatment plan for patients with PMP owing to
the small number of cases. As peritoneal spread and local invasion
are the main treatment problems, a number of studies suggest
routine peritoneal chemotherapy (13,25).
Previous studies have also revealed that the application of
intraperitoneal thermochemotherapy has important advantages in
treatment for PMP (14,19,27).
In early cases of PMP with low-grade malignant histology, a
previous study has recommended abandoning abdominal chemotherapy
(28). One patient with PMP
received 5-fluorouracil chemotherapy after surgery (17), as in the present case, and no
recurrence was observed. A total of 18 patients (including 3
patients with PMP) were followed-up for 1-84 months. No recurrence
or metastasis occurred after treatment, suggesting good prognosis
of MCTLMP. Due to the limitations of current studies, including
insufficient sample size, potential diagnostic biases and
inaccessible/limited data, the true incidence of MCTLMP combined
with PMP, the optimal treatment and the actual prognosis remain
unclear. Therefore, observation of further cases is required. The
follow-up of the present patient is ongoing.
In summary, the present study presented a rare case
of urachal MCTLMP that required histopathological confirmation.
MCTLMP can also cause PMP as in the present case. This finding
suggests that when PMP is present, it should be considered that the
PMP may originate from urachal MCTLMP. Treatment involves complete
surgical excision, and postoperative adjuvant perfusion
chemotherapy may be necessary for patients with PMP. Because MCTLMP
is rare, more cases must be examined to further reveal its clinical
characteristics and biological nature.
Supplementary Material
Tumor cells are positive for
cytokeratin 20. Magnification, x100; scale bar, 200 μm.
Tumor cells are positive for CDX2.
Magnification, x100; scale bar, 200 μm.
Tumor cells are positive for Villin.
Magnification, x40; scale bar, 625 μm.
Tumor cells are membrane positive for
β-catenin. Magnification, x100; scale bar, 200 μm.
Tumor cells are positive for wild type
p53. Magnification, x100; scale bar, 200 μm.
Tumor cells are positive for special
AT rich sequence binding protein 2. Magnification, x100; scale bar,
200 μm.
Tumor cells are positive for DNA
mismatch repair protein MLH1. Magnification, x100; scale bar, 200
μm.
Tumor cells are positive for MutS
homolog 2. Magnification, x100; scale bar, 200 μm.
Tumor cells are positive for MutS
Homolog 6. Magnification, x100; scale bar, 200 μm.
Tumor cells are positive for PMS1
homolog 2, mismatch repair system component. Magnification, x100;
scale bar, 200 μm.
Tumor cells are negative for
cytokeratin 7. Magnification, x40; scale bar, 625 μm.
Tumor cells are negative for GATA 3.
Magnification, x100; scale bar, 200 μm.
Tumor cells are negative for Spalt
like transcription factor 4. Magnification, x40; scale bar, 625
μm.
Tumor cells are negative for prostate
specific antigen. Magnification, x100; scale bar, 200
μm.
Tumor cells are negative for α
methylacyl CoA racemase. Magnification, x40; scale bar, 625
μm.
Tumor cells are positive for Ki 67.
Magnification, x100; scale bar, 200 μm.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LC and QF drafted the manuscript and analyzed
patient data. LS was in charge of the case data collection, imaging
of immunohistochemical staining and participated in data analysis.
QF and MD revised the manuscript and interpreted the data. LC and
QF confirm the authenticity of all the raw data. All authors agreed
to be accountable for all aspects of the work. All authors have
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided written informed consent for
the case study to be published.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nguyen M, Addicott B, Chu J, Parham D and
Kim E: Congenital cyst of the umbilical cord. Fetal Pediatr Pathol.
35:344–347. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Netto GJ, Amin MB, Berney DM, Compérat EM,
Gill AJ, Hartmann A, Menon S, Raspollini MR, Rubin MA, Srigley JR,
et al: The 2022 world health organization classification of tumors
of the urinary system and male genital organs-part B: Prostate and
urinary tract tumors. Eur Urol. 82:469–482. 2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Humphrey PA, Moch H, Cubilla AL, Ulbright
TM and Reuter VE: The 2016 WHO classification of tumours of the
urinary system and male genital organs-part B: Prostate and bladder
tumours. Eur Urol. 70:106–119. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Amin MB, Smith SC, Eble JN, Rao P, Choi
WW, Tamboli P and Young RH: Glandular neoplasms of the urachus: A
report of 55 cases emphasizing mucinous cystic tumors with proposed
classification. Am J Surg Pathol. 38:1033–1045. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wang D and Sule N: Mucinous cystadenoma of
the urachus and review of current classification of urachal
mucinous cystic neoplasms. Arch Pathol Lab Med. 143:258–263.
