Introduction
Acute coronary syndromes (ACS) represent a spectrum
of events ranging from unstable angina (UA) to acute myocardial
infarction (AMI) with or without ST elevation, (1) which is the most common manifestation
of coronary artery disease (CAD) and the main cause of mortality
worldwide (2,3). The timely identification of AMI is
crucial for determining the prognosis of a patient. In contrast to
the relatively clear-cut diagnosis of AMI with ST segment elevation
through electrocardiogram (ECG) analysis, the identification of
patients with AMI without ST segment elevation from UA poses a
significant diagnostic dilemma (4). Clinical assessment and ECG alone are
insufficient for definitive confirmation or excluding the diagnosis
of AMI in most patients. Consequently, troponin continues to serve
as the fundamental element for promptly establishing a diagnosis
and facilitating appropriate treatment (5). However, a substantial percentage of
these patients will ultimately have a ‘normal’ invasive
angiography. Therefore, there is an urgent need for alternative
diagnostic strategies to avoid multiple unnecessary and invasive
examinations.
Coronary computed tomography angiography (CCTA) has
become a fast, accurate, reliable and noninvasive method for
assessing CAD in recent decades. Compared with invasive coronary
angiography (ICA), CCTA is highly accurate in assessing coronary
stenosis. However, the presence of obstructive CAD on CCTA does not
always result in myocardial ischemia. Numerous studies have
revealed that the degree of stenosis of lesions and their effects
on myocardial ischemia are often inconsistent (6,7). The
ROMICAT-I trial demonstrated that only 46% of patients with
obstructive CAD who were diagnosed via CCTA had abnormal single
photon emission computed tomography (SPECT) perfusion findings
during stress testing. Therefore, CCTA revealing >50% stenosis
has limited diagnostic value for ACS (8).
CT-derived fractional flow reserve (CT-FFR) is a
method that was developed for noninvasive calculation of the
hemodynamic consequences of stenosis. This can be explained in the
same manner as the invasive fractional flow reserve (FFR), which is
the gold standard clinical method for determining the functional
significance of coronary stenosis (9). CT-FFR has combined the advantages of
non-invasive CCTA and traditional invasive FFR. This processing
technology derives hemodynamic parameters from CCTA image data, in
order to quantify the hemodynamic impact of coronary artery
stenosis (10,11). A previous study revealed that
CT-FFR can detect the absence of hemodynamically significant
lesions in patients with high-risk ACS without ST segment elevation
who are admitted to the emergency department due to chest pain
(5). To date, the use of CT-FFR
for risk stratification in patients with ACS has not been evaluated
in any studies. Therefore, the present study aimed to assess the
ability of CT-FFR to identify patients with AMI and to develop a
comprehensive multiparameter AMI model with ‘one-stop’ CCTA.
Patients and methods
Study population
The present study involving human participants was
reviewed and approved by the Institutional Review Board of the
First Affiliated Hospital of Hebei North University (Zhangjiakou,
China). In view of the retrospective nature of the present study,
the local institutional review board waived the informed consent
requirements in accordance with the national legislation and
institutional requirements.
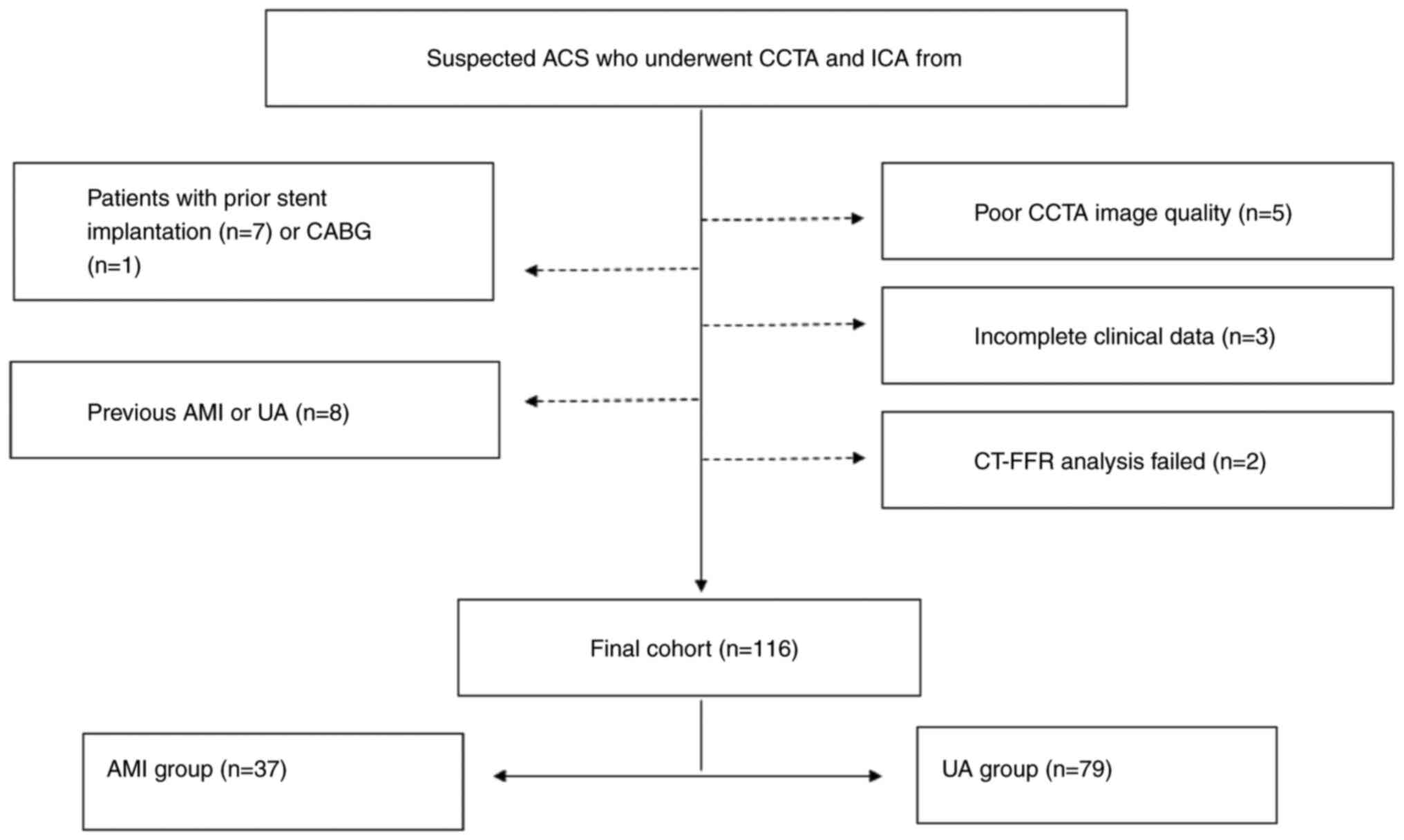
Patients admitted with suspected ACS who underwent
CCTA examinations followed by ICA at the First Affiliated Hospital
of Hebei North University (Zhangjiakou, China) from January 2019 to
July 2020 were included in the present study. ICA is widely
recognized as the gold standard in imaging for CAD. A total of 116
participants were finally enrolled in this study. The AMI group
comprised 37 patients (27 males; 10 females), with an average age
of 62.06±7.74 years, whereas the UA group comprised 79 patients (59
males; 20 females), with an average age of 58.11±10.0 years.
Adjudication of AMI and UA was performed by a panel
of two cardiologists. AMI was defined as an increase and/or
decrease in cardiac troponin (cTnI) levels with at least 1 value
above the 99th percentile and more than one of the following
clinical evidence criteria: i) Symptoms of acute myocardial
ischemia (e.g., chest pain or dyspnea); ii) new ischemic ECG
changes; iii) development of pathological Q waves; iv) imaging
evidence of new loss of viable myocardium or new regional wall
motion abnormality in a pattern consistent with an ischemic
etiology; and v) identification of a coronary thrombus by
angiography including intracoronary imaging or by autopsy (12). UA was described as a symptom of
myocardial ischemia, and ischemia-related ECG abnormalities were
identified at rest or with minimal exertion without cardiomyocyte
necrosis (13). Patients with UA
had no abnormalities in their myocardial enzymes until they were
discharged. In both groups, fasting blood samples were collected
within 24 h of admission, followed by CCTA examinations which were
performed within 3 days. cTnI was verified at admission and
rechecked at 2-h intervals if negative. cTnI levels with at least
one value above the 99th percentile were considered positive.
The exclusion criteria were as follows: Percutaneous
coronary intervention or coronary artery bypass graft (CABG) prior
to CCTA; previous AMI or UA; patients who directly underwent
invasive angiography without CCTA examinations; incomplete clinical
data; poor CCTA image quality; and failed CT-FFR analyses.
Based on medical records, the risk factors and
baseline characteristics of patients were determined. The present
study design and method for patient selection are described in
Fig. 1.
CCTA acquisition
In the present study, the CCTA procedure was
performed using Aquilion One 320-row volume CT (Canon Medical
Systems Corporation). The ECG data of each patient was continuously
monitored throughout the process. Patients with heart rate values
of 75 beats/min or greater before scanning were orally administered
20-60 mg of metoprolol tartrate tablets at 1 h prior to the
scanning. All of the patients were injected with 0.8 ml/kg isotonic
contrast agent (iodixanol, 320 mg iodine per ml; Yangtze River
Pharmaceutical Group) at a flow rate of 5.0 ml/sec with a dual-shot
injector (OptiVantage DH; Mallinckrodt Tyco Healthcare). The tube
current was determined by using automatic exposure control on the
basis of X-ray attenuation on anterior-posterior and lateral scout
images and the reconstruction kernel. By default, the tube voltage
was set at 100 kVp and was manually increased to 120 kVp when the
maximum automatic current was reached. With a rotation time of 350
msec and a z-coverage value of 140-160 mm, the scan range included
the whole heart. In addition, the scan plan of low-dose
retrospective ECG-gated technology was performed.
Two doctors with expertise in cardiovascular imaging
diagnoses who were blinded to the information of the participants
assessed the data to assure objectivity. In cases of disagreement,
the third senior chief physician made the final evaluation. The
degree of luminal stenosis was visually estimated by using a
vascular diameter percentage. The degree of stenosis was noted as
follows: 1-24%, minimal; 25-49%, mild; 50-69%, moderate; 70-99%,
severe; and 100%, total obstruction.