2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wu J, Liu A, Chen A and Zhang P: Urachal
borderline mucinous cystadenoma: A rare case report and literature
review. Medicine (Baltimore). 96(e8740)2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chen L, Wei N, Zhou G, Hou Y, Cheng J and
Lu C: Low malignant potential mucinous cystic tumor of urachus:Case
report. Chin J Med Imaging Technol. 37(1254)2021.(In Chinese).
|
|
8
|
Miao J, Shang P, Huang Y and Zhao X: Low
malignant potential mucinous cystic tumor of urachus-Case report.
Chin J Diffic and Compl Cas. 20:1263–1265. 2021.(In Chinese).
|
|
9
|
Brennan K, Johnson P, Curtis H and Arnason
T: Urachal mucinous cystic tumor of low malignant potential with
concurrent sigmoid colon adenocarcinoma. Case Rep Gastrointest Med.
2019(1434838)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Schmeusser B, Wiedemer J, Obery D, Buckley
K and Yu M: Urachal mucinous cystic tumor of low malignant
potential in a polymorbid female: A case report and review of the
literature. Int Cancer Conf J. 11:104–108. 2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
de Bree E, Witkamp A, Van De Vijver M and
Zoetmulde F: Unusual origins of Pseudomyxoma peritonei. J Surg
Oncol. 75:270–274. 2000.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Mukherjee A, Parvaiz A, Cecil TD and Moran
BJ: Pseudomyxoma peritonei usually originates from the appendix: A
review of the evidence. Eur J Gynaecol Oncol. 25:411–414.
2004.PubMed/NCBI
|
|
13
|
Carr NJ, Finch J, Ilesley IC,
Chandrakumaran K, Mohamed F, Mirnezami A, Cecil T and Moran B:
Pathology and prognosis in pseudomyxoma peritonei: A review of 274
cases. J Clin Pathol. 65:919–923. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yan TD, Sugarbaker PH and Brun EA:
Pseudomyxoma peritonei from mucinous adenocarcinoma of the urachus.
J Clin Oncol. 24:4944–4946. 2006.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Agrawal AK, Bobiński P, Grzebieniak Z,
Rudnicki J, Marek G, Kobielak P, Kazanowski M, Agrawal S and Hałoń
A: Pseudomyxoma peritonei originating from urachus-case report and
review of the literature. Curr Oncol. 21:e155–e165. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Stenhouse G, McRae D and Pollock AM:
Urachal adenocarcinoma in situ with pseudomyxoma peritonei: A case
report. J Clin Pathol. 56:152–153. 2003.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Shinohara T, Misawa K, Sano H, Okawa Y and
Takada A: Pseudomyxoma peritonei due to mucinous cystadenocarcinoma
in situ of the urachus presenting as an inguinal hernia. Int J Clin
Oncol. 11:416–419. 2006.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Paner GP, Lopez-Beltran A, Sirohi D and
Amin MB: Updates in the pathologic diagnosis and classification of
epithelial neoplasms of urachal origin. Adv Anat Pathol. 23:71–83.
2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sugarbaker PH, Verghese M, Yan TD and Brun
E: Management of mucinous urachal neoplasm presenting as
pseudomyxoma peritonei. Tumori. 94:732–736. 2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Gupta S, Singh G, Gupta A, Singh H, Arya
AK, Shrotriya D and Kumar A: Pseudomyxoma peritonei: An uncommon
tumor. Indian J Med Paediatr Oncol. 31:58–61. 2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Adams E, Sepich-Poore GD,
Miller-Montgomery S and Knight R: Using all our genomes:
Blood-based liquid biopsies for the early detection of cancer. View
(Beijing). 3(20200118)2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Cao J, Wang Y, Zhang Y and Qian K:
Emerging applications of mass spectrometry-based metabolic
fingerprinting in clinics. Adv Int Syst. 4(2100191)2022.
|
|
23
|
Li Y, Bao Q, Yang S, Yang M and Mao C:
Bionanoparticles in cancer imaging, diagnosis, and treatment. View.
3(20200027)2022.
|
|
24
|
Wang L, Zhang M, Pan X, Zhao M, Huang L,
Hu X, Wang X, Qiao L, Guo Q, Xu W, et al: Integrative serum
metabolic fingerprints based multi-modal platforms for lung
adenocarcinoma early detection and pulmonary nodule classification.
Adv Sci (Weinh). 9(e2203786)2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Martínez A, Ferron G, Mery E, Gladieff L,
Delord JP and Querleu D: Peritoneal pseudomyxoma arising from the
urachus. Surg Oncol. 21:1–5. 2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Bartoška P, Antoš F, Vítek P, Marx J,
Kopic J and Holečková P: Pseudomyxoma Peritonei. Klin Onkol.
32:329–332. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Mohamed F, Cecil T, Moran B and Sugarbaker
P: A new standard of care for the management of peritoneal surface
malignancy. Curr Oncol. 18:e84–e96. 2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Nozaki T, Yasuda K, Watanabe A and Fuse H:
Laparoscopic management of urachal mucinous borderline tumor
associated with pseudomyxoma peritonei. Surg Laparosc Endosc
Percutan Tech. 21:e152–e155. 2011.PubMed/NCBI View Article : Google Scholar
|