The CT-FFR values were calculated from diastolic
CCTA images based on the online DEEPVESSEL-FFR platform by applying
the deep learning technique (Keya Medical). Coronary stenosis was
deemed to be hemodynamically significant if CT-FFR was ≤0.80, which
was similar to invasive FFR results (14).
The following criteria were used for determination
of a culprit vessel. i) A single significant stenosis that was
treated by ICA was identified as the culprit vessel. ii) The
revascularization treatment vessel was the culprit vessel if
multiple vessels had ≥50% luminal stenosis on the ICA. iii) The
culprit lesions were located based on ECG findings, aberrant wall
motion on echocardiography, or angiographic appearance during ICA,
as previously documented (15).
Statistical analysis
The SPSS software program (version 25.0; SPSS, Inc.)
and MedCalc for Windows (version 20.113; MedCalc Software) were
used for all of the statistical analyses, with P <0 .05,
considered to indicate a statistically significant difference.
Continuous data are expressed as the mean ± standard deviation. To
compare the baseline characteristics, CCTA stenosis, and CT-FFR
between the AMI group and UA group, an independent-samples unpaired
Student's t-test was employed for continuous variables, while a
Fisher's exact test was utilized for categorical variables.
Univariate and multivariate logistic regressions were used to
analyze the independent influencing factors of AMI, and the degree
of association was expressed by odds ratios (ORs) and 95%
confidence intervals (95% CIs). Three diagnostic models of AMI were
established: Model 1, CCTA stenosis; model 2, CT-FFR; and model 3,
CCTA stenosis combined with CT-FFR. The effectiveness of the three
models for differentiating between AMI and UA was assessed by using
the receiver operating characteristic (ROC) curve and
Hosmer-Lemeshow goodness-of-fit test. The difference in the area
under the ROC curve (AUC) of the three models was compared via the
DeLong method (16).
Results
Patient characteristics
A total of 116 patients were finally included in the
analysis. There were 37 cases in the AMI group (27 males and 10
females), with an average age of 62.06±7.74 years. There were 79
cases in the UA group (59 males and 20 females), with an average
age of 58.11±10.0 years. There was no significant difference in age
or sex (P>0.05). Moreover, there was no significant difference
in smoking history, hypertension, diabetes history, hyperlipidemia
history or CAD family history between the AMI group and the UA
group (P>0.05). There was no significant difference in chest
tightness, difficulty breathing, or chest pain between the AMI
group and the UA group (P>0.05). In the AMI group, more patients
exhibited myocardial ischemia on the ECG than in the UA group, and
the difference was statistically significant (P<0.001). The
number of patients undergoing percutaneous coronary intervention
was greater in the AMI group than in the UA group, and the
difference was statistically significant (P<0.001). Patient
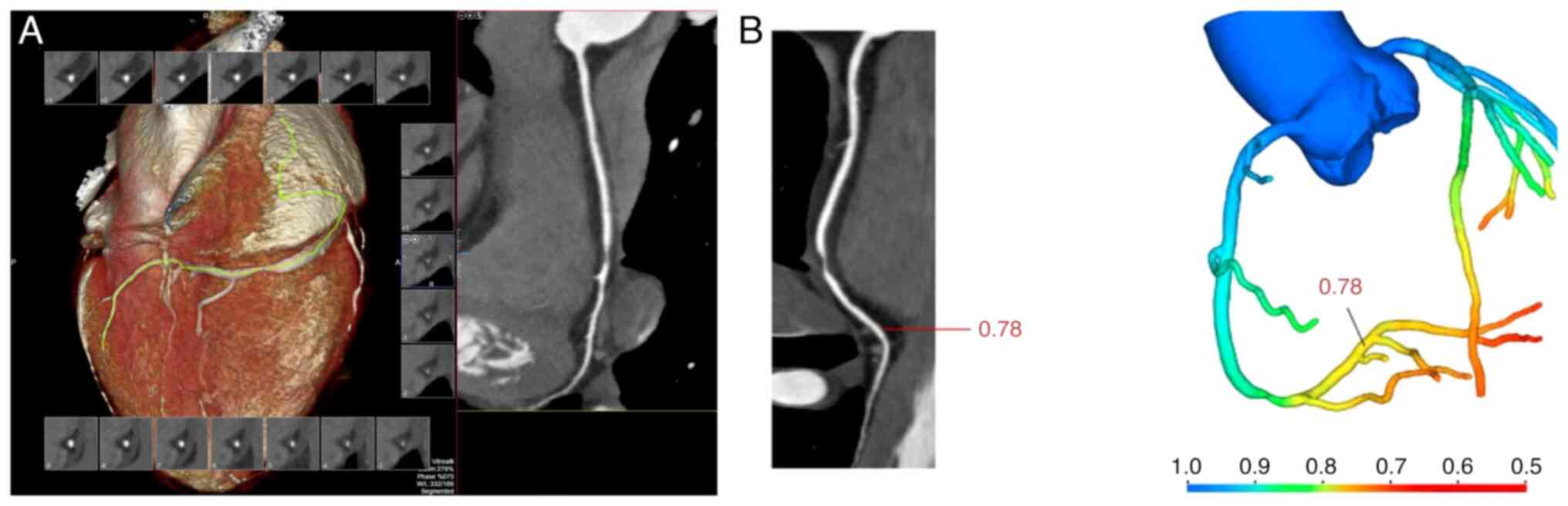
demographics and baseline characteristics are presented in Table I. The CCTA and CT-FFR images of a
67-year-old male patient are shown in Fig. 2. The CCTA revealed a hypodense
plaque with severe luminal stenosis at the second turn of the right
coronary artery (RCA). The CT-FFR value measured at the distal end
of the plaque was 0.78.
 | Table IBaseline characteristics and clinical
details of the study population. |
Table I
Baseline characteristics and clinical
details of the study population.
| Parameters | AMI group (n=37) | UA group (n=79) |
t/χ2 | P-value |
|---|
| Male | 27 (72.97) | 59 (74.68) | 0.038 | 0.845 |
| Age, years | 58.65±9.53 | 61.75±7.42 | 1.909 | 0.059 |
| Smoking | 20 (54.05) | 42 (53.16) | 0.008 | 0.929 |
| Diabetes | 11 (29.73) | 24 (30.38) | 0.005 | 0.943 |
| Hypertension | 20 (54.05) | 43 (54.43) | 0.001 | 0.97 |
| Hyperlipidemia | 9 (24.32) | 15 (18.99) | 0.437 | 0.508 |
| CAD family | 3 (8.11) | 10 (12.66) | 0.167 | 0.683 |
| ECG suggests
myocardial ischemia | 15 (40.54) | 5 (6.33) | 20.669 | <0.001 |
| Chest
tightness/difficulty breathing | 13 (35.14) | 42 (53.16) | 3.285 | 0.070 |
| Chest pain | 22 (59.46) | 32 (40.51) | 3.638 | 0.056 |
| Percutaneous coronary
intervention | 26 (70.27) | 26 (32.91) | 14.220 | <0.001 |
Comparison of CCTA stenosis and CT-FFR
between the AMI group and UA group
There were 34 patients (34/37) with ≥70% CCTA
stenosis in the AMI group and 21 patients (21/79) in the UA group.
Statistical analyses demonstrated a disparity between the two
groups (χ2=43.107; P<0.001) (data not shown).
The overall CT-FFR value of the AMI group was
0.713±0.079, whereas for the UA group it was 0.833±0.061. There was
a statistically significant difference between the two groups
(t=8.925; P<0.001) (data not shown).
Correlations between ≥70% CCTA
stenosis, ≤0.80 CT-FFR and AMI
The univariate logistic regression analysis showed
that ≥70% CCTA stenosis and ≤0.80 CT-FFR affected the diagnosis of
AMI. Among these factors, the effect of ≤0.80 CT-FFR was the most
significant (OR=4.156; P<0.001). When considering all of these
factors, the multivariate logistic regression analysis found that
≥70% CCTA stenosis and ≤0.80 CT-FFR were independent predictors of
AMI (Table II).
 | Table IIUnivariate and multivariable logistic
regression analyses of clinical characteristics, ≥70% CCTA stenosis
and ≤0.80 CT-FFR for AMI. |
Table II
Univariate and multivariable logistic
regression analyses of clinical characteristics, ≥70% CCTA stenosis
and ≤0.80 CT-FFR for AMI.
| | Univariate logistic
regression | Multivariable
logistic regression |
|---|
| Parameters | OR (95% CI) | P-value | OR (95% CI) | P-value |
|---|
| CCTA stenosis,
≥70% | 31.302 (8.689,
112.767) | <0.001 | 10.796 (2.566,
45.425) | 0.001 |
| CT-FFR, ≤0.80 | 4.156 (13.922,
292.585) | <0.001 | 28.074 (5.712,
137.973) | <0.001 |
| Sex | 0.915 (0.378,
2.218) | 0.915 | N/A | N/A |
| Age | 0.956 (0.911,
1.002) | 0.062 | N/A | N/A |
| Smoking | 1.036 (0.474,
2.268) | 0.929 | N/A | N/A |
| Hypertension | 0.985 (0.450,
2.156) | 0.985 | N/A | N/A |
| Diabetes | 0.970 (0.413,
2.274) | 0.970 | N/A | N/A |
| Hyperlipidemia | 1.371 (0.537,
3.504) | 0.509 | N/A | N/A |
Comparison of the diagnostic efficacy
of ≥70% CCTA stenosis, ≤0.80 CT-FFR and their combined application
in AMI
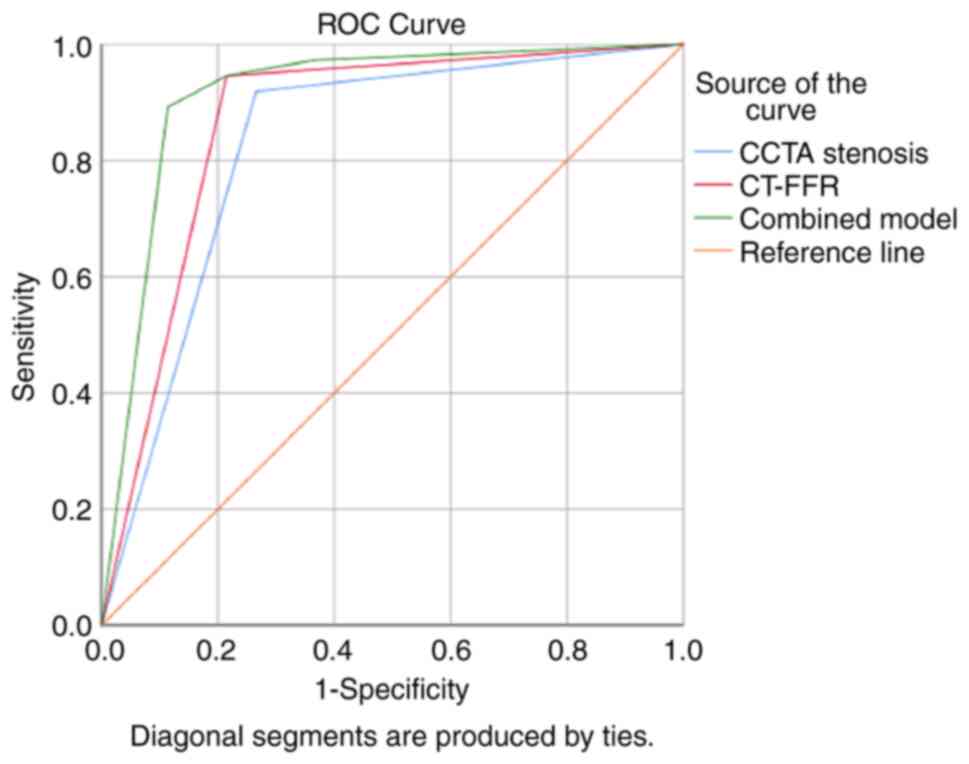
The discrimination performance of each prediction
model was demonstrated by the ROC curves (Fig. 3). The Hosmer-Lemeshow test showed
that the model fit was good (P>0.05). Additionally, the combined
application of ≥70% CCTA stenosis and ≤0.80 CT-FFR had a
significantly higher diagnostic performance for AMI than either
factor alone (P<0.001), which was mainly due to the improvement
of specificity (Table III). The
pairwise comparison revealed that the AUC of the combined
application model of ≥70% CCTA stenosis and ≤0.80 CT-FFR was the
highest (AUC=0.914; 95% CI: 0.847-0.958), which was greater than
that of the ≥70% CCTA stenosis model (AUC=0.827; 95% CI:
0.745-0.891; P=0.0008) and the ≤0.80 CT-FFR model (AUC=0.865; 95%
CI: 0.790-0.922; P=0.0060). Moreover, there was no significant
difference between the ≥70% CCTA stenosis model and the ≤0.80
CT-FFR model (P=0.2926) (data not shown).
 | Table IIIDiagnostic efficacy of ≥70% CCTA
stenosis, ≤0.80 CT-FFR and their combined model for AMI. |
Table III
Diagnostic efficacy of ≥70% CCTA
stenosis, ≤0.80 CT-FFR and their combined model for AMI.
| Model | AUC | Sensitivity, % | Specificity, % | P-value |
|---|
| CCTA stenosis,
≥70% | 0.827 | 91.89 | 73.42 | <0.001 |
| CT-FFR, ≤0.80 | 0.865 | 94.59 | 78.48 | <0.001 |
| CCTA stenosis +
CT-FFR | 0.914 | 89.19 | 88.61 | <0.001 |
Discussion
The present brief study developed the diagnostic
utility of CCTA stenosis and hemodynamic CT-FFR for AMI and
explored a corresponding combination model. According to the
findings, significant risk factors for AMI included a CT-FFR of
0.80 and a CCTA stenosis of 70%. A reliable diagnostic model for
AMI with independent risk factors for ≥70% CCTA stenosis (OR:
10.796; P=0.001) and ≤0.80 CT-FFR (OR: 28.074; P<0.001) could be
achieved by using a combined model, which increased the diagnostic
efficacy (AUC=0.914; P<0.001) of single parameters.
The present study found that ≥70% CCTA stenosis was
an independent predictor of AMI. Multiple relevant studies have
demonstrated that the utilization of CCTA can effectively rule out
AMI in patients presenting with suspected ACS in the emergency
department. These studies have consistently shown that normal CCTA
findings possess a remarkably high negative predictive value in
excluding AMI during the initial hospitalization (17-19).
However, this strategy is being challenged by the increasing
recognition of the limitations of coronary stenosis severity in
recent years. A threshold of ≥50% exhibits limited sensitivity in
identifying patients and lesions that will ultimately lead to AMI
(20). Moreover, despite the
utilization of quantitative methodologies, the efficacy of coronary
stenosis severity in accurately detecting lesion-specific ischemia
has not been significantly enhanced (21). More importantly, in addition to
coronary stenosis severity, numerous other factors may collectively
influence flow dynamics in the vessel. Thus, it has become
necessary to identify additional or improved markers to aid in the
risk assessment of AMI.
In addition to coronary stenosis, CCTA can also
obtain plaque characteristics, pericoronary adipose tissue (PCAT),
and various parameters of hemodynamics, which may also affect the
diagnosis and prognosis of patients; however, further research on
this aspect is required. The SCOT-HEART study showed that
low-attenuation plaque burden was the most potent predictor of AMI
[adjusted hazard ratio: 1.60 (95% CI: 1.10-2.34) per doubling;
P=0.014], regardless of coronary artery calcium score, coronary
artery area stenosis, or cardiovascular risk score (22). These results elicit doubts about
the dominance of the traditional risk factors for myocardial
infarction, such as the degree of coronary stenosis. A recent study
reported that the fat attenuation index (FAI) could not distinguish
patients with AMI from patients with UA. In addition, the
CCTA-based radiomics phenotype of PCAT performed better than the
FAI model in differentiating AMI from UA. The combined model of
PCAT radiomics and FAI can improve the effectiveness of AMI
identification (23). Therefore,
the radiomic characteristics of PCAT may enhance the diagnostic
utility of AMI (23,24). However, the hemodynamic parameter
of CT-FFR was not used in the aforementioned studies.
CT-FFR utilizes computational fluid dynamics or
machine learning to derive noninvasive FFR and assesses the
hemodynamic importance of coronary artery stenosis in a noninvasive
manner (25,26). Previous research has confirmed that
noninvasive CT-FFR is a feasible alternative to invasive FFR for
detecting and excluding ischemic coronary artery lesions (27). In multiple clinical studies, CT-FFR
has demonstrated high diagnostic accuracy for myocardial ischemia
caused by coronary stenosis (28,29).
However, there are few reports on the application of CT-FFR for
evaluating AMI. The present study demonstrated that the CT-FFR
value of the AMI group was lower than that of the UA group
(P<0.001), and ≤0.80 CT-FFR was an independent predictor of AMI.
As in the study by Meier et al (5), patients with high-risk ACS without ST
segment elevation (NSTE-ACS) could be noninvasively identified by
CCTA and CT-FFR, avoiding the need for coronary angiography and
thereby reducing surgery-related risks and medical costs.
Furthermore, Arena et al (30) assessed a combined strategy of FFR
and angiography in stratifying cardiovascular risk in patients with
type 1 myocardial infarction (T1MI) or T2MI non-ST elevation acute
myocardial infarction, and they found that the combined strategy
allowed the treatment of nonfunctional significant lesions to be
safely deferred and patient cardiovascular risk to be identified.
Therefore, it was deduced that CT-FFR is valuable in predicting
risk stratification of patients with ACS.
However, the clinical value of the combined
application of CCTA stenosis and CT-FFR in the diagnosis of AMI is
not very clear. The results of the present study revealed that the
CT-FFR of the AMI group was lower than that of the UA group, and
its AUC for diagnosing AMI was 0.865. Additionally, the AUC for
diagnosing AMI via CCTA stenosis was 0.827. In the present study,
CT-FFR and CCTA stenosis were combined to evaluate their diagnostic
efficacy for AMI, and it was found that the combined model of
CT-FFR and CCTA stenosis was superior to the CT-FFR model and CCTA
stenosis model, as well as the fact that the AUC increased to
0.914, suggesting that combining anatomical, morphological, and
functional data may improve its ability to diagnose AMI, guide the
diagnosis and treatment strategy of patients with suspected or
confirmed CAD and reduce unnecessary invasive examinations.
Compared to using CT-based anatomical evaluation, adding CT-FFR
further improves the model performance for identifying patients
with AMI. It consequently helps to guide an appropriate therapeutic
strategy and reduce unfavorable outcomes.
There were certain limitations to the present study.
First, it was a single-center, retrospective case-control study
with a small positive sample size, which may have resulted in a
selection bias; therefore, studies with larger sample sizes are
needed in the future. Second, due to the fact that this study
investigated a cohort planning ICA, the incidence rate of CAD in
this population was high. To validate the findings of the present
study, it is necessary to conduct prospective studies in a larger
study cohort. Finally, there was no invasive FFR as a control, but
it has been widely confirmed that CT-FFR has a good correlation
with FFR (31).
In short, the present study revealed that ≤0.80
CT-FFR and ≥70% CCTA stenosis are independent risk factors for AMI,
and that the combined model of CT-FFR and CCTA stenosis could
further improve the performance of AMI identification, which may
guide the diagnosis and treatment strategy of patients with
suspected or confirmed CAD and reduce unnecessary invasive
examinations.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Project of Hebei
Medical Science Research (grant no. 20210342) and the Project of
Zhangjiakou Science and Technology Bureau of Hebei Province (grant
no. 2021030D).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FY and DW contributed to the conception and design
of the study. YY, ZY, ZP, PJ and YW conducted the data collection
and the statistical analysis. FY wrote the first draft of the
manuscript. SC revised the manuscript, managed the project,
coordinated the study and gave final approval of the version to be
published. All of the authors contributed to manuscript revision,
and read and approved the final version of the manuscript. FY and
DW confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The present study involving human participants was
reviewed and approved by the Institutional Review Board of the
First Affiliated Hospital of Hebei North University (approval no.
k2020274; Zhangjiakou, China). In view of the retrospective nature
of the present study, the local institutional review board waived
the informed consent requirements in accordance with the national
legislation and institutional requirements.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dudas K, Björck L, Jernberg T, Lappas G,
Wallentin L and Rosengren A: Differences between acute myocardial
infarction and unstable angina: A longitudinal cohort study
reporting findings from the register of information and knowledge
about swedish heart intensive care admissions (RIKS-HIA). BMJ Open.
3(e002155)2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Benjamin EJ, Virani SS, Callaway CW,
Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling
FN, Deo R, et al: Heart disease and stroke statistics-2018 update:
A Report from the american heart association. Circulation.
137:e67–e492. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Koskinas KC, Ughi GJ, Windecker S, Tearney
GJ and Räber L: Intracoronary imaging of coronary atherosclerosis:
Validation for diagnosis, prognosis and treatment. Eur Heart J.
37(524-535a-c)2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Grech ED and Ramsdale DR: Acute coronary
syndrome: Unstable angina and non-ST segment elevation myocardial
infarction. BMJ. 326:1259–1261. 2003.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Meier D, Skalidis I, De Bruyne B, Qanadli
SD, Rotzinger D, Eeckhout E, Collet C, Muller O and Fournier S:
Ability of FFR-CT to detect the absence of hemodynamically
significant lesions in patients with high-risk NSTE-ACS admitted in
the emergency department with chest pain, study design and
rationale. Int J Cardiol Heart Vasc. 27(100496)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ahmadi A, Stone GW, Leipsic J, Serruys PW,
Shaw L, Hecht H, Wong G, Nørgaard BL, O'Gara PT, Chandrashekhar Y
and Narula J: Association of coronary stenosis and plaque
morphology with fractional flow reserve and outcomes. JAMA Cardiol.
1:350–357. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ciccarelli G, Barbato E, Toth GG, Gahl B,
Xaplanteris P, Fournier S, Milkas A, Bartunek J, Vanderheyden M,
Pijls N, et al: Angiography versus hemodynamics to predict the
natural history of coronary stenoses: fractional flow reserve
versus angiography in multivessel evaluation 2 substudy.
Circulation. 137:1475–1485. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ahmed W, Schlett CL, Uthamalingam S,
Truong QA, Koenig W, Rogers IS, Blankstein R, Nagurney JT, Tawakol
A, Januzzi JL and Hoffmann U: Single resting hsTnT level predicts
abnormal myocardial stress test in acute chest pain patients with
normal initial standard troponin. JACC Cardiovasc Imaging. 6:72–82.
2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Raza S and Efstathiou M: CT coronary
angiogram with FFR CT-A revolution in the diagnostic flow of
coronary artery disease. J Ayub Med Coll Abbottabad. 33:376–381.
2021.PubMed/NCBI
|
|
10
|
De Bruyne B, Fearon WF, Pijls NH, Barbato
E, Tonino P, Piroth Z, Jagic N, Mobius-Winckler S, Rioufol G, Witt
N, et al: Fractional flow reserve-guided PCI for stable coronary
artery disease. N Engl J Med. 371:1208–1217. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Fairbairn TA, Nieman K, Akasaka T,
Nørgaard BL, Berman DS, Raff G, Hurwitz-Koweek LM, Pontone G,
Kawasaki T, Sand NP, et al: Real-world clinical utility and impact
on clinical decision-making of coronary computed tomography
angiography-derived fractional flow reserve: Lessons from the
ADVANCE registry. Eur Heart J. 39:3701–3711. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Thygesen K, Alpert JS, Jaffe AS, Chaitman
BR, Bax JJ, Morrow DA and White HD: Executive Group on behalf of
the Joint European Society of Cardiology (ESC)/American College of
Cardiology (ACC)/American Heart Association (AHA)/World Heart
Federation (WHF) Task Force for the Universal Definition of
Myocardial Infarction. Fourth universal definition of myocardial
infarction (2018). J Am Coll Cardiol. 72:2231–2264. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Collet JP, Thiele H, Barbato E, Barthélémy
O, Bauersachs J, Bhatt DL, Dendale P, Dorobantu M, Edvardsen T,
Folliguet T, et al: 2020 ESC guidelines for the management of acute
coronary syndromes in patients presenting without persistent
ST-segment elevation. Eur Heart J. 42:1289–1367. 2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Matsumura-Nakano Y, Kawaji T, Shiomi H,
Kawai-Miyake K, Kataoka M, Koizumi K, Matsuda A, Kitano K, Yoshida
M, Watanabe H, et al: Optimal cutoff value of fractional flow
reserve derived from coronary computed tomography angiography for
predicting hemodynamically significant coronary artery disease.
Circ Cardiovasc Imaging. 12(e008905)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Dey D, Achenbach S, Schuhbaeck A,
Pflederer T, Nakazato R, Slomka PJ, Berman DS and Marwan M:
Comparison of quantitative atherosclerotic plaque burden from
coronary CT angiography in patients with first acute coronary
syndrome and stable coronary artery disease. J Cardiovasc Comput
Tomogr. 8:368–374. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
DeLong ER, DeLong DM and Clarke-Pearson
DL: Comparing the areas under two or more correlated receiver
operating characteristic curves: A nonparametric approach.
Biometrics. 44:837–845. 1988.PubMed/NCBI
|
|
17
|
Hoffmann U, Bamberg F, Chae CU, Nichols
JH, Rogers IS, Seneviratne SK, Truong QA, Cury RC, Abbara S,
Shapiro MD, et al: Coronary computed tomography angiography for
early triage of patients with acute chest pain: The ROMICAT (rule
out myocardial infarction using computer assisted tomography)
trial. J Am Coll Cardiol. 53:1642–1650. 2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hollander JE, Chang AM, Shofer FS, Collin
MJ, Walsh KM, McCusker CM, Baxt WG and Litt HI: One-year outcomes
following coronary computerized tomographic angiography for
evaluation of emergency department patients with potential acute
coronary syndrome. Acad Emerg Med. 16:693–698. 2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Rubinshtein R, Halon DA, Gaspar T, Jaffe
R, Karkabi B, Flugelman MY, Kogan A, Shapira R, Peled N and Lewis
BS: Usefulness of 64-slice cardiac computed tomographic angiography
for diagnosing acute coronary syndromes and predicting clinical
outcome in emergency department patients with chest pain of
uncertain origin. Circulation. 115:1762–1768. 2007.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chang HJ, Lin FY, Lee SE, Andreini D, Bax
J, Cademartiri F, Chinnaiyan K, Chow BJW, Conte E, Cury RC, et al:
Coronary atherosclerotic precursors of acute coronary syndromes. J
Am Coll Cardiol. 71:2511–2522. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Tonino PAL, De Bruyne B, Pijls NH, Siebert
U, Ikeno F, van 't Veer M, Klauss V, Manoharan G, Engstrøm T,
Oldroyd KG, et al: Fractional flow reserve versus angiography for
guiding percutaneous coronary intervention. N Engl J Med.
360:213–224. 2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Williams MC, Kwiecinski J, Doris M,
McElhinney P, D'Souza MS, Cadet S, Adamson PD, Moss AJ, Alam S,
Hunter A, et al: Low-attenuation noncalcified plaque on coronary
computed tomography angiography predicts myocardial infarction:
Results from the multicenter SCOT-HEART trial (scottish computed
tomography of the HEART). Circulation. 141:1452–1462.
2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Si N, Shi K, Li N, Dong X, Zhu C, Guo Y,
Hu J, Cui J, Yang F and Zhang T: Identification of patients with
acute myocardial infarction based on coronary CT angiography: The
value of pericoronary adipose tissue radiomics. Eur Radiol.
32:6868–6877. 2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lin A, Kolossváry M, Yuvaraj J, Cadet S,
McElhinney PA, Jiang C, Nerlekar N, Nicholls SJ, Slomka PJ,
Maurovich-Horvat P, et al: Myocardial infarction associates with a
distinct pericoronary adipose tissue radiomic phenotype: A
prospective case-control study. JACC Cardiovasc Imaging.
13:2371–2383. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Benton SM Jr, Tesche C, De Cecco CN,
Duguay TM, Schoepf UJ and Bayer RR II: Noninvasive derivation of
fractional flow reserve from coronary computed tomographic
angiography: A review. J Thorac Imaging. 33:88–96. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Tang CX, Wang YN, Zhou F, Schoepf UJ,
Assen MV, Stroud RE, Li JH, Zhang XL, Lu MJ, Zhou CS, et al:
Diagnostic performance of fractional flow reserve derived from
coronary CT angiography for detection of lesion-specific ischemia:
A multi-center study and meta-analysis. Eur J Radiol. 116:90–97.
2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhuang B, Wang S, Zhao S and Lu M:
Computed tomography angiography-derived fractional flow reserve
(CT-FFR) for the detection of myocardial ischemia with invasive
fractional flow reserve as reference: Systematic review and
meta-analysis. Eur Radiol. 30:712–725. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Liu X, Wang Y, Zhang H, Yin Y, Cao K, Gao
Z, Liu H, Hau WK, Gao L, Chen Y, et al: Evaluation of fractional
flow reserve in patients with stable angina: Can CT compete with
angiography? Eur Radiol. 29:3669–3677. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Liu X, Mo X, Zhang H, Yang G, Shi C and
Hau WK: A 2-year investigation of the impact of the computed
tomography-derived fractional flow reserve calculated using a deep
learning algorithm on routine decision-making for coronary artery
disease management. Eur Radiol. 31:7039–7046. 2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Arena M, Caretta G, Gistri R, Tonelli G,
Scardigli V, Rezzaghi M, Ragazzini A and Menozzi A: Fractional flow
reserve in patients with type 1 or type 2 non-ST elevation acute
myocardial infarction. J Cardiovasc Med (Hagerstown). 23:119–126.
2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Tesche C, De Cecco CN, Albrecht MH, Duguay
TM, Bayer RR II, Litwin SE, Steinberg DH and Schoepf UJ: Coronary
CT angiography-derived fractional flow reserve. Radiology.
285:17–33. 2017.PubMed/NCBI View Article : Google Scholar
|